Graded IR Filters: Distinguishing Between Single and Multipoint NO2···I Halogen Bonded Supramolecular Synthons (P, Q, and R Synthons)
Subhankar Saha A , Somnath Ganguly A and Gautam R. Desiraju A BA Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore 560012, India.
B Corresponding author. Email: desiraju@sscu.iisc.ernet.in
Australian Journal of Chemistry 67(12) 1840-1848 https://doi.org/10.1071/CH14361
Submitted: 5 June 2014 Accepted: 17 July 2014 Published: 4 September 2014
Abstract
The NO2···I supramolecular synthon is a halogen bonded recognition pattern that is present in the crystal structures of many compounds that contain these functional groups. These synthons have been previously distinguished as P, Q, and R types using topological and geometrical criteria. A five step IR spectroscopic sequence is proposed here to distinguish between these synthon types in solid samples. Sets of known compounds that contain the P, Q, and R synthons are first taken to develop IR spectroscopic identifiers for them. The identifiers are then used to create graded IR filters that sieve the synthons. These filters contain signatures of the individual NO2···I synthons and may be applied to distinguish between P, Q, and R synthon varieties. They are also useful to identify synthons that are of a borderline character, synthons in disordered structures wherein the crystal structure in itself is not sufficient to distinguish synthon types, and in the identification of the NO2···I synthons in compounds with unknown crystal structures. This study establishes clear differences for the three different geometries P, Q, and R and in the chemical differences in the intermolecular interactions contained in the synthons. Our IR method can be conveniently employed when single crystals are not readily available also in high throughput analysis. It is possible that such identification may also be adopted as an input for crystal structure prediction analysis of compounds with unknown crystal structures.
Introduction
The concept of the supramolecular synthon is finding greater importance in crystal engineering, with applications in many diverse fields.[1–5] Synthons are constituted with non-covalent interactions, which continue to be a subject of intensive research in the study of engineered solids.[6] Intermolecular interactions where the positively charged polar regions[7] of halogen atoms form contacts with electron rich regions of other atoms, are known as halogen bonds.[8,9] Halogen bonded systems are characterised by well defined, specific, and directional interactions that may be used to assemble other complex supramolecular architectures in solids.[10–12] Halogen bonds are seen to be implicated in many branches of structural chemistry from materials science[13–16] to biological chemistry[17,18] and drug design.[19]
The NO2···I Synthon
The halogen bond, like other interactions, has been employed in solid state crystal design to develop solids with tailored properties.[20–22] In 1993, Desiraju and co-workers discussed the NO2···I synthon geometry in crystals.[23] On the basis of a Cambridge Structural Database (CSD) study, they mentioned the existence of three (Fig. 1) different patterns of this synthon that they denoted P, Q, and R. The first of these, P, is a two-point symmetrical unit where the iodine atom interacts with both oxygen atoms of the nitro group. The two O···I distances, D1 and D2, are comparable with D1 – D2 ≤ 0.2 Å. R is a single-point synthon with only one O···I contact while Q is a two-point unsymmetrical arrangement, topologically similar to P but geometrically dissimilar. For the Q synthon, the distance restriction is D1 – D2 ~0.6 Å. This classification was proposed on the basis of scatter plots of D1 versus D2 in which a demarcation was observed for the three populations. Fig. 1 is an updated scatter plot of this type wherein it is seen that the demarcation between P, Q, and R regions persists although the number of structures considered is nearly 70 times larger. A question arises as to whether this classification has any chemical basis or whether it is based just on arbitrary geometric criteria. There are, for example, some reported structures where the NO2···I synthons lie in a hazy structural zone between the P and Q geometries (and to a lesser extent between Q and R).[24] Some other systems[2] are known that have NO2/I positional disorder which is common in such systems, and which makes synthon assignment quite problematic.
