The incidence of cardiac surgery in adults with treated kidney failure in Australia: a retrospective cohort study
Dominic Keuskamp A B * , Christopher E. Davies A B , Robert A. Baker C D , Kevan R. Polkinghorne E F G , Christopher M. Reid F H , Julian A. Smith I J , Lavinia Tran F , Jenni Williams-Spence F , Rory Wolfe F and Stephen P. Mcdonald A B KA
B
C
D
E
F
G
H
I
J
K
Abstract
Kidney failure increases people’s risk of cardiovascular disease, sometimes requiring cardiac surgery. The aim of this study was to estimate the risk of cardiac surgery for adults with treated kidney failure in comparison with the general population in Australia.
We performed a population-based retrospective cohort study by linking data between the Australia and New Zealand Dialysis and Transplant Registry and the Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database, for 2010–2019. Age–sex-standardised surgery risk relative to the general population was estimated for adults receiving long-term dialysis and kidney transplant recipients, and subpopulations defined by procedure type, comorbidity, clinical status and dialysis-related factors.
Among 1541 adults receiving treatment for kidney failure at the time of cardiac surgery in 2010–2019, the prevalence of comorbidity and risk factors was usually highest in those receiving dialysis, followed by transplant recipients and the general population (n = 113,126). For all major cardiac surgical procedure types, the incidence of surgery for adults receiving dialysis and transplant recipients exceeded that for the general population (e.g. isolated coronary artery bypass grafting relative rates 15.3 [95% CI 13.7–17.0] and 2.0 [1.6–2.6] respectively). Relative incidence was especially high for the dialysis cohorts with insulin-treated diabetes and those with body mass index <25 kg/m2.
Adults with treated kidney failure had a higher risk of cardiac surgery than the general population in Australia in 2010–2019, especially when associated with diabetes. Data linkage between clinical quality registries enabled estimation of the extent of cardiac surgical burden.
Keywords: aortic valve replacement, cardiac surgery, coronary artery bypass graft, dialysis, incidence, kidney failure, kidney replacement therapy, kidney transplant.
Introduction
In Australia, chronic kidney disease is a risk for one in three adults and if untreated may lead to kidney failure (KF).1 People with KF live with an increased burden of cardiovascular disease.2 A high prevalence of traditional (e.g. diabetes and hypertension) and non-traditional factors (e.g. inflammation and oxidative stress) contribute to their excess risk.3–5 Cardiovascular disease remains their leading cause of mortality.6 For coronary artery disease (CAD), coronary artery bypass grafting (CABG) is an important strategy for revascularisation in this cohort.7 Mitral annular and aortic valve calcifications are common and progress to stenosis or regurgitation more rapidly for adults with KF than in the general population.8 Surgical interventions carry a substantial risk, especially for mitral valve (MV) replacement or repair.8
The prevalence of adults with KF treated with either dialysis or kidney transplantation (collectively, kidney replacement therapy [KRT]) in Australia is projected to increase to approximately 37,000 by 2030, most rapidly among people over 60 years of age.9 Greater comorbidity is also anticipated in the KRT population, associated with the rise of diabetes and other diseases in the general population.10,11 However, the frequency and nature of cardiac surgeries for these cohorts and their preoperative risk profiles are not well documented. Postoperative outcomes for adults with KF in Australia suggest they are a high risk for cardiac surgery12–14 and this is reflected in risk prediction models.15,16 Estimating the recent burden and risk of cardiac surgery is therefore critical to inform patients and clinicians, and facilitate accurate risk assessment and peri- and postoperative planning.
Limited data are available on cardiac surgery trends for KRT cohorts17,18 and the specific consideration of kidney transplant recipients is rare. This study aimed to bridge this gap by estimating the incidence of cardiac surgeries among adults receiving KRT in a population-based retrospective cohort based on data linkage between two binational clinical quality registries.
Methods
Study design and data source
The study population comprised those aged ≥18 years undergoing cardiac surgery in Australia between January 2010 and December 2019, as reported to the Australian and New Zealand Society of Cardiac and Thoracic Surgeons (ANZSCTS) Cardiac Surgery Database. The ANZSCTS Cardiac Surgery Database was established in 2001 to collect adult surgery data and by the end of 2019 included 40 hospital contributors across Australia.19 All Australian public hospital units have contributed since 2015. Those adults receiving KRT at the time of surgery were identified from data linkage between the ANZSCTS Cardiac Surgery Database and the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. The ANZDATA Registry was established in 1977 and captures data from all people commencing and receiving dialysis or kidney transplantation in Australia and New Zealand.
