Inhibitor mixture for reducing bacteria growth and corrosion on marine steel†
Rainier A. Catubig A , Agnes Michalczyk B , Wayne C. Neil C , Grant McAdam C , John Forsyth A B , Mahdi Ghorbani A , Ruhamah Yunis A , M. Leigh Ackland B , Maria Forsyth A and Anthony E. Somers A *
A *
A Institute for Frontier Materials, Deakin University, Burwood, Vic. 3125, Australia.
B Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
C Defence Science and Technology Group, Fishermans Bend, Vic. 3207, Australia.
Australian Journal of Chemistry 75(9) 619-630 https://doi.org/10.1071/CH21266
Submitted: 11 October 2021 Accepted: 17 January 2022 Published: 15 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
High strength steel in marine environments suffers from severe corrosion susceptibility and the presence of bacteria can exacerbate the effect, accelerating degradation via microbiologically influenced corrosion (MIC). Here we propose a novel approach to MIC inhibition by designing a system capable of limiting the effects of both bacterial growth and corrosion. The combination of a newly synthesised compound, cetrimonium 4-hydroxycinnamate (Cet-4OHCin), with lanthanum 4-hydroxycinnamate was the only system tested to date that could both inhibit abiotic corrosion in artificial seawater and minimise bacteria consortium densities over an exposure period of 24 h. This success was proposed to be due to them having the same anion, making them stable when mixed in the solution of the test environment. Furthermore, we confirmed from cytotoxicity testing that Cet-4OHCin demonstrated similarly limited toxicity towards human cells as the commercially available cetrimonium bromide, a known safe additive to cosmetic products. This new system shows promise as a safe and effective multifunctional inhibitor mixture to reduce the effects of MIC.
Keywords: anti-microbial, corrosion, inhibitor, microbiologically influenced corrosion, profilometry, rare-earth, seawater, steel.
Introduction
The susceptibility of high strength steel to corrosion in marine environments due to chloride ions is well established.[1,2] Corrosion attack on steel can be exacerbated in the presence of microbiological colonies, falling under the effects referred to as microbiologically influenced corrosion (MIC).[3,4] A common mechanism for accelerated corrosion attack due to MIC involves the formation of a biofilm to protect the numerous individual bacteria strains within from the external environment.[5] The bacteria can change the potential distribution on the steel surface, such as through the formation of anaerobic areas, which can result in localised corrosion attack on the steel. Considering that MIC accounts for approximately 20% of all corrosion costs, it is a significant issue to address in addition to degradation in the absence of microbes (referred to as abiotic corrosion).[4,6,7]
A simple method for controlling MIC is to eliminate bacteria with effective biocides before they have a chance to form biofilms.[4] In the past various biocides have been incorporated into coating systems to limit microbial adhesion, including copper, tin, mercury and arsenic. However, such systems were found to be toxic to the environment.[8] In a similar way, abiotic corrosion was effectively diminished with chromate-based inhibitors, which are carcinogenic and environmentally toxic. Due to the economic and environmental issues associated with MIC, we are in dire need of more environmentally friendly inhibitor alternatives.[9]
To effectively combat MIC, both microbial and abiotic corrosion effects need to be limited. We hypothesise that either a single multifunctional inhibitor, or more likely, a mixture of two or more inhibitors is needed to eliminate bacterial growth and mitigate corrosion. Our previous work reported the mixture of lanthanum 4-hydroxycinnamate (La(4OHCin)3) and 2-methylimidazolinium 4-hydroxycinnamate (IMI-4OHCin), referred to as La + IMI, provided more significant abiotic corrosion inhibition on the HY80 high strength steel in artificial seawater than La(4OHCin)3 tested separately, suggesting the La + IMI synergistically works together to provide a much more efficient corrosion inhibitor.[10] La(4OHCin)3 has previously been shown to inhibit abiotic corrosion to a similar level as sodium dichromate but at a quarter of the concentration.[11] In the current work, we exposed cultures of single bacterial species and a consortium of planktonic bacteria to the La + IMI mixture to verify the bactericidal properties of the inhibitors, in addition to their already confirmed abiotic corrosion properties.
Cetrimonium nalidixate (CetNal) has previously demonstrated significant antimicrobial properties to single bacterial strains on coated mild steel in 0.01 M NaCl solution.[12] However, in artificial seawater all systems containing CetNal displayed accelerated corrosion attack on HY80 steel.[10] We included this compound in the current study to confirm the antimicrobial properties on a consortium of planktonic bacteria in addition to individual bacterial strains.
This study investigated the potential of each inhibitor and mixture as a biocide by means of anti-microbial testing and investigated their cytotoxicity. Additionally, the data obtained for each inhibitor system tested thus far has prompted the synthesis of a new compound, cetrimonium 4-hydroxycinnamate (Cet-4OHCin). As the mechanism proposed for rare earth carboxylates is that the carboxylate group initially attaches to the iron surface to protect against corrosion by bridging between the rare earth and the iron surface,[13] we propose that having the same cinnamate cation on both a corrosion inhibiting compound and an anti-microbial compound will result in enhanced protection. Thus, a carboxylate was substituted for the nalidixate anion in CetNal to give Cet-4OHCin with the aim of retaining biocide properties whilst also improving the protection against corrosion.
This work focuses on the elimination of planktonic bacteria from high strength steel by inhibitors prior to formation of the biofilm. Additionally, the initial processes of corrosion inhibition were also examined to better understand the mechanisms by which the Cet-4OHCin inhibitor and its mixtures provide protection. For these reasons the exposure periods were purposely limited to 24 h. Longer exposure times would have hindered examination due to the presence of excessive biofilms or corrosion product. This study will be followed up by a subsequent study on sessile consortia within the biofilm and MIC studies over longer times for promising systems identified here.
Materials and methods
Material properties
All abiotic corrosion experiments were performed on the high strength steel alloy, 80HLES, with the composition shown in Supplementary Table S1, determined by inductively coupled plasma atomic emission spectroscopy. This alloy is commonly used in marine applications.
Abiotic corrosion experiments were performed under standard laboratory conditions (23 ± 1°C) at neutral pH in artificial seawater (details of artificial seawater presented in Supplementary Table S2). Prior to encasing into cold mounted epoxy, samples were cleaned by immersion in concentrated hydrochloric acid 32% (ChemSupply Australia, ≥31.5% w/w) for 5 min, thoroughly rinsed with deionised water and dried with argon gas. The epoxy mounted alloy surface was polished to P1200 grit finish with silicon carbide polishing paper and deionised water, then rinsed with deionised water, dried with argon gas and stored in a desiccator for 1 h prior to testing.
