Isotope and Temperature Effects on the Electronic Spectra of Large Carbonaceous Molecular Ions of Interstellar Relevance
Ewen K. Campbell A B C , Corey A. Rice A , Francois X. Hardy A and John P. Maier AA Department of Chemistry, University of Basel, Klingelbergstrasse 80, 4056 Basel, Switzerland.
B Present address: School of Chemistry, University of Edinburgh, Joseph Black Building, Kings Buildings, David Brewster Road, Edinburgh, EH9 3FJ, UK.
C Corresponding author. Email: e.k.campbell@ed.ac.uk
Australian Journal of Chemistry 72(11) 856-859 https://doi.org/10.1071/CH19206
Submitted: 7 May 2019 Accepted: 17 June 2019 Published: 23 July 2019
Abstract
The electronic spectra of isotopologues of protonated coronene in the gas phase were measured at a vibrational and rotational temperature of ~10 K in a 22-pole ion trap. The (1) 1A′←X 1A′ transition of these polycyclic aromatic hydrocarbon cations with one to three carbon-13 have origin band maxima that blue-shift successively by 0.03 nm. All isotopologues show distinct vibrational structure in the (1) 1A′ state. These results are compared with the effect of 13C substitution on the near infrared electronic absorptions of C60+. The (1) 1A←X 1A electronic transition of monodeuterated coronene was also recorded and its origin band is red-shifted to that of protonated coronene by 0.8 nm. The implications for astronomical observations are considered.
Introduction
Astronomical spectra have been recorded along many lines-of-sight and towards different binary star systems in the 400–900 nm region.[1,2] The stationary absorption features are due to neutral and ionic molecules in interstellar clouds that have electronic transitions in the visible. These are referred to as the diffuse interstellar bands (DIBs) and they resemble unresolved rotational profiles of gaseous polyatomics. Observations of DIBs in the spectra of different stars reveal similar wavelengths; however, the intensities vary because of concentration and stellar luminosity.[3]
Carbon-containing species such as polycyclic aromatic hydrocarbons (PAHs) have been proposed as possible candidate molecules responsible for the DIBs.[3,4] PAHs or related structures may even comprise up to 20 % of the interstellar carbon budget[5] and it is supposed that the unidentified infrared bands are due to vibrational emission of aromatic species.[6] Despite this, no individual PAH has been identified in the interstellar medium (ISM). PAHs composed of more than around 50 carbon atoms are photostable and are less likely to fragment upon UV irradiation.[7] This could be one of the reasons why smaller PAHs have not been detected as only their photodissociation products may be present in the diffuse ISM. However, cold gas phase data have now been recorded in the laboratory for PAHs as large as hexabenzocoronene,[8] C42H18, and its cation (HBC+).[9] No DIBs have been observed at the wavelengths of the strongest absorptions in the electronic spectra of these and led to upper limits of 1012 cm−2 on their column densities.
On the other hand, the infrared signatures of the fullerenes C60 and C70 were identified in the emission spectrum of a young planetary nebula.[10] Those of C60 were also observed in a reflection nebula,[11] and since then from a variety of stellar environments; see, for example, Roberts et al.[12] and references therein. A recent success has been the attribution of several DIBs to C60+ between 930–970 nm.[13–16] Due to the number of carbon atoms in C60+ there is a high probability for 13C to be present in the structure. In general, both rotational temperature and isotopologues will influence the full-width at half-maximum (fwhm) and central wavelength of DIBs.[17,18] In the case of C60+, it was determined that most of the spectroscopic broadening at the low temperature of the laboratory measurement is due to the excited-state lifetime.[14] However, clear shifts in the wavelengths of absorption bands involving transitions to vibrational levels with one quanta in the excited electronic state were observed experimentally for 13C12C59+ and 13C212C58+.[19]
The possibility that the appearance of observed DIB profiles is due to isotopes has been considered previously. For example, the substructure in the 6614 DIB led to the hypothesis that it could be due to a 13C effect.[18] This particular interstellar absorption band was found to consist of peaks separated in energy by around 0.7 cm−1. Under the assumption that it is caused by a molecule containing between 30 and 100 carbon atoms, such a profile could be explained by a vibrational isotope effect in the excited electronic state, as shown in the modelling by Webster.[18] In addition, subsequent work on the narrow[20] 6196 DIB led to the discussion that its broadening could arise because of a zero-point isotope effect.[21] Although high-resolution observational work has pointed to an alternative explanation for the structure in the 6614 interstellar absorption,[22] the influence of isotopes on the electronic spectra of DIB candidates is now important, particularly in view of the assignment of near infrared bands to C60+.
