An Institutional Approach to Solar Fuels Research
Johannes Messinger AA Department of Chemistry, Chemistry Biology Center (KBC), Umeå University, Linnaeus väg 6, 901 87 Umeå, Sweden. Email: Johannes.Messinger@chem.umu.se
Australian Journal of Chemistry 65(6) 573-576 https://doi.org/10.1071/CH12020
Submitted: 18 January 2012 Accepted: 25 February 2012 Published: 17 May 2012
Abstract
This account gives a brief overview of various directions in current solar fuels research. On that basis, the necessity for an interdisciplinary approach is argued, and an institutional way for promoting this development is presented using the example of the Chemistry Biology Centre (KBC) at Umeå University in Sweden.
Introduction
Current energy consumption of mankind is largely based on fossil fuels, i.e. on solar energy that was stored as chemical (free) energy by photosynthetic bacteria and plants over a period of 2–3 billion years. It is almost universally agreed that the heavy reliance on fossil fuels must be reduced in favour of CO2 neutral processes in order to slow global warming and to conserve parts of the remaining natural oil and gas reserves for higher value applications.
The only long-term (biological time scales) sustainable free energy input of global significance in Earth’s ecosystem is solar light. The free energy of solar radiation reaching Earth exceeds by far the present world energy consumption,[1] and the potential of solar energy exploitation for mankind is exemplified by the tremendous global success of oxygenic (O2-evolving) photosynthetic organisms and their impact on the course of evolution.[2]
Challenges in using solar energy arise at different levels. While the sun shines at almost constant intensity, the light that arrives on a particular place on Earth varies strongly due to the Earth’s rotation (day–night), the tilt of the Earth’s rotation axis with respect to the plane of the Earth’s orbit around the sun (seasonal variation), and cloud and dust formation. This situation is made worse by the fact that in general most free energy is needed when the local solar radiation is lowest, i.e. during nights and in winter.
A second challenge is the low energy density (free energy per area) of solar radiation at the surface of the Earth. For most applications this implies that relatively large areas are required for harvesting sufficient amounts of solar energy. Finally, it should be remarked that the free energy of sunlight can in most cases not be directly used, but must be converted into electricity or fuels.
As a consequence, highly efficient, decentralized methods for solar energy harvesting, conversion, and storage are required for meeting future energy needs. Presently, a diverse set of methods are being developed and implemented that range from fully biological approaches to man-made devices, and involve conversion of solar energy into heat, electricity, fuels, biomass, or high value products.[3,4] Implementation of such methods or devices on global scale requires that they are environmentally benign, i.e. that they are non-toxic and based on earth abundant elements.
The employment of solar energy systems will contribute to the development of decentralized energy systems that reduce the dependence on central energy grids. This new technology will allow a faster development of areas currently not connected to central fuel and electricity supplies, and thereby may have its biggest impact in developing countries.
Biological Approaches to Solar Energy Conversion
Biological solar energy usage can be divided into several subcategories:
-
Production of solid biomass (wood, sugars) for direct combustion and/or extraction of valuable products such as cellulose and fine chemicals,
-
Production of liquid fuels, such as biodiesel and low chain alcohols (methanol, ethanol),
-
Production of free energy rich gases such as H2 or methane.
