An autoantigen profile from Jurkat T-Lymphoblasts provides a molecular guide for investigating autoimmune sequelae of COVID-19
Julia Y. Wang A * , Wei Zhang B , Michael W. Roehrl A , Victor B. Roehrl A and Michael H. Roehrl
A * , Wei Zhang B , Michael W. Roehrl A , Victor B. Roehrl A and Michael H. Roehrl  C D *
C D *
A Curandis, Boston, MA, USA.
B Department of Gastroenterology, Affiliated Hospital of Guizhou Medical University, Guizhou, China.
C Department of Pathology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
D Harvard Medical School, Boston, MA, USA.
Handling Editor: John Wade
Australian Journal of Chemistry 76(8) 508-524 https://doi.org/10.1071/CH22268
Submitted: 20 December 2022 Accepted: 6 June 2023 Published: 20 July 2023
Abstract
In order to understand autoimmune phenomena contributing to the pathophysiology of COVID-19 and post-COVID syndrome, we have been profiling autoantigens (autoAgs) from various cell types. Although cells share numerous autoAgs, each cell type gives rise to unique COVID-altered autoAg candidates, which may explain the wide range of symptoms experienced by patients with autoimmune sequelae of SARS-CoV-2 infection. Based on the unifying property of affinity between autoAgs and the glycosaminoglycan dermatan sulfate (DS), this paper reports 140 candidate autoAgs identified from proteome extracts of human Jurkat T-cells, of which at least 105 (75%) are known targets of autoantibodies. Comparison with currently available multi-omic COVID-19 data shows that 125 (89%) DS-affinity proteins are altered at protein and/or RNA levels in SARS-CoV-2-infected cells or patients, with at least 94 being known autoAgs in a wide spectrum of autoimmune diseases and cancer. Protein alterations by ubiquitination and phosphorylation during the viral infection are major contributors of autoAgs. The autoAg protein network is significantly associated with cellular response to stress, apoptosis, RNA metabolism, mRNA processing and translation, protein folding and processing, chromosome organization, cell cycle, and muscle contraction. The autoAgs include clusters of histones, CCT/TriC chaperonin, DNA replication licensing factors, proteasome and ribosome proteins, heat shock proteins, serine/arginine-rich splicing factors, 14-3-3 proteins, and cytoskeletal proteins. AutoAgs, such as LCP1 and NACA, that are altered in the T cells of COVID patients may provide insight into T-cell responses to viral infection and merit further study. The autoantigen-ome from this study contributes to a comprehensive molecular map for investigating acute, subacute, and chronic autoimmune disorders caused by SARS-CoV-2.
Keywords: autoantibodies, autoantigens, autoimmunity, COVID-19, long COVID, dermatan sulfate, SARS-Cov-2, T cell immunity.
Introduction
The COVID-19 pandemic has been devastating. After initial recovery from acute SARS-CoV-2 infection, many people continue to suffer from lingering health problems (so called ‘long COVID’ or post-COVID syndrome), such as fatigue, shortness of breath, joint pain, chest pain, muscle pain, loss of smell or taste, and other neurological problems. Although the underlying causes are unclear, autoimmune effects are likely important contributors to chronic post-COVID disorders. To understand how SARS-CoV-2 infection may induce autoimmune responses, we are establishing a comprehensive COVID autoantigen (autoAg) atlas, i.e. all possible endogenous autoAgs that may be rendered immunogenic by the viral infection. Because different tissues or cells may give rise to distinct pools of autoAgs, we have been profiling autoAgs from multiple human tissues and cell types, including human lung fibroblast HFL1 cells, human lung epithelial-like A549 cells, and B-lymphoblast HS-Sultan cells.[1–3] In this study, we report an autoantigen-ome identified from human Jurkat T-lymphoblast cells.
Our autoAg discovery is based on a unifying mechanism of autoantigenicity that we have uncovered.[4–6] AutoAgs are the targets of autoantibodies (autoAbs) and T-cell autoimmune responses. Typically, self-molecules are naturally tolerated by the immune system and do not provoke autoimmune responses. However, certain self-molecules transform into autoAgs and become targets of autoimmune attacks. Thus far, hundreds of autoAgs with seemingly no obvious structural or functional commonality have been identified across various autoimmune diseases and cancers. Our studies have demonstrated that autoAgs do, in fact, share common properties. AutoAgs are commonly released by apoptotic cells, and we found that the glycosaminoglycan dermatan sulfate (DS) has peculiar affinity to apoptotic cells and their autoAgs.[4,6] DS and autoAgs can form affinity complexes and cooperatively stimulate autoreactive B1 cells and autoantibody production.[4,6] Based on autoAg–DS affinity, we have identified several hundred autoAgs from various cells and tissues.[1–3,7–9]
A variety of autoAbs have been identified in COVID-19 patients.[10–20] Children infected with SARS-CoV-2 who develop the rare multisystem inflammatory syndrome show multiple autoAbs, including classical antinuclear antigen (ANA) autoAbs and specific autoAbs recognizing endothelial, gastrointestinal, or immune cell autoAgs.[10,11] ANA autoAbs are also frequently detected in COVID-19 patients with acute respiratory syndrome or other critical conditions,[12–14] and in COVID patients with no previous clinical record of autoimmune diseases.[15] A high frequency of cerebrospinal fluid autoAbs is found in COVID patients with neurological symptoms.[16] New-onset autoAbs were detected in a significant proportion of hospitalized COVID-19 patients and were positively correlated with immune responses to SARS-CoV-2 proteins.[18] Overall, an increasing number of observations suggest a positive correlation between emergence of autoAbs and an adverse clinical course of COVID-19.
As revealed by our prior studies, SARS-CoV-2 infection may induce numerous molecular changes in the host and transform naturally non-antigenic self-molecules to antigenic autoAgs.[1–3] In order to better understand the possible extent of autoimmune disorders caused by SARS-CoV-2, we are building a comprehensive catalog of all possible intrinsic autoAgs across cell and tissue types related to the viral infection. Herein, we report a profile of autoAgs identified from human Jurkat T-cells using our DS-affinity enrichment approach, which will provide valuable molecular targets for understanding the diverse autoimmune sequelae of COVID-19.
Experimental
Jurkat T-cell culture
The human T lymphoblast Jurkat cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in complete RPMI-1640 medium. Short tandem repeat DNA identity was confirmed, and mycoplasma testing was negative. The growth medium was supplemented with 10% fetal bovine serum and a penicillin–streptomycin–glutamine mixture (Thermo Fisher). The cells were grown at 37°C in a CO2 incubator.
Protein extraction
Protein extraction was performed as previously described.[5] In brief, Jurkat cells were lysed with 50 mM phosphate buffer (pH 7.4) containing the Roche Complete Mini protease inhibitor cocktail, and then homogenized on ice with a microprobe sonicator until the turbid mixture turned nearly clear with no visible cells left. The homogenate was centrifuged at 10 000g at 4°C for 20 min, and the total protein extract in the supernatant was collected. Protein concentration was measured by absorbance at 280 nm using a NanoDrop UV-Vis spectrometer (Thermo Fisher).
DS-Sepharose resin preparation
The DS-affinity resins were synthesized as previously described.[5,7] In brief, 20 mL of EAH Sepharose 4B resins (GE Healthcare Life Sciences) were washed with distilled water three times and mixed with 100 mg of DS (Sigma-Aldrich) in 10 mL of 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES) buffer, pH 5.0. About 100 mg of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (Sigma-Aldrich) powder was added, and another 100 mg was added after 8 h of reaction. The reaction proceeded by mixing on a rocker at 25°C for 16 h. The coupled resins were washed with water and equilibrated with 0.5 M NaCl in 0.1 M acetate (pH 5.0) and 0.5 M NaCl in 0.1 M Tris (pH 8.0).
DS-affinity fractionation
The total proteomes extracted from Jurkat cells were fractionated in a DS-Sepharose column.[5] About 40 mg of proteins in 40 mL of 10 mM phosphate buffer (pH 7.4; buffer A) was loaded onto the DS-affinity column at a rate of 1 mL/min. Unbound and weakly bound proteins were removed with 60 mL of buffer A and then 40 mL of 0.2 M NaCl in buffer A. The remaining bound proteins were eluted in step gradients of 40 mL each of 0.4, 0.6, and 1.0 M NaCl in buffer A. Fractions were desalted and concentrated with 5 kDa cut-off Vivaspin centrifugal filters (Sartorius). Fractionated proteins were separated in 1-D SDS-PAGE in 4–12% Bis-Tris gels, and each gel lane was divided into two or three sections for sequencing.
Mass spectrometry sequencing
Protein sequencing was performed at the Taplin Biological Mass Spectrometry Facility at Harvard Medical School. Proteins in gels were digested with sequencing-grade trypsin (Promega) at 4°C for 45 min. Tryptic peptides were separated in a nanoscale C18 HPLC capillary column and analyzed in an LTQ linear ion-trap mass spectrometer (Thermo Fisher). Peptide sequences and protein identities were assigned by matching the measured fragmentation pattern with proteins or translated nucleotide databases using Sequest. All data were manually inspected. Proteins with ≥ 2 peptide matches were considered positively identified.
COVID data comparison
DS-affinity proteins were compared with currently available COVID-19 multi-omic data compiled in the Coronascape database (as of 2 February 2021).[21–42] These data have been obtained with proteomics, phosphoproteomics, interactome, ubiquitome, and RNA-seq techniques. Up- and down-regulated proteins or gene transcripts were identified by comparing cells infected vs uninfected by SARS-CoV-2 or COVID-19 patients vs healthy controls. Similarity searches were conducted to identify DS-affinity proteins that are up- and/or down-regulated in viral infection at any omic level.
Protein network analysis
Protein–protein interactions were analyzed by STRING.[43] Interactions included both direct physical interaction and indirect functional associations, which were derived from genomic context predictions, high-throughput lab experiments, co-expression, automated text mining, and previous knowledge in databases. Each interaction was annotated with a confidence score from 0 to 1, with 1 being the highest, indicating the likelihood of an interaction to be true. Pathways and processes enrichment were analyzed with Metascape,[21] which utilizes various ontology sources such as KEGG Pathway, GO Biological Process, Reactome Gene Sets, Canonical Pathways, CORUM, TRRUST, and DiGenBase. Terms with a P-value <0.01, a minimum count of three, and an enrichment factor (ratio between the observed counts and the counts expected by chance) >1.5 were collected and grouped into clusters based on their membership similarities. The most statistically significant term within a cluster was chosen to represent the cluster.
