Lithium: From Bipolar to Batteries
Victoria L. Blair AA Department of Chemistry and Biochemistry, Monash University, Melbourne, Vic. 3800, Australia. Email: victoria.blair@monash.edu
Australian Journal of Chemistry 72(12) 931-932 https://doi.org/10.1071/CH19596
Submitted: 18 November 2019 Accepted: 19 November 2019 Published: 29 November 2019
Known as the lightest metal in the periodic table, lithium’s natural existence is rare compared with its two siblings helium and hydrogen, all created during the Big Bang.[1] It was discovered in its mineral form petalite (LiAlSi4O10) by the Brazilian Jozè Bonifácio Andralda e Silvia, who also first observed its crimson flame when the mineral was thrown into fire. Not surprising then that its name stems from the Greek word for stone: lithos. Swedish chemist Johan August Arfvedson deduced in 1817 that this new lighter alkali metal (Na was discovered first by Humphry Davy in 1807) was lithium, although he was unsuccessful in separating it from its ore. It was not until 1855 that an actual sample of lithium metal (Fig. 1) was able to be extracted by the chemists Robert Bunsen and Augustus Matthiessen through electrolysis of molten lithium chloride.[2]
Lithium represents 0.005 % of the earth’s crust, making it the 25th most abundant element. It is primarily mined in Australia from the lithium aluminium inosilicate Spodumene [LiAl(SiO3)2] with over 1.5 million tonnes extracted in 2017 alone, with Chile and China following next as top producers.[3] Due to its mass extraction, it is unsurprising to find lithium used in a variety of everyday materials; for instance, as an alloy with magnesium or aluminium allowing the production of strong lightweight aircraft, as lithium oxide in ceramics and glass manufacture, and as the vital component in high temperature grease (lithium stearate) that also works in Antarctic conditions (below −60°C). Most catastrophically, it is the key element in hydrogen bombs – with lithium-deuteride, in which the 6 Li isotope is used as the fusion fuel.
From a biological perspective, lithium is not considered an essential trace element. In fact, it was discovered to be moderately toxic when lithium chloride was substituted in place of common salt. However, possibly one of the most notable and life-changing discoveries in lithium salt chemistry of the 20th century is lithium carbonate, which has been used in the treatment of millions of sufferers of bipolar affective disorder. Discovered by Australian psychiatrist John Cade in 1948, he noticed considerable calming effects on normally active guinea pigs upon injection of a 0.5 % solution of lithium carbonate. With no ethics committees in those days, Cade progressed to taking it himself for a week, to govern it was ‘safe’ before trialling it in one of his most manic patients at Bundoora Repatriation Mental Hospital.[4] The extraordinary calming effects were so great that the patient was transferred out of psychiatric care and was soon able to return to work. Unpatentable due to it being a natural salt, it still remains the single most effective treatment for bipolar disorder today, making lithium the ‘penicillin of mental health’.[5]
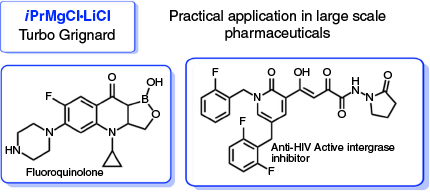
Not only is lithium effective as a drug, but its use in organometallic chemistry has been fundamental. It is salient to recognise that Johanna Holtz and Wilhelm Schlenk discovered the now ubiquitous synthetic reagents, MeLi, EtLi, and PhLi, just over 100 years ago, in the process giving the organometallic chemist their lithium toolkit.[6] With nearly 95 % of drug syntheses relying upon lithium-based reagents at some point in their preparation, the value of their discovery cannot be underestimated.[7] Reflecting its human health effects, the power of ‘salt effects’ in organometallic chemistry can have drastically different reactivity and selectivity outcomes compared with the analogous salt-free reactions.[8] One of the most common and commercially available reagents is Knochel’s Turbo Grignard [iPrMgCl LiCl][9] which exhibits enhanced halogen-magnesium exchange power towards a wide range of functionalised aromatic and heterocyclic synthons, usually inert in the absence of the lithium chloride.[10] Only 15 years old, its commercial availability and unique reactivity has seen the reagent successfully utilised in the industrial production of numerous marketed pharmaceutical drugs and other up-scaled industrial processes (Fig. 2).[11] Another synergistic approach, where lithium plays a vital role, is the pairing of an alkali metal (Li, Na or K) with a more subordinate metal partner (e.g. Mg, Zn, Al, Ga) to give a bimetallic super base reagent capable of transforming inert C-H bonds to reactive, useful M-C bonds (Scheme 1). Coined alkali metal mediated metallators (AMMM) by Mulvey[12] the alkali metal is essential to enforce reactivity, while the other spectator partner is often the one that performs the synthetic transformation (metalation). Lithium dominates these mixed-metal systems, pairing up with multiple main group partners.[13] Continued development of such super base systems, through isolation of key reactive intermediates and probing of substrate scope, will allow better guidance in their synthetic applications in the future, of which lithium, as either a salt or metal partner, will ultimately continue to play a pivotal role.
