First report of Tuberose mild mosaic potyvirus from tuberose (Polianthes tuberosa L.) in India
S. K. Raj A C , S. K. Snehi A , S. Kumar A , T. Ram B and A. K. Goel BA Plant Molecular Virology, National Botanical Research Institute, Lucknow 226 001, U.P., India.
B Botanic Gardens, National Botanical Research Institute, Lucknow 226 001, U.P., India.
C Corresponding author. Email: skraj2@rediffmail.com
Australasian Plant Disease Notes 4(1) 93-95 https://doi.org/10.1071/DN09040
Submitted: 18 February 2009 Accepted: 28 August 2009 Published: 9 September 2009
Abstract
A potyvirus was detected molecularly by reverse-transcription polymerase chain reaction (RT-PCR) and serologically by Ouchterlony’s double diffusion test from a tuberose (Polianthes tuberosa L.) plant with mild mosaic disease. Potyvirus group-degenerate primers specific to the core region of coat protein gene were used in RT-PCR and the subsequent amplicon was sequenced. The sequence shared highest 98% and 99% identities at nucleotide and amino acid level, respectively and had a close phylogenetic relationship with the sequence of Tuberose mild mosaic potyvirus (TuMMV). This is the first report of TuMMV being present in India.
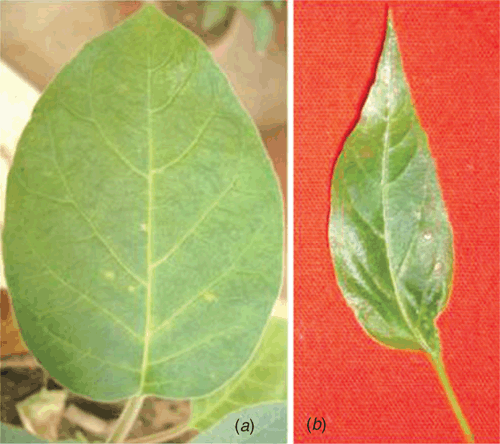
Tuberose (Polianthes tuberosa L.) of family Agavaceae is a bulbous ornamental plant, native of Mexico. It is grown commercially for cut flowers and occupies a prime position in the floriculture industry of India. Two cultivars of P. tuberosa namely, Rajat Rekha and Svarna Rekha, were bred and maintained at NBRI, Lucknow (Sharga 1999). During the survey in May–June 2007, a mild mosaic disease was observed on several commercial plants of cultivar Rajat Rekha at the botanic gardens of NBRI, Lucknow, India. The observed symptoms of the disease were green stripes and a mild mosaic on leaves (Fig. 1a, b) as compared to healthy one (Fig. 1c, d). Small sized floral spikes typically develop in affected plants as compared to healthy ones. Based on the reports of potyviruses on tuberose in India and abroad (Pearson and Horner 1986; Chen and Chang 1998; Kulshrestha et al. 2005; Krishnareddy et al. 2007) and mild mosaic symptoms similar with those of potyvirus (Kulshrestha et al. 2005; Krishnareddy et al. 2007), the infection of a potyvirus was suspected.