Autoantigen literature text mining
Every DS-affinity protein identified in this study was searched for specific autoAbs reported in the PubMed literature. Search keywords included the MeSH keyword ‘autoantibodies’, the protein name or its gene symbol, or alternative names and symbols. Only proteins for which specific autoAbs are reported in PubMed-listed journal articles were considered ‘confirmed’ or ‘known’ autoAgs in this study.
Results and discussion
Autoantigen-ome of Jurkat cells identified by DS-affinity
Total proteins were extracted from Jurkat T-cells and fractionated in a DS-Sepharose affinity column. Proteins with increasing DS-affinity were eluted from the column with increasing ionic strength of salt. Fractions eluted with 0.4, 0.6, and 1.0 M NaCl correspond to proteins with intermediate, strong, and very strong DS-affinity, respectively. Mass spectrometry sequencing identified a total of 140 proteins from these three DS-affinity fractions (Table 1). The majority of proteins (120/140) were eluted with 0.4 M NaCl, 31 proteins were found in the 0.6 M NaCl elution, and 11 proteins were identified in the 1.0 M NaCl elution. Three proteins were detected redundantly in all three fractions (HIST4H4, H2AC1, and RPLP2), 1H2BC1 was detected in both 0.6 and 1.0 M fractions, C1QBP was detected in both 0.4 and 1.0 M NaCl fractions, and 13 proteins were detected in both 0.4 and 0.6 M fractions.
| Symbol | Protein name | DS-affinity | SARS-CoV-2 effect | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| VS | S | M | Up | Dn | Interact | ||||
| ACTC1 | Actin, alpha 1, skeletal muscle | 2 | 6 | u | d | [44] | |||
| ACTG1 | Actin, cytoplasmic 2 | 4 | u | d | [45] | ||||
| ACTN1 | Alpha-actinin-1, f-actin cross linking protein | 8 | u | d | [46] | ||||
| ALDH18A1 | Delta 1-pyrroline-5-carboxylate synthetase | 2 | d | ||||||
| ANP32A | Acidic leucine-rich nuclear phosphoprotein 32 family member a | 9 | u | d | |||||
| ANP32B | Acidic leucine-rich nuclear phosphoprotein 32 family member b | 6 | d | [47] | |||||
| ANXA6 | Annexin a6 (chromobindin-20) | 9 | u | d | [48] | ||||
| ATP5F1B | ATP synthase subunit beta, mitochondrial precursor | 7 | u | d | Nsp6 | [49] | |||
| BZW2 | Basic leucine zipper and W2 domain-containing protein 2 | 2 | M | ||||||
| C1QBP | Complement component 1 q subcomponent-binding protein | 2 | 2 | d | [47] | ||||
| CALM1 | Calmodulin-1 | 4 | d | [13] | |||||
| CALM3 | Calmodulin-3 | 2 | u | [50] | |||||
| CALR | Calreticulin precursor | 11 | u | d | [51] | ||||
| CAND1 | Cullin-associated nedd8-dissociated protein 1, TIP120 | 6 | |||||||
| CAPRIN1 | Membrane component chromosome 11 surface marker 1 | 3 | d | ||||||
| CAPZA1 | F-actin capping protein alpha-1 subunit | 2 | d | [52] | |||||
| CCT2 | T-complex protein 1 subunit beta | 8 | d | [53] | |||||
| CCT3 | T-complex protein 1 subunit gamma | 12 | u | [54] | |||||
| CCT4 | T-complex protein 1 subunit delta (stimulator of tar rna-binding) | 3 | u | [54] | |||||
| CCT5 | T-complex protein 1 subunit epsilon | 7 | u | d | [53] | ||||
| CCT6A | T-complex protein 1 subunit zeta | 5 | u | d | [53] | ||||
| CCT7 | T-complex protein 1 subunit eta | 9 | [53] | ||||||
| CCT8 | T-complex protein 1 subunit theta | 18 | u | d | [54] | ||||
| CDC37 | Hsp90 chaperone protein kinase-targeting subunit | 6 | u | d | |||||
| DDB1 | Damage-specific DNA-binding protein 1 | 2 | u | d | [6] | ||||
| DDX39A | ATP-dependent RNA helicase ddx39 | 7 | u | d | |||||
| DDX39B | Spliceosome RNA helicase bat1 | 2 | d | ||||||
| DHX15 | Pre-mRNA-splicing factor ATP-dependent RNA helicase | 2 | d | ||||||
| EEF1B2 | Elongation factor 1-beta | 2 | d | ||||||
| EEF1G | Elongation factor 1-gamma | 5 | u | d | |||||
| EIF4A1 | Eukaryotic initiation factor 4A-I | 14 | u | d | |||||
| EIF5A2 | Eukaryotic translation initiation factor 5a isoform 2 | 2 | d | [55] | |||||
| FASN | Fatty acid synthase | 5 | u | d | [56] | ||||
| HDGF | Hepatoma-derived growth factor | 3 | u | d | [57] | ||||
| HIST1H1A | Histone h1.1, H1-1 | 3 | 2 | u | d | [58] | |||
| HIST1H1B | Histone h1.5 (histone h1a), H1-5 | 5 | 3 | u | d | [59] | |||
| HIST1H1C | Histone h1.2 (histone h1d), H1-2 | 3 | 3 | u | d | [60] | |||
| HIST1H2AA | Histone h2a type 1-a, H2AC1, H2AFR | 3 | 2 | 2 | [59] | ||||
| HIST1H2BA | Histone h2b type 1-a (testis-specific histone h2b), H2BC1 | 5 | 4 | [58] | |||||
| HIST1H2BB | Histone h2b type 1-b (h2b.f) H2BC3 | 2 | [61] | ||||||
| HIST3H3 | Histone h3.4, H3-4 | 3 | [58] | ||||||
| HIST4H4 | Histone h4, H4C1 | 5 | 6 | 8 | u | [61] | |||
| HMGB1 | High mobility group protein 1-like 10 (hmg-1l10) | 10 | d | [57] | |||||
| HMGCS1 | Hydroxymethylglutaryl-coa synthase | 2 | u | d | |||||
| HNRNPA1 | hnRNP core protein A1 | 2 | u | d | [62] | ||||
| HNRNPCL1 | hnRNP core protein C-like 1 | 2 | [63] | ||||||
| HNRNPK | hnRNP K | 3 | u | [64] | |||||
| HNRNPU | hnRNP U (scaffold attachment factor a) | 2 | u | d | [65] | ||||
| HSP90AA1 | Heat shock protein hsp 90-alpha (hsp 86) | 2 | 38 | u | d | [66] | |||
| HSP90AB1 | Heat shock protein hsp 90-beta (hsp 84) (hsp 90) | 16 | u | d | [67] | ||||
| HSP90B1 | Heat shock protein 90 kDa beta member 1 (grp94) | 23 | u | d | [68] | ||||
| HSPA4 | Heat shock 70 kDa protein 4 | 14 | u | d | [69] | ||||
| HSPA5 | GRP78, BiP | 8 | u | d | Nsp2 Nsp4 | [70] | |||
| HSPD1 | Hsp60 (mitochondrial matrix protein p1) | 30 | u | d | [71] | ||||
| HSPH1 | Heat-shock protein 105 kDa | 13 | u | [72] | |||||
| HYOU1 | Hypoxia up- regulated 1, ORP150 | 2 | u | Orf8 | [73] | ||||
| IPO5 | Importin beta-3, ranbp5 | 7 | [74] | ||||||
| KPNB1 | Importin beta-1 subunit (nuclear factor p97) | 5 | [74] | ||||||
| LCP1 | Plastin-2 | 8 | u | [75] | |||||
| LMNB1 | Lamin-b1 | 2 | u | d | [76] | ||||
| LSM8 | U6 snRNA-associated Sm-like protein LSm8 | 2 | |||||||
| MAPRE1 | Microtubule-associated protein rp/eb family member 1 | 3 | Orf3 | ||||||
| MCM2 | DNA replication licensing factor mcm2 | 6 | d | [77] | |||||
| MCM3 | DNA replication licensing factor mcm3 | 7 | u | d | [77] | ||||
| MCM4 | DNA replication licensing factor mcm4, CDC21 | 5 | u | d | [77] | ||||
| MCM5 | DNA replication licensing factor mcm5, CDC46 | 3 | u | d | [77] | ||||
| MCM6 | DNA replication licensing factor mcm6 | 9 | u | d | [77] | ||||
| MYL6 | Myosin light polypeptide 6 | 2 | u | [78] | |||||
| NACA | Nascent polypeptide-associated complex subunit alpha | 3 | u | d | [51] | ||||
| NASP | Nuclear autoantigenic sperm protein | 4 | u | d | [79] | ||||
| NCL | Nucleolin | 23 | u | d | [80] | ||||
| NPM1 | Nucleophosmin | 6 | 6 | u | d | [81] | |||
| NUDT5 | ADP-sugar pyrophosphatase | 2 | d | ||||||
| P4HB | Protein disulfide-isomerase precursor (thyroid hormone-binding protein) | 7 | u | d | [82] | ||||
| PABPC3 | Polyadenylate-binding protein 3 | 3 | d | ||||||
| PCNA | Proliferating cell nuclear antigen | 8 | u | d | [83] | ||||
| PDIA4 | Protein disulfide-isomerase a4 precursor | 12 | u | d | [84] | ||||
| PDIA6 | Protein disulfide-isomerase a6 precursor | 4 | u | d | [82] | ||||
| PFDN3 | Prefoldin subunit 3, VBP1 | 3 | d | ||||||
| POTEKP | Putative beta-actin-like protein 3, kappa actin, ACTBL3 | 2 | 2 | u | |||||
| PPP1R7 | Protein phosphatase 1 regulatory subunit 7 | 2 | u | ||||||
| PPP2R1A | Serine/threonine-protein phosphatase 2a (pp2a) regulatory subunit A | 7 | d | [85] | |||||
| PRKCSH | Glucosidase 2 subunit beta (protein kinase c substrate heavy chain) | 4 | d | Orf3 | |||||
| PRMT1 | Protein arginine n-methyltransferase 1 | 3 | d | [82] | |||||
| PSMA1 | Proteasome subunit alpha type 1 | 3 | u | [86] | |||||
| PSMA2 | Proteasome subunit alpha type 2 | 2 | d | ||||||
| PSMA3 | Proteasome subunit alpha type 3 | 2 | u | d | [87] | ||||
