Binder-Free TiO2 Monolith-Packed Pipette Tips for the Enrichment of Phosphorylated Peptides*
Chang Lei A , Liang Zhou B , Chun Xu A , Xiaoran Sun A , Amanda Nouwens A C and Chengzhong Yu A DA Australian Institute for Bioengineering and Nanotechnology, The University of Queensland, Brisbane, Qld 4072, Australia.
B State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China.
C School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Qld 4072, Australia.
D Corresponding author. Email: c.yu@uq.edu.au
Australian Journal of Chemistry 69(12) 1396-1401 https://doi.org/10.1071/CH16443
Submitted: 29 July 2016 Accepted: 26 August 2016 Published: 21 September 2016
Abstract
A macroporous TiO2 monolith-entrapped pipette-tip was developed through a binder-free packing method for convenient phosphorylated peptide enrichment. A detection limit of 1 ng mL–1 for phosphorylated peptide is achieved, showing a better enrichment efficiency compared with the commercial pure TiO2-embedded NuTip.
Introduction
Phosphorylation is an important post-translational modification (PTM) of proteins in biological processes.[1] The existence and extent of phosphorylation usually play essential roles in the development of diseases.[2] For example, the phosphorylation of extracellular signal-regulated kinases (ERK) is responsible for the activation and development of osteoarthritis.[3,4] In recent years, mass spectrometry has been widely used for low-abundance biomolecule analysis, including phosphopeptides and phosphoproteins (p-peptide or p-protein).[5] However, it is difficult to directly determine phosphoproteomes owing to their low abundance and difficult ionization.[6] To address the challenge, efficient pretreatment is essential to reduce sample complexity and concentrate the targeted molecules.
In recent years, metal oxides have been used in many studies for p-peptide and protein enrichment,[7] and titanium dioxide (TiO2) has been shown to be one of the most effective materials.[8] It is reported that TiO2 can selectively bind to the phosphate group of p-peptides and proteins.[9] TiO2 and other metal oxides have been packed in pipette tips as a convenient and frequently used device for in situ sample pretreatment.[8,10–12] There are commercial pipette tips on the market (e.g. NuTip, Titansphere Phos-TiO Kit) employing embedded TiO2 for p-peptide and protein enrichment. The use of a functional tip in pretreatment is beneficial for minimizing sample loss, simplifying the operation procedures, and reducing the contamination risk. However, the packing of TiO2 into the tip in literature reports is generally assisted by polymers for adhesion[10,12] or a plug[8] to prevent materials from leaking. The mix of adhesive polymer would reduce the functional TiO2 content, which may result in a decrease in enrichment efficiency, and the use of a plug makes the packing procedure complicated. In order to increase the functional TiO2 content to ensure high enrichment efficiency, directly packing TiO2 without binder is a promising yet challenging approach. The design of a pure TiO2-embedded NuTip is limited by the small amount of solid TiO2 spheres (0.003 mg) in each tip. It is expected that increasing the effective surface area of TiO2 in a tip could further improve the enrichment efficacy.
In the present study, macroporous TiO2 monolith-entrapped pipette tips (T-tip) were developed through a binder-free packing method for p-peptide enrichment (Scheme 1). The macroporous TiO2 monolith was synthesized through a simple and facile method using titanium(iv) bis(ammonium lactato)dihydroxide (TiBALDH) as the precursor and 3-aminophenol/formaldehyde (APF) resin spheres as the templates (Scheme 1a). The TiO2 monolith with large particle sizes (>0.6 mm) was directly entrapped in the pipette tips without any additives, which ensures a pure TiO2 composition (Scheme 1b). The macroporous monolithic structure is beneficial for increased surface area and reduced flow resistance, which allows specific entrapment of TiO2 monolith inside the tips for efficient sample pretreatment (Scheme 1c).[13] The use of T-tip for p-ERK1 peptide enrichment enables a detection limit of 1 ng mL–1, showing a better enrichment efficiency compared with the commercial NuTip.

