An Efficient One-pot Two-component Protocol for Regio- and Chemoselective Synthesis of 5-Aryloyl-1,3,7,9-tetraalkyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-diones
Mehdi Rimaz A D , Hossein Rabiei A , Behzad Khalili B and Rolf H. Prager CA Department of Chemistry, Payame Noor University, PO Box 19395-3697, Tehran, Iran.
B Department of Chemistry, Faculty of Sciences, University of Guilan, PO Box 41335-1914, Rasht, Iran.
C School of Chemistry, Physics and Earth Sciences, Flinders University, Adelaide 5001, Australia.
D Corresponding author. Email: rimaz.mehdi@gmail.com
Australian Journal of Chemistry 67(2) 283-288 https://doi.org/10.1071/CH13438
Submitted: 27 August 2013 Accepted: 8 October 2013 Published: 12 November 2013
Abstract
Novel symmetric fused pyrano[2,3-d]pyrimidine derivatives were synthesized in 75–92 % yield by a one-pot two-component reaction of arylglyoxals and 1,3-dialkyl-2-thiobarbituric acids in ethanol at room temperature. This is the first protocol to be reported for the synthesis of 5-aryloyl-1,3,7,9-tetraalkyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d′]dipyrimidine-4,6(5H,7H)-diones and the significant features of the present protocol are simplicity, high yields, a simple isolation procedure, and high chemoselectivity.
Introduction
Pyrane and its derivatives have attracted great interest owing to the incorporation of this ring system in compounds showing antimicrobial,[1–3] influenza inhibition, virus sialidase,[4] mutagenic,[5] antiviral,[6] antiproliferation,[7] sex-pheromone,[8] antitumour,[9] and anti-inflammatory activity.[10] Moreover, pyrane derivatives are well known for their antihistaminic activity.[11] Also, pyrimidines and their fused derivatives play an essential role in several biological processes of chemical and pharmacological importance. In particular, the pyrimidine nucleus can be found in a broad variety of antibacterial, antiviral, NAR (nicotinic acid receptor), and antitumour agents as well as in agrochemical and veterinary products.[12–15] Examples of such compounds are emivirine and 5-ethyl-2-thioxo-2,3-dihydro-1H-pyrano[2,3-d]pyrimidine pyrano[2,3-d]pyrimidine-4,7-dione (Fig. 1.).[16,17]

|
Designing new multicomponent reactions (MCRs) as well as improving known MCRs constitutes a research area of immense interest in contemporary organic synthesis.[18] In contrast to classical multistep linear synthetic protocols, MCRs enable expedient and efficient assembly of molecules of structural complexity and diversity in one-pot operations with facile execution, and high atom economy and selectivity.[18b,18c,19,20] These reactions obviate the isolation and purification of intermediates and diminish waste generation, thereby enhancing the greenness of transformations. Consequently, MCRs have emerged as a powerful tool for delivering molecular libraries needed in combinatorial approaches for the assembly and lead identification of bioactive compounds.[21]
Based on the above considerations and in continuance of our previous research into the arylglyoxal-mediated synthesis of various heterocycles,[22–32] we have found that 1,3-dialkyl thiobarbituric acids undergo a tandem Knoevenagel–Michael condensation with arylglyoxals leading to the formation of a substituted pyranopyrimidine skeleton. Herein, we report an efficient two-component strategy for synthesis of symmetrically substituted 5-aryloyl-1H-pyrano[2,3-d]pyrimidine 1. The retrosynthesis of this novel strategy is shown in Scheme 1.

|
Results and Discussion
During our recent work on the synthesis of new pyrimidopyridazine derivatives,[22] we found that in some cases stirring the mixture of 1,3-diethyl-2-thiobarbituric acid or 1,3-dimethyl-2-thiobarbituric acid and arylglyoxals with hydrazine in ethanol at room temperature led to the formation of insoluble intermediates that did not react with the hydrazine. We have now investigated these reactions in the absence of hydrazine and have characterized the products as previously unreported pyranopyrimidine derivatives.
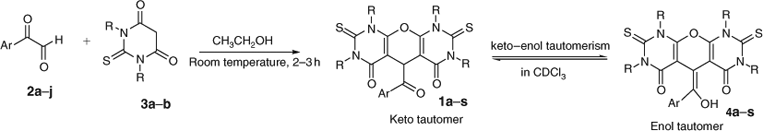
The arylglyoxal derivatives (2a–j) react with two equivalents of 1,3-diethyl-2-thiobarbituric acid (3a) or 1,3-dimethyl-2-thiobarbituric acid (3b) in ethanol at room temperature to produce symmetric pyrano[2,3-d]pyrimidine derivatives 1a–s that partially exist as the enol tautomers 4a–s in solution (Scheme 2, Table 1).

