Advanced inorganic chemistry laboratory curricula in Australian universities: investigating the major topics and approaches to learning
Alex C. Bissember A * , Timothy U. Connell
A * , Timothy U. Connell  B * , Rebecca O. Fuller
B * , Rebecca O. Fuller  A * , Reyne Pullen
A * , Reyne Pullen  C * and Alexandra Yeung D *
C * and Alexandra Yeung D *
A School of Natural Sciences – Chemistry, University of Tasmania, Hobart, Tas., Australia.
B School of Life and Environmental Sciences, Deakin University, Geelong, Vic., Australia.
C School of Chemistry, University of Sydney, Sydney, NSW, Australia.
D School of Molecular and Life Sciences, Curtin University, Perth, WA, Australia.
Handling Editor: George Koutsantonis
Australian Journal of Chemistry 75(9) 698-707 https://doi.org/10.1071/CH21334
Submitted: 15 December 2021 Accepted: 7 February 2022 Published: 17 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The teaching laboratory remains an important environment for developing undergraduate chemists, but the inherent diversity of inorganic chemistry results in less standardised undergraduate curricula than other sub-disciplines. This study surveys the content of advanced (third-year) inorganic chemistry across Australia and reviews experimental materials from 15 universities that offer inorganic laboratory programmes at this level. All institutions offer at least one traditional inorganic experiment, the most common being the preparation and acetylation of ferrocene, spectroscopy and magnetochemistry of nickel coordination compounds and palladium-catalysed cross-couplings. These inorganic classics are complemented by a breadth of non-traditional offerings that often align with institutional research strengths. Academic unit coordinators were also surveyed and their responses interpreted using ASELL (Advancing Science and Engineering through Laboratory Learning) tools. Advanced inorganic laboratory programmes were found to develop students’ practical and transferrable skills. Students generally receive guidance from teaching staff in all aspects of experimental work, including planning, development, analysis and communicating conclusions. Academic unit coordinators identified potential improvements that included diversifying student activities in the lab and how they are being assessed.
Keywords: Australia, curriculum, inorganic chemistry, practical laboratory, third-year, undergraduate.
Introduction
The opening sentence of Michael Faraday’s seminal 1827 text entitled ‘Chemical Manipulation’ still elegantly captures the significance of the laboratory in our discipline: ‘Chemistry is necessarily an experimental science’.[1] Teaching experimental skills is therefore as critical and relevant now as almost 200 years ago; the origins of the chemistry teaching laboratory trace back to 1824 when Justus von Liebig transformed the then standard format, of coupling demonstration with lectures, to incorporate laboratory-based learning.[2] The resulting paradigm shift from passive, content-driven delivery to a focus on hands-on experience and learning by doing represented a drastic change in how to train chemists; von Liebig envisaged a systematic programme focused on the rigorous development of laboratory techniques and scientific thinking.[2,3] While the teaching laboratory became the cornerstone of university-level programmes across the world, the model of teaching in practical laboratories began to change towards the end of the 19th century as enrolments increased.[4] Instead of von Liebig’s more open inquiry approach, laboratory programs increasingly focused on expository or ‘recipe-based’ experiments with known parameters and outcomes.[5] In their seminal 1982 review of the laboratory in science teaching, Hofstein and Lunetta described the broad goals for laboratory learning as: (1) understanding scientific concepts; (2) interest and motivation; (3) scientific practical skills and problem-solving skills; (4) scientific habits of mind; and (5) understanding the nature of science.[6]
Despite the predominance of the expository approach in the teaching laboratory, this more prescriptive inquiry model is not without limitations.[7–9] Several prominent concerns regarding expository-style experiments include the inadequate development of critical thinking skills,[10] failure to equip students with an understanding of scientific thinking,[11] and their increased susceptibility to student academic misconduct.[12] Furthermore, when questioned after a laboratory session, students are often unable to identify the intended purpose of an experiment.[13] In response, transferable or generic skills have emerged as a new wave of potential learning outcomes. Key examples such as ‘team-work, time-management, and problem-solving’ are increasingly defined as goals of the chemistry teaching laboratory, although exclusively associating these generic skills with a laboratory environment should be avoided.[14] Even though the modern teaching laboratory might look different to that of von Liebig’s time, its importance and intended learning outcomes remain similar.[14,15] In their review of the laboratory’s role in university chemistry, Reid and Shah summarised the four broad learning outcomes of a practical programme: (1) skills related to learning chemistry; (2) practical skills; (3) scientific skills; and (4) general skills.[16] Notably, these learning outcomes still closely resemble those of both Hofstein and Lunetta in 1982 and even von Liebig in 1824.[2,3]
There is no prescribed undergraduate chemistry curriculum in the Australian higher education sector; instead, the Australian Council of Deans of Science developed a set of Threshold Learning Outcomes (TLOs) for undergraduate chemistry (Table 1).[17] These outcomes are implemented by academics at the institutional-level and evaluated via degree accreditation overseen by the Royal Australian Chemical Institute (RACI).[18] Developing practical skills is integral to the third of these outcomes but the common themes and skills explored in the undergraduate laboratory align with all five, thus reflecting what the overwhelming majority of chemical practitioners recognise – chemistry education is reinforced, enabled and extended though practical experimentation.[19] Despite these important learning outcomes, there exist numerous challenges to implementing a teaching laboratory programme. Most prominent are the substantial costs and resources required to deliver practical experiments, including: teaching staff, physical space, equipment, laboratory consumables and the acquisition and maintenance of scientific equipment.[12,15] The true value of the teaching laboratory remains difficult to quantify[15] and is often further confounded by the competing opinions of staff and students (and, increasingly, university administrators).[19]

|
Here we report a surface-level analysis of the advanced (third-year) inorganic chemistry laboratory curricula across Australian universities and the prevailing approaches to learning. Previous studies exploring Australian undergraduate laboratory curricula overwhelmingly focus on general chemistry programs (typically first-year or equivalent) with the largest cohorts.[20] While we accept that practical techniques are built up incrementally at each stage of the undergraduate programme, ‘discovery research’ closer to von Liebig’s ideal is generally associated with more advanced levels. Recognising this limitation and the fundamental importance of experimental laboratory programmes in training young chemists, this snapshot was prepared with the assistance of our academic peers; teaching staff from 19 national universities answered questionnaires about laboratory-based learning outcomes and provided student materials for review. Inorganic chemistry curricula are less uniform between institutions compared to other, more standardised areas of undergraduate chemistry, such as organic and physical chemistry.[21] The breadth of experimental content we reviewed matched the diversity of the (mostly) inorganic elements that comprise the periodic table. Finally, scientific teaching and research is built on our predecessors’ advances and we believe this Special Issue honouring Prof. Glen Deacon, and his manifest contributions to inorganic chemistry,[22] is the perfect forum for this discussion.
