Effect of Alkyl Chain Length in Anions on the Physicochemical Properties of Cellulose-Dissolving Protic Ionic Liquids
Hideki Hanabusa A , Yuko Takeoka A , Masahiro Rikukawa A and Masahiro Yoshizawa-Fujita A BA Department of Materials and Life Sciences, Sophia University, 7-1 Kioi-cho, Chiyoda-ku, Tokyo 102-8554, Japan.
B Corresponding author. Email: masahi-f@sophia.ac.jp
Australian Journal of Chemistry 72(2) 55-60 https://doi.org/10.1071/CH18170
Submitted: 16 April 2018 Accepted: 6 July 2018 Published: 20 August 2018
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
A protic ionic liquid (PIL) composed of 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) and acetic acid can dissolve cellulose under mild conditions and catalyse its transesterification. To investigate the relationship between physicochemical properties and chemical structures, PILs composed of DBU and carboxylic acids with varying alkyl chain lengths were prepared as cellulose-dissolving solvents. The thermal behaviours of the PILs were analysed by thermogravimetry and differential scanning calorimetry, and their viscosities, ionic conductivities, and cellulose-dissolution abilities were determined. The effect of the alkyl chain length in the carboxylate ion on the physicochemical properties of the PILs was investigated. With increasing chain length, the thermal stability and ionic conductivity increased, whereas the melting point (Tm), glass-transition temperature (Tg), cellulose solubility, and viscosity decreased. The cellulose solubility increased as the difference between the pKa values of the DBU and carboxylic acid (ΔpKa) increased. In addition, the cellulose solubility increased with the increasing density of the PIL. It was revealed that PILs with a high ΔpKa value and a carboxylate ion with a short alkyl chain are suitable for cellulose dissolution.
Introduction
In recent years, ionic liquids (ILs) have attracted a great deal of attention across many research fields.[1] Defined as organic salts that melt below 100°C, ILs exhibit unique properties, including non-volatility, non-flammability, high ionic conductivity, and high cellulose solubility. Protic ionic liquids (PILs), synthesised by a simple proton transfer reaction from an acid to a base, can be inexpensively obtained in high yield. In general, bulky bases and strong acids are used for the synthesis of PILs.[2–4] The neutralisation reactions are reversible, and therefore, not only ions but also acids and bases are present in the PILs. PIL formation is best achieved when the difference between the pKa values of the acid and base (ΔpKa) is large. Angell and co-workers found that PILs with large ΔpKa values (>10) showed behaviours similar to those of aprotic ILs (AILs).[5]
PILs can be distilled when their ΔpKa value is low (<10). By Le Chatelier’s principle, the equilibrium is shifted to the reverse reaction under vacuum, and therefore, the boiling points of the PILs become lower than the decomposition temperature.[6] According to this phenomenon, King et al. reported the PIL 1,1,3,3-tetramethylguanidinium propionate ([TMGH][OPr]) as a ‘reusable cellulose solvent’ via a distillation process.[7]
Recently, we reported that PILs composed of amidines and carboxylic acids could be used as cellulose solvents and low-cost catalysts for the direct transesterification reaction of cellulose.[8] It has already been shown that combinations of 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) and organic acids dissolve a range of synthetic and biological polymers, but no mention of distillation of the solvents has been made.[9] We succeeded in reusing the PILs, not only as cellulose solvents but also as cellulose transesterification catalysts, after their recovery by distillation. PILs composed of DBU and carboxylic acids were suitable for cellulose transesterification.[8] There are few reports on the physicochemical properties of amidinium carboxylates. For example, Miran et al. found that the properties of 1,8-diazabicyclo[5.4.0]-undec-7-enium ([DBUH]+)-based PILs, such as thermal stability and ionicity, were controlled by the differences in the pKa values between the bases and acids (ΔpKa) due to the generation of N–H hydrogen bonds during cation formation.[10] In addition, Parviainen et al. found that several PILs composed of amidines and carboxylic acids, such as 1,5-diazabicyclo[4.3.0]non-5-enium propionate ([DBNH][OPr]), were good cellulose solvents because of their cellulose dissolution ability and low viscosity. Furthermore, [DBNH][OPr] was distillable and recyclable, and thus, was regarded as a ‘green solvent’ for cellulose.[11] Considering PILs for cellulose transesterification, an investigation of their physicochemical properties is of paramount importance.
