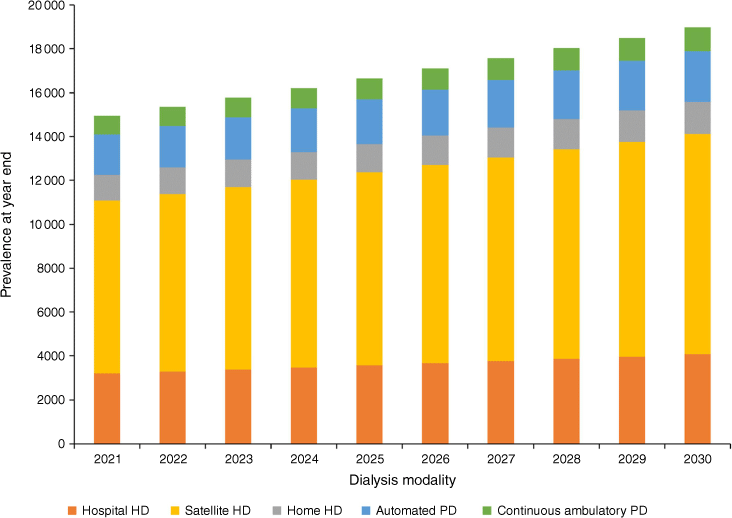Projecting the future: modelling Australian dialysis prevalence 2021–30
Dominic Keuskamp A B * , Christopher E. Davies A B , Georgina L. Irish A B C , Shilpanjali Jesudason A B C and Stephen P. McDonald A B CA Australia & New Zealand Dialysis & Transplant Registry, South Australian Health & Medical Research Institute, Adelaide, SA, Australia.
B Faculty of Health & Medical Sciences, University of Adelaide, Adelaide, SA, Australia.
C Central Northern Adelaide Renal & Transplantation Service, Royal Adelaide Hospital, Adelaide, SA, Australia.
Australian Health Review 47(3) 362-368 https://doi.org/10.1071/AH22291
Submitted: 22 December 2022 Accepted: 22 April 2023 Published: 16 May 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of AHHA. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Objectives To project the prevalence of people receiving dialysis in Australia for 2021–30 to inform service planning and health policy.
Methods Estimates were based on data from 2011 to 2020 from the Australia & New Zealand Dialysis & Transplant (ANZDATA) Registry and the Australian Bureau of Statistics. We projected dialysis and functioning kidney transplant recipient populations for the years 2021–30. Discrete-time, non-homogenous Markov models were built on probabilities for transition between three mutually exclusive states (Dialysis, Functioning Transplant, Death), for five age groups. Two scenarios were employed – stable transplant rate vs a continued increase – to assess the impact of these scenarios on the projected prevalences.
Results Models projected a 22.5–30.4% growth in the dialysis population from 14 554 in 2020 to 17 829 (‘transplant growth’) – 18 973 (‘transplant stable’) by 2030. An additional 4983–6484 kidney transplant recipients were also projected by 2030. Dialysis incidence per population increased and dialysis prevalence growth exceeded population ageing in 40–59 and 60–69 year age groups. The greatest dialysis prevalence growth was seen among those aged ≥70 years.
Conclusion Modelling of the future prevalence of dialysis use highlights the increasing demand on services expected overall and especially by people aged ≥70 years. Appropriate funding and healthcare planning must meet this demand.
Keywords: ageing, chronic disease management, chronic kidney disease, dialysis, epidemiology, health services research, kidney failure, population health, registry.