| PSMA5 | Proteasome subunit alpha type 5 | 5 | u | [88] | |||||
| PSMA7 | Proteasome subunit alpha type 7 | 2 | u | d | [89] | ||||
| PSMA8 | Proteasome subunit alpha type 7-like | 2 | [89] | ||||||
| PSMB3 | Proteasome subunit beta type 3 | 2 | d | [87] | |||||
| PSMB4 | Proteasome subunit beta type 4 | 3 | |||||||
| PSMB7 | Proteasome subunit beta type 7 (subunit z) | 2 | d | [87] | |||||
| PSMC1 | 26s Proteasome regulatory subunit 4 | 2 | d | ||||||
| PSME3 | Proteasome activator complex subunit 3 | 3 | d | [90] | |||||
| PTGES3 | Prostaglandin E synthase 3 | 2 | d | ||||||
| PTMA | Prothymosin alpha | 4 | u | d | [91] | ||||
| RBBP7 | Histone-binding protein rbbp7 | 3 | u | d | |||||
| RPA3 | Replication protein A 14 kDa subunit | 2 | [92] | ||||||
| RPL22 | 60s ribosomal protein L22 (heparin-binding protein hbp15) | 2 | d | [93] | |||||
| RPL5 | 60s ribosomal protein L5 | 5 | d | [94] | |||||
| RPL6 | 60s ribosomal protein L6 | 4 | u | d | [77] | ||||
| RPL7 | 60s ribosomal protein L7 | 3 | u | d | [93] | ||||
| RPLP0 | 60s acidic ribosomal protein P0 | 3 | u | d | [95] | ||||
| RPLP2 | 60s acidic ribosomal protein P2 (ny-ren-44 antigen) | 2 | 2 | 2 | u | d | [96] | ||
| RPS3A | Ribosomal protein S3a | 2 | u | d | |||||
| RPS7 | Ribosomal protein S7 | 2 | u | d | |||||
| SET | Protein SET | 4 | u | d | [97] | ||||
| SF3B3 | Splicing factor 3b subunit 3, SAP130 | 3 | u | ||||||
| SNRNP70 | U1 snRNP 70 kDa | 3 | u | d | [98] | ||||
| SNRPD2 | Small nuclear ribonucleoprotein D2 polypeptide | 3 | 2 | d | [99] | ||||
| SNRPD3 | Small nuclear ribonucleoprotein sm d3 | 2 | d | [100] | |||||
| SRRT | Arsenite-resistance protein 2 | 2 | d | ||||||
| SRSF1 | Splicing factor, arginine/serine-rich 1 | 5 | u | d | [46] | ||||
| SRSF3 | Serine/arginine-rich splicing factor 3, SFRS3 | 2 | [101] | ||||||
| SRSF5 | Serine/arginine-rich splicing factor 5, SRP40 | 2 | u | d | [102] | ||||
| SRSF7 | Splicing factor, arginine/serine-rich 7 (9g8) | 2 | u | ||||||
| SRSF8 | Serine/arginine-rich splicing factor 8 | 2 | d | ||||||
| SSB | Lupus La protein (Sjogren syndrome type b antigen] | 3 | 5 | u | d | [103] | |||
| ST13 | Hsc70-interacting protein (suppression of tumorigenicity protein 13) | 6 | u | [104] | |||||
| SYNCRIP | hnRNP Q (synaptotagmin-binding, cytoplasmic rna-interacting protein) | 3 | d | ||||||
| TCP1 | T-complex protein 1 subunit alpha | 7 | d | [53] | |||||
| TPM1 | Tropomyosin 1 alpha chain | 3 | u | d | [105] | ||||
| TPM3 | Tropomyosin alpha-3 chain | 5 | u | d | [106] | ||||
| TPM4 | Tropomyosin alpha-4 chain | 5 | u | d | [107] | ||||
| TUBA1C | Tubulin alpha-6 chain | 2 | 2 | u | d | [108] | |||
| TUBA3C | Tubulin alpha-2 chain | 3 | 10 | ||||||
| TUBB | Beta-tubulin | 2 | 7 | u | d | [109] | |||
| UBA1 | Ubiquitin-activating enzyme E1 | 2 | u | d | [110] | ||||
| VCP | Transitional endoplasmic reticulum ATPase | 14 | u | d | [111] | ||||
| VIM | Vimentin | 4 | 10 | u | d | [112] | |||
| VPS35 | Vacuolar protein sorting 35 | 2 | u | d | [113] | ||||
| XRCC5 | ATP-dependent dna helicase 2 subunit 2 (lupus ku86) | 8 | d | [114] | |||||
| XRCC6 | ATP-dependent dna helicase 2 subunit 1 (lupus ku70) | 6 | 11 | u | d | [115] | |||
| YWHAB | 14-3-3 protein beta/alpha | 12 | u | d | |||||
| YWHAE | 14-3-3 protein epsilon | 8 | u | d | [116] | ||||
| YWHAG | 14-3-3 protein gamma | 5 | u | [116] | |||||
| YWHAH | 14-3-3 protein eta | 3 | d | [117] | |||||
| YWHAQ | 14-3-3 protein theta | 3 | u | d | [118] | ||||
| YWHAZ | 14-3-3 protein zeta/delta | 3 | u | d | [119] | ||||
Abbreviations from left to right: VS (very strong DS-affinity, eluted with 1.0 M NaCl), S (strong DS-affinity, eluted with 0.6 M NaCl), M (medium DS-affinity, eluted with 0.4 M NaCl), Up (up-regulated in SARS-CoV-2 infection), Dn (down-regulated in SARS-CoV-2 infection), Interact (found in the protein interactomes of listed SARS-CoV-2 viral proteins), Ref. (representative literature references in which autoantibodies to specific autoAgs are reported). Numbers in the ‘DS-affinity’ columns denote numbers of proteins identified.
Remarkably, among the 140 DS-affinity proteins identified from Jurkat T-cells, at least 105 (75%) are known autoAgs, i.e. the existence of specific autoAbs against these proteins has been reported in the literature (see references in Table 1). These autoAb/autoAg pairs are found in a wide spectrum of autoimmune diseases as well as a variety of cancers. Although 36 of the DS-affinity proteins have not yet been reported as autoAgs, we suspect that most, if not all, are putative autoAgs awaiting serological confirmation. For example, six serine/arginine-rich splicing factors were identified by DS-affinity, but only three of them (SRSF1, SRSF3, and SRSF5) have thus far been individually reported as autoAgs (Table 1). A serine/arginine-rich repeating octapeptide of Arg-Ser-Arg-Ser-Arg(Lys)-Glu(Asp)-Arg-Lys(Arg) has been found in several nuclear autoAgs such as U2AF 35 and 65 kDa splicing factors and 70 kDa U1 snRNP,[120] and many other splicing factors have been reported as autoAgs, such as SF3B1 and SRSF2. Therefore, we suspect that the other three splicing factors (SRSF3B3, SRSF7, and SRSF8) identified by DS-affinity in this study are likely true autoAgs that are yet to be confirmed.
Proteins eluted with 1.0 M NaCl possess the strongest DS-affinity and, strikingly, 10/11 (90.9%) are known autoAgs (Table 1), indicating that increasing affinity to DS increases the propensity of a protein to be an autoAg, consistent with our prior findings.[1–5,7–9] These include histones (H4, H2B types 1-a and 1-b, and H2A type 1-a), 60S ribosomal proteins (P0, P2, L6, and L7), ACTC1 (skeletal muscle actin), C1QBP, and PABPC3 (polyadenylate-binding protein 3). Histones and ribosomal P proteins are hallmark autoAgs used in routine clinical tests of autoimmune diseases. Histone autoAbs are nearly always present in drug-induced systemic lupus erythematosus, and ribosomal P autoAbs are tested for to aid in the differential diagnosis of lupus patients with neuropsychiatric symptoms. C1QBP has been repeatedly identified as a putative autoAg in several of our prior studies,[1,2,7,8] and was recently confirmed as an autoAg in the neurodegenerative disorder primary open-angle glaucoma.[121] Poly(A)-binding proteins bind the poly(A) tail of messenger RNAs and control mRNA stability and translation initiation. Although PABPC3 has not yet been reported as an autoAg, its paralog PABPC1 has been found to be an autoAg.[122]
Proteins eluted with 0.6 M NaCl possess strong DS-affinity and 26/31 (83.9%) are known autoAgs (Table 1). Several well-known autoAgs are identified in this strong DS-affinity fraction, including six histone autoAgs, SSB (lupus La autoAg), XRCC6 (lupus Ku70 autoAg), three snRNP autoAgs (Sm D2, Sm D3, and U1 70kD). Other autoAgs identified with strong DS-affinity include ANP32B, nucleolin, nucleophosmin, SET, HNRNPCL1, HSP90AA1, three ribosomal proteins (L22, L5, and S3a), three serine/arginine-rich splicing factors, three tropomyosin subunits, prothymosin alpha, three tubulin subunits, vimentin, and T-complex protein 1 alpha. A few have not yet been confirmed as autoAgs, including ANP32A, kappa actin, and ribosomal protein 3A.
We see evidence that the 140 candidate autoAgs identified from Jurkat T-cells are not a random collection but are highly enriched in a few groups of proteins. Among them, there are 11 proteasomal proteins, eight ribosomal proteins, eight histones, eight T-complex protein (CCT/TriC) subunits, seven heat shock proteins, six splicing factors, six 14-3-3 proteins, five DNA replication licensing factors (or minichromosome maintenance proteins), five DNA or RNA helicases, and four hnRNPs.
