Physicians’ legal duty to disclose more cost-effective treatment options: an examination of Australian civil law applied to personal importation
Narcyz Ghinea A *
A *
A Philosophy Department, Faculty of Arts, Centre for Agency, Values and Ethics, Macquarie University, North Ryde, NSW 2109, Australia.
Australian Health Review 47(3) 314-321 https://doi.org/10.1071/AH23008
Submitted: 16 January 2023 Accepted: 29 March 2023 Published: 24 April 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of AHHA. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Objective A significant proportion of Australians defer or do not fill prescriptions they require due to cost. This article explores whether, and under what circumstances, physicians have a duty to assist these patients by disclosing how they can access more affordable medicines via personal importation.
Methods This study involved a critical examination of Australian statutory and case law pertaining to physicians’ duty to disclose material information to identify key principles applicable to the context of cost-motivated personal importation.
Results There are several legal principles that suggest that physicians have a duty to advise patients of options for accessing more affordable medicines, including via personal importation. These include a duty to warn of inherent and non-inherent risks, a duty to disclose treatments that offer clear advantages, and a duty to facilitate access to the means for achieving patients’ health goals. However, it is unclear whether, and on what grounds, responsibility for harm arising from a patient's inability to afford prescribed medicines should be attributed to the prescribing physician. Arguments supporting attribution of such a responsibility are proposed to motivate further legal, policy and ethical debate.
Conclusions Physicians have a duty to take reasonable steps to mitigate foreseeable harm to their patients, however the law is silent on whether this duty extends to taking steps to help patients access medicines that they can afford. This investigation provides a framework to guide the development of sound policy and law on informed financial consent and economically motivated prescribing.
Keywords: affordability, clinical ethics, duty of care, informed consent, legal duty, negligence, pharmaceuticals, Personal Importation Scheme.
Introduction
In Australia a significant proportion of patients do not adhere to prescribed treatment due to cost and medicine affordability, and this is a growing concern.1 Cost-related non-adherence to prescribed medicines is therefore an important healthcare challenge that needs to be addressed to avoid iatrogenic harm.2,3
Physicians can help their patients to access cheaper medicines in a number of ways: they can prescribe generics rather than brand name products; they can enrol their patients in clinical trials; or they can lobby for subsidised access from the manufacturer or a hospital. More controversially, they may prescribe cheaper off-label alternatives to the on-label treatment, or suggest that patients import cheaper medicines from abroad.4,5 Importantly, although Australian physicians can access unapproved medicines from abroad via the Special Access Scheme, it can be used only for clinical reasons (e.g. because no equivalent treatment is available in Australia), not financial reasons.5 Therefore, if cost is the primary motivation for importation patients must do it themselves via the Personal Importation Scheme.5 It is this phenomenon that is the focus of this article.
Importation can be facilitated by buyer clubs, for example the FixHepC Buyers Club, which helped thousands of Australians import curative hepatitis C medicines that otherwise cost $100 000 per treatment,6 and the Cystic Fibrosis Buyers Club, which helped patients to import medicines that otherwise would cost hundreds of thousands of dollars per year.7 It can also be facilitated by commercial online platforms such as PharmacyChecker, which provides consumers with cost comparisons across global pharmacies that meet the organisation's safety and quality criteria.8 Although the sourcing of medicines not regulated in the patients’ home country raises safety and quality concerns, in some cases it may be a patient’s only option.
This article examines physicians’ legal duty to inform patients of such options to access medicines that they otherwise cannot afford. It presents valuable conceptual insights for the development of informed financial consent policy and law.
The duty to take precautions against harm
Australia is party to multiple international human rights treaties, including the International Covenant on Economic and Social Rights 1976, which recognises a right to health.9 However, international law is not automatically enforceable in Australia, and no domestic law protecting this right exists. Nevertheless, certain legal duties do enable the surreptitious realisation of this right to a degree.