Data linkage and outcomes
While the ANZSCTS Cardiac Surgery Database captures whether a patient has preoperative dialysis, the duration of dialysis is not captured. Likewise, whether a patient has undergone a prior renal transplant is captured but not the status of that transplant. Data linkage with the ANZDATA Registry was therefore undertaken to determine adults receiving either long-term dialysis, or those with a functioning kidney transplant at the time of surgery. Probabilistic data linkage was undertaken by SA-NT DataLink based on names, dates of birth and death, sex and postcode. Subsequently, manual review was undertaken of those cases coded for preoperative dialysis and/or kidney transplant in the ANZSCTS Cardiac Surgery Database. Data on surgery date, type, demographics, comorbidity and body mass index (BMI) were extracted from the ANZSCTS Cardiac Surgery Database. Data on dialysis modality at the time of surgery and dates of KRT commencement were extracted from the ANZDATA Registry.
Analysis
Cohorts were defined as either of two KRT modalities – adults receiving long-term dialysis at the time of surgery (hereafter ‘dialysis’) or those with a functioning kidney transplant at the time of surgery (‘transplant’) – and the surgery population not receiving KRT (‘non-KRT’). All rates were calculated for the period 2010–2019, with the denominator being the sum of the annual prevalent populations for the respective cohorts. Data were sourced from the Australian Bureau of Statistics for calculation of the non-KRT denominators.20
Demographics, comorbidities and other characteristics at the time of surgery were described with percentages or medians and chi-squared or Kruskal–Wallis testing, as appropriate to the type and distribution of the data. Unstandardised incidence rates (relative to the non-KRT population) were calculated within the dialysis and transplant populations by sex and age group (18–39, 40–49, 50–59, 60–69 and ≥70 years) for major cardiac surgical procedure types: isolated CABG, isolated aortic valve replacement (AVR), CABG with AVR, isolated MV surgery (replacement or repair) and others. Incidence rate ratios were estimated using a binomial model.
Incidence rates were standardised to the adult Australian estimated resident population in 200121 and stratified by groups defined by procedure type, comorbidity, BMI (<25, 25–30, ≥30 kg/m2) dialysis vintage (commencement <30 days before surgery, ≥30 days–1 year, ≥1–2 years, ≥2–3 years and ≥3 years) and dialysis modality (facility haemodialysis, home haemodialysis and peritoneal dialysis). Relative rates were calculated for the dialysis and transplant population (or subpopulation) to the respective non-KRT population, except for: (a) the dialysis vintage group, where rates were calculated relative to the 30 days to 1 year cohort; and (b) the dialysis modality group, where rates were calculated relative to the facility haemodialysis cohort. Confidence intervals for all standardised rates were based on the gamma distribution.22 Analyses were performed in STATA version 17.1 (StataCorp).
Ethics
Ethics approval was obtained from the University of Adelaide Human Research Ethics Committee (H-2020-060). Data from ANZDATA were used with approval from the Central Adelaide Local Health Network Human Research Ethics Committee (HREC Reference number HREC/17/RAH/408, CALHN Reference number R20170927). Data from the ANZSCTS Cardiac Surgery Database were used with approval of the ANZSCTS Cardiac Surgery Database Research Committee.
Results
A total of 114,667 surgeries could be assigned to either of the three study cohorts for the period 2010–2019, with 1243 (1.08%) for adults receiving dialysis and 298 (0.26%) for those with a functioning kidney transplant. Excluded from analyses were surgeries for which the patient was coded in the ANZDATA Registry as in renal recovery (n = 11), lost to follow-up (n = 1) or deceased (n = 3).