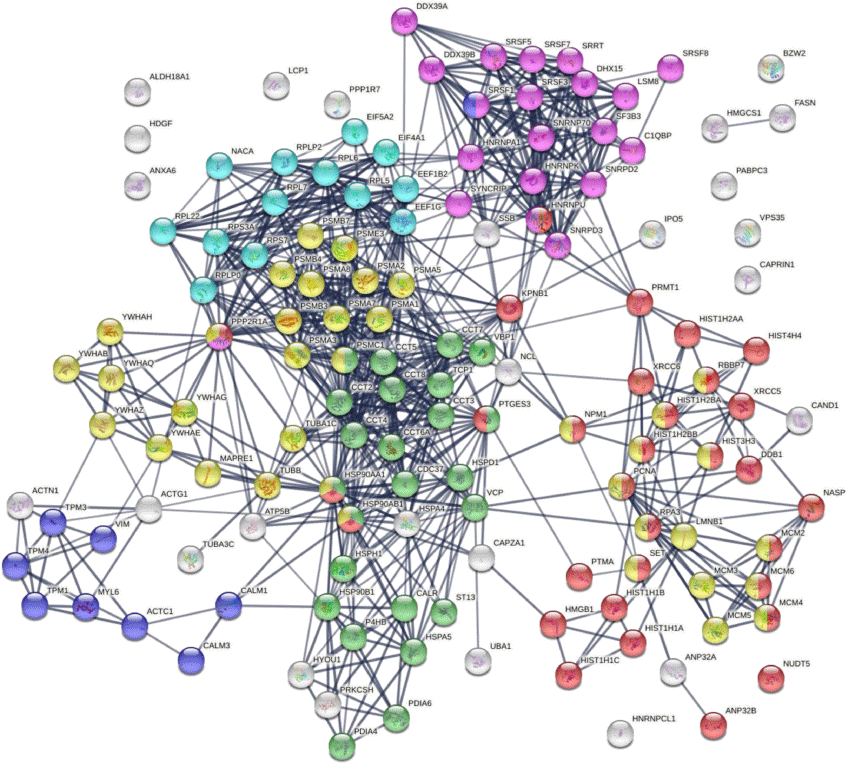
Protein–protein interaction network analysis by STRING[43] reveals that the DS-affinity autoantigen-ome is highly connected (Fig. 1). There are 787 interactions at high confidence level (vs 284 expected; enrichment P-value <10−16). These DS-affinity proteins are enriched in several clusters and significantly associated with the cell cycle, protein folding, chromosome organization, RNA splicing, translation, and muscle contraction (Fig. 1). There are 36 DS-affinity proteins associated with the cell cycle, particularly the G2/M checkpoints (26 proteins), the G2/M DNA damage checkpoint, and the G1/S and G2/M transitions.
The autoantigen-ome from Jurkat T-cells identified by DS affinity. Lines represent protein–protein interactions at high confidence levels. Marked proteins are associated with cell cycle (37 proteins, yellow), chromosome organization (31 proteins, red), RNA splicing (20 proteins, pink), translation (13 proteins, aqua), protein folding (24 proteins, green), and muscle contraction (nine proteins, blue).
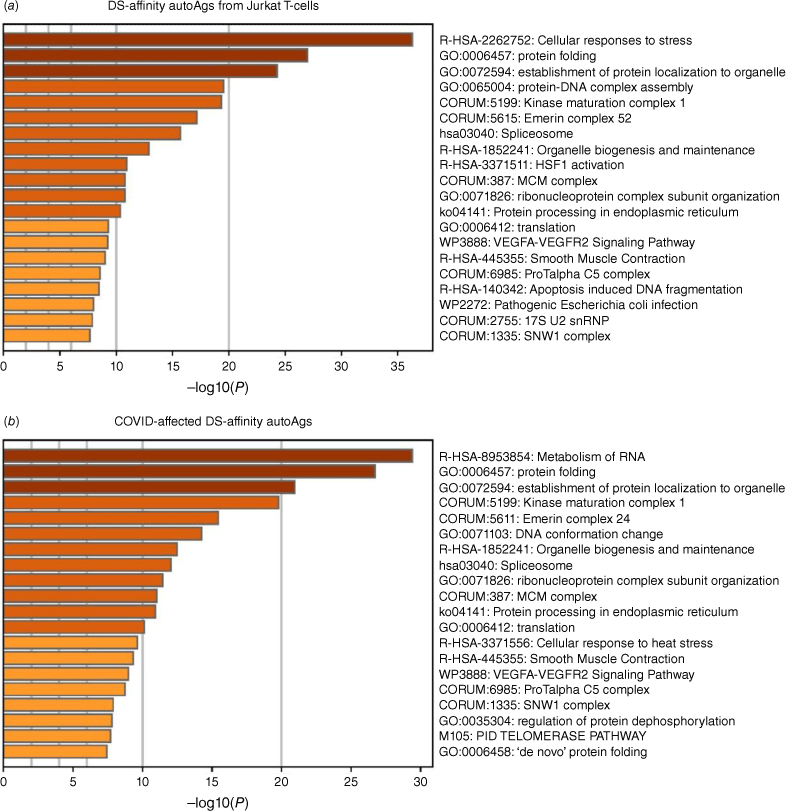
Pathway and process enrichment analyses by Zhou et al.[21] also reveal that proteins of the DS-affinity autoantigen-ome are significantly associated with cellular response to stress, protein folding, and protein localization to organelles (Fig. 2a). In addition, they are associated with kinase maturation complex 1, spliceosome, HSF1 activation (activates gene expression in response to a variety of stresses), protein processing in the endoplasmic reticulum, VEGFA-VEGFR2 signaling (major pathway that activates angiogenesis), apoptosis-induced DNA fragmentation, and 17S U2 snRNP.
Top 20 enriched pathways and processes among COVID-altered DS-affinity proteins. (a) 140 proteins identified by DS-affinity from Jurkat T-cells. (b) 125 DS-affinity proteins that are altered in SARS-CoV-2 infection. The x-axes show the negative decadic logarithm of the respective pathway’s enrichment P-value.
DS-affinity autoantigen-ome related to COVID-19
To determine how many of the DS-affinity autoAgs identified from Jurkat T-cells are affected by SARS-CoV-2 infection, we searched for them in a multi-omic COVID database compiled by Coronascape.[21–41] Among the 140 DS-affinity proteins identified in our study, 125 (89.3%) are affected by SARS-CoV-2 infection, and at least 94 (of the 125; 75.2%) are known autoAgs (Table 1 and Supplementary Table S1). Among the COVID-altered DS-affinity proteins, 17 are up-regulated only, 35 are down-regulated only, and 71 are altered (up or down depending on study conditions) at protein and/or RNA levels in SARS-CoV-2 infected cells. The COVID database was assembled from different cell and patient tissue types by multiple research laboratories using different technologies, including proteomics, phosphoproteomics, ubiquitinomics, and bulk and single-cell RNA sequencing.
Six DS-affinity proteins are found in the interactomes of SARS-CoV-2 viral proteins, i.e. these host proteins interact directly or indirectly with the viral proteins.[23,34,38] Specifically, HSPA5 (GRP78/BiP) interacts with Nsp2 and Nsp4, HYOU1 interacts with Orf8, PRKCSH and MAPRE1 interact with Orf3, and BZW2 interacts with the viral M protein. HSPA5/BiP (binding immunoglobulin protein) has been consistently identified by DS-affinity in our previous studies, and we have recently reported that DS-BiP association plays important roles in regulating precursor autoreactive B1 cells.[6] HYOU1 (hypoxia up-regulated protein 1) was also found overexpressed at protein level in the urine of COVID-19 patients and up-regulated at mRNA level in B cells from four patients out of a cohort of seven hospitalized COVID-19 patients.[27,42] HYOU1 belongs to the heat shock protein 70 family, accumulates in the endoplasmic reticulum under hypoxic conditions, and has been shown to be up-regulated in tumors. PRKCSH (glucosidase 2 subunit beta) is an N-linked glycan processing enzyme in the endoplasmic reticulum, and mutations of this gene have been associated with autosomal dominant polycystic liver disease. MAPRE1 (microtubule-associated protein RP/EB family member 1) binds the plus-end of microtubules and regulates microtubule cytoskeleton dynamics. BZW2 (basic leucine zipper and W2 domain 2) may be involved in neuronal differentiation and is associated with congenital hypomyelinating neuropathy.
Similar to the 140 DS-affinity protein autoantigen-omes, the 125 COVID-altered DS-affinity proteins are most significantly associated with RNA metabolism and protein folding (Fig. 2b). In addition, they are associated with establishment of protein localization to organelles, kinase maturation complex 1, emerin complex 24, DNA conformation change, spliceosome, cellular response to heat stress, smooth muscle contraction, VEGFA-VESFR2 signaling pathway, prothymosin alpha C5 complex, regulation of protein dephosphorylation, and telomerase pathway (Fig. 2b). Protein–protein interaction network analysis also confirms that the COVID-altered DS-affinity protein network is strongly associated with mRNA processing, translation, chromosome organization, protein processing in the endoplasmic reticulum, CCT/TriC chaperonin, and apoptosis (Fig. 3).
DS-affinity proteins that are altered by SARS-CoV-2 infection. Lines represent protein–protein interactions at high confidence levels. Marked proteins are associated with chromosome organization (25 proteins, red), mRNA processing (17 proteins, pink), translation (13 proteins, aqua), protein processing in endoplasmic reticulum (green, 17 proteins), muscle contraction (nine proteins, blue), TCP-1/cpn60 chaperonin (yellow, eight proteins), and apoptosis (21 proteins, brown).
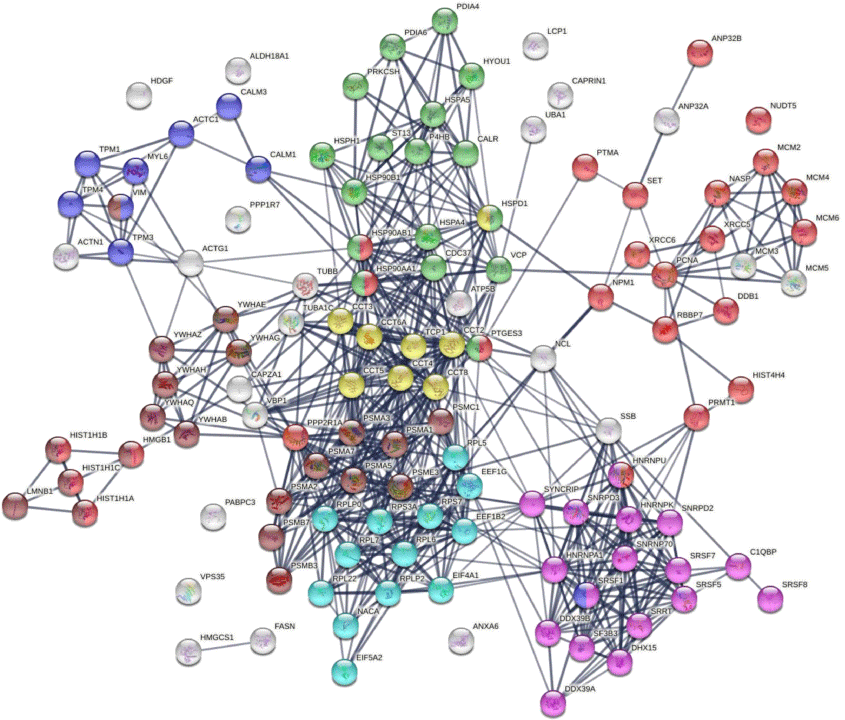
Nine COVID-altered DS-affinity proteins are associated with muscle contraction, including ACTC1, CALM1, CALM3, MYL6, TPM1, TPM3, TPM4, SRSF1, and VIM. All of these proteins are known autoAgs (Table 1). CALM1 has recently been identified as one of the autoAgs in multisystem inflammatory syndrome in children from SARS-CoV-2 infection.[11] Six 14-3-3 proteins are identified, all of which are autoAgs. The presence of 14-3-3 proteins in cerebrospinal fluid, a marker of ongoing neurodegeneration, has been detected in COVID-19 patients.[123]
AutoAgs from altered phosphorylation and ubiquitination
Thirty-eight of the 125 COVID-affected DS-affinity proteins have phosphorylation changes in SARS-CoV-2 infection (Fig. 4). Their molecular functions include histone binding (six proteins), RNA binding (10 proteins), helicase activity (five proteins), ATP binding (12 proteins), DNA binding (14 proteins), and hydrolase activity (11 proteins). These COVID-altered phosphoproteins are significantly associated with gene expression, chromosome organization, and mRNA metabolism. Chromosome-associated proteins are particularly related to DNA conformation change (XRCC6, SET, NPM1, HIST1H1C, HIST1H1B, RBBP7, NASP, and MCMs) and DNA replication (MCM2, MCM3, MCM4, NASP, RBBP7, and SET). mRNA-associated proteins are related to mRNA splicing (SRSF1, SRSF7, SRRT, HNRNPA1, HNRPNK, HNRNPU, and DDX39A) and RNA 3′-end processing (DDX39A, SRSF7, SRSF1, and SSB). In addition, nuclear matrix protein lamin-B1, nucleolar protein nucleolin, vacuolar protein sorting-associated protein VPS35, vimentin, fatty acid synthetase FASN, protein phosphatase 1 regulatory subunit PPP1R7, and HDGF (hepatoma-derived growth factor) are altered by phosphorylation.