Australian jurisdictions impose civil duties on professionals with regards to their clients (Table 1). Physicians must take reasonable precautions against a risk of harm to their patient if the harm is significant, is likely foreseeable, and a reasonable person in that position would have taken those precautions. In assessing what is reasonable the court may consider the probability of harm if care were not taken, the likely seriousness of the harm, and the burden of taking precautions. Most Australian jurisdictions have provisions for a defence against negligence claims if physicians act in accordance with competent professional practice. The meaning of professional practice for the purposes of this defence has been controversial,10 but at present is understood to be a widely accepted pre-existing pattern of behaviour (Table 2). The fact that professional peers would consider an act reasonable is not sufficient grounds for a defence. Importantly, this defence does not apply to a breach in the duty to warn patients of risks, highlighting the importance of disclosing relevant information to patients (Table 1). In what follows, particular duties that arise from this general responsibility are explored (summarised in Table 2).
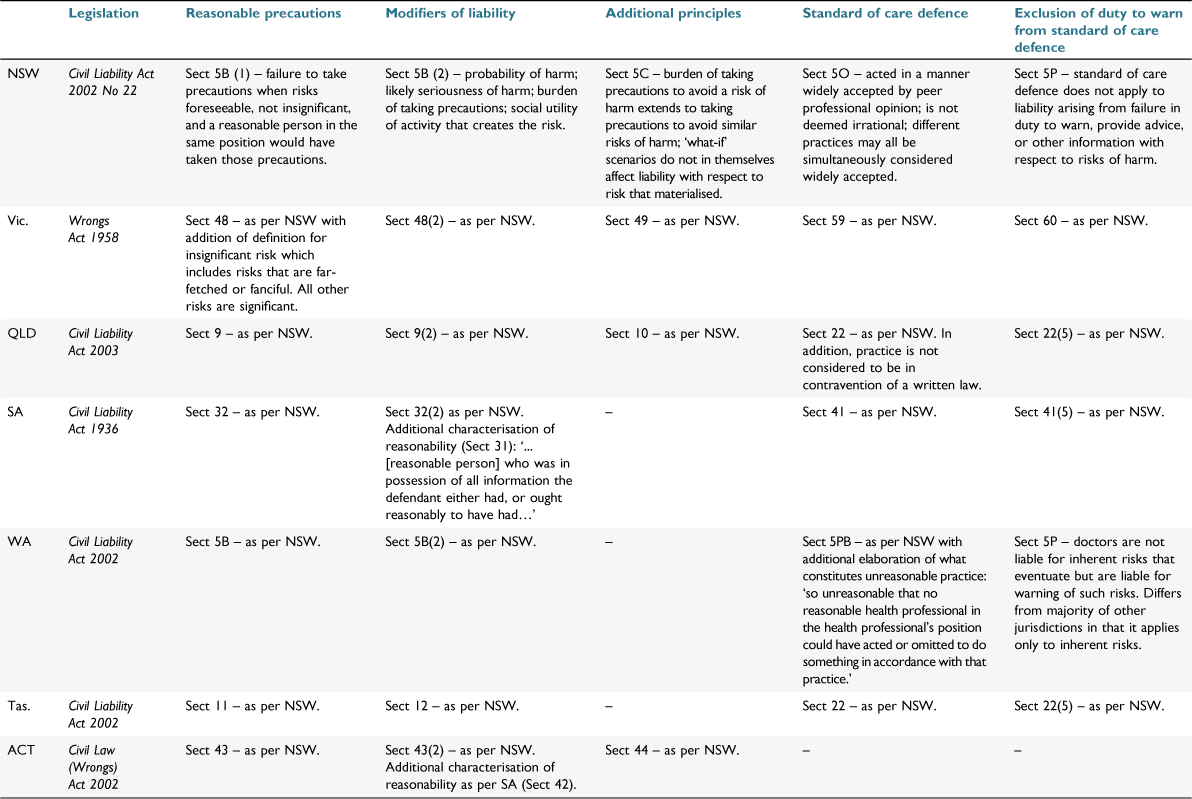
|

|
The duty to warn of inherent risks and non-inherent risks
Physicians are required to warn patients of the inherent risks of treatment, that is, treatment-related risks that are unavoidable by the exercise of reasonable skill and care.11,12 Information to which a reasonable patient would attach significance, and which a specific patient would consider significant, must be disclosed.12 This latter condition is the more demanding because it requires physicians to set aside their own perceptions of what constitutes a significant risk. Furthermore, physicians have a duty to respond truthfully to patients’ questions.13 Hence, if a patient discloses that they cannot afford treatment and asks for advice about how to access more affordable medicines, their physician may be obliged to divulge what they know about more affordable treatment options.