While single crystal X-ray diffraction (SCXRD) is one of the most suitable techniques to obtain accurate information on the structures of molecular assemblies in the solid state, it has also been found that obtaining a single crystal is in itself often a challenge. Computational crystal structure prediction (CSP) is an alternative approach in such cases.[25] It is noteworthy that despite the large number of crystal structures known today, CSP is a challenging task that is fraught with difficulties.[26] Synthon-assisted CSP is a promising strategy in this overall endeavour.[27] There is accordingly a need to obtain basic synthon information in polycrystalline samples. Powder XRD (PXRD), although helpful in determining bulk phase purity, cannot be applied for routine synthon identification. A simple, robust, and accurate method that can identify supramolecular synthons is of importance especially in a high throughput, rapid analysis protocol. Infrared spectroscopy is a well established technique if a process can be identified that can effectively remove overlapping bands and thereby identify and mark bands due to the interacting groups of the synthon under consideration.[28] Such IR methods for supramolecular synthon identification have been recently reported by Mukherjee et al.[29] and Chakraborty et al.[30] for hydrogen-bonded synthons. In those cases, the strong nature of the interaction makes it easy to identify new band positions caused by interacting groups. This could however, prove to be a challenge in a halogen bonded assembly where the interactions are somewhat weaker. Halogen bonding has been studied by IR and Raman spectroscopy but identification of subtle variations in the halogen bonds has not been tried with IR and Raman methods,[31] because the interactions are basically weak, and therefore the expected spectroscopic variations are small. In this study, we attempt to study characteristic changes in IR spectra for P, Q, and R NO2···I synthons using mid-IR spectroscopy and assign them to the synthon in question using SCXRD as a benchmark. We have employed a five step method that incorporates a gradation in the identification protocol to distinguish between the synthons in samples with unknown structures.
Experimental
Synthesis
Amides 1, 2, 3, 5, and 9 (Chart 1) were synthesised according to known procedures[32] and purified by crystallisation from acetone for 1, from THF for 9, and from ethanol for 2, 3 and 5. Esters 6, 10, 11, 12, 13, and 14 (Chart 1) were prepared by following the reported procedure[33] and purified by crystallisation from ethanol for 6 and 10, from THF for 11, from methanol for 13, and from chloroform for 12 and 14. The sulfonamide 4 was synthesised according to a reported procedure[34] and purified through crystallisation from 1,2-dichloromethane. Imine 7 was synthesised according to a known procedure[24] and purified by crystallisation from ethanol. The urea derivative 8 was prepared by following the reported procedure[2] and purified by crystallisation from THF. Syntheses and melting points of 9 to 14 are given in Supplementary Material S1and S2, respectively.

|
FTIR Spectroscopy
Solid state IR spectra of the compounds were recorded on a Perkin Elmer frontier infrared spectrometer in ATR mode from 1800 to 600 cm–1 with a resolution of ± 2 cm–1. Solution (0.1 M in THF) IR spectra were collected in a CaF2 liquid cell using a polytetraflouroethylene (PTFE) spacer of diameter 25 mm and path length 0.05 mm from 1800 to 1200 cm–1 with a resolution of ± 2 cm–1. Complete solid state IR spectra of all compounds are given in the Supplementary Material along with solution IR spectra.
SCXRD
SCXRD data were collected on a Rigaku Mercury 375R/M CCD (XtaLAB mini) diffractometer using graphite monochromatic MoKα radiation, with a Rigaku low temperature gas spray cooler facility. Data were processed with the Rigaku CrystalClear 2.0 software.[35,36] Structure solution and refinements were performed using SHELX97[37] implemented in the WinGX suite.[38] The crystallographic data and ORTEP diagrams are given in the Supplementary Material (Table S12 and Fig. S13).
Results and Discussion
In the present study an attempt is made to identify the P, Q, and R NO2···I synthons using a unique IR spectroscopic marker method: A five step process (Fig. 2) has been developed. The first step involves identification of IR characteristics for the P, Q, and R synthons of model compounds. The identified IR characteristics are inputs into the second step where the spectroscopic characteristics are verified in another set of compounds. Once the spectroscopic characteristics are established, they are adopted to formulate a ‘graded IR filter’ protocol. We employ the term ‘graded’ because we sort out compounds with the R synthon first, then compounds with the P synthon, and finally the Q synthon. Compounds that still remain do not contain any of these three synthon varieties. The IR filter is then used in establishing synthon structures in cases where ambiguity exists. Finally the IR filter is applied in the unambiguous identification of P, Q, or R synthons in unknown structures.
