Mapping regulatory models for medicinal cannabis: a matrix of options
Vendula Belackova A , Marian Shanahan A and Alison Ritter A BA Drug Policy Modelling Program, National Drug and Alcohol Research Centre, UNSW, 22–32 King Street, Randwick, NSW 2052, Australia. Email: vendulabelackova@gmail.com; m.shanahan@unsw.edu.au
B Coresponding author. Email: Alison.ritter@unsw.edu.au
Australian Health Review 42(4) 403-411 https://doi.org/10.1071/AH16257
Submitted: 17 November 2016 Accepted: 4 April 2017 Published: 30 May 2017
Journal compilation © AHHA 2018 Open Access CC BY-NC-ND
Abstract
Objective The aim of the present study was to develop a framework for assessing regulatory options for medicinal cannabis in Australia.
Methods International regulatory regimes for medicinal cannabis were reviewed with a qualitative policy analysis approach and key policy features were synthesised, leading to a conceptual framework that facilitates decision making across multiple dimensions.
Results Two central organising dimensions of medicinal cannabis regulation were identified: cannabis supply and patient authorisation (including patient access). A number of the different supply options can be matched with a number of different patient authorisation options, leading to a matrix of possible regulatory regimes.
Conclusions The regulatory options, as used internationally, involve different forms of cannabis (synthetic and plant-based pharmaceutical preparations or herbal cannabis) and the varying extent to which patient authorisation policies and procedures are stringently or more loosely defined. The optimal combination of supply and patient authorisation options in any jurisdiction that chooses to make medicinal cannabis accessible will depend on policy goals.
What is known about the topic? Internationally, regulation of medicinal cannabis has developed idiosyncratically, depending on formulations that were made available and local context. There has been no attempt to date in the scientific literature to systematically document the variety of regulatory possibilities for medicinal cannabis.
What does this paper add? This paper presents a new conceptual schema for considering options for the regulation of medicinal cannabis, across both supply and patient authorisation aspects.
What are the implications for practitioners? The design of regulatory systems in Australia, whether for pharmaceutical or herbal products, is a vital issue for policy makers right now as federal and state and territory governments grapple with the complexities of medicinal cannabis regulation. The conceptual schema presented herein provides a tool for more systematic thinking about the options.
Introduction
Cannabis is globally the most widely used illicit psychoactive substance.1 The cannabis plant, and/or its constituents, also has medicinal properties for treating specific health conditions.2,3 Due to the way cannabis has historically been understood politically, socially and legally, designing schemes that regulate access to medicinal cannabis for patients (but not access for other purposes) represents a complex and controversial policy challenge.
Australia is now embarking on the path of medicinal cannabis. In the past 2 years we have seen multiple state and federal initiatives.4 For example, there have been initiatives in several Australian states, including in New South Wales (NSW), with the establishment of the Medicinal Cannabis Compassionate Use Scheme; in Victoria, the Access to Medicinal Cannabis Act was passed in April 2016 and, in June 2016, a new bill was presented before the Queensland government (the Public Health (Medicinal Cannabis) Bill). At the federal level, in February 2016 an amendment was passed to the Narcotic Drugs Act to enable controlled cultivation of cannabis for medical and scientific purposes. As of 30 October 2016, the Therapeutic Goods Administration (TGA) has rescheduled medicinal cannabis from a Schedule (S) 9 (prohibited) to S8 (therapeutic use) drug: ‘medicinal cannabis’ here standing only for that cannabis defined under the Narcotic Drugs Act. Import of medicinal cannabis from abroad under the TGA Special Access Scheme is possible, given all necessary permissions are obtained in advance.
The regulatory regime for medicinal cannabis and program design features can make a significant difference to the extent to which medicinal cannabis programs are widely adopted, target and address the needs of specific patient groups and/or contribute to unintended effects on recreational use among adults. As reported previously,5–8 the specific features of medicinal cannabis programs can affect population health outcomes. Thus, the ways in which Australia designs and develops medicinal cannabis regimes will have important implications.
Clarity regarding policy goals is also essential. An Australian program may be designed with the primary goal of maximising patient access, including enabling those with a large variety of medical conditions to access medicinal cannabis. Alternatively, the policy goal may reflect a more restricted view about access, which is restricted only to patients with certain prescribed conditions for which there is a substantial evidence base regarding the efficacy and/or effectiveness of medicinal cannabis. Other important considerations include the extent of integration of medicinal cannabis treatment programs with routine healthcare.
