Oil Spill Source Identification Using Colorimetric Detection
Walmiria Woodland A , Richard Lim A , Cherie Motti B C , Paul Irving D , Jun Wang A , Mark Payne A , Peter C. Junk A and George Vamvounis
A and George Vamvounis  A E
A E
A College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia.
B AIMS@JCU, Division of Research and Innovation, James Cook University, Townsville, Qld 4811, Australia.
C Australian Institute of Marine Science, PMB no. 3, Townsville MC, Qld 4810, Australia.
D Australian Maritime Safety Authority, Braddon, ACT 2612, Australia.
E Corresponding author. Email: george.vamvounis@jcu.edu.au
Australian Journal of Chemistry 72(11) 874-880 https://doi.org/10.1071/CH19336
Submitted: 19 July 2019 Accepted: 15 August 2019 Published: 26 September 2019
Abstract
The colorimetric detection of polycyclic aromatic hydrocarbons (PAHs) was investigated for the quick and easy identification of likely oil spill offenders. In this new technology, photochromic compounds were used to sense PAHs by varying their photoswitching capacity. To that end, three photochromes were designed and showed varying degrees of photoswitching inhibition, depending on PAH analyte, photochrome, and excitation wavelength. PAH mixtures that mimic oil spills showed the same varying response and demonstrated the accuracy of this technology. To prove the applicability of this technology, an array was assembled, using the three photochromes at three excitation wavelengths, and tested against authentic crude oil samples. Not only could these samples be differentiated, but also weathering of two distinctly different oil samples showed limited variation in response, demonstrating that this may be a viable technique for in situ oil identification.
Introduction
Oil spills in marine environments can have detrimental long-term effects on marine ecosystems.[1] With shipping (and other industries) under greater financial and timetable constraints, the number of worldwide illegal pollution events (both intentional and unreported accidents) is growing,[1] in particular, in crowded ports and harbours. For instance, since the year 2000 there have been more than 3000 oil pollution incident reports recorded for Australian waters – most of which have occurred in harbours.[1] Therefore, a quick, portable, robust, and reliable oil screening and identification technology is needed to analyse and identify the source of the spill.
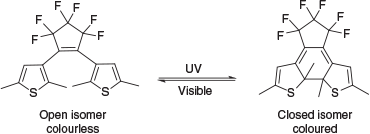
The current available analytical processes to identify oils are cumbersome laboratory-based technologies, requiring extensive, expensive, and time-consuming evidential quality sampling and analysis of all possible oily spaces (fuel, waste, cargo, and oil tanks and pipes) in each vessel. One characteristic of oils frequently used to discriminate oils is the relative composition of various polycyclic aromatic hydrocarbons (PAHs).[2,3] The relative amounts of PAHs can be determined through various chromatographic techniques, such as gas chromatography (GC).[4–6] Although highly effective, GC is not portable and requires hazardous hydrogen and expensive helium gases. Therefore, spectroscopic determination provides the opportunity to revolutionise this methodology, especially as light sources and sensors are becoming smaller and more powerful.[6–8] Spectroscopic methods for identifying and quantifying PAHs have been developed and include fluorescence, absorption, infrared, and Raman spectroscopies.[2,5–10] To that end, we recently reported a new colorimetric method for the detection of individual PAHs and mixtures thereof using a photochromic compound.[11] Photochromic compounds are materials that reversibly switch between two isomeric forms through photoexcitation (Scheme 1), which present distinct optical properties (often colourless and coloured).[12,13] In this technology, the degree of photoisomerisation varied according to the molar absorptivity (ϵ) of the PAHs present and the photochromic compound at a given excitation wavelength.[11] In general, the more the PAH absorbed the excitation light, the more it inhibited the photoisomerisation reaction, and hence the difference in the colour intensity. This is a powerful technique; the simple visible change in colour could allow non-specialist operators to use this technology and to make a quick and accurate assessment of PAH content.
|
In the present work, we extend our previous study,[11] where herein we investigated the effect of the photochrome structure on the PAH sensing capability and developed an array that could further distinguish individual PAHs. Furthermore, this array technology was applied to four crude oil samples to demonstrate its feasibility. Finally, we analysed two weathered oil samples to establish the reliability of this technology.