Introduction
Chronic kidney disease is a risk for one in three adult Australians and if untreated may lead to kidney failure.1 Kidney failure (KF) may be treated with either dialysis or kidney transplant, collectively termed kidney replacement therapy (KRT), or managed with a conservative care pathway. Treatment is expensive, exceeding a total cost of A$1.2 billion to the Australian Government when last assessed, and requires a specialised infrastructure and workforce.2 Dialysis capacity in all jurisdictions is severely limited, although rarely publicly recognised.3
Projecting future dialysis prevalence and thus demand on capacity is essential for healthcare funders and providers to estimate consumer burden and effective future management of infrastructure, staffing and resources. Projections are especially important given the increased prevalence of older people with treated KF, with dialysis users aged ≥70 years growing by 37% from 2011 to 2020 to account for almost 40% of prevalence.4 The impact of the ageing population on KF care is likely to be substantial given the more complex and costly renal needs.5 Older people are more likely to use more costly facility haemodialysis than self-care options (home haemodialysis or peritoneal dialysis) or receive a kidney transplant (a description of these modalities can be found in Supplementary Table S1).
Projecting transfers between dialysis and transplant modalities must supplement incidence and mortality modelling, as the life expectancy, cost and quality differ among treatment modalities.6 Markov modelling is commonly used for projecting disease prevalence in such situations with multiple mutually exclusive states.7 The most recently published projections, for 2012–20, employed Markov modelling and data from the Australia & New Zealand Dialysis & Transplant (ANZDATA) Registry.8 Those age-specific dialysis prevalence projections were generally within 5% of subsequently reported actual prevalence.4
To inform Australian health policy and funding and optimise health care service delivery, a detailed understanding of future trends in dialysis prevalence is vital, considering the effects of population growth and ageing. We used Australian Bureau of Statistics (ABS) population data and recent trends sourced from ANZDATA data to project demand for dialysis (and kidney transplantation) for 2021–30, to evaluate the future burden on individuals, community and the health system.
Methods
Models
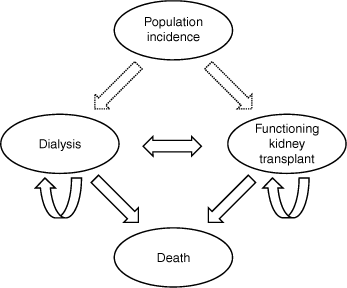
The dialysis and functioning kidney transplant recipient populations from 2021 to 2030 were projected using discrete-time, non-homogeneous Markov models (Fig. 1). Time was incremented in calendar years, with the prevalent numbers projected being those at the end of that year. Age (at the end of each year) was categorised into 0–19, 20–39, 40–59, 60–69 and ≥70 years. The estimated transition rates were based on inspection of models of the observed ANZDATA data from 2011 to 2020. Estimations were verified for consistency with current clinical experiences by authors with clinical expertise (GLI, SJ, SPM). All transition rates are listed in Supplementary Tables S2–S4.
Incident KRT and dialysis rates
Incident KRT and dialysis-as-first-KRT numbers were estimated relative to the size of the Australian population in that age group. For incident KRT, a constant age-specific rate was assumed at the mean of the yearly rate for 2016–20 for all but the 60–69 year age group, where linear growth over time was assumed based on a linear regression fit to the data for 2016–20. For dialysis, linear growth was assumed based on a regression of 2016–20 for the 40–59-year and 60–69-year age groups. For the 0–19-year, 20–39 and ≥70 year age groups, a constant age-specific rate was assumed at the mean of the yearly rate for 2016–20.
Rates of return to dialysis, mortality and ageing
Loss of transplant function (‘graft failure’) and return to dialysis is another transition, which augments incidence to increase prevalence (Fig. 1). Rates of return to dialysis after transplantation were estimated as a proportion of the prevalent transplant population in each age group. Constant age-specific rates were assumed at the mean of the yearly rate for 2016–20.
Mortality rates for both the dialysis and transplant populations were estimated as proportions of the prevalent populations in that age group. A constant rate was assumed at the mean of the yearly rate for 2016–20, or – where the rate was zero – for 2011–20 (0–19-year age group). The probabilities of people ‘ageing’ into the older category within the dialysis and transplant groups were estimated according to the mean proportion of people that changed categories from each group over 2011–20.
