Effect of a state hospital formulary on medicines utilisation in Australia
Joel Iedema A B C DA Department of Medicine, Redland Hospital, Metro South Hospital and Health Service, Weippin Street, Cleveland, Qld 4163, Australia.
B PA-Southside Clinical Unit, Faculty of Medicine, University of Queensland, Princess Alexandra Hospital, Woolloongabba, Qld 4102, Australia.
C Present address: Metro South Health COVID Vaccination Program, Metro South Hospital and Health Service, Building 19, Garden City Office Park, 2404 Logan Road, Eight Mile Plains, Qld 4113, Australia.
D Email: joel.iedema@health.qld.gov.au
Australian Health Review 45(6) 704-717 https://doi.org/10.1071/AH20330
Submitted: 17 November 2020 Accepted: 11 January 2021 Published: 8 November 2021
Journal Compilation © AHHA 2021 Open Access CC BY-NC-ND
Abstract
Objective The provision of medicines through state public hospitals is comparatively restrictive compared with the federally funded Pharmaceutical Benefits Scheme (PBS). Individual states are progressively moving towards statewide medicines formularies. Although a statewide formulary has existed in Queensland for some time. The effects of hospital formularies on medicines utilisation and policy in Australia has not been quantified. Thus, the aim of the present study was to quantify the effects of the Queensland Health List of Approved Medicines (LAM) on medicines utilisation in Queensland at a state and PBS-purchasing level and describe the implications for medicines policy.
Methods This study used a quasi-experimental design with an interrupted time series (with control for PBS) examining utilisation effects of medicines within the therapeutic classes of proton pump inhibitors and non-vitamin K oral anticoagulants with LAM listing or delisting.
Results The LAM was demonstrated to be highly effective at controlling utilisation within Queensland Health purchasing. Effects on PBS utilisation were evident, resulting in increases in generic utilisation (where available) and associated reduced total costs both within Queensland Health and to the PBS. The full benefit is likely underestimated due to limitations in the PBS datasets.
Conclusion The LAM is a highly effective state medicines policy tool with demonstrable effects on PBS utilisation. With increased use of statewide medicines formularies, this will be an increasingly relevant aspect of Australia’s overall medicines policy.
What is known about the topic? State medicines policy is comparatively restrictive compared with the federal PBS. Most Australian states have, or are developing, statewide medicines formularies.
What does this paper add? By examining several classes of medicines, a substantial quantitative effect of the Queensland state formulary on both state and PBS medicines utilisation can be demonstrated. Increased use of generic medicines and reduced costs are seen.
What are the implications for practitioners? With increased use of state medicines formularies, state medicines formularies will become increasingly relevant to medicines policy makers and advocates at both the state and federal level.
Keywords: epidemiology, health economics, health funding and financing, health policy, pharmaceuticals, medicines formularies, Pharmaceutical Benefits Scheme, policy makers.
Introduction
The Pharmaceutical Benefits Scheme (PBS) is a foundational component of the Australian social health insurance system.1,2 The national formulary, the Schedule of Pharmaceutical Benefits (https://www.pbs.gov.au/browse/publications), defines which medicines are subsidised by the federal government. The Pharmaceutical Benefits Advisory Committee (PBAC), established in 1953,1 recommends to the Minister of Health which medicines should be listed on the formulary.
The current expenditure of the PBS is A$11 690 million per annum.3 Like all social health insurance schemes, the value and affordability of the PBS is questioned.4,5 Much of the debate is levelled at price-setting mechanisms. Comparisons to other jurisdictions have demonstrated higher per capita expenditure and a higher mean price of pharmaceuticals, particularly compared with more restrictive schemes, such as New Zealand’s Pharmaceutical Management Agency (PHARMAC).6,7 There is considerably commentary on mechanisms to reduce medicine prices on the PBS.7–11
Comparatively less commentary is devoted to the effect of medicines utilisation on expenditure within the PBS. Analyses of statin purchasing suggest that relative utilisation, rather than price, accounts for most of the increased expenditure within the PBS.12,13 Mechanisms on the supply and demand side exist to control utilisation in the PBS. These mechanisms include educational support through the National Prescribing Service, Therapeutic Group Premium copayments for higher-priced products and generic switching at the pharmacy level.5,12
The provision of public hospital services in Australia is the jurisdiction of state governments. Access to the PBS was restricted for public hospital services, but changes to legislation have allowed access for general out-patient and discharge prescriptions (in most states) and medicines listed in the Highly Specialised Drug (HSD) program.14 Most in-patient medicine supply remains outside the PBS. In contrast with the PBS, state medicines policy is comparatively restrictive through medicines formularies (Table 1). Formularies were historically curated at individual hospital level, as remains the process in the two most populous states, New South Wales and Victoria, as well as in the Australian Capital Territory. Other states have introduced and continue to develop statewide medicines formularies. For example, the South Australian Medicines Formulary framework was first published in March 2013, but the inclusion of all therapeutic groups was not completed until 2018. The formulary is currently less restrictive than the formulary committee intends long term.16 Similarly, development of the Western Australia Statewide Medicines Formulary (SMF) commenced in 2015 with the supporting SMF Policy enacted January 2018.14,17 Tasmania commenced early implementation of The Tasmanian Electronic Medicines Formulary in June 2013,18 with the formulary maturing after early implementation. The Northern Territory also has a statewide medicines formulary list.