|
Results and Discussion
APF spheres were used as templates to prepare TiO2 monolith with a macroporous structure. During the centrifugation and drying process, the APF spheres became arranged to form a close-packing structure. As the water evaporated, the TiBALDH precursor filled the packing voids. After calcination at 400°C, the APF spheres were removed and the TiO2 monolith was obtained (Scheme 1a). Previously, many templates (e.g. block polymer surfactants) have been used to prepare porous TiO2 monoliths.[14,15] However, in most cases, prepacking of templates is necessary. The use of APF spheres as templates allows a convenient synthesis of macroporous TiO2 monolith with a uniform pore size. This approach has the potential to be applied to the synthesis of macroporous monoliths of metal oxides with other compositions.[16–20]
Scanning electron microscope (SEM) images were taken to show the porous structure of the TiO2 monolith (Fig. 1a, b). The average diameter of macropores was measured to be ~400 nm. The interconnected pores can be seen as indicated by red arrows. The average diameter of the as-prepared APF spheres is 710 nm, characterized by both transmission electron microscopy (TEM) and dynamic light scattering (DLS) analysis (Fig. S1a and b, Supplementary Material). The pore size of the monolith (400 nm) is smaller than the template size (710 nm) owing to the shrinkage of the packed macrostructure during the calcination process.[21]

|
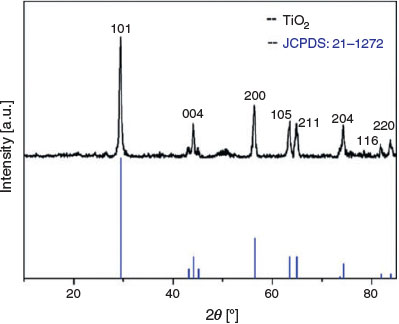
To identify the crystalline structure of calcined TiO2 monolith, the X-ray diffraction (XRD) pattern was recorded. From the XRD pattern shown in Fig. 2, diffractions at 2θ = 29.4, 44.1, 56.3, 63.5, 64.8, 74.2, 81.8, and 83.8° are observed, which can be assigned to the 101, 004, 200, 105, 211, 204, 116 and 220 diffractions of anatase (JCPDS no. 21–1272). The full width at half-maximum of the 101 diffraction is 0.473 nm (obtained from JADE software). The mean crystallite size of anatase in the TiO2 monolith was calculated to be 20 nm using Scherrer’s equations,[22] indicating good crystallinity. Highly crystalline anatase with a porous structure is desirable for p-peptide enrichment as demonstrated in previous reports.[23–25]
|
A nitrogen adsorption test was used to investigate the texture of synthesized TiO2 monolith. The N2 adsorption–desorption isotherm of TiO2 monolith shows a typical type II isotherm with the capillary condensation step occurring at relative pressure (P/P0) > 0.9. The Brunauer–Emmett–Teller (BET) surface area and pore volume were measured to be 22 m2 g–1 and 0.09 cm3 g–1, which are consistent with a previous report using polystyrene spheres as hard templates to synthesize TiO2 with a similar pore size.[21]
Thirty T-tips were prepared and weighted. The average amount of TiO2 monolith in each T-tip was measured to be 0.150 ± 0.020 mg, indicating that the TiO2 monolith amount in each packed T-tip is comparable. In order to check stability during the pipetting process, every T-tip was tested by liquid adsorption. As shown in Fig. S2 (Supplementary Material), the packed monoliths (Fig. S2a) are still fixed in the tip after pipetting 10 times (Fig. S2b).
The phosphorylation of ERK1 has significance in osteoarthritis development; thus, the sensitive detection of p-ERK1 is essential.[3,4] The T-tip was applied to enrich p-ERK1 peptide from digested ERK1 solution, and the enrichment efficacy was evaluated with a mass spectrometer. Without enrichment, several peaks can be seen in the mass spectrum of 0.5 μg mL–1 tryptic-digested p-ERK1 (Fig. 3a). The most obvious peaks represent non-phosphorylated peptides. In contrast, a single peak at m/z 2252.25 is clearly seen in the mass spectrum after T-tip enrichment with a high intensity above 1100 (Fig. 3b), which is not observed in the spectrum before enrichment. In the meantime, almost no peak for non-p-peptide is present after enrichment, suggesting an excellent performance of the T-tip on selective p-peptide enrichment.