If medium-sized or larger PAHs are present in the ISM, isotopically substituted derivatives must play a role in their astronomical spectroscopy. In this contribution the electronic spectrum of protonated coronene (H+Cor) in the gas phase was recorded in a cryogenic radio-frequency (rf) quadrupole trap by means of a photofragmentation technique. Using the terrestrial abundance of carbon-13 in protonated coronene, the electronic spectrum of mass-selected isotopologues was obtained.
Experimental
The setup has been described by Hardy et al.[23] Cations are created in a chemical ionisation source containing coronene heated to ~150°C. Ions are accumulated in a hexapole, where collisions with residual gas from the source narrow the kinetic energy distribution. The exit potential is lowered, and cations are released into a quadrupole mass selector, discriminating the desired mass-to-charge ratio. The ion beam is deflected 90°, injected into an rf-only octupole, and transported to a cryogenic ion trap, confining positive species in the radial and axial direction.
A 36 mm long 22-pole trap[24] mounted on the second stage of a closed-cycle helium cryostat at 4 K is filled with 105 ions per cycle. Within the ion trap, the temperature can be controlled from 4 to 300 K, influencing the population of rotational and vibrational states. A piezo-valve introduces helium buffer gas at the beginning of the trapping cycle, and after a few milliseconds, a density of 5 × 1015 cm−3 is achieved. Positive species exit the trap and are collimated by electrostatic electrodes, deflected by 90°, and transported into a quadrupole mass spectrometer. The latter selects m/z for H/D-loss, and an increase in the number of ions on the associated m/z is monitored as a function of laser wavelength (bandwidth of 0.01 nm) to obtain an electronic spectrum.
Results and Discussion
Temperature Effect
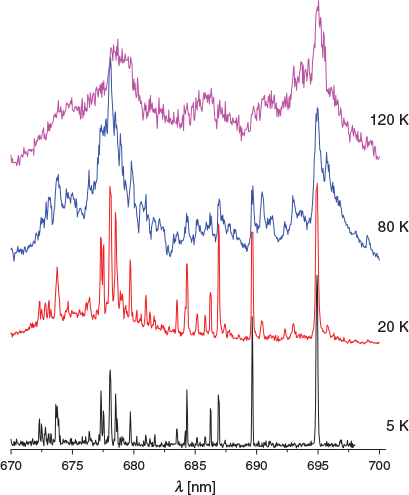
Experimental conditions similar to those in the ISM are appropriate when comparing laboratory and astronomical data in the visible region. This requires rotational temperatures of 10–80 K for non-polar molecules. Normally, it is advantageous to obtain spectra around 10 K such that congestion is avoided, i.e. only the vibrational ground state and the lowest rotational levels are populated. At higher temperatures more states are occupied and hot bands become apparent. In Fig. 1, the temperature of the trap (Tnom) was varied from 5 to 120 K to show the effect on the (1) 1A′ ← X 1A′ transition of H+Cor. At Tnom = 5 K, there are no absorptions to the red of the origin band at 694.93 nm due to hot bands. Already at Tnom = 20 K, new features are observed in the electronic spectrum; the maximum wavelength of the origin does not change but a weak hot band at 695.78 nm is apparent. As Tnom increases the origin maximum red-shifts to 695.03 nm at 120 K. The vibrational progression at higher Tnom smears such that an assignment of the transitions is difficult.
|
It appears that the widths of the bands (fwhm) monotonically increase with Tnom, reflecting the enhancement of vibrational motion. The fwhm of the origin band of the (1) 1A′ ← X 1A′ transition changes from 0.1 nm for Tnom = 5 K to > 2 nm at 120 K (Fig. 1).
13C Substitution
The natural abundance of 13C is 1.109 % on Earth but does vary between galactic environments. This should be taken into consideration for carboneous species of the size of protonated coronene when comparing laboratory and astronomical spectra. For example, 13Cx12C24−xH13+ (x = 1, 2, and 3) is present in 26.2, 3.3, and 0.3 % abundance relative to x = 0, respectively. In the context of potential detection in astrophysical environments it should be emphasised that random 13C inclusion would result in a statistical ensemble of isotopomers.