|
The reactions proceeded to completion in 2–3 h, and the pure products were obtained simply by recrystallization from methanol; no chromatographic purification was necessary. As mentioned above, the enol forms exist only in solution and all compounds were obtained as keto forms from the reaction mixture. Notably, there is no signal for the OH group in Fourier-transform (FT)IR spectra because these spectra were recorded in the solid state. The keto/enol ratio was ~1 : 1 in all cases in chloroform, except with the strongly electron withdrawing nitro substituent in 1o, where none of the enol tautomer could be detected. However, when the N-alkyl group was changed from methyl to ethyl, as in 1e, the general 1 : 1 keto/enol ratio in solution was restored. The novel compounds listed in Table 1 were characterized by IR, 1H NMR and 13C NMR spectra, and elemental analyses.
As shown in Table 1, all the obtained pyranopyrimidine derivatives exist as mixtures of keto and enol tautomers in solution. In the 1H NMR spectra of the products, the OH group of the enol tautomer shows a broad singlet at δ 8.21–13.18 ppm, and in keto tautomers, the α CH adjacent to the carbonyl of the aryloyl group shows a sharp singlet at δ 4.91–5.69 ppm, which was used to calculate the keto/enol ratio.
As suggested in Scheme 3, the formation of the product involves the initial regioselective formation of a Knoevenagel adduct 6 by condensation between 2 and 5, followed by regioselective Michael addition between 6 and 5 to give the intermediate 7, dehydration of which leads to the formation of products 1a–s, and their enol tautomers 4a–s.

|
As noted in our previous work, the initial attack of the 1,3-dialkyl-2-thiobarbituric acid occurred exclusively on the formyl group of the arylglyoxal and subsequent Michael addition also occurred regioselectively and chemoselectively.
The possible utility of the products described in the present paper is enhanced by noting that the high enol content of the products in solution allows their facile alkylation on carbon or oxygen, allowing modification of their shape or transport properties. This aspect of the work is being actively pursued.
Conclusion
In summary, we have developed an efficient high-yielding protocol for the regio- and chemoselective synthesis of substituted symmetric pyrano[2,3-d]pyrimidines that does not require any catalyst. The protocol involves a two-component reaction of arylglyoxals with 1,3-dialkyl-2 thiobarbituric acids in ethanol at room temperature, leading to chemoselective synthesis of 5-aryloyl-1,3,7,9-tetraalkyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d′]dipyrimidine-4,6(5H,7H)-diones. The advantages offered by this strategy are: simple operation, mild reaction conditions, ease of product isolation, and good to excellent yields.
Experimental
General Procedures
Arylglyoxals and 1,3-dimethyl-2-thiobarbituric acid were prepared by reported procedures.[33,34] Other starting materials and solvents were obtained from Merck (Germany) and Fluka (Switzerland) and were used without further purification. The methods used to monitor the reactions were TLC and NMR. Melting points were measured on an Electrothermal 9200 apparatus. IR spectra were obtained on a Nexus-670 FTIR spectrometer. 1H and 13C NMR spectra (CDCl3) were recorded on a Bruker DRX-300 Avance spectrometer at 300 and 75.5 MHz respectively. Elemental analyses were performed using a Leco Analyzer 932.
General Procedure for the Synthesis of 5-Aryloyl-1,3,7,9-tetraalkyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-diones
A mixture of 1,3-diethyl (or dimethyl)-2-thiobarbituric acid (1 mmol) and arylglyoxal (1 mmol) in absolute ethanol (10 mL) was stirred at room temperature for 2–3 h. After the appropriate time, the resulting precipitate was filtered and washed with ethanol. The crude products were recrystallized from methanol.
5-Benzoyl-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1a
Cream powder, 79 %, mp 197°C (dec.). δH 1.12 (t, J 6.9, 6H, 2 × CH3), 1.36 (t, J 6.9, 6H, 2 × CH3), 4.49 (q, J 6.9, 4H, 2 × CH2), 4.62 (q, J 6.9, 4H, 2 × CH2), 5.57 (s, 1H, CH in keto tautomer), 7.37 (t, J 7.5, 2H, Ar), 7.49 (t, J 7.5, 1H, Ar), 7.67 (d, J 7.5, 2H, Ar), 10.06 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.5, 44.5, 44.9, 95.9, 127.6, 128.2, 132.7, 136.0, 162.3, 162.9, 174.5, 194.4. νmax (KBr)/cm–1 2981, 2935, 2520, 1694, 1622, 1444, 1384, 1269, 1110, 785. Anal. Calc. for C24H26N4O4S2: C 57.81, H 5.26, N 11.24, S 12.86. Found: C 57.90, H 5.29, N 11.30, S 12.90 %.