Synthesis of inhibitors
Sodium hydroxide (LR, ChemSupply Australia), trans-4-hydroxycinnamic acid or p-Coumaric acid (97%, Sigma Aldrich Australia), silver nitrate (LR, ChemSupply Australia), cetrimonium bromide (≥99%, Sigma Aldrich Australia), acetonitrile (HPLC, ChemSupply Australia), lanthanum (III) chloride heptahydrate (≥99, Sigma Aldrich Australia) and nalidixic acid (≥98%, Sigma Aldrich Australia) were used as received. Distilled water was used in synthesis and to make solutions where required. The synthesis of lanthanum trans-4-hydroxycinnamate (La(4OHCin)3), 2-methylimidazolinium trans-4-hydroxycinnamate (IMI-4OHCin) and cetrimonium nalidixate (CetNal) were performed by previously reported procedures.[14–16] Note that La(4OHCin)3 is in a hydrated state and should be written as La(4OHCin)3·5H2O, but for simplicity the associated water is not written here in the short hand notation. For further details on the structure of this complex see the work by Deacon et al.[15]
1H NMR spectra were collected on a Bruker Avance III instrument operating at 400 MHz in CD3OD by referencing the solvent peak. Mass spectrometry was performed on an Agilent 1200 series HPLC system. All samples were dried for at least 72 h on a Schlenk line. All samples were sent to The Campbell Microanalytical Laboratory, New Zealand for elemental analysis.
Synthesis of Cet-4OHCin
The synthetic scheme for the synthesis of Cet-4OHCin is presented in Fig. 1. Sodium trans-4-hyroxycinnamate was synthesised according to Deacon et al.[15] Sodium trans-4-hydroxycinnamate (1.506 g, 8.1 mmol) was then dissolved in 10 mL of distilled water and silver nitrate (1.38 g, 8.1 mmol) was dissolved in 10 mL of distilled water.[15] Upon mixing these two clear solutions, a white precipitate formed instantaneously. The solution with white precipitate was left to stir at room temperature for an hour in the dark. The white precipitate was washed with water (5 × 20 mL) to obtain silver trans-4-hydroxycinnamate.
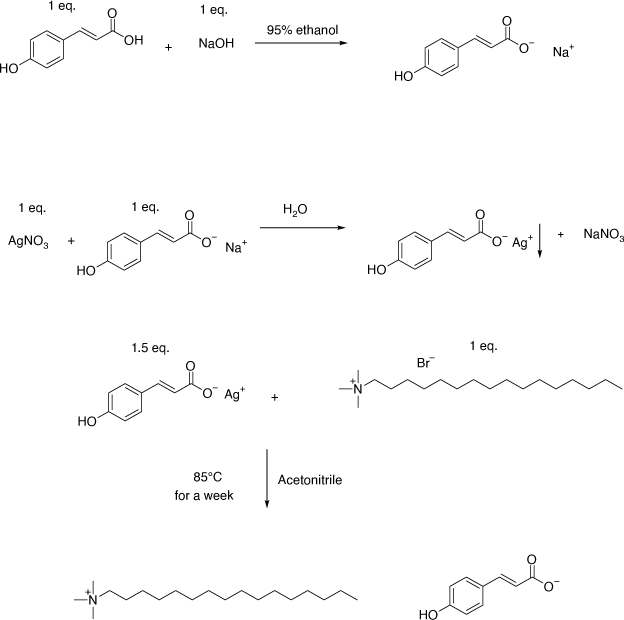
|
Freshly prepared silver trans-4-hydroxycinnamate (2.19 g, 8.1 mol) and cetrimonium bromide (1.9786 g, 5.4 mmol) were added to acetonitrile solution (20 mL), a yellow precipitate of AgBr formed instantaneously. The solution was left to stir at 85°C in the dark for a week. The solution was filtered by using filter paper and later filtered over celite to remove silver bromide and unreacted silver trans-4-hydroxycinnmate completely. The organic solvent was removed in vacuo and later dried for 48 h in vacuo again to get Cet-4OHCin (1.6 g, 66%).
1H NMR (400 MHz, CD3OD); 7.40 (m, J = 16 Hz, J = 8.2 Hz, ring, CCH–COO, 3H), 6.69 (d, J = 8.25 Hz, ring, 2H), 3.35 (d, J = 16 Hz, HCC–COO, 1H), 3.30 (m, NCH2, 2H), 3.11 (s, N(CH3)3, 9H), 1.78 (m, NCH2CH2, 2H), 1.34 (m, CH2, 26H), 0.92 (t, J = 7 Hz, CH2CH3, 3H) ppm. 13C NMR (100 MHz, CD3OD); 173.98 (COO), 158.53 (ring carbon attached to OH), 140.30 (ring C), 128.81 (ring C, C–C(OH)–C), 127.18 (CCH–COO), 120.99 (CC–COO), 115.19 (ring C), 66.50 (NCH2), 52.07 (N(CH3)3), 31.67 (CH2), 29.38 (CH2), 29.32 (CH2), 29.23 (CH2), 29.14 (CH2), 29.07 (CH2), 28.83 (CH2), 25.97 (CH2), 22.53 (CH2), 22.33 (CH2), 13.04 (CH3) ppm. ES+ m/z 284.5 ((CH3)3N(C16H33)+, ES− m/z 163.0 (trans-4-hydroxycinnmate)−. Anal. calculated for C28H53N1O5; C, 70.09; H, 11.04: N, 2.89. Found: C, 70.09; H, 11.09: N, 2.68.
The residual silver and bromide content were determined by an Ionode IJ-Ag or IJ-Br selective electrode after calibration with 10 and 100 ppm solutions of the silver (from silver nitrate) or bromide ion (potassium bromide). Bromide content measured by ion selective electrode (ISE) was 600 ppm. Silver content measured by ISE was below the detection limit of the electrode.