Transplant rates
Living donor pre-emptive transplant rates (people receiving a kidney transplant without prior dialysis) were calculated as a proportion of the incident KRT cohort, and living donor transplants following dialysis were calculated as a proportion of the prevalent dialysis population in the previous year. Living donor transplant rates and deceased donor pre-emptive transplant rates were assumed to be constant at the mean of the yearly rate for 2015–19. Deceased donor transplant rates were estimated relative to the total size of the Australian population, for both pre-emptive transplants and transplants following dialysis. Two scenarios were evaluated for deceased donor transplantation following dialysis. The ‘transplant stable’ model assumed a steady state at the mean of the yearly rate for 2015–19. The ‘transplant growth’ model used linear growth over time based on a linear regression fit to the data for 2015–19, or for 2010–19 where the rate was negative (for the 40–59-year age group). To estimate the number of deceased donors required to provide the projected number of deceased donor kidney transplants, the mean ratio of deceased donor kidney transplants to actual deceased donors for 2016–20 was calculated. For all transplant rate calculations, 2020 data were omitted due to coronavirus disease 2019 (COVID-19) driven pauses in transplantation that year.
Assumptions
Dialysis and transplant vintage were not accounted for in modelling. Inspection of observed data for transitions by vintage revealed few clear trends; the decision to omit vintage also served to minimise the number of transitions and hence assumptions and errors. For similar reasons, models were also not stratified by dialysis modality. Instead, to project future prevalence by dialysis modality, the relative proportions of each modality were held constant in each age group based on the 2016–20 mean prevalence.
Results
Models projected that dialysis and functioning kidney transplant prevalences would increase markedly from 2021 to 2030, regardless of the scenario employed (Table 1). Projected dialysis (by modality) and transplant prevalences for all years and both scenarios are in Supplementary Table S5.
| Age in years | 2020 | ‘Transplant stable’ | ‘Transplant growth’ | ||
| Projected | Projected | ||||
| 2025 | 2030 | 2025 | 2030 | ||
| Dialysis | |||||
| 0–19 | 70 | 75 | 80 | 68 | 61 |
| 20–39 | 1022 | 1088 | 1154 | 1024 | 1003 |
| 40–59 | 4218 | 4561 | 5054 | 4427 | 4709 |
| 60–69 | 3460 | 4087 | 4661 | 3916 | 4273 |
| ≥70 | 5784 | 6849 | 8025 | 6740 | 7783 |
| Total | 14 554 | 16 660 | 18 973 | 16 174 | 17 829 |
| Transplant | |||||
| 0–19 | 312 | 356 | 393 | 364 | 415 |
| 20–39 | 1692 | 1915 | 2106 | 1981 | 2269 |
| 40–59 | 5371 | 6231 | 7015 | 6383 | 7446 |
| 60–69 | 3573 | 4307 | 4974 | 4507 | 5498 |
| ≥70 | 2182 | 2906 | 3626 | 3039 | 3986 |
| Total | 13 130 | 15 714 | 18 113 | 16 275 | 19 614 |
Dialysis population projections
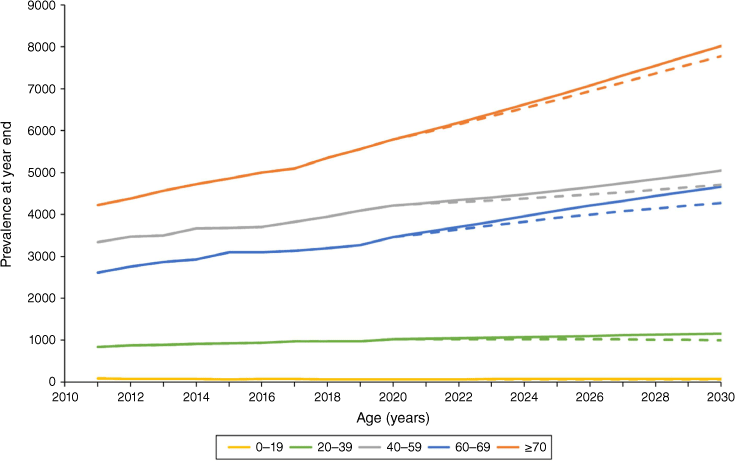
Estimated dialysis incidence rates for 2021–30 were highest among those aged ≥70 years (349 per million persons per year). The ‘transplant stable’ model projected that dialysis prevalence would increase from 14 554 to 18 973 people from 2020 to 2030 (4419 people or 30.4%) and the ‘transplant growth’ model projected an increase to 17 829 (22.5%). Projected prevalence increased for the three oldest age groups, especially for people aged ≥70 years, with a 38.7% rise in the ‘transplant stable’ model (Fig. 2). The 30.4% increase in dialysis prevalence from 2020 to 2030 in the ‘transplant stable’ model well exceeded the ABS-projected underlying population change of 16.5% (Table 2).9 Comparisons by age group revealed that the excess was driven largely by growth in people aged 40–69 years, despite the prevalence increase being largest for those aged ≥70 years. Population growth exceeded dialysis prevalence growth for the youngest two age groups in the ‘transplant stable’ model.
