Disclosure of adverse events: a data linkage study reporting patient experiences among Australian adults aged ≥45 years
Merrilyn Walton A , Reema Harrison B G , Jennifer Smith-Merry C , Patrick Kelly A , Elizabeth Manias D E , Christine Jorm A and Rick Iedema FA School of Public Health, University of Sydney, NSW 2006, Australia. Email: merrilyn.walton@sydney.edu.au; p.kelly@sydney.edu.au; christine.jorm@sydney.edu.au
B School of Public Health and Community Medicine, UNSW Sydney, NSW 2052, Australia.
C School of Health Sciences, University of Sydney, NSW 2006, Australia. Email: jennifer.smith-merry@sydney.edu.au
D The University of Melbourne, Parkville, Vic. 3052, Australia. Email: emanias@unimelb.edu.au
E Deakin University, Faculty of Health, School of Nursing and Midwifery, Melbourne Burwood Campus, Vic. 3215, Australia.
F School of Health Sciences, Monash University, Vic. 3800, Australia. Email: rick.iedema@kcl.ac.uk
G Corresponding author. Email: reema.harrison@unsw.edu.au
Australian Health Review 43(3) 268-275 https://doi.org/10.1071/AH17179
Submitted: 2 August 2017 Accepted: 23 November 2017 Published: 26 April 2018
Journal Compilation © AHHA 2019 Open Access CC BY-NC-ND
Abstract
Objective Since Australia initiated national open disclosure standards in 2002, open disclosure policies have been adopted in all Australian states and territories. Yet, research evidence regarding their adoption is limited. The aim of the present study was to determine the frequency with which patients who report an adverse event had information disclosed to them about the incident, including whether they participated in a formal open disclosure process, their experiences of the process and the extent to which these align with the current New South Wales (NSW) policy.
Methods A cross-sectional survey about patient experiences of disclosure associated with an adverse event was administered to a random sample of 20 000 participants in the 45 and Up Study who were hospitalised in NSW, Australia, between January and June 2014.
Results Of the 18 993 eligible potential participants, completed surveys were obtained from 7661 (40% response rate), with 474 (7%) patients reporting an adverse event. Of those who reported an adverse event, a significant majority reported an informal or bedside disclosure (91%; 430/474). Only 79 patients (17%) participated in a formal open disclosure meeting. Most informal disclosures were provided by nurses, with only 25% provided by medical practitioners.
Conclusions Experiences of open disclosure may be enhanced by informing patients of their right to full disclosure in advance of or upon admission to hospital, and recognition of and support for informal or bedside disclosure for appropriate types of incidents. A review of the open disclosure guidelines in relation to the types of adverse events that require formal open disclosure and those more suitable to informal bedside disclosure is indicated. Guidelines for bedside disclosure should be drafted to assist medical practitioners and other health professionals facilitate and improve their communications about adverse events. Alignment of formal disclosure with policy requirements may also be enhanced by training multidisciplinary teams in the process.
What is known about the topic? While open disclosure is required in all cases of serious adverse events, patients’ experiences are variable, and lack of, or poor quality disclosures are all too common.
What does this paper add? This paper presents experiences reported by patients across New South Wales in a large cross-sectional survey. Unlike previous studies of open disclosure, recently hospitalised patients were identified and invited using data linkage with medical records. Findings suggest that most patients receive informal disclosures rather than a process that aligns with the current policy guidance.
What are the implications for practitioners? Experiences of open disclosure may be enhanced by informing patients of their right to full disclosure in advance of or upon admission to hospital, and recognition of and support for informal or bedside disclosure for appropriate types of incidents.
Additional keywords: ethics, incident disclosure, medical error, open disclosure, patient satisfaction.
