Microfluidic Radiosynthesis of the Muscarinic M2 Imaging Agent [18F]FP-TZTP*
Lidia Matesic A D , Ivan Greguric A B and Giancarlo Pascali A CA Australian Nuclear Science and Technology Organisation, Locked Bag 2001, Kirrawee DC, NSW 2232, Australia.
B Australian Centre of Nanomedicine, School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia.
C Brain and Mind Centre, The University of Sydney, Mallett Street, Camperdown, NSW 2050, Australia.
D Corresponding author. Email: lidia.matesic@ansto.gov.au
Australian Journal of Chemistry 71(10) 811-817 https://doi.org/10.1071/CH18266
Submitted: 1 June 2018 Accepted: 1 August 2018 Published: 31 August 2018
Abstract
3-(4-(3-[18F]Fluoropropylthio)-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,6-tetrahydropyridine ([18F]FP-TZTP) is a selective 18F-radiotracer for the muscarinic acetylcholine receptor subtype M2, which can be used to perform positron emission tomography (PET) scans on patients with neurological disorders such as Alzheimer’s disease. [18F]FP-TZTP was produced using continuous-flow microfluidics, a technique that uses reduced amounts of chemical reagents, shorter reaction times and in general, results in higher radiochemical yields compared to currently used techniques. The optimal 18F-radiolabelling conditions consisted of a total flow rate of 40 µL min−1 and 190°C, which produced [18F]FP-TZTP in 26 ± 10 % radiochemical yield with a molar activity of 182 ± 65 GBq µmol−1 and >99 % radiochemical purity.
Introduction
Alzheimer’s disease (AD) affects 35–40 million patients worldwide and currently is incurable.[1] The quest to develop diagnostic tools for AD is currently an intense area of research in neuroscience in order to diagnose AD patients before they become symptomatic owing to already well-established structural and functional changes in the brain. Currently, the diagnosis of this disease can be achieved through the use of positron emission tomography (PET) using molecules radiolabelled with carbon-11 or fluorine-18, although some of the lead candidates display undesired off-target binding in the brain, which could compromise their utility in molecular imaging.[2]
A promising target for the diagnosis of AD involves the muscarinic acetylcholine subtype M2 receptor, as a selective loss in the cerebral cortex of M2 receptors over the M1 subtype receptors has been linked to AD.[3] Therefore, earlier detection of AD through a selective M2 receptor imaging agent would provide a more favourable prognosis and earlier intervention at presymptomatic stages that could slow disease progression. Derivatives of thiadiazolyltetrahydropyridine (TZTP) were evaluated in the past for their muscarinic receptor selectivity.[4] Further investigation by preparing fluorinated analogues led to the most promising M2 selective compound being the fluoropropylthio-TZTP (FP-TZTP) derivative.[5] Radiolabelling the analogue with fluorine-18 to form [18F]FP-TZTP allowed in vivo imaging in animals that demonstrated favourable blood–brain barrier transport and rapid tissue uptake, and confirmed M2 receptor selectivity.[5–7] Owing to these promising results, [18F]FP-TZTP is nowadays the only 18F-radiotracer available for human in vivo imaging of the M2 receptor.
Initial attempts to produce [18F]FP-TZTP involved a two-step procedure, starting with the 18F-radiolabelling of propyl ditosylate to produce [18F]fluoropropyl tosylate, followed by alkylation onto a sulfide to produce the thioalkyl chain of [18F]FP-TZTP.[5] This convoluted radiosynthesis produced [18F]FP-TZTP in 34 ± 4 % radiochemical yield (RCY) in 85 min with a molar activity of 85 GBq µmol−1 decay-corrected to end-of-bombardment (EOB). The radiolabelling procedure was improved through the use of an automated radiosynthesis module and a new one-step radiolabelling precursor featuring a mesylate salt.[8] Although the entire synthesis time was shorter (60 min), the RCY was lower (19 ± 2 % decay-corrected to end-of-synthesis (EOS)); however, this may be attributed to the mesylate precursor containing a 10 % impurity of 2-propanol. Similarly, an [18F]FP-TZTP one-step precursor incorporating a tosylate leaving group was proposed.[9] Using the automated TRACERlab FXFN radiosynthesis module, [18F]FP-TZTP was produced in 29 ± 4 % uncorrected RCY, in 35 min, with a molar activity of 138 ± 41 GBq µmol−1.