Most surgical procedures were in males (n = 74%), with no difference in sex ratio among the three cohorts (Table 1). Median age at the time of surgery varied significantly, with the dialysis and transplant cohorts younger than the non-KRT cohort. Almost all other risk factors varied significantly (P < 0.01) among the sample; risk for the dialysis cohort tended to be highest, followed by transplant and non-KRT cohorts. Isolated CABG procedures exceeded 60% of the procedures for adults on dialysis, but were only 36.2% for transplant recipients, as compared with 51.7% for the general population.
| Dialysis (n = 1243) | Transplant (n = 298) | Non-KRT (n = 113,126) | P-value | ||
|---|---|---|---|---|---|
| Male sex, n (%) | 927 (74.6) | 225 (75.5) | 83,507 (73.8) | 0.67 | |
| Age (years), median (IQR) | 63 (54–70) | 63 (54–69) | 67 (58–75) | <0.001 | |
| Age group (years), n (%) | <0.001 | ||||
| 18–39 | 40 (3.2) | 10 (3.4) | 4714 (4.2) | ||
| 40–49 | 143 (11.5) | 31 (10.4) | 7268 (6.4) | ||
| 50–59 | 294 (23.7) | 75 (25.2) | 18,940 (16.7) | ||
| 60–69 | 435 (35.0) | 117 (39.3) | 33,792 (29.9) | ||
| 70–79 | 284 (22.8) | 61 (20.5) | 35,798 (31.6) | ||
| ≥80 | 47 (3.8) | 4 (1.3) | 12,614 (11.2) | ||
| Aboriginal/Torres Strait Islander peoples, n (%) | 194 (15.6) | 8 (2.7) | 3292 (2.9) | <0.001 | |
| BMI (kg/m2), median (IQR) | 27.8 (24.2–32.0) | 27.2 (24.2–30.8) | 28.0 (24.9–31.6) | 0.014 | |
| BMI (kg/m2), n (%) | 0.006 | ||||
| <25 | 364 (29.4) | 87 (29.2) | 29,326 (26.0) | ||
| 25–30 | 428 (34.6) | 120 (40.3) | 43,689 (38.7) | ||
| ≥30 | 446 (36.0) | 91 (30.5) | 39,809 (35.3) | ||
| Diabetes status, n (%) | <0.001 | ||||
| Insulin-treated | 466 (37.6) | 85 (28.5) | 9134 (8.1) | ||
| Non-insulin-treated | 295 (23.8) | 50 (16.8) | 23,756 (21.0) | ||
| None | 479 (38.6) | 163 (54.7) | 80,033 (70.8) | ||
| Hypertension, n (%) | 1118 (90.1) | 250 (83.9) | 81,245 (71.9) | <0.001 | |
| Hypercholesterolaemia, n (%) | 932 (75.3) | 210 (70.5) | 73,740 (65.3) | <0.001 | |
| Peripheral vascular disease, n (%) | 283 (22.8) | 47 (15.8) | 9028 (8.0) | <0.001 | |
| Respiratory disease, n (%) | 211 (17.0) | 29 (9.7) | 15,446 (13.7) | 0.001 | |
| Cerebrovascular disease, n (%) | 169 (13.6) | 31 (10.4) | 11,554 (10.2) | 0.001 | |
| Previous myocardial infarction, n (%) | 654 (52.7) | 106 (35.6) | 37,939 (33.6) | <0.001 | |
| Infective endocarditis, n (%) | 74 (6.0) | 14 (4.7) | 3534 (3.1) | <0.001 | |
| Arrhythmia, n (%) | 229 (18.5) | 57 (19.1) | 19,590 (17.3) | 0.78 | |
| Smoking status, n (%) | <0.001 | ||||
| Current | 137 (11.4) | 16 (5.5) | 15,815 (14.2) | ||
| Former | 550 (45.8) | 129 (44.5) | 47,786 (42.9) | ||
| Never | 515 (42.8) | 145 (50.0) | 47,917 (43.0) | ||
| Congestive heart failure, n (%) | 401 (32.3) | 106 (35.6) | 21,444 (19.0) | <0.001 | |
| Previous cardiothoracic intervention, n (%) | 277 (22.3) | 76 (25.5) | 22,001 (19.5) | <0.001 | |
| Clinical status, n (%) | <0.001 | ||||
| Elective | 769 (62.0) | 216 (72.5) | 79,659 (70.5) | ||
| Urgent | 423 (34.1) | 70 (23.5) | 28,256 (25.0) | ||
| Emergency/salvage | 49 (3.9) | 12 (4.0) | 5122 (4.5) | ||
| Procedure type, n (%) | <0.001 | ||||
| Isolated CABG | 752 (60.5) | 108 (36.2) | 58,489 (51.7) | ||
| Isolated AVR | 120 (9.7) | 54 (18.1) | 12,260 (10.8) | ||
| AVR and CABG | 111 (8.9) | 35 (11.7) | 7768 (6.9) | ||
| Isolated MV surgery | 39 (3.1) | 8 (2.7) | 6670 (5.9) | ||
| Others | 221 (17.8) | 93 (31.2) | 27,918 (24.7) | ||
| Dialysis vintage (years), median (IQR) | 2.2 (0.8, 4.6) | ||||
| Dialysis vintage, n (%) | |||||
| <30 days | 89 (7.2) | ||||