Introduction
Honest communication with patients, an enduring ethical tenet, today includes additional disclosure obligations for iatrogenic injuries. The strong evidence of system-related harm to patients gathered over the past two decades underpins the specific guidance to health professionals to be open and honest about what happened, why and what will be done to address the problem.1 This principle of being honest with patients after a health care incident underpins open disclosure.2 Open disclosure is defined as ‘an open discussion with a patient about an incident(s) that resulted in harm to that patient while receiving health care’.3
In 2008, The National Open Disclosure Standard was published by the Australian Commission on Safety and Quality in Health Care.3 The Standard required healthcare organisations to provide an expression of regret, an explanation of what happened and a description of the action being taken to manage the incident and prevent reoccurrence.3 Formal open disclosure involves an exchange of information that may take place over one or several meetings.3–7 Each jurisdiction has published open disclosure guidelines whose principles align with the national standard. The requirements of the NSW Health Open Disclosure Policy are shown in Box 1.7
| Box 1. NSW Health Open Disclosure Policy7 | ||
| ||
Most open disclosure policies concern adverse events that either have the potential for or have caused actual patient harm.7–9 A 2012 review of the Australian open disclosure standard found many patients were dissatisfied with open disclosure because of the lack of timeliness, openness and transparency.10 Similarly, a national study of the UK open disclosure policy guidance ‘Being Open’ revealed tension between the principles documented and the reality in practice.9
A 2015 literature review showed disclosure to be a significant topic of debate.8 The few primary research studies of patient experiences of open disclosure processes9,11–14 have consistently found that open disclosure did not meet patient expectations. In Australia, the 100 Patient Stories project found that although patients welcomed open disclosure, they were not adequately followed-up with tangible support or information about changes in practice, were not being offered an apology and were not being given the opportunity to meet with staff directly involved in the event.11
Studies of disclosure have previously relied upon hypothetical rather than real-life experiences or included small patient samples.9,15 A recognised research barrier is contacting patients who have suffered adverse events, primarily because of medicolegal and confidentiality restrictions.16,17 Patient samples identified through health services, Internet research companies or by advertising in national print media are limited by the potential to generate a biased sample of patients with particularly positive or negative experiences.11,18
Using data linkage, we identified a large cohort of recently hospitalised patients to survey regarding their experiences in New South Wales (NSW) hospitals in an attempt to reduce the biases noted above.19 Respondents who reported an adverse event were asked questions about how they were informed about it. The aim of the present study was to determine the frequency with which patients (who experienced an adverse event) were engaged in a formal open disclosure process, their experiences and the extent to which these align with the NSW Health Open Disclosure Policy (Box 1).
Methods
The study methods are reported in brief because they have been detailed elsewhere.19
Ethics approval
The conduct of the 45 and Up Study was approved by the NSW Population and Health Services Research Ethics Committee. The patient experiences study received additional ethics approval from the same committee.
Design
The 45 and Up Study is a mixed-methods study involving data collection via cross-sectional survey and data linkage between The Centre for Health Record Linkage (CHeReL), the Admitted Patient Data Collection (APDC), the Register of Births, Deaths and Marriages (RBDM) and the 45 and Up Study databases.
Setting and participants
The study used the Sax Institute’s 45 and Up Study cohort of older adults in Australia, which includes a database of 267 153 citizens aged ≥45 years. Prospective 45 and Up participants were randomly sampled from the Department of Human Services (formerly Medicare Australia) enrolment database, which provides near-complete coverage of the population. People aged ≥80 years and residents of rural and remote areas were oversampled. Those agreeing to participate in the study completed a baseline questionnaire (between January 2006 and December 2009) and consented for follow-up and linkage of their information to routine health databases. Evidence suggests that the 45 and Up population gives results that are consistent with other population-based health-related studies in NSW.20,21 Respondents in the present study were randomly selected by the 45 and Up Study team using the SAS PROC SURVEYSELECT function in SAS (SAS Institute Inc., Sydney, NSW, Australia) from a sample of 20 000 individuals from the 45 and Up cohort who were hospitalised in NSW between January and June 2014. The group, who provided additional consent to take part in this substudy, were identified using data linkage via the CHeReL with the APDC administered by NSW Health. This dataset captures patients in public district and tertiary hospitals, as well as in private hospitals.
