Natural products isolation studies of native Australian fern species
Thinley Gyeltshen A , Jason A. Smith A * and Alex C. Bissember
A , Jason A. Smith A * and Alex C. Bissember  A *
A *
A School of Natural Sciences – Chemistry, University of Tasmania, Hobart, Tas., 7001, Australia.
Handling Editor: Craig Hutton
Australian Journal of Chemistry 75(6) 422-437 https://doi.org/10.1071/CH22108
Submitted: 16 May 2022 Accepted: 7 June 2022 Published: 26 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Natural products isolation studies of 16 native Australian fern species have been undertaken, facilitated by pressurised hot water extraction (PHWE). Fourteen of these fern species have not been the subject of natural products isolation research previously. In total, 14 different compounds were isolated from 12 of these 16 different fern species. This included γ- and δ-lactones; flavonoid glycosides, a dihydrobenzofuran neolignan, in addition to hydroxycinnamate/caffeic acid esters. More specifically, the lactones 5,6-dihydro-5-hydroxy-6-methyl-2H-pyran-2-one, 5-(1-hydroxyethyl)-2(5H)-furanone and osmundalin were obtained from Todea barbara, while a dihydrobenzofuran neolignan, (−)-trans-blechnic acid were found in Austroblechnum penna-marina subsp. alpina, and the shikimate ester 5-O-caffeoylshikimic acid was isolated from Parablechnum wattsii. In addition, flavonoids and their glycoside derivatives, kaempferol 3-O-glucopyranoside, 4β-carboxymethyl-(−)-epicatechin, (2R)-eriodictyol-7-O-β-d-glucopyranoside, naringin, quercitrin, quercetin 3-O-(6″-acetyl)-β-d-glucopyranoside, rutin, and tiliroside were isolated from seven other fern species.
Keywords: ferns, flavonoid, glycoside, natural products, natural products isolation, neolignan, Polystichum, Todea.
Introduction
Ferns are a group of vascular plants bearing complex leaves called megaphylls. These plants do not produce flowers or seeds and reproduce via spores. There are more than 12 000 species of ferns that are widely distributed across the globe, with the greatest diversity typically found in the tropics.[1] Ferns represent the phylogenetic bridge between the lower and higher plants in the plant kingdom. For centuries, ferns have been used in many different contexts: as food, medicines, and ornaments. The fiddleheads (or croziers) of many fern species often feature in Asian cuisine. Indeed, it is reported that 52 species feature in Chinese food and it is estimated that the actual number of edible ferns may extend to 144 species.[2] In Japan, ostrich (Matteuccia struthiopteris (L.) Tod.), bracken (Pteridium aquilinum (L.) Kuhn), and royal ferns (Osmunda japonica Thunb.) are the most popular edible ferns that are harvested.[3] In Australia, the sporocarps of the small freshwater fern, nardoo (Marselia drummondii A. Braun) are consumed as baked cakes by first nations people following proper and extensive preparation.[4] In addition to their use in cuisine, many fern species feature in traditional pharmacopoeias and are used to treat an array of ailments.[5–8] In this context, relative to other species of vascular plants, ferns and lycophytes are poorly represented.[9]
Ferns and lycophytes, like angiosperms, are a rich source of phytochemicals with interesting biological properties. Natural products isolation studies reveal that they contain flavonoids, terpenoids (including steroids), and polyphenols (Fig. 1).[5,10] They also contain more distinctive alkaloid secondary metabolites. For example, lycopodium alkaloids such as lycopodine (1), lycodine (2), fawcettimine (3), and phlegmarine (4) skeletons have been isolated from Lycopodiaceae and Huperziaceae.[10–12] Flavonoids are commonly isolated from numerous fern species. For example, species of the genus Pteris are rich in flavonoids with mainly α- and β-glucosides, galactosides, rhamnosides or arabinosides present.[13,14] Distinctive flavonoids that have been isolated include neoflavonoids, calomelanols A–J (5–14) from farinose of Pityrogramma calomelanos (L.) Link,[15,16] bioflavonoids such as hinokiflavone (15), 7″-O-methylhinokiflavone (16) amentoflavone (17) and 7,7″-di-O-methylamentoflavone (18) from Selaginella tamariscina (P.Beauv.) Spring,[17] involvenflavones A–F (19–24) from S. involven (Sw.) Spring[18] and prenylated flavonoids (25–28) from Helminthostachys zeylanica (L.) Hook.[19] Many sesquiterpenoid compounds with indane or cadinene skeletons are found in ferns.[10] Sesquiterpenyl indanones, known as pterosins, and their glycosides (pterosides) have been isolated from bracken fern species and polypodiaceous ferns.[20] For example, multifidosides A–C (29–31) have been isolated from Pteris multifida Poir.[14] and the carcinogenic pteroside, ptaquiloside (32) has been isolated from Pteridium aquilinium (L.) Kuhn.[21] The terpenoids obtained from ferns are typically ent-kaurane-, ent-atisane- and ent-primarane-type diterpenoids, which are present in Pteris species.[22] Labdane- and clerodane-type diterpenoids, diterpenoid glycosides and triterpenoids are major constituents in the Gleicheniaceae family,[23–26] and ecdysteroids have been isolated from species of genera Microsorum and Diplopterygium in the family Polypodiaceae.[27–31] Phenolic compounds are another class of secondary metabolites widely distributed in ferns. Commonly isolated molecules of this type include caffeic (33), chlorogenic (34) and vanillic (35) acids.[10,32,33] The glycosylated phenolic acid, 7-O-caffeoylhydroxymaltol-3-β-d-glucopyranoside (36) was isolated from Pteris ensiformis Burm.,[33–35] in addition to a chalcone derivative, licoagrochalcone D (37) from Pteris multifida Poir.[36]
Natural products research concerning ferns native to Australia are mainly restricted to toxicity studies. For example, the sporocarps of the freshwater fern nardoo, Marsilea drummondii A. Braun, which are used for food by Australian Aborigines,[4] are reportedly toxic to humans, cattle, and sheep.[37] Studies on this waterfern has revealed that its toxicity derives from high levels of the enzyme thiaminase which breaks down thiamine (vitamin B1).[38] Similarly, bracken ferns Pteridium aquillinum (L.) Kuhn and P. esculentum (G.Forst.) Nakai have been the subject of many phytochemical and pharmacological studies in order to elucidate the mechanism of toxicity involving the carcinogenic norsesquiterpene glucoside, ptaquiloside (32).[39–42] Consequently, many sesquiterpenoid compounds have been isolated from P. aquillinum (L.) Kuhn.[20,43–45] The presence of ptaquiloside has been reported in fern species from the genera Pteris, Microlepia, Hypolepis and Chelianthes.[43,44,46] Beyond their toxicity, very limited information regarding the phytochemistry of ferns found in Australia exists. Nevertheless, due to their wide geographic distribution, many native Australian ferns are also found in Asia and South America and natural products isolation studies of species found in these locations have been undertaken. For example, Helminthostachys zeylanica (L.) Hook which is found widely distributed in tropical parts of Asia, the Pacific region, and Australia,[1] contains prenylated flavonoids, ugonins,[47,48] cyclised geranyl stilbenes, and ugonstiblenes.[49] Similarly, Salvinia species have a global distribution and the species, S. auriculata Aubl. and a hybrid, S.xmolesta D.S.Mitch. are found in Australia.[1] Bioactivity-guided phytochemical investigation of these two Australian species allowed for the isolation of more than sixty different secondary metabolites that include diterpenes, polyphenols, fatty acids, triterpene, apocarotenoids, acyclic sesquiterpenoids, monoterpenes, jasmonates, steroids and coumarins.[50–52] However, a large number of native Australian ferns, particularly endemic species, have not been the subject of natural products isolation studies.
In this report, a total of 16 Australian native fern species formed the basis of natural products isolation studies. Specifically, our research concerned Todea barbara (L.) T. Moore, Alsophila australis (R.Br.) Domin, Dicksonia antarctica Labill., Calochlaena dubia (R.Br.) M. D. Turner & R. A. White, Polystichum proliferum (R.Br.) C. Presl, P. vestitum (G. Forst.) C. Presl, Pellaea falcata (R.Br.) Fée, Lecanopteris pustulata subsp. pustulata (G. Forst.) Testo & A. R. Field, Oceaniopteris cartilaginea (Sw.) Gasper & Salino, Lomaria nuda (Labill.) Willd., Doodia australis (Parris) Parris, Austroblechnum penna-marina (Poir.) Gasper & V. A. O. Dittrich subsp. alpina (R.Br.) S. Jess. & L. Lehm., Parablechnum wattsii (Tindale) Gasper & Salino, Gleichenia alpina R.Br., Histiopteris incisa (Thunb.) J.Sm. and Pteridium esculentum (G. Forst.) Nakai subsp. esculentum. Two of these species, Polystichum vestitum (G. Forst.) C. Presl and Gleichenia alpina R.Br., were previously thought to be endemic to Tasmania,[53] however, these species were recently found in New Zealand.[1] We isolated a total of 14 different compounds (38–51) from 12 native Australian fern species, including γ- and δ-lactones; flavonoid glycosides, a dihydrobenzofuran neolignan, in addition to hydroxycinnamate/caffeic acid esters (Fig. 2).

|
Results and discussion
For each of the 16 native Australian fern species that we investigated, PHWE of leaf material, followed by liquid–liquid extraction of the aqueous PHWE extract with ethyl acetate provided a crude organic extract after concentration under reduced pressure. In each case, the remaining aqueous phase was concentrated under reduced pressure to afford a crude aqueous extract. The combined yield (% w/w) of the respective crude extracts thus obtained is shown in Table 1. With the exception of P. vestitum (0.38% w/w), yields of crude extracts were >0.5% w/w in all cases. The crude extracts were then subjected to various standard flash column chromatography and preparative thin layer chromatography procedures.

|
Dicksonia antarctica and Pteridium esculentum are the only 2 of these 16 fern species that have been the subject of previous natural products isolation studies. Previously, the phenolic compounds (5S,6S,9S,10S)-15-hydroxycadina-3,11-dien-2-one and p-hydroxystyrene β-vicianoside, in addition to p-hydroxystyrene β-d-glucoside, kaempferol 3-O-β-d-glucoside, kaempferol 3-O-(2-O-β-d-xylosyl)-β-d-glucoside, kaempferol 3-O-(6-p-coumaroyl)-β-d-glucoside and chlorogenic acid have been isolated from P. esculentum fronds (1.2 kg of dry plant material).[54] 4-O-Caffeoylshikimic acid and 4-O-(p-coumaroyl) shikimic acid were isolated from croziers of D. antarctica (2.4 kg of fresh plant material) in 1997.[55,56] In this present study, secondary metabolites were not isolated from D. antarctica, Doodia australis, Oceaniopteris cartilaginea and Histiopteris species. However, 14 different compounds were isolated from the remaining 12 fern species we investigated. A combination of standard 1H, 13C and 2D (COSY, HMBC and HSQC) NMR spectroscopic techniques were employed to elucidate the structures of these compounds. In each case, these data were consistent with equivalent data reported in the literature. Specifically, we obtained 5,6-dihydro-5-hydroxy-6-methyl-2H-pyran-2-one (38), 5-(1-hydroxyethyl)-2(5H)-furanone (39), osmundalin (40), astragalin (41), 4β-carboxymethyl-(−)-epicatechin (42), (2R)-eriodictyol-7-O-β-d-glucopyranoside (43), naringin (44), (−)-trans-blechnic acid (45), (p-hydroxybenzyl)malonic acid (46), quercitrin (47), quercetin 3-O-(6″-acetyl-β-d-glucopyranoside) (48), 5-O-caffeoylshikimic acid (49), rutin (50), and tiliroside (51) (Fig. 2).