Non-inherent risks arise from a physician’s lack of training, lack of education, lack of experience, or lack of skills rather than the treatment.14 Failure to disclose a deficit of this type can also constitute a breach of duty.15 In fact, the law extends a duty of care to all ‘similar risks’ to those presently recognised (Table 1). Hence, if risks arising from failing to advise patients of effective and affordable medicines can be demonstrated to be similar to those arising from failing to inform patients of plainly superior treatment options – which represents a clear breach – a duty could be adduced.
Physicians must disclose options that offer clear advantages
The law does not recognise a patient's right to information for its own sake, nor does it protect a patient's right to the best possible care, because doing so would impose an unreasonable burden on physicians.16 Therefore physicians are not responsible for telling patients everything they would like to know, or to advise patients of all options available.17 This is contrary to maximalist constructions of informed consent that suggest that patients should be told as much as possible, a position that has widely been recognised as impractical.18 A breach in the duty to disclose material information occurs only if non-disclosure deprived a patient from a reasonable standard of care, or adversely influenced the patient’s decision.11 For this reason the law requires physicians to inform patients of treatment options that offer clear advantages over others, even if they consider the risk–benefit of treatment unfavourable.19,20 The corollary is that physicians are not required to disclose all ‘legitimate’ treatment options when no clear advantage of one treatment over the other is apparent.17 In other words, in the presence of clinical ambiguity, physicians cannot absolve themselves of responsibility by appeal to informed consent, especially when patients are not equipped to make the choice (Table 2; Row 3). This suggests that a duty to disclose the option of importing more affordable medicines may exist in cases where it represents a clearly better alternative (e.g. versus potentially no treatment at all).
Physicians have a duty to facilitate access to the means for achieving health goals
Physicians not only have a duty to treat patients, they also have a duty to facilitate access to the means by which patients’ health goals may be achieved.21 This can include referring patients to diagnostic services, specialist services, or other clinical services, and encouraging the acceptance of such services. Under certain circumstances physicians also have a duty to follow up their patients to ensure that they have accessed the services that they have been referred to.22 This demonstrates that a physician’s duty does not end when the patient leaves the consulting room, but extends to setting ‘in train steps for’ a treatment to be administered.23 Therefore, insofar as importing affordable medicines will help patients to achieve health goals that they cannot otherwise attain, a duty may exist for physicians to support, or at least disclose, this option. However, the enforcement of such a duty would be likely to depend upon knowledge the physician had, or the courts deem they ought to have had, regarding such options (Table 1).
Challenges of interpreting affordability as a risk
The risks that physicians are legally responsible for arise either directly from the treatments they offer (inherent risks), or from their own capabilities or lack thereof (non-inherent risks). Cost-motivated personal importation highlights another category of risk arising from patients’ socioeconomic circumstances. This is neither a risk that rests with the treating physician, nor the treatment, but rather with the patient. While the law does not limit responsibility for taking reasonable precautions to a specific category of risk, there is no evidence that courts would hold physicians liable for harms arising from not prescribing (or advising patients of) affordable treatment when it was within their power to do so. To the contrary, a patient’s financial struggles could be characterised as an intervening cause of harm that breaks the chain of causality and therefore absolves the physician of blame.24
However, if the primary duty of a physician is to diagnose and treat,17 then some responsibility for helping patients to access treatments follows. To dissociate a physician’s duty to prescribe appropriate treatment from all responsibility for accessing it would reduce their role to merely the act of prescribing. This would be inconsistent with both the duty to facilitate access to the means for achieving health goals, and the code of conduct governing medical professionals in Australia,25 which states that physicians have a responsibility for ‘[u]pholding the patient’s right to gain access to the necessary level of healthcare and, whenever possible, helping them to do so’ (section 7.2).