Step 1: Identification of IR Characteristics for P, Q, and R Synthons from Model Compounds
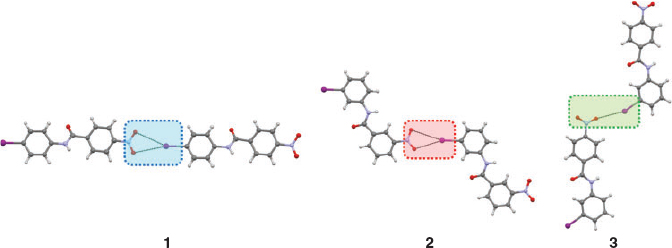
In order to study the P, Q, and R NO2···I synthons, a set of three chemically very similar compounds, 1, 2, and 3 (Chart 1) was selected,[32] where changes in the substitution position of the nitro and iodo groups result in a change in the pattern of the NO2···I synthons. The synthon structures of the compounds are given in Fig. 3.
Method of the IR Spectroscopic Analysis
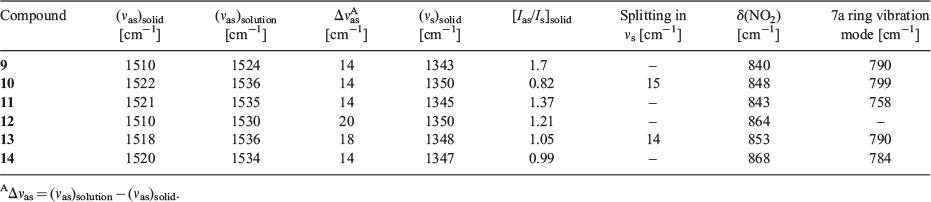
Fig. 4 shows IR spectra of model compounds in the solid state. IR characteristics are tabulated in Table 1. Vibrational assignments of 4-iodonitrobenzene are used to mark the NO2 group bands.[39] Solution IR spectra of the compounds in THF (Supplementary Material S8) were taken to mark the liquid state frequency of the nitro group. Typically, the spectroscopic shifts between solution and the solid were examined to assess the nature of the intermolecular interactions. The vibrations studied are the shift of the asymmetric stretching mode of the NO2 group, intensity ratio of the asymmetric to symmetric stretching band, the splitting of the symmetric stretch, and finally the positions of some low frequency vibrations. Notations employed in the study are, νas: asymmetric stretch; νs: symmetric stretch; δ(NO2): NO2 scissoring vibration, and γ(NO2): NO2 wagging vibration. Also, Ias and Is are the intensities of NO2 group asymmetric and symmetric stretching bands in the solid.

|
Spectroscopic Observations for Synthons P, Q, and R in Model Compounds (Table 1)
Significantly different spectroscopic characters are observed among the P, Q and R synthons, based on their IR features:
-
A large frequency shift (Δνas) of νas on going from solution to solid in the case of the P synthon is observed.
-
The ratio of intensity, [Ias/Is]solid of νas upon νs for the solid state gives a value ~1 for R-type and ~1.3 for P, and Q-type synthons.
-
A clear splitting is found in the νs band for the R-type synthon due to asymmetry in the O···I interaction.
-
A 7a ring vibration mode of the aromatic ring to which the NO2 is attached appears at ~785 cm–1[40] for the Q and R synthons.
-
The NO2 scissoring mode is found at different positions for the different synthons and these are given in Table 1.
These features allow the development of a process to differentiate the synthons. The next step involves transfer of these IR characteristics to another set of molecules to confirm the above spectroscopic observations.