5,6-Dihydro-5-hydroxy-6-methyl-2H-pyran-2-one (38) and 5-(1-hydroxyethyl)-2(5H)-furanone (39) were obtained as a mixture from Todea barbara in a ~2:1 ratio, as judged by NMR spectroscopic and GC-MS analysis (see Supplementary Material). Osmundalin (40) was also isolated from T. barbara. Lactones 38–40 have been isolated from Osmunda japonica, a common Japanese fern species, and are reported to exhibit antifeedant properties against the larvae of yellow butterfly, Eurema hecabe mandarina.[57,58] In addition, all three natural products have also been isolated from Angiopteris caudatiformis, a fern species used in Chinese folk medicine for the treatment of a broad range of ailments.[59] Natural products 38 and 39 have been isolated from the fern species A. esculenta[60] and angiopteroside, an epimer of osmundalin (40), was isolated from Angiopteris evecta.[61]
4β-Carboxymethyl-(−)-epicatechin (42),[62] was isolated from Polystichum vestitum. This secondary metabolite has been isolated from Davallia divaricata,[62] D. solida[63] and Dryopteris crassirhizoma.[64] We also obtained molecule 42 from Polystichum proliferum and Lecanopteris pustulata subsp. pustulata. All of these fern species are members in the order Polypodiales. Dihydrobenzofuran neolignane, (−)-trans-blechnic acid (45) was isolated from Austroblechnum penna-marina subsp. alpina; a species formerly classified within the genus Blechnum. (−)-trans-Blechnic acid (45) and its epimer, epiblechnic acid, represent characteristic constituents of the family Blechnaceae.[65] Blechnic acid has been isolated from various fern species, including Blechnopsis orientalis, Spicantopsis amabilis, S. niponica, Woodwardia orientalis, W. prolifera, Brainea insignis[65] and Struthiopteris spicant.[66] We isolated 5-O-caffeoylshikimic acid (49) (0.5% w/w yield) from Parablechnum wattsii (also formerly in the genus Blechnum). Compound 49 is a major secondary metabolite present in this fern and a known enzymatic browning agent present in dates, Phoenix dactylifera.[55,67] It is a major phytochemical and an anti-thiamine factor isolated from Pteridium aquillinum var. latiusculum and reportedly causes depression of leucocytes and thrombocytes in calves.[68] 5-O-Caffeoylshikimic acid is found widely distributed in Equisetaceae family and in ferns from the families Adiantaceae, Dryopteridaceae, Athyriaceae, Dennstaedtiaceae, Osmundaceae and Thelypteridaceae.[69] Interestingly, we isolated p-hydroxybenzylmalonic acid (46) and 4β-carboxymethyl-(–)-epicatechin (42) from Lecanopteris pustulata subsp. pustulata. Natural product 46 has been isolated from liquorice previously.[70] Liquorice primarily derives from three species, Glycyrrhiza glabra, Glycyrrhiza uralensis and Glycyrrhiza inflata and the presence of p-hydroxybenzylmalonic acid (46) has been reported from all three.[71]
Flavonoid glycosides 41, 43, 44, 47, 48, 50, and 51 were also isolated in our study. Specifically, we obtained the common flavonoid glucoside astragalin (41) from Alsophila australis, Calochalaena dubia and Pteridium esculentum. It has been isolated from many plant species including from the bracken fern P. aquilinum.[72–76] We isolated (2R)-eriodictyol-7-O-β-d-glucopyranoside (43) from P. vestitum. This natural product has been isolated from a wide range of flowering plants.[77–85] In ferns, its presence has been identified in species of Pyrrosia.[86] Molecule 43 is a reported Nrf2 activator and confers protection against cisplatin-induced toxicity and cerebral ischemic injury.[84,87] Naringin (44) and rutin (50) were isolated from Pellaea falcata and Gleichenia alpina, respectively. Both are commonly reported flavonoid glycosides found in citrus, and exhibit a broad range of pharmacological activity.[88–95] Compounds 44 and 50 have also been found in fern species.[96–99] Trace amounts of quercitrin (47) and quercetin 3-O-(6″-acetyl)-glucoside (48) were isolated from Lomaria nuda. Both molecules are present in an array of plant species and possess wide ranging biological properties. Tiliroside (51), a kaempferol flavonoid glucoside with a coumaroyl moiety, was isolated from Pteridium esculentum. Its presence has been identified in various plant species, including P. aquilinum; and compound 51 is a reported carcinogen found in bracken fern.[100–103] However, tiliroside also exhibits profound anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects, and has potential therapeutic applications for the treatment of diabetes.[100,104–106]
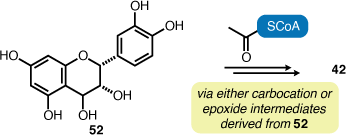
Among the 14 secondary metabolites isolated in this study, osmundalin (40), 4β-carboxymethyl-(−)-epicatechin (42) and trans-blechnic acid (45) have only been isolated from fern sources to date (Table 2). This reveals that various Australian fern species investigated in our study contain secondary metabolites that are consistent with other members in the genera, families or orders that are found distributed beyond Australia. For example, Todea barbara is exclusively a southern hemisphere species and both Osmunda japonica and Angiopteris caudatiformis are species found in eastern Asia regions. trans-Blechnic acid (45) has only been obtained from fern species of the family Blechnaceae. The remaining natural products are regularly isolated from angiosperms, including various fern species. 4β-Carboxymethyl-(−)-epicatechin (42) is a particularly rare natural product and its isolation solely from ferns suggests that its formation might derive from a biosynthetic pathway unique to certain fern species (Scheme 1).

|
|
Conclusions
Our natural products isolation studies provide further evidence that fern species represent a source of structurally diverse phytochemicals with interesting biological properties. Phytochemical screening of 16 native Australian ferns enabled the isolation of 14 previously reported compounds. The structures of these compounds were primarily elucidated via 1D (1H and 13C) and 2D (COSY, HSQC and HMBC) NMR spectroscopy. In each case, the characterisation data were consistent with equivalent data reported in the literature. These isolated compounds included flavonoid glycosides, caffeic acid esters, lactones, and a dihydrobenzofuran neolignan. Lactone molecules, 5,6-dihydro-5-hydroxy-6-methyl-2H-pyran-2-one, 5-(1-hydroxyethyl)-2(5H)-furanone and osmundalin were isolated from Todea barbara. 4β-Carboxymethyl-(−)-epicatechin was isolated from both species of Polystichum investigated in this study. Our isolation of (−)-trans-blechnic acid from Austroblechnum penna-marina subsp. alpina is consistent with this natural product representing a characteristic compound in the family Blechnaceae.
Experimental
Plant material
Leaf material from 13 ferns Todea barbara, Alsophila australis, Dicksonia antarctica, Calochlaena dubia, Polystichum proliferum, P. vestitum, Pellaea falcata, Lecanopteris pustulata subsp. pustulata, Oceaniopteris cartilaginea, Lomaria nuda, Doodia australis, Austroblechnum penna-marina subp. alpina and Parablechnum wattsii were collected from the Royal Botanical Gardens of Tasmania in Hobart during February 2020. Aerial parts of Gleichenia alpina was collected from Wombat Moor at Mt Field National Park (42.6829°S, 146.6174°E; 1073 m above sea level) in February 2021. Histiopteris incisa was collected from Mt Field National Park (42.7806°S, 146.5844°E; 461 m above sea level) in February 2021. Aerial parts of Pteridium esculentum were collected from five healthy plants growing at Marion Bay (42.8218°S, 147.8671°E) in October 2021. Voucher specimens have been provided to the Tasmanian Herbarium, Tasmanian Museum and Art Gallery (no. HO607603–HO607618). With the exception of Pteridium esculentum, all plant material was air-dried for 2 weeks and stored prior to extraction. P. esculentum plant material was dried in an oven at 45°C for 3 days prior to extraction.
General
Solvents used in all experiments were of analytical grade or purified by standard laboratory procedures. Plant material was ground using a Sunbeam spice grinder. Pressurised hot water extraction (PHWE) was performed using Breville Expresso Machine Model 800ES. This PHWE method is a well-established natural products extraction technique.[107–109] The extracted organic solvents were dried using anhydrous MgSO4 and Na2SO4. Solvents were removed under reduced pressure on a rotary evaporator. Flash column chromatography was performed using flash grade silica gel (Kieselgel 60). Automated flash chromatography was performed using a Grave Reveleris X2 flash column chromatography system or a Büchi Flash Pure system with 40 µm silica gel cartridges. TLC analysis was performed using Merck silica gel 60-F254 plates. NMR spectroscopy was performed on a Bruker Avance III NMR spectrometer operating at 400 MHz (1H) and 100 MHz (13C) or Bruker AscendTM 600 NMR spectrometer operating at 600 MHz for (1H) and 150 MHz (13C). The deuterated solvents used were D2O, CDCl3, acetone-d6, CD3OD and DMSO-d6. Spectra were calibrated by assignment of the residual solvent peak to δH 7.26 and δC 77.16 for CDCl3; δH 2.50 and δC 39.52 for DMSO-d6; δH 3.31 and δC 49.00 for CD3OD; δH 4.79 for D2O; and δH 2.05 and δC 29.84 for acetone-d6.[110] Infrared spectroscopy was performed using a Shimadzu FTIR 8400s spectrometer, with samples prepared as thin films on NaCl plates. Gas chromatography mass spectrometry (GC-MS) experiments were performed on Agilent 6850 GC and Agilent 5975C mass spectrometers. HRESIMS analyses were conducted on a Thermo LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific).
Extraction and isolation
PHWE of Todea barbara
T. barbara dried leaflets (29 g) were finely ground using a spice grinder, mixed with sand (~4 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (646 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (10 g). Extract A: Extract A (646 mg) was redissolved in EtOAc, adsorbed onto silica/Celite® (1:1 mixture by mass), and subjected to flash column chromatography {silica; hexanes (50 mL), 10% acetone/hexanes (50 mL), 20% acetone/hexanes (100 mL), 30% acetone/hexanes (150 mL), 40% acetone/hexanes (100 mL), 50% acetone/hexanes (50 mL), 60% acetone/hexanes (50 mL) and acetone (150 mL)} to provide a mixture of compounds 38 and 39 (23 mg, 0.8% w/w yield in a ~2:1 ratio) as colourless crystalline solids. This mixture was analysed by GC-MS. Extract B: Extract B (10 g) was soaked in MeOH (~200 mL) for 1 h, repeated five times, combined, and concentrated to provide extract B.1 (4 g). Extract B.1 was then soaked in acetone (~200 mL) for 1 h, repeated 5 times, combined, and concentrated to provide extract B.2 (~2 g). Extract B.2 (~1 g) was redissolved in acetone, absorbed onto silica/Celite® (1:1 mixture by mass), and subjected to flash column chromatography {silica; hexanes (50 mL), 10% acetone/hexanes (100 mL), 50% acetone/hexanes (50 mL), acetone (50 mL) and 50% MeOH/acetone (50 mL)}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to afford six fractions, F.1–6. Fraction F.5 (709 mg) was again adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; hexanes (50 mL), 10% acetone/hexanes (50 mL), 30% acetone/hexanes (200 mL), 50% acetone/hexanes (200 mL), and acetone (30 mL)}, which provided compound 40 (570 mg, 2.0% w/w yield) as an off-white solid. Extract B.1 (1.5 g) was absorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (75 mL), 13% MeOH/CH2Cl2 (150 mL), 27% MeOH/CH2Cl2 (75 mL), 53% MeOH/CH2Cl2 (150 mL) and MeOH (100 mL)}. Following TLC and 1H NMR spectroscopic analysis the resulting fractions were combined to afford four fractions, F.1–4. Fraction F.3 (641 mg) was redissolved in MeOH, absorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 25% MeOH/CH2Cl2 (100 mL), 66% MeOH/CH2Cl2 (75 mL) and MeOH (50 mL)}, which provided compound 40 (30 mg, 2.0% w/w yield) as an off-white solid.
Osmundalactone (38).[57] Colourless crystalline solid. 1H NMR (600 MHz, CDCl3) δ 6.78 (1H, dd, J = 9.9 and 2.3 Hz; H-4), 5.92 (1H, dd, J = 9.9 and 1.9 Hz; H-3), 4.31 (1H, m; H-6), 4.18 (1H, d, J = 8.7 Hz; H-5), 1.42 (3H, d, J = 6.4 Hz; 7-CH3) ppm; 13C NMR (150 MHz, CDCl3) δ 163.2 (C-2), 148.5 (C-4), 120.7 (C-3), 79.0 (C-6), 67.7 (C-5), 18.2 (7-CH3) ppm.