Biological systems, which depending on the process can be higher plants, algae, or cyanobacteria, have the great advantage that they are self replicating. To increase the yield of the desired product they can be modified by breeding or by molecular genetics. Presently, the overall solar-to-fuel energy efficiency for most biological organisms is in the range of 1–3 % at best, which is in striking contrast to the estimated 16 % efficiency of the initial solar-to-chemical energy efficiency of photosystem II.[5–7] The aim of current research is therefore to understand biological pathways leading to product formation, and to maximize production by downregulation of non-essential competing processes. Theoretically, by minimizing photorespiration, increasing efficiency of photosynthesis, and improving stress tolerance, the combined efficiency in biomass production could be doubled.[8]
In addition to the direct use of biological systems for energy production, the detailed study of the biological catalysts involved in solar-to-chemical energy conversion can give valuable hints for the design of artificial catalysts. Prominent examples are the only biological catalyst for solar water-splitting, photosystem II, and hydrogenases that reduce protons to molecular hydrogen.[9] A combination of both enzymes leads in principle to a direct solar-to-H2 conversion via photolytic water-splitting, resulting in molecular oxygen (O2) as by-product. In practice however, this has proven very difficult for many reasons, including enzyme instability and O2-sensitivity of many hydrogenases.[9]
Man-made Devices for Solar Energy Conversion (Artificial Photosynthesis)
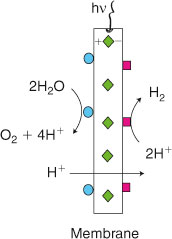
In bio-inspired artificial photosynthesis approaches researchers are trying to understand the underlying principles and details of biological energy-conversion enzymes, and to copy various parts within chemical mimics. Both homogeneous and heterogeneous catalysts have been tested for individual reactions. A big challenge is still the combination of individual solutions for light absorption or catalysts for specific reactions into one working device, such as an artificial leaf (Fig. 1). Additional challenges are long-term stability and overall efficiency.[3]
|
Although this rapidly expanding field of research is relatively young, a first working artificial leaf was recently presented.[10] However, significant improvements in catalyst stability (turnover number), reaction speed (turnover frequency), solar-to-H2 efficiency, and production costs have to be achieved before larger scale implementations become feasible.
Structural similarities between the natural water-splitting complex in photosystem II and current synthetic catalysts such as metal-oxides are indeed striking.[11–15] Other aspects that can still be learned from the biological systems are, for example, the efficient coupling of electron and proton transfer and the optimal geometry and electronic structure for O–O bond formation. Therefore, a tight interplay between researchers working on the biological processes and those developing man-made devices will contribute significantly to a faster development and implementation of devices for artificial photosynthesis.
An Institutional Approach to Solar Energy Conversion
The direct conversion of solar energy into chemical energy is a complex process, and to be successful an interdisciplinary approach must be applied. Traditional departmental and faculty borders can limit such efforts and a first step to success may therefore be to build an organization that stimulates contacts and collaborations across traditional disciplinary borders. The development at Umeå University over the last two decades may serve as a case study for how this can be achieved.
Physical distance between people is not per se a limitation for (ongoing) collaborations. However, it is a restriction for spontaneous meetings and interactions, which are important for establishing new interdisciplinary collaborations. At the Umeå University campus a new house was built in the mid-1990s connecting the existing Chemistry and Biology buildings. The new combined building was named ‘Chemistry Biology Centre’ but the immediate effects on interdepartmental interactions were limited. However, several reorganizations made during the following years boosted collaborations. In 2001 the two plant physiology departments at Umeå University and the neighbouring Swedish University for Agricultural Sciences (SLU) moved together to form the Umeå Plant Science Centre (UPSC). The centre is now recognized as an internationally leading constellation in plant and forest biotechnology. A few years later, the Chemistry department was reorganized and coupled to this eight new professors were recruited, mainly to Chemistry, but some also to other KBC departments. At the same time the university appointed a KBC group with representatives from all participating departments to actively work for increased coordination and collaborations within the environment. The group has organized common technical platforms and other infrastructure, a post-doc program for collaborative projects between departments, support for workshops, common activities, and improved internal and external information. In addition, a common graduate school was installed. The KBC idea has proven so attractive that over the years additional departments and units have joined the initiative, so that KBC now includes Chemistry, UPSC, Ecology and Environmental Sciences, Medical Biochemistry and Biophysics, Physics, the Unit of Biomass Technology and Chemistry (BTC) at SLU, and the Energy Technology and Thermal Process Chemistry (ETPC) unit.
Present examples for common technological platforms include electron microscopy, vibrational spectroscopy, NMR, mass spectrometry facilities for metabolomics and proteomics, biochemical imaging, greenhouse facilities, and the Computational Life Science Cluster (CLiC). These platforms, which are being constantly further developed, are equipped with top of the line instrumentation that can be booked and used by anyone within KBC at cost level. Training courses for graduate students and postdocs are an integral part of this concept.