Results and Discussion
The diarylethene (DAE) class of photochromic compounds used in this study have a unique combination of properties, including thermal stability, fatigue resistance, fast response, and high cyclisation quantum yields.[13–17] Materials with these properties are routinely used in the optoelectronic industry for molecular switches, optical memory devices, and chemical sensors.[13,18] In this study, three DAEs were designed to vary the extent of conjugation in order to expand the optical window of the array, for which the conjugation length is DAE1 < DAE2 < DAE3 (Fig. 1).
Optical Characterisation
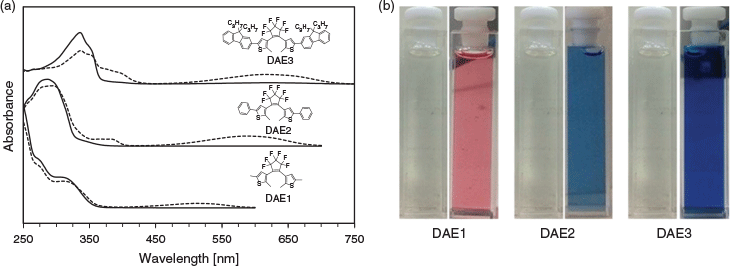
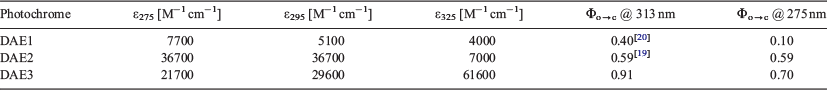
The absorption spectra of the three photochromes in the ring-opened and -closed forms are depicted in Fig. 2a. The three photochromic compounds are colourless in the open form (Fig. 2b). The open form of DAE1 has a similar absorption spectrum to that of DAE2, whereas DAE3 revealed a red-shifted absorption spectrum. The photochromic compounds follow the same trend in the closed form. An important optical property for PAH sensing is the molar absorptivity of the DAE, which dictates how well the DAE absorbs light at a particular wavelength. Table 1 summarises the absorption characteristics of the three DAEs and their corresponding molar absorptivities at particular wavelengths. DAE1 has a low molar absorptivity at all three wavelengths. DAE2, on the other hand, has a low molar absorptivity at 325 nm but a high molar absorptivity at both 275 and 295 nm. Conversely, DAE3 has a very high molar absorptivity at 325 nm and lower molar absorptivity at 275 and 295 nm, although still higher than that of DAE1. Therefore, this series of photochromic compounds span the UV range where PAHs absorb.
|
The photoconversion quantum yield (Φ) (from ring opened to closed) dictates the number of excited molecules that convert from the open to the closed form; the larger the quantum yield of photoconversion, the higher the probability that the PAH will affect the photoconversion process. That is, if the quantum yield of photoconversion is unity, then every photon absorbed by the opened DAE will cyclise the DAE to its closed form. The presence of a light-absorbing PAH may therefore have the most pronounced effect on this photoconversion process, as compared with a DAE with a low photoconversion. In this series of photochromes, the quantum yield at 313 nm excitation of DAE1 is 0.40, of DAE2 is 0.59, and of DAE3 is 0.91.[19,20] The high photoconversion of DAE3 is likely due to the extra stabilisation that occurs through the extended conjugation imparted by the fluorenyl moieties.