| Age in years | 2020–30 percentage increase | |
| Projected dialysis prevalence | Australian population prevalence | |
| 0–19 | 14.7 | 16.6 |
| 20–39 | 12.9 | 13.9 |
| 40–59 | 19.8 | 13.4 |
| 60–69 | 34.7 | 11.3 |
| ≥70 | 38.7 | 34.4 |
| Total | 30.4 | 16.5 |
Functioning kidney transplant population projections
Age-specific transplant incidence rates in general had stabilised and were modelled conservatively with constant rates in both scenarios, apart from the positive linear trend for deceased donor transplants from dialysis employed for all age groups in the ‘transplant growth’ model. The ‘transplant growth’ model projected higher transplant (and lower dialysis) prevalence than the ‘transplant stable’ model for all age groups (Supplementary Fig. S1). Overall, increases in transplant prevalence were projected from 13 130 to 18 113 from 2020 to 2030 (4983 transplant recipients or 38.0%) for the ‘transplant stable’ model and to 19 614 (6484 and 49.4%) for the ‘transplant growth’ model (Table 1). A deceased donor rate of 20.2–26.3 donors per million population (dpmp) by 2030 was projected, based on a transplant−donor ratio of 1.59.
Dialysis location
The mean proportion of people receiving facility haemodialysis (i.e. hospital and satellite) over 2016–20 varied from 39% for 0–19 years to 80% for those aged ≥70 years (Table 3). Projected dialysis prevalence by dialysis modality for the ‘transplant stable’ model is shown in Fig. 3. Notably, of the projected additional 4419 people receiving dialysis in 2030 over 2020, 3238 of them (73.3%) required facility haemodialysis. Only 327 of the remaining projected people required home haemodialysis, at current utilisation rates.
Discussion
The enclosed projections depicted substantial future growth in the prevalence of treated KF in Australia. By 2030, up to 4416 additional dialysis users and 6484 additional kidney transplant recipients were projected to require care. Incidence rates have stabilised such that the projected increase in dialysis prevalence of 71% for 2011–30 is lower than the three-fold increase seen in the two decades prior.8 Nonetheless, the excess increase in dialysis prevalence over underlying population growth and the rising proportion of older people among the prevalent cohort should demand attention from healthcare policymakers and funders.
The projected increase in facility haemodialysis users of 3238 people would require over 800 additional dialysis chairs by 2030, assuming three dialysis treatments per consumer per week.4 Dialysis capacity, already limited in all jurisdictions, would be further strained.3 The projected additional annual direct cost to the health system was A$235 million for a total of over A$1 billion for facility haemodialysis alone (using notional costings in AUD 2019–20).10 A more thorough economic evaluation than ours would require high-quality information about the actual cost of providing dialysis treatment in a variety of contexts.11 Although notional funding allocations exist, as do more detailed data from satellite facilities in the Northern Territory,12 no detailed ‘bottom up’ costings of actual treatment are publicly available to our knowledge. Moreover, accurate and recent costing data are not available to calculate these without a large margin of error. The significant age-based variation in the enclosed modelling also highlights due consideration be given to renal funding models that are adjusted by age and not merely activity-based.