Owing to our laboratory’s interest in producing 18F-radiotracers using microfluidic systems, we envisaged that the production of [18F]FP-TZTP could be readily translated from a batch process in a vessel radiosynthesis module to a continuous-flow microfluidic process. The advantages of using microfluidic systems to produce 18F-radiotracers, namely shorter reaction times, reduced amounts of chemical reagents, and higher radiochemical yields,[10,11] are contributing to the rising interest in the use of this technology.[12–14] Using the commercially available Advion NanoTek microfluidic synthesis system, we attached a custom-made electrical board to modify the system to incorporate HPLC and solid phase extraction (SPE) purification and subsequent formulation.[15] This customised system has produced a range of 18F-radiotracers including [18F]CB102, [18F]fluoroalkylcholines,[15] and [18F]MEL050.[16] More recently, it was demonstrated that this system was capable of producing up to three different 18F-radiotracers sequentially, using the same starting batch of [18F]fluoride.[17] Herein, we discuss the first account of the microfluidic radiolabelling optimisation and production of the muscarinic M2 imaging agent [18F]FP-TZTP.
Results and Discussion
18F-Fluorination Optimisation
Previously, the mesylate salt of FP-TZTP was initially disregarded as the preferred radiolabelling precursor owing to the presence of a 10 % impurity of 2-propanol.[8] Nonetheless, as a commercial source of the precursor in excellent purity is now available, in our study, we radiofluorinated the mesyl FP-TZTP precursor using the Advion NanoTek system to produce [18F]FP-TZTP (Scheme 1). Microfluidic radiolabelling conditions for [18F]FP-TZTP were rapidly optimised (~30 reactions in 4 h) by varying parameters such as temperature (Fig. 1), flow rate (hence, residence time; Fig. 2), and stoichiometric ratio (amount of precursor mass; Fig. 3), which were altered in the Discovery Mode of the NanoTek software.[18] Initial radiolabelling optimisation using acetonitrile as a solvent led to the maximum 18F-incorporation yield of 39 % (unisolated, determined by radio-HPLC as percentage of product peak area over entire radiochromatogram area) at 190°C. The use of DMSO facilitated greater solubility of the mesyl FP-TZTP precursor, which may have contributed to an increase in 18F-incorporation yield at 190°C (53 %). Interestingly, using a non-dried potassium [18F]fluoride-Kryptofix [2.2.2] (K[18F]F.K222) complex, i.e. without the aid of azeotropic distillation to remove water from K2.2.2/K2CO3, resulted in decreased, but not completely supressed, 18F-incorporation yield (35 %) when using DMSO as the reaction solvent. Although it is generally regarded that radiofluorinations should occur under anhydrous conditions, there have been recent reports of radiotracers being radiolabelled using non-azeotropically dried K[18F]F.K222 complex.[19–22] In the case of [18F]FP-TZTP, it appeared that anhydrous radiolabelling conditions were vital to achieve high levels of 18F-incorporation. By altering the reaction solvent to DMF, the optimal 18F-incorporation yield of 78 % at 190°C was achieved (Fig. 1). There was no significant difference in 18F-incorporation yield when K2.2.2/K2CO3 or tetraeathylammonium bicarbonate were used as the activating agents.