Survey tool
A five-part survey was administered to patients. This paper analyses Part 4 of the survey, which captured patient communications with health professionals after an adverse event.22 To assist patients in completing the survey, we provided them with the key terms outlined in Box 2.
| Box 2. Key definitions provided to survey respondents | |
| |
Data analysis
Frequencies and percentages were calculated using Stata Release 13 (StataCorp, College Station, TX, USA). Pearson Chi-squared tests were used to assess the significance of differences between those who did and did not receive formal open disclosure. A significance level of 0.05 was used for analyses. Free-text data were managed using NVivo 10 (QSR International, Melbourne, Vic., Australia), as described previously.23 Two researchers (RH, JSM) separately read the free-text responses and identified key themes. Researcher discussions identified groups of themes that were merged into categories and labelled. A third researcher (MW) rechecked the categories and themes.
Results
Preliminary analysis
There were no significant differences between responders and non-responders in age (the distribution for non-responders was the same as for responders; P = 0.95), gender (49% of non-responders were male; P = 0.49), English as their main language (92% of non-responders; P = 0.63), local government area (the distribution for non-responders was within 1% of responders in each category; P = 0.84) or level of education (the distribution for non-responders was the same as for responders; P = 0.93).
Of the 20 000 potential respondents from the 45 and Up study who were invited to participate, 18 993 were eligible. Completed surveys were received from 7661 (40% response rate). Potential respondents were ineligible if the postal survey was ‘returned to sender’ (n = 640), they were reported as deceased (n = 189) or they responded to say the data linkage was not correct and they had not been in hospital (n = 178).
Of the 7661 respondents, 474 reported experiencing an adverse event (7%). Table 1 provides the demographic breakdown of the sample of respondents who reported an adverse event.

|
Table 2 identifies the position of the health professional who told the patients about the incident regardless of whether this was via a formal or informal disclosure. Of those who reported an adverse event, the majority reported an informal or bedside disclosure (91%; 430/474), with only 79 patients (17%) participating in a formal open disclosure meeting(s). Just under half the informal disclosures were provided by nurses (n = 205; 48%), followed by medical practitioners (n = 109; 25%) and then a multidisciplinary team (17%).

|
Of the 474 respondents who experienced an adverse event, 428 (90%) responded to the items regarding their experiences of disclosure (Table 3). Of this group, 79 respondents (18%) had at least one formal open disclosure meeting and 349 (82%) reported no meetings. For those who experienced an adverse event, being female (22% vs 15%; P = 0.05), severe versus non-severe event (24% vs 16%; P = 0.04) and a weekend admission (25% vs 16%) were all more likely to have a formal open disclosure meeting (see Table 3). No significant differences were identified in age (P = 0.35), admission status (P = 0.30), language other than English (P = 0.30), education level (P = 0.06) or local health district (P = 0.48).

|
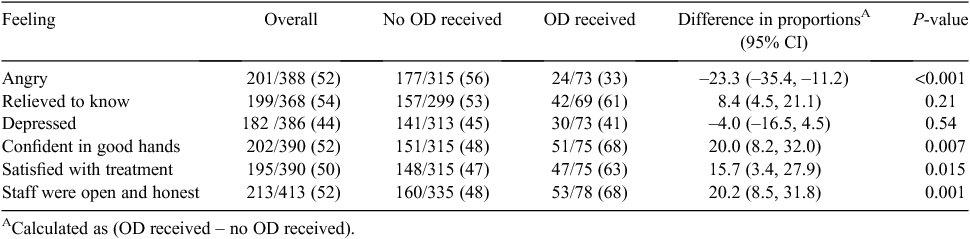
Table 4 summarises the feelings of those who experienced an adverse event and whether or not they had an open disclosure meeting. Patients participating in a formal open disclosure meeting were less likely to be angry (33% vs 56%; P < 0.001), were more likely to be confident they were in good hands (68% vs 48%; P = 0.002), were more satisfied with how they were treated (63% vs 47%; P = 0.015) and were more likely to feel that doctors or nurses were open and honest (68% vs 48%; P = 0.001).