| ≥30 days to 1 year | 280 (22.5) | ||||
| ≥1–2 years | 211 (17.0) | ||||
| ≥2–3 years | 176 (14.2) | ||||
| ≥3 years | 487 (39.2) | ||||
| Dialysis modality, n (%) | |||||
| Facility haemodialysis | 901 (72.5) | ||||
| Home haemodialysis | 84 (6.8) | ||||
| Peritoneal dialysis | 258 (20.8) | ||||
| Transplant vintage (years), median (IQR) | 7.8 (3.2–13.2) | ||||
| Transplant vintage, n (%) | |||||
| <30 days | 17 (5.7) | ||||
| ≥30 days to 1 year | 18 (6.0) | ||||
| ≥1–5 years | 62 (20.8) | ||||
| ≥5–10 years | 79 (26.5) | ||||
| ≥10 years | 122 (40.9) | ||||
| Total | 1243 | 298 | 113,126 | ||
AVR, aortic valve replacement; BMI, body mass index; CABG, coronary artery bypass graft; IQR, interquartile range; KRT, kidney replacement therapy; MV, mitral valve.
The unstandardised cardiac surgery rate was highest for adults receiving dialysis, 10.1 per 1000 persons per year (95% CI 9.6–10.7) followed by transplant recipients (2.9 [2.6–3.3]) and those not receiving KRT (0.62 [0.61–0.62]). Incidence rate ratios (IRRs), relative to the non-KRT cohort, exceeded one for all combinations of modality, sex and age except for 18–39-year-old transplant recipients (Fig. 1); IRRs tended to be higher for females than for males, for both dialysis and transplant cohorts. For both sexes, IRRs were higher for adults receiving dialysis than for transplant recipients, and for all ages except for those over 70 years (Supplementary material Table S1).
Incidence rate ratios for dialysis and transplant cohorts relative to the non-KRT population for 2010–2019, by modality at the time of surgery, sex and age group.
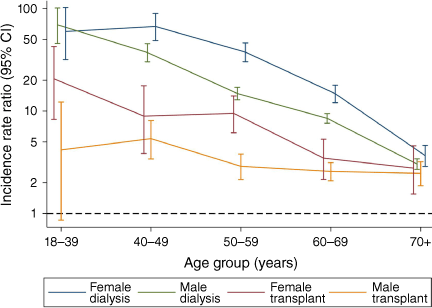
The age–sex-standardised incidence rates for dialysis and transplant cohorts relative to the non-KRT cohort significantly exceeded one for all procedure types except for isolated MV surgeries in transplant recipients (n = 8) (Table 2). The procedure type with the highest relative rate for the dialysis and transplant cohorts was isolated CABG and isolated AVR respectively. For all the cohorts defined by diabetes status, BMI and clinical status, the dialysis rate tended to exceed the respective transplant rate and both were significantly higher (P < 0.05) than the non-KRT rate (Tables 3–5). The highest relative rates for any measured group were for adults with insulin-treated diabetes: for adults receiving dialysis (65.2 per 1000 persons per year [95% CI 56.6–74.7]) and those with a transplant (11.4 [8.3–15.5]). Other elevated relative rates included those for adults receiving dialysis with BMI < 25 kg/m2 and undergoing non-elective surgeries.
| Dialysis | Transplant | Non-KRT | |||
|---|---|---|---|---|---|
| Isolated CABG | N | 752 | 108 | 58489 | |
| Rate (CI) | 4.39 (3.93–4.89) | 0.58 (0.46–0.74) | 0.29 (0.29–0.29) | ||
| Relative rate (CI) | 15.25 (13.65–16.99) | 2.02 (1.58–2.58) | |||
| Isolated AVR | N | 120 | 54 | 12260 | |
| Rate (CI) | 0.64 (0.47–0.85) | 0.37 (0.24–0.54) | 0.061 (0.060–0.062) | ||
| Relative rate (CI) | 10.55 (7.74–14.08) | 6.04 (3.99–8.85) | |||
| AVR and CABG | N | 111 | 35 | 7768 | |
| Rate (CI) | 0.36 (0.29–0.47) | 0.17 (0.12–0.27) | 0.037 (0.036–0.038) | ||
| Relative rate (CI) | 9.73 (7.71–12.6) | 4.62 (3.12–7.15) | |||
| Isolated MV surgery | N | 39 | 8 | 6670 | |
| Rate (CI) | 0.50 (0.30–0.76) | 0.08 (0.02–0.19) | 0.034 (0.033–0.035) | ||
| Relative rate (CI) | 14.56 (8.71–22.38) | 2.37 (0.72–5.72) |
AVR, aortic valve replacement; CABG, coronary artery bypass graft; CI, 95% confidence interval; KRT, kidney replacement therapy; MV, mitral valve.