|

|

|

|
The optimal flow rate for the reaction was 10 µL min−1 of the precursor solution and of the K[18F]F.K222 complex (total flow rate of 20 µL min−1; residence time 47 s), and there was no significant difference in 18F-incorporation yield when a total flow rate of 40 µL min−1 was employed (residence time 23.6 s, Fig. 2). Interestingly, higher amounts of precursor mass, obtained by modifying the flow ratio of the reagents, resulted in decreased yields of [18F]FP-TZTP (Fig. 3). The modification of flow ratios to achieve higher precursor mass was performed using the same bolus volume of K[18F]F.K222 complex (10 µL) reacted with varying bolus volumes (10–50 µL) of 2 mg mL−1 DMF solution of precursor. Using the same bolus volume of precursor solution (10 µL) and varying bolus volumes of K[18F]F.K222 complex (10–50 µL) resulted in lower overall precursor mass. Using a precursor bolus volume of 10 µL (i.e. 20 µg) resulted in 45 % yield [18F]FP-TZTP at 190°C, as determined by radio-TLC. In contrast, using a 50 µL aliquot of the 2 mg mL−1 solution (i.e. 100 µg) led to only 3 % 18F-incorporation. (The difference in 18F-incorporation yield from previous experiments was due to the different analytical method selected to expedite the collection of results. Additionally, whereas radio-HPLC yield tends to overestimate the amount of organic radio-products, radio-TLC yield provides complete recovery of unreacted [18F]fluoride, but owing to their volatility, some organic radio-products may be lost. The use of newer monolithic or polymeric HPLC stationary phases reduces this discrepancy.[23]) The precursor concentration used in the present study (2 mg mL−1) was significantly decreased from 6 mg mL−1 used in a vessel radiosynthesis module.[8,9] Although these studies both used 3 mg of the precursor molecule in 500 µL of solvent, the entire solution including the K[18F]F.K222 complex was consumed during the radiosynthesis. In contrast, using a microfluidic method, only a 100 µL subsample of the 2 mg mL−1 solution (i.e. 200 µg) and 100 µL subsample of the K[18F]F.K222 complex were required for each full production of [18F]FP-TZTP.
Production of [18F]FP-TZTP
The entire production process for the radiosynthesis of [18F]FP-TZTP was performed using custom-written macro sequences in the NanoTek software. Prior to the production-scale experiment, up to three ‘dummy’ (i.e. mock) reactions were performed using a 20 µL subsample of the precursor solution and K[18F]F.K222 complex. These ‘dummy’ reactions ensure the homogeneity, reliability, and reproducibility of use of the reagents before the larger-scale reaction and, additionally, the RCY of the isolated [18F]FP-TZTP was extrapolated based on the radioactivity recovered from the ‘dummy’ reactions, i.e. RCY = product activity/estimated starting activity based on ‘dummy’ reaction, corrected to EOS.[17] Although three ‘dummy’ reactions may not always be necessary, it is recommended that at least two are performed to ensure reproducibility. Performing ‘dummy’ reactions also increases the overall total amount of precursor used in the radiosynthesis (in our case, an additional 40 µg of precursor is used per ‘dummy’ reaction) and the time required to perform these reactions is additional to the synthesis time required to produce the 18F-radiotracer. The production of [18F]FP-TZTP was achieved using 100 µL subsamples of the precursor solution and of the K[18F]F.K222 complex solution and heating at 190°C. Although the optimal flow rate from the radiolabelling optimisation was a total flow rate of 20 µL min−1 (54 % as determined by radio-TLC, residence time 47 s, Fig. 2), a total flow rate of 40 µL min−1 (residence time 23.6 s) was selected owing to similar 18F-incorporation yield (52 %, Fig. 2) in order for the time required to react the entire bolus (200 µL) to decrease from 10 to 5 min.
