Element 44 – Ruthenium
George Koutsantonis AA Chemistry, M310, School of Molecular Sciences, The University of Western Australia, Crawley, WA 6009, Australia. Email: george.koutsantonis@uwa.edu.au
Australian Journal of Chemistry 72(3) 161-163 https://doi.org/10.1071/CH18622
Submitted: 18 December 2018 Accepted: 20 December 2018 Published: 17 January 2019
This year has been proclaimed as the International Year of the Periodic Table of Chemical Elements (IYPT 2019) by the United Nations General Assembly. The motivation for this initiative is the relationship of chemistry to society and its role in promoting sustainable development and societal challenges in ‘energy, education, agriculture and health’.[1] IYPT 2019 will coincide with the 150th anniversary of the formulation of the Periodic System by Dmitry Mendeleev in 1869. It is remarkable that we are still adding new elements to the periodic table with the four newest named and added to the table in 2016.
This first essay of a series to be published in the Australian Journal of Chemistry concerns this co-Editor in Chief’s favourite element, ruthenium. A large proportion of my scientific output has centred on this most versatile element, clearly initially inspired by my mentors and lately as a matter of choice. Scifinder® has over 300 000 references relating to the element when a chemistry search is run.
Ruthenium is a chemical chameleon and its position in the periodic table, centrally located, confers upon it a mix of late and early transition metal properties. This fact has led to the development of several seminal compounds in inorganic and organometallic chemistry. In what can be considered a landmark discovery in inorganic chemistry, the structure of ferrocene,[2] the very next metallocene to be prepared was that of ruthenium.[3]
Henry Taube’s pioneering work on electron transfer reactions and the realisation of the role that π-backbonding plays in the chemistry of ruthenium[4] have ensured the metal continues to be prominent in chemistry. Nobel laureate Robert H. Grubbs recounts his fascination with the element and its penchant for catalysis,[5] noting that Geoffrey Wilkinson referred to ruthenium as ‘an element for the connoisseur’. Grubbs’ innovations in the chemistry of ruthenium-carbon multiple bonding has given us the collection of highly active, well defined ruthenium metathesis catalysts, leading to his sharing the 2005 Nobel prize.[6]
Ruthenium is often found with the other platinum group metals, osmium, rhodium, iridium, platinum, and palladium. Ruthenium is a hard white metal resistant to oxidation at ambient temperature, but oxidises in air at ~800°C. It is used in microelectronics in the manufacture of resistors and electrical contacts. Ruthenium oxide is robust enough to coat the anodes of electrochemical cells for chlorine production. The metal provides efficacious catalysts for ammonia and acetic acid production.[7]
It is hard to imagine a chemical life without the periodic table, so the recognition of periodicity and the construction of the table is a tremendous achievement. However, it is one on which is relied the steady discovery of the elements and the study of their properties, compounds, and chemistry. The small Swedish island of Ytterby gave the world four elements and is an example of the dedicated effort that was required to identify these hard-to-separate elements.