5-(1-Hydroxyethyl)-2(5H)-furanone (39).[57,58] Colourless crystalline solid. 1H NMR (600 MHz, CDCl3) δ 4.35 (1H, m; H-5), 4.08 (1H, m; H-6), 2.53 (1H, m; H-3a), 2.48 (1H, m; H-3b), 2.19 (1H, m; H-4a), 2.12 (1H, m; H-4b), 1.13 (3H, d, J = 6.5 Hz; 7-CH3) ppm; 13C NMR (150 MHz, CDCl3) δ 177.5 (C-2), 83.5 (C-5), 67.4 (C-6), 28.6 (C-3), 20.9 (C-4), 17.7 (7-CH3) ppm.
Osmundalin (40) (CAS# 54835-71-1).[57] Off-white solid. [α] –65.1° (c 0.075, MeOH), lit. [α] –107° (c 1.0, MeOH).[58] 1H NMR 600 MHz, CD3OD) δ 7.04 (1H, dd, J = 9.9 and 2.9 Hz; H-3), 5.98 (1H, dd, J = 9.9 and 1.5 Hz; H-2), 4.48 (1H, quintet, J = 7.4 Hz; H-5), 4.45 (1H, d, J = 7.8 Hz; H-1′), 4.43 (1H, ddd, J = 7.6, 2.8 and 1.5 Hz; H-4), 3.85 (1H, dd, J = 11.8 and 5.6 Hz; H-6′b), 3.79 (1H, dd, J = 11.8 and 1.9 Hz; H-6′a), 3.33 (1H, t, J = 8.9 Hz; H-3′), 3.26 (2H, m; H-4′,5′), 3.16 (1H, t, J = 9.1 Hz; H-2′), 1.42 (3H, d, J = 6.5 Hz; 6-CH3) ppm; 13C NMR (150 MHz, CD3OD) δ 165.14 (C-1), 147.71 (C-3), 121.55 (C-2), 102.81 (C-1′), 79.29 (C-5), 78.16 (C-5′), 77.95 (C-3′), 74.82 (C-2′), 73.36 (C-4), 71.49 (C-4′), 62.72 (C-6′), 18.57 (C-6) ppm.
PHWE of Alsophila australis
A. australis dried leaflets (30 g) were finely ground using a spice grinder, mixed with sand (~6 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (2 × 200 mL and 150 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (268 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (7 g). Extract A: Extract A (268 mg) was redissolved in EtOAc and MeOH, absorbed onto silica/Celite® (1:1 mixture by mass), and subjected to flash column chromatography {silica; hexanes (100 mL), 50% EtOAc/hexanes (100 mL), EtOAc (100 mL), 10% MeOH/EtOAc (100 mL) and 20% MeOH/EtOAc (100 mL)}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to afford five fractions, F.1–5. Fraction F.3 (146 mg) was redissolved in EtOAc and MeOH, adsorbed onto absorbed onto silica/Celite® (1:1 mixture by mass), and subjected to flash column chromatography {silica; CH2Cl2 (150 mL), 20% EtOAc/CH2Cl2 (100 mL), 40% EtOAc/CH2Cl2 (100 mL), 60% EtOAc/CH2Cl2 (100 mL), 80% EtOAc/CH2Cl2 (100 mL), EtOAc (100 mL) and 20% MeOH/EtOAc (100 mL)}, which afforded compound 41 (12 mg, 0.04% w/w) as a pale-yellow solid.
Astragalin (41) (CAS# 480-10-4).[73,75] Pale-yellow solid. 1H NMR (600 MHz, CD3OD) δ 8.06 (2H, d, J = 8.9 Hz; H-2′,6′), 6.90 (2H, d, J = 8.9 and 1.9 Hz; H-3′, 5′), 6.41 (1H, d, J = 2.1 Hz; H-8), 6.21 (1H, d, J = 2.1 Hz; H-6), 5.26 (1H, d, J = 7.4 Hz; H-1″), 3.69 (1H, dd, J = 11.9 and 2.3 Hz; H-6a″), 3.53 (1H, dd, J = 11.9 and 5.6 Hz; H-6b″), 3.40–3.46 (2H, m; H-2″,3″), 3.32 (1H, m; H-4″), 3.21 (1H, m; H-5″) ppm; 13C NMR (150 MHz, CD3OD) δ 179.54 (C-4), 165.98 (C-7), 163.10 (C-5), 161.58 (C-4′), 159.09 (C-2), 158.52 (C-9), 135.45 (C-3), 132.28 (C-2′,6′), 122.80 (C-1′), 116.07 (C-3′,5′), 105.75 (C-10), 104.04 (C-1″), 99.87 (C-6), 94.73 (C-8), 78.43 (C-5″), 78.04 (C-3″), 75.73 (C-2″), 71.36 (C-4″), 62.62 (C-6″) ppm.
PHWE of Calochlaena dubia
C. dubia leaflets (30 g) were finely ground using a spice grinder, mixed with sand (~6 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (3 × 200 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide dark green extract A (575 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (10 g). Extract A: Extract A (315 mg) was redissolved in MeOH and EtOAc, absorbed onto silica/Celite® (1:1 mixture by mass), and subjected to automated flash chromatography {silica cartridge (24 g); 0–100% EtOAc/hexanes and 0–50% MeOH/EtOAc, for 12 min with flow rate of 28 mL/min}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to give nine fractions, F.1–9. Fraction F.8 (41 mg) was subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 10% MeOH/CH2Cl2 (200 mL) and 50% MeOH/CH2Cl2 (20 mL)}, which provided compound 41 (4 mg, 0.014% w/w).
PHWE of Polystichum vestitum
P. vestitum dried leaflets (30 g) were finely ground using a spice grinder, mixed with sand (~6 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (Na2SO4), filtered, and concentrated under reduced pressure to provide extract A (114 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (7 g). Extract A: Extract A (114 mg) was redissolved in EtOAc and MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass), and subjected to automated flash column chromatography {silica cartridge (4 g); 0–100% EtOAc/hexanes and 0–50% MeOH/EtOAc, for 26 min with flow rate 12 mL/min)} and afforded compound 42 (30 mg, 0.1% w/w) as a light brown solid. Following TLC and 1H NMR spectroscopic analysis, the fractions were combined to give six fractions, F.1–6. Fractions F.3–5 (27 mg) were combined and subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 5% MeOH/CH2Cl2 (50 mL), 15% MeOH/CH2Cl2 (50 mL) and 40% MeOH/CH2Cl2 (30 mL)} to provide compound 43 (4 mg) as a yellow solid. Extract B: Extract B (4 g) was redissolved in MeOH and water, adsorbed onto silica/Celite® (1:1 mixture by mass), and fractionated through a silica plug (150 mL of EtOAc, 10% MeOH/EtOAc, 20% MeOH/EtOAc, 30% MeOH/EtOAc, 40% MeOH/EtOAc and 50% MeOH/EtOAc) which afforded six fractions, F.1–6. Following 1H NMR spectroscopic analysis, fractions F.1 and 2 (145 mg) were recombined, absorbed onto silica and subjected to flash column chromatography {silica; CH2Cl2 (100 mL), 2.5% MeOH/CH2Cl2 (100 mL), 5% MeOH/CH2Cl2 (100 mL), 10% MeOH/CH2Cl2 (100 mL), 15% MeOH/CH2Cl2 (100 mL), 20% MeOH/CH2Cl2 (100 mL) and 35% MeOH/CH2Cl2 (100 mL)} to provide compound 42 (10 mg, 0.035% w/w).
4β-Carboxymethyl-(–)-epicatechin (42) (CAS# 146805-53-0).[62] Light-brown solid. 1H NMR (600 MHz, acetone-d6) δ 7.08 (1H, d, J = 1.7 Hz; H-2′), 6.87 (1H, dd, J = 8.2 and 1.8 Hz; H-6′), 6.81(1H, d, J = 2.2 Hz; H-5′), 6.04 (1H, d, J = 2.2 Hz; H-8), 5.94 (1H, d, J = 2.2 Hz; H-6), 4.93 (1H, s; H-2), 4.01 (1H, s; H-3), 3.46 (1H, d, J = 6.2 Hz; H-4), 3.05 (1H, dd, J = 16.4 and 3.5 Hz; H-1b″), 2.45 (1H, dd, J = 16.4 and 11.2 Hz; H-1a″) ppm. 13C NMR (150 MHz, acetone-d6) δ 173.9 (2″-COOH), 157.9 (C-7,9), 156.3 (C-5), 145.5 (C-4′), 145.3 (C-3′), 132.2 (C-1′), 119.3 (C-6′), 115.6 (C-5′), 115.3 (C-2′), 102.7 (C-10), 96.6 (C-8), 95.8 (C-6), 75.4 (C-2), 70.2 (C-3), 39.1 (C-1″), 36.0 (C-4) ppm.
(2R)-Eriodictyol-7-O-β-d-glucopyranoside (43) (CAS# 38965-51-4).[80] Yellow solid. 1H NMR (600 MHz, CD3OD) δ 6.82 (1H, s, H-2′), 6.69 (2H, t, J = 10.9 Hz, H-5′,6′), 6.11 (1H, s, H-6), 6.09 (1H, s, H-8), 5.23 (1H, d, J = 12.5 Hz, H-2), 4.87 (1H, t, J = 6.6 Hz, H-1″), 3.88 (1H, d, J = 12.1 Hz, H-6a″), 3.69 (1H, m, H-6b″), 3.42–3.47 (3H, m, H-2″,3″,5″), 3.39 (1H, m, H-4″), 3.13 (1H, dd, J = 17.5 Hz, H-3a), 2.75 (1H, d, J = 17.0 Hz, H-3b) ppm. 13C NMR (150 MHz, CD3OD) δ 198.5 (C-4), 167.0 (C-7), 164.9 (C-9), 164.6 (C-5), 146.9 (C-3′), 146.5 (C-4′), 131.5 (C-1′), 119.3 (C-6′), 116.3 (C-5′), 114.8 (C-2′), 104.9 (C-10), 101.2 (C-1″), 97.9 (C-8), 96.9 (C-6), 80.7 (C-2), 78.3 (C-3″), 77.8 (C-5″), 74.7 (C-2″), 71.2 (C-4″), 62.3 (C-6″), 44.1 (C-3) ppm.
PHWE of Polystichum proliferum
P. proliferum leaves (30 g) were finely ground using a spice grinder, mixed with sand (7 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (Na2SO4), filtered, and concentrated under reduced pressure to provide extract A (240 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to afford a dark brown extract B (4 g). Extract A: Extract A (240 mg) was redissolved in CH2Cl2 and MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass), and subjected to flash column chromatography {silica; CH2Cl2 (200 mL), 10% MeOH/CH2Cl2 (100 mL), 20% MeOH/CH2Cl2 (100 mL) and 40% MeOH/CH2Cl2 (200 mL)} which provided compound 42 (16 mg, 0.06% w/w).
PHWE of Pellaea falcata
P. falcata dried leaflets (30 g) were finely ground using a spice grinder, mixed with sand (~8 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 3 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (1.0 g). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (8 g). Extract A: Extract A (1.0 g) was redissolved in EtOAc and MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to automated flash chromatography {silica cartridge (12 g); (0–100% EtOAc/hexanes and 0–50% MeOH/EtOAc for 21 min with a flow rate of 28 mL/min)}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to afford seven larger fractions, F.1–7. Fraction F.5 was redissolved in MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 10% MeOH/CH2Cl2 (100 mL), 20% MeOH/CH2Cl2 (50 mL) and 40% MeOH/CH2Cl2 (50 mL)} which provided compound 44 (21 mg, 0.7% w/w) as a yellow solid.