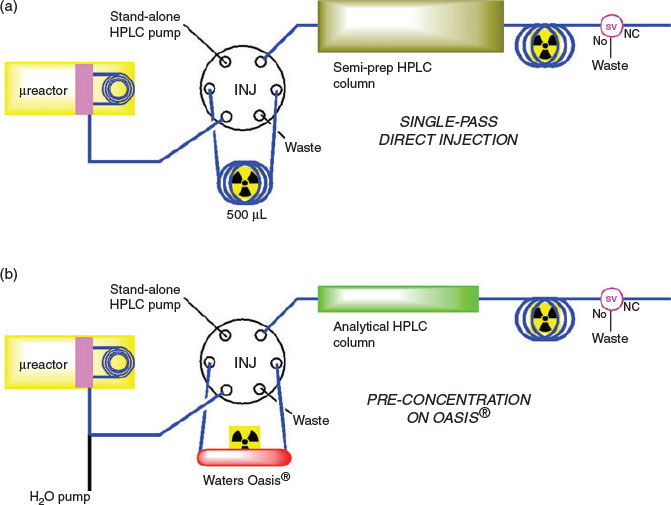
Once the 18F-fluorination reaction was complete, it was washed out of the microreactor with additional DMF and loaded onto an HPLC injector loop containing a radiodetector. The sample was then loaded onto a semipreparative HPLC column (Fig. 4a) and the product peak collected (at 12–15 min) into a two-necked round-bottom vial with the aid of another radiodetector. Additional water (15 mL) was added to the collected fraction before it was loaded onto a Sep-pak C18 Light SPE cartridge and eluted with 1.5 mL EtOH into the vial of an additional NanoTek Concentrator module. The EtOH was evaporated at 90°C for 15 min, before the residue was reconstituted in 1 mL of 10 % ethanolic saline solution as described previously.[16,17] Under these conditions, [18F]FP-TZTP was obtained in 26 ± 10 % RCY (n = 15), with a molar activity of 182 ± 65 GBq µmol−1 at EOS. The entire radiosynthesis was completed in 49 ± 2 min and radiochemical purity was >99 %. The identification of [18F]FP-TZTP was confirmed through the co-injection with the non-radioactive analogue (Fig. 5).

|
In order to decrease the purification time, it was envisaged that an analytical HPLC column could be employed instead. Once the 18F-fluorination was complete, the reaction output from the microreactor was diluted online with water using an external syringe pump at a flow rate of 1 mL min−1 (1 : 5 dilution factor product/water) and loaded onto a Waters Oasis® column to preconcentrate the [18F]FP-TZTP and other organic impurities (Fig. 4b), while the aqueous stream was directed to waste. This method is efficient owing to the use of smaller HPLC columns and therefore smaller volumes of the desired product being collected. If biocompatible HPLC eluents are used (i.e. ethanol/saline), this method may negate the need for further reformulation of the final product.[24] In our hands, the HPLC preconcentration step was effective as the aqueous waste from this process was evaluated by radio-HPLC to contain >99.5 % of the [18F]fluoride ion and <0.5 % of [18F]FP-TZTP. Once the [18F]FP-TZTP had been trapped onto the Oasis® cartridge and washed, the HPLC pump and eluent were switched to the appropriate mobile phase and the contents of the Oasis® cartridge were injected onto an analytical monolithic HPLC column. Following collection of the desired product peak (at 3–5 min), the solution was reformulated using SPE as described above. Using this method of purification led to the production of [18F]FP-TZTP in 18 ± 8 % RCY (number of runs, n = 5), with a molar activity of 218 ± 182 GBq µmol−1 at EOS. The entire radiosynthesis was produced in a shorter period of time (41 min) owing to faster purification of [18F]FP-TZTP.
The decrease in RCY obtained when purifying the product via the Oasis® method was further investigated. First, a total bolus volume of 20 µL was dispensed into the microreactor at 190°C at a total flow rate of 40 µL min−1, and the reaction output was collected, analysed by radio-HPLC, and found to contain 69 % [18F]FP-TZTP (Fig. 6). The reaction was then repeated; however, the solution was diluted online with water at 1 mL min−1, collected, and analysed by radio-HPLC, and found to contain 25 % [18F]FP-TZTP (Fig. 7). These results indicated that the online dilution with water led to a significant decrease in the RCY of [18F]FP-TZTP and therefore this method of purification was unsuitable for this 18F-radiotracer. Further evidence of decreased RCY due to hydrous conditions was observed when the radiosynthesis of [18F]FP-TZTP was performed using conventional semipreparative HPLC but starting with a non-azeotropically dried K[18F]F.K222 complex (17 ± 2 % RCY, n = 4). Although not fully understood, it is speculated that the presence of water and [18F]FP-TZTP at elevated temperatures, either at 190°C during the reaction or as it is exiting the heated microreactor towards the Oasis® cartridge, leads to degradation of the product. [18F]FP-TZTP does not decompose when it is subjected to HPLC containing an aqueous mobile phase, or if it is diluted with water at room temperature.