| Dialysis | Transplant | Non-KRT | |||
|---|---|---|---|---|---|
| Diabetes: insulin-treated | N | 466 | 85 | 9134 | |
| Rate (CI) | 2.95 (2.57–3.38) | 0.52 (0.38–0.70) | 0.045 (0.044–0.046) | ||
| Relative rate (CI) | 65.18 (56.59–74.73) | 11.43 (8.28–15.52) | |||
| Diabetes: non-insulin-treated | N | 295 | 50 | 23,756 | |
| Rate (CI) | 1.64 (1.37–1.95) | 0.35 (0.22–0.52) | 0.12 (0.12–0.12) | ||
| Relative rate (CI) | 14.09 (11.78–16.74) | 2.96 (1.85–4.50) | |||
| No diabetes | N | 479 | 163 | 80,033 | |
| Rate (CI) | 3.21 (2.74–3.74) | 1.05 (0.85–1.29) | 0.40 (0.40–0.40) | ||
| Relative rate (CI) | 8.04 (6.85–9.36) | 2.64 (2.14–3.24) |
CI, 95% confidence interval; KRT, kidney replacement therapy.
| Dialysis | Transplant | Non-KRT | |||
|---|---|---|---|---|---|
| <25 kg/m2 | N | 364 | 87 | 29,326 | |
| Rate (CI) | 2.58 (2.16–3.05) | 0.70 (0.50–0.96) | 0.15 (0.15–0.15) | ||
| Relative rate (CI) | 17.58 (14.71–20.80) | 4.82 (3.44–6.56) | |||
| 25–30 kg/m2 | N | 428 | 120 | 43,689 | |
| Rate (CI) | 2.55 (2.17–2.98) | 0.64 (0.51–0.80) | 0.21 (0.21–0.22) | ||
| Relative rate (CI) | 11.85 (10.08–13.83) | 2.98 (2.37–3.75) | |||
| ≥30 kg/m2 | N | 446 | 91 | 39,809 | |
| Rate (CI) | 2.71 (2.35–3.11) | 0.57 (0.43–0.75) | 0.20 (0.20–0.20) | ||
| Relative rate (CI) | 13.63 (11.80–15.66) | 2.86 (2.16–3.75) |
CI, 95% confidence interval; KRT, kidney replacement therapy.
| Dialysis | Transplant | Non-KRT | |||
|---|---|---|---|---|---|
| Elective | N | 769 | 216 | 79,659 | |
| Rate (CI) | 4.77 (4.26–5.32) | 1.42 (1.17–1.71) | 0.40 (0.39–0.40) | ||
| Relative rate (CI) | 12.06 (10.78–13.45) | 3.59 (2.97–4.32) | |||
| Urgent | N | 49 | 12 | 5122 | |
| Rate (CI) | 0.41 (0.24–0.64) | 0.063 (0.031–0.139) | 0.026 (0.025–0.027) | ||
| Relative rate (CI) | 15.90 (9.39–24.78) | 2.40 (1.19–5.36) | |||
| Emergency/salvage | N | 423 | 70 | 28,256 | |
| Rate (CI) | 2.67 (2.26–3.12) | 0.43 (0.30–0.61) | 0.14 (0.14–0.14) | ||
| Relative rate (CI) | 18.96 (16.05–22.23) | 3.08 (2.13–4.35) |
CI, 95% confidence interval; KRT, kidney replacement therapy.