Tandem mass spectrometry (MS/MS) was carried out to analyse the sequence of the identified peak (m/z 2252.25). In the first stage of MS/MS (MS1), ions are separated by mass-to-charge ratio (e.g. m/z 2252.25). Then, the ions of the selected mass-to-charge ratio are fragmented and detected at the second stage of mass spectrometry (MS2), showing the sequence of the selected precursor ion. The result indicated that the selected peptide (m/z 2252.25) was a phosphorylated peptide with a sequence of IADPEHDHTGFLTEYVATR by matching with the database using ExPASy-PeptideMass and Proteomics Toolkit (Fig. S3, Supplementary Material), in accordance with a previous report.[12]
Considering the ERK level in biological samples is usually very low, tips with sufficient enrichment ability at low concentrations are essential. The enrichment test was further conducted with two lower concentrations, 50 (Fig. 4a) and 1 ng mL–1 (Fig. 4b). At both concentrations, p-ERK1 peptide (m/z 2252.25) was detectable with high intensities and low interference from non-phosphopeptides, showing the excellent ability of the T-tip for low-abundance p-peptide enrichment.
The signal intensity of peak in matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) spectra cannot be used to directly determine the peptide concentration because the ionization behaviour is analyte-dependent and difficult to predict. Thus, a synthetic peptide was applied as an internal standard (IS) to enable label-free quantification.[26] The sequence of the IS is IADPEHDHTGFLTEYVVTR (molecular mass 2200.4 Da), which differs from p-ERK1 peptide (IADPEHDHTGFLTEYVATR) by only one single amino acid; thus, the IS should have similar desorption-ionization behaviour to the target peptide.[27] To obtain a standard curve for peptide quantification, a series of enriched p-ERK1 peptide solutions (10 µL of 0.02–0.1 µg mL–1) mixed with 10 µL (0.06 µg mL–1) of IS were prepared, and the signal ratio of p-ERK1 peptide/IS was measured. The standard curve shows a linear relationship with a regression coefficient (R2) of 0.978 (Fig. S4, Supplementary Material), indicating that using T-tip for p-peptide enrichment is highly reliable for semiquantification in a low concentration range.
There are several brands of TiO2 tips on the market owing to the great demand of functional tips for sample pretreatment. Most of them are fabricated with the assistance of binder or extra support; only NuTip (Fig. S5A, Supplementary Material) is made from pure TiO2. A comparison between T-tip and NuTip towards p-ERK1 peptide enrichment was conducted. When NuTip was applied to enrich peptides, the limit of detection for p-ERK1 peptide was 50 ng mL–1 (Fig. 4c), and peaks from non-phosphopeptides were observed. The results from NuTip enrichment are in contrast to those from the T-tip (Fig. 4a) at the same concentration (50 ng mL–1), demonstrating the excellent selectivity and enrichment efficiency of T-tip compared with commercial NuTip.
When the sample concentration is as low as 1 ng mL–1, the p-ERK1 peptide cannot be detected using NuTip for enrichment (Fig. 4d). The contrast between Fig. 4b and 4d further confirms that the homemade T-tip has better selective enrichment efficiency than the NuTip. The higher TiO2 amount in the T-tip (0.150 ± 0.020 mg) with a porous structure may be the reason for the better performance than NuTip (0.003 mg of solid TiO2 spheres).[28]
To confirm the advantage of high TiO2 content (100 %) in the T-tip, a reported polymer assisted-packing method (Fig. S6, Supplementary Material) was used to fabricate tips (Fig. S5B, Supplementary Material) with a lower TiO2 content (3.5 %) as a control. The limit of detection of the polymer-assisted tip is 5 ng mL–1 (Fig. 4e) with a low intensity under 200. When the sample concentration decreases to 1 ng mL–1, no peak can be detected using the polymer-assisted tip (Fig. 4f). The results indicate that the selective enrichment efficiency of the polymer-assisted tip towards p-peptide is lower than binder-free T-tip. The use of polymer in tip packing reduces the content of functional TiO2; moreover, the polymer matrix may partially cover the TiO2 surface and thus reduce the effective surface area for p-peptide binding. Compared with the binder (polymer)-assisted tip in the current study and previous reports,[12] the binder-free T-tip shows a lower detection limit because there is a higher availability of functional groups on the surface of the anatase in the absence of surface-bound binder, which enables the sensitive detection of p-peptides in a trace amount.