|
Mechanical inoculations, using sap of naturally infected tuberose, induced necrotic local lesions on Chenopodium amaranticolor, Datura metal (Fig. 2a) and Capsicum annuum (Fig. 2b) leaves, 10 days post-inoculation. However, neither local nor systemic symptoms developed on similarly inoculated plants of Lycopersicom esculentum, Nicotiana glutinosa, N. rustica and N. tabacum cv. White Burley even after 30 days post-inoculation. Mechanical inoculation on tuberose also resulted in the similar mild mosaic symptoms after 30 days.
|
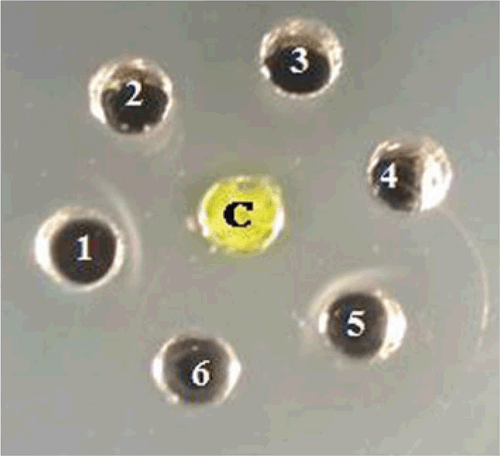
The Ouchterlony’s double diffusion test (ODDT) was as described by (Ouchterlony 1958). Briefly, crude sap from leaf tissue of naturally infected tuberose was prepared by homogenising 1 g of leaf tissue in 10 mL of sodium phosphate buffer (0.1 M, pH 7.0) and used as the test antigen in the ODDT. The crude sap was reacted against antiserum of three Potyviridae species, Cardamom mosaic virus, Bean yellow mosaic virus and an unknown potyvirus from Narcissus tazetta (Aminuddin et al. 1999) and two species from unrelated virus families, Cucumber mosaic virus (Bromoviridae) and Carnation mottle virus (Tombusviridae). The antigen only reacted positively with antiserum to Cardamom mosaic virus and the unknown potyvirus from N. tazetta as observed by precipitin lines in the media (Fig. 3). Based on results of the ODDT the presence of a potyvirus was suspected.
|
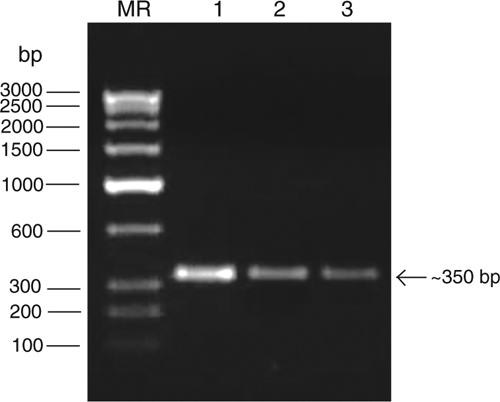
For molecular identification of the putative potyvirus, total RNA was extracted from infected leaf samples following the method described by Pawlowski et al. (1994). RT-PCR was performed as described earlier (Raj et al. 2009) using the degenerate forward primer ‘oligo1n’ encoding the MVWCIEN motif and reverse primer ‘oligo2n’ corresponding to the reverse complimentary of the nucleotide sequence encoding the QMKAAA motif in the core of the coat protein gene of potyviruses (Marie-Jeanne et al. 2000). Complementary DNA (cDNA) synthesis was carried out in a 20 μL reaction mixture containing 1.0 μg of RNA template, 1× reverse transcriptase buffer, 1.0 mm each dNTPs, 25 p.m. reverse primer, 25 U RNA guard (MBI Fermentas, USA) and 200 U of MMuLV Reverse transcriptase Revert Aid H-minus (MBI Fermentas, USA). The reaction was performed at 42°C as per manufacturers’ instruction. PCR was carried in a 50 μL reaction mixture containing: 50 ng cDNA template, 1× Pfu DNA polymerase buffer, 200 μM dNTPs, 25 p.m. each of forward and reverse primers and 3U Pfu DNA Polymerase (MBI Fermentas, USA) in a Peltier thermal cycler PTC200 engine (MJ Research, USA). The PCR condition were an initial template denaturation at 94°C for 5 min then 29 cycles consisting of denaturation at 94°C for 45 s, primer annealing at 50°C for 2 min and extension at 72°C for 50 s and a final extension at 72°C for 10 min. The amplified PCR products were electrophoresed with a known low range DNA ruler (Bangalore Genei Pvt. Ltd, India) using a 1.2% agarose gel to assess the size of amplicons. The electrophoresis of PCR products resulted in the expected size (ca. 350 bp) amplicons derived from leaf samples of diseased tuberose collected from three locations (Fig. 4), but not from two healthy plants.
|
Three amplicons were obtained, sequenced and the 328 bp consensus sequence deposited in GenBank under accession number EU032486. National Center for Biotechnology Information Basic local alignment search tool (NCBI/BLAST/blastn suite) analysis of the deposited nucleotide sequence revealed that it was 98% identical with Tuberose mild mosaic virus (AF062926 from China, Chen et al. 2002), 94% with Tuberose mild mottle virus (EU042759 from The Netherlands) and only 80–79% with Tuberose mild mottle virus (EU362634, AJ581528, Lin et al. 2004; AJ888228, Kulshrestha et al. 2005; AY833736, Krishnareddy et al. 2007). The putative amino acid sequence (108 aa) of virus isolate was also analysed by BLAST which revealed 99% identities with the polyprotein of Tuberose mild mosaic virus (AAL71892), 97% with Tuberose mild mottle virus (ABU41879) and 88% with other isolates of Tuberose mild mottle virus (CAE46329, AAX24131 and CAI59781). Based on highest 99% identities, the potyvirus under study have been considered as a strain of Tuberose mild mosaic virus.
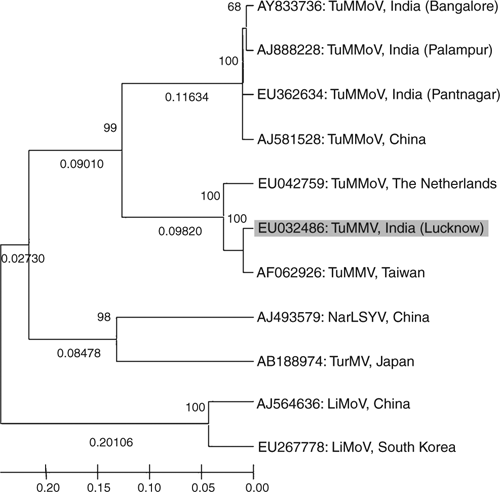
Phylogenetic analysis of nucleotide sequence of the core of the coat protein of the virus isolate with the most related potyviruses or potyvirus-like sequences was performed using molecular evolutionary genetics analysis (MEGA) neighbour joining version 4.0 tool (Tamura et al. 2007). The analyses revealed the virus isolate was most closely related to Tuberose mild mosaic virus (Chen et al. 2002) of Taiwan and has distant relationship with Tuberose mild mottle virus isolates of Indian origin (Fig. 5). The Tuberose mild mottle virus of The Netherlands (EU042759) which showed 94% sequence identity also shared a close relationship with the virus isolate under study, may be misidentified and has been considered as an strain of Tuberose mild mosaic virus based on its highest 92% identities with Tuberose mild mosaic virus (AF062926, reported from Taiwan by Chen et al. 2002) and low identities 74% with strains of Tuberose mild mottle virus (AY833736, AJ581528, AJ888228). Furthermore, the sequence (EU042759) is available onto GenBank as an unpublished record so there are no other details provided to verify its identity. Based on highest 98% and 99% sequence identity at nucleotide and amino acid respectively of core region of coat protein, and close phylogenetic relationships, the virus under study was identified as a strain of Tuberose mild mosaic virus.
|
This is the first report on the occurrence of Tuberose mild mosaic virus on tuberose in India. Tuberose mild mottle virus (TuMMoV) from India (Kulshrestha et al. 2005; Krishnareddy et al. 2007), New Zealand (Pearson and Horner 1986) and Tuberose mild mosaic virus (TuMMV) from Taiwan have been reported earlier (Chen and Chang 1998; Chen et al. 2002) and China (Lin et al. 2004). Spread of these viruses in planting material is a concern for the Indian floriculture industry as propagation and commercial cultivation of tuberose is through bulbs and/or tissue culture. Screening of this propagation material for viruses would be useful to establish and maintain a virus-free tuberose industry in India.
Acknowledgements
The authors would like to thank the Director, N. B. R. I., Lucknow, for facilities and Council of Scientific and Industrial Research, New Delhi, for fellowship to S. Kumar.
Aminuddin
,
Khan JA, Raj SK
(1999) Association of an unknown potyvirus isolate with severe mosaic of Narcissus tazetta L. Indian Journal of Experimental Biology 37, 1034–1036.