For isolated CABG, the incidence rate for adults commencing dialysis less than 30 days prior to surgery was higher relative to those 30 days to <1 year receiving dialysis, and relative rates for those receiving dialysis for ≥2 years were lower (Table 6). Adults on home haemodialysis had a lower cardiac surgery incidence than those on facility haemodialysis (Table 7).
| Isolated CABG | Others | |||
|---|---|---|---|---|
| <30 days | N | 71 | 18 | |
| Rate (CI) | 16.80 (11.97–23.24) | 6.92 (2.72–13.84) | ||
| Relative rate (CI) | 2.91 (1.95–4.31) | 2.41 (0.89–5.30) | ||
| ≥30 days to 1 year | N | 186 | 91 | |
| Rate (CI) | 5.78 (4.68–7.07) | 2.87 (2.02–3.94) | ||
| Relative rate (CI) | ||||
| ≥1–2 years | N | 133 | 70 | |
| Rate (CI) | 4.73 (3.55–6.17) | 2.03 (1.42–2.83) | ||
| Relative rate (CI) | 0.82 (0.57–1.15) | 0.71 (0.44–1.16) | ||
| ≥2–3 years | N | 100 | 70 | |
| Rate (CI) | 3.55 (2.66–4.69) | 2.81 (1.83–4.13) | ||
| Relative rate (CI) | 0.61 (0.43–0.88) | 0.98 (0.57–1.66) | ||
| ≥3 years | N | 251 | 210 | |
| Rate (CI) | 3.01 (2.53–3.57) | 3.85 (2.98–4.86) | ||
| Relative rate (CI) | 0.52 (0.40–0.69) | 1.34 (0.89–2.05) |
CABG, coronary artery bypass graft; CI, 95% confidence interval.
| Facility haemodialysis | Home haemodialysis | Peritoneal dialysis | ||
|---|---|---|---|---|
| N | 901 | 84 | 258 | |
| Rate (CI) | 8.51 (7.59–9.50) | 5.44 (3.96–7.39) | 7.56 (6.25–9.07) | |
| Relative rate (CI) | 0.64 (0.46–0.89) | 0.89 (0.71–1.10) |
CI, 95% confidence interval.
Discussion
This is the first population-based Australian study to estimate the incidence of cardiac surgeries for adults receiving KRT relative to the general population. Although people receiving KRT are approximately 0.1% of the population, they comprised over 1.3% of cardiac surgeries in 2010–2019. The relative incidence of each major cardiac surgical procedure type was elevated for the KRT cohorts: at least 9- and 2-fold higher for adults receiving dialysis and transplant recipients, respectively. Given the observed clustering of comorbidities and risk, especially for adults receiving dialysis, that finding was expected. Unmeasured, non-traditional risk factors are also likely to have accelerated arterial and valve disease.4 Those undergoing cardiac surgery were predominantly males aged 50–69 years. Decreased relative incidence of surgery for those over 70 years of age receiving dialysis likely reflects a reduction in referral for cardiac work-up pre-kidney transplantation in those older adults.
Relative surgery risk for adults with KF who were comorbid with insulin-treated diabetes was substantial: over 65-fold for those receiving dialysis. Postoperative outcomes are also known to be worse for this cohort.7,23 The projected increase in the diabetic kidney disease population (even accounting for widespread treatment limiting its progression) raises concern at the consequent increase in surgical burden for the individual and the health system.24 In contrast to the relationship with diabetes, adults with a BMI <25 kg/m2 receiving KRT had a higher relative rate than those with a higher BMI. Those findings, while contradictory at face value, may illustrate the competing factors of surgical disease burden and comorbidity versus risk stratification and patient selection in determining who ultimately undergoes a procedure. Notably, high BMI is associated with lower postoperative risk.25
Despite significant elevation over the general population risk, the relative incidence of cardiac surgery was lower for transplant recipients compared with adults receiving dialysis. They had less comorbidity and a lowered risk profile, and incidence rates were not as strongly associated with diabetes status. Those findings were anticipated given the routine cardiovascular evaluation and treatment that occurs prior to kidney transplantation. Adults receiving home haemodialysis had a lower incidence of cardiac surgery than those receiving facility haemodialysis. While peritoneal dialysis and haemodialysis both accelerate vascular disease, findings remain mixed on any differences in their cardiovascular outcomes.26
Over a similar period to this study, the CABG case volume for adults receiving dialysis (for those with stable CAD) decreased in the USA, reflecting a broader downward trend reported from the USA and internationally.17,27,28 A combination of improved results of non-invasive treatment, a shift in patient selection, and the advent of percutaneous coronary intervention (PCI) have contributed, despite a trend toward an older and more morbid underlying population. Trends owing to advances in CAD revascularisation strategies with PCI, and the use of transcatheter aortic valve replacement for older and/or more comorbid adults are likely to continue to influence cardiac surgery rates in Australia, for the general and KRT populations.