Conclusion
In the present study, a binder-free method was applied to fabricate macroporous TiO2 monolith-packed pipette tips for high-efficiency p-ERK1 peptide enrichment. The binder-free method allows a high TiO2 content, which exhibits a low limit (1 ng mL–1) of p-ERK1 peptide detection. It was demonstrated that the homemade T-tip is more sensitive than the commercial NuTip and polymer-assisted TiO2 tips, showing great potential in applications for p-peptide- and protein-based biomarker detection.
Experimental
Chemicals
ERK1 (active, human), TiBALDH, (50 wt-% in water), acetonitrile (ACN, 99.9 %), ammonium hydroxide solution, 2,5-dihydroxybenzoic acid (DHB), α,α,-dimethoxy-α-phenylacetophenone (DPA), ethylene glycol dimethacrylate (EDMA), 3-aminophenol, formaldehyde solution, ammonium bicarbonate (NH4HCO3), dithiothreitol (DTT), and iodoacetamide (IAA) were purchased from Sigma–Aldrich. Trypsin was ordered from Promega. Trifluoroacetic acid (TFA, 99.8 %) was purchased from Merck. Ethanol was received from ChemSupply. All reagents were used as received without further purification. Deionized water (DI water) used for all experiments was obtained from a Milli-Q system (Millipore, Bedford, MA).
Materials Synthesis
The synthesis protocol of APF spheres was adopted from the literature.[29] In a typical synthesis, 0.18 g of 3-aminophenol (final concentration 98.1 mM) was dissolved in ammonia (0.054 mL, 47.7 mM)–water (12 mL)–ethanol (4.8 mL) solution at 30°C. Then, 0.243 mL of formaldehyde solution was added to form a final concentration of 196 mM and the mixture was stirred at 30°C for 4 h. The resulting resin spheres were collected by centrifugation and washed with deionized water 3 times.
Synthesis of TiO2 monolith: 0.6 g APF spheres were dispersed in 30 mL of TiBALDH solution (50 wt-% in water) and stirred at room temperature for 24 h. After centrifugation, the supernatant was discarded and the mixture was calcined at 400°C for 5 h to remove the APF spheres. The final TiO2 monolith was collected and the particles of different sizes were separated with sieves.
Materials Characterization
SEM images were obtained using a Jeol JSM 7800 field-emission scanning electron microscope (FE-SEM) operated at 5 kV using gentle beam mode. Before the SEM test, the sample was placed on a conductive carbon tape and dried in a vacuum oven at 60°C for 12 h. TEM images were taken with a Jeol 1010 microscope operating at 100 kV by dispersing the samples on a Cu grid covered with carbon film. DLS measurements were carried out at 25°C using a Zetasizer Nano-ZS (Malvern Instruments). The XRD pattern was recorded on a Rigaku Miniflex X-ray diffractometer with Co-Kα radiation (wavelength 0.179 nm). Nitrogen sorption isotherms of the samples were obtained with a Micromeritics Tristar II 3020 system at 77 K. Before the measurements, the samples were degassed at 100°C for at least 8 h under vacuum.