DS-affinity proteins that show changes in phosphorylation or ubiquitination in SARS-CoV-2 infection. (a) Phosphorylation: marked proteins are associated with gene expression (15 proteins, red), chromosome organization (13 proteins, green), and ATP binding (12 proteins, blue). (b) Ubiquitination: marked proteins are associated with protein folding (12 proteins, pink), chromosome organization (15 protein, green), translation (six protein, aqua), cytoskeleton (eight proteins, yellow), and apoptosis (10 proteins, brown).
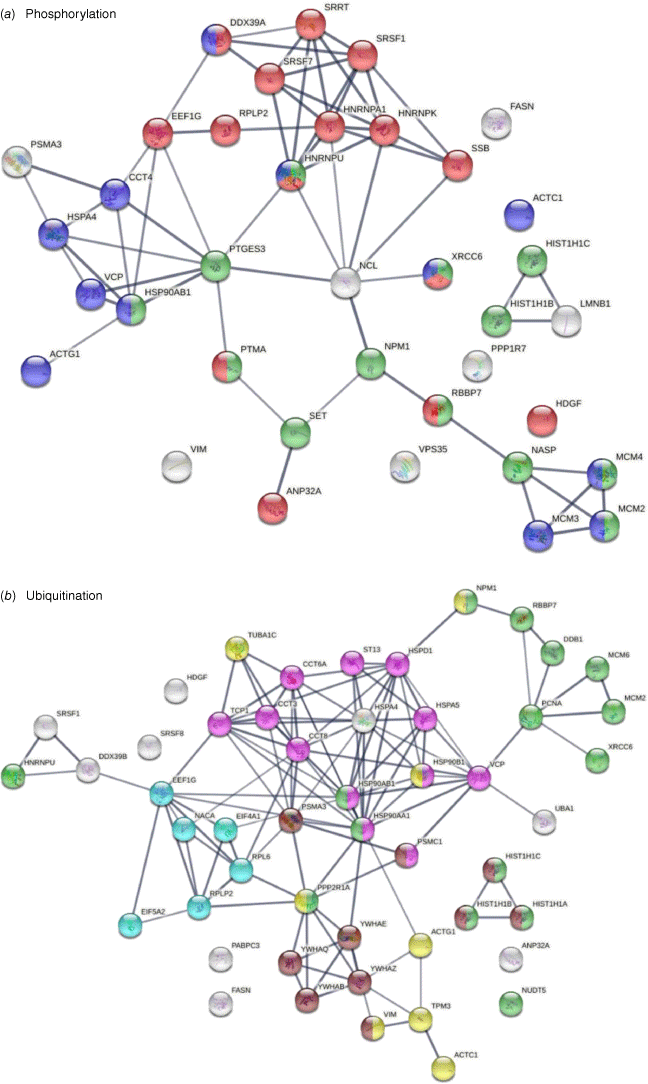
Among the 125 COVID-affected DS-affinity proteins, 50 are altered by ubiquitination in SARS-CoV-2 infection (Fig. 4). These proteins are associated with apoptosis, chromosome organization, protein folding, translation, cell cycle, and cytoskeleton. Proteins related to apoptosis include linker histones (HIST1H1A, HIST1H1B, and HIST1H1C), 14-3-3 proteins (YWHAB, YWHAE, YWHAQ, and YWHAZ), and proteasome proteins (PSMA3and PSMC1). Proteins related to the cell cycle include PNCA, MCM2, MCM6, and 14-3-3 proteins. Five heat shock proteins and four subunits of chaperonin CCT/TriC are ubiquitinated. Other interesting ubiquitinated proteins include NACA (nascent polypeptide-associated complex subunit alpha), DDB1 (DNA damage-binding protein 1), NUDT5 (ADP-sugar pyrophosphatase), and NPM1 (nucleophosmin). Ubiquitination is typically the ‘kiss of death’ modification that marks proteins destined for degradation by the proteasome, although ubiquitination may also modulate protein interaction and activity. Intriguingly, we identified UBA1 (ubiquitin-like modifier-activating enzyme 1), which catalyzes the first step in ubiquitin conjugation and plays a central role in ubiquitination, as a ubiquitination-altered DS-affinity autoAg, which is consistent with our previous studies.[1,2]
DS-affinity proteins altered in T cells of COVID-19 patients
Because Jurkat cells were established from human T-cell lymphoblastic leukemia, we searched for DS-affinity proteins that were altered in T cells of seven COVID-19 patients.[27] Five proteins (LCP1, CALR, HSPA5, HSP90AA1, and HSP90AB1) were up-regulated in CD4+ T cells, and 13 proteins (LCP1, CALR, HSPA5, HSP90AA1, HSP90AB1, HSPD1, HSPH1, MCM4, VIM, PTMA, TUBB, H1-2, and LMNB1) were up-regulated in CD8+ T cells of COVID-19 patients. Three proteins (ACTG1, EEF1B2, and SRSF5) were down-regulated in the CD4+ T cells, and three proteins (ATCG1, EEF1B2, and NACA) were down-regulated in CD8+ T cells. Remarkably, all up-regulated DS-affinity proteins are known autoAgs (Table 1). NACA, ACTG1, and SRSF5, which were down-regulated at the mRNA level, are also known autoAgs. EEF1B2 (or EEF1B, elongation factor 1-beta) has not been identified as an autoAg, although other similar elongation factors such as EEF1A and EF2 are known autoAgs (see references in Table 1).
Among the up-regulated proteins, LCP1 was up-regulated in CD4+ T cells of two patients (out of four patients with available data) and in CD8+ T cells of two patients (out of five patients with available data), with one of the patients having LCP1 up in both CD4+ and CD8+ T cells. Up-regulation of heat shock proteins, particularly HSPA5 and HSP90AA1, was detected in CD4+ T cells of two patients and CD8+ T cells of one patient. MCM4 up-regulation was detected in CD8+ T cells of three out of six patients. Among down-regulated proteins, NACA was detected in CD4+ T cells of one patient and CD8+ T cells of all three patients whose data were available. EEF1B2 was down in CD4+ T cells of three patients (out of five with available data) and down in CD8+ T cells of two out of three patients. ACTG1 down-regulation was detected in CD4+ T cells of two patients and CD8+ T cells of one patient. SRSF5 was down in CD4+ T cells of three out of five patients.
Among these T-cell-altered proteins, LCP1 and NACA are perhaps most interesting. LCP1 (plastin-2, an actin binding protein) has been found to play a significant role in T cell activation in response to co-stimulation through TCR/CD3 and regulates the stability of the immune synapse of naïve and effector T cells.[124] NACA (nascent polypeptide-associated complex subunit alpha) binds to newly synthesized polypeptide chains as they emerge from the ribosome, blocks their interaction with the signal recognition particle, and prevents inappropriate targeting of non-secretory polypeptides to the endoplasmic reticulum. NACA is an IgE autoAg in atopic dermatitis patients with chronic skin manifestations.[125] The significance of these T-cell proteins in COVID-19 and autoimmunity merits further study.
Conclusion
In order to establish a comprehensive COVID-19 autoantigen-ome, we have been profiling autoAgs from different cell and tissue types. Compared to other cells we have examined, Jurkat T-cells contain relatively fewer DS-affinity autoAgs than HFL1 lung fibroblasts, A549 lung epithelial cells, HS-Sultan B-lymphoblasts, and HEp-2 fibroblasts. Although cells share numerous autoAgs, each cell type gives rise to unique COVID-altered autoAg candidates, which may explain the wide range of symptoms experienced by patients with autoimmune sequelae of SARS-CoV-2 infection. We believe that our effort of discovering autoAgs across different cell types provides a comprehensive and valuable autoAg database for better understanding of autoimmune diseases and post-COVID-19 health problems.
Data availability
The data that support this study are available in the article and accompanying online supplementary material. An earlier version of this paper is available as a preprint on bioRxiv (https://doi.org/10.1101/2021.07.05.451199).
Conflicts of interest
JYW is the founder and Chief Scientific Officer of Curandis. MHR is a member of the Scientific Advisory Boards of Trans-Hit Bio (Azenta Life Sciences), Proscia, and Universal DX, but these companies have no relation to the study. MWR and VBR are volunteers for Curandis and have no commercial or financial relationships that could be construed as a potential conflict of interest. WZ has no conflicts of interest to declare.
Declaration of funding
This work was partially supported by Curandis. MHR acknowledges grants from the NIH/NCI (R21 CA251992, R21 CA263262, and U01 CA263986), a Cycle for Survival Equinox Innovation Grant, an Investigator Grant from the Neuroendocrine Tumor Research Foundation (NETRF), and MSKCC Cancer Center Support Grant P30 CA008748. The funding bodies were not involved in the design of the study and the collection, analysis, and interpretation of data.
Acknowledgements
The authors dedicate this paper to Prof. Ed Nice as part of a special issue for the occasion of his 75th birthday. The authors thank Dr. Jung-hyun Rho for technical assistance with experiments. They thank Ross Tomaino and the Taplin Biological Mass Spectrometry facility of Harvard Medical School for expert service with protein sequencing.