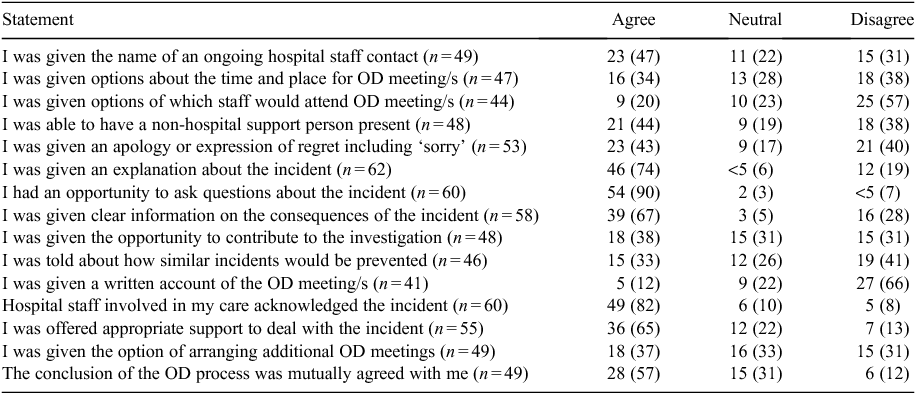
Table 5 summarises comments from respondents who were offered formal open disclosure. The questions reflect the steps that are outlined in the NSW Health Open Disclosure Policy7 and the responses from patients indicate whether they had experienced the activity. Those who said the question was not applicable to their situation were removed from the analysis and the number of valid responses for each item is given in each table.
|
For those having at least one formal open disclosure meeting, the meeting occurred within 48 h of the incident in 60% of cases. Approximately half of those (who had at least one formal open disclosure meeting) had an experience that complied with the policy, specifically: being given the name of a hospital contact to liaise with (23/49; 47%); being offered the opportunity to have a support person present (21/48; 44%); and being given an apology or expression of regret (23/53; 43%). Most were provided with an explanation about the incident (46/62; 74%), asked questions (54/60; 90%) and were given clear information about the consequences of the incident (39/58; 67%). Almost half (n = 19; 40%) had no information as to how similar events would be prevented in the future. Few were given options about the staff who would be attending the open disclosure meeting (9/44; 20%) or were provided with written information about what was discussed (5/41; 12%).
Qualitative findings
Positive aspects of open disclosure meetings identified by respondents fell into three categories: (1) a human approach; (2) openness and honesty; and (3) reciprocal discussion and resolution.
A human approach describes the impact of staff who were caring, friendly, helpful and good listeners on patient experiences. This approach is evidenced in the following quotation:
The hospital representative was honest and caring. She made my husband, daughter and myself welcome and was a good listener.
Openness and honesty with patients about adverse events are central to any disclosure process. Participants expressed a positive experience of disclosure when discussions were genuine and frank, with staff taking the time to address their questions and concerns. This is represented by the following comments:
Questions were answered frankly & openly.
They came to the point and there was no attempt to down play the incident.
Positive experiences were also associated with disclosures that were consultative and with clear explanations about what happened. Respondents were satisfied when they understood what had happened to them but also when they had a mutually agreed resolution to the event.
Their explanations and assistance and treatment were clear and helpful.
The openness of the information given and the treatment recommended.
Negative experiences of open disclosure were also identified, with three categories emerging: (1) lack of an open disclosure process; (2) inadequate implementation of open disclosure; and (3) non-responsive staff.
The lack of an open disclosure process was a key feature of negative patient responses, with many reporting open disclosure was either not offered or involved one meeting that was insufficient.
I was never offered open disclosure.
There were no meetings only my follow up visit with [my doctor] who abused me for writing a letter of complaint.