In an ideal world, regulation arrangements (e.g. regulation of supply and patient authorisation) would be informed by a regulatory evidence base. As Scott9 noted in relation to regulation more generally, ‘more empirical work…would likely yield better understanding of the conditions under which regulatory regimes deliver the effects which are intended’. For medicinal cannabis, empirical data regarding regulatory regimes are lacking. As a first step, the aim of the present study was to review international regulatory models for medicinal cannabis and thus generate a framework that could be used to analyse regulatory regimes pertaining to medicinal cannabis. By reviewing the international regulatory systems, this study aims to help Australian decision makers distinguish between the different models and options, and provide a conceptual schema to identify the potential of different options to address policy goals.
Methods
In the first phase, available documentation on regulatory regimes from countries with medicinal cannabis programs was reviewed. Six countries were reviewed in detail (Australia, Canada, Czech Republic, Israel, the Netherlands and the US). Additional information was gathered from aggregate sources that pertain to medicinal cannabis accessibility in other countries (e.g. National Organization for the Reform of Marijuana Laws (NORML), International Association for Cannabinoid Medicines (IACM), Americans for Safe Access (ASA)) and confirmed through information from producers, media and other publicly available sources.
The review included documenting the variety of sources for medicinal cannabis (domestic production, importation etc.), the ways in which patients gain access (through medical prescription, via authorisations and recommendations) and the type of cannabis available (pharmaceutical and/or herbal preparations). This phase involved analysing the data to establish the key distinguishing features of the regulatory regimes. The third phase involved developing a conceptual schema that could be used to aid consideration of the options simultaneously. The final phase of the study was to identify the potential policy goals, as well as the advantages and limitations of the regulatory features with regard to the variety of goals.
Results
Internationally, there has been a proliferation of medicinal cannabis programs. These programs differ widely in their regulatory features: whether the medicinal cannabis is a pharmaceutical or herbal preparation, the ways in which patients can access the program, the dispensing or distribution mechanisms, the extent of domestic supply, the availability of home-growing provisions etc. The specific features of these programs are discussed below. In the first instance we examined the regulatory regimes using prescription, special access and clinical trials for pharmaceutical preparations and for medicinal-grade herbal cannabis, followed by an analysis of models using domestically cultivated herbal cannabis for medicinal purposes.
Prescription, special access and clinical trials with pharmaceutical preparations and with medicinal-grade herbal cannabis
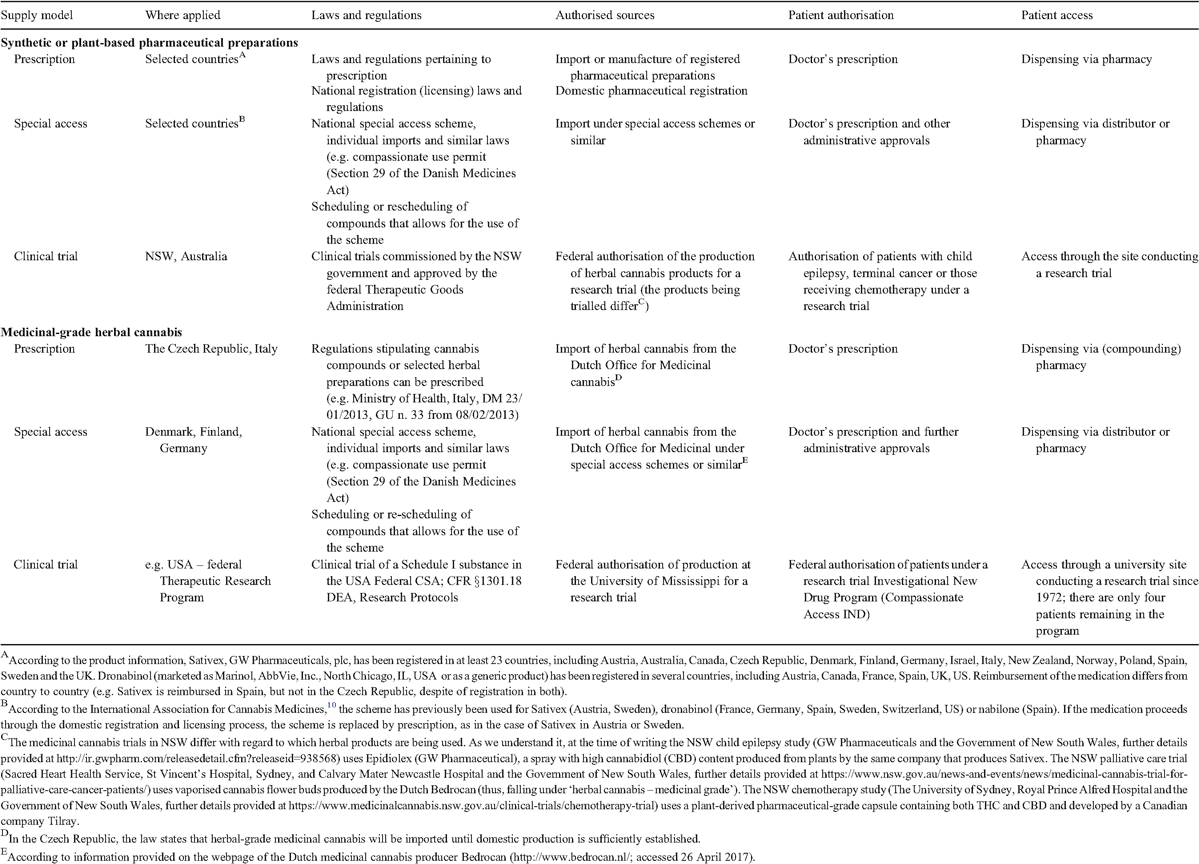