Due to the relatively low solubility of some inhibitors, each was sonicated separately in analytical grade ethanol (Chem-Supply, ≥96.5%) until fully dispersed before being added into the artificial seawater solution. The final solutions were heated and stirred at approximately 50°C for 3 h to allow the ethanol to evaporate while not being too hot to cause the degradation of the inhibitors. The chemical structures of the molecular ions are shown in Fig. 2, while the various combinations and solution concentrations are presented in Table 1. When mixed together into solution CetNal and La(4OHCin)3 would immediately form a precipitate. This solution was still tested, however all steel coupons were tested in a vertical position to avoid any precipitate resting on the surface and potentially influencing inhibition performance.
|
Antimicrobial assay of inhibitors
The bacterial species: Shewanella chilikensis, Pseudomonas balearica and Proteus mirabilis (CDC PR14) were used in this study due to their known association with biofilm formation on steel and their potential association with MIC.[17] The silt bacterial consortium was obtained from Port Adelaide River silt in South Australia, where it was associated with an aerobic, corroding environment. The Escherichia coli strain D-037, while not commonly associated with MIC, was used as a control organism. Bacterial strains were inoculated from fresh Tryptone Soya Agar plates into Tryptone Soya Broth (TSB) (ThermoFisher Scientific, Australia) and grown aerobically overnight at 30°C with shaking at 200 rpm. Each bacterial culture was then diluted in TSB to 0.5 on the McFarland Standard (OD600 nm = 0.1–0.15) and used for inhibitor testing as described below.
A high-throughput method for testing the inhibitors was adapted from the ‘Methods for the Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition (Clinical and Laboratory Standards Institute, 2012)’ utilised by Seter et al.[16] Briefly, inhibitors were tested in 96-well plates, where the first column was use as TSB blank, second one as a pure inhibitor blank, followed by the inhibitors that were serially diluted in TSB by a factor of 2, starting from column 3 until column 11. Column 12 contained ‘no inhibitor’ control for bacterial growth. The same volume (100 µL) of diluted bacterial stock was added into columns 3–12 and plates were incubated for 24 h at 30°C with shaking at 150 rpm. All samples were run in quadruplicate. Optical density readings (OD600 nm) were collected using a POLARstar Omega (BMG Labtech, Australia) microplate reader and MARS data analysis software (BMG Labtech, Australia).
Data were firstly analysed by subtracting the averaged TSB blank readings, followed by subtraction of the average of the inhibitor blank reading then recalculated to reflect the concentration of the inhibitors in each diluted sample. The transformed data was then normalised to provide the bacterial survival percentage by dividing each of the sample results by the average from the ‘no inhibitor’ control that represented 100% growth.
Immersion abiotic corrosion testing
Coupons (10 mm × 10 mm) were immersed in separate 80 mL solutions for 30 min and 24 h with and without each inhibitor. The relatively large volume was used to ensure full immersion was achieved throughout the immersion periods and to exceed the recommended solution-to-specimen area ratio of 0.2 mL mm−2 according to ASTM G31 72 (reapproved 2004) ‘Standard practice for laboratory immersion corrosion testing of metals’ Clause 8.9.2.[18] Solutions for abiotic corrosion tests were all exposed to artificial seawater prepared with analytical grade NaCl (Chem-Supply, ≥99.7%), MgCl2 (Sigma, ≥98.0%), MgSO4 (Merck, ≥96.0%), CaCl2 (Sigma, ≥96.0%) and KCl (Univar, ≥99.8%) as shown in Supplementary Table S2. Artificial seawater was used so that the composition of the electrolyte was known and constant throughout all testing and consistent with previous testing. Upon removal the coupons were subsequently gently rinsed with deionised water and dried with argon gas.
SEM/EDS on abiotic full immersion samples
Each sample was carefully removed from its epoxy and mounted onto an aluminium stub using double-sided adhesive carbon tape for analysis. Surface characterisation was performed with a JEOL JSM-IT300-LV scanning electron microscope (SEM) with an Oxford 50 mm2 X-Max energy dispersive X-ray spectroscopy (EDS) detector.
Electrochemical testing on abiotic solutions
All polarisation experiments were performed with a standard three-electrode polarisation cell, using 80HLES as the working electrode, a titanium mesh (approximately 700 mm2 in occupied space of the mesh) as the counter electrode and silver–silver chloride (Ag/AgCl, Ionode) as the reference electrode. A glass Luggin capillary containing the test solution held the Ag/AgCl reference electrode and enabled the tip of the Luggin (and by extension the reference electrode) to an approximate distance of 2D from the steel sample, whereby D is the diameter of the Luggin tip (2.5 mm).[19] The surface area of the working electrode was 10 mm × 10 mm. Electrochemical testing was performed using a BioLogic 16 Channel-VMP3 (variable multichannel potentiostat) with the EC-lab software version 11.03.
Linear polarisation tests were performed at 30 min and every 4 h thereafter (for 24 h tests). A potential was applied to the steel from −10 to +10 mV relative to the open circuit potential (OCP) at a scan rate of 0.167 mV/s.
Potentiodynamic polarisation scans were performed after 30 min and 24 h on separate samples. The scans were conducted from −150 to +250 mV relative to OCP, at a scan rate of 0.167 mV/s. The uninhibited sample was scanned from −60 to +250 mV instead since polarising to more negative potentials without an inhibitor resulted in significant changes in the measured corrosion potential as compared to the OCP prior to the test. This was thought to be due to changes in the environment near the working electrode when polarising further than −60 mV relative to OCP leading to inconsistent results. This effect was not seen for the inhibited solutions. The solution volume was 300 mL and was left open to air over the duration of the experiments. A minimum of triplicate tests was performed for each condition.
Removal of corrosion product and optical profilometry on immersion samples
Once the corrosion product and inhibitor deposits were examined with SEM/EDS, they were removed chemically to characterise the localised corrosion statistics for each sample. The corrosion product was removed by immersing each sample for 3 min in a solution of analytical grade HCl (25 mL), deionised water (25 mL) and analytical grade hexamethylene tetramine (0.175 g, Sigma, ≥99.0%). This was adopted from ASTM G1-03 (reapproved 2017) ‘Standard practice for preparing, cleaning and evaluating test specimens’ designation C.3.5.[20] Following corrosion product removal each sample was rinsed with deionised water and dried with argon gas.
Three dimensional surface profilometry was conducted with a Bruker Contour GT-K1 3D optical profilometer running Vision 64 software. The samples were analysed with the optical profilometer within 1 h of corrosion product removal.