On this point, consider we take seriously the proposition that physicians have no responsibility for considering the affordability of treatments they prescribe. As a practical example, in 2015 the UK General Medical Council issued controversial guidance stating that physicians should not prescribe an off-label or unlicensed medicine when an on-label alternative is available.26 This was in response to physicians prescribing the cancer medicine Avastin for the treatment of age-related macular degeneration as an alternative to the approved product Lucentis, which cost 50 times more. If taken at face value, the Council’s advice seemed to suggest that cost is irrelevant, and it is better to not prescribe anything than prescribe a cheaper off-label product. It is difficult to see how this could be considered in the best interests of patients unable to afford up to $2000 for Lucentis.27 By extension, the Council’s position would suggest that advising patients of the option to import unlicensed generic medicines from abroad would also be a violation of good clinical practice. This would mean that all the physicians who assisted thousands of patients to source affordable hepatitis C medicines via buyer clubs (at up to a 99% discount) could be accused of violating their professional duties.6
The recent introduction of policies on informed financial consent in Australia and internationally are a partial response to this paradox.28–31 These policies recognise that out-of-pocket costs, particularly if unexpected, have a significant impact on patients’ wellbeing (giving rise to the new concept ‘financial toxicity’), and that therefore disclosing cost information is critical to gaining valid consent.32 However, the focus has primarily been on disclosure of planned treatment costs rather than disclosure of more affordable alternatives. One exception is the Australian Cancer Council’s Standard for Informed Financial Consent, which explicitly requires physicians to advise patients of ‘care options such as where the same or similar benefit can be provided at less cost’.28 Understandably, such demands are controversial because it is unclear whether physicians are equipped to offer such information, especially for complex care episodes. Nevertheless, evidence suggests that there is support from segments of the medical community for the idea that costs should be discussed with patients.33
Although there may be additional risks associated with importing medicines from abroad, these risks would be analogous to those that arise from physicians prescribing provisionally approved products that pose greater risk of harm, but are justified on the basis of unmet need.34,35 There is no moral basis for distinguishing between unmet need arising from lack of availability of a medicine, and that arising from lack of affordability.4 Where these alternative pathways for accessing treatment are utilised, including importation, the risks may be characterised as inherent risks of the treatment plan and therefore not risks for which physicians are responsible as long as they warn patients of them.
Discussion
Cost-motivated personal importation of medicines is politically contentious. Cross-border competition undermines the ability of pharmaceutical companies to maintain high prices in their most lucrative markets, it challenges the business model of local pharmacies, and can perpetuate a de facto unregulated market that threatens the integrity of the regulatory system. However, at stake is the health of patients who the system and vested interests have failed. Non-subsidised medicines represent the largest component of out-of-pocket healthcare spending in Australia,36 the market is being inundated with high-cost medicines that few can afford without support,37 and pharmaceutical companies implement aggressive strategies to maintain high medicine prices for as long as possible.38,39 In addition the prices charged for medicines are often not correlated to the added benefits provided.40 It is difficult to countenance that the medical profession, the only institution with a duty to put patients’ interests first, has no role in combating financial toxicity by directing patients to cheaper yet effective treatments. Many patients face the very real prospect of being priced out of the market for medicines that they need, and what this means for the only profession that has a duty to put patients’ interests first must be addressed.
This study has shed light on the potential duty that physicians have to disclose treatment options that are not only effective, but also affordable to the patient, whether through personal importation or other means. Official acknowledgement of such a duty would represent a significant policy shift with far reaching implications and therefore requires careful thought. One specific issue that deserves particular attention is the epistemic conditions antecedent to this duty, given that physicians cannot be held accountable for disclosing information that they are not obliged to know. Two critical questions in this regard are: to what extent are physicians required to learn about the relative costs of different medicines available, or different ways in which cheaper medicines can be accessed?; and, to what extent are physicians responsible for soliciting, and considering, non-clinical information when prescribing medicines (e.g. relating to a patient’s financial circumstances)? Little is known about the answer to the former question, however, empirical evidence shows that many medical professionals believe that discussing the costs of care is important, but they struggle to engage in such conversations.33,41 Developing guidance and resources to assist physicians to navigate their epistemic responsibilities would be essential to implementing effective policies on informed financial consent.