5-(4-Bromobenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1b
Pink powder, 81 %, mp 193°C (dec.). δH 1.15 (t, J 6.9, 6H, 2 × CH3), 1.35 (t, J 6.9, 6H, 2 × CH3), 4.50 (q, J 6.3, 4H, 2 × CH2), 4.61 (q, J 6.3, 4H, 2 × CH2), 5.52 (s, 1H, CH in keto tautomer), 7.57–7.48 (m, 4H, Ar), 11.58 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.4, 44.6, 45.0, 95.6, 127.7, 129.1, 131.5, 134.8, 162.4, 162.9, 174.4, 193.4. νmax (KBr)/cm–1 2981, 2934, 2521, 1700, 1621, 1585, 1437, 1383, 1269, 1110, 798. Anal. Calc. for C24H25BrN4O4S2: C 49.91, H 4.36, N 9.70, S 11.10. Found: C 49.84, H 3.31, N 9.88, S 11.14 %.
5-(4-Chlorobenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1c
Yellow powder, 85 %, mp 202°C (dec.). δH 1.16 (t, J 6.3, 6H, 2 × CH3), 1.33 (t, J 6.3, 6H, 2 × CH3), 4.51 (q, J 6.3, 4H, 2 × CH2), 4.62 (q, J 6.3, 4H, 2 × CH2), 5.53 (s, 1H, CH in keto tautomer), 7.36 (d, J 8.4, 2H, Ar), 7.63 (d, J 8.1, 2H, Ar), 9.47 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.4, 44.6, 45.0, 95.7, 128.6, 129.0, 134.3, 139.1, 162.4, 162.9, 174.4, 193.2. νmax (KBr)/cm–1 2982, 2935, 2875, 2516, 1699, 1621, 1435. Anal. Calc. for C24H25ClN4O4S2: C 54.08, H 4.73, N 10.51, S 12.03. Found: C 54.12, H 4.70, N 10.60, S 12.10 %.
5-(4-Fluorobenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1d
Pink powder, 80 %, mp 204°C (dec.). δH 1.15 (bt, J 6.9, 6H, 2 × CH3), 1.35 (bt, J 6.3, 6H, 2 × CH3), 4.50 (q, J 6.3, 4H, 2 × CH2), 4.62 (q, J 6.3, 4H, 2 × CH2), 5.54 (s, 1H, CH in keto tautomer), 7.08–7.02 (m, 2H, Ar), 7.73–7.69 (m, 2H, Ar), 9.78 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.4, 44.6, 45.0, 95.8, 115.3, 115.6, 130.2, 130.3, 132.2, 162.4, 162.9, 163.5, 166.9, 174.4, 192.8. νmax (KBr)/cm–1 3075, 2984, 2520, 1698, 1623, 1597, 1440, 1383, 1269, 1110, 849, 783. Anal. Calc. for C24H25FN4O4S2: C 55.80, H 4.88, N 10.85, S 12.41. Found: C 55.78, H 4.89, N 10.96, S 12.50 %.
5-(4-Nitrobenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1e
Grey powder, 88 %, mp 223°C (dec.). δH 1.15 (t, J 6.6, 6H, 2 × CH3), 1.36 (t, J 6.6, 6H, 2 × CH3), 4.49 (q, J 6.6, 4H, 2 × CH2), 4.62 (q, J 6.6, 4H, 2 × CH2), 5.58 (s, 1H, CH in keto tautomer), 7.76 (d, J 8.7, 2H, Ar in enol tautomer), 7.84 (d, J 8.7, 2H, Ar in keto tautomer), 8.25 (d, J 8.7, 2H, Ar in keto tautomer), 8.28 (d, J 8.7, 2H, Ar in enol tautomer), 9.92 (bs, 1H, OH in enol tautomer). δC 11.6, 11.9, 41.8, 44.6, 44.9, 95.1, 123.4, 125,6, 128.6, 141.4, 149.8, 161.5, 162.3, 163.0, 174.4, 193.2. νmax (KBr)/cm–1 3078, 2976, 2934, 2521, 1709, 1619, 1528, 1430, 1382, 1271, 1110, 857, 798. Anal. Calc. for C24H25N5O6S2: C 53.03, H 4.64, N 12.88, S 11.80. Found: C 53.00, H 4.68, N 13.01, S 11.83 %.