Sensing Individual PAHs
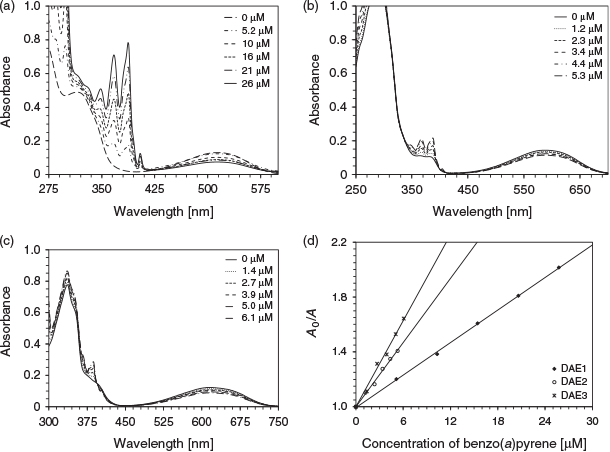
The photoswitching inhibition of DAE1, DAE2, and DAE3 was performed using chrysene, pyrene, benzo(a)pyrene, phenanthrene, naphthalene, and toluene. Fig. 3 depicts the absorption spectra due to photoisomerisation of the three DAEs as a function of benzo(a)pyrene concentration. The degree of photoswitching inhibition can be represented by a linear relationship between the PAH concentration and the change in the closed-form photochrome absorbance (Eqn 1). This relationship is analogous to the Stern–Volmer fluorescence quenching (Fig. 3d); A0 and A are the absorption intensity in the absence and presence of analyte (PAH) respectively, Kpsv is the pseudo Stern–Volmer constant, and [PAH] is the analyte concentration.[21]
|

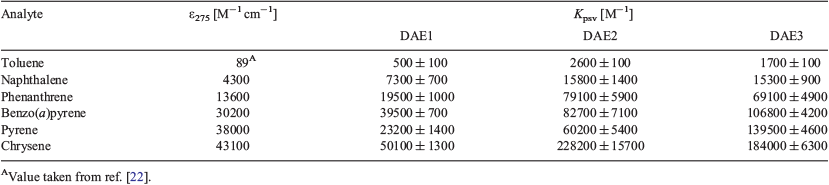
Table 2 summarises the Kpsv values of the various DAEs with the corresponding discrete PAH when excited at 275 nm. For DAE3, the trend in the analyte sensitivity is toluene < naphthalene < phenanthrene < benzo(a)pyrene < pyrene < chrysene, which follows exactly the molar absorptivity of the PAH at 275 nm. This trend indicates that the analyte molar absorptivity directly correlates to the photoswitching inhibition, where the analyte attenuates the number of exciting photons for photoisomerisation. DAE1 and DAE2 behave similarly except in the presence of pyrene, a response attributed to the sharpness of the analyte absorption at the excitation wavelength.[11]
|
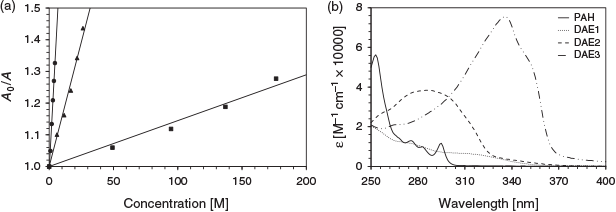
The effect of the excitation wavelength on the photoswitching inhibition of DAE3 using phenanthrene as the analyte is depicted in Fig. 4a. The photoswitching inhibition was greatest at 275 nm excitation and lowest at 325 nm. This excitation wavelength dependency of the photoswitching inhibition correlates well with the optical density of the analyte at the excitation wavelength. Fig. 4b illustrates the absorption spectrum of phenanthrene compared with that of the photostationary state of the three DAEs. The analyte absorbs well at 275 and 295 nm, whereas the absorption at 325 nm is negligible. Therefore, the more the analyte absorbs at the excitation wavelength, the higher the photoswitching inhibition, which is consistent with the above analyte molar absorptivity and photoswitching inhibition trend. These DAEs in combination have the potential to provide selectivity for differentiating PAH fingerprints.[11]
|
The effect of the DAE structure on the photoswitching inhibition was analysed. The PAH sensitivity follows the general trend of DAE1 < DAE2 ≈ DAE3 (Fig. 5), which is likely due to the quantum yield of photoconversion from the open to the closed state at the excitation wavelength. At 275 nm, DAE1 has the lowest photoconversion efficiency, hence the lowest Kpsv. DAE2 and DAE3 have a similar photoconversion efficiency at 275 nm (Table 1), and therefore their Kpsv values are similar. Hence, the response of the photoswitching for a particular PAH can vary based on the DAE and the excitation wavelength used. This property may be helpful in further differentiating PAH fingerprints in oil spills.