LiCl][9] which exhibits enhanced halogen-magnesium exchange power towards a wide range of functionalised aromatic and heterocyclic synthons, usually inert in the absence of the lithium chloride.[10] Only 15 years old, its commercial availability and unique reactivity has seen the reagent successfully utilised in the industrial production of numerous marketed pharmaceutical drugs and other up-scaled industrial processes (Fig. 2).[11] Another synergistic approach, where lithium plays a vital role, is the pairing of an alkali metal (Li, Na or K) with a more subordinate metal partner (e.g. Mg, Zn, Al, Ga) to give a bimetallic super base reagent capable of transforming inert C-H bonds to reactive, useful M-C bonds (Scheme 1). Coined alkali metal mediated metallators (AMMM) by Mulvey[12] the alkali metal is essential to enforce reactivity, while the other spectator partner is often the one that performs the synthetic transformation (metalation). Lithium dominates these mixed-metal systems, pairing up with multiple main group partners.[13] Continued development of such super base systems, through isolation of key reactive intermediates and probing of substrate scope, will allow better guidance in their synthetic applications in the future, of which lithium, as either a salt or metal partner, will ultimately continue to play a pivotal role.

|
|
It is hard to imagine a modern-day world without portable electronics, mobile phones, and laptops; ultimately all this and more would not be possible without the protagonist lithium. Its fundamental role in the development of lithium-ion batteries, due to its lightweight and reactive nature, was pioneered by Stanley Whittingham who developed the first functional lithium battery in the 1970s.[15] Shortly after this, both John Goodenough, who doubled its power capacity implementing cobalt oxide in the cathode,[16,17] and Akira Yoshino, who made it safer by using petroleum coke to intercalate the lithium ions in the anode,[18] collectively created the lithium-ion rechargeable battery we know and rely upon today. This significant achievement was recognised this year with all three pioneers awarded the Nobel Prize in Chemistry.[19] Looking to the future, lithium has the potential to pave the way to cleaner energy technologies and electric vehicles, by allowing the ability to store energy which will have a dramatic impact on society and the planet.
So, from bipolar to batteries, lithium really is the element that just keeps on giving.
Conflicts of Interest
The author declares no conflicts of interest.
Acknowledgements
This research did not receive any specific funding.
References
[1] A. M. Boesgaard, G. Steigman, Annu. Rev. Astron. Astrophys. 1985, 23, 319.| Crossref | GoogleScholarGoogle Scholar |
[2] J. Emsley, Elements 1–112, 114, 116 and 117 2012 (Oxford University Press: Oxford).
[3] T. J. Brown, N. E. Idoine, E. R. Raycraft, S. F. Hobbs, R. A. Shaw, P. Everett, C. Kresse, E. A. Deady, T. Bide, World Mineral Production 2013–17 2019 (British Geological Survey: Keyworth, Nottingham).
[4] J. F. J. Cade, Med. J. Aust. 1949, 2, 349.
| Crossref | GoogleScholarGoogle Scholar |
[5] A. Westmoore, G. de Moore, Finding Sanity: John Cade, Lithium and the Taming of Bipolar Disorder 2016 (Allen and Unwin: Sydney).
[6] W. Schlenk, J. Holtz, Chem. Ber. 1917, 50, 262.
| Crossref | GoogleScholarGoogle Scholar |
[7] D. B. Collum, Acc. Chem. Res. 1993, 26, 227.
| Crossref | GoogleScholarGoogle Scholar |
[8] E. Hevia, R. E. Mulvey, Angew. Chem. Int. Ed. 2011, 50, 6448.
| Crossref | GoogleScholarGoogle Scholar |
[9] A. Krasovskiy, P. Knochel, Angew. Chem. Int. Ed. 2004, 43, 3333.
| Crossref | GoogleScholarGoogle Scholar |
[10] D. S. Ziegler, B. Wei, P. Knochel, Chem. – Eur. J. 2019, 25, 2695.
| Crossref | GoogleScholarGoogle Scholar | 30230067PubMed |
[11] R. L.-Y. Bao, R. Zhao, L. Shi, Chem. Commun. 2015, 51, 6884.
| Crossref | GoogleScholarGoogle Scholar |
[12] R. E. Mulvey, Acc. Chem. Res. 2009, 42, 743.
| Crossref | GoogleScholarGoogle Scholar | 19348412PubMed |
[13] S. D. Robertson, M. Uzelac, R. E. Mulvey, Chem. Rev. 2019, 119, 8332.
| Crossref | GoogleScholarGoogle Scholar | 30888154PubMed |
[14] S. E. Baillie, V. L. Blair, D. C. Blakemore, D. Hay, A. R. Kennedy, D. C. Pryde, E. Hevia, Chem. Commun. 2012, 48, 1985.
| Crossref | GoogleScholarGoogle Scholar |
[15] M. S. Whittingham, Science 1976, 192, 1126.
| Crossref | GoogleScholarGoogle Scholar | 17748676PubMed |
[16] J. B. Goodenough, K. Mizushima, U.S. Patent No. 4,357, 215 1982.
[17] K. Mizushima, P. C. Jones, P. J. Wiseman, J. B. Goodenough, Mater. Res. Bull. 1980, 15, 783.
| Crossref | GoogleScholarGoogle Scholar |
[18] A. Yoshino, Angew. Chem. Int. Ed. 2012, 51, 5798.
| Crossref | GoogleScholarGoogle Scholar |
[19] The Nobel Prize in Chemistry 2019. Nobel Media AB 2019. 3 November 2019. Available at: NobelPrize.org



