Floral constituents of the Australian tar tree, Semecarpus australiensis
Soo Jean Park A B * , Jodie Cheesman C , Donald N. S. Cameron A B , Stefano G. De Faveri C and Phillip W. Taylor A B
A B * , Jodie Cheesman C , Donald N. S. Cameron A B , Stefano G. De Faveri C and Phillip W. Taylor A B
A Applied BioSciences, Macquarie University, Sydney, 2109, Australia.
B Australian Research Council Centre for Fruit Fly Biosecurity Innovation, Macquarie University, North Ryde, NSW 2109, Australia.
C Horticulture and Forestry Science, Queensland Department of Agriculture and Fisheries, Mareeba, Qld, Australia.
Australian Journal of Chemistry 75(12) 945-952 https://doi.org/10.1071/CH22097
Submitted: 27 April 2022 Accepted: 22 September 2022 Published: 23 November 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Floral constituents of the Australian tar tree, Semecarpus australiensis, distributed in Melanesia and Northern Australia, were extracted with solvent, and analyzed by gas chromatography-mass spectrometry. The main constituents were 16- and 18-carbon fatty acids and their ethyl esters. Amongst the 67 identified compounds, zingerone was detected in minute quantity, providing the chemical basis for previous observations of fruit fly attraction to the flowers. The present study is the first to report the chemical profile of tar tree flowers.
Keywords: fatty acids, floral volatiles, fruit fly, GC-MS, isoeugenol, native cashew, salicylates, Tephritidae.
Introduction
The flowers of many orchid species of the genus Bulbophyllum contain phenylpropanoids or phenylbutanoids that attract male Bactrocera and Zeugodacus fruit flies (Diptera, Tephritidae).[1] Their most common responses are to raspberry ketone and methyl eugenol, although many species do not respond to either of these compounds or respond only weakly.[2] More recent studies have found that some Bactrocera and Zeugodacus species respond to zingerone,[1a,1d,3] which occurs as a floral scent in Bu. patens and Bu. baileyi.[1a,1b,1d] In addition, isoeugenol and methyl isoeugenol have been found to attract some species that are non-responsive or weakly responsive to raspberry ketone and methyl eugenol.[3c,3d,4] Isoeugenol and methyl isoeugenol occur in the essential oils of various plant taxa, including different species of Citrus, Clusia, Pimenta, and Etlingera.[5] Reasons for the attraction of male fruit flies to phenylpropanoids and phenylbutanoids are incompletely understood,[6] and vary across compounds and fruit fly species. For example, males of some species of Bactrocera acquire methyl eugenol as a pheromone precursor[7] or as a metabolic enhancer to increase mating competitiveness. [8] In return, Bulbophyllum orchids benefit through pollination,[1a,1b,1f] hence the interaction between the orchids and fruit flies is mutually beneficial.