Fabrication of TiO2 Entrapped Pipette Tips (T-Tip)
The binder-free packing was achieved by choosing the monolith sizes. The TiO2 monoliths were separated into three groups: (I) 0.60–0.85 mm, (II) <0.60 mm, and (III) >0.85 mm. A monolith was chosen from group I, and placed at the lower end of the tip. The particle size is larger than the inner diameter of the 10 µL tip (~0.58 mm), and can be fixed at the bottom of the tip. Then, monoliths with smaller sizes (group II) were filled into the tip followed by the entrapment of one large piece (group III) on the top. The packed tips were washed with methanol 4 times to remove any loose small pieces and check the stability of the entrapped monoliths. The TiO2 loading amount was measured for three groups of tips (10 tips in each group) to get the mean loading of TiO2 in one tip.
The protocol of polymer-assisted packing was adapted from the literature.[10] Briefly, 1 mg of TiO2 monolith was ground into small pieces and dispersed in 40 µL of methanol. Then, 0.2 µL of DPA and 25 µL of EDMA were added as the initiator and monomer. The mixture was stirred for 5 min to form a suspension. The suspension (1 µL) was aspirated into a pipette tip and then a capillary tubing template was inserted into the tip to create a main channel through the immobilized bed. The tip containing the mixture was irradiated with 365 nm UV light to activate the polymerization. After 1 min exposure, the capillary tube was removed from the polymer monolithic tip, and the tip was further exposed to UV light for 20 min to form a TiO2-entrapped pipette tip with a main channel. Before use, the tip was aspirated with 10 µL of methanol and washed 10 times to remove unbound TiO2 monoliths and unreacted monomer.
P-ERK Peptide Enrichment
p-ERK1 protein solution (10 µL, 0.1 µg µL–1) was mixed with 8 µL of 100 mM NH4HCO3 and 0.5 µL of 10 mM DTT, and the mixture was incubated at 50°C in thermomixer for 15 min. After cooling down to room temperature, 1.25 µL of 10 mM IAA was added and the mixture incubated in the dark for 15 min. Next, 0.05 µg of trypsin was added to the solution to give a protein/enzyme ratio of 20 : 1. After incubation at 37°C for 14 h, the p-ERK1 protein was digested into peptides. The p-ERK peptide solution was diluted into different concentrations for the enrichment study.
Before sample loading, the T-tip was wet with 100 % ACN (3 × 10 µL) and equilibrated with 5 % ACN/0.1 %TFA (3 × 10 µL). The sample (100 µL) was loaded by pipetting the peptide solution up and down 20 times. Then, the T-tip was washed with 5 % ACN/0.1 %TFA (3 × 10 µL). In the final step, the loaded sample was eluted with 10 µL of 100 mM NH4OH (pH 11.5) into a new tube and ready for the mass spectrometry test. For quantification, 10 µL of IS (0.06 µg mL–1) was added to 10 µL of eluted p-ERK1 peptide solution (0.02–0.1 µg mL–1). Samples were run in triplicate.
The mass spectrum of tryptic-digested ERK1 after isolation with TiO2 tips was acquired on a Bruker Autoflex TOF/TOF III Smart beam. Eluted sample (1 µL) was deposited on a MALDI MTP 384 plate and mixed with 1 µL of DHB (10 mg mL–1 in 50 % ACN/0.1 %TFA). The reflector mode via an accumulation of 500 laser shots under an intensity of 39 % was applied to collect mass spectra at 10 different sites for each sample. Tryptic digested ERK1 solution (1 µL) was spotted on the MALDI plate without T-tip enrichment and mixed with 1 µL of DHB as a control. A Bruker peptide calibration standard, angiotensin II (1046.5 Da), angiotensin I (1296.7 Da), Substance P (1347.7 Da), bombesin (1619.8 Da), ACTH-Clip 1–17 (2093.1 Da), ACTH-Clip 18–39 (2465.2 Da), and somatostatin 28 (3147.5 Da), was used for calibration purposes to reduce variability. The sequence analysis was conducted in the MS/MS (LIFT) mode, and identified by matching the result with database analysis using ExPASy-PeptideMass and Proteomics Toolkit.
Supplementary Material
Figs S1–S6 (images of T-tip, sequence analysis, etc.) are available on the Journal’s website.
Acknowledgements
The authors acknowledge the Australian Research Council, Queensland Cancer Council, and Queensland Government for financial support. We thank the Australian National Fabrication Facility and Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy and Microanalysis, The University of Queensland.