Inhibitor cytotoxicity test
HaCaT (immortalised human keratinocytes) and HuTu80 (human duodenum adenocarcinoma) were used to test cytotoxicity of the inhibitors, as they represent a biologically relevant model of skin and intestine for studies of chemical product safety in human health, an alternative to in vivo tests.[21,22]
HaCaT or HuTu80 cells were cultured to 85% confluency in 75 cm2 culture flasks in culture media: Dulbecco’s Modified Eagle medium (DMEM) (Life Technology, Australia) with 10% fetal bovine serum (FBS) for HaCaT cells and Roswell Park Memorial Institute medium (RPMI) (Life Technology, Australia) with 10% FBS for HuTu80 cells. Cells were trypsinised, counted and seeded at 5 × 103/well in 96-well plates in 200 µL of growth medium and incubated for 24 h at 37°C with 5% CO2. A range of inhibitor concentrations were added to the cells, which were incubated at predetermined time intervals from 15 min to 5 days. At the end of incubation 50 µL of MTT (3(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, Australia) solution (5 mg mL−1) was added to each well and plates were incubated at 37°C with 5% CO2 for 2 h. Media were then removed and 150 µL of dimethyl sulfoxide (Sigma-Aldrich, Australia) was pipetted into each well. Plates were agitated for 20 min on a shaker and absorbances (OD550 nm) were measured on a POLARstar Omega (BMG Labtech, Australia) microplate reader with MARS data analysis software (BMG Labtech, Australia). Data were firstly analysed by subtracting the averaged cell culture media readings, followed by normalisation to ‘no inhibitor’ control. This will be referred to the cell density.
Results
Bacterial testing of inhibitors in solution
The significant reductions in abiotic corrosion attack by the mixture La + IMI from our previous work and its potential surfactant properties made it a good candidate system to investigate against MIC.[10] The CetNal inhibitor was also included as it has been reported to have a strong effect on bacterial growth[12] and will likely give a good anti-microbial baseline compared to the other inhibitors.
For evaluating the anti-microbial properties of the compounds and their mixtures, each inhibitor was dissolved in solution containing the Port silt (collected at a location from the Port River in Adelaide, South Australia) that is known to contain a mixture of bacteria strains. Since the microbial tests are purely to observe the effect on the bacterial density in the presence of the various inhibitors, no steel was present.
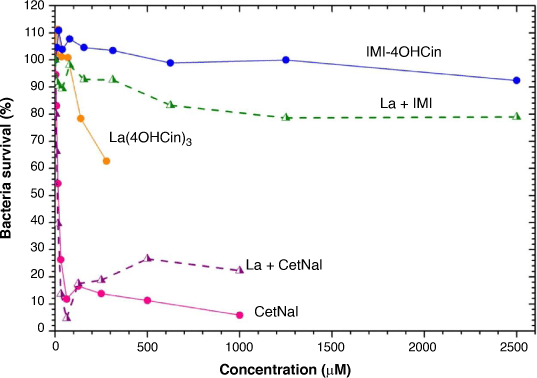
Fig. 3 shows the bacteria density curve (%) from the mixed Port silt culture as a function of inhibitor concentration for La(4OHCin)3, IMI-4OHCin, CetNal and their respective combined inhibitor mixtures. Lower percentages correspond to a reduction in the bacteria survival. The maximum concentration tested for La(4OHCin)3 was much lower than the CetNal or IMI-4OHCin systems because it has a much lower saturation limit. The maximum concentrations for the La + CetNal and La + IMI mixtures correspond to the saturation concentrations of CetNal and IMI-4OHCin respectively.
|
La(4OHCin)3 was added based on its capacity to inhibit abiotic corrosion and was not expected to provide any anti-microbial properties. The lack of effect on the bacteria can be seen in Fig. 3, which shows bacterial survival of approximately 60% at the highest concentration tested (almost 300 µM) after 24 h.
In contrast, even at very small concentrations (<100 µM), CetNal limited the bacteria survival irrespective of the presence of La(4OHCin)3, demonstrating its effectiveness as an anti-microbial. Unfortunately, the mixture La + CetNal accelerated abiotic corrosion, as previously demonstrated, therefore it is not a viable option for this project.[10] We present it here as an example of an efficient anti-microbial compound as compared with La(4OHCin)3, IMI-4OHCin and the La + IMI mixture.
La + IMI, which showed the most effective abiotic inhibition from previous work, had almost no effect on the bacteria, having less reduction in density compared to La(4OHCin)3 alone (Fig. 3).[10] Thus, it too cannot combat both abiotic corrosion and MIC simultaneously.
As the initial inhibitor mixtures were only effective in minimising either abiotic corrosion or bacteria density but not both, the new compound, Cet-4OHCin, was synthesised. It was hypothesised that the cetrimonium cation will retain its anti-microbial properties while the 4OHCin− anion will allow for corrosion inhibitive speciation, either on its own or to complement the inhibition mechanism of La(4OHCin)3. Previous studies have shown that initial corrosion protection from La(4OHCin)3 alone is due to the carboxylate bridging to the surface to form a protective film on anodic sites, while the rare earth is hydrolysed with time to cover cathodic sites, thus if more 4OHCin is present, initial protection could be more effective.[13]
Initially this new Cet-4OHCin compound was tested against several single strain bacteria as well as the Port silt (Fig. 4a). Shewanella chilikensis, E. coli and the mixed bacteria Port silt, were very sensitive to the presence of Cet-4OHCin, resulting in bacterial survival of less than 10% between concentrations of 10–20 µM. Proteus mirabilis and Pseudomonas balearica showed a more gradual decrease as concentration increased.
The inset on the top right corner of Fig. 4a compares the Port silt microbial consortium density of CetNal and Cet-4OHCin. The similar survival percentages demonstrated that the anti-microbial properties of CetNal were retained when the nalidixate anion was replaced with the 4OHCin− anion.
The mixture of La(4OHCin)3 and Cet-4OHCin, referred to as La + Cet, was exposed to the same bacterial strains (shown in Fig. 4b). At concentrations lower than 40 µM for the individual bacterial strains and 8.8 µM for the Port silt, the bacteria survival remained relatively constant around 100%, which is indicative of no significant bacteria death. Beyond these respective concentrations, the bacteria survival dramatically drops to less than 8%, at least for the Port silt, E. coli and S. chilikensis. This indicates the presence of a minimum inhibitor concentration to cause drastic reductions in the bacteria density and also shows the reduced sensitivity of the La + Cet towards the bacteria strains compared to Cet-4OHCin alone (Fig. 4a), which was effective against these strains at lower concentrations.