|
In contrast, Queensland has a long history (Fig. 1) of state formulary management supported by the only central government procurement and warehouse facility.14,20 The formulary, the List of Approved Medicines (LAM), is maintained by the Queensland Health Medicines Advisory Committee (QHMAC). The LAM is limited: listing one or two pharmaceuticals within a therapeutic group is typical.20 As is common with restrictive medicines formularies, access to non-listed medicines for patients with specific clinical need is available through a prior approval process (‘individual patient approval’).

|
Despite the shift towards state hospital formularies, their effects on medicines utilisation have not been described. The purpose of this study was to describe and quantify the effects of the Queensland Health (QH) LAM on medicine utilisation within Queensland.
Methods
Two therapeutic groups of medicines, namely proton pump inhibitors (PPIs) and non-vitamin K oral anticoagulants (NOACs), were selected based on having several competitive agents, with at least some considered to be substitutable based on contemporary clinical guidelines. A third class, statins, was also reviewed, but opportunity for an interrupted time series (ITS) analysis was limited due to a lack of relevant LAM listing alteration within the available data periods. Substitutability was established by a review of Australian and international clinical guidelines. For PPIs the three most commonly used medicines (pantoprazole, omeprazole and esomeprazole) were selected for specific review, whereas for NOACs the two Factor Xa inhibitors (rivaroxaban and apixaban) were selected for review.
Purchasing data (available from August 2003) were extracted from the QH pharmacy information system i.Pharmacy version 8.1.0 (Computer Sciences Corporation). Dates of LAM listing were extracted from QHMAC minutes. PBS utilisation (available from January 1992) was extracted from Medicare Statistics – PBS item reports (https://www.humanservices.gov.au/corporate/statistical-information-and-data/medicare-statistics). PBS item numbers, including historical item numbers, and PBS listing dates were determined by searching PBS schedules (http://www.pbs.gov.au/info/publication/schedule/archive; available online from 2003). Utilisation was converted to defined daily dose (DDD; https://www.whocc.no/atc_ddd_index) per 1000 persons.15 Data were considered from time of market entry (or data availability, where applicable) through to December 2018.
For PPIs, four periods of analysis were identified. For pantoprazole: Period 1, November 1995–January 2001 (off LAM); Period 2, February 2002–April 2005 (on LAM); Period 3, May 2005–May 2013 (off LAM); and Period 4, June 2013–December 2018 (on LAM). For omeprazole: Period 1, January 1992–January 2001 (on LAM); Period 2, February 2001–May 2005 (off LAM); Period 3, May 2005–June 2013 (on LAM); and Period 4, June 2013–December 2018 (off LAM).
For NOACs, specifically rivaroxaban, two periods were identified: pre- (January 2013–June 2013) and post-LAM (June 2013–December 2018) listing.
Data were analysed with Stata SE Version 15.1 (StataCorp). A quasi-experimental design using ITS analyses was used with ordinary least squares segmented regression with one autocorrelation lag and Newey–West standard errors (given evidence of heteroscedasticity in some datasets) using the itsa Stata module.21 The significance of slope and level changes was evaluated using t-tests. PBS utilisation was tested using multiple-group methodology to construct controlled ITS. A quasi-control group was constructed including all states and territories of Australia excluding Queensland. Preintervention trends were inspected visually to ensure comparable trends between the control and intervention groups. Exclusion of one or more individual states was considered to improve the match, but not required.
Autocorrelation testing for PBS claims using Cumby–Huizinga testing was performed. Where seasonality presence was detected, AutoRegressive Integrated Moving Average (ARIMA) modelling with segmented regression of an exogenous indicator variable, LAM listing, was performed.
Given cost savings from closed formulary medicines management systems are typically realised in settings where one or more on-patent medications competes against one or more substitutable generic medications, a relevant time period where this condition was met was selected for PPIs to illustrate potential cost savings. Historical pricing for QH purchasing was extracted from i.Pharmacy. Historical PBS government purchasing costs were estimated from PBS Expenditure and Prescription reports3 (annual government cost divided by script volume for the relevant medication). Additional patient costs were not included.
Given state hospital purchasing data outside of Queensland were not available, a robust comparator group could not be constructed. Hence, to illustrate potential total cost savings at the state hospital level, it was naïvely assumed that relative within-class PPI utilisation would match Australian (excluding Queensland) PBS utilisation and that total PPI volume would remain fixed during the relevant period. Illustrative cost savings were estimated based on these assumptions and historical price estimates.
Similar naïve assumptions were made to determine potential government PBS cost savings within Queensland, this time assuming relative within-class PPI PBS utilisation in Queensland would match Australian (excluding Queensland) PBS utilisation and that total PPI volume would again remain fixed. Given a constructed comparator group existed for this analysis, a second analysis using the estimated slope effects (mean price differences multiplied by slope, time and population) from the controlled ITS analysis was used to estimate illustrative cost savings attributable to the LAM once potential confounding factors had been considered.
Ethics approval for the study was provided by the Metro South Human Research Ethics Committee and permission to use QH data was granted by the Chief Executive Officer, Health Support Queensland.
Results
Proton pump inhibitors
PPIs are approved for use by the Australian Therapeutics Good Administration (TGA) for symptomatic relief of gastro-oesophageal reflux disease, peptic ulcer disease and Zollinger–Ellison syndrome. Australian and international guidelines do not preference PPIs, providing class recommendations.22,23 Dates of first availability are listed in Table 2 and PBS utilisation is shown in Fig. 2.