|

|
Conclusion
We have demonstrated the first method for the microfluidic radiolabelling of the muscarinic M2 imaging agent [18F]FP-TZTP. Using continuous-flow microfluidics allowed the fluorine-18 radiolabelling conditions to be rapidly optimised. Through this process, it was found that higher concentrations of the mesyl FP-TZTP precursor led to decreased 18F-incorporation, which is in contrast to most examples of 18F-radiotracers. Using a commercially available mesylate salt precursor obviated purity issues that had resulted in decreased RCY in the past. Therefore, the microfluidic production of [18F]FP-TZTP from the mesylate salt was an improvement in terms of RCY, molar activity, amount of precursor mass used, and total synthesis time. Our results were comparable with what had been achieved previously by using a tosylate precursor, although in our case, we were also able to significantly decrease the amount of precursor required. The production of [18F]FP-TZTP is susceptible to hydrolysis under aqueous conditions and therefore purification using a preconcentration step and online dilution with water was unsuitable. To further decrease purification times for [18F]FP-TZTP, it is recommended that HPLC purification be performed with biocompatible solvents, or other methods of purification be investigated.
Experimental
General
All reagents and solvents were purchased from ABX (Radeberg, Germany) or Sigma–Aldrich. The [18F]FP-TZTP precursor and reference standards were purchased from IsoSciences (King of Prussia, PA, USA). Nucleophilic [18F]fluoride was produced on an IBA Cyclone 18 Twin cyclotron (ANSTO, Sydney, NSW). Radiosynthesis was performed using the NanoTek Microfluidic Synthesis System with NanoTek v1.4.1 software (Advion, Ithaca, NY, USA) with in-house modifications as described previously.[15] MP-1 resins (mini-cartridge) were obtained from ORTG (Oakdale, TN, USA). (These are no longer available through ORTG; however, micro-QMA cartridges can be purchased from MedChem Imaging (Boston, MA, USA).) Sep-Pak C18 Light SPE cartridges were purchased from Waters (Sydney, NSW). Radio-HPLC was performed using a Waters 515 pump and radioactivity was measured using a Capintec R15C dose calibrator. Radio-TLC was performed using silica plates (100 % ethyl acetate) and analysed using a Cyclone Plus phosphor imager (PerkinElmer, Melbourne, Vic.).
Radiochemistry
Aqueous [18F]fluoride (15–110 GBq) was trapped on an MP-1 resin (activated with 1 mL of EtOH and 2 mL H2O), eluted with 15 mg of K2.2.2 in 1 mL of CH3CN and 80 µL of 10 % w/v aqueous K2CO3, and azeotropically dried with CH3CN under vacuum and N2 flow in the vial of the Concentrator Module of the NanoTek system. The dried K[18F]F.K222 complex was reconstituted with 700 µL of DMF, before being loaded into the storage loop of P3 (Pump 3). The FP-TZTP precursor (2 mg mL−1 in DMF) was loaded into the storage loop of P1 (Pump 1). DMF was used as the sweeping solvent.
For the microfluidic radiolabelling optimisation of [18F]FP-TZTP, aliquots (10–20 µL) of the precursor and K[18F]F.K222 complex were reacted at various temperatures, flow rates, and stoichiometries according to our laboratory protocol[18] using the Discovery Mode of the NanoTek software. For the microfluidic production of [18F]FP-TZTP, 100 µL of the precursor solution and 100 µL of the K[18F]F.K222 complex were dispensed into the microreactor (100 µm × 2 m, 15.7 µL internal volume) at a total flow rate of 40 µL min−1 and at 190°C. The reaction mixture was swept out of the microreactor with 120 µL of DMF. The [18F]FP-TZTP was purified by HPLC using a Rheodyne MX series autoinjector (Grace Discovery Sciences, Melbourne, Vic.) and:
-
The reaction mixture from the microreactor was loaded into a 500 µL HPLC loop, monitored by a radiodetector viewable in the NanoTek software. The product was purified by semipreparative HPLC using a Phenomenex Luna C18(2) 250 × 10 mm 5-µm column with 65 % CH3CN/35 % H2O + 5 mM Et3N + 5 mM NaH2PO4 at 4 mL min−1 as the eluent (tR 12–15 min); or
-
The reaction mixture from the microreactor was diluted online with water at 1 mL min−1 and loaded onto a Waters Oasis® HLB 20 × 3.9 mm 25 µm column monitored by a radiodetector viewable in the NanoTek software. The precolumn was connected to an analytical Merck Chromolith high-resolution RP-18e 50 × 4.6 mm column for further purification using 25 % CH3CN/75 % 0.1 M ammonium formate at 1 mL min−1 as the eluent (tR = 3–5 min).