Our projections confirm that older Australians are a significant and increasing proportion of dialysis prevalence and suggest that population ageing (rather than disease incidence) appears to be driving prevalence growth. However, the anticipated greater demand for dialysis chairs does assume that current treatment regimens continue. The incidence rates among older age groups seen prior to 2015 have plateaued, possibly related to stabilisation of clinical practice supported by data on outcomes of older people receiving dialysis and availability of conservative care pathways.13
The uptake of home-based modalities (PD and home haemodialysis) has been actively supported by several state-based schemes, on the basis of better outcomes and lower costs.14,15 Some hospitals’ funding incentives are aligned with the proportion of PD and home haemodialysis users. However, the effectiveness of these is uncertain, especially given the ‘opt-in’ approach, and there has been little sign of growth in home dialysis proportions in national data.4 Appropriate workforce planning and support that addresses volume as well as case mix – and evolving technologies in dialysis machines and remote support – must meet the projected growth in demand for care. The specialised dialysis workforce is scarce and ageing, and requires innovation to address recruitment shortfalls.16,17
Although a necessary component of the modelling, transplant prevalence is more difficult to project accurately. The number of deceased organ donors is determined by several factors, including changes in policy and practice around deceased organ donation, intrinsic biological trends, and recent fluctuations in donor numbers due to COVID-19. Our deceased donation rate (based on projected transplant activity) of 20.2–26.3 dpmp, is consistent with the current national target of 25 dpmp set by the Australian Organ and Tissue Authority.18 The donor rate was set constant in those calculations, while acknowledging that recent years have witnessed a downward trend. Whether the transplantation rates projected here are feasible – especially in the ‘transplant growth’ model – is beyond the scope of the present study. Organ availability could increase with more marginal donor utilisation, or as machine preservation becomes more available. However, recent COVID-19-related trends emphasise the considerable uncertainty about donor availability. What is clear is the cost-effectiveness of increasing transplantation rates,2,14 which are notably higher in some other countries (e.g. Spain and the US) where deceased donation rates exceeded 50 dpmp prior to the COVID-19 pandemic.19 A hypothetical increase of 90% in deceased donor transplants over the ‘transplant growth’ model was needed to elevate the Australian donor rate to this standard by 2030. This depressed dialysis prevalence to negative growth, well below ABS-projected population growth.
Our projections reflect recent trends in the stability of factors influencing KF/KRT incidence including stable prevalence of diabetes and other primary kidney diseases, competing mortality, referral rates, access to dialysis, the propensity to treat KF with KRT, and survivorship.20 Recent modelling has suggested that diabetes prevention with sodium–glucose cotransporter 2 inhibitors could have substantial implications for KF incidence alone or in combination with other interventions, although their impact on prevalent dialysis numbers is likely to be delayed beyond the time frame of these projections.21 Likewise, wider uptake of antihypertensive therapy – such as renin-angiotensin blockers – could also impact KF incidence, as has been likely to date.22
Several factors were not controlled for in our modelling that may influence future prevalence of treated KF: dialysis type, therapy period and diabetes status. However, even at a national level, numbers offered little opportunity to stratify and ensure model stability and accurate projections. For the same reason, transitions for Indigenous people were not included (tailored projections are available elsewhere for the Northern Territory).23 Moreover, our exploratory analyses revealed negligible differences in mortality rates among modalities that would justify stratification by dialysis type. Our modelling, which integrated dialysis and transplant prevalences, also masked some subtleties. For example, an increase in transplants would likely increase the dialysis mortality rate as healthier dialysis users are more likely to receive a transplant. Finally, uncertainty in our projections has been addressed by use of two scenarios, rather than more explicit modelling of error. Projected dialysis prevalence in 2030 only differed by 6.4% between ‘transplant stable’ and ‘transplant growth’ scenarios, suggesting projections were not overly sensitive to the variation in 2011–20 transplant rates informing the models. The suitability of our methodology, and more broadly Markov modelling, for projecting prevalence had been demonstrated by the alignment between previously published projections8 and data subsequently reported by ANZDATA.