Step 2: Verifying the IR Characteristics with Other Molecules Having Known Synthons
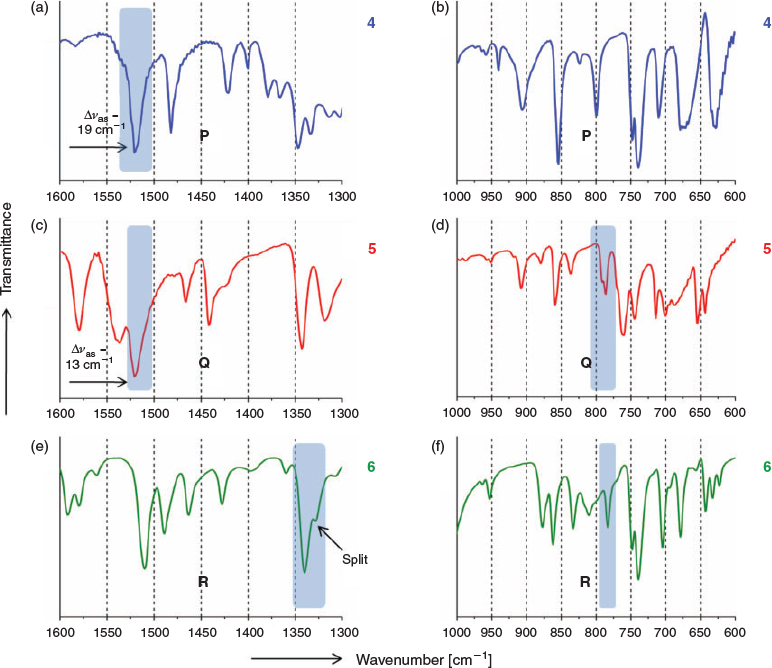
The IR characteristics mentioned in Step 1 are now mapped onto a second set of iodo and nitro group containing molecules (4, 5, and 6)[33,34,41] (Fig. 5), with known crystal structures but also having other functional groups. IR spectra of these compounds are summarised in Fig. 6 and Table 2.

|
|
The IR analysis of this set shows good overlap of spectroscopic features with the model compounds. These first two steps are then merged to form the general IR characteristics table (Table 3) for P, Q, and R synthons. This result shows that synthons can be tagged independently in the IR spectra.

|
Step 3: Graded IR Filter
Table 3 shows common but also some unique spectroscopic features for synthons P, Q, and R. Sometimes more than one feature has to be used in identification of a synthon. This helped us to build our graded IR filter protocol (Fig. 7) that can be employed in identification of synthons in unknown systems. Since the R-type has very different IR characteristics (such as splitting in νs and [Ias/Is]solid ratio), molecules with unknown structure are first scanned with the R filter having IR characteristics of an R-type synthon. This operation effectively sorts out molecules with the R synthon. After that, the remaining molecules are scanned with P and Q filters to separate each set of molecules.

|
Step 4: Graded IR Filter to Clarify Synthon Ambiguities in Known Crystal Structures
Till now we have considered IR spectra of compounds with no ambiguity in the definition of P, Q, and R patterns. However, CSD analysis shows some NO2···I interactions that lie in the region of haziness between P and Q or Q and R (7, Fig. 8)[24] with respect to the D1 and D2 values. In some other cases there is the existence of NO2 and iodo disorder (8, Fig. 8),[2] increasing the complication involved in NO2···I synthon pattern identification. To solve this, we analysed the IR spectra (Fig. 9) of 7 and 8 with our graded IR filter. The solution IR spectrum is given in Supplementary Material S10.

|

|
The IR spectroscopic analysis (Table 4) clearly indicates that while 7 is R, compound 8 has a Q-type synthon.
Step 5: Graded IR Filter for Synthon Identification in Compounds with Unknown Crystal Structures
In order to identify synthons in unknown structures, a set of random molecules 9–14 (Chart 1) were prepared and their IR spectra analysed. The solid state and solution IR spectra are given in Supplementary Material S6 and S11, respectively. IR spectroscopic features are tabulated in Table 5.