In concluding, it is important to note that the aim of this study is not to further burden physicians but rather to overcome defensive medical practices. If a legal duty to consider costs of care can be established, the hope is that physicians may be more willing to actively help patients to explore options to overcome financial barriers to treatment without fear of legal repercussions, rather than leaving patients to try and find affordable medicines themselves, which could potentially expose them to greater risk of harm.4
Conclusion
Cost-related non-adherence to prescribed treatment is a significant issue in Australia. There is no precedent in law that explores doctors’ duty to disclose more affordable treatment options to patients, whether via personal importation or other mechanisms. This is a significant lacuna that needs to be addressed. The current legal framework has the potential to support cost-motivated personal importation and other economically motivated prescribing. This investigation provides a framework to guide further research and the development of sound policy and law on informed financial consent both in Australia and abroad.
Data availability
Data sharing is not applicable because no new data were generated or analysed during this study.
Conflicts of interest
NG is a board member of the non-profit Prescription Justice Institute, which seeks to improve access to medicines.
Declaration of funding
This research is made possible via a Macquarie University Research Fellowship.
References
[1] Healthengine and Australian Patients Association. Australian Healthcare Index. Report 2. October. 2021. Available at https://australianhealthcareindex.com.au/wp-content/uploads/2021/10/Australian-Healthcare-Index-Report-2-October-2021.pdf[2] Van Alsten SC, Harris JK. Cost-Related Nonadherence and Mortality in Patients With Chronic Disease: A Multiyear Investigation, National Health Interview Survey, 2000–2014. Prev Chronic Dis 2020; 17 200244
| Cost-Related Nonadherence and Mortality in Patients With Chronic Disease: A Multiyear Investigation, National Health Interview Survey, 2000–2014.Crossref | GoogleScholarGoogle Scholar |
[3] Seaman K, Sanfilippo F, Bulsara M, Roughead L, Kemp-Casey A, Bulsara C, Watts GF, Preen D. Predictors of ceasing or reducing statin medication following a large increase in the consumer copayment for medications: a retrospective observational study. Public Health Res Pract 2020; 30 e29121905
| Predictors of ceasing or reducing statin medication following a large increase in the consumer copayment for medications: a retrospective observational study.Crossref | GoogleScholarGoogle Scholar |
[4] Ghinea N. Do doctors have a responsibility to help patients import medicines from abroad? J Med Ethics 2023; 49 131–5.
| Do doctors have a responsibility to help patients import medicines from abroad?Crossref | GoogleScholarGoogle Scholar |
[5] Ghinea N. Personal Importation and the law: protecting patients who import medicines for legitimate health care needs. J Law Med 2022; 29 829–46.
[6] Ghinea N, Lipworth W, Day R, Hill A, Dore GJ, Danta M. Importation of generic hepatitis C therapies: bridging the gap between price and access in high-income countries. Lancet 2017; 389 1268–72.
| Importation of generic hepatitis C therapies: bridging the gap between price and access in high-income countries.Crossref | GoogleScholarGoogle Scholar |
[7] Cohen D. NHS England explores funding options for cystic fibrosis drug. BMJ 2019; 366 l5600
| NHS England explores funding options for cystic fibrosis drug.Crossref | GoogleScholarGoogle Scholar |
[8] PharmacyChecker.com. Drug Price Comparisons & Online Pharmacy Safety. Available at https://www.pharmacychecker.com/verification-portal/[cited 14 February 2023].
[9] Harper C, Ghinea N, Lipworth W. The right to health: Implications for the funding of medicines in Australia. J Law Med 2017; 24 640–55.
[10] Sparks v Hobson; Gray v Hobson. 2018. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/nsw/NSWCA/2018/29.html?context=1;query=Sparks%20v%20Hobson%20;mask_path= [cited 12 January 2023].