|

|
LAM and QH purchasing
In effect, several therapeutic switches between omeprazole and pantoprazole have occurred on the LAM. QH purchasing of the three most used PPIs in Australia is shown in Fig. 3.

|
Interrupted time series analysis of QH purchasing confirms a highly significant effect of LAM listing on QH purchasing (Figs 4, 5). Near complete therapeutic switches (evidenced by a change in levels of between 26.5 and 46.6 DDD per 1000 persons) were seen with LAM change.

|

|
LAM and PBS
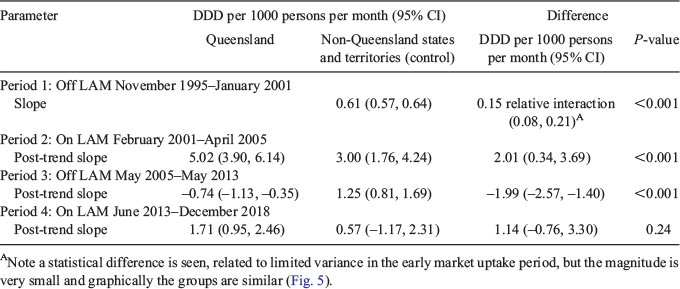
Interrupted time series analysis is shown in Fig. 6 and presented in Table 3. Treatment groups were comparable in the preintervention period (Period 1). Pantoprazole utilisation increased continuously across the four periods of analysis in the non-Queensland states, with a large increase in utilisation (early market uptake) at the commencement of Period 2 (97.3 DDD per 1000 persons; 95% confidence interval (CI) 54.56–140.03 DDD per 1000 persons), also seen in Queensland with no significant interaction with Queensland and the control states (P = 0.577). After this point, utilisation differed in Queensland, with changes in slope occurring with LAM change breakpoints (statistically significant except for Period 4). Post-trend coefficients for Queensland and non-Queensland (control) states are listed in Table 4. LAM listing between February 2001 and April 2005 was associated with an increase in pantoprazole use in Queensland of 2.01 DDD per 1000 persons per month (95% CI 0.34–3.69 DDD per 1000 persons per month), and delisting between May 2005 and February 2013 was associated with a decline of –1.99 DDD per 1000 persons per month (95% CI –2.57, –1.40 DDD per 1000 persons per month).

|
|

|
Assessing the LAM effect on omeprazole utilisation was confounded by a decline in overall utilisation commencing in 2002, associated with esomeprazole market entry (Fig. 7). The use of non-Queensland states as a control allows the effect of the LAM to be estimated (Fig. 8). Treatment groups were comparable in the preintervention period (Period 1). Although omeprazole was LAM listed at this point, similarity with the control group likely reflects the lack of competitive agents available at the time.

|

|
Significant changes to slope were noted at each LAM change breakpoint (P < 0.01 for all). A significant change in level was noted at Period 2 (171 DDD per 1000 persons in the control groups; 95% CI 133–209 DDD per 1000 persons), but the interaction between Queensland and non-Queensland (control) states was not significant (P = 0.84). Post-trend coefficients for Queensland and non-Queensland (control) states are provided in Table 4. Delisting of omeprazole was associated with declines of –2.40 DDD per 1000 persons per month (95% CI –4.21, –0.55 DDD per 1000 persons per month; P = 0.01) DDD between February 2001 and May 2005 and–0.95 DDD per 1000 persons per month (95% CI –1.89, –0.16 DDD per 1000 persons per month; P = 0.046) between June 2013 and December 2018, whereas listing was associated with an increase of 2.06 DDD per 1000 persons per month (95% CI 1.58–2.55 DDD per 1000 persons per month; P < 0.001).
Given the apparent effect of the LAM on omeprazole utilisation appears to be a reduction in the decline associated with therapeutic switching to esomeprazole, the utilisation of esomeprazole was explored. A significant reduction of 1.18 DDD per 1000 persons per month (95%CI –1.80, –0.56 DDD per 1000 persons per month; P < 0.001), or 18.4% less than the control states, in esomeprazole uptake on the PBS was seen in Queensland compared with non-Queensland states (Fig. 9).