The physicochemical properties (thermal behaviour, viscosity, and ionic conductivity) and cellulose dissolution abilities of [DBUH]+-based PILs with various carboxylate ions are investigated in this study. The effect of the alkyl chain length in the carboxylate ion on cellulose solubility is discussed.
Results and Discussion
Thermal Properties of PILs
The chemical structures of the PILs synthesised in this study are shown in Fig. 1. [DBUH][OFo], [DBUH][OAc], and [DBUH][OPr] were obtained as white solids at room temperature. In contrast, the remaining PILs were transparent colourless liquids at room temperature. The DSC curves are shown in Fig. S1 (Supplementary Material). [DBUH][OFo] exhibits the highest melting temperature (Tm) value at 89°C among the seven PILs in this study. In the case of [DBUH][OFo], the endothermic Tm peak is observed in both the first and second heating scans, suggesting that the PIL has high crystallinity. In the cases of [DBUH][OAc] and [DBUH][OPr], the endothermic peaks corresponding to Tm are observed in the first heating scan only. [DBUH][OAc] and [DBUH][OPr] exhibit glass-transition (Tg) values of −43 and −45°C, respectively, in the second heating scan, and maintain a liquid state at room temperature. Their supercooled states are stable at room temperature. In the cases of [DBUH][OBu], [DBUH][OHe], [DBUH][OOc], and [DBUH][ODe], Tg signals are observed in both the first and second heating scans. The Tg values of the PILs decrease with increasing alkyl chain length in the carboxylate ion.
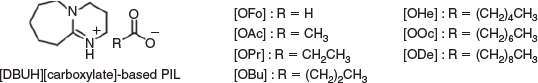
|
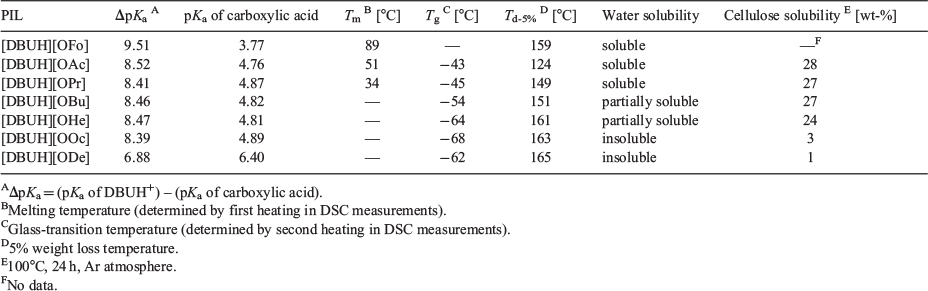
To evaluate the thermal stability of the PILs, the Td-5% values, corresponding to 5 % weight loss, were measured using thermogravimetric analysis (TGA). The TGA curves are shown in Fig. S2 (Supplementary Material). The Td-5% values are between 120 and 170°C for all the PILs used herein (see Table 1), and are lower than those of aprotic ionic liquids (AILs) with carboxylate ions. For example, the thermal decomposition temperatures (Td) of 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) and 1-butyl-3-methylimidazolium acetate ([C4mim][OAc]) are 221 and 216°C, respectively.[12] Angell and co-workers reported that the boiling points of PILs increased to over 300°C when their ΔpKa values were above 10. This was attributed to the formation of strong ionic bonds after proton transfer from the Brønsted acid to the Brønsted base.[5] In addition, Miran et al. found that PILs whose ΔpKa values exceeded 15 were superior in terms of thermal stability. For the case in which the ΔpKa value was above 20, the Td exceeded 450°C under an N2 atmosphere.[10] Table 1 summarises the ΔpKa values for this study, which were determined via the following equation: ΔpKa = pKa of DBUH+ (13.28) – pKa of carboxylic acid[13–15] (see Table 1). Since the ΔpKa values of the PILs synthesised in this study are between 6.88 and 9.51, it is likely that proton transfer (neutralisation) insufficiently occurs during the synthesis of PILs. Lower ΔpKa values (<10) are responsible for lower thermal stability.