The discovery of ruthenium is a little problematic in its assignation. The element was named after the country of its discovery, Russia, or the Latin version Ruthenia, and the discoverer has been the source of some contention.[8,9] What is not contentious is the source of the ore from which it was identified, the area north of Ekaterinburg and west of Nizhniy Tagil in the Russian Urals. The area had proved to be a bountiful source of minerals and was the centre of Russian steel manufacturing during the Second World War. By the mid-nineteenth century there were numerous platinum mines that led to a glut of the metal, so much so, that it was used as general currency between 1828 and 1844.[8]
The main protagonists in this play were Gottfried Wilhelm Osann (1797–1866) and Karl Karlovich Klaus (1796–1864), both of whom were associated in some way with the University of Dorpat (later Tartu) in Estonia. Osann was provided with a sample of Pt wastes by the Russian minister of Finance, Count Yegor Frantsevich Kancrin, whose remit also included the St Petersburg Mint,[8] and was successively providing interested scientists with samples of Pt ore to study. The size of sample that Osann was provided somewhat hampered his investigations, but he was able to identify the main minor components of the ore, viz. Pd (0.27 %), Ir (1.30 %), Rh (0.27 %), and Os and the typical analysis for the major constituent, Pt (83.06 %).[9] He then turned to the indissoluble portion of the acid extract of the crude ore. Base treatment of this residue and subsequent melting, followed by the addition of water and nitric acid to the melt, gave a precipitate with an ‘unpleasant smell’[9] which, according to Osann, was indicative of the presence of Os (presumably OsO4!). His further research led him to conclude that he had discovered a new element.[9] Osann had been collaborating with renowned Swedish chemist, Baron Jöns Jacob Berzelius, who had confirmed his observations and suggested that he had indeed come across a new element, which he named ruthenium.[9] Historical reassessments of the claims made by Osann suggest that he was perhaps a little premature in his claims[8] but he had certainly sown the seeds that there was a new element to be found. The great effort that was required to extract this putative new element is evident in the detailed steps required.[9] Modern analysis suggests that Osann had only managed to prepare a crude mixture of Ir and Ru, at best.[9]
The other player to strut and fret his hour upon the stage was Karl Karlovich Klaus who was a Dorpat local and studied at the University of Dorpat, going on to become a pharmacist and subsequently returning to the university to take a chemistry degree. While there, he became acquainted with the research that Osann was undertaking on platinum ores. Klaus moved to the University of Kazan, first in pharmacy, but then in chemistry, where he also started exploring the nature of the residues obtained after extraction of the Pt. In this endeavour he was greatly aided by the Russian Finance Minister who supplied him with a much larger quantity of material; most importantly, this material was processed ore – that is, after the extraction of the Pt – clearly an advantage over Osann who had less material with the major constituent still present. Klaus went on to say that the residue he had obtained contained ‘in addition to 10 % of platinum, quite a lot of iridium, osmium, some palladium and ... a new body’.[10] Several sources characterise Klaus as being ‘methodical and careful’[9] and capable of ‘careful observation and diligence’[10] which undoubtedly served him well in the tedious procedures needed to extract the ‘new body’.
His procedure involved the well known step, even at that time, of dissolving the other platinum group metals in aqua regia and then working with the remanent black residue, known to contain Os and Ir. The subsequent procedure (Fig. 1) allowed the isolation of gram quantities of the pure element.
Interestingly, Klaus was a pioneer of the use of microscopes and astoundingly ‘he tasted and smelt his preparations, so discovering that the ammines of ruthenium have a more caustic taste than alkalis, while the taste of osmium tetroxide is acute pepper-like’.[10] Clearly, an experimental practice that has long since been discontinued!