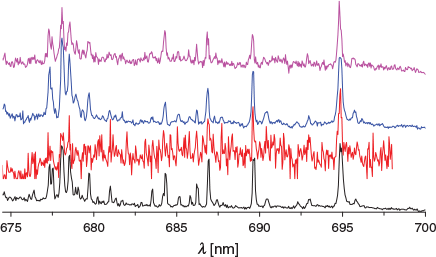
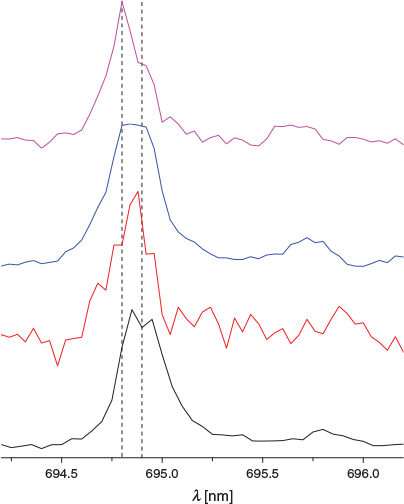
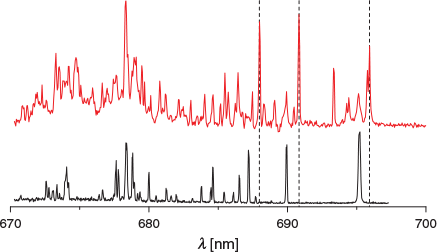
In Fig. 2, the (1) 1A′ ← X 1A′ transition of H+Cor with increasing 13C content is presented. The bottom trace is C24H13+ and the top one 13C312C21H13+. At first glance, no significant changes can be attributed to the addition of 13C. This is probably because of the retention of Cs symmetry upon incorporation of one or more 13C. On closer inspection of the origin band (Fig. 3), a slight change in the band contour with one to three 13C atoms present is apparent. The maximum of the origin shifts to shorter wavelengths by 0.03 nm per 13C.
|
|
Spectra of 12C60+, 13C12C21+, and 13C212C58+ were recently obtained by helium tagging for the lowest energy electronic transition.[19] Unlike the case of H+Cor, 13C substitution was found to have negligible effect on the origin band at 957.7 nm, with absorption maxima for 12C60+ and 13C12C59+ found to lie within the uncertainties of the two measurements. This was taken to indicate that substitution of one of sixty 12C atoms by a 13C results in only small differences in the zero-point energies. However, observable changes to the wavelengths of the absorption bands were found for transitions involving excitation of a vibrational mode in the upper electronic state. Measurement of the 936.5 nm band, which involves a 236 cm−1 vibrational mode in the excited state, with two 13C atoms gives rise to a red-shift of 0.03 nm relative to 12C60+. These results, due to the vibrational isotope effect, were interpreted using a perturbation theory treatment that was previously applied to describe the Raman spectrum of C60.[25] These observations led to the conclusion that the isotopic effect will play only a minor role in the C60+ absorptions identified as DIBs, but larger wavelength shifts are expected for transitions involving higher energy vibrational modes.
Deuteration versus Protonation
The cosmic D/H ratio is 2.5 × 10−5, which is too low to have a significant effect on the electronic spectroscopy for possible species in the ISM.[26] However, interstellar molecules, e.g. PAHs, can become D-enriched by gas-phase ion–molecule reactions at low temperatures,[27,28] gas–grain interactions,[29–31] unimolecular photodissociation,[6,32] and UV photolysis in D-enriched ice grains.[33] Each of these processes is expected to leave a distinct signature in the distribution of D enrichment in the PAH population. Fractionation is more likely to occur in the ISM than in the solar nebula because this effect falls off rapidly with increasing temperature.