In an evolutionarily unrelated interaction, the flowers of Passiflora maliformis release zingerone from their filaments and attract Bactrocera jarvisi.[9] Earlier records report that B. jarvisi is also attracted to flowers of the tar tree, also known as native cashew, Semecarpus australiensis, as well as to Bu. baileyi.[10] Zingerone in Bu. baileyi is responsible for attraction of B. jarvisi.[10b]
The tar tree grows naturally in rainforests, including the northeast part of Queensland and Northern Territory in Australia, and across a wide range in Melanesia including Torres Strait Islands, New Guinea, New Britain, and Aru Islands.[11] The tar tree produces cream-colored flowers in spring. Staminate (male) flowers, about 1.5 mm long, are sessile, while pistillate (female) flowers, about 4 mm long, are on pedicels. To date, the chemistry of tar tree flowers is unknown. The present study aims to (1) characterize the compounds in the tar tree flowers and (2) confirm the chemical basis of previous observations on the attraction of B. jarvisi males. The present study describes constituents in the solvent extracts of both staminate and pistillate tar tree flowers analyzed by gas chromatography-mass spectrometry (GC-MS) and identifies the chemical basis for the attraction of fruit flies to these flowers.
Results and discussion
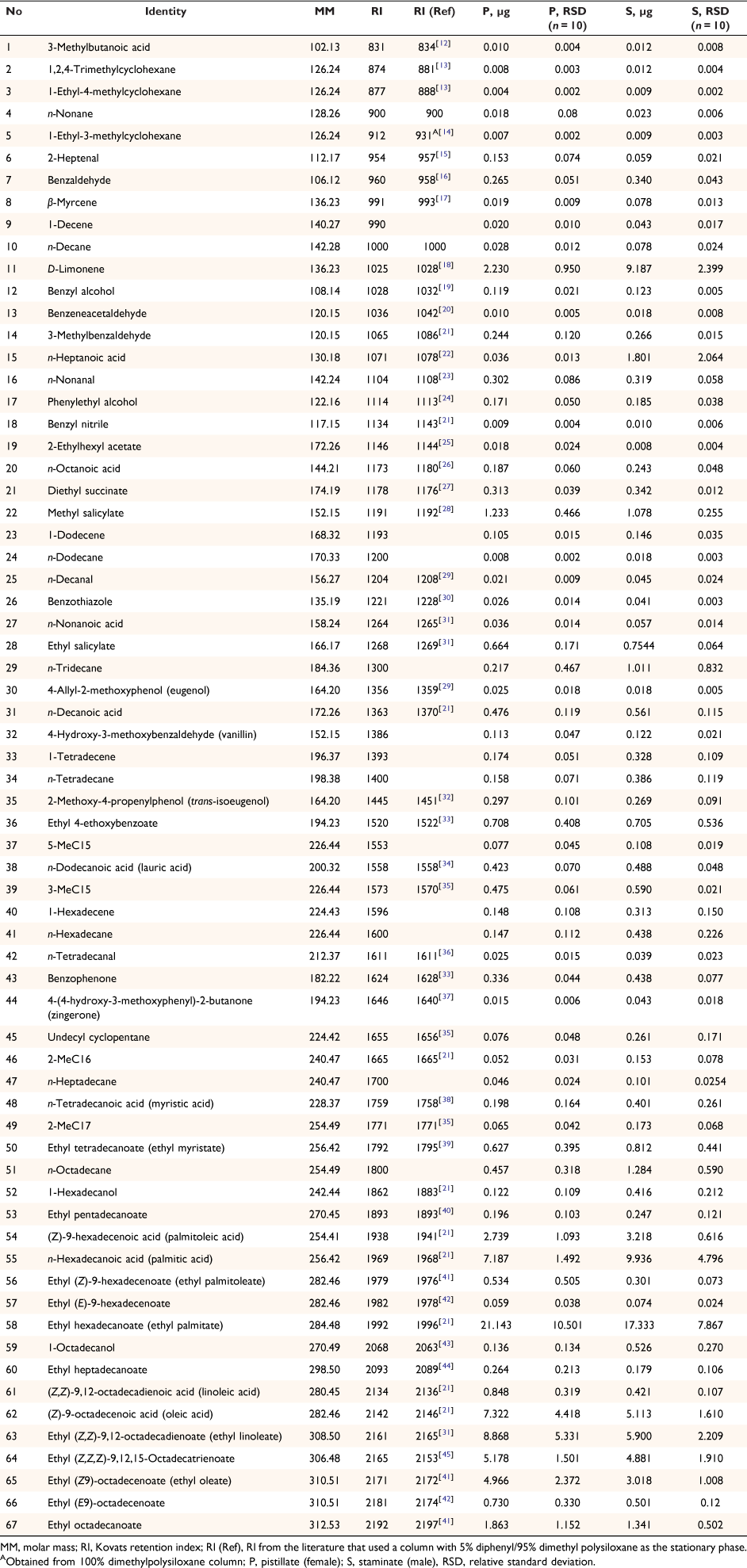
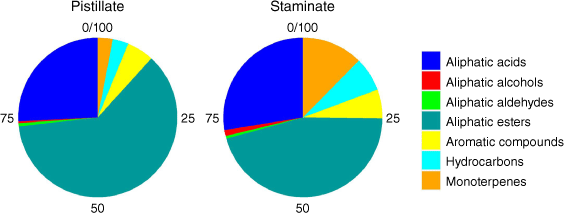
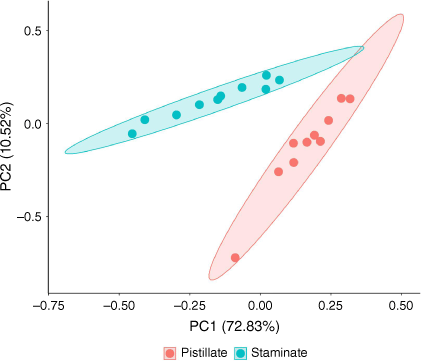
Extracts of tar tree flowers contained a diverse suite of 67 compounds, including 11 aliphatic acids, 13 aliphatic esters, 2 aliphatic alcohols, 2 monoterpenes, 4 aldehydes, 15 aromatic compounds, and 20 hydrocarbons (Table 1). The proportions of the classes of the detected compounds in pistillate and staminate flowers are illustrated in Fig. 1. The aliphatic esters had the highest proportions in both sexes (51.9% in pistillate flowers and 35.2% in staminate flowers), followed by the aliphatic acids (21.8% in pistillate and 21.3% in staminate flowers). While many hydrocarbons were detected, their proportions were small (2.7% in pistillate flowers and 5.4% in staminate flowers). The proportions of aromatic compounds were 4.7 and 4.5% in pistillate flowers and staminate flowers, respectively. The remainder consisted of monoterpenes (2.6% in pistillate flowers and 9.6% in staminate flowers), aliphatic aldehydes (0.6% in pistillate flowers and 0.4% in staminate flowers), and aliphatic alcohols (0.3% in pistillate flowers and 0.9% in staminate flowers). D-limonene is noticeably higher in staminate flowers than in pistillate flowers (Table 1). Principal component analysis identified notable differences in the compositions of pistillate and staminate flowers (Fig. 2). Further detailed investigation will be required to identify the functional or physiological role of such compositional differences in these flowers.