At least 25 countries provided access to synthetic or plant-based pharmaceutical preparations and our analysis revealed that at least 10 countries (including Australia) adopted or used pre-existing legislation through which medicinal cannabis in herbal form or in preparations derived from it can be accessed (Table 1).
|
The most straightforward option used in medicinal cannabis programs has been the supply of pharmaceutical preparations following standard production and registration protocols for therapeutic goods. Pharmaceutical preparations containing synthetically derived tetrahydrocannabinol (THC), such as dronabinol, nabilone) are registered medications in at least six countries (Austria, Canada, France, Spain, UK, US), whereas pharmaceutical preparations that isolate pure THC (Δ9-THC) and cannabidiol (CBD) from the cannabis plant (e.g. Sativex by GW Pharmaceuticals, plc) have been registered (according to GW Pharmaceuticals, plc) in at least 23 countries (including Australia) to date.10 Alternatively, medicinal-grade herbal cannabis is available in several countries. For example, in the Czech Republic and in Italy, herbal cannabis of medicinal grade (imported from the Dutch Office for Medicinal Cannabis) is prescribed and available in pharmacies as a ‘compounding’ medication (see Table 1 for details).11
Synthetic or plant-based pharmaceutical preparations and compounded cannabis medications can be prescribed by a physician and dispensed in pharmacies, which can guarantee high adherence to standard health care as well as tight control over the supply chain and the groups of patients receiving the medication. The registered (licenced) pharmaceutical preparations have the advantage of having being assessed for efficacy and safety by the national regulatory agencies (however, this can be a lengthy and costly process). In instances where no product containing cannabinoids is available domestically and/or the scheduling precludes its prescription, compassionate exemptions to a ‘named patient’ have been applied;12 this stems from the World Health Organization (WHO) recommendations on the import of pharmaceutical preparations that are not (yet) domestically registered.13 These ‘personal import’ or ‘special access’ schemes have been used in several countries with regard to Sativex before its registration on the domestic market (e.g. in the UK, Sweden and New Zealand12) and ‘compounding’ herbal cannabis sourced from the Netherlands (e.g. in Denmark, Finland and Germany).14 Another means of accessing pharmaceutical preparations has been through clinical trials (Table 1).
Models using domestically cultivated herbal cannabis for medicinal purposes
The rationale for pursuing the herbal form of medicinal cannabis is the synergistic (entourage) treatment effects of the combination of relevant (phyto)cannabinoids present in the cannabis plant15 and the rapid onset of effect associated with vaporisation of the herbal product. Clinical evidence on the effectiveness of herbal cannabis preparations is growing, but remains scarce;16 there are few studies on smoked cannabis compared with the number of trials on pharmaceutical preparations. The various regulatory approaches for herbal cannabis are summarised in Table 2.