Following HPLC purification, the product peak was collected and diluted with 15 mL of H2O before being loaded onto a Sep-Pak C18 Light SPE cartridge. The cartridge was washed with 2 mL of H2O and eluted with 1.5 mL EtOH into the vial of an additional NanoTek concentrator module, which acted as the reformulation reservoir. This vial was heated at 90°C for 15 min and the dried residue was subsequently redissolved in 1 mL of 10 % ethanolic saline solution. Quality control radio-HPLC was performed by recording absorbance at λ = 254 nm using a Phenomenex Luna C18 150 × 4.6 mm 5-µm column and 30 % CH3CN/70 % 0.1 M ammonium formate at 2 mL min−1 (tR = 5–7 min). [18F]FP-TZTP was obtained in 26 ± 10 % RCY (n = 15) in 49 ± 2 min from the start of bolus delivery, with a molar activity of 182 ± 65 GBq µmol−1 at EOS. Radiochemical purity was >99 %. The identification of [18F]FP-TZTP was confirmed through co-injection with the non-radioactive analogue.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
The authors gratefully acknowledge Nikolas Paneras for the cyclotron production of fluorine-18. This research did not receive any specific funding.
References
[1] Y. Biran, C. L. Masters, K. J. Barnham, A. I. Bush, P. A. Adlard, J. Cell. Mol. Med. 2009, 13, 61.| Crossref | GoogleScholarGoogle Scholar |
[2] A. V. Mossine, A. F. Brooks, B. D. Henderson, B. G. Hockley, K. A. Frey, P. J. H. Scott, EJNMMI Radiopharmacy and Chemistry 2017, 2, 7.
| Crossref | GoogleScholarGoogle Scholar |
[3] R. Rodríguez‐Puertas, J. Pascual, T. Vilaró, Á. Pazos, Synapse 1997, 26, 341.
| Crossref | GoogleScholarGoogle Scholar |
[4] P. Sauerberg, P. H. Olesen, S. Nielsen, S. Treppendahl, M. J. Sheardown, T. Honore, C. H. Mitch, J. S. Ward, A. J. Pike, J. Med. Chem. 1992, 35, 2274.
| Crossref | GoogleScholarGoogle Scholar |
[5] D. O. Kiesewetter, J. Lee, L. Lang, S. G. Park, C. H. Paik, W. C. Eckelman, J. Med. Chem. 1995, 38, 5.
| Crossref | GoogleScholarGoogle Scholar |
[6] R. E. Carson, D. O. Kiesewetter, E. Jagoda, M. G. Der, P. Herscovitch, W. C. Eckelman, J. Cereb. Blood Flow Metab. 1998, 18, 1130.
| Crossref | GoogleScholarGoogle Scholar |
[7] E. M. Jagoda, D. O. Kiesewetter, K. Shimoji, L. Ravasi, M. Yamada, J. Gomeza, J. Wess, W. C. Eckelman, Neuropharmacology 2003, 44, 653.
| Crossref | GoogleScholarGoogle Scholar |
[8] D. O. Kiesewetter, B. K. Vuong, M. A. Channing, Nucl. Med. Biol. 2003, 30, 73.