References
[1] B. Bodenmiller, L. N. Mueller, M. Mueller, B. Domon, R. Aebersold, Nat. Methods 2007, 4, 231.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitFChsbs%3D&md5=bac404df9979539744adaf5187c085d1CAS | 17293869PubMed |
[2] C. J. Marshall, Cell 1995, 80, 179.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXjtlGnsr8%3D&md5=e8b1b6d9f7fad59dee3cc5f6f5f63d9aCAS | 7834738PubMed |
[3] I. Prasadam, T. Friis, W. Shi, S. van Gennip, R. Crawford, Y. Xiao, Bone 2010, 46, 226.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFynsrrE&md5=bec9d685aa09a8c0db68fdc48cf62ed1CAS | 19853676PubMed |
[4] I. Prasadam, S. van Gennip, T. Friis, W. Shi, R. Crawford, Y. Xiao, Arthritis Rheum. 2010, 62, 1349.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGnu7nL&md5=cc3ff3cc0fe0492f8b4c65a222cfbb15CAS | 20155832PubMed |
[5] C. Lei, K. Qian, O. Noonan, A. Nouwensa, C. Z. Yu, Nanoscale 2013, 5, 12033.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhvVejur7O&md5=52060078349502d1a7b26df6afb23356CAS | 24162102PubMed |
[6] L. Qiao, C. Roussel, J. J. Wan, P. Y. Yang, H. H. Girault, B. H. Liu, J. Proteome Res. 2007, 6, 4763.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlOgsrzK&md5=da296200d14eab4394569fc5acb622d6CAS | 18047269PubMed |
[7] A. Leitner, TrAC, Trends Anal. Chem. 2010, 29, 177.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhslWqsr0%3D&md5=3f8cc4f598f91fe2a187f02e63e7301aCAS |
[8] T. E. Thingholm, T. J. D. Jorgensen, O. N. Jensen, M. R. Larsen, Nat. Protoc. 2006, 1, 1929.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFagt77N&md5=c0fa84eb2c922f5336e8cd13505ff7c0CAS | 17487178PubMed |
[9] H. Matsuda, H. Nakamura, T. Nakajima, Anal. Sci. 1990, 6, 911.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXnsVCqtg%3D%3D&md5=a217d7cca804789aa5042dc1d853595fCAS |
[10] H. C. Hsieh, C. Sheu, F. K. Shi, D. T. Li, J. Chromatogr. A 2007, 1165, 128.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXpvFKns7o%3D&md5=4273eeb0a23b0669adb57bbdc281222dCAS | 17714720PubMed |
[11] S. Miyazaki, K. Morisato, N. Ishizuka, H. Minakuchi, Y. Shintani, M. Furuno, K. Nakanishi, J. Chromatogr. A 2004, 1043, 19.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVSlu7c%3D&md5=18aa7dcf97bfeb058b18909bebc8aa0fCAS | 15317408PubMed |
[12] M. Rainer, H. Sonderegger, R. Bakry, C. W. Huck, S. Morandell, L. A. Huber, D. T. Gjerde, G. K. Bonn, Proteomics 2008, 8, 4593.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVCjtrvK&md5=ccfb2e185133548d974bb21f0152adcaCAS | 18837466PubMed |
[13] R. H. Sui, S. Y. Liu, G. A. Lajoie, P. A. Charpentier, J. Sep. Sci. 2010, 33, 1604.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmvVGgsL8%3D&md5=ad5ad6eff76af179409e7b103b68bdc4CAS |
[14] T. Kubo, N. Tsujioka, N. Tanaka, K. Hosoya, Mater. Lett. 2010, 64, 177.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVGntb3N&md5=1730ac5b36857040c76cae93401ba595CAS |
[15] L. Wan, M. C. Long, D. Y. Zhou, L. Y. Zhang, W. M. Cai, Nano-Micro Lett. 2012, 4, 90.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVenurvK&md5=ef456f52ee1f6402b49d23c73b431d67CAS |