Some respondents found the open disclosure process inadequate, reporting lack of privacy for discussions, unsuitable staff attending the meetings, lack of opportunity to have a support person, unplanned meetings without time for preparation and lack of written confirmation of the discussions. These factors contributed to a poor patient experience of the process, as exemplified in the following quotations:
…[open disclosure should have been] given privately and not in front of patients in 4 bed ward.
I would prefer someone higher up would have been present and a copy of the report given to me.
Respondents also identified negative experiences involving staff who failed to attend to their concerns or feelings during open disclosure, did not to listen to them, did not use clear language and were patronising and/or uncaring.
The doctor and nurse were verbally angry with each other, ignored me.
We were patronised, lied to, treated with arrogance & disrespect.
Respondents suggested staff listening and attending to patient concerns would improve the experience. Notably, although only two patients discussed the need for an apology or the desire for compensation, the overall focus of comments was on the importance of having the opportunity to have open disclosure meetings and the approach taken by staff to these meetings.
Discussion
The present study provides new knowledge about disclosure after an adverse event among a large cohort of recently hospitalised patients. Most respondents, who were aware of their adverse event, were informally told of the event by doctors and nurses. Most practitioners would be aware of their ethical obligation to disclose an adverse event to their patient but may fear that disclosure according to the guidelines exposes them to more than just the patient’s response to the event.7 This ethical obligation refocuses practitioners to long-standing traditions that underpin trust in the doctor–patient relationship: the duty of candour.1,24 The results of the present study indicate that ‘informal’ bedside disclosure may be an area for further exploration. More than half the patients experiencing an adverse event rated the incident as moderate to severe. NSW Health requires an open disclosure process for serious adverse events, yet nearly half of those who reported adverse events with moderate to serious effects were told about the event informally. Further research is required to better understand why there is a preference for informal disclosure even when an adverse event is moderate or serious and to explore the implications of increasing emphasis on multidisciplinary team in the disclosure of adverse events.25
Open disclosure is a prominent policy leaver and comprehensively promoted in Australia. The evidence demonstrates disclosure is the ethical and appropriate course of action following an adverse health care event.3,4,7,9 The present study showed that only a small proportion of respondents engaged in a formal open disclosure process. Although there is evidence in research and policy literature of the value of formal open disclosure processes,3–5,11 our data suggest implementation across the health system remains a problem, despite extensive training during the introduction of open disclosure in NSW. Challenges include introducing policies in large-scale organisations, matching patient expectations with practice, reconciling legal privilege associated with quality improvement initiatives and open disclosure requirements, understanding of open disclosure and liability compensation and how to measure disclosure.4 Uncertainty about what and how to disclose has been identified in the research literature as a further barrier.26
Informal disclosure occurs when information about an adverse event is shared with a patient (usually at the bedside) and outside the policy framework. There is usually no prior planning, leaving the patient unprepared for a meaningful and detailed discussion about what happened to them. Informal open disclosure, particularly for serious incidents, may fulfil an ethical duty to disclose harm, but is less transparent and may leave patients with incomplete information about their well being and future care. The results of the present study show that health professionals are committed to disclosure and engage in discussions with patients and carers, but shy away from using the open disclosure guidelines, even when there are clear guidelines that they should. One explanation is the different appreciation by staff of what constitutes a ‘moderate’ or ‘serious’ event, and thus whether formal open disclosure was required. In other cases, staff may not have recognised the patient’s experience as an adverse event. The results confirm staff attention to their duty of candour and may satisfy patients suffering less serious adverse events. The challenge is to ensure that when a patient suffers a serious adverse event they are supported by health professionals who are familiar and experienced in providing open disclosure that conforms with the national standard.