5-(4-Methoxybenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1f
Pink powder, 82 %, mp 202°C (dec.). δH 1.52–1.02 (m, 12H, 4 × CH3), 3.84 (s, 3H, OCH3), 4.77–4.27 (m, 8H, 4 × CH2), 5.55 (s, 1H, CH in keto tautomer), 6.85 (d, J 8.7, 2H, Ar), 7.69 (d, J 8.7, 2H, Ar), 9.44 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.5, 44.5, 45.0, 55.4, 96.0, 112.5, 119.3, 119.9, 129.1, 137.2, 159.4, 162.4, 162.8, 174.4, 194.1. νmax (KBr)/cm–1 2981, 2935, 2521, 1688, 1620, 1437, 1383, 1263, 1109, 780. Anal. Calc. for C25H28N4O5S2: C 56.80, H 5.34, N 10.60, S 12.13. Found: C 56.84, H 5.36, N 10.69, S 12.18 %.
5-(3-Bromobenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1g
Pink powder, 75 %, mp 179°C (dec.). δH 1.18 (t, J 6.9, 6H, 2 × CH3), 1.37 (t, J 6.9, 6H, 2 × CH3), 3.91 (q, J 6.9, 4H, 2 × CH2), 3.94 (q, J 6.9, 4H, 2 × CH2), 5.59 (s, 1H, CH in keto tautomer), 7.03 (d, J 6.9, 1H, Ar), 7.32–7.25 (m, 3H, Ar), 11.63 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.6, 44.6, 45.0, 95.6, 122.4, 125.9, 129.7, 130.9, 135.5, 137.8, 162.3, 162.9, 174.5, 193.0. νmax (KBr)/cm–1 2955, 2878, 2559, 1708, 1633, 1592, 1459, 1375, 1110, 878, 795. Anal. Calc. for C24H25BrN4O4S2: C 49.91, H 4.36, N 9.70, S 11.10. Found: C 49.93, H 4.37, N 9.81, S 11.17 %.
5-(3-Methoxybenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1h
White powder, 77 %, mp 179°C (dec.). δH 1.15 (t, J 6.3, 6H, 2 × CH3), 1.35 (t, J 6.3, 6H, 2 × CH3), 3.81 (s, 3H, OCH3), 4.51 (q, J 6.3, 4H, 2 × CH2), 4.61 (q, J 6.3, 4H, 2 × CH2), 5.56 (s, 1H, CH in keto tautomer), 7.04 (d, J 7.8, 1H, Ar in enol tautomer), 7.30–7.18 (m, 3H, Ar), 10.95 (bs, 1H, OH in enol tautomer). δC 11.6, 12.0, 41.5, 44.6, 45.0, 55.4, 96.0, 112.5, 119.3, 119.9, 129.1, 137.2, 159.4, 162.4, 162.8, 174.5, 194.1. νmax (KBr)/cm–1 2979, 2934, 2523, 1702, 1621, 1445, 1380, 1269, 1110, 790. Anal. Calc. for C25H28N4O5S2: C 56.80, H 5.34, N 10.60, S 12.13. Found: C 56.79, H 5.39, N 10.71, S 12.16 %.
5-(3,4-Dimethoxybenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1i
Grey powder, 84 %, mp 201°C (dec.). δH 1.47–0.90 (m, 12H, 4 × CH3), 3.87 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 4.72–4.33 (m, 8H, 4 × CH2), 5.57 (s, 1H, CH in keto tautomer), 6.77 (d, J 8.4, 1H, Ar in),7.26 (d, J 8.4, 1H, Ar in enol tautomer), 7.34 (s, 1H, Ar), 12.01 (bs, 1H, OH in enol tautomer). δC 11.9, 41.4, 44.3, 44.7, 55.9, 56.0, 96.3, 109.8, 110.8, 121.7, 128.4, 148.7, 153.0, 162.6, 174.4, 192.6. νmax (KBr)/cm–1 2987, 2898, 2542, 1692, 1623, 1561, 1472, 1385, 1270, 1112, 771. Anal. Calc. for C26H30N4O6S2: C 55.90, H 5.41, N 10.03, S 11.48. Found: C 55.95, H 4.44, N 10.10, S 11.53 %.
5-(3,4-Methylenedioxybenzoyl)-1,3,7,9-tetraethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1j
Pink powder, 83 %, mp 161°C (dec.). δH (CDCl3, 300 MHz) 1.23 (t, J 7.5, 6H, 2 × CH3), 1.34 (t, J 7.5, 6H, 2 × CH3), 4.71–4.29 (m, 8H, 4 × CH2), 5.53 (s, 1H, CH in keto tautomer), 6.03 (s, 2H, CH2), 6.76 (d, J 8.1, 1H, Ar in), 7.26–7.14 (m, 2H, Ar), 7.34 (s, 1H, Ar), 8.21 (bs, 1H, OH in enol tautomer). δC 11.8, 11.9, 44.7, 44.8, 96.1, 101.8, 123.4, 130.1, 147.8, 151.5, 163.1, 174.5, 192.4. νmax (KBr)/cm–1 2982, 2935, 2527, 1701, 1620, 1440, 1383, 1269, 1250, 1110, 1038, 793, 731. Anal. Calc. for C25H26N4O6S2: C 55.34, H 4.83, N 10.33, S 11.82. Found: C 55.31, H 4.81, N 10.45, S 11.88 %.