[16] M. Sturm, A. Leitner, J. H. Smatt, M. Linden, W. Lindner, Adv. Funct. Mater. 2008, 18, 2381.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVyltbvM&md5=d12ba8ef951917b1e16912c5d53f88eaCAS |
[17] A. Leitner, M. Sturm, J. H. Smatt, M. Jarn, M. Linden, K. Mechtler, W. Lindner, Anal. Chim. Acta 2009, 638, 51.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjs1amsLc%3D&md5=b7173039911d1cb91b7982b36a6f1645CAS | 19298879PubMed |
[18] A. Leitner, M. Sturm, O. Hudecz, M. Mazanek, J. H. Smatt, M. Linden, W. Lindner, K. Mechtler, Anal. Chem. 2010, 82, 2726.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXislyqsro%3D&md5=4cf0976672ef8899e9828ff452b097f3CAS | 20201521PubMed |
[19] J. H. Smatt, N. Schuwer, M. Jarn, W. Lindner, M. Linden, Microporous Mesoporous Mater. 2008, 112, 308.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmtVKgs78%3D&md5=d80ef9ec7f4986a05f436ac550c6383fCAS |
[20] Y. A. Nor, L. Zhou, A. K. Meka, C. Xu, Y. Niu, H. Zhang, N. Mitter, D. Mahony, C. Yu, Adv. Funct. Mater. 2016, 26, 5408.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhtVahtbjI&md5=d50a8a96acc3b529c0d919fd3d1cb161CAS |
[21] M. Wu, Y. Li, Z. Deng, B. L. Su, ChemSusChem 2011, 4, 1481.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOmsrbN&md5=25e63e808fe1fb505d16f8887246cb6fCAS | 21994156PubMed |
[22] H. Borchert, E. V. Shevehenko, A. Robert, I. Mekis, A. Kornowski, G. Grubel, H. Weller, Langmuir 2005, 21, 1931.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXosFWisg%3D%3D&md5=84734f203d49dffb5db53d887d5da738CAS | 15723491PubMed |
[23] S. S. Liang, H. Makamba, S. Y. Huang, S. H. Chen, J. Chromatogr. A 2006, 1116, 38.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XksFaltbw%3D&md5=fc0ec1108add0066b055bbdc6743e6aaCAS | 16580007PubMed |
[24] J. Konishi, K. Fujita, K. Nakanishi, K. Hirao, K. Morisato, S. Miyazaki, M. Ohira, J. Chromatogr. A 2009, 1216, 7375.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1Gks7bF&md5=418774f0da3d43615ccf21d8ebb035f3CAS | 19580973PubMed |
[25] J. Nawrocki, C. Dunlap, A. McCormick, P. W. Carr, J. Chromatogr. A 2004, 1028, 1.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltVGnsw%3D%3D&md5=6641c2531b916ab6943778d41c42aeabCAS | 14969280PubMed |
[26] K. Qian, W. Y. Gu, P. Yuan, F. Liu, Y. H. Wang, M. Monteiro, C. Z. Yu, Small 2012, 8, 231.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFGrsrzO&md5=09ed4cb8aba9dae7a51b999b362643e4CAS | 22135224PubMed |
[27] M. Cuollo, S. Caira, O. Fierro, G. Pinto, G. Picariello, F. Addeo, Rapid Commun. Mass Spectrom. 2010, 24, 1687.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmt12lsr8%3D&md5=4911446491e9d3f761148b59fc9fc33eCAS | 20486267PubMed |
[28] GlySci, NuTipTM. Available at: http://www.glysci.com/products/NuTip.html (accessed 2 September 2016).
[29] J. M. Zhao, W. X. Niu, L. Zhang, H. R. Cai, M. Y. Han, Y. L. Yuan, S. Majeed, S. Anjum, G. B. Xu, Macromolecules 2013, 46, 140.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhvVGrtbbL&md5=674b18c4e67eac8e9a6fabbcb16b8c40CAS |
* Chengzhong Yu is the recipient of the 2015 Le Fèvre Memorial Prize of the Council of the Australian Academy of Science.