As such KBC provides a very interactive and interdisciplinary environment for many research areas. One of the more successful areas is solar energy related research. This research covers projects involving the synthesis and application of nanomaterials such as carbon nanotubes[16–18]; the production of organic optoelectronic devices[19–21]; synthesis of industrial catalysts[22–24]; fundamental studies on the mechanism of enzymes (such as photosystem II)[9,11,25–27]; elucidation of the genome sequence, metabolism and biomass formation of various species[28–30]; combustion, gasification and torrefaction of biomass including ash behaviour[31–37]; the improvement of biomass (wood) quantity and quality by breeding and genetic modification[38,39]; and implementation of a biorefinery concept at industrial scale.[40–45]
For building and maintaining these research facilities and efforts, internal and external funding is required. Umeå University made significant initial investments, which were accompanied by generous support by private foundations (K&A Wallenberg, Kempe). Thereby new professors could be hired and given excellent research facilities and several years of very low teaching loads. This, together with the already largely established KBC research environment, allowed them to build strong interdisciplinary connections and to write large projects grant applications for outside funding.
To further support this process Umeå University had in 2010 an internal competition for interdisciplinary research projects. As a result, support was given to 14 ‘Strong Research Environments’. This prioritized research at Umeå University has in several cases resulted already in external follow-up grants from the Swedish government and private organizations such as the K&A Wallenberg and Kempe foundations. Below the main solar energy projects at KBC are briefly presented.
Bio4Energy
The Bio4Energy constellation was formed in 2010 and is a joint research project between Umeå University, Luleå University of Technology, the Swedish University for Agricultural Sciences (SLU), and partners from industry. The research of this consortium covers improvements of forest feedstock to production of advanced fuels and chemicals in an environmentally friendly way.[24,35,46–55] Bio4Energy is led by Stellan Marklund and is funded with 20 million euros by the Swedish government for the initial 5-year period 2010–14. Further constellations at Umeå University that deal with forest feedstock optimization include Formas-financed Strong Research Environments BioImprove (2.5 million euros) and FuncFiber (2.5 million euros),[56–63] the Wallenberg-financed Conifer Genome Consortium (7.5 million euros), and the UPSC Berzelii Centre for Forest Biotechnology (10 million euros)[47,64,65] financed by the Swedish Research councils VR and Vinnova.
ETPC
The main focus of the ETPC research group lies within the area of thermochemical conversion (e.g. combustion and gasification) of biomass and other solid fuels. This research covers fundamental and applied aspects of fuel conversion, ash transformation, trace metals, thermochemical modelling, aerosols formation and health effects, combustion- and gasification behaviour of different fuels, torrefaction, whole process development, and optimization.[31–37] This group thereby contributes to a successful conversion to a sustainable energy system based on renewable fuels such as forest and agricultural waste products.
Solar Fuels Strong Research Environment
This group is supported with 0.9 million euros by Umeå University and was awarded an additional grant of 4.4 million euros in October 2011 from the K&A Wallenberg Foundation for the project: ‘The artificial leaf: light-driven water-splitting into H2 and O2’. This environment (Fig. 2) connects 15 group leaders that cover the fields of plant physiology and natural photosynthesis (including photosynthetic water-splitting), catalysis, photochemistry, spectroscopy, crystallography, nano-materials, physics, mathematics, and device-building. The approach is two-fold: (a) understanding and improving natural photosynthesis,[9,11,25–27] and (b) applying this knowledge to the construction of low cost, printable artificial membranes for the direct (wireless) conversion of solar energy into fuels.[12,66] This environment is led by the author.

|
To further increase interaction and information exchange between these different solar fuels-related projects at KBC, the ‘Umeå Renewable Energy Meetings’ were started in 2009, which since then became an annual event in February/March. This meeting, which thanks to the support by the KBC graduate school has so far been free to attend, has brought many world experts to Umeå University and has contributed much to the education and inspiration of our students in the solar fuels area.
Conclusion
Interdisciplinary research is required for solving many of today’s problems, including the need for clean, sustainable energy. Institutional measures can be taken to facilitate communication across traditional departmental borders. However, it needs more than a onetime event like connecting two buildings; rather a continuous process over several years is required for such a transformation. The described research projects are a direct consequence of this development at Umeå University. Since most of the projects started only recently, a final evaluation of this institutional approach towards solar fuels research is currently not possible. Nevertheless it is clear that this development has created an unusually lively, positive, and interactive research environment.
