Firefly Bioluminescence-Based Detection of ATP
Angie M. Jarrad A
A
A Department of Chemical Biology, Helmholtz Centre for Infection Research, Inhoffenstrasse 7, 38124, Braunschweig, Germany. Email: angie.jarrad@helmholtz-hzi.de

Dr Angie M. Jarrad has expertise in medicinal chemistry, organic synthesis, and microbiology, and has experience in a broad range of antimicrobial drug development projects. She received her Ph.D. from the University of Queensland for her thesis on novel nitroimidazole and glycopeptide antibiotics targeting the enteric pathogens Clostridium difficile, Giardia lamblia, and Entamoeba histolytica. In 2018, Dr Jarrad was the recipient of the RACI Graham Johnston Best Thesis Award and an Alexander von Humboldt Postdoctoral Fellowship. |
Australian Journal of Chemistry 72(8) 644-645 https://doi.org/10.1071/CH19127
Submitted: 19 March 2019 Accepted: 13 May 2019 Published: 4 June 2019
Background
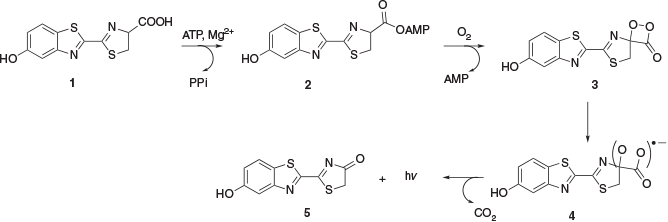
Adenosine triphosphate (ATP) bioluminescence is a powerful light-producing phenomenon that occurs in nature in a variety of organisms, with ATP bioluminescence of fireflies one of the most well-known examples. The firefly ATP bioluminescence reaction has been adapted to the laboratory with a wide range of applications that include monitoring cellular processes, antimicrobial susceptibility testing, and the detection of bacterial contamination of environmental surfaces. ATP bioluminescence occurs through a multistep reaction between firefly luciferase, ATP, magnesium salt, and oxygen (Scheme 1).[1] As a simplified overview, luciferyl adenylate 2 is first formed from luciferin 1 and Mg2+-ATP. The luciferyl adenylate 2 is then oxidised with molecular oxygen to form a dioxetanone cyclic peroxide intermediate 3. Following intramolecular conversion to produce electronically excited states of oxyluciferin, the dioxetanone is decarboxylated. Finally, the return of excited oxyluciferin to the ground state 5 results in emission of visible light. For more detailed insights into the reaction mechanism, including alternative reactions and different tautomers of oxyluciferin at varying pH values, readers are referred to additional literature.[2–4]
|
Different ATP bioluminescence kits are available. The two main types of kits include flash-based and stable glow-based reagents.[5] Flash-based kits typically have higher sensitivity but require injection of the luminescence substrate by the instrument and then immediate measurement of the luminescence signal.[5] In contrast, stable glow-based kits can be set up on the bench and then transferred to the instrument for measurement.[5] Other types of kits include swab-based kits for collecting samples from environmental surfaces that can then be tested for luminescence in a portable luminometer.
Research Advances Utilising ATP Bioluminescence
Jarrad et al. recently reported the use of ATP bioluminescence as a high-throughput method to detect the Eagle Effect, a type of bacterial persistence.[6] In their study, Clostridium difficile bacteria resisted supra minimum inhibitory concentrations of vancomycin, above optimal bactericidal concentrations of antibiotic. The Eagle Effect is typically measured through quantification of colony-forming units. However, the endpoint ATP bioluminescence assay provided an alternative, rapid means to assess the Eagle Effect that did not require subsequent growth of the bacteria. In this study, BacTitre-GloTM with a simple add–mix–measure format was utilised, which additionally facilitated the detection of the Eagle effect outside the anaerobic chamber that was required for growth of C. difficile.
Adenosine triphosphate bioluminescence has also been used to develop a fast antimicrobial susceptibility test of combinations of antibiotics against carbapenem-resistant Gram-negative bacteria (CR-GNB).[7] In this study, effective antibiotic combinations against CR-GNB could be determined within 6 h as opposed to conventional viable plating, which requires 48 to 72 h. This type of assay could facilitate targeted treatment earlier than when using traditional culture-based assays, thus potentially improving patient outcomes and reducing health care costs.