|
|
|
Flowers release a more diverse suite of volatiles at higher levels than other plant parts.[46] The floral volatiles are by-products of plant secondary metabolism, and some volatiles function to attract pollinators or as a defence against florivores and pathogens.[46] The fatty acids, 16- and 18-carbon species, i.e. C16:1 (palmitoleic acid), C16:0 (palmitic acid), C18:1 (oleic acid) and C18:2 (linoleic), and their ethyl esters are the predominant constituents in the tar tree flower samples. The fatty acids are important compounds in plants. For example, most cutin monomers are derived from the 16- and 18-carbon fatty acids to form the macromolecules that are the framework of the plant cuticles. [47] The 18-carbon unsaturated fatty acids can be nitrated to act as signalling mediators in the plant-defence system in oxidative stress situations.[48] It is also known that linoleic acid serves as a hydroperoxyl intermediate for the biosynthesis of green leaf volatiles.[49] The other detected compounds are also commonly found in plants. For example, D-limonene inhibits spore germination of the rice blast fungus (Magnaporthe oryzae) and is expressed at higher levels in response to the up-regulation of a terpene synthase gene when rice plants have the fungal infection.[50] Methyl salicylate is widespread as a herbivore-induced volatile to communicate herbivore attacts in plants. For example, barley exposed to deuterated methyl salicylate showed significant qualitative and quantitative changes in the chemical profile of the headspace.[51] Methyl salicylate is also known as a mobile signal to induce systemic acquired resistance against biotrophic pathogens in the tobacco plant.[52]
Ethyl and methyl salicylates are the major aromatic constituents in solvent extracts of the tar tree flowers, while phenylpropanoids and phenylbutanoids are present only in minute amounts. Although the amount of zingerone is minute (Table 1), the presence of this compound explains the attraction of B. jarvisi.[3a,53] The attraction of B. jarvisi is specific to zingerone and has been confirmed by systematic modification of zingerone and testing the synthesized analogs in the field.[54] In the study, >99% of flies captured by traps containing zingerone and its analogs were B. jarvisi. Hence, it is likely that zingerone is responsible for the observed interaction between the plant and fruit fly species. There appears to be no record of other fruit fly species being attracted to tar tree flowers. Along the tar tree natural distribution range, it is likely that different local fruit fly species are attracted to one or more of the phenylpropanoids and phenylbutanoids. For example, tar tree flowers contain trans-isoeugenol which is attractive to males of a New Caledonian fruit fly, B. curvipennis.[3d]
In summary, the present study addressed the chemical basis of the interaction between B. jarvisi and the native tar tree. Solvent extracts from tar tree flowers were dominated by unsaturated fatty acids and their ethyl esters. Amongst the 67 identified compounds, zingerone, a fruit fly attractant, was detected in minute amounts, providing a likely explanation for reported attraction of B. jarvisi fruit flies. Co-evolutionary interactions between plants and their insect pollinators mediated by plant secondary metabolites are known.[55] The commonly used fruit fly attractants are plant secondary metabolites or derivatives, which have been deployed for decades to monitor and control horticultural pest fruit flies.[56] There are many unexplored plant species, but future investigations will greatly benefit from a targeted approach, for example, studies on species with previous observations and records will increase the chances of new attractant discovery and address the chemistry of a species of interest.
Experimental
Chemicals
3-Methylbutanoic acid, n-nonane, 2-heptenal, benzaldehyde, β-myrcene, 1-decene, n-decane, D-limonene, benzyl alcohol, 3-methylbenzaldehyde, n-heptanoic acid, n-nonanal, phenethyl alcohol, n-octanoic acid, methyl 2-hydroxybenzoate, 1-dodecene, n-dodecane, n-decanal, benzothiazole, n-nonanoic acid, ethyl 2-hydroxybenzoate, n-tridecane, 3-alyl-6-methoxyphenol, n-decanoic acid, 4-hydroxy-3-methoxybenzaldehyde, 1-tetradecene, n-tetradecane, 3-allyl-6-methoxyphenol, 1-tetradecene, tetradecane, (E)-2-methoxy-4-(1-propenyl)phenol, n-dodecanoic acid, n-hexadecane, n-tetradecanal, benzophenone, 4-(4-hydroxy-3-methoxyphenyl)-2-butanone, n-heptadecane, n-tetradecanoic acid, ethyl tetradecanoate, n-octadecane, 1-hexadecanol, ethyl pentadecanoate, (Z)-9-hexadecenoic acid, n-hexadecanoic acid, ethyl (Z)-9-hexadecenoate, ethyl (E)-9-hexadecenoate, ethyl hexadecanoate, (Z,Z)-9,12-octadecadienoic acid, ethyl (Z,Z)-9,12-octadecadienoate, ethyl (Z,Z,Z)-9,12,15-octadecatrienoate, 1-octadecanol, (Z,Z)-9,12-octadecadienoic acid, (Z)-9-octadecenloic acid, ethyl (Z)-9-octadecenoate, and ethyl octadecanoate were purchased from Sigma-Aldrich. 3-Ethylhexyl acetate was purchased from Tokyo Chemical Industry. All compounds were analytical grade with 98% purity or higher.
Collection of flowers
The flowers (Queensland Herbarium voucher number: AQ952605) were collected from male and female tar trees located in Silver Crescent Park, Palm Cove, Queensland, Australia (−16.758420, 154.669235) in October 2018. Branches with staminte or pistillate flowers were cut from the trees. Ten flowers of each sex were separately cut into fine pieces in a ceramic bowl using microscissors. The fine flower pieces were transferred to a 2.0 mL clear vial containing 1.0 mL of absolute ethanol (six replicates for each sex). The samples were transported to Macquarie University, Australia, and stored at 4°C for 2–3 weeks until extracted.
Extraction of flowers
Milli-Q water (1.0 mL) (Millipore) was added to each sample vial, vortexed for 30 s, and then ultrasonicated for 30 min. The aqueous extract was transferred to a 10 mL separating funnel. The aqueous phase was extracted with the organic solvents (3 × 2.0 mL, 10% ethyl acetate in hexane). The organic layers were combined, washed with Milli-Q water (6.0 mL), and dried over Na2SO4. The solvents were evaporated using a Rotary evaporator (Buchi), and the residue was re-dissolved in 200 µL of 10% ethyl acetate (v/v) in hexane. The extracted samples were stored at −30°C until analyzed. An aliquot (5 μL) of the three internal standards, 1-octanol, methyl n-dodecanoate, and methyl n-hexadecanoate, were incorporated to give 2.6, 2.5, and 2.6 µg/mL, respectively.