Methodology
This study utilised a mixed methods design employing both qualitative and quantitative data as part of a survey of inorganic laboratory directors.[23] Two key perspectives were analysed: that of the directors and their perceptions and that of the physical materials provided to students. We approached this study with a pragmatic paradigm, a world view commonly associated with mixed methods methodology dictating a set of beliefs that guided the actions of the researchers and analysis of this study.[24]
Ethics approval was obtained for this study through Curtin University (HRE2021-0597) and unless otherwise stated, all authors equally contributed to the collection and analysis of data.
Sample recruitment
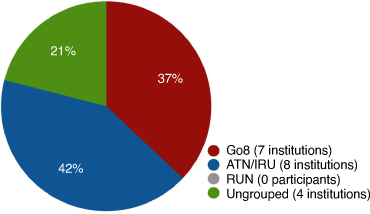
Potential participants were identified via publicly available staff profiles and the authors’ professional networks. Participants were contacted using a mail-out, digital invitation including an informal explanation of the study and their potential role in it. Consent was obtained by completing an online questionnaire and providing a copy of laboratory teaching materials. Information was requested from staff at 23 of the 43 Australian Universities, representing those that offer undergraduate degrees with a Chemistry major. We received 19 responses; 15 of these offered substantive advanced inorganic laboratory programmes. While individual institutions are not specifically identified, we have reported the percentage of total respondents comprising the larger Australia-wide networks of research-intensive universities, including: the Group of Eight (Go8), Australian Technology Network (ATN), Innovation Research Universities (IRU) and ungrouped universities (Fig. 1). We received responses from Universities located in almost all Australian states and territories and despite no respondents from the Regional University Network (RUN), two of the ungrouped universities are located in regional Australia.
|
Data collection
The ASELL (Advancing Science and Engineering through Laboratory Learning) project, formerly funded by the late Office of Learning and Teaching and now continued without funding, is a national project focused on providing professional development for academics to improve learning outcomes in the teaching laboratory. Australian academics have benefited from peer feedback and the formal ASELL instruments to reflect on, evaluate and improve their laboratory programmes, creating a culture of continuous laboratory growth that matches graduate needs. We collected data through an online questionnaire constructed from two previously validated instruments of this project, the ASELL Laboratory Programme Evaluation (ALPE) and ASELL Inquiry Slider.[25–27] Questionnaires were administered using Qualtrics and teaching laboratory materials received via email.
The original ALPE questionnaire contains fourteen questions employing a Likert scale with the options: Strongly Disagree, Disagree, Neutral, Agree, and Strongly Agree. It also includes four open-response items to probe the best and worst experiments students experience during the semester, as well as student suggestions for improving the laboratory programme. Participants in this study completed a version of the ALPE questionnaire adapted for the educator’s point of view (see Supplementary material for full questionnaire). This adaptation required only minor textual changes by adding a common prompt used for each question (‘The laboratories completed as part of this laboratory programme have helped students to…’) and switching perspective by replacing instances of ‘my’ with ‘their’ to reflect a laboratory director commenting on student experience.