5-Benzoyl-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1k
Cream powder, 83 %, mp 202°C (dec.) δH (CDCl3, 300 MHz) 3.84–3.58 (m, 12H, 4 × CH3), 5.69 (s, 1H, CH in keto tautomer), 7.40 (t, J 7.5, 2H, Ar), 7.53 (t, J 7.5, 1H, Ar), 7.73 (d, J 7.5, 2H, Ar), 8.55 (bs, 1H, OH in enol tautomer). δC 35.3, 36.6, 41.5, 95.9, 127.8, 128.5, 133.0, 135.7, 162.8, 163.2, 175.4, 194.2. νmax (KBr)/cm–1 2952, 2869, 2484, 1702, 1621, 1467, 1394, 1339, 1295, 1339, 1110, 789. Anal. Calc. for C24H26N4O4S2: C 57.81, H 5.26, N 11.24, S 12.86. Found: C 57.90, H 5.29, N 11.30, S 12.90 %.
5-(4-Bromobenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1l
Pink powder, 87 %, mp 237°C (dec.). δH 3.62 (s, 6H, 2 × CH3), 3.84 (s, 6H, 2 × CH3), 5.63 (s, 1H, CH in keto tautomer), 7.55 (d, J 8.4, 2H, Ar), 7.61 (d, J 8.1, 2H, Ar), 9.49 (bs, 1H, OH in enol tautomer). δC 36.4, 36.6, 41.5, 95.7, 128.1, 129.3, 131.9, 134.5, 162.7, 163.2, 175.4, 193.2. νmax (KBr)/cm–1 2947, 2556, 1706, 1619, 1586, 1461, 1391, 1342, 1111, 810. Anal. Calc. for C20H17BrN4O4S2: C 46.07, H 3.29, N 10.75, S 12.30. Found: C 46.10, H 3.31, N 10.66, S 12.35 %.
5-(4-Chlorobenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1m
Yellow powder, 86 %, mp 227°C (dec.). δH 3.71 (s, 6H, 2 × CH3), 3.82 (s, 6H, 2 × CH3), 5.63 (s, 1H, CH in keto tautomer), 7.46 (d, J 6.9, 2H, Ar), 7.67 (d, J 6.9, 2H, Ar), 8.58 (bs, 1H, OH in enol tautomer). δC 36.9, 37.1, 41.7, 96.3, 128.7, 129.8, 132.8, 135.1, 161.6, 163.1, 177.3, 192.8. νmax (KBr)/cm–1 2951, 2932, 2551, 1708, 1620, 1590, 1465, 1387, 1226, 1110, 790. Anal. Calc. for C20H17ClN4O4S2: C 50.36, H 3.59, N 11.75, S 13.45. Found: C 50.32, H 3.57, N 11.60, S 13.50 %.
5-(4-Fluorobenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1n
Pink powder, 84 %, mp 210°C (dec.). δH 3.72 (s, 6H, 2 × CH3), 3.83 (s, 6H, 2 × CH3), 5.65 (s, 1H, CH in keto tautomer), 7.11–7.05 (m, 2H, Ar), 7.79–7.75 (m, 2H, Ar), 11.21 (bs, 1H, OH in enol tautomer). δC 36.6, 41.5, 95.8, 115.6, 115.9, 130.4, 130.5, 131.9, 132.0, 162.7, 163.1, 163.7, 167.1, 175.4, 192.6. νmax (KBr)/cm–1 3069, 2981, 2538, 1692, 1618, 1588, 1472, 1381, 1264, 856, 778. Anal. Calc. for C20H17FN4O4S2: C 52.16, H 3.72, N 12.17, S 13.93. Found: C 52.14, H 3.75, N 12.01, S 13.97 %.
5-(4-Nitrobenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1o
Beige powder, 92 %, mp 228°C (dec.). δH 3.70 (s, 6H, 2 × CH3), 3.85 (s, 6H, 2 × CH3), 5.69 (s, 1H, CH in keto tautomer), 7.89 (d, J 8.7, 2H, Ar in keto tautomer), 8.27 (d, J 8.7, 2H, Ar in keto tautomer). δC 36.4, 36.7, 42.0, 95.1, 123.7, 124.6, 128.7, 141.0, 150.1, 162.7, 175.4, 202.5. νmax (KBr)/cm–1 2951, 2541, 1713, 1621, 1526, 1394, 1345, 1109, 789. Anal. Calc. for C20H17N5O6S2: C 49.27, H 3.51, N 14.37, S 13.15. Found: C 49.30, H 3.55, N 14.30, S 13.19 %.