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis was performed on a Shimadzu GCMS TQ8040 spectrometer equipped with a split/splitless injector, fused silica capillary column (SH-Rtx-5MS, 30 m × 0.25 mm I.D. × 0.25 μm film thickness) with cross-bond 5% diphenyl/95% dimethyl polysiloxane as the stationary phase and integrated mass spectrometry (MS). Helium gas (BOC, North Ryde, NSW, Australia) (99.999%) was used as a carrier gas with a constant flow of 1.5 mL/min. An aliquot of 1 μL of a sample was injected in splitless mode, and the injection port temperature was 270°C. The initial column temperature was set at 60°C and held for 4 min, increased to 220°C at a rate of 2°C/min, then increased to 320°C at a rate of 30°C/min and held for 3 min. The interface and ion source box temperatures were set at 250 and 200°C, respectively. The ionization method was electron impact at a voltage of 70 eV. The spectra were obtained over a mass range of m/z 41–600. The data were processed by Shimadzu GCMS Post-run software. The retention indices were obtained by analyzing a run of a standard mixture of C8 to C40 alkane with a sample. For identifications, mass spectra were compared with the NIST library (NIST17-1, NIST17-2, NIST17s) to identify related molecules. Fragmentation patterns and retention indices published in the literature were used to determine candidate molecules. The identity of a candidate molecule was confirmed by comparing retention time and fragmentations of the authentic molecule. The solvents used, including absolute ethanol, hexane, and ethyl acetate, were routinely analyzed by GC-MS to identify any impurities. The percentage of each compound was calculated from the quantification or semi-quantification of the constituents.
Standard solutions of five known concentrations of the individual compounds were prepared to quantify individual compounds. These contained three internal standards with the same concentration used in the extracted samples, in 5 mL volumetric flasks. The standard solutions were analyzed by GC-MS along with the flower samples. The standard curves of the authentic samples were generated by linear regression of peak area ratios of a compound to the internal standard against concentration ratios of a compound to that of the internal standard. Equations obtained by linear regression were used to calculate the concentrations of the compounds in a sample, and the amount of a compound per flower was subsequently estimated by taking account of the final sample volume of a sample prepared from ten flowers.
Several compounds that are not commercially available were estimated by the use of a surrogate.[57] The response factor of 1,2,4-trimethyl cyclohexane was used to estimate the quantities of 1-ethyl-4-methylcyclohexane and 1-ethyl-3-methylcyclohexane. The response factor of benzaldehyde was used to estimate the quantity of benzeneacetaldehyde. The response factor of methyl hexadecanoate was used to estimate the quantities of 3-methyl pentadecane, undecyl cyclopentane, 2-methyl hexadecane, 2-methyl heptadecane. The response factor of ethyl (Z)-9-hexadecenoate was used to estimate the quantity of ethyl (E)-9-hexadecenoate. The response factor of ethyl (Z)-9-octadecenoate was used to estimate the quantity of ethyl (E)-9-octadecenoate.
Data analysis
Principal component analysis was carried out to compare the compositions of pistillate and staminate flowers.
Data availability
Not applicable.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by the Australian Research Council Industrial Transformation Training Centre (ITTC) for Fruit Fly Biosecurity Innovation (Project IC50100026), funded by the Australian Government.
Supplementary material
Supplementary material is available online.
Acknowledgements
The authors thank Prof Darren Crayn and Mr Stuart Worboys for helping the identification of the Australian tar trees and locating them.
References
[1] (a) K-H Tan, R Nishida, Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J Chem Ecol 2000, 26, 533.| Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone.Crossref | GoogleScholarGoogle Scholar |
(b) K-H Tan, R Nishida, Synomone or kairomone? – Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J Chem Ecol 2005, 31, 497.
| Synomone or kairomone? – Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies.Crossref | GoogleScholarGoogle Scholar |
(c) K-H Tan, R Nishida, Y-C Toong, Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination. J Chem Ecol 2002, 28, 1161.
| Floral synomone of a wild orchid, Bulbophyllum cheiri, lures Bactrocera fruit flies for pollination.Crossref | GoogleScholarGoogle Scholar |
(d) KH Tan, R Nishida, Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination. Biochem Syst Ecol 2007, 35, 334.
| Zingerone in the floral synomone of Bulbophyllum baileyi (Orchidaceae) attracts Bactrocera fruit flies during pollination.Crossref | GoogleScholarGoogle Scholar |
(e) KH Tan, R Nishida, editors. Pollination of bactrocerophilous Bulbophyllum orchids. Proceedings of the 20th World Orchid Conference. Singapore: Singarpore Botanic Gardens; 2011.
(f) KH Tan, LT Tan, R Nishida, Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination. J Chem Ecol 2006, 32, 2429.
| Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination.Crossref | GoogleScholarGoogle Scholar |
(g) T Katte, KH Tan, Z-H Su, H Ono, R Nishida, Floral fragrances in two closely related fruit fly orchids, Bulbophyllum hortorum and B. macranthoides (Orchidaceae): assortments of phenylbutanoids to attract tephritid fruit fly males. Appl Entomol Zool 2020, 55, 55.
| Floral fragrances in two closely related fruit fly orchids, Bulbophyllum hortorum and B. macranthoides (Orchidaceae): assortments of phenylbutanoids to attract tephritid fruit fly males.Crossref | GoogleScholarGoogle Scholar |
[2] (a) RAI Drew, The response of fruit fly species (Diptera: Tephritidae) in the South Pacific area to male attractants. Aust J Entomol 1974, 13, 267.
| The response of fruit fly species (Diptera: Tephritidae) in the South Pacific area to male attractants.Crossref | GoogleScholarGoogle Scholar |
(b) BS Fletcher, The biology of dacine fruit flies. Annu Rev Entomol 1987, 32, 115.
| The biology of dacine fruit flies.Crossref | GoogleScholarGoogle Scholar |
[3] (a) HAC Fay, A highly effective and selective male lure for Bactrocera jarvisi (Tryon) (Diptera: Tephritidae). Aust J Entomol 2012, 51, 189.
| A highly effective and selective male lure for Bactrocera jarvisi (Tryon) (Diptera: Tephritidae).Crossref | GoogleScholarGoogle Scholar |
(b) M Nakahira, H Ono, SL Wee, KH Tan, R Nishida, Floral synomone diversification of Bulbophyllum sibling species (Orchidaceae) in attracting fruit fly pollinators. Biochem Syst Ecol 2018, 81, 86.