The ASELL Inquiry Slider is based on the National Research Council’s essential features of inquiry.[28] This framework can be used by educators in the laboratory to identify the level of inquiry within an experiment. Each experimental feature can be further expanded and range from fully teacher directed (demonstrated inquiry) to fully student directed (open inquiry). For example, demonstrated inquiry involves a teacher performing a technique or experiment while the students observe, while open inquiry sees a student given minimal guidance, similar to an Honours or PhD project. In this study, participants were asked to think about their institution’s laboratory programme as a whole when determining the level of inquiry. A summary of each feature is provided below (for the full questionnaire and expanded responses for each feature, see the Supplementary Table S2):
Learner engages in scientifically oriented questions and predictions. (Questions and predictions)
Learner plans how to carry out investigation and collect data. (Plans investigations)
Learner conducts investigation, recording data. (Conducts investigations)
Learner processes and analyses data. (Processes and analyses)
Learner uses scientific reasoning and problem solving to link evidence to science concepts. (Problem solving)
Learners communicate, and justify findings based on evidence and scientific reasoning. (Communicates and justifies conclusions)
Data analysis
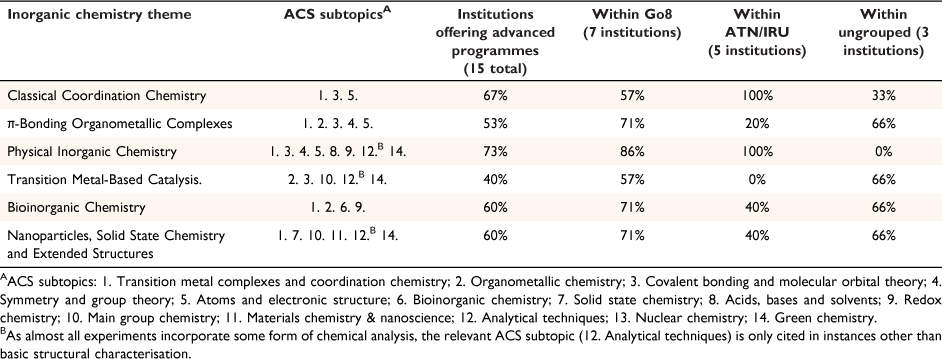
We used descriptive statistics to indicate the mean response to questions as the sample size is not appropriate for statistical tests for significance. The qualitative-type questions within the questionnaire were independently analysed by two authors highly experienced in qualitative research. A thematic analysis was employed to determine common themes with ~95% agreement. Laboratory manuals were independently analysed by two authors and experiments sorted into inorganic chemistry themes, with ~85% agreement in initial assignment. Discrepancies were mediated by a third member of the team. Themes were further referenced to a framework of inorganic chemistry subtopics developed by the American Chemical Society (ACS),[29,30] along with an anecdotal understanding of the academic community’s views on relevant inorganic chemistry content (Table 2).[21,31]
|
Limitations
Given the sample size of this study, drawing quantitative statistical significance is not possible and our analysis and conclusions should be viewed with this in mind. It is highly possible that there are ‘on-the-ground’ factors that contribute significantly to the experience students receive when undertaking these advanced inorganic chemistry laboratory programmes. Similarly, only perspectives from the laboratory coordinators have been collected which provides no insight from either casual teaching staff or students. Finally, the minimal qualitative feedback collected only goes some way towards gaining a deeper understanding of the questionnaire responses. We reiterate that this study is not meant to be conclusive but offer an overview of the current state of advanced inorganic teaching laboratories in Australia and to serve as a platform for future research and educational development.
Results and discussion
The inherent diversity within undergraduate inorganic chemistry curricula has been observed in the USA,[32] and the variety of topics covered in the advanced inorganic laboratory programmes we reviewed support this observation in the Australian context. We noted that the varied advanced-level experiments on offer were all well developed, often using published literature. This matches a similar diversity in inorganic theory taught at an advanced level; the higher degree of flexibility within these offerings results from a lower dependence on prior content knowledge.[21] The range of inorganic content, exemplified by the ACS subtopics (Table 2), also increase the likelihood of aspects being taught in other units that won’t be accurately captured in the present analysis. We ordered all experiments offered into six broad Inorganic Chemistry themes: Classical Coordination Chemistry, π-Bonding Organometallic Complexes, Physical Inorganic Chemistry, Transition Metal-Based Catalysis, Bioinorganic Chemistry and Nanoparticles, Solid State Chemistry and Extended Structures. Each of these themes was represented in the advanced inorganic laboratory programme of more than half the participating institutions (except for Transition Metal-Based Catalysis, Table 2), and no two universities offered the exact same content mix (Supplementary Table S1). Content was often influenced by the research strengths of a specific department;[33] for example, several institutions provided laboratory programmes more focused towards organometallic chemistry. Some institutions have moved away from laboratory programmes focused on specific disciplines (i.e. organic/inorganic/physical) and instead offer broader general programmes. What follows is a summary of the practical skills and experiments (grouped by Inorganic Chemistry Theme) before an analysis of results from the teaching staff questionnaire.
Developing practical skills
The importance of methodology and standard experimental techniques are key parts of undergraduate laboratory programmes.[34] Building on fundamental synthetic skills developed at lower levels, we found that advanced inorganic chemistry experiments introduce new techniques not common in other disciplines.[35] Students are often trained in handling air-sensitive materials, including several specialised techniques: solvent purification using stills, drying agents, degassing or modern systems, transferring solids and Schlenk techniques.[36] These experiences provide students with fundamental practical skills and help them better understand the principles behind air-free chemistry. Synthesis on the small scale is also commonly incorporated in advanced inorganic laboratories; sub-gram syntheses are perhaps much more common than in organic programmes due to the higher cost of many metal reactants and reagents.
Classical coordination chemistry
Coordination chemistry is fundamental to inorganic chemistry and is often taught at all undergraduate levels. At the advanced stage, students often explore the nuance of the relationship between the structure and function of metal complexes using more detailed explanations of valence bond theory, crystal and ligand field theory and molecular orbital theory. Focus also shifts towards mechanism and the molecular principles that guide reactivity (e.g. inert vs labile complexes, inner- and outer-sphere mechanisms) as well as in-depth interpretation of electronic spectra using term symbols, selection rules, and Tanabe–Sugano diagrams. We found that 10 of 15 participating institutions offer classic coordination experiments distinct from π-bonding organometallic, bioinorganic and extended structures. These encompass a variety of ligand types often used to illustrate key concepts, including: simple σ-donors (e.g. NH3, halides), strong multi-dentate donors [N,N-bis(salicylidene)ethylenediamines, i.e. salen]; and σ-donor and π-acceptor ligands (e.g. imines, phosphines). Nickel coordination complexes (containing salen, halides, aqua, NH3 ligands) are particularly common around the country; these experiments use absorption spectroscopy and magnetochemistry to provide context for crystal and ligand field theory. They also provide opportunities to investigate selection rules and spin multiplicity. Other common coordination experiments involve aminoacetates, acetylacetonates and hydrotris(1-pyrazolyl)borate ligands that support a variety of metals (e.g. Co, Cr, Al, Cu). We did not review any experiments investigating lanthanoid chemistry, where Prof. Deacon’s contribution is undeniable, which we attribute to the prohibitive cost of rare-earth metal salts.