Acknowledgements
Per Gardeström, Hannele Tuominen, Göran Samuelsson and the ETPC team are acknowledged for their help with this manuscript. I thank Werner Messinger (Messinger Design) for the Logo types for the Solar Fuels and Artificial Leaf environments. This work was supported by the strong research environment Solar Fuels (Umeå University) and the Artificial Leaf Project (K&A Wallenberg Foundation).
References
[1] N. S. Lewis, D. G. Nocera, Proc. Natl. Acad. Sci. USA 2006, 103, 15729.| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFymtbrJ&md5=e4252961598780ced30bcd2df19c9437CAS |
[2] J. Messinger, D. Shevela, in Fundamentals of Materials for Energy and Environmental Sustainability (Eds D. S. Ginley, D. Cahen) 2012, pp. 302–314 (Materials Research Society: Cambridge).
[3] Molecular Solar Fuels (Eds T. J. Wydrzynski, W. Hillier) 2012 (RSC Publishing: Cambridge).
[4] Fundamentals of Materials for Energy and Environmental Sustainability (Eds D. S. Ginley, D. Cahen) 2012 (Materials Research Society: Cambridge).
[5] H. Dau, I. Zaharieva, Acc. Chem. Res. 2009, 42, 1861.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVSgur%2FO&md5=a955f8d8116974ec49b5105edbc69e80CAS |
[6] R. E. Blankenship, D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, et al. Science 2011, 332, 805.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXlslylsLk%3D&md5=7fef5a2be0a1e90cdea051d4e8001fa9CAS |
[7] L. Hammarström, J. R. Winkler, H. B. Gray, S. Styring, Science 2011, 333, 288.
| Crossref | GoogleScholarGoogle Scholar |
[8] S. P. Long, X. G. Zhu, S. L. Naidu, D. R. Ort, Plant Cell Environ. 2006, 29, 315.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xktlyltrw%3D&md5=397f7930635954786c22b03b064eed0bCAS |
[9] W. Lubitz, E. J. Reijerse, J. Messinger, Energy Environ. Sci. 2008, 1, 15.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtV2htrvE&md5=b3cb1a4cb93656028c8b994f205f19a8CAS |
[10] S. Y. Reece, J. A. Hamel, K. Sung, T. D. Jarvi, A. J. Esswein, J. J. H. Pijpers, et al. Science 2011, 334, 645.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlyqu7vF&md5=e0298161b92e218b9b45c56538dc6eb5CAS |
[11] W. Ames, D. A. Pantazis, V. Krewald, N. Cox, J. Messinger, W. Lubitz, et al. J. Am. Chem. Soc. 2011, 133, 19743.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsV2ju7%2FL&md5=148b2005d852cb0d83ac63edfcefae10CAS |
[12] D. Shevela, S. Koroidov, M. M. Najafpour, J. Messinger, P. Kurz, Chemistry 2011, 17, 5415.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXkvF2iu74%3D&md5=e0d2d0b5cda8f85979cd170838dd2e06CAS |
[13] I. Zaharieva, M. M. Najafpour, M. Wiechen, M. Haumann, P. Kurz, H. Dau, Energy Environ. Sci. 2011, 4, 2400.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFWrtrrM&md5=8c2d92f1dd89bcaf6b82fd227f33e71aCAS |
[14] M. Wiechen, H. M. Berends, P. Kurz, Dalton Trans. 2012, 41, 21.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsFOisLjJ&md5=00da333268814fd8640977ea9d46f9e8CAS |
[15] M. W. Kanan, J. Yano, Y. Surendranath, M. Dincă, V. K. Yachandra, D. G. Nocera, J. Am. Chem. Soc. 2010, 132, 13692.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGru7zM&md5=540cc424d400d53fa31ddfbaffecfcd3CAS |
[16] E. Abou-Hamad, Y. Kim, T. Wågberg, D. Boesch, S. Aloni, A. Zettl, et al. ACS Nano 2009, 3, 3878.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsVWhsbbF&md5=04206c30596526387237d8b56013abbaCAS |
[17] G. Z. Hu, F. Nitze, T. Barzegar, T. Sharifi, T. Wågberg, J. Mater. Chem. 2012, 22, 8541.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XltVWru7c%3D&md5=c055616835556d9384b19b708069d5ddCAS |
[18] Y. G. Zou, B. B. Liu, L. C. Wang, D. D. Liu, S. D. Yu, P. Wang, et al. Proc. Natl. Acad. Sci. USA 2009, 106, 22135.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXmtlWkug%3D%3D&md5=51ac52a40e19951295f7d92d842d6f7cCAS |
[19] P. Matyba, K. Maturova, M. Kemerink, N. D. Robinson, L. Edman, Nat. Mater. 2009, 8, 672.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXovFSisr4%3D&md5=dfc422a449cf6aa9c07ccccacc63a6b9CAS |
[20] P. Matyba, H. Yamaguchi, G. Eda, M. Chhowalla, L. Edman, N. D. Robinson, ACS Nano 2010, 4, 637.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsFams7k%3D&md5=4858eba30243aa1c87da47cac2accbd2CAS |
[21] T. Wågberg, P. R. Hania, N. D. Robinson, J. H. Shin, P. Matyba, L. Edman, Adv. Mater. 2008, 20, 1744.