5-(4-Methoxybenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1p
Pink powder, 87 %, mp 201°C (dec.). δH 3.14 (s, 6H, 2 × CH3), 3.16 (s, 6H, 2 × CH3), 3.83 (s, 3H, OCH3), 4.91 (s, 1H, CH of keto tautomer), 6.87 (d, J 8.4Hz, 2H, Ar), 7.32 (d, J 8.1Hz, 2H, Ar), 9.49 (s, 1H, OH in enol tautomer). δC 36.6, 41.3, 55.4, 96.1, 113.8, 114.2, 128.1, 130.2, 131.0, 162.8, 163.5, 175.4, 192.8. νmax (KBr)/cm–1 2947, 2560, 1691, 1595, 1466, 1395, 1338, 1171, 1110, 1023, 787. Anal. Calc. for C21H20N4O5S2: C 53.38, H 4.27, N 11.86, S 13.57. Found: C 53.41, H 4.25, N 11.77, S 13.61 %.
5-(3-Bromobenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1q
Pink powder, 79 %, mp 152°C (dec.). δH 3.72 (s, 6H, 2 × CH3), 3.82 (s, 6H, 2 × CH3), 5.67 (s, 1H, CH in keto tautomer), 7.06 (d, J 5.4, 1H, Ar), 7.32–7.25 (m, 3H, Ar), 13.18 (bs, 1H, OH in enol tautomer). δC 36.7, 41.6, 96.0, 112.8, 119.5, 119.9, 129.7, 136.9, 159.6, 162.7, 175.4, 193.9. νmax (KBr)/cm–1 2941, 2548, 1700, 1625, 1579, 1470, 1365, 1111, 875, 790. Anal. Calc. for C20H17BrN4O4S2: C 46.07, H 3.29, N 10.75, S 12.30. Found: C 46.12, H 3.32, N 10.68, S 12.39 %.
5-(3-Methoxybenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1r
Pink powder, 80 %, mp 187°C (dec.). δH 3.72 (s, 3H, OCH3), 3.83 (s, 12H, 2 × CH3), 5.68 (s, 1H, CH in keto tautomer), 7.32–7.26 (m, 3H, Ar), 7.70 (d, J 7.5, 1H, Ar), 9.38 (bs, 1H, OH in enol tautomer). δC 36.5, 41.6, 55.3, 96.0, 112.7, 119.5, 119.9, 129.4, 136.9, 159.6, 163.0, 175.4, 193.9. νmax (KBr)/cm–1 2946, 2557, 1697, 1607, 1455, 1340, 1168, 1110, 1025, 752. Anal. Calc. for C21H20N4O5S2: C 53.38, H 4.27, N 11.86, S 13.57. Found: C 53.37, H 4.24, N 11.83, S 13.60 %.
5-(3,4-Dimethoxybenzoyl)-1,3,7,9-tetramethyl-2,8-dithioxo-2,3,8,9-tetrahydro-1H-pyrano[2,3-d:6,5-d']dipyrimidine-4,6(5H,7H)-dione 1s
Grey powder, 82 %, mp 207°C (dec.). δH 3.67 (s, 6H, 2 × CH3), 3.79 (s, 6H, 2 × CH3), 3.90 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.70 (s, 1H, CH in keto tautomer), 6.80 (d, J 8.4, 1H, Ar), 7.30 (d, J 8.7, 1H, Ar), 7.44 (s, 1H, Ar), 8.90 (bs, 1H, OH in enol tautomer). δC 36.6, 41.2, 55.9, 56.0, 96.3, 110.9, 121.9, 128.1, 149.0, 153.3, 162.8, 175.4, 192.7. νmax (KBr)/cm–1 3001, 2932, 2874, 2834, 2529, 1696, 1618, 1516, 1465, 1338, 1265, 1111, 1026, 790. Anal. Calc. for C22H22N4O6S2: C 52.58, H 4.41, N 11.15, S 12.76. Found: C 52.55, H 4.40, N 11.10, S 12.85 %.
Supplementary Material
IR, 1H NMR and 13C NMR spectra of pyranopyrimidine derivatives are available on the Journal’s website.
Acknowledgements
Financial support from the Research Council of Payame Noor University is gratefully acknowledged.