This study showed a significant reduction in planktonic bacteria after 24 h exposure to Cet-4OHCin and Cet Nal on their own and their mixtures with La(4OHCin)3. This time was selected on the basis that the bacteria should be killed prior to them having the opportunity to establish a biofilm. Bacterial growth reduction by CetNal seen in the anti-microbial tests (Fig. 3) strongly supports the observations by Seter et al.[12] A similarly strong anti-microbial effect was observed with the new Cet-4OHCin compound (as seen in the inset of Fig. 4a). This indicates the substitution of nalidixate for the 4OHCin− anion had little influence on its anti-microbial effect, and that the anti-microbial functionality resides mainly with the cetrimonium cation. The minor reduction in sensitivity of the La + Cet mixture compared to Cet-4OHCin towards each bacterial strain became negligible beyond 70 µM at which point the bacteria survival percentages converge towards zero (at least for the S. chilikensis strain, E. coli and Port silt consortium shown in Fig. 4b).
IMI-4OHCin was proposed as a possible MIC inhibitor and was reported to provide significant abiotic corrosion inhibition at acidic (pH 2) through to relatively alkaline (pH 8) conditions on mild steel.[14,23,24] Furthermore, due to its similarity to imidazolium compounds, which are known to have anti-microbial properties, we hypothesised that IMI-4OHCin would also provide some level of anti-microbial effects. However, this is typical for structures with longer alkyl chains of 6–8,[25] which is much longer than on the IMI-4OHCin and likely explains the lack of anti-microbial properties observed in Fig. 3.
Electrochemical analysis of corrosion inhibition
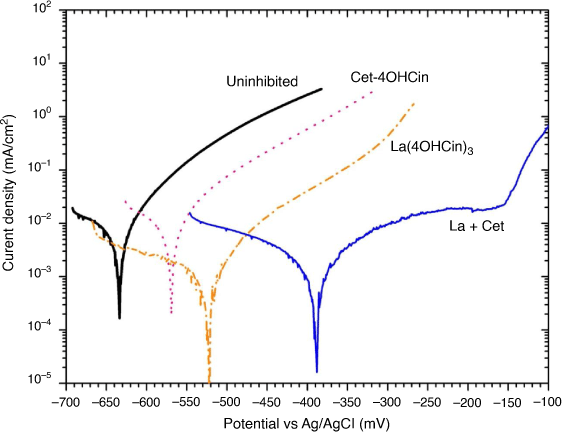
Beyond the anti-microbial properties demonstrated by the new compound Cet-4OHCin and the La + Cet mixture, it is important to determine if these inhibitor systems can also limit abiotic corrosion. Fig. 5 shows representative potentiodynamic polarisation curves for 80HLES steel after 24 h immersion in the artificial seawater solution. The average corrosion current density (icorr) values and the corrosion potential (Ecorr) are shown in Table 2.
|

|
All inhibitors were predominantly anodic relative to the uninhibited samples, as indicated by reductions in anodic current densities and anodic shifts in their respective corrosion potentials, as seen in Fig. 4, Table 2. The most significant reduction in anodic current densities was with the La + Cet mixture with over two orders of magnitude reduction at approximately −380 mV vs Ag/AgCl. Additionally only the La + Cet mixture showed a region between −250 and −150 mV which is characteristic of a passive region like behaviour, with an inflection characteristic of a pitting potential at approximately −150 mV vs Ag/AgCl.
It is also interesting to note that the icorr for La(4OHCin)3 was initially higher than La + Cet after 30 min but was lower after 24 h. This may suggest that, as proposed, the La + Cet mixture initially forms a more protective film after 30 min due to higher amounts of 4OHCin present, however over time the La(4OHCin)3 becomes comparatively more protective. As previously reported, La(4OHCin)3 behaved as a mixed inhibitor by also reducing cathodic current densities over 24 h.[11] The icorr of Cet-4OHCin tested alone was similar to the uninhibited sample indicative of limited inhibition for this HY80 steel in artificial seawater.
Considering this dependence of inhibition effectiveness with time (i.e. 30 min vs 24 h), the polarisation resistance (Rp) was measured throughout a 24-h period using a non-destructive technique. This resistance is indicative of corrosion inhibition. Linear Polarisation Resistance (LPR) was conducted at 30 min and 4 h intervals thereafter during the 24 h exposure and is shown in Supplementary Fig. S1. Similar to the average icorr data, the LPR measurements for the uninhibited and the Cet-4OHCin inhibitor were almost coincident throughout the exposure period. Given Rp is inversely proportional to icorr, this is to be expected.
The Rp for the La(4OHCin)3 inhibitor steadily increased over 12 h, then plateaued up to 24 h. In contrast, the La + Cet mixture showed a higher Rp at 30 min, but over the 24 h period the Rp fluctuated around that of the La(4OHCin)3. The scatter in the data is evident in the error bars on Supplementary Fig. S1 and may be indicative of a less stable protective film compared to La(4OHCin)3 on its own.
When exposed to artificial seawater, Cet-4OHCin alone still performed poorly as an abiotic corrosion inhibitor in this aggressive environment. This is consistent with the observations of Kalinowska et al.[26] and Seter et al.[27] that having the 4OHCin− anion incorporated into an inhibitor does not guarantee an effective inhibitor.
However, there was a marked improvement in abiotic corrosion inhibition with the La + Cet mixture compared to the La + CetNal mixture. Upon mixing La(4OHCin)3 with CetNal (to form the mixture La + CetNal), a precipitate would immediately form (likely to be a lanthanum-nalidixate salt) and it is presumed that the remaining ions may form Cet-4OHCin and remain dissolved in solution. We now know from this current work that Cet-4OHCin is a poor corrosion inhibitor when dissolved in solution by itself and so we have an explanation for accelerated corrosion attack for the La + CetNal mixture, as it likely only consists of Cet-4OHCin in solution.
In contrast, both La + IMI and La + Cet mixtures did not result in precipitation upon mixing and additionally yielded significant abiotic corrosion inhibition. This suggests that preserving the general structure of the inhibitor (in this case the La(4OHCin)3) should be one of the priorities when mixing multiple inhibitors to maintain a high level of abiotic corrosion inhibition. One way this can be achieved is by mixing inhibitors with identical anions, as with the 4OHCin− anion in the current work. Since there is only one type of anion present then there is limited possibility of new compounds forming and precipitating out.