| Crossref | GoogleScholarGoogle Scholar |
[22] S. Ajaikumar, J. Ahlkvist, W. Larsson, A. Shchukarev, A. R. Leino, K. Kordas, et al. Appl. Catal. A Gen. 2011, 392, 11.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXjsV2jtA%3D%3D&md5=8d86d5998722b870a8b7aaa4a5281617CAS |
[23] V. Eta, P. Maki-Arvela, A. R. Leino, K. Kordas, T. Salmi, D. Y. Murzin, et al. Ind. Eng. Chem. Res. 2010, 49, 9609.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtF2qsrvN&md5=d3909366a35262a4e8c4fa804e8d5594CAS |
[24] M. C. Wu, J. Hiltunen, A. Sapi, A. Avila, W. Larsson, H. C. Liao, et al. ACS Nano 2011, 5, 5025.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsFCru7g%3D&md5=0103ddd3e37d2e407d651c7d4cd10c9dCAS |
[25] D. Shevela, K. Beckmann, J. Clausen, W. Junge, J. Messinger, Proc. Natl. Acad. Sci. USA 2011, 108, 3602.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXivFKltL4%3D&md5=f96fad109a7f81c7d001a3e1b27091edCAS |
[26] N. Cox, L. Rapatskiy, J.-H. Su, D. A. Pantazis, M. Sugiura, L. Kulik, et al. J. Am. Chem. Soc. 2011, 133, 3635.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitlams7w%3D&md5=4008aca56fbc7bf4971567c7c1df3f7aCAS |
[27] J. Yano, J. Kern, K. Sauer, M. J. Latimer, Y. Pushkar, J. Biesiadka, et al. Science 2006, 314, 821.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtFKit73N&md5=347b86e74bdd04fa290532ebbe2c7265CAS |
[28] M. E. Eriksson, M. Israelsson, O. Olsson, T. Moritz, Nat. Biotechnol. 2000, 18, 784.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXltFCmsrc%3D&md5=d423ef3aa8c8893741a07762b01cd127CAS |
[29] T. Näsholm, A. Ekblad, A. Nordin, R. Giesler, M. Högberg, P. Högberg, Nature 1998, 392, 914.
| Crossref | GoogleScholarGoogle Scholar |
[30] G. A. Tuskan, S. DiFazio, S. Jansson, J. Bohlmann, I. Grigoriev, U. Hellsten, et al. Science 2006, 313, 1596.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpsVOktLk%3D&md5=498edddd3d38bed5ec6379b0f061c35aCAS |
[31] C. Boman, E. Pettersson, R. Westerholm, D. Boström, A. Nordin, Energy Fuels 2011, 25, 307.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtFymsQ%3D%3D&md5=7b1c8a96f1df46294bc13ea71cf2ae51CAS |
[32] E. Brus, M. Öhman, A. Nordin, Energy Fuels 2005, 19, 825.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXjslWmtbs%3D&md5=0d359583573e6815807d0cea8499ca70CAS |
[33] E. Natarajan, A. Nordin, A. N. Rao, Biomass Bioenergy 1998, 14, 533.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjvFSrtrk%3D&md5=54a859864a2bf11890feaee3ddeed5c5CAS |
[34] A. Nordin, Biomass Bioenergy 1994, 6, 339.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXjt1GnsLs%3D&md5=678708e57ecce42774588c46790e80c3CAS |
[35] D. Boström, N. Skoglund, A. Grimm, C. Boman, M. Öhman, M. Broström, et al. Energy Fuels 2012, 26, 85.