D+Cor was generated in a chemical ionisation source using the reaction: C7D9+ + C24H12 → C24H12D+ + C7D8. All peripheral carbons are equivalent and D-attachment to the inner ring was not considered. The molecular symmetry of D+Cor is C1, whereas that of H+Cor is Cs. In Fig. 4, the (1) 1A ← X 1A transition of D+Cor is presented. Two major effects are observed: a red-shift of the origin band in the electronic spectrum by 0.8 nm and an increase in the number of vibrations in the excited state. The measured red-shift, due to differences in zero-point energies of C24H12D+ and 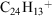 , is similar to the 14.6 cm−1 energy difference found for C10H8D+ relative to C10H9+.[34] The appearance of further bands in the spectrum of C24H12D+ is reminiscent of recent work on the C14H10D and C14H11 systems, where quantum-induced symmetry-breaking is reported in the deuterated analogue and additional features ascribed to excitation of out-of-plane modes.[35]
, is similar to the 14.6 cm−1 energy difference found for C10H8D+ relative to C10H9+.[34] The appearance of further bands in the spectrum of C24H12D+ is reminiscent of recent work on the C14H10D and C14H11 systems, where quantum-induced symmetry-breaking is reported in the deuterated analogue and additional features ascribed to excitation of out-of-plane modes.[35]
|
Conclusions
The electronic spectrum of the protonated coronene cation was measured in an ion trap at a range of temperatures between 5 and 120 K. The effect of increasing temperature on the shape and widths of the vibronically resolved absorptions is discussed and at temperatures above 20 K hot bands are observed. Such a range is relevant to the internal temperature of non-polar molecules in the ISM which can take values from 30–80 K.[36]
By exploiting the terrestrial abundance of carbon-13, the electronic spectrum of H+Cor with 1–3 13C atoms were investigated, leading to small shifts in the wavelength of the origin band of the (1) 1A′ ← X 1A′ transition. If large neutral and/or ionic PAHs are present in the ISM, these results indicate that carbon isotope effects should be considered in the comparison between laboratory and astronomical observations. Monodeuteration of planar PAHs leads to spectroscopic complexity because of the breaking of the Cs symmetry, as demonstrated here in the case of D+Cor. The probability to form peri-deuterated species in the ISM is less likely given the D/H ratio; however, chemical processes in ices or on grains cannot be disregarded.[37]
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the European Research Council (ERC-AdG-ElecSpecIons: 246998), Swiss National Science Foundation (Project No. 200020-124349/1), and the University of Basel.
References
[1] L. M. Hobbs, D. G. York, T. P. Snow, T. Oka, J. A. Thorburn, M. Bishof, S. D. Friedman, B. J. McCall, B. Rachford, P. Sonnentrucker, D. E. Welty, Astrophys. J. 2008, 680, 1256.| Crossref | GoogleScholarGoogle Scholar |
[2] L. M. Hobbs, D. York, J. Thorburn, T. Snow, M. Bishof, S. Friedman, B. McCall, T. Oka, B. Rachford, P. Sonnentrucker, Astrophys. J. 2009, 705, 32.
| Crossref | GoogleScholarGoogle Scholar |
[3] The Diffuse Interstellar Bands, Vol. 202 (Eds A. G. G. M. Tielens, T. P. Snow) 1995 (Kluwer: Dordrecht).
[4] W. Duley, Faraday Discuss. 2006, 133, 415.
| Crossref | GoogleScholarGoogle Scholar | 17191461PubMed |
[5] P. Ehrenfreund, S. B. Charnley, Annu. Rev. Astron. Astrophys. 2000, 38, 427.
| Crossref | GoogleScholarGoogle Scholar |
[6] L. J. Allamandola, A. G. G. M. Tielens, J. R. Barker, Astrophys. J. Suppl. Ser. 1989, 71, 733.
| Crossref | GoogleScholarGoogle Scholar | 11542189PubMed |
[7] A. G. G. M. Tielens, Annu. Rev. Astron. Astrophys. 2008, 46, 289.
| Crossref | GoogleScholarGoogle Scholar |
[8] D. L. Kokkin, T. P. Troy, M. Nakajima, K. Nauta, T. D. Varberg, G. F. Metha, N. T. Lucas, T. W. Schmidt, Astrophys. J. 2008, 681, L49.
| Crossref | GoogleScholarGoogle Scholar |
[9] E. K. Campbell, J. P. Maier, Astrophys. J. 2017, 850, 69.
| Crossref | GoogleScholarGoogle Scholar |
[10] J. Cami, E. Bernard-Salas, E. Peeters, S. E. Malek, Science 2010, 329, 1180.
| Crossref | GoogleScholarGoogle Scholar | 20651118PubMed |
[11] K. Sellgren, M. W. Werner, J. G. Ingalls, J. D. T. Smith, T. M. Carleton, C. Joblin, Astrophys. J. 2010, 722, L54.
| Crossref | GoogleScholarGoogle Scholar |
[12] K. R. G. Roberts, K. T. Smith, P. J. Sarre, Mon. Not. R. Astron. Soc. 2012, 421, 3277.
| Crossref | GoogleScholarGoogle Scholar |
[13] B. H. Foing, P. Ehrenfreund, Nature 1994, 369, 296.
| Crossref | GoogleScholarGoogle Scholar |
[14] E. K. Campbell, M. Holz, D. Gerlich, J. P. Maier, Nature 2015, 523, 322.