Surface characterisation with SEM/EDS
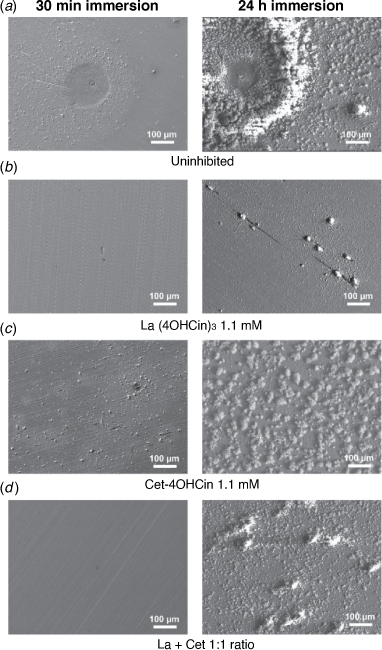
Characterisation of 80HLES surfaces immersed in the solutions for 30 min and 24 h was conducted to compare their appearance and relate to the electrochemical data and overall corrosion inhibition. Representative SEM micrographs are presented in Fig. 6, with 24 h EDS data shown in Supplementary Fig. S3.
|
After 30 min immersion, Fig. 6c shows corrosion product deposition as well as localised attack on the steel surface in the presence of the Cet-4OHCin inhibitor alone. This was visibly similar to the uninhibited sample surface shown in Fig. 5a. However, in the presence of either La(4OHCin)3 (Fig. 6b) or the La + Cet mixture (Fig. 6d), the steel surface was relatively pristine, as shown by the lack of locally attacked sites, lack of corrosion product deposits and visibility of original polishing marks. These results support the observations from the electrochemical data, whereby Cet-4OHCin alone in solution offered little change in icorr compared to the uninhibited control whilst the La(4OHCin)3 and La + Cet systems both provided significant inhibition.
Immersion for 24 h in the uninhibited solution resulted in greater corrosion product deposits on the general surface that increased significantly around locally attacked sites (Fig. 6a). EDS in Supplementary Fig. S2 shows increased sulfur present at these sites, suggesting localised corrosion is initiated at sulfide inclusions.
The La(4OHCin)3 sample surface had isolated deposits and EDS showed the presence of increased amount of La present at these (Supplementary Fig. S2b), most likely as mixed oxide/hydroxide and La(4OHCin)x species.[28] The surfaces for samples exposed to either the La + Cet mixture or the La(4OHCin)3 were similar in appearance, however more fine deposits can be seen in Fig. 6d for the mixture. Again, these deposits contain higher amounts of La and so are likely to be a reaction of the inhibitor with the surface as opposed to common corrosion product. In both cases there is an increased presence of sulfur at these deposits, suggesting that these La deposits predominantly form in the vicinity of localised corrosion occurring at sulfide inclusions. Such areas are cathodic compared to the bulk and become active sites of localised corrosion. The presence of La at these sites is consistent with the mechanism previously proposed and the 24 h polarisation presented here, whereby the La part of the molecule predominantly acts as a cathodic inhibitor.[28] The increased number of deposits in the mixture may be indicative of increased surface activity e.g. corrosion initiation which is quickly stifled as a result of inhibitor compound being deposited. This may also explain the less stable electrochemical response of the La + Cet as compared to the La(4OHCin)3 seen in the polarisation resistance (Supplementary Fig. S1).
While the samples immersed in the La(4OHCin)3 and La + Cet solutions had a consistent level of deposition over the entire steel surface, those immersed in the uninhibited solution and the Cet-4OHCin solution resulted in non-uniform corrosion. Optical images of the whole surface for both samples after 24 h are shown in Supplementary Fig. S3. More heavily deposited regions can be observed as rust-coloured areas on the uninhibited sample in Supplementary Fig. S3a and a dark grey colour on the Cet-4OHCin (Supplementary Fig. S3b). Away from these heavily deposited regions, representative SEM micrographs for the uninhibited (Supplementary Fig. S3c) and the Cet-4OHCin (Supplementary Fig. S3d) show significantly less corrosion product as compared to their counterparts in the heavily deposited regions shown in Fig. 6a and c.
Localised attack analysed by optical profilometry
To quantify the penetration of corrosion into the steel, corrosion product was chemically removed from samples immersed for 30 min and 24 h and the underlying corroded steel were examined with optical profilometry to measure damage depths and diameters at locally corroded sites. The large deposits of corrosion product tended to act as ‘caps’ for underlying localised corrosion. While the surface of the steel was not in a passive state and thus classical pitting did not occur, the removal of the corrosion product confirmed the presence of pit-like attack beneath the corrosion product. With this in mind, it was assumed that all sites undergoing localised corrosion were cone shaped. This allowed the approximation for the volume of corroded material using the maximum corroded depth (average of the 10 deepest sites), corroded diameter (average of the 10 widest sites) and the average number of corroded sites per square micrometre.
Supplementary Fig. S4 presents the approximate corroded volume results vs the maximum corroded depth for each inhibitor after 30 min and 24 h immersion periods. The visual difference observed across the samples for both the uninhibited and Cet-4OHCin (Supplementary Fig. S3), appears to only influence the surface deposit. This is assumed since subsequent to the removal of these surface deposit, the differences in the maximum corroded depth, width or number of sites between the two zones in both cases were insignificant. Therefore, the average values from the zones that appeared rusted and less rusted were combined in Supplementary Fig. S4.
The greatest differences between the samples is in the corroded volume, which is presented on a log scale, in comparison to the depths, which are relatively similar. After 30 min, the corroded volume of the Cet-4OHCin sample was greater than the uninhibited sample, both of which had greater corroded volume than La(4OHCin)3 and La + Cet.
After 24 h, the corroded volume has substantially increased for the uninhibited and Cet-4OHCin, with the latter having the largest volume, indicating accelerated localised corrosion compared to the uninhibited sample. While remaining low compared to the control, the La(4OHCin)3 yielded slightly higher corroded volumes than the La + Cet. These results are mostly consistent with the electrochemistry, with the increased localised corrosion in the case of Cet-4OHCin after 24 h being the exception.