| Crossref | GoogleScholarGoogle Scholar |
[9] E. M. van Oosten, A. A. Wilson, K. A. Stephenson, D. C. Mamo, B. G. Pollock, B. H. Mulsant, A. K. Yudin, S. Houle, N. Vasdev, Appl. Radiat. Isot. 2009, 67, 611.
| Crossref | GoogleScholarGoogle Scholar |
[10] M.-W. Wang, W.-Y. Lin, K. Liu, M. Masterman-Smith, C. K.-F. Shen, Mol. Imaging 2010, 9, 175.
| Crossref | GoogleScholarGoogle Scholar |
[11] G. Pascali, P. Watts, P. A. Salvadori, Nucl. Med. Biol. 2013, 40, 776.
| Crossref | GoogleScholarGoogle Scholar |
[12] S. H. Liang, D. L. Yokell, M. D. Normandin, P. A. Rice, R. N. Jackson, T. M. Shoup, T. J. Brady, G. E. Fakhri, T. L. Collier, N. Vasdev, Mol. Imaging 2014, 13, 1.
| Crossref | GoogleScholarGoogle Scholar |
[13] H. Kimura, K. Tomatsu, H. Saiki, K. Arimitsu, M. Ono, H. Kawashima, R. Iwata, H. Nakanishi, E. Ozeki, Y. Kuge, H. Saji, PLoS One 2016, 11,
| Crossref | GoogleScholarGoogle Scholar |
[14] S. Pfaff, C. Philippe, V. Pichler, M. Hacker, M. Mitterhauser, W. Wadsak, Dalton Trans. 2018, 5997.
| Crossref | GoogleScholarGoogle Scholar |
[15] G. Pascali, A. Berton, M. DeSimone, N. Wyatt, L. Matesic, I. Greguric, P. A. Salvadori, Appl. Radiat. Isot. 2014, 84, 40.
| Crossref | GoogleScholarGoogle Scholar |
[16] L. Matesic, A. Kallinen, N. A. Wyatt, T. Q. Pham, I. Greguric, G. Pascali, Aust. J. Chem. 2015, 68, 69.
| Crossref | GoogleScholarGoogle Scholar |
[17] L. Matesic, A. Kallinen, I. Greguric, G. Pascali, Nucl. Med. Biol. 2017, 52, 24.
| Crossref | GoogleScholarGoogle Scholar |
[18] G. Pascali, L. Matesic, T. L. Collier, N. Wyatt, B. H. Fraser, T. Q. Pham, P. A. Salvadori, I. Greguric, Nat. Protoc. 2014, 9, 2017.
| Crossref | GoogleScholarGoogle Scholar |
[19] G. Pascali, M. Simone, L. Matesic, I. Greguric, P. Salvadori, J. Flow Chem. 2014, 4, 86.
| Crossref | GoogleScholarGoogle Scholar |
[20] J.-H. Chun, S. Telu, S. Lu, V. W. Pike, Org. Biomol. Chem. 2013, 11, 5094.
| Crossref | GoogleScholarGoogle Scholar |
[21] L. Matesic, N. A. Wyatt, B. H. Fraser, M. P. Roberts, T. Q. Pham, I. Greguric, J. Org. Chem. 2013, 78, 11262.
| Crossref | GoogleScholarGoogle Scholar |
[22] M. A. Klenner, G. Pascali, B. Zhang, T. R. Sia, L. K. Spare, A. M. Krause‐Heuer, J. R. Aldrich‐Wright, I. Greguric, A. J. Guastella, M. Massi, B. H. Fraser, Chem. – Eur. J. 2017, 23, 6499.
| Crossref | GoogleScholarGoogle Scholar |
[23] D. Ory, J. Van den Brande, T. de Groot, K. Serdons, M. Bex, L. Declercq, F. Cleeren, M. Ooms, K. Van Laere, A. Verbruggen, G. Bormans, J. Pharm. Biomed. Anal. 2015, 111, 209.
| Crossref | GoogleScholarGoogle Scholar |
[24] T. Collier, M. Akula, S. Liang, J. Nucl. Med. 2014, 55, 1245.
* Lidia Matesic was awarded the 2017 RACI Rita Cornforth Lectureship.