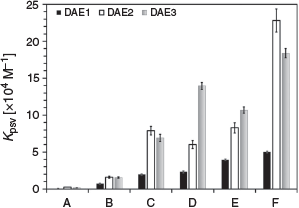
|
Oil Spill Mimics
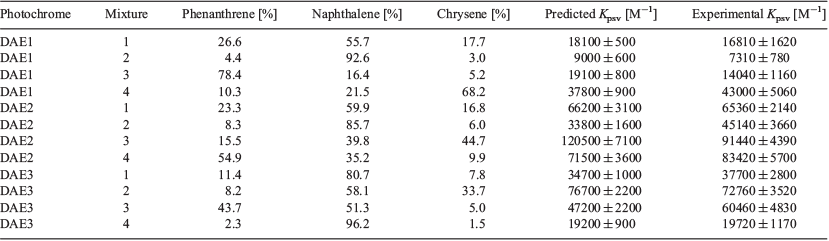
Four oil spill mimics were created by simply mixing various compositions of phenanthrene, naphthalene, and chrysene, thereby generating unique known PAH fingerprints. As for individual PAHs, the colorimetric analysis of these mixtures was performed in the presence of DAE1, DAE2, and DAE3 (Table 3). The Kpsv of each mixture was predicted by multiplying the Kpsv values of the analytes by the mole ratio. Comparison of the theoretical Kpsv values with the experimental Kpsv values provided a means by which the accuracy of the response could be determined (Table 3). The theoretical and experimental results are similar, indicating this technology can reliably differentiate unique combinations of PAHs, as is present in oil samples.
|
Oil Spill Assessment
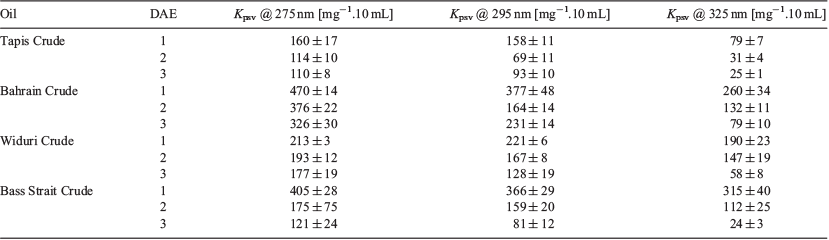
To determine the efficacy of this technology, the response of four different crude oil samples (Tapis Crude, Bahrain Crude, Widuri Crude, and Bass Strait Crude) was analysed. The photoswitching inhibition experiment was repeated using these oils to determine the colorimetric response as a function of the excitation wavelength and DAE to generate a 3 × 3 array (Table 4). Each of the four oil samples exhibited a unique response according to the excitation wavelength and DAE (similar to that of the individual PAHs). The 3 × 3 array is necessary because the selectivity would be limited without it. For instance, Bass Strait Crude and Bahrain Crude would have the same output if DAE1 was only used at three excitation wavelengths. DAE2 and DAE3 respond differently at those wavelengths (driven by the quantum yield of photoconversion), whereby providing a unique colorimetric response for both Bass Strait Crude and Bahrain Crude. This colorimetric array is therefore a fast, easy, and cheap method to identify oils, and when combined with pattern recognition software could be particularly powerful.[23]
|
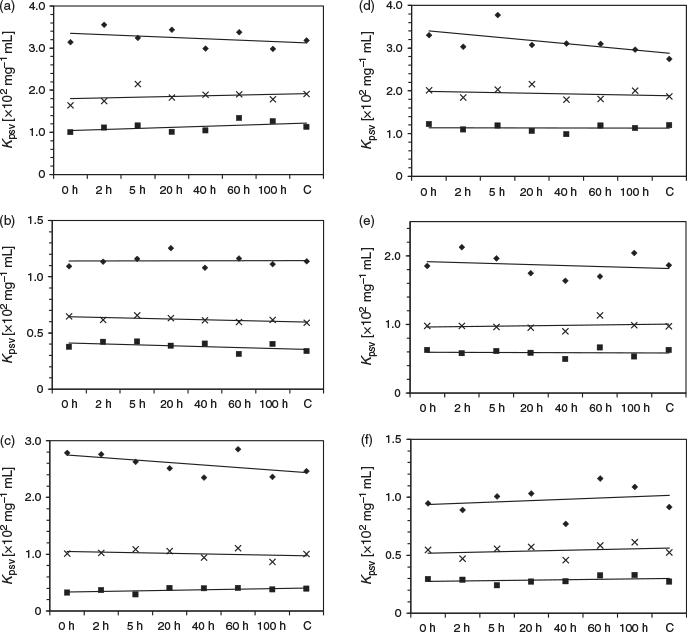
Oil spills may be discovered (or uncovered) days after the initial polluting event, exposing the oil to reactive UV light that can alter the relative PAH composition of the oil.[24] Therefore, the stability of the photometric response is critical for a field-based analytical device. The photometric response with respect to the aging time was therefore investigated by using two different oils: a heavy crude oil (H sample) and a distillate (M sample). These oil samples were aged according to methods developed by Guedes et al. where 5 mL of the oil was placed over 20 mL of seawater, and exposed to sunlight on cloudless days from 9 a.m. to 3 p.m.