π-Bonding organometallic complexes
Experiments that investigate the nature of π-donor and π-acceptor ligands fall into two main categories: metallocenes and related half-sandwich structures and metal carbonyls. The classic experiment involving the synthesis of ferrocene and its subsequent conversion to acetylferrocene remains a mainstay of many laboratory programmes (6 of 15).[37,38] Students purify these sandwich complexes by complementary techniques: sublimation (ferrocene), flash column chromatography (acetylferrocene) and recrystallisation (acetylferrocene, in one instance). One institution also offers an experiment where acetylferrocene is reduced with sodium borohydride. Two of 15 universities task students with investigating the chemistry of nickelocenes and their related half-sandwich complexes. In one case, students transform supplied nickelocene via two pathways: the preparation of a nickel half-sandwich complex; and a Diels–Alder cycloaddition with the dienophile dimethyl acetylenedicarboxylate. Another laboratory programme explores the synthesis of a nickel–(η-C6H6) half-sandwich complex from [NiCl2(PPh3)2]. Beyond exploring metallocene chemistry, the synthesis of various metal carbonyl complexes also features in many laboratory programmes around the country (6 of 15). Two laboratory programmes contain experiments where students prepare molybdenum carbonyl complexes that also bear cycloheptatrienyl ligands, whilst other carbonyl experiments prepare ruthenium, nickel and cobalt complexes. It is unsurprising that infrared spectroscopy features prominently in these experiments given the sensitivity of carbonyl stretching bands towards complex structure and geometry.
Physical inorganic chemistry
Employing spectroscopic and analytical methods for characterising the physical properties of materials and coordination complexes is a key component of all advanced inorganic laboratory programmes. Topics include: diffraction methods (X-ray, neutron), absorption spectroscopy (FT-IR, UV-Vis and Raman), resonance spectroscopy (NMR and electron spin), chemical analysis (photoelectron, mass spectrometry), magnetometry, electrochemical and computational techniques. A broad selection of these techniques is represented across the surveyed institutions, although FT-IR spectroscopy remains the most utilised technique, likely a result of relatively inexpensive instrumentation and ready sample preparation that allows for more student-based inquiry. While NMR spectroscopy and mass spectrometry are also common, in many instances these techniques are taught with a degree of separation; where students either submit samples to be run by technical staff or are provided spectra for analysis. In-depth experiments that focus on studying the physical properties of inorganic materials are also a common feature of national laboratory programmes (11 of 15). These experiments typically focus on magnetochemistry, electrochemistry, chemical kinetics and spectrochemical measurements. Transition metal polypyridyl complexes are the most common systems employed for electrochemical, kinetic, X-ray diffraction, and gravimetric studies. Our experience also suggests that specific experiments focused on exploring these techniques are often found in laboratory programmes associated directly with physical and analytical units and may not be fully captured by this study.
Transition metal-based catalysis and C–C bond-forming reactions
Many laboratory programmes (6 of 15) also contain experiments exploring aspects of transition-metal-based homogenous catalysis. In this regard, the primary focus is on palladium-catalysed cross-couplings; principally the Suzuki–Miyaura reaction (3 of 15), although we also noted individual experiments investigating Heck and Sonogashira cross-couplings. In all cases, students are tasked with the preparation and characterisation of products obtained via one of these carbon–carbon bond-forming reactions. Beyond exploring the fundamental principles that underpin classical organometallic catalysis, a number of these experiments also highlight aspects of green chemistry. One notable experiment investigates photoredox catalysis and while students do not perform a photoredox reaction, they prepare the prototypical catalyst [Ru(bpy)3]2+ and explore its key physical properties via absorption spectroscopy and electrochemistry. Synthetic photoredox catalysis has enjoyed a renaissance over the past decade[39,40] and we anticipate analogous experiments will be incorporated into more undergraduate laboratory programmes in the future. Despite the ubiquity of handling alkyllithium reagents with the Schlenk techniques commonly introduced in advanced inorganic laboratory programmes, only one institution includes an experiment using n-butyllithium in the lithiation of 2-chloropyridine. However, the ensuing organolithium is not employed in a C–C bond-forming reaction.
Bioinorganic chemistry
Bioinorganic experiments in undergraduate laboratory programmes exclusively focus on the chemistry of metalloenzymes. This is achieved using two main approaches: kinetic studies employing purified full proteins (2 of 15) or the synthesis and characterisation of biomimetic coordination complexes as model systems (7 of 15). Many full proteins are prone to denaturing and too expensive to purchase at a scale suitable for undergraduate courses, but cheaper, more robust proteins, including myoglobin and carbonic anhydrase, still catalyse meaningful chemical reactions. Protein mechanism may be readily analysed using absorption spectroscopy to monitor a representative substrate or inhibition studies. It is worth noting that protein-based laboratories teach students skills otherwise lacking in most inorganic laboratory programmes, including handling sensitive biological chemicals and preparing buffer solutions. Biomimetic models are more commonly used to teach aspects of bioinorganic chemistry, likely due to the similar skill set with the other common themes of inorganic laboratory programmes. In these experiments, students typically synthesise and characterise coordination complexes that either mimic a metalloenzyme active site, such as cobaloximes and vitamin B12, or are prevalent in a range of natural systems, e.g. porphyrins (3 of 15). One experiment offered by several institutions (3 of 15) is the classic cobalt(II) salen complex as a model of the oxygen binding in haemoglobin and myoglobin.[41] This coordination complex undergoes reversible oxygen binding that is readily observed by eye, but also monitored using cyclic voltammetry.