Cet-4OHCin and Cet-Br toxicity analysis in human cell models
While Cet-4OHCin has been shown here to be an effective anti-microbial, it is also important to investigate its toxicity to humans. As an initial indication of toxicity the effect of Cet-4OHCin on various human cell cultures was compared to the well-established antimicrobial, cetrimonium bromide (Cet-Br). Cet-Br is a known safe additive in numerous cosmetic products and is a starting material for synthesising Cet-4OHCin.[29]
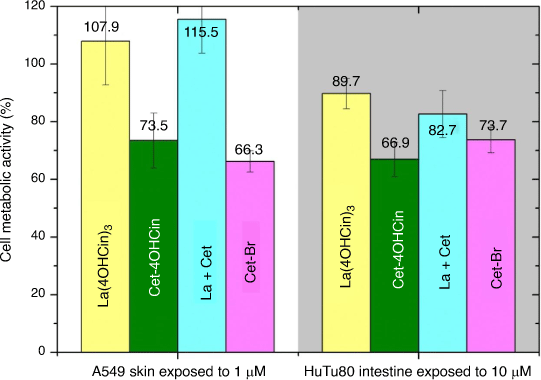
Cytotoxicity tests were conducted on the cultured skin and intestine cell models at predetermined time intervals from 15 min to 3 h exposure, in the presence of La(4OHCin)3, Cet-4OHCin, the La + Cet mixture and Cet-Br. Skin and intestine cell cytotoxicity tests model the potential effect on skin exposure and ingestion of the compounds. The absorption of Cet-Br into skin and intestine mucosa has been reported at 0.59 and 10% respectively.[29] To approximate this, we present the results for skin and intestine cells exposed to inhibitor concentrations of 1 and 10 µM respectively in Fig. 7.
|
The high metabolic activity of the cells in the presence of La(4OHCin)3 demonstrates its relatively low toxicity expected towards humans at least through skin or ingestion. Increased sensitivity is observed for both Cet-4OHCin and Cet-Br, however, the reductions in cell metabolism can still be considered relatively small.
Interestingly the cells are not as sensitive to the La + Cet mixture compared to Cet-4OHCin alone, implying reduced sensitivity of the mixture towards the human cells compared to Cet-4OHCin tested alone. Given that the results for the Cet-4OHCin containing samples were very similar or better than that of the Cet-Br it is an excellent indication that it is safe for human contact.
Discussion
Corrosion inhibition of the La + Cet mixture compared to La(4OHCin)3 alone
As shown from the electrochemical and optical profilometry data, the 30 min test results indicated La + Cet was initially more effective at preventing abiotic corrosion than La(4OHCin)3 alone, with a considerably lower icorr (Table 2, 0.68 and 1.14 µA cm−2 respectively) and similar average localised corrosion volume, as calculated from profilometry analysis (Supplementary Fig. S4a). By 24 h of exposure however, the La + Cet mixture maintained its low icorr while the La(4OHCin)3 now exhibited a lower icorr (0.47 and 0.28 µA cm−2 respectively). As expected with ongoing corrosion the localised corrosion volume increased slightly, but at a similar rate for both (Supplementary Fig. S4b). Considering the La + Cet mixture is predominantly an anodic inhibitor, within the first 30 min of immersion there is a higher concentration of inhibitor in solution to deposit and inhibit corrosion attack on the steel, resulting in the lower icorr of La + Cet relative to La(4OHCin)3. Since initial deposition onto the steel for the La(4OHCin)3 inhibitors has been proposed to occur via the carboxyl functional group, an increased amount of this anion present is likely the reason for this improved performance after 30 min.[30] This suggests that Cet-4OHCin improves the robustness of the deposited film within the first 30 min of immersion through increased film deposition due to the higher local concentration of 4OHCin− anions. Since the La(4OHCin)3 is at its solubility limit, adding the Cet-4OHCin is a way of getting more of the 4OHCin− anion into solution and subsequently onto the surface. By being able to by-pass the solubility limit in this way along with not being detrimental to the chemical speciation, an improved level of inhibition compared to the La + CetNal mixture has been facilitated.
The process whereby the carboxyl functional group attaches to the metal surface would also bring the La3+ and the Cet+ cations towards the steel surface. However, as more Cet+ cations complex into the film over the steel, steric hinderance and competition to other inhibitor species, such as La, may occur. This may effectively limit the deposition of the inhibitor, resulting in reduced protection over time. Forêt et al.[31] reported a possible steric obstruction effect from multiple nitrogen atoms limiting film formation of amines and steric hindrance has also been shown to reduce corrosion inhibition by the nitrogen containing piperidin-4-one derivatives.[32] However, when in a mixture (such as the La + IMI system, which again both include the cinnamate), better corrosion inhibition was observed when the inhibitors had matching chain lengths.[33] This may explain why the previously tested La + IMI[10] showed a slightly lower icorr and corroded volume than the La + Cet system as the imidazolinium cation has a similar chain length to the 4OHCin− anion, unlike the cetrimonium cation. Additionally, it has previously been shown that the La present in La(4OHcin)3 takes time to deposit and reduce cathodic reactions,[34] which would explain the increased protection over 24 h. In the La+Cet mixture however, the cetrimonium cation present may compete with the La, reducing its ability to affect the cathodic reaction.
Cytotoxicity of Cet-4OHCin versus Cet-Br
The MTT test that measures cellular metabolic activity is a simple and relatively inexpensive method to estimate toxicity of a chemical towards specific human cells. It can act as a precursor for expensive animal testing and has been shown to correlate to toxicity in the form of LD50 values.[35] We expect Cet-4OHCin will be relatively safe for exposure to the cell lines tested in this work due to the similar cell metabolic activity after 15 min for Cet-4OHCin and Cet-Br (Fig. 7).
Additionally, exposing the inhibitors directly to cultured human cells is not equivalent to an in vivo situation where the layers of the skin provide an increased resistance to chemicals. In vitro data for dermal penetration is expected to be overestimated compared to in vivo.[36] We therefore expect the reductions in cell density in our work to be overestimated, suggesting minimal effect on human skin cells. These factors may explain why Cet-Br is considered safe in cosmetic products (between 1 and 30 g L−1) even though cell density was reduced by just over 30% on cultured skin cells in Fig. 6.[29] We assume the intestinal cells will behave in a similar fashion and sensitivity towards the inhibitors will be less under in vivo conditions.
The La(4OHCin)3 inhibitor was non-toxic to HaCaT cells and had very limited sensitivity towards intestinal cells. In addition to the low sensitivity to bacteria, this provides evidence that La(4OHCin)3 is a strong alternative to the toxic chromate inhibitors.
Interestingly the La + Cet mixture yielded similar cell densities as La(4OHCin)3 alone in solution, indicating the presence of La(4OHCin)3 in solution can nullify the sensitivity of Cet-4OHCin towards human cells whilst still providing significant reductions in bacteria density and abiotic corrosion. This makes the La + Cet mixture a very strong candidate as a more environmentally friendly MIC inhibitor for high strength steel while simultaneously being relatively safe to human skin and intestine exposure.