[25,26] Oil samples exposed to sunlight for 100 h in an amber flask acted as controls to eliminate the effect of UV light. Fig. 6 depicts the response of the oils exposed for 0, 2, 5, 20, 40, 60, and 100 h to the 3 × 3 array. The slope of the lines, measured at 295 and 325 nm, approximated to zero, indicating no change in response within that timeframe. Although the data at 275 nm varied with time, they were all within experimental error of the measurement. The general lack of significant change in the chronological response is attributed to the relatively low rate of photoreaction of PAHs[24] and the minimal response of the more volatile PAHs,[11] as demonstrated in the individual PAH study (Table 2). Any loss of low-molecular-weight PAHs will have limited effect on the photoswitching inhibition; therefore, this colorimetric array offers a high level of reproducibility over a wide timeframe.
Conclusion
The potential of three diarylethene photochromic compounds towards the development of a quick and simple methodology for identifying oil spill offenders was investigated. The commercially available diarylethenes DAE1 and DAE2 were compared with DAE3, which was designed with greater conjugation, to further understand the effect of the photochrome characteristics on the PAH sensing behaviour. DAE2 and DAE3 showed the highest sensitivity, whereas DAE1 showed the lowest. This difference was rationalised by the different quantum yield of photoconversion. Oil spill mimics established the accuracy of this technology; the observed photoswitching inhibition was similar to that of the predicted values. Furthermore, the technique was found to be applicable to, and able to readily differentiate between, authentic oil samples. The variation of the response of aged oil samples to the DAEs was similar to the standard deviation of the initial non-aged response. Based on this systematic study, this technology represents a simple approach to the quick identification of oils and as such is highly amenable to in situ applications.
Experimental
Materials
The commercially available PAHs (Sigma-Aldrich) were used as received, and solutions were prepared in freshly distilled dichloromethane (DCM; Univar). DAE1 and DAE2 were prepared according to literature methods.[19,20] DAE3 was prepared by using direct arylation of 3-bromo-2-methylthiophene,[27] followed by metal–halogen exchange,[28] as described in the Supplementary Material. The crude oil samples Tapis Crude (Malaysian), Bahrain Crude (Bahrain), Widuri Crude (Indonesian), and Bass Strait Crude (Australian) were provided by the Australian Institute of Marine Science (AIMS) and used as received.
Characterisation
Absorption spectra were recorded by using a Shimadzu UV1800 or a Cary 5E UV-Vis-NIR spectrophotometer. A PerkinElmer LS-50B luminescence spectrometer was used as a source of light for photoswitching the photochromic solutions. All experiments were performed in 1 cm quartz cuvettes. The relative quantum yields of photoconversion were performed, as previously described, at 313 nm.[19,20] The quantum yields at 275 nm were estimated using DAE2 = 0.59 as a reference.