Nanoparticles, solid state chemistry and extended structures
Experiments that investigate inorganic nanoparticles and materials chemistry are relatively rare in advanced inorganic laboratory programmes (5 of 15) despite the rapid growth of these research areas over the last 30 years. These materials exhibit many unusual properties and provide opportunities for students to use experimental techniques not commonly taught in conventional laboratory programmes; sizing these materials (typically between the molecular and bulk scale) requires either electron microscopy (TEM, SEM) or dynamic light scattering. The versatility of these inorganic materials also extends to their application; with experiments covering catalysis, superconductors, plasmonics and solar cells in the laboratory programmes we reviewed. In particular, the preparation of dye-sensitised solar cells using fruit or plant extracts is offered by several universities (3 of 15) and provides students with unique experiences in applied chemistry and device fabrication. We also predict that that many experiments investigating solid state chemistry may instead be found in physical chemistry laboratory programmes (or even Chemical Engineering) which our review may not accurately capture.
Despite Australia’s rich research history in supramolecular chemistry, coordination polymers and metal–organic frameworks,[42,43] only limited examples (2 of 15) are taught in advanced inorganic laboratory programmes. Both institutions offer a similar multiweek experiment where students prepare several extended coordination frameworks containing copper(II) and pyrazine in different stoichiometric ratios. Thermogravimetric analysis is used to demonstrate the inherent porosity of these structures, but otherwise minimal characterisation is possible with the equipment available in most undergraduate laboratories. Along with often considerable crystallisation time, this may account for the low representation of this inorganic sub-discipline; although we predict it to increase in coming years. Supramolecular chemistry fares no better and even though discrete inorganic macromolecules are intrinsically easier to characterise than infinite coordination polymers, only one experiment we reviewed investigated the properties of a dinuclear iron cluster. The classic Sargeson[44] template synthesis of the macrocyclic cage [Co(diNOsar)]3+ is still offered at some institutions (2 of 15) while another specifically investigates the electrochemistry of this complex.
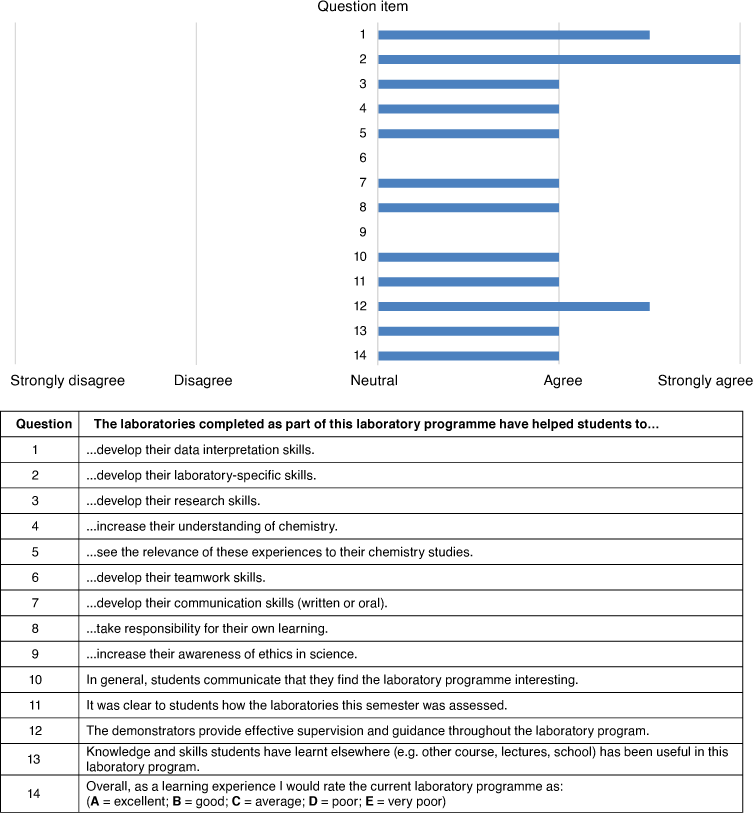
ASELL Laboratory Programme Evaluation (ALPE)
Likert items 1–13 from the ALPE were scored as +2 (strongly agree) to −2 (strongly disagree), with a 0 (neutral) midpoint, while item 14 was scored using a +2 (excellent) to −2 (very poor) scale, with a 0 (average) midpoint. The median response for each item is shown in Fig. 2, with the analysis of the ordinal data guided by the literature.[45] The three laboratory programme items rated highest by participants were ‘develop data interpretation skills’, ‘develop laboratory-specific skills’, and ‘demonstrators provide effective supervision and guidance’. This is not surprising given that a major learning outcome of many advanced laboratory programmes is to enhance the skills (both theory and practical) students developed in earlier years with assistance from demonstrators. Q9 scored low indicating that participants believed their laboratory programme did not help increase student awareness of scientific ethics, a topic usually indirectly taught (i.e. through preparation of laboratory reports) and especially not in the laboratory. Developing teamwork (Q6) also scored low, perhaps consistent with an increased focus on individual experiments in advanced laboratory programmes.