References
1 Wang JY, Zhang W, Roehrl MW, Roehrl VB, Roehrl MH. An Autoantigen Atlas from Human Lung HFL1 Cells Offers Clues to Neurological and Diverse Autoimmune Manifestations of COVID-19. Front Immunol 2022; 13: 831849.
| Crossref | Google Scholar | PubMed |
2 Wang JY, Zhang W, Roehrl MW, Roehrl VB, Roehrl MH. An Autoantigen Profile of Human A549 Lung Cells Reveals Viral and Host Etiologic Molecular Attributes of Autoimmunity in COVID-19. J Autoimmun 2021; 120: 102644.
| Crossref | Google Scholar | PubMed |
3 Wang JY, Zhang W, Roehrl VB, Roehrl MW, Roehrl MH. An Autoantigen-ome from HS-Sultan B-Lymphoblasts Offers a Molecular Map for Investigating Autoimmune Sequelae of COVID-19. Aust J Chem 2023;
| Crossref | Google Scholar |
4 Wang JY, Lee J, Yan M, Rho J-h, Roehrl MHA. Dermatan sulfate interacts with dead cells and regulates CD5+ B-cell fate: implications for a key role in autoimmunity. Am J Pathol 2011; 178(5): 2168-76.
| Crossref | Google Scholar |
5 Rho J-h, Zhang W, Murali M, Roehrl MHA, Wang JY. Human proteins with affinity for dermatan sulfate have the propensity to become autoantigens. Am J Pathol 2011; 178(5): 2177-90.
| Crossref | Google Scholar |
6 Lee J, Rho J-h, Roehrl MH, Wang JY. Dermatan Sulfate Is a Potential Master Regulator of IgH via Interactions with Pre-BCR, GTF2I, and BiP ER Complex in Pre-B Lymphoblasts [Preprint]. bioRxiv 2021; 12: 680212.
| Crossref | Google Scholar |
7 Wang JY, Zhang W, Rho J-h, Roehrl MW, Roehrl MH. A proteomic repertoire of autoantigens identified from the classic autoantibody clinical test substrate HEp-2 cells. Clin Proteomics 2020; 17: 35.
| Crossref | Google Scholar |
8 Zhang W, Rho J-h, Roehrl MH, Wang JY. A comprehensive autoantigen-ome of autoimmune liver diseases identified from dermatan sulfate affinity enrichment of liver tissue proteins. BMC Immunol 2019; 20(1): 21.
| Crossref | Google Scholar |
9 Zhang W, Rho J-h, Roehrl MW, Roehrl MH, Wang JY. A repertoire of 124 potential autoantigens for autoimmune kidney diseases identified by dermatan sulfate affinity enrichment of kidney tissue proteins. PLoS One 2019; 14(6): e0219018.
| Crossref | Google Scholar |
10 Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020; 183(4): 968-81.e7.
| Crossref | Google Scholar |
11 Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020; 183(4): 982-95.e14.
| Crossref | Google Scholar |
12 Gagiannis D, Steinestel J, Hackenbroch C, Schreiner B, Hannemann M, Bloch W, et al. Clinical, Serological, and Histopathological Similarities Between Severe COVID-19 and Acute Exacerbation of Connective Tissue Disease-Associated Interstitial Lung Disease (CTD-ILD). Front Immunol 2020; 11: 587517.
| Crossref | Google Scholar |
13 Zhou Y, Han T, Chen J, Hou C, Hua L, He S, et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin Transl Sci 2020; 13(6): 1077-86.
| Crossref | Google Scholar |
14 Fujii H, Tsuji T, Yuba T, Tanaka S, Suga Y, Matsuyama A, et al. High levels of anti-SSA/Ro antibodies in COVID-19 patients with severe respiratory failure: a case-based review. Clin Rheumatol 2020; 39(11): 3171-5.
| Crossref | Google Scholar |
15 Sacchi MC, Tamiazzo S, Stobbione P, Agatea L, De Gaspari P, Stecca A, et al. SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci 2021; 14: 898-907.
| Crossref | Google Scholar |
16 Franke C, Ferse C, Kreye J, Reincke SM, Sanchez-Sendin E, Rocco A, et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun 2021; 93: 415-9.
| Crossref | Google Scholar |
17 Zuo Y, Yalavarthi S, Navaz S, Hoy C, Harbaugh A, Gockman K, et al. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. JCI Insight 2021; 6(15): e150111.
| Crossref | Google Scholar | PubMed |
18 Chang SE, Feng A, Meng W, Apostolidis SA, Mack E, Artandi M, et al. New-Onset IgG Autoantibodies in Hospitalized Patients with COVID-19. Nat Commun 2021; 12(1): 5417.
| Crossref | Google Scholar | PubMed |
19 Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (New York, NY) 2020; 370(6515): eabd4585.
| Crossref | Google Scholar |
20 Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol 2020; 268: 751-7.
| Crossref | Google Scholar |
21 Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 2019; 10(1): 1523.
| Crossref | Google Scholar |
22 Zhang JY, Wang XM, Xing X, Xu Z, Zhang C, Song JW, et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol 2020; 21(9): 1107-18.
| Crossref | Google Scholar |
23 Davies JP, Almasy KM, McDonald EF, Plate L. Comparative Multiplexed Interactomics of SARS-CoV-2 and Homologous Coronavirus Nonstructural Proteins Identifies Unique and Shared Host-Cell Dependencies. ACS Infect Dis 2020; 6(12): 3174-89.
| Crossref | Google Scholar |
24 Klann K, Bojkova D, Tascher G, Ciesek S, Münch C, Cinatl J. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Mol Cell 2020; 80(1): 164-74.e4.
| Crossref | Google Scholar |
25 Sun J, Ye F, Wu A, Yang R, Pan M, Sheng J, et al. Comparative Transcriptome Analysis Reveals the Intensive Early Stage Responses of Host Cells to SARS-CoV-2 Infection. Front Microbiol 2020; 11: 593857.
| Crossref | Google Scholar |
26 Bojkova D, Klann K, Koch B, Widera M, Krause D, Ciesek S, et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020; 583(7816): 469-72.
| Crossref | Google Scholar |
27 Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martínez-Colón GJ, McKechnie JL, et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med 2020; 26(7): 1070-6.
| Crossref | Google Scholar |
28 Lieberman NAP, Peddu V, Xie H, Shrestha L, Huang ML, Mears MC, et al. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLoS Biol 2020; 18(9): e3000849.
| Crossref | Google Scholar |
29 Riva L, Yuan S, Yin X, Martin-Sancho L, Matsunaga N, Pache L, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 2020; 586(7827): 113-9.
| Crossref | Google Scholar |
30 Bouhaddou M, Memon D, Meyer B, White KM, Rezelj VV, Correa Marrero M, et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell 2020; 182(3): 685-712.e19.
| Crossref | Google Scholar |
31 Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020; 181(5): 1036-45.e9.
| Crossref | Google Scholar |
32 Shen B, Yi X, Sun Y, Bi X, Du J, Zhang C, et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020; 182(1): 59-72.e15.
| Crossref | Google Scholar |
33 Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Science (New York, NY) 2020; 369(6499): 50-4.
| Crossref | Google Scholar |
34 Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020; 583(7816): 459-68.
| Crossref | Google Scholar |
35 Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 2020; 9(1): 761-70.
| Crossref | Google Scholar |
36 Vanderheiden A, Ralfs P, Chirkova T, Upadhyay AA, Zimmerman MG, Bedoya S, et al. Type I and Type III Interferons Restrict SARS-CoV-2 Infection of Human Airway Epithelial Cultures. J Virol 2020; 94(19): e00985-20.
| Crossref | Google Scholar |
37 Appelberg S, Gupta S, Svensson Akusjärvi S, Ambikan AT, Mikaeloff F, Saccon E, et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg Microbes Infect 2020; 9(1): 1748-60.
| Crossref | Google Scholar |
38 Stukalov A, Girault V, Grass V, Bergant V, Karayel O, Urban C, et al. Multilevel proteomics reveals host perturbations by SARS-CoV-2 and SARS-CoV. Nature 2021; 594(7862): 246-252.
| Crossref | Google Scholar |
39 Wyler E, Mösbauer K, Franke V, Diag A, Gottula LT, Arsiè R, et al. Bulk and single-cell gene expression profiling of SARS-CoV-2 infected human cell lines identifies molecular targets for therapeutic intervention. iScience 2021; 24(3): 102151.
| Crossref | Google Scholar |
40 Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26(6): 842-4.
| Crossref | Google Scholar |
41 Laurent EMN, Sofianatos Y, Komarova A, Gimeno J-P, Tehrani PS, Kim D-K, et al. Global BioID-based SARS-CoV-2 proteins proximal interactome unveils novel ties between viral polypeptides and host factors involved in multiple COVID19-associated mechanisms [Preprint]. bioRxiv 2020; 2020.08.28.272955.
| Crossref | Google Scholar |
42 Li Y, Wang Y, Liu H, Sun W, Ding B, Zhao Y, et al. Urine proteome of COVID-19 patients. URINE (Amst) 2020; 2: 1-8.
| Crossref | Google Scholar |
43 Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019; 47(D1): D607-13.
| Crossref | Google Scholar |
44 Chatterjee D, Pieroni M, Fatah M, Charpentier F, Cunningham KS, Spears DA, et al. An autoantibody profile detects Brugada syndrome and identifies abnormally expressed myocardial proteins. Eur Heart J 2020; 41(30): 2878-90.
| Crossref | Google Scholar |
45 Vainio E, Lenoir GM, Franklin RM. Autoantibodies in three populations of Burkitt's lymphoma patients. Clin Exp Immunol 1983; 54(2): 387-96.
| Google Scholar |
46 Pandey S, Dioni I, Lambardi D, Real-Fernandez F, Peroni E, Pacini G, et al. Alpha actinin is specifically recognized by Multiple Sclerosis autoantibodies isolated using an N-glucosylated peptide epitope. Mol Cell Proteomics 2013; 12(2): 277-82.
| Crossref | Google Scholar |
47 Pott MC, Frede N, Wanders J, Hammarström L, Glocker EO, Glocker C, et al. Autoantibodies against BAFF, APRIL or IL21 - an alternative pathogenesis for antibody-deficiencies? BMC Immunol 2017; 18(1): 34.
| Crossref | Google Scholar |
48 Seko Y, Matsumoto A, Fukuda T, Imai Y, Fujimura T, Taka H, et al. A case of neonatal lupus erythematosus presenting delayed dilated cardiomyopathy with circulating autoantibody to annexin A6. Int Heart J 2007; 48(3): 407-15.