Naringin (44) (CAS# 10236-47-2).[111,112] Yellow solid. 1H NMR (600 MHz, CD3OD) δ 7.32 (2H, d, J = 8.5 Hz; H-2′,6′), 6.82 (2H, d, J = 8.5 Hz; H-3′,5′), 6.18 (1H, d, J = 2.0 Hz; H-8), 6.16 (1H, d, J = 2.1 Hz; H-6), 5.37 (1H, dd, J = 12.9 and 2.7 Hz; H-2), 5.25 (1H, s; H-1″), 5.09 (1H, d, J = 7.6 Hz; H-1″), 3.93 (1H, s; H-2″), 3.85–3.90 (2H, m; H-6a″,5‴), 3.62–3.69 (2H, m; H-3‴,6b″), 3.57-3.60 (2H, m; H-2″,5″), 3.43–3.46 (1H, m; H-3″), 3.39 (2H, t, J = 9.5 Hz; H-4″,4‴), 3.16 (1H, dd, J = 17.2 and 12.9 Hz; H-3a), 2.75 (1H, dd, J = 17.2 and 2.8 Hz; H-3b), 1.28 (3H, d, J = 6.2 Hz; 6‴-CH3) ppm. 13C NMR (150 MHz, CD3OD) δ 198.5 (C-4), 166.6 (C-7), 164.9 (C-5), 164.6 (C-9), 159.1 (C-4′), 130.8 (C-1′), 129.1 (C-2′,6′), 116.3 (C-3′,5′), 104.9 (C-10), 102.5 (C-1‴), 99.4 (C-1″), 97.8 (C-6), 96.7 (C-8), 80.7 (C-2), 79.0 (C-2″), 78.9 (C-3″), 78.1 (C-5″), 73.9 (C-4″‘), 72.2 (C-3‴), 71.2 (C-2‴), 71.2 (C-4″), 69.9 (C-5‴), 62.3 (C-6″), 44.1 (C-3), 18.2 (6‴-CH3) ppm.
PHWE of Austroblechnum penna-marina subsp. alpina
A. penna-marina subsp. alpina dried leaflets (29 g) were finely ground using a spice grinder, mixed with sand (~7.5 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further three times to provide a combined extract (800 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (409 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (6 g). Extract A. Extract A (400 mg) was redissolved in MeOH and EtOAc, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; hexanes (100 mL), 20% EtOAc/hexanes (200 mL), 40% EtOAc/hexanes (100 mL), 60% EtOAc/hexanes (100 mL), 80% EtOAc/hexanes (100 mL), EtOAc (50 mL), 10% MeOH/EtOAc (100 mL) and MeOH/EtOAc (100 mL)} that provided compound 45 (8.9 mg, 0.3% w/w) as a pale-green solid.
Blechnic acid (45) (CAS# 146805-53-0).[113] Pale-green solid. [α] –23.2° (c 0.0345, MeOH), lit. [α] –28° (c = 1.0, MeOH).[65] 1H NMR (600 MHz, CD3OD) δ 7.47 (d, J = 15.9 Hz; H-7′), 7.03 (d, J = 8.5 Hz; H-6′), 6.86 (d, J = 2.0 Hz; H-2), 6.74 (dd, J = 8.2, 1.9 Hz; H-6), 6.71 (d, J = 8.4 Hz; H-5), 6.65 (d, J = 8.2 Hz; H-5′), 6.16 (d, J = 15.9 Hz; H-8′), 5.83 (d, J = 9.3 Hz; H-7), 4.50 (d, J = 9.3 Hz; H-8) ppm. 13C NMR (150 MHz, CD3OD) δ 173.6 (C-9), 170.6 (C-9′), 149.6 (C-3′), 146.5 (C-4), 146.0 (C-3), 145.1 (C-4′), 143.3 (C-7′), 129.3 (C-1), 129.1 (C-2′), 124.5 (C-1′), 122.6 (C-6′), 119.7 (C-6), 118.0 (C-5′), 117.8 (C-8′), 115.9 (C-5), 115.1 (C-2), 88.4 (C-7), 55.3 (C-8) ppm. HRESIMS m/z calcd for C18H14O8Na [M + Na]+ 381.0586; found 381.0581.
PHWE Lecanopteris pustulata subsp. pustulata
L. pustulata subsp. pustulata dried leaflets (30 g) were finely ground using a spice grinder, mixed with sand (~7 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL) and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (154 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (5.6 g). Extract A. Extract A (118 mg) was redissolved in MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; hexanes (30 mL), 30% EtOAc/hexanes (50 mL), 60% EtOAc/hexanes (100 mL), 80% EtOAc/hexanes (50 mL), EtOAc (50 mL), 20% MeOH/EtOAc (50 mL) and 50% MeOH/EtOAc (25 mL)}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to afford six larger fractions, F.1–6. Fractions F.2 (19.5 mg) was redissolved in CH2Cl2 and MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (25 mL), 5% MeOH/CH2Cl2 (25 mL) and 20% MeOH/CH2Cl2 (25 mL)}. Following TLC analysis, the resulting fractions were combined to afford three fractions F.1–3; and F.2 provided compound 42 (12 mg, 0.04% w/w). Extract B. Extract B (5.6 g) was redissolved in MeOH and water, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash chromatography {silica; EtOAc (200 mL), 10% MeOH/EtOAc (200 mL), 20% MeOH/EtOAc (200 mL) and 30% MeOH/EtOAc (200 mL)} that provided five fractions, F.1–5. Fraction F.1 (127 mg) was redissolved in MeOH and CH2Cl2, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 5% MeOH/CH2Cl2 (100 mL), 10% MeOH/CH2Cl2 (50 mL), 30% MeOH/CH2Cl2 (100 mL) and MeOH (30 mL)} that provided compound 46 (4.8 mg, 0.016% w/w) as an off-white solid.
(p-Hydroxybenzyl)malonic acid (46).[70] Off-white solid. 1H NMR (600 MHz, acetone-d6) δ 7.05 (2H, d, J = 8.3 Hz; H-2′,6′), 6.68 (2H, d, J = 8.3 Hz; H-3′,5′), 3.53 (1H, t, J = 7.6 Hz; H-2), 3.06 (2H, d, J = 7.6 Hz; H-3); 1H NMR (600 MHz, DMSO-d6) δ 6.99 (2H, d, J = 8.4 Hz; H-2′,6′), 6.63 (2H, d, J = 8.4 Hz; H-3′,5′), 3.39 (1H, m, H-2), 2.92 (2H, d, J = 7.5 Hz; H-3) ppm. 13C NMR (150 MHz, acetone-d6) δ 172.9 (C-1), 157.1 (C-4′), 130.9 (C-2′,6′), 130.9 (C-1′), 116.2 (C-3′,5′), 55.4 (C-2), 35.2 (C-3); 13C NMR (150 MHz, DMSO-d6) δ 170.7 (C-1), 155.7 (C-4′), 129.6 (C-2′,6′), 128.7(C-1′), 114.9 (C-3′,5′), 53.5 (C-2), 33.5 (C-3) ppm. HRESIMS m/z calcd for C10H10O5Na [M + Na]+ 233.0426; found 233.0423.
PHWE of Lomaria nuda
L. nuda fronds (30 g) were finely ground using a spice grinder, mixed with sand (~6 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (254 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark brown extract B (5 g). Extract A. Extract A (254 mg) was redissolved in MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to automated flash chromatography {silica cartridge (4 g); 0–40% MeOH/EtOAc for 17 min}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined into seven large fractions, F.1–7. Fraction F.3 (14.5 mg) was redissolved in MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 5% MeOH/CH2Cl2 (50 mL), 10% MeOH/CH2Cl2 (50 mL), 20% MeOH/CH2Cl2 (50 mL) and MeOH (20 mL)} which provided compound 47 (2.5 mg, 0.01% w/w) as a yellow solid and compound 48 (5 mg, 0.016% w/w) as a yellow solid.
Quercetin-3-O-(6′-O-acetyl)glucoside (47) (CAS# 54542-51-7).[114] Yellow solid. 1H NMR (600 MHz, CD3OD) δ 7.81 (1H, d, J = 2.2 Hz; H-6′), 7.63 (1H, dd, J = 8.5 and 2.2 Hz; H-5′), 6.88 (1H, d, J = 8.5 Hz; H-2′), 6.44 (1H, d, J = 2.0 Hz; H-8), 6.23 (1H, d, J = 2.0 Hz; H-6), 5.08 (1H, d, J = 7.9 Hz; H-1″), 4.18 (1H, dd, J = 11.4 and 7.8 Hz; H-6a″), 4.07 (1H, dd, J = 11.4 and 4.5 Hz; H-6b″), 3.83 (1H, dd, J = 9.6 and 7.4 Hz; H-2″), 3.80 (1H, d, J = 3.3 Hz; H-3″), 3.70 (1H, dd, J = 7.7 and 4.6 Hz; H-5″), 3.58 (1H, dd, J = 9.4 and 3.1 Hz; H-4″), 1.83 (3H, s; 2‴-COCH3) ppm. 13C NMR (150 MHz, CD3OD) δ 179.4 (C-4), 172.5 (1‴-CO), 167.3 (C-7), 162.9 (C-5), 158.6 (C-9), 157.8 (C-2), 150.0 (C-4′), 145.8 (C-3′), 135.7 (C-3), 123.1 (C-6′), 122.8 (C-1′), 117.6 (C-5′), 116.0 (C-2′), 105.8 (C-1″), 105.1 (C-10), 100.4 (C-6), 95.1 (C-8), 74.9 (C-3″), 74.5 (C-5″), 72.9 (C-2″), 70.2 (C-4″), 64.5 (C-6″), 20.4 (2‴-COCH3) ppm.
Quercitrin (48) (CAS# 522-12-3).[115] Yellow solid. 1H NMR (600 MHz, CD3OD) δ 7.33 (1H, d, J = 2.1 Hz; H-2′), 7.30 (1H, dd, J = 8.3 and 2.1 Hz; H-6′), 6.91 (1H, d, J = 8.3 Hz; H-5′), 6.34 (1H, d, J = 2.1 Hz; H-6), 6.18 (1H, d, J = 2.1 Hz; H-8), 5.35 (1H, s; H-1″), 4.22 (1H, m; H-2″), 3.75 (1H, m; H-3″), 3.42 (1H, m; H-5″), 3.35 (1H, m; H-4″), 0.95 (3H, d, J = 6.2 Hz; 6″-CH3) ppm. 13C NMR (150 MHz, CD3OD) δ 179.5 (C-4), 167.3 (C-7), 163.1 (C-5), 159.1 (C-2), 158.6 (C-9), 149.9 (C-4′), 146.5 (C-3′), 136.1 (C-3), 122.9 (C-6′), 122.8 (C-1′), 116.9 (C-5′), 116.4 (C-2′), 105.5 (C-10), 103.5 (C-1″), 100.3 (C-6), 95.0 (C-8), 73.3 (C-4″), 72.1 (C-3″), 72.0 (C-5″), 71.9 (C-2′), 17.6 (6′-CH3) ppm.
PHWE of Parablechnum wattsii
P. wattsii dried leaf material (30 g) was finely ground using a spice grinder, mixed with sand (~6 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 120 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (523 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark-brown extract B (7 g). Extract A. Extract A (523 mg) was redissolved in MeOH, adsorbed onto silica and subjected to automated flash chromatography {silica cartridge (12 g); 0–100% EtOAc/hexanes and 0–40% MeOH/CH2Cl2 for 13 min at a flow rate of 25 mL/min} which provided compound 49 (143 mg, 0.5% w/w) as a dark-brown solid. Approximately 60 mg of compound 49 was absorbed onto silica and further purified by flash column chromatography {silica, 20% MeOH/CH2Cl2 (150 mL) then 40% MeOH/CH2Cl2 (100 mL)} which provided compound 49 as a pale-yellow/-green solid (20 mg).
5-O-Caffeoylshikimic acid (49) (CAS# 73263-62-4).[55] Pale-yellow/-green solid. 1H NMR (600 MHz, CD3OD) δ 7.56 (1H, d, J = 15.9 Hz; H-7′), 7.04 (1H, d, J = 1.7 Hz; H-2′), 6.95 (1H, dd, J = 8.2 and 1.6 Hz; H-6′), 6.83 (br s; H-2), 6.78 (1H, d, J = 8.2 Hz; H-5′), 6.28 (1H, dd, J = 15.9 Hz; H-8′), 5.25 (1H, dd, J = 13.2 and 5.6 Hz; H-5), 4.40 (1H, s; H-3), 3.90 (1H, dd, J = 7.9 and 4.1 Hz; H-4), 2.87 (1H, dd, J = 18.8 and 4.9 Hz; H-6a), 2.32 (1H, dd, J = 18.4 and 5.1 Hz; H-6b) ppm. 13C NMR (150 MHz, CD3OD) δ 170.3 (C-7), 168.6 (C-9′), 149.6 (C-4′), 147.2 (C-7′), 146.8 (C-3′), 138.2 (C-2), 131.1 (C-1), 127.7 (C-1′), 123.0 (C-6′), 116.5 (C-5′), 115.2 (C-2′), 115.1 (C-8′), 71.4 (C-5), 70.1 (C-4), 67.4 (C-3), 29.4 (C-6) ppm. HRESIMS m/z calcd for C16H16O8Na [M + Na]+ 359.0845; found 359.0738.