|
Seasonality was detected on autocorrelation testing for PBS utilisation for PPIs. Segmented regression using ARIMA modelling is described and discussed in Supplementary File S1.
NOACs
NOACs, oral medications that have largely replaced the use of warfarin, are TGA listed for the prevention of stroke in atrial fibrillation and for the treatment and prevention of deep venous thrombosis and pulmonary embolism. Additional indications, venous thromboembolism prophylaxis after hip or knee replacement, exist but were not included because they are short-term, low-use indications.
Three agents are available in Australia. Dabigatran is a direct thrombin inhibitor and rivaroxaban and apixaban are direct Factor Xa inhibitors. Based on available evidence, the three are generally considered therapeutically comparable (Table 5) in published guidelines. Guidelines typically highlight differences in pharmacokinetics, pharmacodynamics and dosing schedules and often indicate the importance of individual patient factors in determining appropriate therapy. Indirect comparisons and observational post-marketing studies of NOACs exist, and some suggest possible differences in efficacy (stroke and embolism incidence) and safety (bleeding incidence) within their study’s cohort.29 In the absence of controlled head-to-head studies, a clear consensus is yet to be reached in clinical guidelines. A discussion of the existing evidence is beyond the scope of the present study, other than to highlight that NOACs are best considered as imperfect substitutable medicines, with uncertainty regarding the magnitude and relevance of clinical outcome differences.
The dates of PBS and LAM availability for the included indications are listed in Table 6.

|
LAM and QH purchasing of NOACs
QH purchasing is shown in Fig. 10. The interrupted time series demonstrates a significant effect (P < 0.001) of LAM listing, but attributing causality is difficult given coexisting PBS listing. Visual inspection of the graph indicates less relative use of apixaban compared with Australian PBS utilisation (Fig. 11).

|

|
LAM and PBS utilisation of NOACs
The interrupted time series analysis is shown in Fig. 12 and presented in Table 7. Limited preintervention data are available because this was a new therapeutic agent to market. A relative increase in rivaroxaban use in Queensland compared with non-Queensland states is demonstrated and estimated at 1.04 DDD per 1000 persons per month (95% CI 0.50–1.58 DDD per 1000 persons per month). This equates to 38% of the Queensland PBS utilisation growth in the post-LAM listing period. Reciprocal lower utilisation of apixaban is evident in Queensland (Fig. 13).

|

|

|
Implications for generic utilisation and overall cost
A 9-year period (July 2005–June 2014) was chosen where a generically available PPI was the sole PPI listed on the LAM and a competitive on-patent substitute (esomeprazole) was available within the Australian pharmaceutical market.
Historical PPI cost estimates for the three most used PPIs are shown in Fig. 14.