| Crossref | GoogleScholarGoogle Scholar |
[36] K. Lundholm, A. Nordin, R. Backman, Fuel Process. Technol. 2007, 88, 1061.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtFamurvJ&md5=d4fa293df712c0a45a5f0a6cbf4bace1CAS |
[37] M. Gabra, A. Nordin, M. Öhman, B. Kjellström, Biomass Bioenergy 2001, 21, 461.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnvFWgsb0%3D&md5=788539b817d1e6bfbaf7c0e688bf0d30CAS |
[38] J. Love, S. Björklund, J. Vahala, M. Hertzberg, J. Kangasjarvi, B. Sundberg, Proc. Natl. Acad. Sci. USA 2009, 106, 5984.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXkvFeiu7w%3D&md5=94f16079c2030ca9885335fdb5ee8f19CAS |
[39] M. Roach, L. Gerber, D. Sandquist, A. Gorzsás, M. Hedenström, M. Kumar, M. C. Steinhauser, R. Feil, G. Daniel, M. Stitt, B. Sundberg, T. Niittyla, Plant J. 2012,
| Crossref | GoogleScholarGoogle Scholar |
[40] I. Anugwom, P. Mäki-Arvela, P. Virtanen, P. Damlin, R. Sjoholm, J. P. Mikkola, RSC Advances 2011, 1, 452.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtF2hs7rO&md5=96ec24d63e73fea0c28f577432d55585CAS |
[41] H. Grenman, J. Warna, J. P. Mikkola, V. Sifontes, P. Fardim, D. Y. Murzin, et al. Ind. Eng. Chem. Res. 2010, 49, 9703.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGhsbzF&md5=42febf7ca471d621b17ee39836e3c111CAS |
[42] A. V. Kirilin, A. V. Tokarev, E. V. Murzina, L. M. Kustov, J. P. Mikkola, D. Y. Murzin, ChemSusChem 2010, 3, 708.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXnsFymsbg%3D&md5=72cbc5f0d81286027f6ef3ed7445f5c8CAS |
[43] E. Leino, P. Mäki-Arvela, K. Eränen, M. Tenho, D. Y. Murzin, T. Salmi, et al. Chem. Eng. J. 2011, 176–77, 124.
| Crossref | GoogleScholarGoogle Scholar |
[44] P. Mäki-Arvela, E. Salminen, T. Riittonen, P. Virtanen, N. Kumar, J.-P. Mikkola, Int. J. Chem. Eng. 2012, 674761.
| Crossref | GoogleScholarGoogle Scholar |
[45] T. Salmi, J. Wärnå, J. P. Mikkola in Chemical Reaction engineering and reactor technology 2010 (Taylor & Francis: Boca Raton).
[46] A. Cavka, B. Alriksson, M. Ahnlund, L. J. Jönsson, Biotechnol. Bioeng. 2011, 108, 2592.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtF2js7nK&md5=77196c265d41d3138b7879699b7f8690CAS |
[47] A. Cavka, B. Alriksson, S. H. Rose, W. H. van Zyl, L. J. Jönsson, J. Ind. Microbiol. Biotechnol. 2011, 38, 891.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXptFymsL0%3D&md5=a8fc4d5d056ece83004f75859ccc6b6fCAS |
[48] R. Chatterjee, K. Johansson, L. Jarnström, L. J. Jönsson, J. Agric. Food Chem. 2011, 59, 5390.