|
Herbal preparations can be described as ‘medicinal grade’ where cultivation and processing occurs under controlled conditions in order to produce predetermined and stable levels of cannabinoids (THC and CBD). The benefits of a medicinal-grade product is that it facilitates dose control by a physician and that it is safe from contamination by adulterants, moulds, heavy metals, pesticides and other chemical residues.17,18
Supply of ‘medicinal-grade’ cannabis in herbal form has relied on specific measures governing controlled national-level production. ‘Tight’, centralised regimes have been introduced in two countries, namely the Netherlands11 and the Czech Republic,19 where a state-level agency is in possession or control of all cannabis produced in the country and distributes it; patients can access the product upon a doctor’s prescription via pharmacies (Table 2). The establishment of a national-level agency follows The United Nations Single Convention on Narcotic Drugs, 1961 (https://www.unodc.org/pdf/convention_1961_en.pdf; accessed 16 April 2017), which was developed with regard to the cultivation of opium poppies.
In Australia, national legislation has been passed that appoints the Federal Department of Health as the agency that issues licences to cultivate medicinal cannabis (Narcotics Amendment Bill, Act. No. 12/2016). Several other countries have used an ‘agency’ to issue cannabis cultivation licenses at the national level, most notably Israel20 and Canada,21 but their cannabis distribution mechanisms appear to be more decentralised (Table 2). Although both jurisdictions have imposed some quality standards on production, cannabis cultivators are, in general, left to supply patients without the agency acting as an intermediary and outside the medical prescription system (Table 2).
In several states in the US, the system is decentralised, with dispersed distribution arrangements driven by state-level regulations. At least 28 US states have passed laws allowing medicinal cannabis programs, and several others have passed laws allowing for the use of CBD, a compound in the cannabis plant that is responsible for some of its therapeutic effects, but not the psychoactive effect.22–24 The diversity of US approaches to medicinal cannabis has been shaped by the lack of regulation from the Federal government (with cannabis listed as a Schedule I substance with no medicinal value) and, at the same time, non-enforcement of Federal laws. The US programs do not adhere to either federal drug control laws or to the healthcare and medical prescription system (using ‘recommendations’ instead).
Finally, there remain programs that simply focus on individual patients being ex ante exempted from criminal prosecution based on their diagnoses as stated by a medical practitioner or by the medical practitioner’s recommendation that the patient would benefit from cannabis use (Table 2). Here the patients and/or their caregivers have been allowed to cultivate a limited amount of cannabis or to access it from the black market, as is the case with the NSW Medicinal Cannabis Compassionate Use Scheme.25 When cannabis is grown by individuals or designated caregivers (whether for medicinal or recreational purposes), there is no certainty about the quality.
Conceptual schema for medicinal cannabis regulation
The previous section documented the variability of features and provisions in medicinal cannabis schemes worldwide. We now turn to a generic conceptual schema that encompasses this diversity. Two central dimensions to the regulation of medicinal cannabis were identified: cannabis supply and patient authorisation. The inclusion of a third possible dimension, patient access, was considered but because the intent was for the most prudent conceptual schema, patient access was managed within the second dimension of patient authorisation. Table 3 provides the conceptual schema.
Dimension A: cannabis supply
Considerations regarding the regulation of the supply of medicinal cannabis need to accommodate the types of medicinal cannabis, ranging from synthetic or plant-based pharmaceutical preparations (A1, A2) to herbal cannabis (the flowering top of the plant or extracts from it). The supply options vary depending on which formulation is being considered. In addition, supply options differ depending on whether the products are imported (A2, A3) or produced domestically by licenced producers (A4), or whether an official supply source has been established at all (A5).
Dimension B: patient authorisation and access
The different patient authorisation and access schemes that have been applied to medicinal cannabis worldwide include exemption from criminal prosecution based on patient diagnosis, medical ‘recommendation’ or formal diagnosis appropriate to the use of cannabis (B1), name-based exemptions to import as yet-unregistered cannabinoid medicines (B2), clinical trials (B3) and standard prescription regimes (B4).
Matrix of possible regulatory regimes – combinations of A and B
Within each of the dimensions of medicinal cannabis regulation, namely supply and patient authorisation and access, several different options have been applied worldwide. The combination of the two dimensions in Table 3 shows the 12 options that have proven feasible internationally and represent regulatory options that could be further pursued by jurisdictions; these are indicated with an ‘X’ in Table 3.
Several countries have used these options in parallel with one another. For example, both A4B1 and A5B1 are currently used in Canada and in some US states, whereas in other US states only one of the two options applies. In the Czech Republic, both A3B4 and A4B4 are available in practice, although if there was sufficient domestic production of herbal cannabis at lower cost, A3B4 could easily be ceased. Clinical trials (represented in B3) are by definition temporary, and may or may not proceed to permanent conventional access. Similarly, A2B2 and A3B2 special access options may be short term and temporary, or they may remain long term and the only option.