|
Near complete generic use occurred within QH (99.99%). Assuming relative class utilisation would have reflected PBS utilisation, in the absence of a QH formulary, a cost saving of A$10 million over 9 years was estimated for the QH budget (based on historical QH purchase prices). Using the same methodology and applying non-Queensland PBS utilisation patterns to Queensland PBS use, there was an additional A$6.3 million in savings to the PBS over this period related to the LAM. PBS cost implications based on slope changes from the controlled ITS estimated a cost saving of A$3.5 million.
Discussion
This study describes the QH LAM and its effects on medicines utilisation within the Queensland public hospital sector, as well as secondary effects on medicines utilisation on the PBS. The LAM is demonstrated to be a highly effective policy tool for the purchasing and utilisation of medicines within Queensland hospitals. The long-standing nature of the LAM and general support among clinicians and hospital managers are likely key determinants of its effectiveness. The system has been examined for its ability to achieve lower pharmaceutical purchase prices, with evidence that QH achieves lower purchasing prices than other states and larger private pharmacy networks (Queensland Health Medicines Advisory Committee, pers. comm.). This analysis demonstrates that the ability to control utilisation is highly contributory to overall cost savings (and may be an important contributor to its success in competitive tendering). The higher use of generics and lower overall costs are consistent with known effects of formulary systems.30–34
This analysis further highlights the effects of the LAM that extend to wider pharmaceutical utilisation in the PBS. This is not surprising, because hospital–primary care interface effects on medicines utilisation have been demonstrated at the patient,35–37 prescriber38–40 and geographic32,41 level. Secondary effects on primary care prescribing through factors such as implicit specialist endorsement and early familiarisation have been described.32,38,42 The present study evaluated the hospital–primary care interface at a large system level and highlights the importance of this interaction for the Australian healthcare system. These effects were clearly seen in the case of PPIs, with the serial changes in listing of PPIs on the LAM having clear effects on PBS utilisation within Queensland. These differences, of around 2 DDD per 1000 persons per month, sustained over time, result in substantial cumulative effects on PBS utilisation. For example, between February 2001 and April 2005, the estimated increase in pantoprazole use related to the LAM was 2.01 DDD per 1000 persons per month, or a difference of 100 DDD per 1000 persons in the final month of this period (equivalent to approximately one-quarter of all pantoprazole use in Queensland at the period end). Similarly, in June 2013, the retention (given overall use was declining) of omeprazole utilisation related to the LAM equated to 200 DDD per 1000 persons (or equivalent to approximately two-thirds of the total Queensland omeprazole utilisation at the period end).
The use of a quasi-experimental approach, namely controlled ITS methodology (a form of difference-in-difference testing), provides more robust estimates of effects by accounting for potential confounding effects, including time-varying effects (assuming they apply equally to the comparator groups), such as the introduction of a new competitor to market or product availability shortages.15,43,44 The demonstration of an effect across several therapeutic classes and different time periods supports the inference of causality of the LAM on medicine utilisation in the Australian pharmaceutical market. The selection of an appropriate control in these analyses is critical.45,46 The comparability of the non-Queensland states in preintervention periods in these analyses is reassuring. However, non-Queensland states are not a perfect control for evaluating the full effect of a hospital formulary, because hospital formulary mechanisms exist in all states (as described in the Introduction), including the maturing statewide formularies towards the later data periods, which influence PBS utilisation. Hence, the effects likely underestimate the full effects of a statewide hospital formulary such as the LAM per se. This study focuses solely on relative utilisation of substitutable medicines within therapeutic classes. Clearly, the total volume of medicines utilisation (and the appropriateness of the underlying prescribing) is of importance to medicines policy. Both PPIs and NOACs demonstrate increasing total utilisation over time within the Australian medicines landscape, seen in both QH and PBS utilisation data in all jurisdictions.
The availability of PBS data is a rich source for pharmacoepidemiological research,47 but limitations exist in the PBS statistics datasets and these have been well described.48,49 Most relevant to the present study is the absence of private and ‘under copayment’ (both unsubsidised) dispensing within the full statistical datasets during the available data periods. These are more prevalent with generic medicines due to their lower cost (and are therefore not relevant to the analysis of NOACs). Currently, approximately 45% of PPIs are dispensed ‘under copayment’.3 Although the ‘under copayment’ dispensing, by definition, does not result in direct government expenditure, its absence in the dataset results in underestimation of the true extent of generic medicine substitution. Again, this limitation is likely to further underestimate, in this case substantially, the full effects of the LAM on PPI utilisation and related cost-savings.