| Floral synomone diversification of Bulbophyllum sibling species (Orchidaceae) in attracting fruit fly pollinators.Crossref | GoogleScholarGoogle Scholar |
(c) JE Royer, S Agovaua, J Bokosou, K Kurika, A Mararuai, DG Mayer, et al. Responses of fruit flies (Diptera: Tephritidae) to new attractants in Papua New Guinea. Aust Entomol 2018, 57, 40.
| Responses of fruit flies (Diptera: Tephritidae) to new attractants in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
(d) JE Royer, C Mille, S Cazeres, J Brinon, DG Mayer, Isoeugenol, a more attractive male lure for the cue-lure-responsive pest fruit fly Bactrocera curvipennis (Diptera: Tephritidae: Dacinae), and new records of species responding to zingerone in New Caledonia. J Econ Entomol 2019, 112, 1502.
| Isoeugenol, a more attractive male lure for the cue-lure-responsive pest fruit fly Bactrocera curvipennis (Diptera: Tephritidae: Dacinae), and new records of species responding to zingerone in New Caledonia.Crossref | GoogleScholarGoogle Scholar |
(e) JE Royer, KH Tan, DG Mayer, Comparative trap catches of male Bactrocera, Dacus, and Zeugodacus Fruit Flies (Diptera: Tephritidae) with four floral phenylbutanoid lures (anisyl aetone, cue-lure, raspberry ketone, and zingerone) in Queensland, Australia. Environ Entomol 2020, 49, 815.
| Comparative trap catches of male Bactrocera, Dacus, and Zeugodacus Fruit Flies (Diptera: Tephritidae) with four floral phenylbutanoid lures (anisyl aetone, cue-lure, raspberry ketone, and zingerone) in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
[4] (a) JE Royer, Responses of fruit flies (Tephritidae: Dacinae) to novel male attractants in north Queensland, Australia, and improved lures for some pest species. Aust Entomol 2015, 54, 411.
| Responses of fruit flies (Tephritidae: Dacinae) to novel male attractants in north Queensland, Australia, and improved lures for some pest species.Crossref | GoogleScholarGoogle Scholar |
(b) JE Royer, M Khan, DG Mayer, Methyl-isoeugenol, a highly attractive male lure for the cucurbit flower pest Zeugodacus diversus (Coquillett) (syn. Bactrocera diversa) (Diptera: Tephritidae: Dacinae). J Econ Entomol 2018, 111, 1197.
| Methyl-isoeugenol, a highly attractive male lure for the cucurbit flower pest Zeugodacus diversus (Coquillett) (syn. Bactrocera diversa) (Diptera: Tephritidae: Dacinae).Crossref | GoogleScholarGoogle Scholar |
[5] (a) JAM Paula, PH Ferri, MTF Bara, LMF Tresvenzol, FAS Sá, JR Paula, Infraspecific chemical variability in the essential oils of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae). Biochem Syst Ecol 2011, 39, 643.
| Infraspecific chemical variability in the essential oils of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae).Crossref | GoogleScholarGoogle Scholar |
(b) KC Wong, Y Sivasothy, PL Boey, H Osman, B Sulaiman, Essential oils of Etlingera elatior (Jack) R. M. Smith and Etlingera littoralis (Koenig) Giseke. J Essent Oil Res 2010, 22, 461.
| Essential oils of Etlingera elatior (Jack) R. M. Smith and Etlingera littoralis (Koenig) Giseke.Crossref | GoogleScholarGoogle Scholar |
(c) L de, PC Nogueira, V Bittrich, GJ Shepherd, AV Lopes, AJ Marsaioli, The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae). Phytochemistry 2001, 56, 443.
| The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae).Crossref | GoogleScholarGoogle Scholar |
(d) H-S Choi, M Sawamura, Composition of the essential oil of Citrus tamurana Hort. ex Tanaka (Hyuganatsu). J Agric Food Chem 2000, 48, 4868.
| Composition of the essential oil of Citrus tamurana Hort. ex Tanaka (Hyuganatsu).Crossref | GoogleScholarGoogle Scholar |
[6] S Raghu, Functional significance of phytochemical lures to dacine fruit flies (Diptera: Tephritidae): an ecological and evolutionary synthesis. Bull Entomol Res 2004, 94, 385.
| Functional significance of phytochemical lures to dacine fruit flies (Diptera: Tephritidae): an ecological and evolutionary synthesis.Crossref | GoogleScholarGoogle Scholar |
[7] R Nishida, KH Tan, M Serit, NH Lajis, AM Sukari, S Takahashi, et al. Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis. Experientia 1988, 44, 534.
| Accumulation of phenylpropanoids in the rectal glands of males of the Oriental fruit fly, Dacus dorsalis.Crossref | GoogleScholarGoogle Scholar |
[8] (a) S-L Wee, K-H Tan, R Nishida, Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae. J Chem Ecol 2007, 33, 1272.
| Pharmacophagy of methyl eugenol by males enhances sexual selection of Bactrocera carambolae.Crossref | GoogleScholarGoogle Scholar |
(b) N Kumaran, PJ Prentis, KP Mangalam, MK Schutze, AR Clarke, Sexual selection in true fruit flies (Diptera: Tephritidae): transcriptome and experimental evidences for phytochemicals increasing male competitive ability. Mol Ecol 2014, 23, 4645.
| Sexual selection in true fruit flies (Diptera: Tephritidae): transcriptome and experimental evidences for phytochemicals increasing male competitive ability.Crossref | GoogleScholarGoogle Scholar |
[9] SJ Park, SG De Faveri, J Cheesman, BL Hanssen, DNS Cameron, IM Jamie, et al. Zingerone in the flower of Passiflora maliformis attracts an Australian fruit fly, Bactrocera jarvisi (Tryon). Molecules 2020, 25, 2877.
| Zingerone in the flower of Passiflora maliformis attracts an Australian fruit fly, Bactrocera jarvisi (Tryon).Crossref | GoogleScholarGoogle Scholar |
[10] (a) AWS May, Queensland host records for the Dacinae (fam. Trypetidae). Queens J Agric Sci 1953, 10, 36.