|
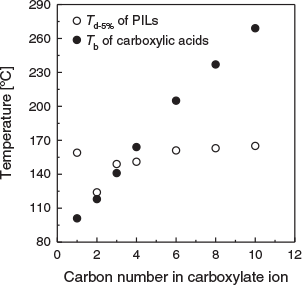
Fig. 2 shows the relationship between the Td-5% values and the carbon number in the carboxylate ion. The boiling points of various carboxylic acids are included for comparison. Except for [DBUH][OFo], the Td-5% values increase with increasing carbon number in the carboxylate ion up to 6, and then reach a plateau. This trend differs from the boiling points (Tb) of the carboxylic acids, which increase monotonously as the carbon number increases. The Td-5% values for [DBUH][OBu], [DBUH][OHe], [DBUH][OOc], and [DBUH][ODe] are lower than the Tb values of the corresponding carboxylic acids. Carboxylic acids form dimers through hydrogen bonds between the two carboxy groups. Therefore, the Tb values of carboxylic acids are higher than would be predicted from their molecular weights. In the case of PILs, proton transfer occurs between DBU and the carboxylic acid, and thus, the carboxylic acids cannot form dimers in the PILs. On the other hand, the ΔpKa value of [DBUH][OFo] is 9.51, which is the highest in this study. Because of its large ΔpKa value, the high thermal stability of [DBUH][OFo] is similar to those of AILs.
|
Cellulose Solubility of PILs
Cellulose solubility tests were performed at 100°C for 24 h under Ar atmosphere, and the results are summarised in Table 1. [DBUH][OAc] dissolves 28 wt-% cellulose at 100°C. The value is similar to those of AILs with acetate anions.[16,17] The cellulose solubility increases with the increase in the ΔpKa values of the PILs. However, several PILs exhibit disparate cellulose solubilities, despite very similar ΔpKa values. [DBUH][OOc] (ΔpKa = 8.39) dissolves 3 wt-% cellulose, in contrast to the 27 wt-% cellulose that dissolves in [DBUH][OPr] (ΔpKa = 8.41). In addition, 24 wt-% cellulose dissolves in [DBUH][OHe] (ΔpKa = 8.47), whereas [DBUH][OBu] (ΔpKa = 8.46) dissolves 27 wt-% cellulose. These differences may be attributed to the alkyl chain of the carboxylate ion, according to the report by Kuzmina et al.[18] The carboxylate ions are thought to disrupt the hydrogen bond network of the hydroxy groups in cellulose, therefore enabling the dissolution of cellulose in carboxylate-based ILs. The longer, hydrophobic alkyl chains would prevent interactions between carboxylate ions and hydroxy groups, thus decreasing cellulose solubility with increasing alkyl chain length in the carboxylate ion. The water miscibility of PILs with longer alkyl chains is low (see Table 1). This tendency is consistent with the solubility of cellulose. Although it is known that the β value of Kamlet–Taft parameters is closely related to the solubility of cellulose in ILs, we cannot determine the β value of Kamlet–Taft parameters for PILs because Reichardt’s dye becomes colourless by the protonation. Abe et al. reported that density is also an important parameter to consider for developing cellulose-dissolving ILs.[19] An increase in the alkyl chain length in the carboxylate ion results in lower cellulose solubility owing to the decrease in density. Thus, we should consider not only the ΔpKa value but also the density of the ILs when assessing cellulose solubility. The densities of the PILs prepared in this study are discussed in the next section. Nevertheless, PILs with a high ΔpKa value and a short alkyl chain in the carboxylate ion are revealed as suitable for cellulose dissolution.
Density of PILs
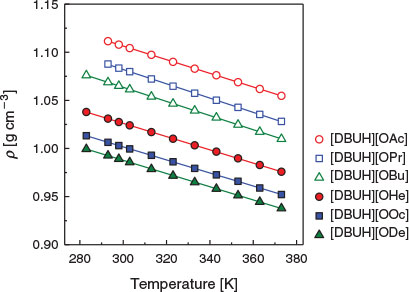
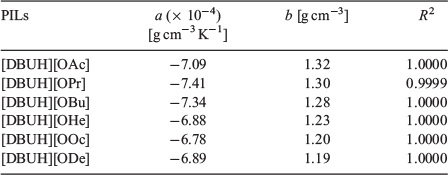
The density of the PILs at various temperatures was investigated. Fig. 3 shows the temperature dependence of the PILs’ densities in the 10–100°C range. The density decreases linearly with increasing temperature. Table 2 summarises the best-fit parameters for the linear fits of density as a function of temperature. The best-fitted results are also depicted as the solid lines in Fig. 3. The a values of the PILs are nearly the same at about −7 × 10−4 g cm−3 K−1. The b values range from 1.19 to 1.32 g cm−3, and increase with the increase in the alkyl chain length. The density values monotonously decrease with decreasing ΔpKa values and increasing alkyl chain lengths. These trends are consistent with the cellulose solubility results.