| Crossref | GoogleScholarGoogle Scholar | 26178962PubMed |
[15] G. A. H. Walker, D. A. Bohlender, J. P. Maier, E. K. Campbell, Astrophys. J. 2015, 812, L8.
| Crossref | GoogleScholarGoogle Scholar |
[16] E. K. Campbell, M. Holz, J. P. Maier, D. Gerlich, G. A. H. Walker, D. A. Bohlender, Astrophys. J. 2016, 822, 17.
| Crossref | GoogleScholarGoogle Scholar |
[17] G. H. Herbig, Annu. Rev. Astron. Astrophys. 1995, 33, 19.
| Crossref | GoogleScholarGoogle Scholar |
[18] A. Webster, Mon. Not. R. Astron. Soc. 1996, 282, 1372.
| Crossref | GoogleScholarGoogle Scholar |
[19] E. K. Campbell, J. P. Maier, Astrophys. J. 2018, 858, 36.
| Crossref | GoogleScholarGoogle Scholar |
[20] G. A. H. Walker, A. S. Webster, D. A. Bohlender, J. Krewlowski, Astrophys. J. 2001, 561, 272.
| Crossref | GoogleScholarGoogle Scholar |
[21] A. Webster, Mon. Not. R. Astron. Soc. 2004, 349, 263.
| Crossref | GoogleScholarGoogle Scholar |
[22] J. Cami, F. Salama, J. Jimenez-Vicente, G. A. Galazutdinov, J. Krelowski, Astrophys. J. 2004, 611, L113.
| Crossref | GoogleScholarGoogle Scholar |
[23] F. X. Hardy, C. A. Rice, O. Gause, J. P. Maier, J. Phys. Chem. A 2015, 119, 1568.
| Crossref | GoogleScholarGoogle Scholar | 25264926PubMed |
[24] D. Gerlich, Phys. Scr. 1995, T59, 256.
| Crossref | GoogleScholarGoogle Scholar |
[25] S. Guha, J. Menendez, J. B. Page, G. B. Adams, G. S. Spencer, J. P. Lehman, P. Giannozzi, S. Baroni, Phys. Rev. Lett. 1994, 72, 3359.
| Crossref | GoogleScholarGoogle Scholar | 10056178PubMed |
[26] M. Pettini, R. Cooke, Mon. Not. R. Astron. Soc. 2012, 425, 2477.
| Crossref | GoogleScholarGoogle Scholar |
[27] A. Dalgarno, S. Lepp, Astrophys. J. 1984, 287, L47.
| Crossref | GoogleScholarGoogle Scholar |
[28] A. G. G. M. Tielens, AIP Conf. Proc. 1997, 402, 523.
[29] A. G. G. M. Tielens, Astron. Astrophys. 1983, 119, 177.
[30] A. G. G. M. Tielens, in Astrochemistry of Cosmic Phenomena. International Astronomical Union (Ed. P. D. Singh) 1992, Vol. 150, pp. 91–95 (Springer: Dordrecht).
[31] A. G. G. M. Tielens, in Dust and Molecules in Evolved Stars (Eds I. Cherchneff, T. J. Millar) 1997, pp. 1–13 (Springer: Dordrecht).
[32] L. J. Allamandola, S. A. Sandford, B. Wopenka, Science 1987, 237, 56.
| Crossref | GoogleScholarGoogle Scholar | 17813622PubMed |
[33] S. A. Sandford, M. P. Bernstein, J. P. Dworkin, Meteorit. Planet. Sci. 2001, 36, 1117.
| Crossref | GoogleScholarGoogle Scholar |
[34] O. Krechkivska, C. M. Wilcox, B. Chan, R. Jacob, Y. Liu, K. Nauta, S. H. Kable, L. Radom, T. W. Schmidt, J. Phys. Chem. A 2015, 119, 3225.
| Crossref | GoogleScholarGoogle Scholar | 25756850PubMed |
[35] O. Krechkivska, C. M. Wilcox, K. Nauta, S. H. Kable, L. Radom, T. W. Schmidt, ChemRxiv 2019, preprint
| Crossref | GoogleScholarGoogle Scholar |
[36] J. Huang, T. Oka, Mol. Phys. 2015, 113, 2159.
| Crossref | GoogleScholarGoogle Scholar |
[37] J. Kalvans, I. Shmeld, J. R. Kalnin, S. Hocuk, Mon. Not. R. Astron. Soc. 2017, 467, 1763.