Table 3 also distinguishes between seemingly identical programs based on licensing herbal cannabis cultivators, but they differ in important aspects regarding patient authorisation and access. For example, because of patient authorisation mechanisms, programs in Israel or Canada (which fall under A4B1) differ from those in the Netherlands, the Czech Republic or Australia (A4B4). In addition, in some countries, patients may be obtaining their medication in a pharmacy after undergoing an extensive and costly process of special access (A2B2, A3B2), whereas in other countries patients would obtain their medication through prescription (A2B4, A3B4) at a lower cost and effort.
The recent Australian federal legislation (Narcotics Bill Amendment 2016 and the TGA interim decision on scheduling cannabis, April 2016) has opened up space for the prescription of herbal cannabis preparations (A4B4, A1B4), potentially replacing the rather limited special access scheme (A2B2, A3B2), and sitting alongside existing initiatives, such as the NSW Medicinal Cannabis Compassionate Access Scheme (A5B1), the clinical trials (A1B3) and possibly A2B3, A3B3, A4B3, as well as the availability of Sativex since 2012 (A2B4). Whether and how these schemes will complement each other and how the federal legislation will be translated into state-level laws, as well as integrated with existing initiatives and prescription practices, is yet to be seen.
Strengths and weaknesses of regulatory regimes and the connection to policy goals
The conceptual schema proposed herein can facilitate discussion about the relative advantages and limitations of different options with reference to policy goals. There are several different policy goals, such as accessibility to patients, costs, quality control and integration with usual health care. Each of these can represent different regulatory priorities. In relation to accessibility of a variety of cannabinoid products, clearly A3–A5 allow for a broader range than A1 and A2 at the moment.
The US dispensary model provides ready access for patients, with both the dispensaries and the producer-to-consumer models offering a range of cannabis varieties that may address different patient needs, although the quality of products remains uncertain. The centralised models (such as in the Czech Republic or the Netherlands) offer less flexibility than the distributed models (e.g. selected US states, Israel or Canada), but the policy goal of accessibility needs to be weighed up against managing the risks of diversion of medicinal cannabis to unapproved conditions and for recreational use (e.g. A5B1 or A4B1 allows for less control than any of the B2–B4 options). The US dispensary model has been shown to be rather permissive to access by people who use cannabis recreationally.6
The relative importance of quality control is another consideration. Where regulatory regimes have an absence of quality control of the product (e.g. in countries where only patient cultivation or cannabis from the black market is allowed), therapeutic value may be compromised. In the conceptual schema, options A1–A3 represent stronger potential for quality control mechanisms than A5. In A4, quality control mechanisms will depend on the regulatory requirements and the ability to enforce them; for example, in combination with B4 these will be guided by the WHO’s Good Manufacturing Practice26 at the minimum.
The costs of the various regulatory options have not been documented to date, and further research is required to assess the relative costliness of different regulatory regimes. The considerations here include the cost to patients. Anecdotally, the special access schemes (B2) are seen to be costly to the patient, and time consuming. Pharmaceutical preparations may also be more costly to patients than (medicinal-grade) herbal cannabis, but this would depend on government prescription subsidisation. The high pharmaceutical standards regarding quality can also lead to higher costs (in terms of costs to the pharmaceutical industry, which are then passed on to governments and patients). Another related cost consideration is the nature of the industry. The creation of monopolies (through centralised systems of licenced growers) may lead to ‘monopolistic’ inefficiencies and higher prices due to reduced competition, notwithstanding a public health goal of higher prices to reduce recreational consumption.27,28 For coordinated, centralised regulatory regimes, where quality control is an important policy goal, the administration and compliance monitoring costs to government (e.g. A4 licenced authorised producers) may be high. Conversely, the involvement of domestic producers in a new agricultural economy and taxation revenue may represent substantial economic benefits to a government. For example, the state of Colorado in the US legalised both medicinal and recreational cannabis and taxed them with a 2.9% sales tax and recreational cannabis with an extra 10% retail tax; from this, the state has been retrieving approximately US$11 million per month in revenue, of which US$1 million was from medicinal cannabis alone (as of December 2016).29–31
Another area of regulatory importance is the extent to which regimes are strict regarding the eligible diagnoses versus more liberal with regard to medical conditions (variations within B1). The existing literature on the efficacy and cost-effectiveness of medicinal cannabis to treat medical conditions remains highly contested, with a substantial discrepancy between the conditions purported to respond to medicinal cannabis (see https://www.medicalmarijuana.com; accessed 26 April 2017) versus the scientific literature derived from randomised controlled trials.3 For example, the qualifying conditions vary between US states from an extremely confined list in North Carolina (intractable epilepsy only) to California, where ‘any other chronic or persistent medical symptom that substantially limits the ability of the person to conduct one or more major life activities’ (California legislation, Health and Safety Code, Div 10, Article 2.5 11362.7) provides eligibility for medicinal cannabis. Regimes with broad patient eligibility may potentially have lower adherence to standard health care practice and have less potential for treatment follow-up by a physician. The extent to which integration into usual health care practice is deemed an important feature of a medicinal cannabis program will determine preference for some regulatory options (such as B4) over others (such as A5). Again, to date, evaluative data on these variables is lacking.