(b) AWS May, Queensland host records for the Dacinae (fam. Trypetidae). Second supplementary lists. Queens J Agric Sci 1960, 17, 195.
[11] Zich FA, Hyland BPM, Whiffin T, Kerrigan RA. Australian tropical rainforest plants, Edition 8. 2020. Available at https://apps.lucidcentral.org/rainforest/
[12] B Huang, L Qin, Q Chu, Q Zhang, L Gao, H Zheng, Comparison of headspace SPME with hydrodistillation and SFE for analysis of the volatile components of the roots of Valeriana officinalis var. latifolia. Chromatographia 2008, 69, 489.
| Comparison of headspace SPME with hydrodistillation and SFE for analysis of the volatile components of the roots of Valeriana officinalis var. latifolia.Crossref | GoogleScholarGoogle Scholar |
[13] Bramston-Cook R. Kovats indices for C2-C13 hydrocarbons and selected oxygenated/halocarbons with 100% dimethylpolysiloxane columns. 2013. Available at http://lotusinstruments.com/wp/wp‐content/uploads/List‐of‐Kovats‐Indices‐for‐C2‐C13‐Hydrocarbons.pdf
[14] S Ohnishi, T Shibamoto, Volatile compounds from heated beef fat and beef fat with glycine. J Agric Food Chem 1984, 32, 987.
| Volatile compounds from heated beef fat and beef fat with glycine.Crossref | GoogleScholarGoogle Scholar |
[15] J Xie, B Sun, F Zheng, S Wang, Volatile flavor constituents in roasted pork of Mini-pig. Food Chem 2008, 109, 506.
| Volatile flavor constituents in roasted pork of Mini-pig.Crossref | GoogleScholarGoogle Scholar |
[16] Y Qiao, B Xie, Y Zhang, Y Zhang, G Fan, XL Yao, et al. Characterization of aroma active compounds in fruit juice and peel oil of jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333.
| Characterization of aroma active compounds in fruit juice and peel oil of jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O.Crossref | GoogleScholarGoogle Scholar |
[17] NK Leela, TM Vipin, KM Shafeekh, V Priyanka, J Rema, Chemical composition of essential oils from aerial parts of Cinnamomum malabatrum (Burman f.) Bercht & Presl. Flavour Fragr J 2009, 24, 13.
| Chemical composition of essential oils from aerial parts of Cinnamomum malabatrum (Burman f.) Bercht & Presl.Crossref | GoogleScholarGoogle Scholar |
[18] A Angioni, A Barra, V Coroneo, S Dessi, P Cabras, Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J Agric Food Chem 2006, 54, 4364.
| Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers.Crossref | GoogleScholarGoogle Scholar |
[19] R Mebazaa, A Mahmoudi, M Fouchet, MD Santos, F Kamissoko, A Nafti, et al. Characterisation of volatile compounds in Tunisian fenugreek seeds. Food Chem 2009, 115, 1326.
| Characterisation of volatile compounds in Tunisian fenugreek seeds.Crossref | GoogleScholarGoogle Scholar |
[20] RA Cole, WA Haber, WN Setzer, Chemical composition of essential oils of seven species of Eugenia from Monteverde, Costa Rica. Biochem Syst Ecol 2007, 35, 877.
| Chemical composition of essential oils of seven species of Eugenia from Monteverde, Costa Rica.Crossref | GoogleScholarGoogle Scholar |
[21] N Radulović, P Blagojević, R Palić, Comparative study of the leaf volatiles of Arctostaphylos uva-ursi (L.) Spreng. and Vaccinium vitis-idaea L. (Ericaceae). Molecules 2010, 15, 6168.
| Comparative study of the leaf volatiles of Arctostaphylos uva-ursi (L.) Spreng. and Vaccinium vitis-idaea L. (Ericaceae).Crossref | GoogleScholarGoogle Scholar |
[22] K Javidnia, R Miri, M Kamalinejad, H Khazraii, Chemical composition of the volatile oil of aerial parts of Valeriana sisymbriifolia Vahl. grown in Iran. Flavour Fragr J 2006, 21, 516.
| Chemical composition of the volatile oil of aerial parts of Valeriana sisymbriifolia Vahl. grown in Iran.Crossref | GoogleScholarGoogle Scholar |
[23] G Fan, W Lu, X Yao, Y Zhang, K Wang, S Pan, Effect of fermentation on free and bound volatile compounds of orange juice. Flavour Fragr J 2009, 24, 219.
| Effect of fermentation on free and bound volatile compounds of orange juice.Crossref | GoogleScholarGoogle Scholar |
[24] RA Pérez, T Navarro, C de Lorenzo, HS–SPME analysis of the volatile compounds from spices as a source of flavour in ‘Campo Real’ table olive preparations. Flavour Fragr J 2007, 22, 265.
| HS–SPME analysis of the volatile compounds from spices as a source of flavour in ‘Campo Real’ table olive preparations.Crossref | GoogleScholarGoogle Scholar |
[25] JD Ramsey, RJ Flanagan, Detection and identification of volatile organic compounds in blood by headspace gas chromatography as an aid to the diagnosis of solvent abuse. J Chromatogr A 1982, 240, 423.
| Detection and identification of volatile organic compounds in blood by headspace gas chromatography as an aid to the diagnosis of solvent abuse.Crossref | GoogleScholarGoogle Scholar |
[26] JA Pino, J Mesa, Y Muñoz, MP Martí, R Marbot, Volatile components from mango (Mangifera indica L.) cultivars. J Agric Food Chem 2005, 53, 2213.