PHWE of Gleichenia alpina
G. alpina dried aerial parts (29 g) were finely ground using a spice grinder, mixed with sand (~4 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated further two times to provide a combined extract (600 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (200 mL and 2 × 150 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (646 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark-brown extract B (10 g). Extract A. Extract A (793 mg) was adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; EtOAc (300 mL), 10% MeOH/EtOAc (150 mL), 20% MeOH/EtOAc (150 mL), 30% MeOH/EtOAc (150 mL), 40% MeOH/EtOAc (150 mL) and MeOH (100 mL)}, to afford six fractions, F.1–6. Fraction F.3 (202 mg) was and adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (50 mL), 10% MeOH/CH2Cl2 (50 mL), 20% MeOH/CH2Cl2 (100 mL), 20% MeOH/CH2Cl2 (150 mL) and MeOH (50 mL)} which provided compound 50 (6.9 mg, 0.02% w/w) as a yellow solid that contained minor impurities.
Rutin (50) (CAS# 153-18-4).[116] Yellow solid. 1H NMR (600 MHz, CD3OD) δ 7.67 (1H, d, J = 2.2 Hz; H-2′), 7.63 (1H, dd, J = 8.4 and 2.2 Hz; H-6′), 6.88 (1H, d, J = 8.4 Hz; H-5′), 6.41 (1H, d, J = 2.1 Hz; H-8), 6.21 (1H, d, J = 2.1 Hz; H-6), 5.11 (1H, d, J = 7.7 Hz; H-1″), 4.52 (1H, d, J = 1.4 Hz; H-1‴), 3.80 (1H, dd, J = 11.2 and 1.5 Hz; H-6a″), 3.63 (1H, dd, J = 3.3 and 1.6 Hz; H-2″‘), 3.54 (1H, dd, J = 9.5 and 3.5 Hz; H-3‴), 3.38–3.49 (4H, m; H-2″,3″,5‴,6b″), 3.25–3.34 (3H, m; H-4″,5″,4‴), 1.12 (3H, d, J = 6.2 Hz; 6‴-CH3) ppm. 13C NMR (150 MHz, CD3OD) δ 179.4 (C-4), 166.1 (C-7), 162.9 (C-5), 159.3 (C-2), 158.5 (9), 149.8 (C-4′), 145.8 (3′), 135.6 (C-3), 123.5 (C-6′), 123.1 (C-1′), 117.7 (C-2′), 116.1 (C-5′), 105.6 (C-10), 104.7 (C-1″), 102.4 (C-1‴), 99.9 (C-6), 94.9 (C-8), 78.2 (C-3″), 77.2 (C-5″), 75.7 (C-2″), 73.9 (C-4‴), 72.2 (C-3‴), 72.1 (C-2‴), 71.4 (C-4″), 69.7 (C-5‴), 68.5 (C-6″), 17.9 (C-6‴) ppm.
PHWE of Dicksonia antarctica
D. antarctica dried leaflets (30.5 g) were finely ground using a spice grinder, mixed with sand (~7 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further three times to provide a combined extract (800 mL). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (150 mL and 2 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (298 g). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark-brown extract B (5 g). No compounds could be isolated by flash column chromatography.
PHWE of Doodia australis
D. australis dried leaflets (31 g) were finely ground using a spice grinder, mixed with sand (~7 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL). The aqueous extract was extracted with EtOAc (150 and 2 × 100 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (617 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark-brown extract B (3.5 g). No compounds could be isolated by flash column chromatography.
PHWE of Oceaniopteris cartilaginea
O. cartilaginea dried leaflets (33 g) were finely ground using a spice grinder, mixed with sand (~8 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further two times to provide a combined extract (600 mL) and extracted with EtOAc (400 mL and 2 × 200 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (1.5 g). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark-brown extract B (~2 g). No compounds could be isolated by flash column chromatography.
PHWE of Histiopteris incisa
H. incisa dried leaflets (35 g) were finely ground using a spice grinder, mixed with sand (~16 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further three times to provide a combined extract (800 mL) and extracted with EtOAc (3 × 250 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (877 mg). The remaining aqueous phase was concentrated under reduced pressure (50°C) to yield a dark-brown extract B (3 g). No compounds could be isolated by flash column chromatography.
PHWE of Pteridium esculentum
Aerial parts of P. esculentum (150 g) were finely ground using a spice grinder, mixed with sand (~75 g), and extracted via PHWE (35% EtOH/H2O). This provided a hot extract (200 mL) that was cooled immediately in an ice bath. This process was repeated a further nine times to provide a combined extract (2 L). The extract was concentrated under reduced pressure (35°C) to remove EtOH and extracted with EtOAc (3 × 500 mL). The combined organic phase was dried (MgSO4), filtered, and concentrated under reduced pressure to provide extract A (2.7 g). The remaining aqueous phase was concentrated under reduced pressure (50°C) to afford brown extract B (32 g). Extract A. Extract A (1.5 g) was redissolved in MeOH, adsorbed onto silica/Celite® (1:1 mixture by mass) and subjected to flash column chromatography {silica; CH2Cl2 (250 mL), 10% MeOH/CH2Cl2 (250 mL), 20% MeOH/CH2Cl2 (250 mL), 30% MeOH/CH2Cl2 (250 mL), 40% MeOH/CH2Cl2 (250 mL) and MeOH (200 mL)}. Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to provide seven fractions, F.1–7. Fraction F.4 (150 mg) was redissolved in 20% MeCN/H2O solution and subjected to automated flash chromatography (12 g C18 cartridge; 0–100% MeCN/H2O for 17 min with a flow rate of 28 mL/min). Following TLC and 1H NMR spectroscopic analysis, the resulting fractions were combined to afford four fractions, F.1–4. Fraction F.2 (19 mg) was redissolved in MeOH/CH2Cl2 solution and subjected to flash column chromatography {silica; 10% MeOH/CH2Cl2 (10 mL)} to provide compound 41 (12 mg, 0.006% w/w) as a pale-yellow solid. The fraction F.4 (42 mg) was subjected to preparative TLC which provided compound 51 (25 mg, 0.02% w/w) as a yellow solid.
Tiliroside (51) (CAS# 20316-62-5).[117,118] Yellow solid. 1H NMR (600 MHz, CD3OD) δ 7.98 (2H, d, J = 8.8 Hz; H-2′,6′), 7.40 (1H, d, J = 15.9 Hz; H-7‴), 7.30 (2H, d, J = 8.5 Hz; H-2‴,6‴), 6.82 (2H, d, J = 8.8 Hz; H-3′,5′), 6.79 (2H, d, J = 8.6 Hz; H-3‴,5‴), 6.30 (1H, d, J = 1.9 Hz; H-8), 6.13 (1H, d, J = 2.0 Hz; H-6), 6.07 (1H, d, J = 15.9 Hz; H-8‴), 5.23 (1H, d, J = 7.3 Hz; H-1″), 4.31 (1H, dd, J = 11.8 and 1.9 Hz; H-6a″), 4.20 (1H, m; H-6b″), 3.41–3.50 (3H, m; H-2″,3″,5″), 3.31 (1H, m; H-4″) ppm. 13C NMR (150 MHz, CD3OD) δ 179.4 (C-4), 168.6 (9‴-CO), 166.3 (C-7), 162.9 (C-5), 161.5 (C-4′), 161.2 (C-4‴), 159.3 (C-2), 158.5 (C-9), 146.6 (C-7‴), 135.2 (C-3), 132.2 (C-2′,6′), 131.2 (C-2‴,6‴), 127.1 (C-1‴), 122.7 (C-1′), 116.8 (C-3‴,5‴), 116.1 (C-3′,5′), 114.7 (C-8‴), 105.5 (C-10), 104.1 (C-1″), 100.1 (C-6), 94.9 (C-8), 78.0 (C-3″), 75.7 (C-2″,5″), 71.7 (C-4″), 64.3 (C-6″) ppm.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
We acknowledge the University of Tasmania (UTAS) School of Natural Sciences – Chemistry for funding. TG thanks the UTAS for a Tasmania Graduate Research Scholarship. ACB’s contributions were supported by an ARC Future Fellowship (FT200100049).
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank Brendon Schollum for assistance with GC-MS, the UTAS Central Science Laboratory for providing access to NMR spectroscopy services, and the Royal Botanical Gardens of Tasmania for generous provision of plant material.
References
[1] Hassler M. World ferns. Synonymic checklist and distribution of ferns and lycophytes of the world. 2022. Available at www.worldplants.de [cited 19 January 2022][2] Y Liu, W Wujisguleng, C Long, Food uses of ferns in China: a review. Acta Soc Bot Pol 2012, 81, 263.
| Food uses of ferns in China: a review.Crossref | GoogleScholarGoogle Scholar |
[3] T Matsuura, K Sugimura, A Miyamoto, N Tanaka, Knowledge-based estimation of edible fern harvesting sites in mountainous communities of northeastern Japan. Sustainability 2014, 6, 175.
| Knowledge-based estimation of edible fern harvesting sites in mountainous communities of northeastern Japan.Crossref | GoogleScholarGoogle Scholar |
[4] LA Wallis, B Stephenson, A nardoo processing grinding stone from a rockshelter in the Pilbara, Western Australia. Aust Archaeol 2020, 86, 112.
| A nardoo processing grinding stone from a rockshelter in the Pilbara, Western Australia.Crossref | GoogleScholarGoogle Scholar |
[5] Xr Baskaran, Av Geo Vigila, Sz Zhang, Sx Feng, Wb Liao, A review of the use of pteridophytes for treating human ailments. J Zhejiang Univ Sci B 2018, 19, 85.
| A review of the use of pteridophytes for treating human ailments.Crossref | GoogleScholarGoogle Scholar |
[6] GQ Su, HT Li, H Sun, et al. [Endemic plants for medicine use in China]. Zhongguo Zhongyao Zazhi 2017, 42, 4329.
| [Endemic plants for medicine use in China].Crossref | GoogleScholarGoogle Scholar |
[7] B Liu, Z-y Guo, R Bussmann, F-f Li, J-q Li, L-y Hong, et al. Ethnobotanical approaches of traditional medicine studies in Southwest China: a literature review. J Ethnopharmacol 2016, 186, 343.
| Ethnobotanical approaches of traditional medicine studies in Southwest China: a literature review.Crossref | GoogleScholarGoogle Scholar |
[8] J Sureshkumar, R Silambarasan, KA Bharati, J Krupa, S Amalraj, M Ayyanar, A review on ethnomedicinally important pteridophytes of India. J Ethnopharmacol 2018, 219, 269.
| A review on ethnomedicinally important pteridophytes of India.Crossref | GoogleScholarGoogle Scholar |
[9] RCPDS Reinaldo, ACP Santiago, PM Medeiros, UP Albuquerque, Do ferns and lycophytes function as medicinal plants? A study of their low representation in traditional pharmacopoeias. J Ethnopharmacol 2015, 175, 39.
| Do ferns and lycophytes function as medicinal plants? A study of their low representation in traditional pharmacopoeias.Crossref | GoogleScholarGoogle Scholar |
[10] H Cao, TT Chai, X Wang, MFB Morais-Braga, JH Yang, FC Wong, et al. Phytochemicals from fern species: potential for medicine applications. Phytochem Rev 2017, 16, 379.
| Phytochemicals from fern species: potential for medicine applications.Crossref | GoogleScholarGoogle Scholar |
[11] F Jiang, B Qi, N Ding, H Yang, F Jia, Y Luo, et al. Lycopodium alkaloids from Huperzia serrata. Fitoterapia 2019, 137, 104277.
| Lycopodium alkaloids from Huperzia serrata.Crossref | GoogleScholarGoogle Scholar |
[12] QF Zhu, QS Zhao, Chemical constituents and biological activities of lycophytes and ferns. Chin J Nat Med 2019, 17, 887.
| Chemical constituents and biological activities of lycophytes and ferns.Crossref | GoogleScholarGoogle Scholar |
[13] L-j Lin, X-b Huang, Z-c Lv, Isolation and identification of flavonoids components from Pteris vittata L. SpringerPlus 2016, 5, 5.