Strengths of this study include the 10-year study period and the generalisability of data extracted from binational clinical quality registries. One limitation is that the population coverage of the ANZSCTS Cardiac Surgery Database changed measurably over the study period owing to an increase in the number of contributing units. To address this, the subset of surgeries from 2015 to 2019 were analysed to elucidate the difference in incidence rates between that period and 2010–2019. No sizeable differences were identified (Table S2). Finally, the actual burden of surgical disease is likely to be greater than we have estimated, owing to selection for surgery and biases in care provider and consumer decision-making in the perioperative period.
Conclusion
In conclusion, data linkage between two binational clinical quality registries allowed an estimation of cardiac surgery incidence rates for adults with treated KF at the time of surgery in 2010–2019 that were significantly higher than in the general population. Adults receiving dialysis, especially those comorbid with diabetes, were at the highest risk. These data will aid in determining how best to prevent the need for surgery and optimise outcomes for the growing population of adults receiving KRT at the time of their surgery.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Declaration of funding
This work was supported by the National Health and Medical Research Council Investigator Grant 1173941.
Acknowledgements
Some of the data reported were supplied by ANZDATA; the interpretation and reporting of these data are the responsibility of the authors and should not be regarded as official policy or an interpretation by ANZDATA. We are grateful to the Australian and New Zealand kidney units, consumers and staff for their cooperation and contributions to the ANZDATA Registry. The ANZSCTS Cardiac Surgery Database Program is funded by the Department of Health (VIC), the Clinical Excellence Commission (NSW), Queensland Health (QLD) and funding from individual units. ANZSCTS Cardiac Surgery Database Research activities are supported by the Monash Centre for Cardiovascular Research and Education. The Database thanks all of the investigators, data managers and institutions that participate in the Program.
References
1 Kidney Health Australia. National strategic action plan for kidney disease. Canberra: Commonwealth of Australia; 2019. Available at https://www.health.gov.au/resources/publications/national-strategic-action-plan-for-kidney-disease [accessed 8 August 2022]
2 Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension 2003; 42(5): 1050-65.
| Crossref | Google Scholar | PubMed |
3 Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 2018; 33(suppl_3): iii28-iii34.
| Crossref | Google Scholar | PubMed |
4 Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74(14): 1823-38.
| Crossref | Google Scholar | PubMed |
5 Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, et al. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013; 382(9889): 339-52.
| Crossref | Google Scholar | PubMed |
6 Sypek MP, Dansie KB, Clayton P, Webster AC, McDonald S. Comparison of cause of death between Australian and New Zealand Dialysis and Transplant Registry and the Australian National Death Index. Nephrology 2019; 24(3): 322-9.
| Crossref | Google Scholar | PubMed |
7 Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation 2002; 106(17): 2207-11.
| Crossref | Google Scholar | PubMed |
8 Marwick TH, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and valvular heart disease: Conclusions from a kidney disease: Improving global outcomes (KDIGO) controversies conference. Kidney Int 2019; 96(4): 836-49.
| Crossref | Google Scholar | PubMed |
9 Keuskamp D, Davies CE, Irish GL, Jesudason S, McDonald SP. Projecting the future: Modelling Australian dialysis prevalence 2021–30. Aust Health Rev 2023; 47(3): 362-8.
| Crossref | Google Scholar | PubMed |
10 Lim WH, Johnson DW, McDonald SP, Hawley C, Clayton PA, Jose MD, et al. Impending challenges of the burden of end-stage kidney disease in Australia. Med J Aust 2019; 211(8): 374-80.e3.
| Crossref | Google Scholar | PubMed |
11 White S, Chadban S. Diabetic kidney disease in Australia: Current burden and future projections. Nephrology 2014; 19(8): 450-8.
| Crossref | Google Scholar | PubMed |
12 Keuskamp D, Davies CE, Baker RA, Polkinghorne KR, Reid CM, Smith JA, et al. National outcomes of cardiac surgery in patients receiving kidney replacement therapy. Ann Thorac Surg 2024;
| Crossref | Google Scholar | PubMed |
13 Jayasekera H, Pinto N, Mundy J, Wood A, Beller E, Griffin R, et al. Cardiac surgery in the presence of dialysis: Effect on mid-term outcomes and quality of life. Heart Lung Circ 2011; 20(2): 105-10.