| Crossref | Google Scholar |
49 Creaney J, Dick IM, Yeoman D, Wong S, Robinson BWS. Auto-antibodies to β-F1-ATPase and vimentin in malignant mesothelioma. PLoS One 2011; 6(10): e26515.
| Crossref | Google Scholar |
50 Ikeda Y, Toda G, Hashimoto N, Maruyama T, Oka H. Antibody that recognizes conformations of calmodulin in the serum from patient with chronic active hepatitis. Biochem Biophys Res Commun 1987; 144(1): 191-7.
| Crossref | Google Scholar |
51 Boehm J, Orth T, Van Nguyen P, Söling H-D. Systemic lupus erythematosus is associated with increased auto-antibody titers against calreticulin and Grp94, but calreticulin is not the Ro/SS-A antigen. Eur J Clin Invest 1994; 24(4): 248-57.
| Crossref | Google Scholar |
52 Matsuo K, Xiang Y, Nakamura H, Masuko K, Yudoh K, Noyori K, et al. Identification of novel citrullinated autoantigens of synovium in rheumatoid arthritis using a proteomic approach. Arthritis Res Ther 2006; 8(6): R175.
| Crossref | Google Scholar |
53 Yokota S-i, Hirata D, Minota S, Higashiyama T, Kurimoto M, Yanagi H, et al. Autoantibodies against chaperonin CCT in human sera with rheumatic autoimmune diseases: comparison with antibodies against other Hsp60 family proteins. Cell Stress Chaperones 2000; 5(4): 337-46.
| Crossref | Google Scholar |
54 Hirai K, Maeda H, Omori K, Yamamoto T, Kokeguchi S, Takashiba S. Serum antibody response to group II chaperonin from Methanobrevibacter oralis and human chaperonin CCT. Pathog Dis 2013; 68(1): 12-9.
| Crossref | Google Scholar |
55 Pagaza-Straffon C, Marchat LA, Herrera L, Díaz-Chávez J, Avante MG, Rodríguez YP, et al. Evaluation of a panel of tumor-associated antigens in breast cancer. Cancer Biomark 2020; 27(2): 207-11.
| Crossref | Google Scholar |
56 Heo CK, Woo MK, Yu DY, Lee JY, Yoo JS, Yoo HS, et al. Identification of autoantibody against fatty acid synthase in hepatocellular carcinoma mouse model and its application to diagnosis of HCC. Int J Oncol 2010; 36(6): 1453-9.
| Crossref | Google Scholar |
57 Rosenberg AM, Cordeiro DM. Relationship between sex and antibodies to high mobility group proteins 1 and 2 in juvenile idiopathic arthritis. J Rheumatol 2000; 27(10): 2489-93.
| Google Scholar |
58 Stemmer C, Tuaillon N, Prieur AM, Muller S. Mapping of B-cell epitopes recognized by antibodies to histones in subsets of juvenile chronic arthritis. Clin Immunol Immunopathol 1995; 76(1): 82-9.
| Crossref | Google Scholar |
59 Wesierska-Gadek J, Penner E, Lindner H, Hitchman E, Sauermann G. Autoantibodies against different histone H1 subtypes in systemic lupus erythematosus sera. Arthritis Rheum 1990; 33(8): 1273-8.
| Crossref | Google Scholar |
60 Kwon YS, Chung J, Shin GT, Lee SY, Jang YJ. Variable region genes of human monoclonal autoantibodies to histones H2A and H2B from a systemic lupus erythematosus patient. Mol Immunol 2005; 42(3): 311-7.
| Crossref | Google Scholar |
61 Dieker J, Berden JH, Bakker M, Briand JP, Muller S, Voll R, et al. Autoantibodies against Modified Histone Peptides in SLE Patients Are Associated with Disease Activity and Lupus Nephritis. PLoS One 2016; 11(10): e0165373.
| Crossref | Google Scholar |
62 Ricotti GCBA, Bestagno M, Cerino A, Negri C, Caporali R, Cobianchi F, et al. Antibodies to hnRNP core protein A1 in connective tissue diseases. J Cell Biochem 1989; 40(1): 43-7.
| Crossref | Google Scholar |
63 Vencovský J, Kafková J, Staněk D, Raška I. Heterogenous nuclear RNP C1 and C2 core proteins are targets for an autoantibody found in the serum of a patient with systemic sclerosis and psoriatic arthritis. Arthritis Rheum 1997; 40(12): 2172-7.
| Crossref | Google Scholar |
64 Zhang J-Y, Chan EKL, Peng X-X, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med 1999; 189(7): 1101-10.
| Crossref | Google Scholar |
65 Britton S, Froment C, Frit P, Monsarrat B, Salles B, Calsou P. Cell nonhomologous end joining capacity controls SAF-A phosphorylation by DNA-PK in response to DNA double-strand breaks inducers. Cell Cycle 2009; 8(22): 3717-22.
| Crossref | Google Scholar |
66 Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, et al. Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheum 2013; 65(4): 869-79.
| Crossref | Google Scholar |
67 Pashov A, Kenderov A, Kyurkchiev S, Kehayov I, Hristova S, Lacroix-Desmazes S, et al. Autoantibodies to heat shock protein 90 in the human natural antibody repertoire. Int Immunol 2002; 14(5): 453-61.
| Crossref | Google Scholar |
68 Qin HY, Mahon JL, Atkinson MA, Chaturvedi P, Lee-Chan E, Singh B. Type 1 diabetes alters anti-hsp90 autoantibody isotype. J Autoimmun 2003; 20(3): 237-45.
| Crossref | Google Scholar |
69 Tishler M, Shoenfeld Y. Anti-heat-shock protein antibodies in rheumatic and autoimmune diseases. Semin Arthritis Rheum 1996; 26(2): 558-63.
| Crossref | Google Scholar |
70 Bläß S, Union A, Raymackers J, Schumann F, Ungethüm U, Müller-Steinbach S, et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum 2001; 44(4): 761-71.
| Crossref | Google Scholar |
71 Horváth L, Cervenak L, Oroszlán M, Prohászka Z, Uray K, Hudecz F, et al. Antibodies against different epitopes of heat-shock protein 60 in children with type 1 diabetes mellitus. Immunol Lett 2002; 80(3): 155-62.
| Crossref | Google Scholar |
72 Minohara M. [Heat shock protein 105 in multiple sclerosis]. Nihon Rinsho 2003; 61(8): 1317-22.
| Google Scholar |
73 Kobayashi T, Yura T, Yanagi H. The increment of anti-ORP150 autoantibody in initial stages of atheroma in high-fat diet fed mice. J Vet Med Sci 2002; 64(2): 177-80.
| Crossref | Google Scholar |
74 Ola TO, Biro PA, Hawa MI, Ludvigsson J, Locatelli M, Puglisi MA, et al. Importin beta: a novel autoantigen in human autoimmunity identified by screening random peptide libraries on phage. J Autoimmun 2006; 26(3): 197-207.
| Crossref | Google Scholar |
75 Ueda K, Nakanishi T, Shimizu A, Takubo T, Matsuura N. Identification of L-plastin autoantibody in plasma of patients with non-Hodgkin's lymphoma using a proteomics-based analysis. Ann Clin Biochem 2008; 45(1): 65-9.
| Crossref | Google Scholar |
76 Senécal J-L, Rauch J, Grodzicky T, Raynauld J-P, Uthman I, Nava A, et al. Strong association of autoantibodies to human nuclear lamin B1 with lupus anticoagulant antibodies in systemic lupus erythematosus. Arthritis Rheum 1999; 42(7): 1347-53.
| Google Scholar |
77 Frampton G, Moriya S, Pearson JD, Isenberg DA, Ward FJ, Smith TA, et al. Identification of candidate endothelial cell autoantigens in systemic lupus erythematosus using a molecular cloning strategy: a role for ribosomal P protein P0 as an endothelial cell autoantigen. Rheumatology (Oxford, England) 2000; 39(10): 1114-20.
| Crossref | Google Scholar |
78 Bledzhyants DA, Muratov RM, Movsesyan RR, Podlubnaya ZA. Autoantibodies to myosin light chains in the blood as early marker of myocardial injury after aortocoronary bypass surgery. Bull Exp Biol Med 2007; 144(2): 241-5.
| Crossref | Google Scholar |
79 Batova IN, Richardson RT, Widgren EE, O'Rand MG. Analysis of the autoimmune epitopes on human testicular NASP using recombinant and synthetic peptides. Clin Exp Immunol 2000; 121(2): 201-9.
| Crossref | Google Scholar |
80 Qin Z, Lavingia B, Zou Y, Stastny P. Antibodies against nucleolin in recipients of organ transplants. Transplantation 2011; 92(7): 829-35.
| Crossref | Google Scholar |
81 Ulanet DB, Torbenson M, Dang CV, Casciola-Rosen L, Rosen A. Unique conformation of cancer autoantigen B23 in hepatoma: a mechanism for specificity in the autoimmune response. Proc Natl Acad Sci U S A 2003; 100(21): 12361-6.
| Crossref | Google Scholar |
82 Nagayama S, Yokoi T, Tanaka H, Kawaguchi Y, Shirasaka T, Kamataki T. Occurrence of autoantibody to protein disulfide isomerase in patients with hepatic disorder. J Toxicol Sci 1994; 19(3): 163-9.
| Crossref | Google Scholar |
83 Takasaki Y, Kaneda K, Matsushita M, Yamada H, Nawata M, Matsudaira R, et al. Glyceraldehyde 3-phosphate dehydrogenase is a novel autoantigen leading autoimmune responses to proliferating cell nuclear antigen multiprotein complexes in lupus patients. Int Immunol 2004; 16(9): 1295-304.
| Crossref | Google Scholar |
84 Gut J, Christen U, Frey N, Koch V, Stoffler D. Molecular mimicry in halothane hepatitis: biochemical and structural characterization of lipoylated autoantigens. Toxicology 1995; 97(1–3): 199-224.
| Crossref | Google Scholar |
85 Mojtahedi Z, Safaei A, Yousefi Z, Ghaderi A. Immunoproteomics of HER2-positive and HER2-negative breast cancer patients with positive lymph nodes. OMICS 2011; 15(6): 409-18.
| Crossref | Google Scholar |
86 Mayo I, Arribas J, Villoslada P, Alvarez DoForno R, Rodriguez-Vilarino S, Montalban X, et al. The proteasome is a major autoantigen in multiple sclerosis. Brain 2002; 125(12): 2658-67.