Three photochrome DCM solutions (0.100 mM DAE1, 0.030 mM DAE2, and 0.011 mM DAE3) were used as stock solutions to prepare the PAH solutions in order to maintain a constant photochrome concentration. The pseudo Stern–Volmer constants were calculated by comparing the integrated absorption area (400 to 600 nm of DAE1, 400 to 700 nm of DAE2, and 425 to 750 nm of DAE3) and dividing by the concentration of the solution, with the coefficients and errors reported based on an average of five measurements.
Four oil solutions were prepared by diluting the samples (2.200 mg for Tapis Crude, 1.600 mg for Bahrain Crude, 1.900 mg for Widuri Crude, and 1.500 mg for Bass Strait Crude) in 10 mL of DCM. The pseudo Stern–Volmer constants of the oils were calculated as per the analyte solutions described above. The coefficients and errors were based on an average of five measurements, and the constants obtained in mg−1.10 mL units.
The weathering was performed in Townsville, Australia on a shade-free rooftop between November and December 2016 (UV index extreme, temperature was 304 K, on average). The protocols were as described in the literature.[25,26] Medium fuel oil (an early distillate containing a higher concentration of low-molecular-weight PAHs; 5 mL) or high fuel oil (a late distillate containing a higher concentration of high-molecular-weight PAHs; 5 mL) was added to 20 mL of seawater sourced from Cleveland Bay (Townsville, Australia). The samples were aged for 0, 2, 5, 20, 40, 60, and 100 h in a 100 mL beaker as an open system to allow for evaporation and full sun exposure (from 9 a.m. to 3 p.m.). After and in between exposures, the samples were stored in a fridge. Control samples were prepared in an amber flask for 100 h. After the exposure, 10 mL of freshly distilled dichloromethane was added to the water/oil mixtures and transferred to a separation funnel. The organic phase was separated and evaporated under reduced pressure (583 mbar) at 42°C, over 30 min.
Supplementary Material
Details on the synthesis and characterisation (including X-ray structure determination) of DAE3 are available on the Journal’s website.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank the Australian Research Council (DP1095404 and LE150100049) for financial support and Shimadzu Scientific Instruments for access to their UV-Vis spectrometer through their ‘Fostering Science in Education’ (FSiE) program.
References
[1] T. Gilbert, Oil Spills in the Australian Marine Environment: Environmental Consequences and Response Technologies 1999. Available at: https://www.researchgate.net/publication/228899633_Oil_Spills_in_the_Australian_Marine_Environment_Environmental_Consequences_and_Response_Technologies (accessed 9 September 2019)[2] V. P. Beškoski, G. Gojgić-Cvijović, B. Jovančićević, M. M. Vrvić, in Gas Chromatography – Biochemicals, Narcotics and Essential Oils (Ed. B. Salih) 2012, pp. 1–28 (IntechOpen: London).
[3] Z. Liu, J. Liu, Q. Zhu, W. Wu, Environ. Res. Lett. 2012, 7, 035302.
| Crossref | GoogleScholarGoogle Scholar |
[4] F. M. Adebiyi, E. A. Oluyemi, A. F. Adeyemi, A. A. Akande, O. S. Ajayi, Petrol. Sci. Technol. 2015, 33, 62.
| Crossref | GoogleScholarGoogle Scholar |
[5] V. Bansal, P. Kumar, E. E. Kwon, K.-H. Kim, Crit. Rev. Food Sci. Nutr. 2017, 57, 3297.
| Crossref | GoogleScholarGoogle Scholar | 26714230PubMed |
[6] E. A. Pena, L. M. Ridley, W. R. Murphy, J. R. Sowa, C. S. Bentivegna, Environ. Toxicol. Chem. 2015, 34, 1946.
| Crossref | GoogleScholarGoogle Scholar | 25867932PubMed |
[7] M. Algarra, V. Jimenez, P. F. de Violet, M. Lamotte, Anal. Bioanal. Chem. 2005, 382, 1103.
| Crossref | GoogleScholarGoogle Scholar | 15895215PubMed |
[8] J. Xu, J. Du, C. Jing, Y. Zhang, J. Cui, ACS Appl. Mater. Interfaces 2014, 6, 6891.