With the move towards statewide formularies in Australia, the potential for cost reduction exists; for example, the listing of off-patent PPIs on the LAM is associated with cost-savings of A$16.3 million on utilisation effects alone (A$10 million for QH and A$6.3 million for the PBS based on naïve assumptions) over a 9-year period. A second estimation for PBS utilisation, based on slopes from the controlled ITS, derives a cost saving of A$3.5 million. This cost saving, although smaller, better reflects illustrative cost savings causally related (i.e. once other potential confounding factors are considered) to the existence of the LAM. Regardless of the methodology, the cost savings are noteworthy, particularly in that they predominately exist through the reduced use of esomeprazole, an aggressively marketed, ever-greened medicine35,50 with equivalent efficacy to its parent racemate omeprazole.51 Considering these cost savings are associated with a single therapeutic group and state, potential savings extrapolated across all therapeutic groups and extended to other states may be very substantial. It should be noted that these cost savings occurred before significant reforms in PBS price disclosure arrangements for generic medicines. Potential cost savings related to increased generic medicine substitution may be greater after these reforms.
This study also demonstrates the effects of the LAM on the PBS for a fully on-patent therapeutic class, namely NOACs. A clear effect of the LAM on rivaroxaban (and reciprocal effects in apixaban) utilisation in Queensland is demonstrated. This effect is demonstrated against the background of increasing utilisation of apixaban, both within the PBS and, to a lesser degree, within QH purchasing. This growth in apixaban utilisation may relate to a perception of clinical superiority by clinicians, although second (or later) to market patent medicines are commonly associated with increasing market share, independent of whether a clinical superiority exists (esomeprazole being an example of the latter). In the case of imperfect substitutable medicines (where small but important differences in clinical outcomes between competitive products exist), additional unfavourable downstream health costs may exist and need to be considered.
Given on-patent medicines within the PBS are priced based on cost minimisation analysis, net cost savings to the health system would theoretically not be expected in this setting based on changes in relative utilisation of competing products. Competitive pricing offers to state hospital purchasers may allow cost savings at this level, although the prevalence of this is unknown. Separated pharmaceutical budgets between state and federal health systems increase the risk of counterproductive cost shifting.35,40 If hospital systems are incentivised through competitive pricing to list more expensive on-patent medicines where generic medicines exist, a detrimental effect on the PBS budget may be seen. Periods of this occurring in QH exist: pantoprazole was solely listed on the LAM between 2001 and 2005, when generic omeprazole was available.
Applying restrictive utilisation controls to the PBS would be expected to have significant cost-saving effects. The historical, legislative and political reality2 is that it would be extremely unlikely that a restrictive medicines policy like QH or New Zealand’s PHARMAC would be applied to Australia’s PBS. Indeed, multiple contextual and implementation factors are relevant to decisions regarding national pharmaceutical purchasing models, rather than to cost alone. Utilisation control mechanisms do currently exist in the PBS, and strengthening of existing mechanisms or the introduction of others have been recommended.5,7,12 Many current and proposed mechanisms are directed towards general practitioner prescribing, but hospital-directed approaches have received less attention. With the trend towards statewide formulary management, ensuring decision-making committees within the state public hospital system have appropriate composition, skills, experience and resourcing will be increasingly relevant to Australia’s overall medicines policy and PBS costs.
Conclusion
Significant interest exists in the value and affordability of the PBS in Australia and mechanisms to improve cost-effective utilisation are frequently debated as part of Australia’s medicines policy. This study provides insight into the important, but previously not well described, relationship between state hospital medicines policy and the wider PBS effects. By using a quasi-experimental methodology, a causal inference can be drawn and the significance, in terms of both volume and potential cost-implications, is substantial.
Two implications of this study should be emphasised. First, this study demonstrates the significant effects of utilisation control mechanisms on medicines cost control. Although these mechanisms may not be translatable to all settings, including the PBS itself, other demonstrated effective utilisation control mechanisms may have benefit in these settings. Second, this study highlights the significant effect that state medicines policy has on overall medicines utilisation in Australia. With increasing use of statewide medicines formularies, the importance of robust and transparent decision making that considers both a state and national perspective will continue to grow in relevance for Australian medicines policy.
Competing interests
Joel Iedema is deputy chair of the Queensland Health Medicines Advisory Committee.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
Kind thanks to Stephanie Boydell for extracting the dates of LAM listing from QHMAC minutes and to Sara Machado, London School of Economics, for providing supervisory assistance of this work for Dr Iedema’s MSc dissertation.
References
[1] Goddard MS. How the Pharmaceutical Benefits Scheme began. Med J Aust 2014; 201 S23–5.| 25047771PubMed |
[2] Biggs A. The Pharmaceutical Benefits Scheme – an overview. Parliament of Australia; 2002. Available at https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/Publications_Archive/archive/pbs
[3] Pharmaceutical Benefits Scheme (PBS). PBS Statistics. Australian Government Department of Health; 2020. Available at http://www.pbs.gov.au/info/browse/statistics [verified September 2019].
[4] Harvey K. The Pharmaceutical Benefits Scheme under threat. Health Issues 2002; 71 11–15.
[5] Rickard M. Current issues brief no. 12 2001–2. The Pharmaceutical Benefits Scheme: options for cost control. Parliament of Australia; 2002. Available at https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/Publications_Archive/CIB/cib0102/02CIB12#CostControl
[6] Babar ZUD, Vitry A. Differences in Australian and New Zealand medicine funding policies. Aust Prescr 2014; 37 150–1.