Conclusions
A range of inhibitors and their mixtures have been investigated for their effects on corrosion damage and microbial populations to identify structural factors required of a multifunctional MIC inhibitor. The inhibitor mixture of La(4OHcin)3 and Cet(4OHCin) (La + Cet) was able to significantly reduce the bacteria growth of strains associated with MIC, while the level of corrosion inhibition for this inhibitor mixture remained similar to the well tested La(4OHCin)3. No other inhibitor combination in this work was able to achieve such definitive multifunctionality, thus validating the potential of this mixture as an MIC inhibitor. The Cet-4OHCin compound and the La + Cet mixture were also relatively non-toxic to HaCaT skin and HuTu80 intestinal cells. These results show the La + Cet system to be an attractive alternative to traditional toxic and/or carcinogenic compounds, such as chromate-based inhibitors.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
DST Group is credited for project funding. A. E. Somers is grateful to Deakin University for funding his Alfred Deakin Post-Doctoral Fellowship. A. E. Somers and M. Forsyth are grateful for funding from the Australian Research Council through a Discovery Project, DP180101465.
Supplementary material
Supplementary material is available online.
Acknowledgements
In recognition of this Special Issue honouring Professor Glen Deacon, we would like to acknowledge his continued support and collaboration. One of the compounds used in this work was initially developed in collaboration with Prof. Deacon, as reflected in the reference list, and this is a collaboration that continues to thrive to this day with work we are doing on new rare earth carboxylates. The Deakin University Electron Microscopy Facilities are acknowledged for use of their SEM/EDS instruments.
References
[1] GS Frankel, J Electrochem Soc 1998, 145, 2186.| Crossref | GoogleScholarGoogle Scholar |
[2] G Wranglen, Corrosion Sci 1974, 14, 331.
| Crossref | GoogleScholarGoogle Scholar |
[3] S Kakooei, MC Ismail, B Ariwahjoedi, World Appl Sci J 2012, 17, 524.
[4] CA Loto, Int J Adv Manuf Technol 2017, 92, 4241.
| Crossref | GoogleScholarGoogle Scholar |
[5] HA Videla, LK Herrera, Int Microbiol 2005, 8, 169.
| 16200495PubMed |
[6] R Jia, T Unsal, D Xu, Y Lekbach, T Gu, Int Biodeterior Biodegradation 2019, 137, 42.
[7] D Xu, R Jia, Y Li, T Gu, World J Microbiol Biotechnol 2017, 33, 1.
[8] C de Carvalho, Frontiers in Marine Science 2018, 5,
| Crossref | GoogleScholarGoogle Scholar |
[9] YF Wang, H Su, YL Gu, X Song, JS Zhao, OncoTargets Ther 2017, 10, 4065.
[10] RA Catubig, WC Neil, G McAdam, R Yunis, M Forsyth, AE Somers, J Electrochem Soc 2020, 167, 021503.
[11] F Blin, SG Leary, K Wilson, GB Deacon, PC Junk, M Forsyth, J Appl Electrochem 2004, 34, 591.
[12] M Seter, MJ Thomson, A Chong, DR MacFarlane, M Forsyth, Aust J Chem 2013, 66, 921.
[13] AE Somers, GB Deacon, BRW Hinton, DR MacFarlane, PC Junk, MYJ Tan, et al. J Indian Inst Sci 2016, 96, 285.
[14] AL Chong, M Forsyth, DR Macfarlane, Electrochim Acta 2015, 159, 219.
[15] GB Deacon, M Forsyth, PC Junk, SG Leary, WW Lee, Z Anorg Allg Chem 2009, 635, 833.
[16] M Seter, MJ Thomson, J Stoimenovski, DR MacFarlane, M Forsyth, Chem Commun 2012, 48, 5983.
[17] N Kip, JA van Veen, Isme J 2015, 9, 542.
| 25259571PubMed |
[18] ASTM. G31-72 (reproved 2004) Standard practice for laboratory immersion corrosion testing of metals. ASTM International; 2004.
[19] S Barnartt, J Electrochem Soc 1952, 99, 549.
[20] ASTM. G1-03 (Reapproved 2017) Standard practice for preparing, cleaning, and evaluating corrosion test specimens. ASTM International; 2017. p. 5.
[21] L Kreja, HJ Seidel, Chemosphere 2002, 49, 105.
| 12243325PubMed |
[22] J Lopez-Garcia, M Lehocky, P Humpolicek, P Saha, J Funct Biomater 2014, 5, 43.
| Crossref | GoogleScholarGoogle Scholar | 24956439PubMed |
[23] D Bajpai, VK Tyagi, J Oleo Sci 2006, 55, 319.
| Crossref | GoogleScholarGoogle Scholar |
[24] AL Chong, JI Mardel, DR MacFarlane, M Forsyth, AE Somers, ACS Sustainable Chem Eng 2016, 4, 1746.
[25] KM Docherty, CF Kulpa, Green Chem 2005, 7, 185.
[26] M Kalinowska, R Swisocka, W Lewandowski, J Mol Struct 2007, 834–836, 572.
[27] M Seter, GMA Girard, WW Lee, G Deacon, P Junk, B Hinton, et al. AIMS Mater Sci 2015, 2, 1.
| Crossref | GoogleScholarGoogle Scholar |
[28] M Forsyth, M Seter, B Hinton, GB Deacon, P Junk, Aust J Chem 2011, 64, 812.
[29] LC Becker, WF Bergfeld, DV Belsito, RA Hill, CD Klaassen, D Liebler, et al. Int J Toxicol 2012, 31, 296S.
| 23283705PubMed |
[30] AE Somers, BRW Hinton, C de Bruin-Dickason, GB Deacon, PC Junk, M Forsyth, Corrosion Sci 2018, 139, 430.
[31] Forêt C, Stoianovici G, Chaussec G, Bache aD, Kolk Cz, Hater WD, Editors. Study of efficiency and stability of film forming amines (FFA) for the corrosion protection of the carbon steel in water circuits. 7/09/09. Edinburgh, United Kingdom: EUROCORR; 2008.
[32] B Thirumalairaj, M Jaganathan, Egypt J Pet 2016, 25, 423.
[33] SY Shiao, V Chhabra, A Patist, ML Free, PDT Huibers, A Gregory, et al. Adv Colloid Interface Sci 1998, 74, 1.
[34] M Forsyth, M Seter, MY Tan, B Hinton, Corros Eng Sci Technol 2014, 49, 130.
[35] AB Frank, C Monica, In Vitro Cell Dev Biol Anim 1998, 34, 631.
| Crossref | GoogleScholarGoogle Scholar |
[36] B van Ravenzwaay, E Leibold, Hum Exp Toxicol 2004, 23, 421.
| 15497817PubMed |
† An earlier form of this article was posted to ChemRxiv, https://doi.org/ 10.26434/chemrxiv.13719649.v1.