Conclusion
The projected growth in dialysis prevalence in this study exceeded population growth by 36–84%, highlighting the growing demand for high-cost specialised healthcare services and the need for cost-effective allocation of resources. The increasing contribution of older people to this demand requires a focus on dialysis and transplant care that is tailored to that more complex cohort. Consequently, age-specific funding and planning, including workforce planning, are essential. Chronic kidney disease remains largely preventable, particularly through modifiable risk factors. Recommendations such as increased access to early chronic condition risk asssessment comprise key priorities of the National Strategic Action Plan for Kidney Disease;1 prevention, detection and early intervention are imperative to moderate progression to kidney failure and the need for dialysis.
Data availability
The data that support this study were publicly available from the ABS or obtained by permission from the ANZDATA Registry. Data will be shared upon reasonable request to the corresponding author with permission from the ANZDATA Registry.
Declaration of funding
DK and CED were supported by the Better Evidence and Translation in Chronic Kidney Disease (BEAT-CKD) Program Grant awarded to SPM and SJ (National Health and Medical Research Council, Australia, APP1092957). GI was supported by a Postgraduate Research Scholarship (National Health and Medical Research Council, Australia).
Acknowledgements
We are grateful to the Australian and New Zealand kidney units, consumers and staff for their cooperation and contributions to the ANZDATA Registry. The data reported here were supplied by the ANZDATA Registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the Registry.
References
[1] Kidney Health Australia. National strategic action plan for kidney disease. Canberra: Commonwealth of Australia; 2019. Available at https://www.health.gov.au/resources/publications/national-strategic-action-plan-for-kidney-disease [accessed 8 August 2022].[2] Cass A, Chadban S, Gallagher M, Howard K, Jones A, McDonald S, et al. The economic impact of end stage kidney disease in Australia: Projections to 2020. Melbourne: Kidney Health Australia; 2010.
[3] ACI Renal Network. NSW dialysis capacity audit 2014. Chatswood Agency for Clinical Innovation; 2014. Available at https://aci.health.nsw.gov.au/resources/renal/dialysis-capacity-audits [accessed 20 October 2022].
[4] Australia & New Zealand Dialysis & Transplant Registry. 45th annual report 2021 (data to 2020). Adelaide: ANZDATA; 2021. Available at https://www.anzdata.org.au/anzdata/publications/reports/ [accessed 12 May 2022].
[5] Jassal SV, Watson D. Dialysis in late life: Benefit or burden. Clin J Am Soc Nephrol 2009; 4 2008–12.
| Dialysis in late life: Benefit or burden.Crossref | GoogleScholarGoogle Scholar |
[6] McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol 2009; 20 155–63.
| Relationship between dialysis modality and mortality.Crossref | GoogleScholarGoogle Scholar |
[7] Sonnenberg FA, Beck JR. Markov models in medical decision making: A practical guide. Med Decis Making 1993; 13 322–38.
| Markov models in medical decision making: A practical guide.Crossref | GoogleScholarGoogle Scholar |
[8] Australian Institute of Health and Welfare. Projections of the prevalence of treated end-stage kidney disease in Australia 2012–2020. Cat. No. Phe 176. Canberra: AIHW; 2014.
[9] Australian Bureau of Statistics. Population projections, Australia. Canberra: ABS; 2022. Available at https://www.abs.gov.au/statistics/people/population/population-projections-australia/2017-base-2066 [accessed 12 May 2022].
[10] Independent Health and Aged Care Pricing Authority. National hospital cost data collection. 2022. Available at https://www.ihacpa.gov.au/health-care/costing/national-hospital-cost-data-collection [accessed 3 August 2022].