| Isolation and identification of flavonoids components from Pteris vittata L.Crossref | GoogleScholarGoogle Scholar |
[14] M Hou, Y Chen, Y Wang, K Hao, Sesquiterpenoids and flavonoids from Pteris multifida Poir. Biochem Syst Ecol 2021, 98, 104320.
| Sesquiterpenoids and flavonoids from Pteris multifida Poir.Crossref | GoogleScholarGoogle Scholar |
[15] F Asai, M Iinuma, T Tanaka, M Mizuno, Complex flavonoids in farinose exudate from Pityrogramma calomelanos. Phytochemistry 1991, 30, 3091.
| Complex flavonoids in farinose exudate from Pityrogramma calomelanos.Crossref | GoogleScholarGoogle Scholar |
[16] (a) M Iinuma, T Tanaka, M Takenaka, M Mizuno, F Asai, Five complex flavonoids in the farinose exudate of Pityrogramma Calomelanos. Phytochemistry 1992, 31, 2487.
| Five complex flavonoids in the farinose exudate of Pityrogramma Calomelanos.Crossref | GoogleScholarGoogle Scholar |
(b) F Asai, M Iinuma, T Tanaka, M Mizuno, Two complex flavonoids in the farinose exudate of Pityrogramma Calomelanos. Heterocycles 1992, 33, 229.
| Two complex flavonoids in the farinose exudate of Pityrogramma Calomelanos.Crossref | GoogleScholarGoogle Scholar |
[17] S-Y Shim, S-g Lee, M Lee, Biflavonoids isolated from Selaginella tamariscina and their anti-inflammatory activities via ERK 1/2 signaling. Molecules 2018, 23, 926.
| Biflavonoids isolated from Selaginella tamariscina and their anti-inflammatory activities via ERK 1/2 signaling.Crossref | GoogleScholarGoogle Scholar |
[18] HP Long, H Zou, FS Li, J Li, P Luo, ZX Zou, et al. Involvenflavones A-F, six new flavonoids with 3′-aryl substituent from Selaginella involven. Fitoterapia 2015, 105, 254.
| Involvenflavones A-F, six new flavonoids with 3′-aryl substituent from Selaginella involven.Crossref | GoogleScholarGoogle Scholar |
[19] LH Su, YP Li, HM Li, WF Dai, D Liu, L Cao, et al. Anti-inflammatory prenylated flavonoids from Helminthostachys zeylanica. Chem Pharm Bull 2016, 64, 497.
| Anti-inflammatory prenylated flavonoids from Helminthostachys zeylanica.Crossref | GoogleScholarGoogle Scholar |
[20] RH Mohammad, M Nur-E-Alam, M Lahmann, I Parveen, GJ Tizzard, SJ Coles, et al. Isolation and characterisation of 13 pterosins and pterosides from bracken (Pteridium aquilinum (L.) Kuhn) rhizome. Phytochemistry 2016, 128, 82.
| Isolation and characterisation of 13 pterosins and pterosides from bracken (Pteridium aquilinum (L.) Kuhn) rhizome.Crossref | GoogleScholarGoogle Scholar |
[21] H Niwa, M Ojika, K Wakamatsu, K Yamada, I Hirono, K Matsushita, Ptaquiloside, a novel norsesquiterpene glucoside from bracken, Pteridium aquilinum var. latiusculum. Tetrahedron Lett 1983, 24, 4117.
| Ptaquiloside, a novel norsesquiterpene glucoside from bracken, Pteridium aquilinum var. latiusculum.Crossref | GoogleScholarGoogle Scholar |
[22] X Ge, G Ye, P Li, WJ Tang, JL Gao, WM Zhao, Cytotoxic diterpenoids and sesquiterpenoids from Pteris multifida. J Nat Prod 2008, 71, 227.
| Cytotoxic diterpenoids and sesquiterpenoids from Pteris multifida.Crossref | GoogleScholarGoogle Scholar |
[23] MM Li, K Wang, J He, LY Peng, XQ Chen, X Cheng, et al. Four new labdane-type diterpenoid glycosides from Diplopterygium laevissimum. Nat Prod Bioprospect 2013, 3, 38.
| Four new labdane-type diterpenoid glycosides from Diplopterygium laevissimum.Crossref | GoogleScholarGoogle Scholar |
[24] XL Li, LM Yang, Y Zhao, RR Wang, G Xu, YT Zheng, et al. Tetranorclerodanes and clerodane-type diterpene glycosides from Dicranopteris dichotoma. J Nat Prod 2007, 70, 265.
| Tetranorclerodanes and clerodane-type diterpene glycosides from Dicranopteris dichotoma.Crossref | GoogleScholarGoogle Scholar |
[25] C Socolsky, Y Asakawa, A Bardón, Diterpenoid glycosides from the bitter fern Gleichenia quadripartita. J Nat Prod 2007, 70, 1837.
| Diterpenoid glycosides from the bitter fern Gleichenia quadripartita.Crossref | GoogleScholarGoogle Scholar |
[26] H Wada, Y Shimizu, T Hakamatsuka, N Tanaka, RC Cambie, JE Braggins, Two new clerodane glycosides from Gleichenia microphylla. Aust J Chem 1998, 51, 171.
| Two new clerodane glycosides from Gleichenia microphylla.Crossref | GoogleScholarGoogle Scholar |
[27] E Snogan, I Vahirua-Lechat, R Ho, G Bertho, JP Girault, S Ortiga, et al. Ecdysteroids from the medicinal fern Microsorum scolopendria (Burm. f.). Phytochem Anal 2007, 18, 441.
| Ecdysteroids from the medicinal fern Microsorum scolopendria (Burm. f.).Crossref | GoogleScholarGoogle Scholar |
[28] R Ho, T Teai, D Loquet, JP Bianchini, JP Girault, R Lafont, et al. Phytoecdysteroids in the genus Microsorum (Polypodiaceae) of French Polynesia. Nat Prod Commun 2007, 2, 1934578X0700200.
| Phytoecdysteroids in the genus Microsorum (Polypodiaceae) of French Polynesia.Crossref | GoogleScholarGoogle Scholar |
[29] M Watanabe, T Miyashita, HP Devkota, Phenolic compounds and ecdysteroids of Diplazium esculentum (Retz.) Sw. (Athyriaceae) from Japan and their chemotaxonomic significance. Biochem Syst Ecol 2021, 94, 104211.
| Phenolic compounds and ecdysteroids of Diplazium esculentum (Retz.) Sw. (Athyriaceae) from Japan and their chemotaxonomic significance.Crossref | GoogleScholarGoogle Scholar |
[30] J Hu, X Shi, X Mao, H Li, J Chen, J Shi, Ecdysteroids from the ethanol extract of Diplopterygium rufopilosum. Phytochem Lett 2014, 8, 73.
| Ecdysteroids from the ethanol extract of Diplopterygium rufopilosum.Crossref | GoogleScholarGoogle Scholar |
[31] RG Savchenko, NA Veskina, VN Odinokov, GV Benkovskaya, LV Parfenova, Ecdysteroids: isolation, chemical transformations, and biological activity. Phytochem Rev 2022,
| Ecdysteroids: isolation, chemical transformations, and biological activity.Crossref | GoogleScholarGoogle Scholar |
[32] J Jiang, L Tian, L Wang, Y Liu, Y Chen, Phenolic compounds from the fern Glaphyropteridopsis erubescens (Hook.) Ching. Biochem Syst Ecol 2013, 50, 136.
| Phenolic compounds from the fern Glaphyropteridopsis erubescens (Hook.) Ching.Crossref | GoogleScholarGoogle Scholar |
[33] Y-H Chen, F-R Chang, Y-J Lin, L Wang, J-F Chen, Y-C Wu, et al. Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.). Food Chem 2007, 105, 48.
| Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.).Crossref | GoogleScholarGoogle Scholar |
[34] Y-H Chen, F-R Chang, M-C Lu, P-W Hsieh, M-J Wu, Y-C Du, et al. New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm. Molecules 2008, 13, 255.
| New benzoyl glucosides and cytotoxic pterosin sesquiterpenes from Pteris ensiformis Burm.Crossref | GoogleScholarGoogle Scholar |
[35] H-A Wei, T-W Lian, Y-C Tu, J-T Hong, M-C Kou, M-J Wu, Inhibition of low-density lipoprotein oxidation and oxidative burst in polymorphonuclear neutrophils by caffeic acid and hispidin derivatives isolated from sword brake fern (Peris ensiformis Burm.). J Agric Food Chem 2007, 55, 10579.
| Inhibition of low-density lipoprotein oxidation and oxidative burst in polymorphonuclear neutrophils by caffeic acid and hispidin derivatives isolated from sword brake fern (Peris ensiformis Burm.).Crossref | GoogleScholarGoogle Scholar |
[36] H-B Hu, X-D Zheng, H Cao, Xanthone O-glycosides from the roots of Pteris multifida. J Chin Chem Soc 2006, 53, 459.
| Xanthone O-glycosides from the roots of Pteris multifida.Crossref | GoogleScholarGoogle Scholar |
[37] JW Finnie, PA Windsor, AE Kessell, Neurological diseases of ruminant livestock in Australia. II: Toxic disorders and nutritional deficiencies. Aust Vet J 2011, 89, 247.
| Neurological diseases of ruminant livestock in Australia. II: Toxic disorders and nutritional deficiencies.Crossref | GoogleScholarGoogle Scholar |
[38] B V McCleary, BF Chick, The purification and properties of a thiaminase I enzyme from nardoo (Marsilea drummondii). Phytochemistry 1977, 16, 207.
| The purification and properties of a thiaminase I enzyme from nardoo (Marsilea drummondii).Crossref | GoogleScholarGoogle Scholar |
[39] K Yamada, M Ojika, H Kigoshi, Ptaquiloside, the major toxin of bracken, and related terpene glycosides: Chemistry, biology and ecology. Nat Prod Rep 2007, 24, 798.
| Ptaquiloside, the major toxin of bracken, and related terpene glycosides: Chemistry, biology and ecology.Crossref | GoogleScholarGoogle Scholar |
[40] MP Agnew, DR Lauren, Determination of ptaquiloside in bracken fern (Pteridium esculentum). J Chromatogr A 1991, 538, 462.
| Determination of ptaquiloside in bracken fern (Pteridium esculentum).Crossref | GoogleScholarGoogle Scholar |
[41] LH Rasmussen, DR Lauren, BL Smith, HCB Hansen, Variation in ptaquiloside content in bracken (Pteridium esculentum (Forst. f) Cockayne) in New Zealand. N Z Vet J 2008, 56, 304.
| Variation in ptaquiloside content in bracken (Pteridium esculentum (Forst. f) Cockayne) in New Zealand.Crossref | GoogleScholarGoogle Scholar |
[42] MT Fletcher, IJ Brock, KG Reichmann, RA McKenzie, BJ Blaney, Norsesquiterpene glycosides in bracken ferns (Pteridium esculentum and Pteridium aquilinum subsp. wightianum) from eastern Australia: reassessed poisoning risk to animals. J Agric Food Chem 2011, 59, 5133.
| Norsesquiterpene glycosides in bracken ferns (Pteridium esculentum and Pteridium aquilinum subsp. wightianum) from eastern Australia: reassessed poisoning risk to animals.Crossref | GoogleScholarGoogle Scholar |
[43] DM Potter, MS Baird, Carcinogenic effects of ptaquiloside in bracken fern and related compounds. Br J Cancer 2000, 83, 914.
| Carcinogenic effects of ptaquiloside in bracken fern and related compounds.Crossref | GoogleScholarGoogle Scholar |
[44] K Saito, T Nagao, S Takatsuki, K Koyama, S Natori, The sesquiterpenoid carcinogen of bracken fern, and some analogues, from the pteridaceae. Phytochemistry 1990, 29, 1475.
| The sesquiterpenoid carcinogen of bracken fern, and some analogues, from the pteridaceae.Crossref | GoogleScholarGoogle Scholar |
[45] N V Kovganko, ZN Kashkan, SN Krivenok, Bioactive compounds of the flora of Belarus. 4. Pterosins A and B from Pteridium aquilinum. Chem Nat Compd 2004, 40, 227.