|
|
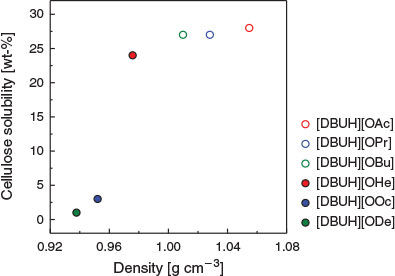
Fig. 4 shows the density dependence of cellulose solubility in the PILs at 100°C. The cellulose solubility decreases with decreasing PIL density. This trend is consistent with the literature.[19] The cellulose solubility of the PILs drastically decreases when the alkyl chain increases from 6 to 8, even though the difference in density is small. According to Table 1, the hydrophilicity of the PILs drastically changes at a carbon number of 8, such that the PILs become hydrophobic. As mentioned in the Cellulose Solubility of PILs section, cellulose solubility increases with the increasing ability of the carboxylate ion to function as a hydrogen-bond acceptor with cellulose. The hydrophilicity of the PIL is an important factor for increasing cellulose solubility.
|
Viscosity of PILs
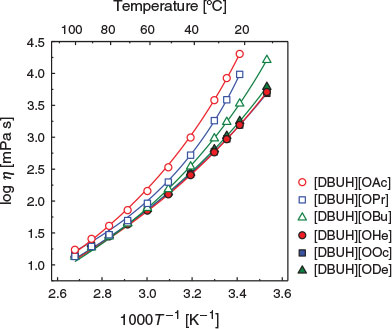
The viscosity (η) of the PILs was investigated by viscometry in the range of 10 to 100°C. Fig. 5 shows the temperature dependence of the viscosity for the PILs, which obeys the Vogel–Fulcher–Tamman (VFT) equation (Eqn 1):
|
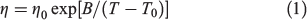
where η0, B, and T0 are adjustable parameters. The best-fit parameters are summarised in Table 3 and depicted as solid lines in Fig. 5. Except for [DBUH][ODe], the viscosities of the PILs decrease with increasing alkyl chain length in the carboxylate ion. This phenomenon is consistent with the trend of Tg values determined by the DSC measurements. Decreases in the polarity, density, and molecular packing of the carboxylate ions are caused by the increases in their alkyl chain lengths.[20] The viscosities of ILs with carboxylate ions, such as imidazolium carboxylate, ammonium carboxylate, and choline carboxylate,[21–23] increase with the increasing bulkiness of the ion species, whether cationic or anionic. The PILs used in this study exhibit a different trend. On the other hand, the viscosity of [DBUH][ODe] is 1.79 × 103 mPa s at 20°C, which is higher than that of [DBUH][OOc]. In general, the viscosity of a molecular liquid increases because the van der Waals force is strengthened as the number of atoms in the molecules increase. Thus, the greater viscosity in the case of [DBUH][ODe] versus [DBUH][OOc] indicates the stronger van der Waals interactions.
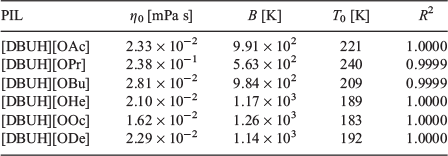
|
Ionic Conductivity of PILs
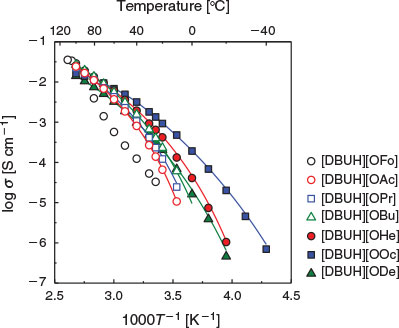
Fig. 6 shows the Arrhenius plots of the ionic conductivity (σ) for the PILs in the cooling process. The plots of the temperature dependence of the ionic conductivity appear as upper convex curves and are well fitted by the VFT equation (Eqn 2):
|
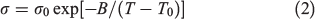
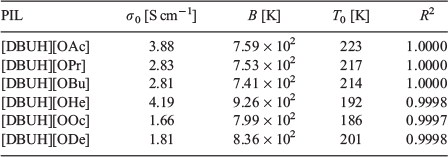
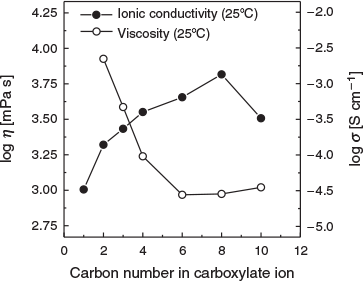
where σ0, B, and T0 are adjustable parameters. The best-fit parameters are summarised in Table 4 and depicted as solid lines in Fig. 6. The ionic conductivity of [DBUH][OFo] drastically increases between 80 and 90°C, because the Tm value of [DBUH][OFo] is 89°C. The ionic conductivity in the low-temperature range increases with the increasing alkyl chain length in the carboxylate ion. Fig. 7 shows the ionic conductivity and viscosity at 25°C as a function of the carbon number in the carboxylate ion. The ionic conductivity increases as the carbon number increases to 8, whereas the viscosity decreases for the same carbon number range. The ionic species can be transported more easily due to the decrease in the viscosity.[24] Thus, the ionic conductivity is correlated with the viscosity.