The IR characteristics (Table 5) of randomly selected compounds 9–14 are analysed with the graded IR filter which indicates that 10 and 13 have the R-type synthon while 12 is found to have P. Compounds 9 and 11 are marked as being Q-type. Interestingly, the IR features of 14 are not in good agreement with any of the P, Q, or R-types. Synthons in these compounds are then confirmed by SCXRD. Fig. 10 represents the synthons in the crystal structures of 9–14. Gratifyingly, SCXRD analysis shows the absence of any type of NO2···I synthon in 14.

|
Conclusions
A five step IR protocol has been developed to distinguish between geometrically and/or topologically distinct NO2···I synthons that are termed P, Q, and R. Known compounds that contain the P, Q, and R synthons have been employed to develop IR spectroscopic identifiers for the synthons. The spectroscopic identifiers are used to generate a graded IR filter. The graded IR filter with signatures of individual NO2···I synthons have been applied in the identification of synthons with ambiguous geometric characteristics, in synthons showing disorder, and in the identification of synthons in compounds with unknown crystal structures. The results show that P, Q, and R synthons are chemically distinct. While the R synthon is clearly different from P and Q, even the latter two synthons are distinctive enough, in a chemical sense. Our work establishes the use of IR spectroscopy in identifying nitro···halogen synthons in solid samples. It may also be possible that such identification can be used as an input for CSP analysis of compounds which do not form good quality single crystals.
Supplementary Material
Compound syntheses, melting points, complete IR spectra, crystallographic information and ORTEP diagrams are available on the Journal website.
Acknowledgements
S. S. thanks the Council of Scientific and Industrial Research for a junior research fellowship. S. G. thanks the Indian Institute of Science for a fellowship. G. R. D. thanks the Department of Science and Technology for the award of a J. C. Bose fellowship.
References
[1] J. A. R. P. Sarma, F. H. Allen, V. J. Hoy, J. A. K. Howard, R. Thaimattam, K. Biradha, G. R. Desiraju, Chem. Commun. 1997, 101.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXos1aktw%3D%3D&md5=b92109fdbe1d0e45623f392c4101c456CAS |
[2] S. George, A. Nangia, C.-K. Lam, T. C. W. Mak, J.-F. Nicoud, Chem. Commun. 2004, 1202.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVamtLg%3D&md5=440202d44247ebf4d1d69d1cbaa6eac0CAS |
[3] P. Dastidar, Chem. Soc. Rev. 2008, 37, 2699.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsVSgsrnE&md5=fc39dd6a8d6135c833ed600357c4e678CAS | 19020683PubMed |
[4] R. Xu, V. Gramlich, H. Frauenrath, J. Am. Chem. Soc. 2006, 128, 5541.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XjtVaqsLg%3D&md5=aaafcc81a2773eff65838602be521cc6CAS | 16620128PubMed |
[5] E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. Int. Ed. 2003, 42, 1210.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXivVSrtbw%3D&md5=604d991eefd82afce2fd73c78c0a927bCAS |
[6] G. R. Desiraju, J. J. Vittal, A. Ramanan, Crystal Engineering: A Textbook 2011 (World Scientific: Singapore).
[7] T. Brinck, J. S. Murray, P. Politzer, Int. J. Quantum Chem. 1992, 44, 57.
| Crossref | GoogleScholarGoogle Scholar |
[8] G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati, K. Rissanen, Pure Appl. Chem. 2013, 85, 1711.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtlSnsLjJ&md5=7cd29b911b690074e2cb694f41cc381cCAS |
[9] A. Mukherjee, S. Tothadi, G. R. Desiraju, Acc. Chem. Res. 2014, in press.
| Crossref | GoogleScholarGoogle Scholar | 25134974PubMed |
[10] K. Merz, Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2003, 59, o65.