|
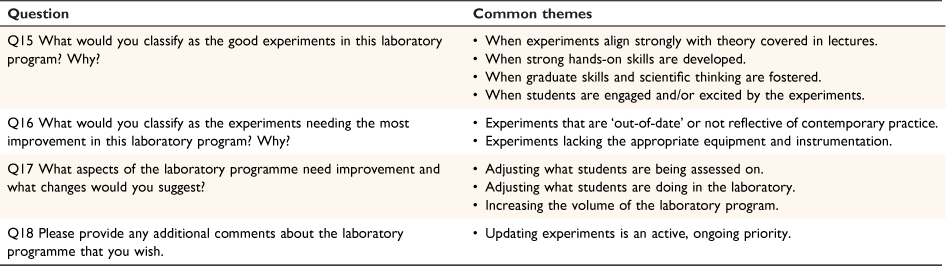
The qualitative response questions (Q15–18) provided further insights into the academic perspective of advanced laboratory programmes (Table 3). Several consistent themes came through strongly when participants were asked how they determined a successful experiment (Q15): (1) when the experiment aligned strongly with theory (‘Experiments 1 and 2 probably work best and integrate well with the lecture material…’ Academic, 2021); (2) and (3) corresponding with the development of hands-on laboratory skills and graduate skills or scientific thinking, respectively (‘In each we are fostering a broad range of graduate attributes, whether it be conceptual, technical & analytical skills, explorative investigation [through an investigative component to each prac], report writing, oral presentation, and teamwork.’ Academic, 2021); and (4) When students are engaged and/or excited by the experiments (‘The first is that [the students] enjoy it, they love the mystery of the unknown, and if we are instilling a love of chemistry and science, that’s a huge victory.’ Academic, 2021). Conversely, when asked what aspects of experiments need most improvement (Q16), participants highlighted ‘out-of-date’ experiments (‘The inorganic coordination chemistry exercises are dated and struggled for relevance.’ Academic, 2021) and limited equipment resources (‘There is a bottleneck caused by lack of instrumentation…’ Academic, 2021).
|
At the programme-level (Q17 and Q18), the majority of participants outlined the need for improvements in two key areas: (1) making improvements to what students are doing (‘Need to shift more focus onto collecting data on materials they make, too much focuses on just making something.’ Academic, 2021) and how students are assessed (‘We aim to improve it continually by balancing the assessment tasks so that it includes a balance between description of experiment observations and their interpretations using theoretical models developed in class.’ Academic, 2021); and (2) increasing the number of hours spent in the laboratory (‘The students need time to learn the technical skills and also need time to repeat the experiments when they do not work, or they fail to get a good outcome.’ Academic, 2021). Most responses were positive and indicated the passion of teaching staff concerning the evaluation, review and updating offered experiments (‘We are currently looking at improving/replacing a couple of labs.’ Academic, 2021).
ASELL Inquiry slider
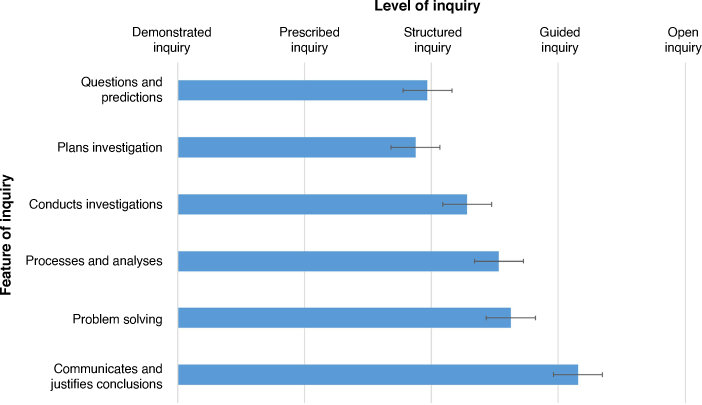
The ASELL Inquiry slider is a continuum that indicates levels of inquiry within an experiment. Each level of inquiry was scored 0 (Demonstrated Inquiry), 1 (Prescribed Inquiry), 2 (Structured Inquiry), 3 (Guided Inquiry) and 4 (Open Inquiry) with participants able to report half scores. The scores for each feature were averaged with error bars indicating the standard error of the mean (Fig. 3, individual participant responses can be found in the Supplementary Fig. S2). Participants scored all features between structured and guided inquiry, with communication and justification of results scored the highest for student-directed inquiry. Experiment questions and planning scored the lowest, just tending towards prescribed inquiry. In all cases our results indicate that students are provided with structure and guidance from teaching staff to develop experimental questions, plan and conduct experiments, process and analyse data and problem solving consistent with the expository nature of most modern laboratory programmes. While students are still guided in how to communicate and justify their conclusions, they are also given more scope in how this information is presented and it is common that students are encouraged to emulate the style of a published scientific manuscript, with proper referencing, at the advanced level. These scores are appropriate in advanced laboratory programmes as it is not expected that all experiments be based at an open inquiry level; that level is usually featured in more open research projects such as Honours and PhD projects. It is worth noting that these scores reflect advanced inorganic laboratory programmes, and some institutions (3 of 15) include ‘capstone’ experiments with greater student-directed (i.e. open) inquiry.
|
Conclusions and outlook
Consistent with observations in the USA, the range of topics and experiments explored in advanced inorganic laboratory programs differ significantly between institutions.[21a,46] We found that most universities offer some conventional experiments (e.g. coordination chemistry, physical inorganic chemistry, bioinorganic) alongside more specialised topics, such as organometallics, nanochemistry and materials chemistry, in line with their institutional expertise and research strengths. The most ubiquitous practicals at a national scale are the synthesis of ferrocene or related sandwich complexes (8 of 15), nickel coordination compounds based on ethylenediamine ligands for interpretation of ligand field and magnetochemistry (6 of 15) and palladium-catalysed cross-couplings (5 of 15). Our survey of teaching staff revealed that participants agreed that advanced inorganic programmes helped students improve both practical and some generic skills, although the development of students’ awareness of ethics could be improved and a greater focus on individual work does limit opportunities for developing teamwork. Qualitative questionnaire responses demonstrated the commitment of teaching staff to constantly improving advanced inorganic laboratory programmes and student outcomes.