A swab-based ATP bioluminescence assay was recently shown to be a useful tool to measure the efficiency of cleaning procedures in operating rooms in public hospitals.[8] In this study, the 3MTM Clean-TraceTM Surface ATP swab device, containing a chemically impregnated reagent, was used to sample a 10 × 10-cm test area. The samples were then immediately analysed with a 3MTM Clean-TraceTM NGi Luminometer. This assay had the advantage of providing rapid feedback of contaminated surfaces, in order to promote immediate remedial cleaning action of critical surfaces in operating theatres. The ATP bioluminescence assay was recommended as an additional tool to support traditional culture-based monitoring methods, which are more time-intensive.
Current methods to detect bacteria on surfaces do not provide information on the specific identification of the target bacteria. To overcome this limitation, biosensors are being developed that target specific bacteria. A recently reported biosensor is an antibody-conjugated gold nanorod-based system.[9] After targeting pathogenic bacteria with the gold nanorod antibody conjugate, bacteria were lysed via localised heating with near-infrared irradiation. The released ATP was then detected with an ATP bioluminescence assay. It could therefore be ascertained whether specific pathogenic bacteria were contaminating the surfaces under investigation.
Conflicts of Interest
The author declares no conflicts of interest.
Acknowledgement
AMJ was supported by a Ludwig Leichhardt Memorial Fellowship of the Alexander von Humboldt Foundation.
References
[1] B. F. Milne, M. A. L. Marques, F. Nogueira, Phys. Chem. Chem. Phys. 2010, 12, 14285.| Crossref | GoogleScholarGoogle Scholar | 20886161PubMed |
[2] H. Fraga, D. Fernandes, J. Novotny, R. Fontes, J. C. G. Esteves da Silva, ChemBioChem 2006, 7, 929.
| Crossref | GoogleScholarGoogle Scholar | 16642538PubMed |
[3] R. Fontes, D. Fernandes, F. Peralta, H. Fraga, I. Maio, J. C. G. Esteves da Silva, FEBS J. 2008, 275, 1500.
| Crossref | GoogleScholarGoogle Scholar | 18279384PubMed |
[4] J. J. Snellenburg, S. P. Laptenok, R. J. DeSa, P. Naumov, K. M. Solntsev, J. Am. Chem. Soc. 2016, 138, 16252.
| Crossref | GoogleScholarGoogle Scholar | 27998082PubMed |
[5] J. Lampinen, M. Raitio, A. Perälä, V. Kytöniemi, R.-R. Harinen, Comparison of Flash and Glow ATP Assays with Thermo Scientific Varioskan Flash Luminometry. Thermo Scientific Application note AP-MIB-Vario12–0108 2008 (Thermo Fisher Scientific Oy: Vantaa, Finland).
[6] A. M. Jarrad, M. A. T. Blaskovich, A. Prasetyoputri, T. Karoli, K. A. Hansford, M. A. Cooper, Front. Microbiol. 2018, 9, 1420.
| Crossref | GoogleScholarGoogle Scholar | 30013531PubMed |
[7] Y. Cai, C. L. Seah, H. Leck, T.-P. Lim, J. Q. Teo, W. Lee, T.-T. Tan, T.-H. Koh, P. L. R. Ee, A. L. Kwa, Antimicrob Agents Chemother. 2018, 62, e00183-18.
| Crossref | GoogleScholarGoogle Scholar | 30181372PubMed |
[8] T. Sanna, L. Dallolio, A. Raggi, M. Mazzetti, G. Lorusso, A. Zanni, P. Farruggia, E. Leoni, BMC Infect. Dis. 2018, 18, 583.
| Crossref | GoogleScholarGoogle Scholar | 30453892PubMed |
[9] S. U. Kim, E.-J. Jo, Y. Noh, H. Mun, Y.-D. Ahn, M.-G. Kim, Anal. Chem. 2018, 90, 10171.
| Crossref | GoogleScholarGoogle Scholar | 30081627PubMed |