When open disclosure was implemented, patients reported the quality of the process as variable. Patients identified the lack of opportunity to decide who should attend open disclosure meetings, a finding reflected in The 100 Patient Stories project.11 Patients understandably want the clinicians directly involved in the event to attend the meeting, along with the patient’s support person, a position supported by the Australian Medical Board’s Code of Conduct requirements.11 The manner in which patients were addressed was another concern, with patients left feeling patronised, rushed and ignored by hospital staff. Respectful treatment of patients, the touchstone of patient-centred care, transcends all areas of health service provision.24,27
Limitations
A potential limitation of the present study is that our sample is from the 45 and Up Study, which may or may not be generalisable to all NSW hospital patients. For example, although only 25% of the 45 and Up Study were born outside of Australia,20 2011 census data puts this figure at 39% for those aged ≥45 years in NSW.28 Given that we studied patient experiences of the NSW Open Disclosure Policy, the extent to which we can generalise our findings outside of the NSW context is limited, although similar policies exist nationally and internationally.9 We did not survey the experiences of some important groups (i.e. patients who died, patients who lacked the capacity to consent and family members or carers of hospitalised patients). Lack of data from family and carers is particularly important in the context of open disclosure. When interpreting these findings, the potential for differences between what patients and health service providers consider an adverse event should be kept in mind. Some of the events identified by patients in the present study may not reflect the formal health service definition of an adverse event or align with the health service categorisation of levels of severity. We did not seek to ‘validate’ patient reports via medical record data due to the inadequate reporting of adverse events in medical records and because the potentially differing perspectives between patients and health providers do not render the patient perspective as invalid. For health care to be truly patient centred, healthcare providers must acknowledge the potential for differences in patient, as well as between patient and health service, definitions of adverse events and not simply dismiss those concerns that do not fall within the scope of the definition of an adverse event they currently use.
Conclusion
The results of the present cross-sectional study show patients are having discussions about their adverse events with health professionals, but mainly informally and therefore outside the recommended formal open disclosure guidelines. Experiences of open disclosure may be enhanced by informing patients of their right to full disclosure in advance of or upon admission to hospital. Recognition of and support for informal or bedside disclosure for appropriate types of incidents may also enhance patient experiences. A review of the open disclosure guidelines in relation to the types of adverse events that require formal open disclosure and those events more suitable to informal bedside disclosure is indicated. Guidelines for bedside disclosure should be drafted to assist medical practitioners and other health professionals to facilitate and improve their communications about adverse events. Alignment of formal disclosure with policy requirements may also be enhanced by training multidisciplinary teams in the process.
Competing interests
None declared.
Acknowledgments
The present study was funded by a National Health and Medical Research Council of Australia (NHMRC) Project Grant (ID: 1049703). This research was completed using data collected through the 45 and Up Study (see www.saxinstitute.org.au, accessed 26 February 2018). The 45 and Up Study is managed by the Sax Institute in collaboration with major partner Cancer Council NSW and other partners: the National Heart Foundation of Australia (NSW Division); NSW Ministry of Health; NSW Government Family & Community Services – Ageing, Carers and the Disability Council NSW; and the Australian Red Cross Blood Service. The authors thank the many thousands of people participating in the 45 and Up Study.
References
[1] Medical Board of Australia. Good medical practice: a code of conduct for doctors in Australia. 2014. Available at: http://www.medicalboard.gov.au/Codes-Guidelines-Policies/Code-of-conduct.aspx [verified 16 February 2017].[2] Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med 1999; 131 963–7.
| Risk management: extreme honesty may be the best policy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FlslClsw%3D%3D&md5=120b04b61579dba10f062d177bdde90dCAS |
[3] Australian Commission on Safety and Quality in Health Care (ACSQHC). Open disclosure standard. Sydney: ACSQHC; 2008. Available at: https://www.safetyandquality.gov.au/wp-content/uploads/2012/01/OD-Standard-2008.pdf [verified 28 March 2017]
[4] Wu AW, McCay L, Levinson W, Iedema R, Wallace G, Boyle DJ, McDonald TB, Bismark MM, Kraman SS, Forbes E, Conway JB, Gallagher TH. Disclosing adverse events to patients: international norms and trends. J Patient Saf 2017; 13 43–9.
| Disclosing adverse events to patients: international norms and trends.Crossref | GoogleScholarGoogle Scholar |
[5] NHS. Serious incident framework: supporting learning to prevent recurrence. 2015. Available at: https://www.england.nhs.uk/patientsafety/wp-content/uploads/sites/32/2015/04/serious-incidnt-framwrk-upd2.pdf [verified 16 February 2017].