In summary, this study was intended to provide a surface-level snapshot of current inorganic, higher-level laboratory programmes. It has inspired several interesting lines of inquiry that need to be pursued in order to better understand this area of chemist training in Australia. We have highlighted three topics that may be of interest to the wider community. (1) Our study relied on the perceptions of the laboratory directors to give insight into these programmes, which neglects the student voice. This is an essential consideration for informing any findings that could influence change beyond localised contexts. (2) While assessment has been extensively researched both inside of and outside of the laboratory, specifically investigating how assessment is employed at a senior level and whether these approaches are effective in measuring intended learning outcomes is an important question. For example, given the focus on practical skills emphasised in the inorganic laboratory curriculum, are these being attained? (3) Finally, these laboratory programmes are intended to prepare students for future research or employment and as such, investigating the usefulness and/or alignment of senior level inorganic laboratory programmes with these aspirations would afford valuable information regarding how we can better prepare students for their future careers.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
There are no conflicts to declare.
Declaration of funding
ROF’s and TUC’s contributions were supported by ARC Discovery Early Career Researcher Awards (DE180100112 and DE210101168, respectively). ACB’s contributions were supported by an ARC Future Fellowship (FT200100049).
Author contributions
All authors contributed equally to the conceptualisation, development and composition of the manuscript and authors names are only ordered alphabetically. ACB, TUC, and ROF oversaw the analysis of laboratory programmes within the context of fundamental inorganic chemistry. RP and AY oversaw the analysis of laboratory programmes within the context of chemical education.
Supplementary material
Supplementary material is available online.
Acknowledgements
Ethics approval for this research was granted by the Curtin University Human Research Ethics Office (Ethics Reference Number: HRE2021-0597). We thank our colleagues at universities around Australia for generously contributing their teaching resources, expertise, and time to assist us with this study.
References
[1] Faraday M. Chemical Manipulation. New York, NY: Wiley; 1827.[2] JB Morrell, Ambix 1972, 19, 1.
| Crossref | GoogleScholarGoogle Scholar | 11615551PubMed |
[3] HG Good, J Chem Educ 1936, 13, 557.
| Crossref | GoogleScholarGoogle Scholar |
[4] JJ Lagowski, J Chem Educ 1990, 67, 185.
| Crossref | GoogleScholarGoogle Scholar |
[5] DS Domin, J Chem Educ 1999, 76, 543.
| Crossref | GoogleScholarGoogle Scholar |
[6] A Hofstein, VN Lunetta, Rev Educ Res 1982, 52, 201.
| Crossref | GoogleScholarGoogle Scholar |
[7] Woolnough B, Allsop T. Practical Work in Science. Cambridge: Cambridge University Press; 1985.
[8] SJ Hawkes, J Chem Educ 2004, 81, 1257.
| Crossref | GoogleScholarGoogle Scholar |
[9] MK Seery, HY Agustian, X Zhang, Isr J Chem 2019, 59, 546.
| Crossref | GoogleScholarGoogle Scholar |
[10] D Hodson, Sch Sci Rev 1990, 71, 33.
[11] Letton KM. A study of the factors influencing the efficiency of learning in an undergraduate chemistry laboratory. M.Phil. thesis, Jordanhill College of Education, Glasgow; 1987.
[12] Carnduff J, Reid N. Enhancing Undergraduate Chemistry Laboratories. Cambridge: The Royal Society of Chemistry; 2003.
[13] PA Kirschner, MAM Meester, High Educ 1988, 17, 81.
| Crossref | GoogleScholarGoogle Scholar |
[14] MK Seery, J Chem Educ 2020, 97, 1511.
| Crossref | GoogleScholarGoogle Scholar |
[15] SL Bretz, J Chem Educ 2019, 96, 193.
| Crossref | GoogleScholarGoogle Scholar |
[16] N Reid, I Shah, Chem Educ Res Pract 2007, 8, 172.
| Crossref | GoogleScholarGoogle Scholar |
[17] The Australian Council of Deans Teaching and Learning Centre. National Chemistry’s TLOs for undergraduate university-level chemistry in Australia; 2011. Available at http://www.chemnet.edu.au/sites/default/files/u39/ChemistryTLOs_withicons.pdf [Accessed 17 November 2021]
[18] M Schultz, D Southam, G O’Brien, Aust J Chem 2020, 73, 825.