| Crossref | GoogleScholarGoogle Scholar | 24720732PubMed |
[9] Y.-H. Kwon, K. Sowoidnich, H. Schmidt, H.-D. Kronfeldt, J. Raman Spectrosc. 2012, 43, 1003.
| Crossref | GoogleScholarGoogle Scholar |
[10] D. L. Poster, M. M. Schantz, L. C. Sander, S. A. Wise, Anal. Bioanal. Chem. 2006, 386, 859.
| Crossref | GoogleScholarGoogle Scholar | 17019586PubMed |
[11] W. Woodland, C. A. Motti, P. Irving, L. Van Herwerden, G. Vamvounis, Aust. J. Chem. 2016, 69, 1292.
| Crossref | GoogleScholarGoogle Scholar |
[12] K. Shibata, K. Muto, S. Kobatake, M. Irie, J. Phys. Chem. 2002, 106, 209.
| Crossref | GoogleScholarGoogle Scholar |
[13] J. Zhang, Q. Zou, H. Tian, Adv. Mater. 2013, 25, 378.
| Crossref | GoogleScholarGoogle Scholar | 22911949PubMed |
[14] S. Cui, S. Pu, Y. Dai, Anal. Methods 2015, 7, 3593.
| Crossref | GoogleScholarGoogle Scholar |
[15] O. Galangau, Y. Kimura, R. Kanazawa, T. Nakashima, T. Kawai, Eur. J. Org. Chem. 2014, 7165.
| Crossref | GoogleScholarGoogle Scholar |
[16] S. Xia, G. Liu, S. Pu, J. Mater. Chem. C Mater. Opt. Electron. Devices 2015, 3, 4023.
| Crossref | GoogleScholarGoogle Scholar |
[17] C. Zhang, C. Fan, S. Pu, G. Liu, Chin. J. Chem. 2015, 33, 1310.
| Crossref | GoogleScholarGoogle Scholar |
[18] I. S. Park, E. Heo, Y.-S. Nam, C. W. Lee, J.-M. Kim, J. Photochem. Photobiol. Chem. 2012, 238, 1.
| Crossref | GoogleScholarGoogle Scholar |
[19] M. Irie, T. Lifka, S. Kobatake, N. Kato, J. Am. Chem. Soc. 2000, 122, 4871.
| Crossref | GoogleScholarGoogle Scholar |
[20] K. Higashiguchi, K. Matsuda, Y. Asano, A. Murakami, S. Nakamura, M. Irie, Eur. J. Org. Chem. 2005, 91.
| Crossref | GoogleScholarGoogle Scholar |
[21] D. Gendron, A. Ansaldo, G. Bubak, L. Ceseracciu, G. Vamvounis, D. Ricci, Compos. Sci. Technol. 2015, 117, 364.
| Crossref | GoogleScholarGoogle Scholar |
[22] J. M. Dixon, M. Taniguchi, J. S. Lindsey, Photochem. Photobiol. 2005, 81, 212.
| Crossref | GoogleScholarGoogle Scholar | 15571431PubMed |
[23] R. A. Potyrailo, Chem. Rev. 2016, 116, 11877.
| Crossref | GoogleScholarGoogle Scholar | 27602947PubMed |
[24] D. Dąbrowska, A. Kot-Wasik, J. Namieśnik, Pol. J. Environ. Stud. 2008, 17, 17.
[25] C. L B. Guedes, E. D. Mauro, V. Antunes, A. S. Mangrich, Mar. Chem. 2003, 84, 1105.
[26] S. Díez, J. Sabaté, M. Viñas, J. M. Bayona, A. M. Solanas, J. Albaigs, Environ. Toxicol. Chem. 2005, 24, 2203.
| Crossref | GoogleScholarGoogle Scholar | 16193747PubMed |
[27] G. Vamvounis, D. Gendron, Tetrahedron Lett. 2013, 54, 3785.
| Crossref | GoogleScholarGoogle Scholar |
[28] D. Gendron, G. Vamvounis, Org. Prep. Proced. Int. 2015, 47, 385.
| Crossref | GoogleScholarGoogle Scholar |