The metal was isolated as a grey powder and Klaus claimed ‘I named the new body, in honour of my Motherland, ruthenium. I had every right to call it by this name because Mr Osann relinquished his ruthenium and the word does not yet exist in chemistry’.[10] This claim caused Osann to claim priority, but the weight of scientific evidence fell on Klaus’ side of the ledger. In a reminder of how papers used to be written, a gentler time, Jas Howe said of the contentious issue, ‘Claus announces the discovery of a new metal, which he calls ruthenium, for the purpose of honoring Osann, whose ruthenium had failed to prove itself an element. It may be mentioned that Osann hardly appreciated the compliment, for he attacked Claus with considerable asperity, accusing him of claiming to discover what Osann himself had discovered. To an impartial critic Osann wholly fails to make out his case. For nearly twenty years Claus continued his work, and his greatest service was in definitely settling the position of the six platinum metals among the elements’.[11]
Before Mendeleev’s momentous work, Klaus was able to discern relationships amongst the platinum group elements, suggesting a special affinity between Ru-Rh-Pd and Os-Ir-Pt, and in fact conceived the notion of ruthenium double salts, a harbinger of the work of the father of coordination chemistry, Alfred Werner.[8,11]
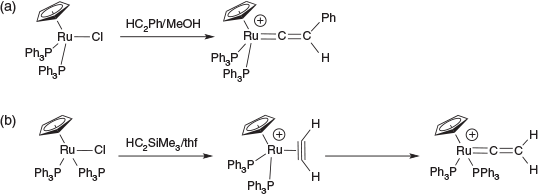
Now to return to the issue of why ruthenium is my favourite element. I, and others, have been fascinated by the capacity of the element to stabilise reactive organic intermediates, a feature that the organometallic community has pursued with lepidopterological zeal. In particular, the synthesis of a ruthenium vinylidene (Fig. 2a) shows the unique capacity that Ru has in forming carbenes,[12] work that had been pioneered by Kolobova in the chemistry of manganese.[13] It took some 12 years more for the simplest member of the vinylidene, :CCH2, family to be captured (Fig. 2b), a task that had occupied a multitude of person years to achieve.[14a,b] The intermediacy of the π-alkyne complex was ably confirmed by a careful structural and NMR study.[14c]
|
The element ruthenium occupies a pivotal place in Mendeleev’s periodic table and has helped to shape the course of inorganic/organometallic chemistry in the 174 years since its definitive discovery by K. K. Klaus. I have no doubt that the unique nature of its reactivity will continue to place it in the forefront of synthetic chemistry for the foreseeable future.
Conflicts of Interest
The author declares no conflicts of interest.
References
[1] https://iupac.org/united-nations-proclaims-international-year-periodic-table-chemical-elements/ (accessed 21 December 2018).[2] G. Wilkinson, J. Am. Chem. Soc. 1952, 74, 6146.
| Crossref | GoogleScholarGoogle Scholar |
[3] G. Wilkinson, J. Organomet. Chem. 1975, 100, 273.
| Crossref | GoogleScholarGoogle Scholar |
[4] C. Creutz, P. C. Ford, T. J. Meyer, Inorg. Chem. 2006, 45, 7059.
| Crossref | GoogleScholarGoogle Scholar | 16933904PubMed |
[5] http://pubs.acs.org/cen/80th/ruthenium.html (accessed 21 December 2018).
[6] K. Wagener, M. Bell, R. Grubbs, R. Waymouth, J. Polym. Sci. A Polym. Chem. 2017, 55, 2863.
| Crossref | GoogleScholarGoogle Scholar |
[7] http://www.rsc.org/periodic-table/element/44/ruthenium (accessed 21 December 2018).
[8] J. Marshall, V. R. Marshall, Rediscovery of the Elements: Ruthenium 2009, Indianapolis, Indiana. University of North Texas Libraries, Digital Library, digital.library.unt.edu; crediting UNT College of Arts and Sciences. Available at: digital.library.unt.edu/ark:/67531/metadc111238/m1/5/ (accessed 16 December 2018).
[9] H. Hödrejärv, Proc. Estonian Acad. Sci. Chem. 2004, 53, 125.
[10] V. N. Pitchkov, Platin. Met. Rev. 1996, 40, 181.
[11] J. L. Howe, Science 1900, 11, 1012.
| Crossref | GoogleScholarGoogle Scholar | 17782532PubMed |
[12] M. I. Bruce, R. C. Wallis, Aust. J. Chem. 1979, 32, 1471.
| Crossref | GoogleScholarGoogle Scholar |
[13] A. N. Nesmeyanov, G. G. AIeksandrov, A. B. Antonova, K. N. Anisimov, N. E. Kolobova, Yu. T. Struchkov, J. Organomet. Chem. 1976, 110, C36.
[14] (a) R. M. Bullock, J. Chem. Soc. Chem. Commun. 1989, 165.
| Crossref | GoogleScholarGoogle Scholar |
(b) M. I. Bruce, G. A. Koutsantonis, Aust. J. Chem. 1991, 44, 207.
| Crossref | GoogleScholarGoogle Scholar |
(c) J. R. Lomprey, J. P. Selegue, J. Am. Chem. Soc. 1992, 114, 5518.
| Crossref | GoogleScholarGoogle Scholar |