Discussion
In our analysis, we aimed to summarise the key features of medicinal cannabis programs across the world. Although several authors have described medicinal cannabis programs at a national level,6,21 a comparative international perspective was lacking in the scientific literature. We found diversity in the types of medicinal cannabis provided, the modes of supply, patient authorisation and patient access and derived a conceptual schema.
Both dimensions of the conceptual schema need to be considered simultaneously. This may not occur because the supply dimensions are controlled by a different agency or jurisdiction from the patient authorisation regimes. Federal and state governments need to work ‘hand in glove’ on medicinal cannabis, designing and evolving the regulatory regimes that represent agreed-upon chosen policy goals. The choice of policy goal will favour some features in Table 3 compared with others.
In an ideal world, some of these choices would be driven by the evidence base from other countries that have had more experience with medicinal cannabis regimes. One example is the extent to which concern about the effects of a medicinal cannabis program on recreational cannabis use (possibly through diversion, as well as through unintended messaging about risks and benefits of cannabis use) is a high priority. Unfortunately, despite growing research, the evidence base remains unclear. For example, in relation to adults, three studies found no changes in adult cannabis use,32 rates of cannabis use disorder33 and cannabis use among arrestees.34 Conversely, two studies found increased rates of both cannabis use and cannabis use disorders35,36 in US states with medicinal cannabis programs.
In addition, other program details play a role in how effective medicinal cannabis regulations are in reaching policy goals. Among them could be the range of diagnoses that make patients eligible for the scheme, the quantities of medicinal cannabis that patients are authorised to possess at a given time, whether the cost of medication is subsidised, the costs and availability of cannabis from other sources and who applies for authorisation (patient vs physician). In addition, it should not be taken for granted that the policy is implemented as per the law, because often practice does not reflect the actual laws.37,38 For example, how quality control is enacted may affect the extent to which the cannabis is medicinal grade and directly impact on prices.
In light of the ambiguity in evidence and the sheer absence of direct comparative research on regulatory regimes, there is a need for ongoing dialogue about cannabis policy goals among all the key players in Australia. In addition, it seems sensible to design scheme(s) that can be readily adjusted and modified over time with growing evidence and experience.
Conclusions
The main aim of the present study was to develop a conceptual schema of the regulatory options for medicinal cannabis in Australia, based on knowledge of the variety of regulatory regimes occurring internationally. There is a wide variety of options to consider across the two dimensions of supply and patient authorisation. The choices largely depend on the form of cannabis (pharmaceutical or herbal) and the related policy goals, such as the extent of patient access restriction, timeliness, integration with usual health care and cost considerations (to regulators and the patient). Given the absence of an evidence base to inform best practice regulation, ongoing dialogue is essential.
Competing interests
None declared.
References
[1] United Nations Office on Drugs and Crime. World drug report. United Nations Publication, No. E.16.XI.7. Vienna: United Nations; 2016.[2] Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract 2015; 10 10
| Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders.Crossref | GoogleScholarGoogle Scholar |
[3] Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015; 313 2456–73.
| Cannabinoids for medical use: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1Ojtr7K&md5=a91f33ee497e03d2d6a6869c1d6016b6CAS |
[4] Freckelton I. Medicinal cannabis law reform in Australia. J Law Med 2016; 23 497–515.