| Differences in Australian and New Zealand medicine funding policies.Crossref | GoogleScholarGoogle Scholar |
[7] Karnon J, Edney L, Sorich M. Costs of paying higher prices for equivalent effects on the Pharmaceutical Benefits Scheme. Aust Health Rev 2017; 41 1–6.
| Costs of paying higher prices for equivalent effects on the Pharmaceutical Benefits Scheme.Crossref | GoogleScholarGoogle Scholar | 26954612PubMed |
[8] Duckett S, Banerjee P. Cutting a better drug deal. Grattan Institute; 2017. Available at https://grattan.edu.au/report/cutting-a-better-drug-deal/
[9] Duckett S, Breadon P. Premium policy? Getting better value from the PBS. Grattan Institute; 2015. Available at https://grattan.edu.au/report/premium-policy-getting-better-value-from-the-pbs/
[10] Duckett S, Breadon P, Ginnivan L, Nolan J. Poor pricing progress: price disclosure isn’t the answer to high drug prices. Grattan Institute; 2013. Available at https://grattan.edu.au/report/poor-pricing-progress-price-disclosure-isnt-the-answer-to-high-drug-prices/
[11] Duckett S, Breadon P, Ginnivan L, Venkataraman P. Australia’s bad drug deal: high pharmaceutical prices. Grattan Institute; 2013. Available at https://grattan.edu.au/report/australias-bad-drug-deal/
[12] Thai LP, Moss JR, Godman B, Vitry A. Cost driver analysis of statin expenditure on Australia’s Pharmaceutical Benefits Scheme. Expert Rev Pharmacoecon Outcomes Res 2016; 16 419–33.
| Cost driver analysis of statin expenditure on Australia’s Pharmaceutical Benefits Scheme.Crossref | GoogleScholarGoogle Scholar | 26707482PubMed |
[13] Clarke PM, Fitzgerald EM. Expiry of patient protections on statins: effects of pharmaceutical expenditure in Australia. Med J Aust 2010; 192 633–6.
| Expiry of patient protections on statins: effects of pharmaceutical expenditure in Australia.Crossref | GoogleScholarGoogle Scholar | 20528715PubMed |
[14] Department of Health, Australian Healthcare Associates. PBS pharmaceuticals in hospitals review report. 2017. Available at https://www.pbs.gov.au/reviews/pbs-pharmaceuticals-in-hospitals-review-files/PBS-Pharmaceuticals-in-Hospitals-Review.pdf
[15] Australian Bureau of Statistics. 3101.0 – Australian demographic statistics, Dec 2018. Australian Government; 2018. Available at https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Dec%202018?OpenDocument
[16] SA Health. South Australian medicines formulary. Government of South Australia; 2019. Available at https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/clinical+resources/clinical+topics/medicines+and+drugs/south+australian+medicines+formulary [verified November 2020].
[17] Government of Western Australia, Department of Health. Statewide medicines formulary policy. 2020. Available at https://ww2.health.wa.gov.au/-/media/Files/Corporate/Policy-Frameworks/Clinical-Governance-Safety-and-Quality/Policy/Statewide-Medicines-Formulary-Policy/MP77-Statewide-Medicines-Formulary-Policy.pdf [verified November 2020].
[18] Lake Maintenance Innovation Awards. The Tasmanian electronic medicines formulary – better hospital medicines information at the click of a button. 2013. Available at https://studylib.net/doc/7761750/the-tasmanian-electronic-medicines-formulary [verified November 2020].
[19] Petrie A, Lynne T, Hurree K. 50 years with a statewide formulary – the Queensland experience. Poster presented at the Society of Hospital Pharmacists of Australia Queensland Branch Conference, 20–22 October 2000; Gold Coast, Qld, Australia.
[20] Queensland Government. Formulary notes for the list of approved medicines. Queensland Government; 2017. Available at https://www.health.qld.gov.au/__data/assets/pdf_file/0019/442432/lam.pdf
[21] Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J 2015; 15 480–500.
| Conducting interrupted time-series analysis for single- and multiple-group comparisons.Crossref | GoogleScholarGoogle Scholar |
[22] Gastroenterological Society of Australia (GESA). Clinical update: Gastro-oesophageal reflux disease in adults. 2011. Available at https://www.gesa.org.au/public/13/files/Education%20%26%20Resources/Clinical%20Practice%20Resources/GORD/Reflux_Disease.pdf
[23] Hunt R, Armstrong D, Katelaris P, Afihene M, Bane A, Bhatia S, Chen MH, Choi MG, Melo AC, Fock KM, Ford A, Hongo M, Khan A, Lazebnik L, Lindberg G, Lizarzabal M, Myint T, Moraes-Filho JP, Salis G, Lin JT, Vaidya R, Abdo A, LeMair A. World Gastroenterology Organisation global guidelines: GERD global perspective on gastroesophageal reflux disease. J Clin Gastroenterol 2017; 51 467–78.
| World Gastroenterology Organisation global guidelines: GERD global perspective on gastroesophageal reflux disease.Crossref | GoogleScholarGoogle Scholar | 28591069PubMed |
[24] Tran HA, Gibbs H, Merriman E, Curnow JL, Young L, Bennett A, Tan CW, Chunilal SD, Ward CM, Baker R, Nandurkar H. New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust 2019; 210 227–35.
| New guidelines from the Thrombosis and Haemostasis Society of Australia and New Zealand for the diagnosis and management of venous thromboembolism.Crossref | GoogleScholarGoogle Scholar | 30739331PubMed |
[25] Kearon C, Akl EA, Omelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149 315–52.
| Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report.Crossref | GoogleScholarGoogle Scholar | 26867832PubMed |
[26] Brieger D, Amerena J, Attia JR. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ 2018; 27 1209–66.
| National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018.Crossref | GoogleScholarGoogle Scholar | 30077228PubMed |
[27] January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update on the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation 2019; 140 e125–51.
| 2019 AHA/ACC/HRS focused update on the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation.Crossref | GoogleScholarGoogle Scholar | 30686041PubMed |
[28] Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37 2893–962.
| 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS.Crossref | GoogleScholarGoogle Scholar | 27567408PubMed |
[29] Liu X, Xu ZX, Yu P, Zhu WG. Non-vitamin K antagonist oral anticoagulants in secondary stroke prevention in atrial fibrillation patients: an updated analysis by adding observational studies. Cardiovasc Drugs Ther 2020; 34 569–78.