| Crossref | GoogleScholarGoogle Scholar |
[11] A. Ranganathan, V. R. Pedireddi, Tetrahedron Lett. 1998, 39, 1803.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhslKqsbs%3D&md5=67076869afa48d113c0e95fbc958fffaCAS |
[12] P. K. Thallapally, G. R. Desiraju, M. Bagieu-Beucher, R. Masse, C. Bourgogne, J.-F. Nicoud, Chem. Commun. 2002, 1052.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XjsFKgt7o%3D&md5=1ef7de96e1d1fa67efcbc01cc9da0eacCAS |
[13] D. Yan, A. Delori, G. O. Lloyd, T. Friščić, G. M. Day, W. Jones, J. Lu, M. Wei, D. G. Evans, X. Duan, Angew. Chem. Int. Ed. 2011, 50, 12483.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVejsrnP&md5=c43c45b020b3e0ea00db3d5dd7cfe13fCAS |
[14] H. L. Nguyen, P. N. Horton, M. B. Hursthouse, A. C. Legon, D. W. Bruce, J. Am. Chem. Soc. 2004, 126, 16.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpsFygtr4%3D&md5=061dffe08b793d1cde7c5b12d7a80556CAS | 14709037PubMed |
[15] L. J. McAllister, C. Prasang, J. P. W. Wong, R. J. Thatcher, A. C. Whitwood, B. Donnio, P. O’Brien, P. B. Karadakov, D. W. Bruce, Chem. Commun. 2013, 49, 3946.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmtVWrtL0%3D&md5=bfeff2c9af06c34a6884dbc6bebc63c7CAS |
[16] X. Pang, X. R. Zhao, H. Wang, H.-L. Sun, W. J. Jin, Cryst. Growth Des. 2013, 13, 3739.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFSns7%2FE&md5=b821bf39bfadfff958b8f6034dcbd401CAS |
[17] A. R. Voth, P. S. Ho, Curr. Top. Med. Chem. 2007, 7, 1336.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXht1SmtbrJ&md5=64bb2fca3200dc3892ab10d02a32010cCAS | 17692024PubMed |
[18] M. R. Scholfield, C. M. V. Zanden, M. Carter, P. S. Ho, Protein Sci. 2013, 22, 139.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsVOnsbw%3D&md5=228b4b509f89847a17928930abb5a732CAS | 23225628PubMed |
[19] E. Parisini, P. Metrangolo, T. Pilati, G. Resnati, G. Terraneo, Chem. Soc. Rev. 2011, 40, 2267.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvVWju7g%3D&md5=6423e06c4c1a7a888b777d9401c58127CAS | 21365086PubMed |
[20] A. Mukherjee, G. R. Desiraju, IUCrJ 2014, 1, 49.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXotFWntg%3D%3D&md5=67eaf72e4f7d347d5e0ee5f023f3b296CAS | 25075319PubMed |
[21] C. M. Reddy, R. C. Gundakaram, S. Basavoju, M. T. Kirchner, K. A. Padmanabhan, G. R. Desiraju, Chem. Commun. 2005, 3945.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXntlaqurw%3D&md5=5d186e08b7e4268e894c56438251245aCAS |
[22] C. M. Reddy, K. A. Padmanabhan, G. R. Desiraju, Cryst. Growth Des. 2006, 6, 2720.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFCqt7%2FL&md5=2e9de1aa091a6a1b52e35776ea18d429CAS |
[23] G. R. Desiraju, V. R. Pedireddi, J. Sarma, D. E. Zacharias, Acta Chim. Hung. 1993, 130, 451.
| 1:CAS:528:DyaK2cXjtlGmtbY%3D&md5=7f8c4f9186a82a332ec9af1a5a0f5dc5CAS |
[24] C. Glidewell, R. A. Howie, J. N. Low, J. M. S. Skakle, S. M. S. V. Wardell, J. L. Wardell, Acta Crystallogr., Sect. B: Struct. Sci. 2002, 58, 864.