[5] Pacula RL, Boustead AE, Hunt P. Words can be deceiving: a review of variation among legally effective medical marijuana laws in the United States. J Drug Policy Anal 2014; 7 1–19.
| Words can be deceiving: a review of variation among legally effective medical marijuana laws in the United States.Crossref | GoogleScholarGoogle Scholar |
[6] Pacula RL, Powell D, Heaton P, Sevigny EL. Assessing the effects of medical marijuana laws on marijuana use: the devil is in the details. J Policy Anal Manage 2015; 34 7–31.
| Assessing the effects of medical marijuana laws on marijuana use: the devil is in the details.Crossref | GoogleScholarGoogle Scholar |
[7] Pacula RL, Sevigny EL. Marijuana liberalizations policies: why we can’t learn much from policy still in motion. J Policy Anal Manage 2014; 33 212–21.
| Marijuana liberalizations policies: why we can’t learn much from policy still in motion.Crossref | GoogleScholarGoogle Scholar |
[8] Hasin DS, Wall M, Keyes KM, Cerdá M, Schulenberg J, O’Malley PM, Galea S, Pacula R, Feng T. Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys. Lancet Psychiatry 2015; 2 601–8.
| Medical marijuana laws and adolescent marijuana use in the USA from 1991 to 2014: results from annual, repeated cross-sectional surveys.Crossref | GoogleScholarGoogle Scholar |
[9] Scott C. Privatization and regulatory regimes. In: Moran M, Rein M, Goodin RE, editors. The Oxford handbook of public policy. Oxford: Oxford University Press; 2006. pp. 651–68.
[10] International Association for Cannabis as Medicine. Law and politics. 2017. Available at: http://www.cannabis-med.org/index.php?tpl=page&id=41&lng=en [verified 15 March 2017].
[11] Hazekamp A, Heerdink ER. The prevalence and incidence of medicinal cannabis on prescription in The Netherlands. Eur J Clin Pharmacol 2013; 69 1575–80.
| The prevalence and incidence of medicinal cannabis on prescription in The Netherlands.Crossref | GoogleScholarGoogle Scholar |
[12] Mead AP. International control of cannabis. In: Pertwee RG, editor. Handbook of cannabis. Oxford: Oxford University Press; 2014. pp. 44–65.
[13] World Health Organization (WHO). WHO Expert Committee on specifications for pharmaceutical preparations. Annex 12: guidelines on import procedures for pharmaceutical products. Geneva: WHO; 1996.
[14] Belackova V, Zabransky T. Pěstování léčebného konopí – analýza zahraničních zkušeností z regulačního a ekonomického hlediska [Medicinal marijuana growing – analysis of international experience from regulative and economic perspective]. Adiktologie 2011; 11 28–39.
[15] Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid–terpenoid entourage effects. Br J Pharmacol 2011; 163 1344–64.
| Taming THC: potential cannabis synergy and phytocannabinoid–terpenoid entourage effects.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXps1Sjtb4%3D&md5=5178c8d1de8f240a52e1e081b1dc5115CAS |
[16] Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2005–2009. Cannabinoids 2010; 5 1–21.
[17] Hazekamp A. An evaluation of the quality of medicinal grade cannabis in the Netherlands. Cannabinoids 2006; 1 1–9.
[18] Pérez-Parada A, Alonso B, Rodríguez C, Besil N, Cesio V, Diana L, Burgueño A, Bazzurro P, Bojorge A, Gerez N, Heinzen H. Evaluation of three multiresidue methods for the determination of pesticides in marijuana (Cannabis sativa L.) with liquid chromatography–tandem mass spectrometry. Chromatographia 2016; 79 1069–83.
| Evaluation of three multiresidue methods for the determination of pesticides in marijuana (Cannabis sativa L.) with liquid chromatography–tandem mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[19] Mravčík VCP, Grohmannová K, Nečas V, Grolmusová L, Kiššová L, Nechanská B, Sopko B, Fidesová H, Vopravil J, Jurystová L. Annual report on drug situation 2012 – Czech Republic. Prague: Office of Government, Czech Republic; 2013.
[20] Mechoulam R. Cannabis – the Israeli perspective. J Basic Clin Physiol Pharmacol 2016; 27 181–7.
| Cannabis – the Israeli perspective.Crossref | GoogleScholarGoogle Scholar |
[21] Fischer B, Kuganesan S, Room R. Medical marijuana programs: implications for cannabis control policy – observations from Canada. Int J Drug Policy 2015; 26 15–9.
| Medical marijuana programs: implications for cannabis control policy – observations from Canada.Crossref | GoogleScholarGoogle Scholar |
[22] Carillo DE. A growing trend: policy diffusion of medical marijuana laws in the American states. San Diego: San Diego State University; 2013.