| 32297024PubMed |
[30] Howard JN, Harris I, Frank G, Kiptanui Z, Qian J, Hansen R. Influencers of generic drug utilization: a systematic review. Res Social Adm Pharm 2018; 14 619–27.
| Influencers of generic drug utilization: a systematic review.Crossref | GoogleScholarGoogle Scholar | 28814375PubMed |
[31] Morgan SG, Leopold C, Wagner AK. Drivers of expenditure on primary care prescription drugs in 10 high-income countries with universal health coverage. CMAJ 2017; 189 E794–9.
| Drivers of expenditure on primary care prescription drugs in 10 high-income countries with universal health coverage.Crossref | GoogleScholarGoogle Scholar | 28606975PubMed |
[32] Vázquez-Mourelle R, Carracedo-Martínez E, Figeiras A. Impact of removal and restriction of me-too-medicines in a hospital drug formulary on in- and outpatient drug prescriptions: interrupted time series design with comparison group. Implement Sci 2019; 14 75
| Impact of removal and restriction of me-too-medicines in a hospital drug formulary on in- and outpatient drug prescriptions: interrupted time series design with comparison group.Crossref | GoogleScholarGoogle Scholar | 31340835PubMed |
[33] Shen X, Stuart BC, Powers CA, Tom SE, Magder LS, Perfeto EM. Impact of formulary restrictions on medication use and costs. Am J Manag Care 2017; 23 e265–74.
| 29087150PubMed |
[34] Dowell JS, Snadden D, Dunbar JA. Changing to generic formulary: how one fundholding practice reduced prescribing costs. BMJ 1995; 310 505–8.
| Changing to generic formulary: how one fundholding practice reduced prescribing costs.Crossref | GoogleScholarGoogle Scholar | 7677830PubMed |
[35] Larsen MD, Schou M, Kristiansen AS, Hallas J. The influence of hospital drug formulary policies on the prescribing policies on the prescribing patterns of proton pump inhibitors in primary care. Eur J Clin Pharmacol 2014; 70 859–65.
| The influence of hospital drug formulary policies on the prescribing policies on the prescribing patterns of proton pump inhibitors in primary care.Crossref | GoogleScholarGoogle Scholar | 24770928PubMed |
[36] Vázquez-Mourelle R, Carracedo-Martínez E. The influence of changes in hospital drug formulary on the prescription of proton pump inhibitors. Farm Hosp 2017; 41 49–67.
| 28045652PubMed |
[37] Himmel W, Tabache M, Kochen MM. What happens to long-term medication when general practice patients are referred to hospital. Eur J Clin Pharmacol 1996; 50 253–7.
| What happens to long-term medication when general practice patients are referred to hospital.Crossref | GoogleScholarGoogle Scholar | 8803514PubMed |
[38] Jones MI, Greenfield SM, Stevenson FA, Naya A, Bradley CP. General practitioners and hospital-initiated prescribing. Eur J Gen Pract 2001; 7 18–22.
| General practitioners and hospital-initiated prescribing.Crossref | GoogleScholarGoogle Scholar |
[39] Davies R. Prescribing at the hospital-general practice interface. BMJ 1992; 304 780
| Prescribing at the hospital-general practice interface.Crossref | GoogleScholarGoogle Scholar | 1637403PubMed |
[40] Jones R, Rawlins MD. Prescribing at the interface between hospitals and general practitioners. BMJ 1992; 304 4–5.
| Prescribing at the interface between hospitals and general practitioners.Crossref | GoogleScholarGoogle Scholar | 1734994PubMed |
[41] Pryce AJ, Heatlie HF, Chapman SR. Buccaling under the pressure: influence of secondary care establishments on the prescribing of glyceryl trinitrate buccal tablets in primary care. BMJ 1996; 313 1621–4.
| Buccaling under the pressure: influence of secondary care establishments on the prescribing of glyceryl trinitrate buccal tablets in primary care.Crossref | GoogleScholarGoogle Scholar | 8991012PubMed |
[42] Florentinus SR, Heerdink ER, van Dijk L, Griens AMGF. Is new drug prescribing in primary care specialist induced? BMC Health Serv Res 2009; 9 6
| Is new drug prescribing in primary care specialist induced?Crossref | GoogleScholarGoogle Scholar | 19134223PubMed |
[43] Bernal JL, Cummins S, Gasparrini A. Difference in difference, controlled interrupted time series and synthetic controls. Int J Epidemiol 2019; 48 2062–63.
[44] Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015; 350 h2750
| Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis.Crossref | GoogleScholarGoogle Scholar | 26058820PubMed |
[45] Benmarhnia T, Rudolph KE. A rose by any other name still needs to be identified (with plausible assumptions). Int J Epidemiol 2019; 48 2061–62.
| A rose by any other name still needs to be identified (with plausible assumptions).Crossref | GoogleScholarGoogle Scholar | 30919891PubMed |
[46] Wing C, Simon K, Bello-Gomez RA. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health 2018; 39 453–69.
| Designing difference in difference studies: best practices for public health policy research.Crossref | GoogleScholarGoogle Scholar | 29328877PubMed |
[47] Pearson SA, Pesa N, Langton JM, Drew A, Faedo M, Robertson J. Studies using Australia’s Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: a systematic review of the published literature (1987–2013). Pharmacoepidemiol Drug Saf 2015; 24 447–55.
| Studies using Australia’s Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: a systematic review of the published literature (1987–2013).Crossref | GoogleScholarGoogle Scholar | 25833702PubMed |
[48] Mellish L, Karanges EA, Litchfield MJ, Schaffer AL, Blanch B, Daniels BJ, Segrave A, Pearson S. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8 634
| The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers.Crossref | GoogleScholarGoogle Scholar | 26526064PubMed |
[49] Page E, Kemp-Casey A, Korda R, Banks E. Using Australian Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: challenges and approaches. Public Health Res Pract 2015; 25 e2541546
| 26536508PubMed |
[50] Sweet M. Website launched to expose ‘tricks’ of drug ads. BMJ 2003; 327 936
| Website launched to expose ‘tricks’ of drug ads.Crossref | GoogleScholarGoogle Scholar |
[51] Asghar W, Pittman E, Jamali F. Comparative efficacy of esomeprazole and omeprazole: racemate to single enantiomer switch. Daru 2015; 23 50
| Comparative efficacy of esomeprazole and omeprazole: racemate to single enantiomer switch.Crossref | GoogleScholarGoogle Scholar | 26573220PubMed |