[6] Canadian Patient Safety Institute. Canadian disclosure guidelines. Being open with patients and families. 2011. Available at: http://www.patientsafetyinstitute.ca/en/toolsResources/disclosure/Documents/CPSI%20Canadian%20Disclosure%20Guidelines.pdf [verified 16 February 2017].
[7] NSW Health. Open disclosure policy. 2014. Available at: http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_028.pdf [verified 16 February 2017].
[8] Birks Y, Entwistle V, Harrison R, Bosanquet K, Watt I, Iedema R. Being open about unanticipated problems in health care: the challenges of uncertainties. J Health Serv Res Policy 2015; 20 54–60.
| Being open about unanticipated problems in health care: the challenges of uncertainties.Crossref | GoogleScholarGoogle Scholar |
[9] Birks Y, Harrison R, Bosanquet K, Hall J, Harden M, Entwistle V, Watt I, Walsh P, Ronaldson S, Roberts D, Adamson J, Wright J, Iedema R. An exploration of the implementation of open disclosure of adverse events in the UK: a scoping review and qualitative exploration. HS&D R 2014; 2
| An exploration of the implementation of open disclosure of adverse events in the UK: a scoping review and qualitative exploration.Crossref | GoogleScholarGoogle Scholar |
[10] Australian Commission on Safety and Quality in Health Care (ACSQHC). Open disclosure standard review report. Sydney: ACSQHC; 2012. Available at: http://www.safetyandquality.gov.au/publications/open-disclosure-standard-review-report/ [verified 16 February 2017]
[11] Iedema R, Allen S, Britton K, Piper D, Baker A, Grbich C, Allan A, Jones L, Tuckett A, Williams A, Manias E, Gallagher TH. Patients’ and family members’ views on how clinicians enact and how they should enact incident disclosure: the ‘100 patient stories’ qualitative study. BMJ 2011; 343 d4423
| Patients’ and family members’ views on how clinicians enact and how they should enact incident disclosure: the ‘100 patient stories’ qualitative study.Crossref | GoogleScholarGoogle Scholar |
[12] Mazor KM, Roblin DW, Greene SM, Lemay CA, Firneno CL, Calvi J, Prouty CD, Horner K, Gallagher TH. Toward patient-centered cancer care: patient perceptions of problematic events, impact, and response. J Clin Oncol 2012; 30 1784–90.
| Toward patient-centered cancer care: patient perceptions of problematic events, impact, and response.Crossref | GoogleScholarGoogle Scholar |
[13] Hobgood C, Tamayo-Sarver JH, Elms A, Weiner B. Parental preferences for error disclosure, reporting, and legal action after medical error in the care of their children. Pediatrics 2005; 116 1276–86.
| Parental preferences for error disclosure, reporting, and legal action after medical error in the care of their children.Crossref | GoogleScholarGoogle Scholar |
[14] Kooienga S, Stewart VT. Putting a face on medical errors: a patient perspective. J Healthc Qual 2011; 33 37–41.
| Putting a face on medical errors: a patient perspective.Crossref | GoogleScholarGoogle Scholar |
[15] Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Choosing your words carefully: how physicians would disclose harmful medical errors to patients. Arch Intern Med 2006; 166 1585–93.
| Choosing your words carefully: how physicians would disclose harmful medical errors to patients.Crossref | GoogleScholarGoogle Scholar |
[16] Iedema R, Allen S, Sorensen R, Gallagher TH. What prevents incident disclosure, and what can be done to promote it? Jt Comm J Qual Patient Saf 2011; 37 409–17.