[23] McKenna GJ. The current status of medical marijuana in the United States. Hawaii J Med Public Health 2014; 73 105–8.
[24] NORML. Medical marijuana. 2016. Available at: http://norml.org/legal/medical-marijuana-2 [verified 15 March 2017].
[25] NSW Government. Medicinal Cannabis Compassionate Use Scheme: fact sheet for adults with a terminal illness and their carers. 2015. Available at: https://www.medicinalcannabis.nsw.gov.au/regulation/medicinal-cannabis-compassionate-use-scheme [verified 15 March 2017].
[26] World Health Organization (WHO). WHO guidelines on good manufacturing practices (GMP) for herbal medicines. Geneva: WHO; 2007.
[27] Pacula RL, Kilmer B, Wagenaar AC, Chaloupka FJ, Caulkins JP. Developing public health regulations for marijuana: lessons from alcohol and tobacco. Am J Public Health 2014; 104 1021–8.
| Developing public health regulations for marijuana: lessons from alcohol and tobacco.Crossref | GoogleScholarGoogle Scholar |
[28] Caulkins JP, Hawken A, Kilmer B, Kleiman MA. High tax states: options for gleaning revenue from legal cannabis. Oregon Law Rev 2012; 91 1041–68.
[29] Hajizadeh M. Legalizing and regulating marijuana in Canada: review of potential economic, social, and health impacts. Int J Health Policy Manag 2016; 5 453–6.
| Legalizing and regulating marijuana in Canada: review of potential economic, social, and health impacts.Crossref | GoogleScholarGoogle Scholar |
[30] Colorado Department of Revenue. Marijuana tax data. 2016. Available at: https://www.colorado.gov/pacific/revenue/colorado-marijuana-tax-data [verified 15 March 2017].
[31] Kilmer B, MacCoun RJ. How medical marijuana smoothed the transition to marijuana legalization in the United States. Annual Rev Law Social Sci 2017; 13
| How medical marijuana smoothed the transition to marijuana legalization in the United States.Crossref | GoogleScholarGoogle Scholar |
[32] Harper S, Strumpf EC, Kaufman JS. Do medical marijuana laws increase marijuana use? Replication study and extension. Ann Epidemiol 2012; 22 207–12.
| Do medical marijuana laws increase marijuana use? Replication study and extension.Crossref | GoogleScholarGoogle Scholar |
[33] Schuermeyer J, Salomonsen-Sautel S, Price RK, Balan S, Thurstone C, Min S-J, Sakai JT. Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003–11. Drug Alcohol Depend 2014; 140 145–55.
| Temporal trends in marijuana attitudes, availability and use in Colorado compared to non-medical marijuana states: 2003–11.Crossref | GoogleScholarGoogle Scholar |
[34] Gorman DM, Huber JC. Do medical cannabis laws encourage medical cannabis use? Int J Drug Policy 2007; 18 160–7.
| Do medical cannabis laws encourage medical cannabis use?Crossref | GoogleScholarGoogle Scholar |
[35] Cerdá M, Wall M, Keyes KM, Galea S, Hasin D. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend 2012; 120 22–7.
| Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence.Crossref | GoogleScholarGoogle Scholar |
[36] Wen H, Hockenberry JM, Cummings JR. The effect of medical marijuana laws on adolescent and adult use of marijuana, alcohol, and other substances. J Health Econ 2015; 42 64–80.
| The effect of medical marijuana laws on adolescent and adult use of marijuana, alcohol, and other substances.Crossref | GoogleScholarGoogle Scholar |
[37] Ritter A, Livingston M, Chalmers J, Berends L, Reuter P. Comparative policy analysis for alcohol and drugs: current state of the field. Int J Drug Policy 2016; 31 39–50.
| Comparative policy analysis for alcohol and drugs: current state of the field.Crossref | GoogleScholarGoogle Scholar |
[38] Belackova V, Ritter A, Shanahan M, Hughes CE. Assessing the concordance between illicit drug laws on the books and drug law enforcement: Comparison of three states on the continuum from ‘decriminalised’ to ‘punitive’. Int J Drug Policy 2017; 41 148–57.
| Assessing the concordance between illicit drug laws on the books and drug law enforcement: Comparison of three states on the continuum from ‘decriminalised’ to ‘punitive’.Crossref | GoogleScholarGoogle Scholar |