Chen CC, Chang CA
(1998) Characterization of a potyvirus causing mild mosaic on tuberose. Plant Disease 82, 45–49.
| Crossref |

Chen CC,
Hsiang T,
Chiang FL, Chang CA
(2002) Molecular characterization of Tuberose mild mosaic virus and preparation of its antiserum to the coat protein expressed in bacteria. Botanical Bulletin Academia Sinica (Taiwan) 43, 13–20.
|
CAS |

Krishnareddy M,
Smitha R,
Devaraju
, Jalali S
(2007) Molecular characterization of a potyvirus infecting tuberose (Polianthes tuberosa) in southern India. Indian Phytopathology 60, 251–258.
|
CAS |

Kulshrestha S,
Mehra A,
Hallan V,
Raikhy G,
Ram R, Zaidi AA
(2005) Molecular evidence for occurrence of Tuberose mild mottle virus infecting tuberose (Polianthes tuberosa) in India. Current Science 89, 870–872.
|
CAS |

Lin L,
Zheng H-Y,
Chen J,
Chen J-P,
Zhang Q-Y,
Zhao M-F,
Antoniw JF, Adams MJ
(2004) A new potyvirus from tuberose (Polianthes tuberosa) in China. Archives of Virology 149, 1107–1116.
|
CAS |
Crossref |
PubMed |

Marie-Jeanne V,
Ioos R,
Peyre J,
Alliot B, Signoret P
(2000) Differentiation of Poaceae potyviruses by reverse transcription-polymerase chain reaction and restriction analysis. Journal of Phytopathology 148, 141–151.
|
CAS |
Crossref |

Ouchterlony O
(1958) Diffusion-in-gel methods for immunological analysis. Progress in Allergy 5, 1–78.
|
CAS |
PubMed |

Pawlowski K,
Kunze R,
Vries SD, Bisseling T
(1994) Isolation of total, poly(A) and polysomal RNA from plant tissues. Plant Molecular Biology Manual D5, 1–13.

Pearson MN, Horner MB
(1986) A potyvirus of Polyanthes tuberose in New Zealand. Australasian Plant Pathology 15, 39.
| Crossref |

Raj SK,
Snehi SK,
Kumar S, Khan MS
(2009) First molecular detection and identification of a Potyvirus associated with severe mosaic disease of amaryllis (Hippeastrum hybridum Hort.) in India. Australasian Plant Disease Notes (in press),

Tamura K,
Dudley J,
Nei M, Kumar S
(2007) MEGA 4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599.
|
CAS |
Crossref |
PubMed |