| Volatile components from mango (Mangifera indica L.) cultivars.Crossref | GoogleScholarGoogle Scholar |
[27] W Fan, MC Qian, Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J Agric Food Chem 2006, 54, 2695.
| Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis.Crossref | GoogleScholarGoogle Scholar |
[28] AE Edris, R Chizzola, C Franz, Isolation and characterization of the volatile aroma compounds from the concrete headspace and the absolute of Jasminum sambac (L.) Ait. (Oleaceae) flowers grown in Egypt. Eur Food Res Technol 2007, 226, 621.
| Isolation and characterization of the volatile aroma compounds from the concrete headspace and the absolute of Jasminum sambac (L.) Ait. (Oleaceae) flowers grown in Egypt.Crossref | GoogleScholarGoogle Scholar |
[29] E Alissandrakis, PA Tarantilis, PC Harizanis, M Polissiou, Comparison of the volatile composition in thyme honeys from several origins in Greece. J Agric Food Chem 2007, 55, 8152.
| Comparison of the volatile composition in thyme honeys from several origins in Greece.Crossref | GoogleScholarGoogle Scholar |
[30] W Fan, MC Qian, Identification of aroma compounds in Chinese ‘Yanghe Daqu’ liquor by normal phase chromatography fractionation followed by gas chromatography[sol]olfactometry. Flavour Fragr J 2006, 21, 333.
| Identification of aroma compounds in Chinese ‘Yanghe Daqu’ liquor by normal phase chromatography fractionation followed by gas chromatography[sol]olfactometry.Crossref | GoogleScholarGoogle Scholar |
[31] A Tava, L Pecetti, M Ricci, MA Pagnotta, L Russi, Volatile compounds from leaves and flowers of Bituminaria bituminosa (L.) Stirt. (Fabaceae) from Italy. Flavour Fragr J 2007, 22, 363.
| Volatile compounds from leaves and flowers of Bituminaria bituminosa (L.) Stirt. (Fabaceae) from Italy.Crossref | GoogleScholarGoogle Scholar |
[32] B Fernández de Simón, E Esteruelas, ÁM Muñoz, E Cadahía, M Sanz, Volatile compounds in acacia, chestnut, cherry, ash, and oak woods, with a view to their use in cooperage. J Agric Food Chem 2009, 57, 3217.
| Volatile compounds in acacia, chestnut, cherry, ash, and oak woods, with a view to their use in cooperage.Crossref | GoogleScholarGoogle Scholar |
[33] J Rohloff, AM Bones, Volatile profiling of Arabidopsis thaliana – Putative olfactory compounds in plant communication. Phytochemistry 2005, 66, 1941.
| Volatile profiling of Arabidopsis thaliana – Putative olfactory compounds in plant communication.Crossref | GoogleScholarGoogle Scholar |
[34] M Miyazawa, T Nishiguchi, C Yamafuji, Volatile components of the leaves of Brassica rapa L. var. perviridis Bailey. Flavour Fragr J 2005, 20, 158.
| Volatile components of the leaves of Brassica rapa L. var. perviridis Bailey.Crossref | GoogleScholarGoogle Scholar |
[35] F Kenig, D-JH Simons, D Crich, JP Cowen, GT Ventura, T Rehbein-Khalily, Structure and distribution of branched aliphatic alkanes with quaternary carbon atoms in Cenomanian and Turonian black shales of Pasquia Hills (Saskatchewan, Canada). Org Geochem 2005, 36, 117.
| Structure and distribution of branched aliphatic alkanes with quaternary carbon atoms in Cenomanian and Turonian black shales of Pasquia Hills (Saskatchewan, Canada).Crossref | GoogleScholarGoogle Scholar |
[36] R Palic, G Stojanovic, S Alagic, M Nikolic, Z Lepojevic, Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of the oriental tobacco, Prilep. Flavour Fragr J 2002, 17, 323.
| Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of the oriental tobacco, Prilep.Crossref | GoogleScholarGoogle Scholar |
[37] FX Garneau, M Bouhajib, GJ Collin, M Gagnon, JW ApSimon, The glycosidically bound volatile compounds of Picea mariana (Mill.) B.S.P. J Essent Oil Res 1994, 6, 43.
| The glycosidically bound volatile compounds of Picea mariana (Mill.) B.S.P.Crossref | GoogleScholarGoogle Scholar |
[38] C Formisano, F Senatore, M Bruno, G Bellone, Chemical composition and antimicrobial activity of the essential oil of Phlomis ferruginea Ten. (Lamiaceae) growing wild in Southern Italy. Flavour Fragr J 2006, 21, 848.
| Chemical composition and antimicrobial activity of the essential oil of Phlomis ferruginea Ten. (Lamiaceae) growing wild in Southern Italy.Crossref | GoogleScholarGoogle Scholar |
[39] J Ledauphin, H Guichard, J-F Saint-Clair, B Picoche, D Barillier, Chemical and sensorial aroma characterization of freshly distilled calvados. 2. identification of volatile compounds and key odorants. J Agric Food Chem 2003, 51, 433.
| Chemical and sensorial aroma characterization of freshly distilled calvados. 2. identification of volatile compounds and key odorants.Crossref | GoogleScholarGoogle Scholar |
[40] J-S Kim, HY Chung, GC-MS analysis of the volatile components in dried boxthorn (Lycium chinensis) fruit. J Korean Soc Appl Biol Chem 2009, 52, 516.
| GC-MS analysis of the volatile components in dried boxthorn (Lycium chinensis) fruit.Crossref | GoogleScholarGoogle Scholar |
[41] M Povolo, V Pelizzola, D Ravera, G Contarini, Significance of the nonvolatile minor compounds of the neutral lipid fraction as markers of the origin of dairy products. J Agric Food Chem 2009, 57, 7387.
| Significance of the nonvolatile minor compounds of the neutral lipid fraction as markers of the origin of dairy products.Crossref | GoogleScholarGoogle Scholar |
[42] Andriamaharavo NR. Retention Data. NIST Mass Spectrometry Data Center; 2014.