| Bioactive compounds of the flora of Belarus. 4. Pterosins A and B from Pteridium aquilinum.Crossref | GoogleScholarGoogle Scholar |
[46] JF Micheloud, LA Colque-Caro, OG Martinez, EJ Gimeno, D da Silva Freitas Ribeiro, BS Blanco, Bovine enzootic haematuria from consumption of Pteris deflexa and Pteris plumula in northwestern Argentina. Toxicon 2017, 134, 26.
| Bovine enzootic haematuria from consumption of Pteris deflexa and Pteris plumula in northwestern Argentina.Crossref | GoogleScholarGoogle Scholar |
[47] YL Huang, PY Yeh, CC Shen, CC Chen, Antioxidant flavonoids from the rhizomes of Helminthostachys zeylanica. Phytochemistry 2003, 64, 1277.
| Antioxidant flavonoids from the rhizomes of Helminthostachys zeylanica.Crossref | GoogleScholarGoogle Scholar |
[48] YL Huang, CC Shen, YC Shen, WF Chiou, CC Chen, Anti-inflammatory and antiosteoporosis flavonoids from the rhizomes of Helminthostachys zeylanica. J Nat Prod 2017, 80, 246.
| Anti-inflammatory and antiosteoporosis flavonoids from the rhizomes of Helminthostachys zeylanica.Crossref | GoogleScholarGoogle Scholar |
[49] CC Chen, YL Huang, PY Yeh, JC Ou, Cyclized geranyl stilbenes from the rhizomes of Helminthostachys zeylanica. Planta Med 2003, 69, 964.
| Cyclized geranyl stilbenes from the rhizomes of Helminthostachys zeylanica.Crossref | GoogleScholarGoogle Scholar |
[50] S Li, P Wang, G Deng, W Yuan, Z Su, Cytotoxic compounds from invasive giant salvinia (Salvinia molesta) against human tumor cells. Bioorg Med Chem Lett 2013, 23, 6682.
| Cytotoxic compounds from invasive giant salvinia (Salvinia molesta) against human tumor cells.Crossref | GoogleScholarGoogle Scholar |
[51] S Lima, G Diaz, MAN Diaz, Antibacterial chemical constituent and antiseptic herbal soap from Salvinia auriculata Aubl. Evid Based Complement Alternat Med 2013, 2013, 480509.
| Antibacterial chemical constituent and antiseptic herbal soap from Salvinia auriculata Aubl.Crossref | GoogleScholarGoogle Scholar |
[52] GA Purgato, S Lima, JVPB Baeta, VR Pizziolo, GN de Souza, G Diaz-Muñoz, et al. Salvinia auriculata: chemical profile and biological activity against Staphylococcus aureus isolated from bovine mastitis. Braz J Microbiol 2021, 52, 2401.
| Salvinia auriculata: chemical profile and biological activity against Staphylococcus aureus isolated from bovine mastitis.Crossref | GoogleScholarGoogle Scholar |
[53] BJ Deans, M De Salas, JA Smith, AC Bissember, Natural products isolated from endemic Tasmanian vascular plants. Aust J Chem 2018, 71, 756.
| Natural products isolated from endemic Tasmanian vascular plants.Crossref | GoogleScholarGoogle Scholar |
[54] N Tanaka, H Yuhara, H Wada, T Murakami, RC Cambie, JE Braggins, Phenolic constituents of Pteridium esculentum. Phytochemistry 1993, 32, 1037.
| Phenolic constituents of Pteridium esculentum.Crossref | GoogleScholarGoogle Scholar |
[55] T Saito, H Yamane, N Murofushi, N Takahashi, BO Phinney, 4-O-Caffeoylshikimic and 4-O-(p-coumaroyl)shikimic acids from the dwarf tree fern, Dicksonia antarctica. Biosci Biotechnol Biochem 1997, 61, 1397.
| 4-O-Caffeoylshikimic and 4-O-(p-coumaroyl)shikimic acids from the dwarf tree fern, Dicksonia antarctica.Crossref | GoogleScholarGoogle Scholar |
[56] BJ Deans, J Just, J Chhetri, LK Burt, JN Smith, NL Kilah, et al. Pressurized hot water extraction as a viable bioprospecting tool: isolation of coumarin natural products from previously unexamined Correa (Rutaceae) species. ChemistrySelect 2017, 2, 2439.
| Pressurized hot water extraction as a viable bioprospecting tool: isolation of coumarin natural products from previously unexamined Correa (Rutaceae) species.Crossref | GoogleScholarGoogle Scholar |
[57] A Numata, K Hokimoto, T Takemura, T Katsuno, K Yamamoto, Plant constituents biologically active to insects. V. Antifeedants for the larvae of the yellow butterfly, Eurema hecabe mandarina, in Osmunda japonica. Chem Pharm Bull 1984, 32, 2815.
| Plant constituents biologically active to insects. V. Antifeedants for the larvae of the yellow butterfly, Eurema hecabe mandarina, in Osmunda japonica.Crossref | GoogleScholarGoogle Scholar |
[58] A Numata, C Takahashi, R Fujiki, E Kitano, A Kitajima, T Takemura, Plant constituents biologically active to insects. VI. Antifeedants for larvae of the yellow butterfly, Eurema hecabe mandarina, in Osmunda japonica. (2). Chem Pharm Bull 1990, 38, 2862.
| Plant constituents biologically active to insects. VI. Antifeedants for larvae of the yellow butterfly, Eurema hecabe mandarina, in Osmunda japonica. (2).Crossref | GoogleScholarGoogle Scholar |
[59] YM Yu, JS Yang, CZ Peng, V Caer, PZ Cong, ZM Zou, et al. Lactones from Angiopteris caudatiformis. J Nat Prod 2009, 72, 921.
| Lactones from Angiopteris caudatiformis.Crossref | GoogleScholarGoogle Scholar |
[60] Y Chen, Y Tao, X Lian, L Wang, Y Zhao, J Jiang, et al. Chemical constituents of Angiopteris esculenta including two new natural lactones. Food Chem 2010, 122, 1173.
| Chemical constituents of Angiopteris esculenta including two new natural lactones.Crossref | GoogleScholarGoogle Scholar |
[61] H Kamitakahara, T Okayama, Praptiwi, A Agusta, Y Tobimatsu, T Takano, Two-dimensional NMR analysis of Angiopteris evecta rhizome and improved extraction method for angiopteroside. Phytochem Anal 2019, 30, 95.
| Two-dimensional NMR analysis of Angiopteris evecta rhizome and improved extraction method for angiopteroside.Crossref | GoogleScholarGoogle Scholar |
[62] T-H Hwang, Y Kashiwada, G-i Nonaka, I Nishioka, 4-Carboxymethyl flavan-3-ols and procyanidins from Davallia divaricata. Phytochemistry 1990, 29, 279.
| 4-Carboxymethyl flavan-3-ols and procyanidins from Davallia divaricata.Crossref | GoogleScholarGoogle Scholar |
[63] Y-H Chen, F-R Chang, Y-J Lin, P-W Hsieh, M-J Wu, Y-C Wu, Identification of antioxidants from rhizome of Davallia solida. Food Chem 2008, 107, 684.
| Identification of antioxidants from rhizome of Davallia solida.Crossref | GoogleScholarGoogle Scholar |
[64] X Chang, W Li, K Koike, L Wu, T Nikaido, Phenolic constituents from the rhizomes of Dryopteris crassirhizoma. Chem Pharm Bull 2006, 54, 748.
| Phenolic constituents from the rhizomes of Dryopteris crassirhizoma.Crossref | GoogleScholarGoogle Scholar |
[65] H Wada, T Kido, N Tanaka, T Murakami, Y Saiki, C-M Chen, Chemical and chemotaxonomical studies of ferns. LXXXI. Characteristic lignans of Blechnaceous ferns. Chem Pharm Bull 1992, 40, 2099.
| Chemical and chemotaxonomical studies of ferns. LXXXI. Characteristic lignans of Blechnaceous ferns.Crossref | GoogleScholarGoogle Scholar |
[66] CZ Wang, LB Davin, NG Lewis, Stereoselective phenolic coupling in Blechnum spicant: formation of 8–2′ linked (−)-cis-blechnic, (−)-trans-blechnic and (−)-brainic acids. Chem Commun 2001, 1, 113.
| Stereoselective phenolic coupling in Blechnum spicant: formation of 8–2′ linked (−)-cis-blechnic, (−)-trans-blechnic and (−)-brainic acids.Crossref | GoogleScholarGoogle Scholar |
[67] VP Maier, DM Metzler, AF Huber, 3-O-Caffeoylshikimic acid (Dactyllifric acid) and its isomers, a new class of enzymic browning substrates. Biochem Biophys Res Commun 1964, 14, 124.
| 3-O-Caffeoylshikimic acid (Dactyllifric acid) and its isomers, a new class of enzymic browning substrates.Crossref | GoogleScholarGoogle Scholar |
[68] M Fukuoka, Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. latiusculum. VI. Isolation of 5-O-caffeoylshikimic acid as an antithiamine factor. Chem Pharm Bull 1982, 30, 3219.
| Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. latiusculum. VI. Isolation of 5-O-caffeoylshikimic acid as an antithiamine factor.Crossref | GoogleScholarGoogle Scholar |
[69] M Veit, C Weidner, D Strack, V Wray, L Witte, FC Czygan, The distribution of caffeic acid conjugates in the equisetaceae and some ferns. Phytochemistry 1992, 31, 3483.
| The distribution of caffeic acid conjugates in the equisetaceae and some ferns.Crossref | GoogleScholarGoogle Scholar |
[70] C Simmler, D Nikolić, DC Lankin, Y Yu, JB Friesen, RB Van Breemen, et al. Orthogonal analysis underscores the relevance of primary and secondary metabolites in licorice. J Nat Prod 2014, 77, 1806.
| Orthogonal analysis underscores the relevance of primary and secondary metabolites in licorice.Crossref | GoogleScholarGoogle Scholar |
[71] G Li, D Nikolic, RB van Breemen, Identification and chemical standardization of licorice raw materials and dietary supplements using UHPLC-MS/MS. J Agric Food Chem 2016, 64, 8062.
| Identification and chemical standardization of licorice raw materials and dietary supplements using UHPLC-MS/MS.Crossref | GoogleScholarGoogle Scholar |
[72] T Nakabayashi, Isolation of astragalin and isoquercitrin from bracken, Pteridium aquilinum. J Agric Chem Soc Jpn 1955, 19, 104.
[73] K Kazuma, N Noda, M Suzuki, Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry 2003, 62, 229.
| Malonylated flavonol glycosides from the petals of Clitoria ternatea.Crossref | GoogleScholarGoogle Scholar |
[74] T Oikawa, K Hosoyama, Y Hiraga, G Kurono, T Takemoto, The constituents of Osmunda spp. II.1) A new flavonoid glycoside of Osmunda asiatica. Chem Pharm Bull 2002, 43, 2091.