| 1:CAS:528:DC%2BC3MXlt1Cntrk%3D&md5=db7a4b7fea050bdd443eef7789b5b3a6CAS |
[49] V. Eta, P. Mäki-Arvela, J. Wärnå, T. Salmi, J. P. Mikkola, D. Y. Murzin, Appl. Catal. A Gen. 2011, 404, 39.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVGmt7fL&md5=f23bfc58eaedd29693e05a90f85e0d13CAS |
[50] N. Halonen, A. Sapi, L. Nagy, R. Puskas, A. R. Leino, J. Maklin, et al. Solid State Phys. 2011, 248, 2500.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtlOjt7zL&md5=802520b6869ca10faca668067bef8e38CAS |
[51] S. Hyvarinen, P. Damlin, J. Grasvik, D. Y. Murzin, J. P. Mikkola, Cell. Chem. Technol. 2011, 45, 483.
| 1:CAS:528:DC%2BC3MXhs1ansL%2FE&md5=3f2428f47e29d61600468a816463735fCAS |
[52] H. Jun, T. Kieselbach, L. J. Jönsson, Microb. Cell Fact. 2011, 10, 68.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXht1WrtrrI&md5=8223fb7b18ac223473844bb0743299aeCAS |
[53] T. A. Lestander, A. Lundström, M. Finell, Can. J. For. Res. 2012, 42, 59.
| Crossref | GoogleScholarGoogle Scholar |
[54] T. A. Lestander, R. Samuelsson, Energ. Fuel. 2010, 24, 5148.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFGjsrzM&md5=5d146e66a802496cf1bb0075a1406c62CAS |
[55] V. P. Soudham, B. Alriksson, L. J. Jönsson, J. Biotechnol. 2011, 155, 244.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVais7fL&md5=65a4e6d54c2500483667439688300060CAS |
[56] I. Bjurhager, A. M. Olsson, B. Zhang, L. Gerber, M. Kumar, L. A. Berglund, et al. Biomacromolecules 2010, 11, 2359.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVSju7vI&md5=f16f3e5caf6b263c4b27c3bae12cbcb4CAS |
[57] M. Hedenström, S. Wiklund-Lindström, T. Öman, F. C. Lu, L. Gerber, P. Schatz, et al. Mol. Plant 2009, 2, 933.
| Crossref | GoogleScholarGoogle Scholar |
[58] M. Mauriat, T. Moritz, Plant J. 2009, 58, 989.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXnvVequ7s%3D&md5=9b0af1c6c8936a680b9a2685df3e746cCAS |
[59] R. Nilsson, K. Bernfur, N. Gustavsson, J. Bygdell, G. Wingsle, C. Larsson, Mol. Cell. Proteomics 2010, 9, 368.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtFWhtrw%3D&md5=1f7ac4a13876c0a6c207bb8d817bc00aCAS |
[60] A. M. Olsson, I. Bjurhager, L. Gerber, B. Sundberg, L. Salmen, Planta 2011, 233, 1277.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmsFGgtbo%3D&md5=9df9c282c90c724b16fc012f6705f701CAS |
[61] E. Pesquet, H. Tuominen, New Phytol. 2011, 190, 138.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXktleltLs%3D&md5=59bd857e30cfcfcaf86311705b6f5f0eCAS |
[62] R. C. Pinto, H. Stenlund, M. Hertzberg, T. Lundstedt, E. Johansson, J. Trygg, Anal. Chim. Acta 2011, 685, 127.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsF2mtLnP&md5=e781722951b454b86fede62587c0493eCAS |
[63] A. M. Wu, C. Rihouey, M. Seveno, E. Hornblad, S. K. Singh, T. Matsunaga, et al. Plant J. 2009, 57, 718.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFGhsbk%3D&md5=3b499141b66c48970f4ab6a1be7a8dffCAS |
[64] J. Onskog, E. Freyhult, M. Landfors, P. Ryden, T. R. Hvidsten, BMC Bioinformatics 2011, 12, 390.
| Crossref | GoogleScholarGoogle Scholar |
[65] N. Street, S. Jansson, T. R. Hvidsten, BMC Plant Biol. 2011, 11, 13.
[66] K. Beckmann, H. Uchtenhagen, G. Berggren, M. F. Anderlund, A. Thapper, J. Messinger, S. Styring, P. Kurz, Energy Environ. Sci. 2008, 1, 668.
| Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXntFWkt7w%3D&md5=799ebbf1e2de5ff525d34d6975a19a9aCAS |


