First report of Pseudocercospora muntingiae on Muntingia calabura in Brazil
D. F. Parreira A B , P. A. Ferreira A , H. S. S. Duarte A , A. S. Capucho A and L. Zambolim AA Universidade Federal de Viçosa, Av. P.H. Rolfs, s/n°, Departamento de Fitopatologia, Campus Universitário, CEP 36570-000 Viçosa, MG, Brazil.
B Corresponding author. Email: douglas.parreira@ufv.br
Australasian Plant Disease Notes 4(1) 110-113 https://doi.org/10.1071/DN09046
Submitted: 6 October 2009 Accepted: 19 October 2009 Published: 4 November 2009
Abstract
Pseudocercospora muntingiae is recorded as the cause of leaf spots on Muntingia calabura (Tiliaceae), for the first time from Brazil and also in South America.
Muntingia calabura L. belongs to the family Tiliaceae. This species is native from the Antilles and is commonly known as calabura or Antilles-cherry. In 1962 the Instituto Agronômico de Campinas (IAC) introduced this plant in Brazil using seed from Egypt. Muntingia calabura shows rapid growth and is used to shade areas. The fruits are appreciated by humans, birds, bats and fish. Because the regular production of fruit lasts almost the whole year, M. calabura is employed in fisheries and among forestry essences to feed the wildlife (Seabra 1999). In May of 2009, in a vicinity of the experimental station of Comissão Executiva de Planejamento da Lavoura Cacaueira (CEPLAC) in Linhares, Espírito Santo, Brazil, plants of M. calabura were found showing leaf spots, angular to irregular, 0.2–2.5 mm in diameter, reddish brown, with a darker border followed by a yellowish margin, on a yellow leaf with a green margin, sometimes coalescing, fruiting almost epiphyllous (Fig. 1). When infected tissue was examined under a stereomicroscope, typical cercosporoid structures were observed on the lesions. Hand-free sections containing the fungal structures and fungal structures scraped with a syringe needle from the plant surfaces were mounted in lactofuchsin and glycerol. Observations and measurements were prepared using an Olympus BX 41. Material examined: Vic. 31213, on Muntingia calabura leaves, Linhares, State of Espírito Santo, Brazil, D.F. Parreira, 05 May 2009.

|
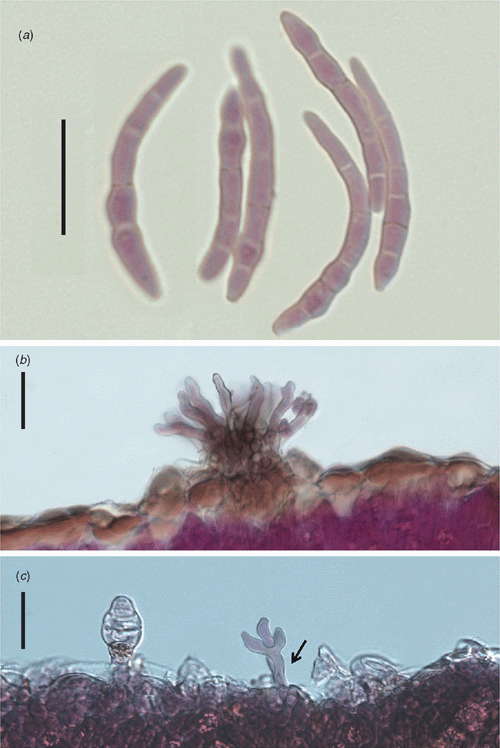
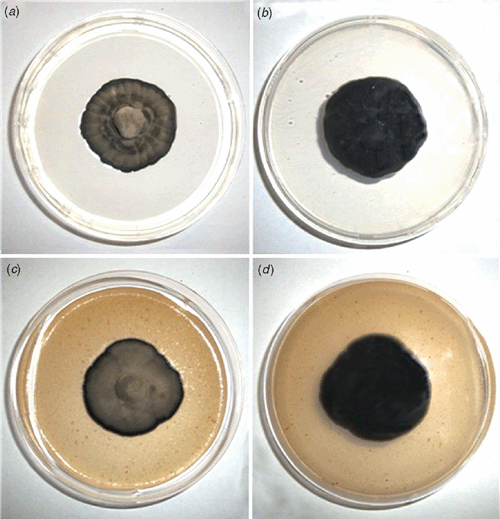
Morphological features of the fungus were: stromata on adaxial surface, 12.5–32.5 × 22.5–45.0 µm, well developed, sub-immersed to erumpent, composed of dark brown, textura angularis cells. Conidiophores in adaxial surface are aggregated in sporodochia, erect, straight to slightly curved, subcylindrical, unbranched, light brown, thin-walled, smooth, in abaxial surface arising to true stomata, branched and sparse, 20.0–50.0 × 3.5–4.0 µm. Conidiogenous cells terminal, integrated, 7.5–22.5 × 2.5–4.0 µm, light brown. Conidiogenous loci inconspicuous, 1–3 per cell, 2.0–2.5 µm diam., non-thickened, not darkened. Conidia solitary, cylindrical to acicular, straight to slightly curved, not geniculate, 25.0–74.0 × 2.5–5.0 µm, 1–8 septate, pale olivaceous, thin-walled, smooth, hila non-thickened and not darkened (Fig. 2). Based on these characteristics the fungus was identified as Pseudocercospora muntingiae (Petr. & Cif.) Deighton (Chupp 1954). On culture: slow growth, 2.75 cm diam. in 30 days, sporulated on potato dextrose agar (PDA) at 25°C and 12 h of light for 15 days, and on V8 agar had little sporulation at 30 days with the same conditions, regular edges, cottonous mycelium, grey, the colony on PDA surface had mild depression, the same was not observed on V8 agar, the culture was deposited in Coleção de Culturas de Fungos Fitopatogênicos ‘Prof. Maria Menezes’ – CMM (CCM-2812) (Fig. 3).
|
|
A conidial suspension with 104 spores/mL, prepared from 20-day-old colonies, was sprayed on both sides of healthy leaves of young plants. After inoculation the plants were maintained for 24 h in a dew chamber, and later transferred to a growing chamber maintained at 26 ± 2°C. After 20 days similar symptoms (leaf spot) were observed, the pathogen structures were observed and reisolated from the leaf lesions fulfilling Koch’s Postulates.
Two Pseudocercospora species are reported on M. calabura: Pseudocercospora muntingiae in Cambodia, Dominican Republic, Guam, Indonesia, Malaysia, Singapore, Solomon Islands and USA and Pseudocercospora muntingiicola (J.M. Yen) J.M. Yen in Taiwan (Yen 1977; Crous and Braun 2003). This is the first record of P. muntingiae in Brazil and also in South America.
Yen JM
(1977) Étude sur champignons parasites du sud-est asiatique. XXVI. Les Cercospora de Formose. II. Bulletin de la Société Mycologique de France 93, 145–164.