[75] JT Han, MH Bang, OK Chun, DO Kim, CY Lee, NI Baek, Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch Pharm Res 2004, 27, 390.
| Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities.Crossref | GoogleScholarGoogle Scholar |
[76] S Deng, Z Deng, Y Fan, Y Peng, J Li, D Xiong, et al. Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (Lotus) by high-speed counter-current chromatography. J Chromatogr B Anal Technol Biomed Life Sci 2009, 877, 2487.
| Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (Lotus) by high-speed counter-current chromatography.Crossref | GoogleScholarGoogle Scholar |
[77] G Mandalari, A Tomaino, T Arcoraci, M Martorana, VL Turco, F Cacciola, et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J Food Compos Anal 2010, 23, 166.
| Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.).Crossref | GoogleScholarGoogle Scholar |
[78] H Yao, ZX Liao, Q Wu, GQ Lei, ZJ Liu, DF Chen, et al. Antioxidative flavanone glycosides from the branches and leaves of Viscum coloratum. Chem Pharm Bull 2006, 54, 133.
| Antioxidative flavanone glycosides from the branches and leaves of Viscum coloratum.Crossref | GoogleScholarGoogle Scholar |
[79] J Malejko, E Nalewajko-Sieliwoniuk, J Nazaruk, J Siniło, A Kojło, Determination of the total polyphenolic content in Cirsium palustre (L.) leaves extracts with manganese (IV) chemiluminescence detection. Food Chem 2014, 152, 155.
| Determination of the total polyphenolic content in Cirsium palustre (L.) leaves extracts with manganese (IV) chemiluminescence detection.Crossref | GoogleScholarGoogle Scholar |
[80] A Mun’im, O Negishi, T Ozawa, Antioxidative compounds from Crotalaria sessiliflora. Biosci Biotechnol Biochem 2003, 67, 410.
| Antioxidative compounds from Crotalaria sessiliflora.Crossref | GoogleScholarGoogle Scholar |
[81] G Cioffi, LM Escobar, A Braca, N De Tommasi, Antioxidant chalcone glycosides and flavanones from Maclura (Chlorophora) tinctoria. J Nat Prod 2003, 66, 1061.
| Antioxidant chalcone glycosides and flavanones from Maclura (Chlorophora) tinctoria.Crossref | GoogleScholarGoogle Scholar |
[82] H Ismaili, S Sosa, D Brkic, S Fkih-Tetouani, A Ilidrissi, D Touati, et al. Topical anti-inflammatory activity of extracts and compounds from Thymus broussonettii. J Pharm Pharmacol 2010, 54, 1137.
| Topical anti-inflammatory activity of extracts and compounds from Thymus broussonettii.Crossref | GoogleScholarGoogle Scholar |
[83] JM Jarrett, AH Williams, The flavonoid glycosides of Salix purpurea. Phytochemistry 1967, 6, 1585.
| The flavonoid glycosides of Salix purpurea.Crossref | GoogleScholarGoogle Scholar |
[84] Q Hu, DD Zhang, L Wang, H Lou, D Ren, Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity. Food Chem Toxicol 2012, 50, 1927.
| Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity.Crossref | GoogleScholarGoogle Scholar |
[85] J Pan, S Zhang, L Yan, J Tai, Q Xiao, K Zou, et al. Separation of flavanone enantiomers and flavanone glucoside diastereomers from Balanophora involucrata Hook. f. by capillary electrophoresis and reversed-phase high-performance liquid chromatography on a C18 column. J Chromatogr A 2008, 1185, 117.
| Separation of flavanone enantiomers and flavanone glucoside diastereomers from Balanophora involucrata Hook. f. by capillary electrophoresis and reversed-phase high-performance liquid chromatography on a C18 column.Crossref | GoogleScholarGoogle Scholar |
[86] C Ma, Y Zhou, AR Liu, Determination of chlorogenic acid and eriodictyol-7-O-β-d-glucuronide in Pyrrosia by RP-HPLC. Yaoxue Xuebao 2003, 38, 286.
[87] X Jing, D Ren, X Wei, H Shi, X Zhang, RG Perez, et al. Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol Appl Pharmacol 2013, 273, 672.
| Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury.Crossref | GoogleScholarGoogle Scholar |
[88] R Chen, QL Qi, MT Wang, QY Li, Therapeutic potential of naringin: an overview. Pharm Biol 2016, 54, 3203.
| Therapeutic potential of naringin: an overview.Crossref | GoogleScholarGoogle Scholar |
[89] S Bharti, N Rani, B Krishnamurthy, DS Arya, Preclinical evidence for the pharmacological actions of naringin: A review. Planta Med 2014, 80, 437.
| Preclinical evidence for the pharmacological actions of naringin: A review.Crossref | GoogleScholarGoogle Scholar |
[90] DC Abeysinghe, X Li, CD Sun, WS Zhang, CH Zhou, KS Chen, Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem 2007, 104, 1338.
| Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species.Crossref | GoogleScholarGoogle Scholar |
[91] MA Alam, N Subhan, MM Rahman, SJ Uddin, HM Reza, SD Sarker, Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr 2014, 5, 404.
| Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action.Crossref | GoogleScholarGoogle Scholar |
[92] S Yusof, HM Ghazali, GS King, Naringin content in local citrus fruits. Food Chem 1990, 37, 113.
| Naringin content in local citrus fruits.Crossref | GoogleScholarGoogle Scholar |
[93] GC Jagetia, VA Venkatesha, TK Reddy, Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis 2003, 18, 337.
| Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow.Crossref | GoogleScholarGoogle Scholar |
[94] S Sharma, A Ali, J Ali, JK Sahni, S Baboota, Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin Investig Drugs 2013, 22, 1063.
| Rutin: Therapeutic potential and recent advances in drug delivery.Crossref | GoogleScholarGoogle Scholar |
[95] LS Chua, A review on plant-based rutin extraction methods and its pharmacological activities. J Ethnopharmacol 2013, 150, 805.
| A review on plant-based rutin extraction methods and its pharmacological activities.Crossref | GoogleScholarGoogle Scholar |
[96] F Imperato, A flavanone glycoside from the fronds of Ceterach officinarum. Phytochemistry 1983, 22, 312.
| A flavanone glycoside from the fronds of Ceterach officinarum.Crossref | GoogleScholarGoogle Scholar |
[97] C Socolsky, A Salvatore, Y Asakawa, A Bardón, Bioactive new bitter-tasting p-hydroxystyrene glycoside and otherconstituents from the fern Elaphoglossum spathulatum. Arkivoc 2003, 2003, 347.
| Bioactive new bitter-tasting p-hydroxystyrene glycoside and otherconstituents from the fern Elaphoglossum spathulatum.Crossref | GoogleScholarGoogle Scholar |
[98] T-H Yang, Y-C Lee, H-N Chung, Constitutents of Drynaria fortunei. Taiwan Yaoxue Zazhi 1966, 18, 38.
[99] J De Britto, VS Manickam, S Gopalakrishnan, T Ushioda, N Tanaka, Determination of aglycone chirality in dihydroflavonol 3-O-a-L-Rhamnosdes by 1H-NMR spectroscopy. Chem Pharm Bull 1995, 43, 338.
| Determination of aglycone chirality in dihydroflavonol 3-O-a-L-Rhamnosdes by 1H-NMR spectroscopy.Crossref | GoogleScholarGoogle Scholar |
[100] DM Grochowski, M Locatelli, S Granica, F Cacciagrano, M Tomczyk, A review on the dietary flavonoid tiliroside. Compr Rev Food Sci Food Saf 2018, 17, 1395.
| A review on the dietary flavonoid tiliroside.Crossref | GoogleScholarGoogle Scholar |
[101] M Kuroyanagi, M Fukuoka, K Yoshihira, S Natori, K Yamasaki, Confirmation of the structure of tiliroside, an acylated kaemferol glycoside, by 13C-nuclear magnetic resonance. Chem Pharm Bull 1978, 26, 3594.
| Confirmation of the structure of tiliroside, an acylated kaemferol glycoside, by 13C-nuclear magnetic resonance.Crossref | GoogleScholarGoogle Scholar |
[102] C-Y Wang, AM Pamukcu, GT Bryan, Isolation of fumaric acid, succinic acid, astragalin, isoquercitrin and tiliroside from Pteridium auilinum. Phytochemistry 1973, 12, 2298.
| Isolation of fumaric acid, succinic acid, astragalin, isoquercitrin and tiliroside from Pteridium auilinum.Crossref | GoogleScholarGoogle Scholar |
[103] S Devi, V Kumar, Comprehensive structural analysis of cis- and trans-tiliroside and quercetrin from Malvastrum coromandelianum and their antioxidant activities. Arab J Chem 2020, 13, 1720.
| Comprehensive structural analysis of cis- and trans-tiliroside and quercetrin from Malvastrum coromandelianum and their antioxidant activities.Crossref | GoogleScholarGoogle Scholar |
[104] W Qiao, C Zhao, N Qin, HY Zhai, HQ Duan, Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis. J Ethnopharmacol 2011, 135, 515.
| Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis.Crossref | GoogleScholarGoogle Scholar |
[105] R Velagapudi, M Aderogba, OA Olajide, Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim Biophys Acta - Gen Subj 2014, 1840, 3311.
| Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia.Crossref | GoogleScholarGoogle Scholar |
[106] Y Zhu, Y Zhang, Y Liu, H Chu, H Duan, Synthesis and biological activity of trans-tiliroside derivatives as potent anti-diabetic agents. Molecules 2010, 15, 9174.
| Synthesis and biological activity of trans-tiliroside derivatives as potent anti-diabetic agents.Crossref | GoogleScholarGoogle Scholar |
[107] J Just, BJ Deans, WJ Olivier, B Paull, AC Bissember, JA Smith, New method for the rapid extraction of natural products: efficient isolation of shikimic acid from star anise. Org Lett 2015, 17, 2428.
| New method for the rapid extraction of natural products: efficient isolation of shikimic acid from star anise.Crossref | GoogleScholarGoogle Scholar |
[108] CC Ho, BJ Deans, J Just, GG Warr, S Wilkinson, JA Smith, et al. Employing pressurized hot water extraction (PHWE) to explore natural products chemistry in the undergraduate laboratory. J Vis Exp 2018, 141, 1.
| Employing pressurized hot water extraction (PHWE) to explore natural products chemistry in the undergraduate laboratory.Crossref | GoogleScholarGoogle Scholar |
[109] BJ Deans, J Just, JA Smith, AC Bissember, Development and applications of water-based extraction methods in natural products isolation chemistry. Asian J Org Chem 2020, 9, 1144.
| Development and applications of water-based extraction methods in natural products isolation chemistry.Crossref | GoogleScholarGoogle Scholar |
[110] GR Fulmer, AJM Miller, NH Sherden, HE Gottlieb, A Nudelman, BM Stoltz, et al. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176.
| NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist.Crossref | GoogleScholarGoogle Scholar |
[111] EJ Chang, WJ Lee, SH Cho, SW Choi, Proliferative effects of flavan-3-ols and propelargonidins from rhizomes of Drynaria fortunei on MCF-7 and osteoblastic cells. Arch Pharm Res 2003, 26, 620.
| Proliferative effects of flavan-3-ols and propelargonidins from rhizomes of Drynaria fortunei on MCF-7 and osteoblastic cells.Crossref | GoogleScholarGoogle Scholar |
[112] T Akiyama, M Yamada, T Yamada, T Maitani, Naringin glycosides α-glucosylated on ring B found in the natural food additive, enzymatically modified naringin. Biosci Biotechnol Biochem 2000, 64, 2246.
| Naringin glycosides α-glucosylated on ring B found in the natural food additive, enzymatically modified naringin.Crossref | GoogleScholarGoogle Scholar |
[113] LB Davin, CZ Wang, GL Helms, NG Lewis, [13C]-Specific labeling of 8-2′ linked (−)-cis-blechnic, (−)-trans-blechnic and (−)-brainic acids in the fern Blechnum spicant. Phytochemistry 2003, 62, 501.
| [13C]-Specific labeling of 8-2′ linked (−)-cis-blechnic, (−)-trans-blechnic and (−)-brainic acids in the fern Blechnum spicant.Crossref | GoogleScholarGoogle Scholar |
[114] H Matsuura, M Amano, J Kawabata, et al. Isolation and measurement of quercetin glucosides in flower buds of japanese butterbur (Petasites japonicus subsp. Gigantea kitam.). Biosci Biotechnol Biochem 2002, 66, 1571.
| Isolation and measurement of quercetin glucosides in flower buds of japanese butterbur (Petasites japonicus subsp. Gigantea kitam.).Crossref | GoogleScholarGoogle Scholar |
[115] JH Lee, CH Ku, N-I Baek, S-H Kim, HW Park, DK Kim, Phytochemical constituents from Diodia teres. Arch Pharm Res 2004, 27, 40.
| Phytochemical constituents from Diodia teres.Crossref | GoogleScholarGoogle Scholar |
[116] M Zor, S Aydin, ND Güner, N Başaran, AA Başaran, Antigenotoxic properties of Paliurus spina-christi Mill fruits and their active compounds. BMC Complement Altern Med 2017, 17, 229.
| Antigenotoxic properties of Paliurus spina-christi Mill fruits and their active compounds.Crossref | GoogleScholarGoogle Scholar |
[117] M Kaouadji, J-M Morand, J Garcia, Further acylated Kaempferol rhamnosides from Platanus acerifolia buds. J Nat Prod 1993, 56, 1618.
| Further acylated Kaempferol rhamnosides from Platanus acerifolia buds.Crossref | GoogleScholarGoogle Scholar |
[118] F Calzada, R Lopéz, M Meckes, R Cedillo-Rivera, Flavonoids of the aerial parts of Helianthemum glomeratum. Pharm Biol 1995, 33, 351.
| Flavonoids of the aerial parts of Helianthemum glomeratum.Crossref | GoogleScholarGoogle Scholar |