|
|
Conclusions
We investigated the physicochemical properties of PILs composed of DBU and various carboxylic acids, which can dissolve cellulose. The thermal stability of the PILs depended on their ΔpKa value and alkyl chain length in the carboxylate ion. The Tg and viscosity values of the PILs decreased with increasing alkyl chain length, following the same trend as cellulose solubility. These trends were closely related to the density of the PILs. [DBUH][OAc] could dissolve 28 wt-% cellulose at 100°C, which was the highest value in this study. The PILs composed of carboxylate ions with short alkyl chains were most suitable for cellulose dissolution.
Experimental
Materials
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU, >98.0 %), propionic acid (>98.0 %), butyric acid (>98.0 %), hexanoic acid (>98.0 %), n-octanoic acid (>98.0 %), and decanoic acid (>98.0 %) were purchased from Tokyo Chemical Industry Co., Ltd. Formic acid (>99.0 %) and acetic acid (>99.7 %) were purchased from Wako Pure Chemical Industries Ltd, and used as obtained. All materials except formic acid and acetic acid were distilled under vacuum before use.
Preparation of PILs
All PILs were prepared by a simple neutralisation reaction without any solvent. The carboxylic acids were added into the amidine under Ar atmosphere in a two-necked round-bottomed flask kept in an ice bath. The mixtures were stirred at room temperature for 24 h, to afford colourless transparent liquids or white solids. The synthesised PILs are designated according to the carbon number in the carboxylate ions ([OFo], [OAc], [OPr], [OBu], [OHe], [OOc], and [ODe]).
Measurements
The 5 % weight loss temperature (Td-5%) was measured by thermogravimetry (TG-DTA; TG-DTA7200, Hitachi High-Technologies). The samples were heated from room temperature to 500°C at a scan rate of 20°C min−1 under a nitrogen atmosphere. The melting temperature (Tm) and glass-transition temperature (Tg) were measured by differential scanning calorimetry (DSC; DSC7020, Hitachi High-Technologies) in the temperature range of −100 to 100°C at heating and cooling rates of 10°C min−1. The samples were tightly sealed in Al pans under an Ar atmosphere in a dry glove box. Density and viscosity were measured with a viscometer (SVM3000, Anton Paar) in the temperature range of 10 to 100°C.
Ionic conductivity was measured with an impedance analyser (VSP-300, BioLogic) and a thermostatic chamber (SU-262, Espec Corp.) in the temperature range of −40 to 110°C. The samples were tightly sealed in cells with two platinum electrodes under an Ar atmosphere in a glove box. After the cells were maintained at each temperature for 20 min, the ionic conductivity was measured (frequency range: 5 Hz to 1 MHz; applied voltage: 10 mV). The cell constant was determined with 0.1 M KCl aq. at 25 and 50°C.
Supplementary Material
The results of differential scanning calorimetry and thermogravimetric analysis of protic ionic liquids used in this study are available on the Journal’s website.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgement
This work was supported by a Sophia University Special Grant for Academic Research.
References
[1] N. V. Plechkova, K. R. Seddon, Chem. Soc. Rev. 2008, 37, 123.| Crossref | GoogleScholarGoogle Scholar |
[2] A. S. Amarasekara, Chem. Rev. 2016, 116, 6133.
| Crossref | GoogleScholarGoogle Scholar |
[3] L. T. Greaves, J. C. Drummond, Chem. Rev. 2008, 108, 206.
| Crossref | GoogleScholarGoogle Scholar |
[4] K. E. Johnson, R. M. Pagni, J. Bartmess, Monatsh. Chem. 2007, 138, 1077.
| Crossref | GoogleScholarGoogle Scholar |
[5] M. Yoshizawa, W. Xu, C. A. Angell, J. Am. Chem. Soc. 2003, 125, 15411.