| Crossref | GoogleScholarGoogle Scholar |
[25] S. L. Price, Acc. Chem. Res. 2009, 42, 117.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1Gnu7%2FM&md5=505321c63bbcff49c4d9d3e56522dc61CAS |
[26] G. M. Day, W. D. S. Motherwell, H. L. Ammon, S. X. M. Boerrigter, R. G. Della Valle, E. Venuti, A. Dzyabchenko, J. D. Dunitz, B. Schweizer, B. P. van Eijck, P. Erk, J. C. Facelli, V. E. Bazterra, M. B. Ferraro, D. W. M. Hofmann, F. J. J. Leusen, C. Liang, C. C. Pantelides, P. G. Karamertzanis, S. L. Price, T. C. Lewis, H. Nowell, A. Torrisi, H. A. Scheraga, Y. A. Arnautova, M. U. Schmidt, P. Verwer, Acta Crystallogr., Sect. B: Struct. Sci. 2005, 61, 511.
| Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2Mrht1Onsg%3D%3D&md5=7bd54563f7c1b6e5bed34a4f0180e976CAS |
[27] J. A. R. P. Sarma, G. R. Desiraju, Cryst. Growth Des. 2002, 2, 93.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFCgtLY%3D&md5=6ad665b5e2465297316dcaf511005445CAS |
[28] L. J. Bellamy, Advances in Infrared Group Frequencies 1968 (Methuen & Co.: London).
[29] A. Mukherjee, S. Tothadi, S. Chakraborty, S. Ganguly, G. R. Desiraju, CrystEngComm 2013, 15, 4640.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXnvV2gtb8%3D&md5=d31229f843565f7e32787b712ca83be1CAS |
[30] S. Chakraborty, S. Ganguly, G. R. Desiraju, CrystEngComm 2014, 16, 4732.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXnvFeqtLY%3D&md5=b76ed55973283ab7afbaa1fa4cb82255CAS |
[31] M. T. Messina, P. Metrangolo, W. Navarrini, S. Radice, G. Resnati, G. Zerbi, J. Mol. Struct. 2000, 524, 87.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXjtVGjsLg%3D&md5=f245d843deea1e2820ce9238a5f86404CAS |
[32] J. L. Wardell, J. N. Low, J. M. S. Skakle, C. Glidewell, Acta Crystallogr., Sect. B: Struct. Sci. 2006, 62, 931.
| Crossref | GoogleScholarGoogle Scholar |
[33] J. L. Wardell, J. M. S. Skakle, J. N. Low, C. Glidewell, Acta Crystallogr., Sect. E: Struct. Rep. Online 2005, 61, o3334.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtVKjurrO&md5=9bdbc2edf973a97963d531966c37bb5dCAS |
[34] C. J. Kelly, J. M. S. Skakle, J. L. Wardell, S. M. S. V. Wardell, J. N. Low, C. Glidewell, Acta Crystallogr., Sect. B: Struct. Sci. 2002, 58, 94.
| Crossref | GoogleScholarGoogle Scholar |
[35] CrystalClear 2.0 (Rigaku Corporation: Tokyo).
[36] J. Pflugrath, Acta Crystallogr., Sect. D: Biol. Crystallogr. 1999, 55, 1718.
| Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FgtlWiug%3D%3D&md5=4cf933e65319d410a38edc9e7d202f87CAS |
[37] G. M. Sheldrick, SHELX-97: Program for the Solution and Refinement of Crystal Structures 1997 (University of Göttingen: Göttingen).
[38] L. J. Farrugia, J. Appl. Crystallogr. 1999, 32, 837.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXlsVSlurk%3D&md5=6ea51a6d337c4eb7b42c7387a614af42CAS |
[39] J. H. S. Green, D. J. Harrison, Spectrochim. Acta, Part A 1970, 26, 1925.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE3cXltVeksLo%3D&md5=457964d539d901cbb481670882327b58CAS |
[40] M. Samsonowicz, R. Swislocka, E. Regulska, W. Lewandowski, Int. J. Quantum Chem. 2007, 107, 480.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlWkt73M&md5=bb3f70e2e8353265b364d18e46a022b7CAS |
[41] J. L. Wardell, J. M. S. Skakle, J. N. Low, C. Glidewell, Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 2005, 61, o634.
| Crossref | GoogleScholarGoogle Scholar |