| Crossref | Google Scholar | PubMed |
14 Zimmet AD, Almeida A, Goldstein J, Shardey GC, Pick AW, Lowe CE, et al. The outcome of cardiac surgery in dialysis-dependent patients. Heart Lung Circ 2005; 14(3): 187-90.
| Crossref | Google Scholar | PubMed |
15 Ariyaratne TV, Billah B, Yap CH, Dinh D, Smith JA, Shardey GC, et al. An Australian risk prediction model for determining early mortality following aortic valve replacement. Eur J Cardiothorac Surg 2011; 39(6): 815-21.
| Crossref | Google Scholar | PubMed |
16 Billah B, Huq MM, Smith JA, Sufi F, Tran L, Shardey GC, et al. AusSCORE II in predicting 30-day mortality after isolated coronary artery bypass grafting in Australia and New Zealand. J Thorac Cardiovasc Surg 2014; 148(5): 1850-5.e2.
| Crossref | Google Scholar | PubMed |
17 Vasudeva R, Mehta H, Chan W-C, Majmundar M, Yarlagadda SG, Downey P, et al. Nationwide trends and outcomes for coronary artery bypass grafting in end-stage kidney disease and stable coronary artery disease. Am J Cardiol 2024; 210: 37-43.
| Crossref | Google Scholar | PubMed |
18 Palamuthusingam D, Hawley CM, Pascoe EM, Johnson DW, Sivalingam P, McDonald S, et al. Trends in rates of surgery and postoperative mortality among patients receiving chronic kidney replacement therapy: A population-based cohort study. Ann Surg 2022; 276(6): 1002-10.
| Crossref | Google Scholar | PubMed |
19 Tran L, Williams-Spence J, Shardey GC, Smith JA, Reid CM. The Australian and New Zealand society of cardiac and thoracic surgeons database program - two decades of quality assurance data. Heart Lung Circ 2019; 28(10): 1459-62.
| Crossref | Google Scholar | PubMed |
20 Australian Bureau of Statistics. National, state and territory population. Canberra: ABS; 2023. Available at https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/jun-2023 [accessed 15 December 2023].
21 Australian Bureau of Statistics. Which population to use for age standardisation? Canberra: ABS; 2013. Available at https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/3101.0Feature+Article1Mar%202013 [accessed 7 March 2024].
22 Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006; 15(6): 547-69.
| Crossref | Google Scholar | PubMed |
23 Hosoda Y, Yamamoto T, Takazawa K, Yamasaki M, Yamamoto S, Hayashi I, et al. Coronary artery bypass grafting in patients on chronic hemodialysis: Surgical outcome in diabetic nephropathy versus nondiabetic nephropathy patients. Ann Thorac Surg 2001; 71(2): 543-8.
| Crossref | Google Scholar | PubMed |
24 Morton JI, McDonald SP, Salim A, Liew D, Shaw JE, Magliano DJ. Projecting the incidence of type 2 diabetes-related end-stage kidney disease until 2040: A comparison between the effects of diabetes prevention and the effects of diabetes treatment. Diabetes Care 2021; 44(7): 1515-23.
| Crossref | Google Scholar | PubMed |
25 Mariscalco G, Wozniak MJ, Dawson AG, Serraino GF, Porter R, Nath M, et al. Body mass index and mortality among adults undergoing cardiac surgery: A nationwide study with a systematic review and meta-analysis. Circulation 2017; 135(9): 850-63.
| Crossref | Google Scholar | PubMed |
26 Ng CH, Ong ZH, Sran HK, Wee TB. Comparison of cardiovascular mortality in hemodialysis versus peritoneal dialysis. Int Urol Nephrol 2021; 53(7): 1363-71.
| Crossref | Google Scholar | PubMed |
27 Blumenfeld O, Na’amnih W, Shapira-Daniels A, Lotan C, Shohat T, Shapira OM. Trends in coronary revascularization and ischemic heart disease-related mortality in Israel. J Am Heart Assoc 2017; 6(2): e004734.
| Crossref | Google Scholar | PubMed |
28 Ohri SK, Benedetto U, Luthra S, Grant SW, Goodwin AT, Trivedi U, et al. Coronary artery bypass surgery in the UK, trends in activity and outcomes from a 15-year complete national series. Eur J Cardiothorac Surg 2022; 61(2): 449-56.
| Crossref | Google Scholar | PubMed |