[43] B Marongiu, A Piras, S Porcedda, E Tuveri, A Maxia, Isolation of Seseli bocconi Guss., subsp. praecox Gamisans (Apiaceae) volatile oil by supercritical carbon dioxide extraction. Nat Prod Res 2006, 20, 820.
| Isolation of Seseli bocconi Guss., subsp. praecox Gamisans (Apiaceae) volatile oil by supercritical carbon dioxide extraction.Crossref | GoogleScholarGoogle Scholar |
[44] HJ Woerdenbag, T Windono, R Bos, S Riswan, WJ Quax, Composition of the essential oils of Kaempferia rotunda L. and Kaempferia angustifolia Roscoe rhizomes from Indonesia. Flavour Fragr J 2004, 19, 145.
| Composition of the essential oils of Kaempferia rotunda L. and Kaempferia angustifolia Roscoe rhizomes from Indonesia.Crossref | GoogleScholarGoogle Scholar |
[45] O Tzakou, A Said, A Farag, K Rashed, Volatile constituents of Ailanthus excelsa Roxb. Flavour Fragr J 2006, 21, 899.
| Volatile constituents of Ailanthus excelsa Roxb.Crossref | GoogleScholarGoogle Scholar |
[46] JK Muhlemann, A Klempien, N Dudareva, Floral volatiles: from biosynthesis to function. Plant Cell Environ 2014, 37, 1936.
| Floral volatiles: from biosynthesis to function.Crossref | GoogleScholarGoogle Scholar |
[47] EA Fich, NA Segerson, JKC Rose, The plant polyester cutin: biosynthesis, structure, and biological roles. Annu Rev Plant Biol 2016, 67, 207.
| The plant polyester cutin: biosynthesis, structure, and biological roles.Crossref | GoogleScholarGoogle Scholar |
[48] (a) C Mata-Pérez, B Sánchez-Calvo, MN Padilla, JC Begara-Morales, R Valderrama, FJ Corpas, et al. Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism. Redox Biol 2017, 11, 554.
| Nitro-fatty acids in plant signaling: New key mediators of nitric oxide metabolism.Crossref | GoogleScholarGoogle Scholar |
(b) M He, N-Z Ding, Plant unsaturated fatty acids: multiple roles in stress response. Front Plant Sci 2020, 11, 562785.
| Plant unsaturated fatty acids: multiple roles in stress response.Crossref | GoogleScholarGoogle Scholar |
[49] A Hammerbacher, TA Coutinho, J Gershenzon, Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ 2019, 42, 2827.
| Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles.Crossref | GoogleScholarGoogle Scholar |
[50] X Chen, H Chen, JS Yuan, TG Köllner, Y Chen, Y Guo, et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol J 2018, 16, 1778.
| The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae.Crossref | GoogleScholarGoogle Scholar |
[51] V Ninkovic, R Glinwood, AG Ünlü, S Ganji, CR Unelius, Effects of methyl salicylate on host plant acceptance and feeding by the aphid Rhopalosiphum padi. Front Plant Sci 2021, 12, 710268.
| Effects of methyl salicylate on host plant acceptance and feeding by the aphid Rhopalosiphum padi.Crossref | GoogleScholarGoogle Scholar |
[52] S-W Park, E Kaimoyo, D Kumar, S Mosher, DF Klessig, Methyl salicylate Is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113.
| Methyl salicylate Is a critical mobile signal for plant systemic acquired resistance.Crossref | GoogleScholarGoogle Scholar |
[53] S-L Wee, T Peek, AR Clarke, The responsiveness of Bactrocera jarvisi (Diptera: Tephritidae) to two naturally occurring phenylbutaonids, zingerone and raspberry ketone. J Insect Physiol 2018, 109, 41.
| The responsiveness of Bactrocera jarvisi (Diptera: Tephritidae) to two naturally occurring phenylbutaonids, zingerone and raspberry ketone.Crossref | GoogleScholarGoogle Scholar |
[54] BL Hanssen, SJ Park, JE Royer, JF Jamie, PW Taylor, IM Jamie, Systematic Modification of Zingerone Reveals Structural Requirements for Attraction of Jarvis’s Fruit Fly. Sci Rep 2019, 9, 19332.
| Systematic Modification of Zingerone Reveals Structural Requirements for Attraction of Jarvis’s Fruit Fly.Crossref | GoogleScholarGoogle Scholar |
[55] PC Stevenson, SW Nicolson, GA Wright, Plant secondary metabolites in nectar: impacts on pollinators and ecological functions. Funct Ecol 2017, 31, 65.
| Plant secondary metabolites in nectar: impacts on pollinators and ecological functions.Crossref | GoogleScholarGoogle Scholar |
[56] Vargas RI, Shelly TE, Leblanc L, Piñero JC. Chapter Twenty-Three - Recent Advances in Methyl Eugenol and Cue-Lure Technologies for Fruit Fly Detection, Monitoring, and Control in Hawaii. In: Litwack G, editor. Vitam Horm. Vol. 83. Academic Press; 2010. pp. 575–95.
[57] D Jenke, A Odufu, Utilization of internal standard response factors to estimate the concentration of organic compounds leached from pharmaceutical packaging systems and application of such estimated concentrations to safety assessment. J Chromatogr Sci 2012, 50, 206.
| Utilization of internal standard response factors to estimate the concentration of organic compounds leached from pharmaceutical packaging systems and application of such estimated concentrations to safety assessment.Crossref | GoogleScholarGoogle Scholar |