[11] Chappel v Hart [1998] HCA 55; 195 CLR 232; 156 ALR 517; 72 ALJR 1344 (2 September 1998). Available at http://classic.austlii.edu.au/cgi-bin/sinodisp/au/cases/cth/HCA/1998/55.html?stem=0&synonyms=0&query=title(Chappel% 20and% 20Hart% 20) [cited 26 July 2022].
[12] Rogers v Whitaker [1992] HCA 58; (1992) 175 CLR 479 (19 November 1992). Available at http://classic.austlii.edu.au/au/cases/cth/HCA/1992/58.html [cited 26 July 2022].
[13] Carver T. Informed consent, Montgomery and the duty to discuss alternative treatments in England and Australia. J Patient Saf Risk Manag 2020; 25 187–93.
| Informed consent, Montgomery and the duty to discuss alternative treatments in England and Australia.Crossref | GoogleScholarGoogle Scholar |
[14] Jambrovic v Day; Jambrovic v Day. 2017. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/nsw/NSWSC/2017/1468.html?context=1;query=Jambrovic%20v%20Day%20;mask_path= [cited 12 January 2023].
[15] McWhirter R. Informed Consent and Performance Data: Clinician Experience as a Material Risk. UNSWLJ 2017; 40 566
| Informed Consent and Performance Data: Clinician Experience as a Material Risk.Crossref | GoogleScholarGoogle Scholar |
[16] UNSW Law Journal. Medical Negligence, Causation and Liability for Non-disclosure of Risk: A Post-Wallace Framework and Critique. 2014. Available at https://www.unswlawjournal.unsw.edu.au/article/medical-negligence-causation-and-liability-for-non-disclosure-of-risk-a-post-wallace-framework-and-critique [cited 12 January 2023].
[17] Richards and Ors v Rahilly and Anor. 2005. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/nsw/NSWSC/2005/352.html?context=1;query=Richards%20and%20Ors%20v%20Rahilly%20and%20Anor;mask_path= [cited 12 January 2023].
[18] Bester J, Cole CM, Kodish E. The Limits of Informed Consent for an Overwhelmed Patient: Clinicians’ Role in Protecting Patients and Preventing Overwhelm. AMA J Ethics 2016; 18 869–86.
| The Limits of Informed Consent for an Overwhelmed Patient: Clinicians’ Role in Protecting Patients and Preventing Overwhelm.Crossref | GoogleScholarGoogle Scholar |
[19] ACT v Gillan; Gillan v ACT. 2018. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/act/ACTSC/2018/223.html?context=1;query=Gillan%20v%20ACT%20;mask_path= [cited 12 January 2023].
[20] Rufo v Hosking [2004] NSWCA 391 (1 November 2004). Available at http://classic.austlii.edu.au/au/cases/nsw/NSWCA/2004/391.html [cited 26 July 2022].
[21] Varipatis v Almario. 2013. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/nsw/NSWCA/2013/76.html?context=1;query=Varipatis%20v%20Almario%20;mask_path= [cited 12 January 2023].
[22] Rubino v Ziaee. 2021. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/act/ACTSC/2021/331.html?context=1;query=Ziaee% 20;mask_path= [cited 12 January 2023].
[23] Tai v Hatzistavrou. 1999. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/nsw/NSWCA/1999/306.html [cited 12 January 2023].
[24] Gould v South Western Sydney Local Health District. 2017. Available at http://www.austlii.edu.au/cgi-bin/viewdoc/au/cases/nsw/NSWDC/2017/67.html?context=1;query=Novus%20actus%20interveniens;mask_path=#_Ref478109736 [cited 8 March 2023].
[25] Medical Board of Australia. Good medical practice: a code of conduct for doctors in Australia. October. 2020. Available at https://www.medicalboard.gov.au/Codes-Guidelines-Policies/Code-of-conduct.aspx
[26] Dickson N. The GMC’s stance on Avastin. BMJ 2015; 350 h2043
| The GMC’s stance on Avastin.Crossref | GoogleScholarGoogle Scholar |
[27] Lipworth W. Wendy Lipworth: Counting the cost of off-label prescribing. InSight+; 2011. Available at https://insightplus.mja.com.au/2011/23/wendy-lipworth-counting-cost-label-prescribing/ [cited 6 March 2023].