[11] Wyld MLR, Lee CMY, Zhuo X, White S, Shaw JE, Morton RL, et al. Cost to government and society of chronic kidney disease stage 1–5: A national cohort study. Intern Med J 2015; 45 741–7.
| Cost to government and society of chronic kidney disease stage 1–5: A national cohort study.Crossref | GoogleScholarGoogle Scholar |
[12] Gorham G, Howard K, Cunningham J, Barzi F, Lawton P, Cass A. Do remote dialysis services really cost more? An economic analysis of hospital and dialysis modality costs associated with dialysis services in urban, rural and remote settings. BMC Health Serv Res 2021; 21 582
| Do remote dialysis services really cost more? An economic analysis of hospital and dialysis modality costs associated with dialysis services in urban, rural and remote settings.Crossref | GoogleScholarGoogle Scholar |
[13] Murtagh FEM, Burns A, Moranne O, Morton RL, Naicker S. Supportive care: Comprehensive conservative care in end-stage kidney disease. Clin J Am Soc Nephrol 2016; 11 1909–14.
| Supportive care: Comprehensive conservative care in end-stage kidney disease.Crossref | GoogleScholarGoogle Scholar |
[14] Howard K, Salkeld G, White S, McDonald S, Chadban S, Craig JC, et al. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology 2009; 14 123–32.
| The cost-effectiveness of increasing kidney transplantation and home-based dialysis.Crossref | GoogleScholarGoogle Scholar |
[15] Marshall MR, Polkinghorne KR, Boudville N, McDonald SP. Home versus facility dialysis and mortality in Australia and New Zealand. Am J Kidney Dis 2021; 78 826–36.e1.
| Home versus facility dialysis and mortality in Australia and New Zealand.Crossref | GoogleScholarGoogle Scholar |
[16] Chow J, Lau B, Gibb C. New ways to approach the recruitment crisis in renal nursing in New South Wales, Australia. J Ren Care 2008; 34 38–42.
| New ways to approach the recruitment crisis in renal nursing in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
[17] Polaschek N, Bennett PN, McNeill L. The Australian and New Zealand dialysis workforce study in the international context. J Ren Care 2009; 35 170–5.
| The Australian and New Zealand dialysis workforce study in the international context.Crossref | GoogleScholarGoogle Scholar |
[18] Australian Organ and Tissue Authority. 2021 Australian donation and transplantation activity report. Canberra: Commonwealth of Australia; 2022. Available at https://www.donatelife.gov.au/all-about-donation/statistics-in-australia [accessed 10 August 2022].
[19] International Registry in Organ Donation and Transplantation. Database. Barcelona: IRODaT - DTI Foundation; 2023. Available at https://www.irodat.org/?p=database [accessed 21 February 2023].
[20] McDonald S, McCredie M, Williams S, Stewart J. Factors influencing reported rates of treated end-stage renal disease. Adv Chronic Kidney Dis 2005; 12 32–8.
| Factors influencing reported rates of treated end-stage renal disease.Crossref | GoogleScholarGoogle Scholar |
[21] Morton JI, McDonald SP, Salim A, Liew D, Shaw JE, Magliano DJ. Projecting the incidence of type 2 diabetes-related end-stage kidney disease until 2040: A comparison between the effects of diabetes prevention and the effects of diabetes treatment. Diabetes Care 2021; 44 1515–23.
| Projecting the incidence of type 2 diabetes-related end-stage kidney disease until 2040: A comparison between the effects of diabetes prevention and the effects of diabetes treatment.Crossref | GoogleScholarGoogle Scholar |
[22] Stewart JH, Disney APS, Mathew TH. Trends in the incidence of end-stage renal failure due to hypertension and vascular disease in Australia, 1972–1991. Aust N Z J Med 1994; 24 696–700.
| Trends in the incidence of end-stage renal failure due to hypertension and vascular disease in Australia, 1972–1991.Crossref | GoogleScholarGoogle Scholar |
[23] You J, Zhao Y, Lawton P, Guthridge S, McDonald SP, Cass A. Projecting demands for renal replacement therapy in the Northern Territory: A stochastic Markov model. Aust Health Rev 2018; 42 380–6.
| Projecting demands for renal replacement therapy in the Northern Territory: A stochastic Markov model.Crossref | GoogleScholarGoogle Scholar |