| What prevents incident disclosure, and what can be done to promote it?Crossref | GoogleScholarGoogle Scholar |
[17] Studdert DM, Mello MM, Gawande AA, Brennan TA, Wang YC. Disclosure of medical injury to patients: an improbable risk management strategy. Health Aff (Milwood) 2007; 26 215–6.
| Disclosure of medical injury to patients: an improbable risk management strategy.Crossref | GoogleScholarGoogle Scholar |
[18] Harrison R, Walton M, Manias E, Smith-Merry J, Kelly P, Iedema R, Robinson L. The missing evidence: a systematic review of patients’ experiences of adverse events in health care. Int J Qual Health Care 2015; 27 424–42.
| The missing evidence: a systematic review of patients’ experiences of adverse events in health care.Crossref | GoogleScholarGoogle Scholar |
[19] Walton M, Smith-Merry J, Harrison R, et al Using patients’ experiences of adverse events to improve health service delivery and practice: protocol of a data linkage study of Australian adults age 45 and above [published errata appear in BMJ Open 2015; 5: e006599corr1 and BMJ Open 2017; 7: e006599corr2]. BMJ Open 2014; 4 e006599
| Using patients’ experiences of adverse events to improve health service delivery and practice: protocol of a data linkage study of Australian adults age 45 and above [published errata appear in BMJ Open 2015; 5: e006599corr1 and BMJ Open 2017; 7: e006599corr2].Crossref | GoogleScholarGoogle Scholar |
[20] 45 and Up Study Collaborators Banks E, Redman S, Jorm L, Armstrong B, Bauman A, Beard J, Beral V, Byles J, Corbett S, Cumming R, Harris M, Sitas F, Smith W, Taylor L, Wutzke S, Lujic S. Cohort profile: the 45 and Up Study. Int J Epidemiol 2008; 37 941–7.
| Cohort profile: the 45 and Up Study.Crossref | GoogleScholarGoogle Scholar |
[21] Mealing NM, Banks E, Jorm LR, Steel DG, Clements MS, Rogers KD. Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs. BMC Med Res Methodol 2010; 10 26
| Investigation of relative risk estimates from studies of the same population with contrasting response rates and designs.Crossref | GoogleScholarGoogle Scholar |
[22] Iedema R, Sorensen R, Manias E, Tuckett A, Piper D, Mallock N, Williams A, Jorm C. Patients’ and family members’ experiences of open disclosure following adverse events. Int J Qual Health Care 2008; 20 421–32.
| Patients’ and family members’ experiences of open disclosure following adverse events.Crossref | GoogleScholarGoogle Scholar |
[23] Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3 77–101.
| Using thematic analysis in psychology.Crossref | GoogleScholarGoogle Scholar |
[24] General Medical Council. Openness and honesty when things go wrong: the professional duty of candour. 2015. Available at: http://www.gmc-uk.org/guidance/ethical_guidance/27233.asp [verified 26 September 2016].
[25] Harrison R, Birks Y, Bosanquet K, Hall J, Iedema R. The contribution of nurses to incident disclosure: a narrative review. Int J Nurs Stud 2014; 51 334–45.
| The contribution of nurses to incident disclosure: a narrative review.Crossref | GoogleScholarGoogle Scholar |
[26] Harrison R, Birks Y, Iedema R, Bosanquet K. Enacting open disclosure in the UK National Health Service: a qualitative exploration. Journal of Evaluation in Clinical Practice 2017; 23 713–18.
| Enacting open disclosure in the UK National Health Service: a qualitative exploration.Crossref | GoogleScholarGoogle Scholar |
[27] Clinical Excellence Commission. Open disclosure. n.d Available at: http://www.cec.health.nsw.gov.au/incident-management/open-disclosure [verified 16 February 2017].
[28] Australian Bureau of Statistics. State and territory composition of country of birth, 2011. 2013. Available at: http://www.abs.gov.au/ausstats/abs@.nsf/Products/3412.0~2011-12+and+2012-13~Chapter~State+and+Territory+Composition+of+Country+of+Birth?OpenDocument [verified 20 March 2018].