[19] SR George-Williams, AL Ziebell, RRA Kitson, P Coppo, CD Thompson, TL Overton, Chem Educ Res Pract 2018, 19, 463.
| Crossref | GoogleScholarGoogle Scholar |
[20] R Pullen, SC Thickett, AC Bissember, Chem Educ Res Pract 2018, 19, 629.
| Crossref | GoogleScholarGoogle Scholar |
[21] (a) JR Raker, BA Reisner, SR Smith, JL Stewart, JL Crane, L Pesterfield, SG Sobel, J Chem Educ 2015, 92, 980.
| Crossref | GoogleScholarGoogle Scholar |
(b) S Srinivasan, BA Reisner, SR Smith, JL Stewart, AR Johnson, S Lin, KA Marek, C Nataro, KL Murphy, JR Raker, J Chem Educ 2018, 95, 726.
| Crossref | GoogleScholarGoogle Scholar |
(c) BA Reisner, SR Smith, JL Stewart, JR Raker, JL Crane, SG Sobel, LL Pesterfield, Inorg Chem 2015, 54, 8859.
| Crossref | GoogleScholarGoogle Scholar |
[22] N Thomas, Chem Aust 2018, 20.
[23] Creswell JW. Educational Research: Planning, Conducting, and Evaluating Quantitative and Qualitative Research, 6th edn. Saddle River, New Jersey: Pearson; 2019.
[24] (a) Treagust DF, Won M, Duit R. In: Lederman NG, Abell SK, editors. Handbook of Research on Science Education, Vol. II. Routledge; 2014. pp. 17–31.
| Crossref |.
(b) V Kaushik, CA Walsh, Soc Sci 2019, 8, 255.
| Crossref | GoogleScholarGoogle Scholar |
[25] SC Barrie, RB Bucat, MA Buntine, K Burke da Silva, GT Crisp, AV George, IM Jamie, SH Kable, KF Lim, SM Pyke, JR Read, MD Sharma, A Yeung, Int J Sci Educ 2015, 37, 1795.
| Crossref | GoogleScholarGoogle Scholar |
[26] A Yeung, SM Pyke, MD Sharma, SC Barrie, MA Buntine, KBD Silva, SH Kable, KF Lim, Int J Innov Sci Math 2011, 19, 51.
[27] S Cornish, A Yeung, SH Kable, M Orgill, MD Sharma, Teaching Science 2019, 65, 4.
[28] Council NR. Inquiry and the National Science Education Standards: A Guide for Teaching and Learning. Washington DC: The National Academies Press; 2000.
[29] JG Verkade, J Chem Educ 1991, 68, 911.
| Crossref | GoogleScholarGoogle Scholar |
[30] JE Huheey, EA Boudreaux, IS Butler, AH Cowley, MY Darensbourg, K Emerson, RE Frost, P Labine, DH McDaniel, DW Meek, RW Neithamer, C Stanitski, LJ Theriot, JG Verkade, J Chem Educ 1972, 49, 326.
| Crossref | GoogleScholarGoogle Scholar |
[31] LL Pesterfield, CH Henrickson, J Chem Educ 2001, 78, 677.
| Crossref | GoogleScholarGoogle Scholar |
[32] S Srinivasan, BA Reisner, SR Smith, JL Stewart, AR Johnson, S Lin, KA Marek, C Nataro, KL Murphy, JR Raker, J Chem Educ 2018, 95, 726.
| Crossref | GoogleScholarGoogle Scholar |
[33] TL McGill, LC Williams, DR Mulford, SB Blakey, RJ Harris, JT Kindt, DG Lynn, PA Marsteller, FE McDonald, NL Powell, J Chem Educ 2019, 96, 35.
| Crossref | GoogleScholarGoogle Scholar |
[34] S DeMeo, J Chem Educ 2001, 78, 373.
| Crossref | GoogleScholarGoogle Scholar |
[35] C Nataro, AR Johnson, J Chem Educ 2020, 97, 3033.
| Crossref | GoogleScholarGoogle Scholar |
[36] TT Tidwell, Angew Chem Int Ed 2001, 40, 331.
| Crossref | GoogleScholarGoogle Scholar |
[37] M Rausch, M Vogel, H Rosenberg, J Chem Educ 1957, 34, 268.
| Crossref | GoogleScholarGoogle Scholar |
[38] RE Bozak, J Chem Educ 1966, 43, 73.
| Crossref | GoogleScholarGoogle Scholar |
[39] MS Galliher, BJ Roldan, CRJ Stephenson, Chem Soc Rev 2021, 50, 10044.
| Crossref | GoogleScholarGoogle Scholar | 34350919PubMed |
[40] CK Prier, DA Rankic, DWC MacMillan, Chem Rev 2013, 113, 5322.
| Crossref | GoogleScholarGoogle Scholar | 23509883PubMed |
[41] TG Appleton, J Chem Educ 1977, 54, 443.
| Crossref | GoogleScholarGoogle Scholar |
[42] R Robson, Dalton Trans 2008, 5113.
| Crossref | GoogleScholarGoogle Scholar | 18813362PubMed |
[43] BF Abrahams, SR Batten, DM D’Alessandro, Aust J Chem 2019, 72, 729.
| Crossref | GoogleScholarGoogle Scholar |
[44] JM Harrowfield, GA Lawrence, AM Sargeson, J Chem Educ 1985, 62, 804.
| Crossref | GoogleScholarGoogle Scholar |
[45] (a) S Jamieson, Med Educ 2004, 38, 1217.
| Crossref | GoogleScholarGoogle Scholar | 15566531PubMed |
(b) GM Sullivan, AR Artino, J Grad Med Educ 2013, 5, 541.
| Crossref | GoogleScholarGoogle Scholar |
[46] JR Raker, BA Reisner, SR Smith, JL Stewart, JL Crane, L Pesterfield, SG Sobel, J Chem Educ 2015, 92, 973.
| Crossref | GoogleScholarGoogle Scholar |