| Crossref | Google Scholar |
87 Feist E, Kuckelkorn U, Dörner T, Dönitz H, Scheffler S, Hiepe F, et al. Autoantibodies in primary Sjögren’s syndrome are directed against proteasomal subunits of the α and β type. Arthritis Rheum 1999; 42(4): 697-702.
| Crossref | Google Scholar |
88 Bohring C, Krause W. Characterization of spermatozoa surface antigens by antisperm antibodies and its influence on acrosomal exocytosis. Am J Reprod Immunol 2003; 50(5): 411-9.
| Crossref | Google Scholar |
89 Sugimoto K, Hiwasa T, Shibuya K, Hirano S, Beppu M, Isose S, et al. Novel autoantibodies against the proteasome subunit PSMA7 in amyotrophic lateral sclerosis. J Neuroimmunol 2018; 325: 54-60.
| Crossref | Google Scholar |
90 Roessler M, Rollinger W, Mantovani-Endl L, Hagmann ML, Palme S, Berndt P, et al. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics 2006; 5(11): 2092-101.
| Crossref | Google Scholar |
91 Vlachoyiannopoulos PG, Frillingos S, Tzioufas AG, Seferiadis K, Moutsopoulos HM, Tsolas O. Circulating antibodies to prothymosin α in systemic lupus erythematosus. Clin Immunol Immunopathol 1989; 53(2): 151-60.
| Crossref | Google Scholar |
92 Yamasaki Y, Narain S, Hernandez L, Barker T, Ikeda K, Segal MS, et al. Autoantibodies against the replication protein A complex in systemic lupus erythematosus and other autoimmune diseases. Arthritis Res Ther 2006; 8(4): R111.
| Crossref | Google Scholar |
93 Luna Coronell JA, Sergelen K, Hofer P, Gyurján I, Brezina S, Hettegger P, et al. The Immunome of Colon Cancer: Functional In Silico Analysis of Antigenic Proteins Deduced from IgG Microarray Profiling. Genom Proteom Bioinform 2018; 16(1): 73-84.
| Crossref | Google Scholar |
94 Guialis A, Patrinou-Georgoula M, Tsifetaki N, Aidinis V, Sekeris CE, Molitsopouios HM. Anti-5S RNA/protein (RNP) antibody levels correlate with disease activity in a patient with systemic lupus erythematosus (SLE) nephritis. Clin Exp Immunol 1994; 95(3): 385-9.
| Crossref | Google Scholar |
95 Gerli R, Caponi L. Anti-ribosomal P protein antibodies. Autoimmunity 2005; 38(1): 85-92.
| Crossref | Google Scholar |
96 Elkon K, Bonfa E, Llovet R, Danho W, Weissbach H, Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A 1988; 85(14): 5186-9.
| Crossref | Google Scholar |
97 Chai Z, Sarcevic B, Mawson A, Toh BH. SET-related cell division autoantigen-1 (CDA1) arrests cell growth. J Biol Chem 2001; 276(36): 33665-74.
| Crossref | Google Scholar |
98 Hof D, Cheung K, de Rooij DJ, van den Hoogen FH, Pruijn GJ, van Venrooij WJ, et al. Autoantibodies specific for apoptotic U1-70K are superior serological markers for mixed connective tissue disease. Arthritis Res Ther 2005; 7(2): R302-9.
| Crossref | Google Scholar |
99 McClain MT, Ramsland PA, Kaufman KM, James JA. Anti-sm autoantibodies in systemic lupus target highly basic surface structures of complexed spliceosomal autoantigens. J Immunol (Baltimore, MD: 1950) 2002; 168(4): 2054-62.
| Crossref | Google Scholar |
100 Brahms H, Raymackers J, Union A, de Keyser F, Meheus L, Lührmann R. The C-terminal RG dipeptide repeats of the spliceosomal Sm proteins D1 and D3 contain symmetrical dimethylarginines, which form a major B-cell epitope for anti-Sm autoantibodies. J Biol Chem 2000; 275(22): 17122-9.
| Crossref | Google Scholar |
101 Iizuka N, Okamoto K, Matsushita R, Kimura M, Nagai K, Arito M, et al. Identification of autoantigens specific for systemic lupus erythematosus with central nervous system involvement. Lupus 2010; 19(6): 717-26.
| Crossref | Google Scholar |
102 Overzet K, Gensler TJ, Kim SJ, Geiger ME, van Venrooij WJ, Pollard KM, et al. Small nucleolar RNP scleroderma autoantigens associate with phosphorylated serine/arginine splicing factors during apoptosis. Arthritis Rheum 2000; 43(6): 1327-36.
| Crossref | Google Scholar |
103 Gordon P, Khamashta MA, Rosenthal E, Simpson JM, Sharland G, Brucato A, et al. Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheumatol 2004; 31(12): 2480-7.
| Google Scholar |
104 Cortini A, Bembich S, Marson L, Cocco E, Edomi P. Identification of novel non-myelin biomarkers in multiple sclerosis using an improved phage-display approach. PLoS One 2019; 14(12): e0226162.
| Crossref | Google Scholar |
105 Geng X, Biancone L, Dai HH, Lin JJ-C, Yoshizaki N, Dasgupta A, et al. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology 1998; 114(5): 912-22.
| Crossref | Google Scholar |
106 Mahesh SP, Li Z, Buggage R, Mor F, Cohen IR, Chew EY, et al. Alpha tropomyosin as a self-antigen in patients with Behçet’s disease. Clin Exp Immunol 2005; 140(2): 368-75.
| Crossref | Google Scholar |
107 Kimura A, Sakurai T, Yamada M, Koumura A, Hayashi Y, Tanaka Y, et al. Anti-endothelial cell antibodies in patients with cerebral small vessel disease. Curr Neurovasc Res 2012; 9(4): 296-301.
| Crossref | Google Scholar |
108 Zhao X, Cheng Y, Gan Y, Jia R, Zhu L, Sun X. Anti-tubulin-α-1C autoantibody in systemic lupus erythematosus: a novel indicator of disease activity and vasculitis manifestations. Clin Rheumatol 2018; 37(5): 1229-37.
| Crossref | Google Scholar |
109 Kimura A, Yoshikura N, Koumura A, Hayashi Y, Kobayashi Y, Kobayashi I, et al. Identification of target antigens of naturally occurring autoantibodies in cerebrospinal fluid. J Proteomics 2015; 128: 450-7.
| Crossref | Google Scholar |
110 Betteridge ZE, Gunawardena H, Chinoy H, North J, Ollier WER, Cooper RG, et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann Rheum Dis 2009; 68(10): 1621-5.
| Crossref | Google Scholar |
111 Miyachi K, Hosaka H, Nakamura N, Miyakawa H, Mimori T, Shibata M, et al. Anti-p97/VCP antibodies: an autoantibody marker for a subset of primary biliary cirrhosis patients with milder disease? Scand J Immunol 2006; 63(5): 376-82.
| Crossref | Google Scholar |
112 Bang H, Egerer K, Gauliard A, Lüthke K, Rudolph PE, Fredenhagen G, et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum 2007; 56(8): 2503-11.
| Crossref | Google Scholar |
113 Mao J, Ladd J, Gad E, Rastetter L, Johnson MM, Marzbani E, et al. Mining the pre-diagnostic antibody repertoire of TgMMTV-neu mice to identify autoantibodies useful for the early detection of human breast cancer. J Transl Med 2014; 12: 121.
| Crossref | Google Scholar |
114 Wen J, Yaneva M. Mapping of epitopes on the 86 kDa subunit of the Ku autoantigen. Mol Immunol 1990; 27(10): 973-80.
| Crossref | Google Scholar |
115 Hoa S, Hudson M, Troyanov Y, Proudman S, Walker J, Stevens W, et al. Single-specificity anti-Ku antibodies in an international cohort of 2140 systemic sclerosis subjects: clinical associations. Medicine (Baltimore) 2016; 95(35): e4713.
| Crossref | Google Scholar |
116 Kistner A, Bigler MB, Glatz K, Egli SB, Baldin FS, Marquardsen FA, et al. Characteristics of autoantibodies targeting 14-3-3 proteins and their association with clinical features in newly diagnosed giant cell arteritis. Rheumatology (Oxford, England) 2017; 56(5): 829-34.
| Crossref | Google Scholar |
117 van Beers-Tas MH, Marotta A, Boers M, Maksymowych WP, van Schaardenburg D. A prospective cohort study of 14-3-3η in ACPA and/or RF-positive patients with arthralgia. Arthritis Res Ther 2016; 18: 76.
| Crossref | Google Scholar |
118 Qiu J, Choi G, Li L, Wang H, Pitteri SJ, Pereira-Faca SR, et al. Occurrence of autoantibodies to annexin I, 14-3-3 theta and LAMR1 in prediagnostic lung cancer sera. J Clin Oncol 2008; 26(31): 5060-6.
| Crossref | Google Scholar |
119 Chakravarti R, Gupta K, Swain M, Willard B, Scholtz J, Svensson LG, et al. 14-3-3 in Thoracic Aortic Aneurysms: Identification of a Novel Autoantigen in Large Vessel Vasculitis. Arthritis Rheumatol (Hoboken, NJ) 2015; 67(7): 1913-21.
| Crossref | Google Scholar |
120 Imai H, Chan EK, Kiyosawa K, Fu XD, Tan EM. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J Clin Invest 1993; 92(5): 2419-26.
| Crossref | Google Scholar |
121 Beutgen VM, Schmelter C, Pfeiffer N, Grus FH. Autoantigens in the trabecular meshwork and glaucoma-specific alterations in the natural autoantibody repertoire. Clin Transl Immunol 2020; 9(3): e01101.
| Crossref | Google Scholar |
122 Becker A, Ludwig N, Keller A, Tackenberg B, Eienbröker C, Oertel WH, et al. Myasthenia gravis: analysis of serum autoantibody reactivities to 1827 potential human autoantigens by protein macroarrays. PLoS One 2013; 8(3): e58095.
| Crossref | Google Scholar |
123 Alexopoulos H, Magira E, Bitzogli K, Kafasi N, Vlachoyiannopoulos P, Tzioufas A, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm 2020; 7(6): e893.
| Crossref | Google Scholar |
124 Wabnitz G, Balta E, Samstag Y. L-plastin regulates the stability of the immune synapse of naive and effector T-cells. Adv Biol Regul 2017; 63: 107-14.
| Crossref | Google Scholar |
125 Mossabeb R, Seiberler S, Mittermann I, Natter S, Kraft D, Valenta R, et al. Characterization of a novel isoform of α-nascent polypeptide-associated complex as IgE-defined autoantigen. J Invest Dermatol 2002; 119(4): 820-9.
| Crossref | Google Scholar |