[28] Cancer Council. Informed financial consent. 2023. Available at https://www.cancer.org.au/health-professionals/resources/informed-financial-consent [cited 26 July 2022].
[29] Australian Medical Association. Informed Financial Consent. 2020. Available at https://www.ama.com.au/articles/informed-financial-consent [cited 6 March 2023].
[30] Australian Commission on Safety and Quality in Health Care. AS18/10: Informed financial consent. 2021. Available at https://www.safetyandquality.gov.au/publications-and-resources/resource-library/as1810-informed-financial-consent [cited 6 March 2023].
[31] Richman B, Hall M, Schulman K. The No Surprises Act and Informed Financial Consent. N Engl J Med 2021; 385 1348–51.
| The No Surprises Act and Informed Financial Consent.Crossref | GoogleScholarGoogle Scholar |
[32] Pisu M, Martin MY. Financial toxicity: a common problem affecting patient care and health. Nat Rev Dis Primers 2022; 8 7
| Financial toxicity: a common problem affecting patient care and health.Crossref | GoogleScholarGoogle Scholar |
[33] Altomare I, Irwin B, Zafar SY, Houck K, Maloney B, Greenup R, et al. ReCAP: Physician Experience and Attitudes Toward Addressing the Cost of Cancer Care. J Oncol Pract 2016; 12 247–8.
| ReCAP: Physician Experience and Attitudes Toward Addressing the Cost of Cancer Care.Crossref | GoogleScholarGoogle Scholar |
[34] Mahase E. FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway. BMJ 2021; 374 n1898
| FDA allows drugs without proven clinical benefit to languish for years on accelerated pathway.Crossref | GoogleScholarGoogle Scholar |
[35] Mostaghim SR, Gagne JJ, Kesselheim AS. Safety related label changes for new drugs after approval in the US through expedited regulatory pathways: retrospective cohort study. BMJ 2017; 358 j3837
| Safety related label changes for new drugs after approval in the US through expedited regulatory pathways: retrospective cohort study.Crossref | GoogleScholarGoogle Scholar |
[36] Australian Institute of Health and Welfare. Health expenditure Australia 2020-21, Non-government sources. 2022. Available at https://www.aihw.gov.au/reports/health-welfare-expenditure/health-expenditure-australia-2020-21/contents/spending-trends-by-sources/non-government-sources [cited 25 January 2023].
[37] Beasley D. U.S. new drug price exceeds $200,000 median in 2022. Reuters; 2023. Available at https://www.reuters.com/business/healthcare-pharmaceuticals/us-new-drug-price-exceeds-200000-median-2022-2023-01-05/ [cited 8 March 2023].
[38] Levy MS. Big Pharma Monopoly: Why Consumers Keep Landing on “Park Place” and How the Game is Rigged. Am Univ Law Rev 2016; 66 247–303.
[39] Fox E. How Pharma Companies Game the System to Keep Drugs Expensive. Harvard Business Review, 6 April 2017. Available at https://hbr.org/2017/04/how-pharma-companies-game-the-system-to-keep-drugs-expensive [cited 2023 Jan 13].
[40] Becker DJ, Lin D, Lee S, Levy BP, Makarov DV, Gold HT, et al. Exploration of the ASCO and ESMO Value Frameworks for Antineoplastic Drugs. J Oncol Pract 2017; 13 e653–65.
| Exploration of the ASCO and ESMO Value Frameworks for Antineoplastic Drugs.Crossref | GoogleScholarGoogle Scholar |
[41] Warsame R, Kennedy CC, Kumbamu A, Branda M, Fernandez C, Kimball B, et al. Conversations About Financial Issues in Routine Oncology Practices: A Multicenter Study. J Oncol Pract 2019; 15 e690–703.
| Conversations About Financial Issues in Routine Oncology Practices: A Multicenter Study.Crossref | GoogleScholarGoogle Scholar |