| Crossref | GoogleScholarGoogle Scholar |
[6] M. J. Earle, J. M. S. S. Esperancüa, M. A. Gilea, J. N. Canongia Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon, J. A. Widegren, Nature 2006, 439, 831.
| Crossref | GoogleScholarGoogle Scholar |
[7] A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi, I. Kilpeläinen, Angew. Chem. Int. Ed. 2011, 50, 6301.
| Crossref | GoogleScholarGoogle Scholar |
[8] H. Hanabusa, E. I. Izgorodina, S. Suzuki, Y. Takeoka, M. Rikukawa, M. Yoshizawa-Fujita, Green Chem. 2018, 20, 1412.
| Crossref | GoogleScholarGoogle Scholar |
[9] G. D’Andola, L. Szarvas, K. Massonne, V. Stegmann, WO 043837 2008.
[10] M. S. Miran, H. Kinoshita, T. Yasuda, M. A. B. H. Susan, M. Watanabe, Phys. Chem. Chem. Phys. 2012, 14, 5178.
| Crossref | GoogleScholarGoogle Scholar |
[11] A. Parviainen, A. W. T. King, I. Mutikainen, M. Hummel, C. Selg, L. K. J. Hauru, H. Sixta, I. Kilpeläinen, ChemSusChem 2013, 6, 2161.
| Crossref | GoogleScholarGoogle Scholar |
[12] Y. Cao, T. Mu, Ind. Eng. Chem. Res. 2014, 53, 8651.
| Crossref | GoogleScholarGoogle Scholar |
[13] M. Namazian, S. Halvani, M. R. Noorbala, Theochem. J. Mol. Struct. 2004, 711, 13.
| Crossref | GoogleScholarGoogle Scholar |
[14] G. Daw, A. C. Regan, C. I. F. Watt, E. Wood, J. Phys. Org. Chem. 2013, 26, 1048.
| Crossref | GoogleScholarGoogle Scholar |
[15] B. A. Wellen, E. A. Lach, H. C. Allen, Phys. Chem. Chem. Phys. 2017, 19, 26551.
| Crossref | GoogleScholarGoogle Scholar |
[16] D. G. Raut, O. Sundman, W. Su, P. Virtanen, Y. Sugano, K. Kordas, J. P. Mikkola, Carbohydr. Polym. 2015, 130, 18.
| Crossref | GoogleScholarGoogle Scholar |
[17] B. Zhao, L. Greiner, W. Leitner, RSC Adv. 2012, 2, 2476.
| Crossref | GoogleScholarGoogle Scholar |
[18] O. Kuzmina, J. Bhardwaj, S. R. Vincent, N. D. Wanasekara, L. M. Kalossaka, J. Griffith, A. Potthast, S. Rahatekar, S. J. Eichhorn, T. Welton, Green Chem. 2017, 19, 5949.
| Crossref | GoogleScholarGoogle Scholar |
[19] M. Abe, K. Kuroda, D. Sato, H. Kunimura, H. Ohno, Phys. Chem. Chem. Phys. 2015, 17, 32276.
| Crossref | GoogleScholarGoogle Scholar |
[20] M. Yoshizawa-Fujita, T. Tamura, Y. Takeoka, M. Rikukawa, Chem. Commun. 2011, 47, 2345.
| Crossref | GoogleScholarGoogle Scholar |
[21] P. Moyer, M. D. Smith, N. Abdoulmoumine, S. C. Chmely, J. C. Smith, L. Petridis, N. Labbé, Phys. Chem. Chem. Phys. 2018, 20, 2508.
| Crossref | GoogleScholarGoogle Scholar |
[22] X. Meng, J. Devemy, V. Verney, A. Gautier, P. Husson, J. M. Andanson, ChemSusChem 2017, 10, 1749.
| Crossref | GoogleScholarGoogle Scholar |
[23] N. Muhammad, M. I. Hossain, Z. Man, M. El-Harbawi, M. A. Bustam, Y. A. Noaman, N. B. M. Alitheen, M. K. Ng, G. Hefter, C. Yin, J. Chem. Eng. Data 2012, 57, 2191.
| Crossref | GoogleScholarGoogle Scholar |
[24] H. Yoon, A. S. Best, M. Forsyth, D. R. MacFarlane, P. C. Howlett, Phys. Chem. Chem. Phys. 2015, 17, 4656.
| Crossref | GoogleScholarGoogle Scholar |