References
[1] A. M. El-Agrody, M. S. Abd El-Latif, N. A. El-Hady, A. H. Fakery, A. H. Bedair, Molecules 2001, 6, 519.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXntleisrs%3D&md5=3633fb6c845bc050538612dd37adbfa6CAS |
[2] A. H. Bedair, N. A. El-Hady, M. S. Abd El-Latif, A. H. Fakery, A. M. El-Agrody, lL Farmaco 2000, 55, 708.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXis1Ojug%3D%3D&md5=30e18e07fc49c28307fba12c61ed170aCAS |
[3] A. M. El-Agrody, M. H. El-Hakim, M. S. Abd El-Latif, A. H. Fakery, E. M. El-Sayed, K. A. El-Ghareab, Acta Pharm. 2000, 50, 111.
| 1:CAS:528:DC%2BD3cXlsFOktLw%3D&md5=9a36cd25c4bf2c652add5af0c85ec962CAS |
[4] R. N. Taylor, A. Cleasby, O. Singh, T. Skarzynski, J. Med. Chem. 1998, 41, 798.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhtF2murY%3D&md5=e09088cde1afa555d5b49652f2adc7c5CAS |
[5] K. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato, K. Kikugawa, Mutat. Res. 1997, 395, 47.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXisl2htA%3D%3D&md5=2e85076febb28f9ecca392ec39cd0d10CAS | 9465913PubMed |
[6] A. Martinez-Grau, L. J. Marco, Bioorg. Med. Chem. Lett. 1997, 7, 3165.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtVGjtQ%3D%3D&md5=93cbe9eb33dbea1f0257965025e51773CAS |
[7] C. P. Dell, C. W. Smith, Chem. Abstr. 1993, 119, 139102d.
[8] G. Bianchi, A. Tava, Agric. Biol. Chem. 1987, 51, 2001.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXhs1Oitrw%3D&md5=c1af1394041eff1a78413d2154962311CAS |
[9] F. Eiden, F. Denk, Arch. Pharm. 1991, 324, 353.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXkvFaisLw%3D&md5=656af1bae14801478fd2eff5603a881bCAS |
[10] C. J. Shishoo, M. B. Devani, G. V. Ullas, S. Ananthan, V. S. Bahadit, J. Heterocycl. Chem. 1981, 18, 43.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXkt1Cqtb4%3D&md5=bedf3c7e75aa4a104d47f02baa25029fCAS |
[11] K. Noda, A. Nakagawa, Y. Nakajima, H. Ide, Chem. Abstr. 1978, 88, 50908q.
[12] Z. H. Ismail, M. M. Ghorab, E. M. A. Mohamed, H. M. Aly, M. S. A. El-Gaby, Phosphorus Sulfur Silicon Relat Elem. 2008, 183, 2541.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtFKlsr3K&md5=6ede2393b08eea374d0463ae47b73185CAS |
[13] S. A. El-Gaby, S. M. Abdel-Gawad, M. M. Ghorab, H. I. Heiba, H. M. Aly, Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 279.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmslSq&md5=301536da6e5dd9f99a8affdc623f4884CAS |
[14] L. P. Prikazchikova, B. M. Khutova, I. F. Vladimirtsev, I. V. Boldyrev, N. I. Zhuravskaya, Chem. Abstr. 1975, 83, 127346m.
[15] D. Brown, A. R. Katritzky, C. W. Rees, Comprehensive Heterocyclic Chemistry, 1984, 3, p. 443 (Pergamon Press: Oxford).
[16] M. Bollini, R. A. Domaoal, V. V. Thakur, R. G. Macias, K. A. Spasov, K. S. Anderson, W. L. Jorgensen, J. Med. Chem. 2011, 54, 8582.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVGkt7rE&md5=f71f02e9088da1b7e39dafea968a7374CAS | 22081993PubMed |
[17] X. Huang, J. Su, A. U. Rao, H. Tang, W. Zhou, X. Zhu, X. Chen, Z. Liu, Y. Huang, S. Degrado, D. Xiao, J. Qin, R. Aslanian, B. A. McKittrick, S. Greenfeder, M. V. Heek, M. Chintala, A. Palani, Bioorg. Med. Chem. Lett. 2012, 22, 854.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XpsFejuw%3D%3D&md5=26d39060589ee08424a9a72ec42a9db2CAS | 22209457PubMed |
[18] (a) A. Domling, I. Ugi, Angew. Chem. Int. Ed. 2000, 39, 3168.
| 1:CAS:528:DC%2BD3cXntleksbk%3D&md5=6b635319ca63db215939c0c19f9a45bdCAS |
(b) J. Zhu, H. Bienayme, Multicomponent Reactions 2005 (Wiley-VCH: Weinheim).
(c) A. Domling, Chem. Rev. 2006, 106, 17.
[19] (a) I. Ugi, Pure Appl. Chem. 2001, 73, 187.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtVeksb8%3D&md5=2b798f220854a093717b05365f7e69bdCAS |
(b) R. R. Nagawade, D. B. Shinde, Acta Chim. Slov. 2007, 54, 642.
(c) D. M. D’Souza, T. Mueller, Chem. Soc. Rev. 2007, 36, 3169.
(d) C. C. A. Cariou, G. J. Clarkson, M. Shipman, J. Org. Chem. 2008, 73, 9762.
| Crossref | GoogleScholarGoogle Scholar |
[20] (a) W. Bannwarth, E. Felder, Combinatorial Chemistry 2000 (Wiley-VCH: Weinheim).
(b) G. Balme, E. Bossharth, N. Monteiro, Eur. J. Org. Chem. 2003, 4101.
| Crossref | GoogleScholarGoogle Scholar |
(c) R. V. A. Orru, M. De Greef, Synthesis 2003, 1471.
| Crossref | GoogleScholarGoogle Scholar |
(d) H. Bienaymé, C. Hulme, G. Oddon, P. Schmitt, Chem. Eur. J. 2000, 6, 3321.
| Crossref | GoogleScholarGoogle Scholar |
(e) G. Vasuki, K. Kumaravel, Tetrahedron Lett. 2008, 49, 5636.
| Crossref | GoogleScholarGoogle Scholar |
[21] (a) For recent reviews, see: I. Ugi, A. Domling, B. Werner, J. Heterocyclic Chem. 2000, 37, 647.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXlt1Ggsrc%3D&md5=6eb7b64c54ed6d221051b9441fbbef11CAS |
(b) I. Ugi, S. Heck, Comb. Chem. High Throughput Screen. 2001, 4, 1.
| Crossref | GoogleScholarGoogle Scholar |
(c) J. Zhu, Eur. J. Org. Chem. 2003, 1133.
| Crossref | GoogleScholarGoogle Scholar |
(d) C. Hulme, V. Gore, Curr. Med. Chem. 2003, 10, 51.
| Crossref | GoogleScholarGoogle Scholar |
(e) L. Weber, Curr. Med. Chem. 2002, 9, 2085.
| Crossref | GoogleScholarGoogle Scholar |
[22] J. Khalafy, M. Rimaz, H. Rabiei, L. Panahi, J. Sulfur Chem. 2013, 34, 395.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhslektLvI&md5=1af24739aa167048b87c259adde7eb2eCAS |
[23] B. Khalili, F. S. Darabi, B. Eftekhari-sis, M. Rimaz, Monatsh. Chem. 2013, 144, 1569.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtV2rt73L&md5=e74f2c32c5f8401ab405052a7ded5972CAS |
[24] J. Khalafy, M. Rimaz, S. Farajzadeh, M. Ezzati, S. Afr. J. Chem. 2013, 66, 176.
[25] J. Khalafy, M. Rimaz, M. Ezzati, A. P. Marjani, Curr. Chem. Lett. 2013, 2, 43.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1Ohsbc%3D&md5=bc51f24bfbc0393753e036e721526fafCAS |
[26] J. Khalafy, M. Rimaz, M. Ezzati, Curr. Chem. Lett. 2012, 1, 115.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhsl2iur%2FL&md5=fe6577eb32d11926825df11da266496cCAS |
[27] J. Khalafy, M. Rimaz, M. Ezzati, R. H. Prager, Bull. Korean Chem. Soc. 2012, 33, 2890.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsVyltb3M&md5=1ef0732b9a7e0b5ea7a440c2bc38c715CAS |
[28] J. Khalafy, M. Rimaz, L. Panahi, H. Rabiei, Bull. Korean Chem. Soc. 2011, 32, 2428.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpslyrsbo%3D&md5=57637f1547c406df148eeac7302f6179CAS |
[29] M. Rimaz, J. Khalafy, N. Noroozi Pesyan, R. H. Prager, Aust. J. Chem. 2010, 63, 507.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvV2rtr0%3D&md5=8f70584fe972a5d0856a68d89f5f1babCAS |
[30] M. Rimaz, J. Khalafy, P. N. Moghadam, Aust. J. Chem. 2010, 63, 1396.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFamurvL&md5=457c32c31570c4205bd167c19d67e088CAS |
[31] M. Rimaz, J. Khalafy, ARKIVOC 2010, ii, 110.
[32] M. Rimaz, N. N. Pesyan, J. Khalafy, Magn. Reson. Chem. 2010, 48, 276.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjt1Giurw%3D&md5=2ddf146653283501a56aa34ae5b4b1eaCAS | 20169579PubMed |
[33] H. A. Riley, A. R. Gray, Organic Syntheses 1943. Vol. II, p 509 (Wiley & Sons: New York, NY).
[34] A. I. Vogel, Practical Organic Chemistry 1974 (Longman Group Limited: London).