Reporting Indigenous status, ethnicity, language and country of birth to build equity in international paediatric clinical trials with Australian sites: a scoping review
Jacqueline Cunninghame A B * , Mari Takashima A C , Lorelle Holland A D E , Linda Nguyen A , Abbey Diaz F , Shuaijun Guo G H , Mitchell Dufficy
A B * , Mari Takashima A C , Lorelle Holland A D E , Linda Nguyen A , Abbey Diaz F , Shuaijun Guo G H , Mitchell Dufficy  A , Craig F. Munns D I and Amanda Ullman A C
A , Craig F. Munns D I and Amanda Ullman A C
A
B
C
D
E
F
G
H
I
Abstract
Ensuring equitable access to clinical trials for children from Indigenous and ethnically and linguistically diverse backgrounds should be central to clinical trial design. This review aims to expansively review the reporting of Indigenous status (Aboriginal and/or Torres Strait Islander origin), ethnicity, culture, location, language and country of birth in paediatric clinical trials with Australian sites.
This scoping review systematically searched PubMed, CINAHL and Embase for international clinical trials with Australian sites conducted between 2018 and 2022 involving children (aged <18 years) to determine the reporting of Indigenous status, race, ethnicity, language and country of birth.
Of the 262 studies included, 154 (58.8%) clinical trials did not report any of the variables of interest. When reported, terms used by authors were heterogeneous. ‘Indigenous status’ was most reported (n = 40, 15.3%) and self-identification was the most common method to determine this (n = 14, 35.9%). International clinical trials had higher rates of reporting for ethnicity, cultural background and race. Overall, more than 60 terms were used to categorise study participants in relation to ‘Indigenous status’, 'ethnicity and cultural background', ‘race’, ‘race and ethnicity’ or ‘natural skin colour’.
This review demonstrated low rates of reporting of demographic variables in paediatric clinical trials. Clear reporting standards, partnering with consumers to co-design trials and self-identification during collection are required. Ensuring adequate access to clinical trials for Indigenous children and children from ethnically, linguistically and geographically diverse backgrounds is essential in building health equity and ensuring patient safety.
Keywords: clinical trial, equity, Indigenous health, minority health, paediatric, reporting, scoping review.
Introduction
Considered by many the gold standard in clinical research, clinical trials utilise rigorous methodologies to explore the therapeutic effectiveness and safety of interventions, which subsequently inform evidence-based practice.1–4 However, it must be recognised that the safety and efficacy of therapeutic interventions evaluated through clinical trials are subject to limitations imposed by the methodological approaches.5,6
Indigenous status, race and ethnicity are formed by social, cultural, spiritual, geographical and generational histories that are well documented to influence access to health care.7 Constructs that categorise human differences may generate marginalisation, drive discrimination and perpetuate racism; however, in research, understanding these constructs is ethically responsible and valuable.8 Failure to capture and report these attributes inadvertently disregards the potential variances observed between racial and ethnic groups regarding incidence, pathophysiology, social determinants and subsequent interventional effectiveness and safety.9–12 For example, the effectiveness of pulse oximetry in racially diverse populations has recently been questioned, with studies demonstrating poor inclusion of participants from diverse backgrounds in initial validation studies.11,13
It is essential for equity and safety that clinical trials include diverse participants.6,10,12,14–18 Globally, there have been increasing efforts to improve accessibility to clinical trials for children from diverse backgrounds and ensure clinical trial demographic information is transparent.19,20 However, the context of demographic reporting and diversity within paediatric clinical trials with Australian sites, a multi-cultural nation with highly increasing numbers of migrants, remains unclear. Therefore, this scoping review aims to review the reporting of Indigenous status, ethnicity, language and country of birth in paediatric clinical trials with Australian sites.
Methods
This scoping review is congruent with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis protocol for Scoping Reviews (PRISMA-ScR).21
Search strategy and eligibility criteria
The search strategy was iteratively designed in consultation with an expert librarian and included three databases: PubMed, Embase and CINAHL (Supplementary Table S1). Searches were refined to include publications from 1 January 2012 to 28 November 2022, with full-text availability in English. Records retrieved were imported into EndNote V.20.2.0.17373 where duplicates were removed. Covidence Systematic Review Software22 was utilised for screening. Screening was conducted by two independent authors (JC and MD), with arbitration by a third author (MT). References of eligible studies were also screened.
Inclusion criteria included population-based clinical trials on health-related interventions in paediatric cohorts (participants aged <18 years) or mixed adult and paediatric cohorts with Australian sites, with results published in the preceding 5 years. Clinical trials were defined as randomised controlled trials (RCTs), non-randomised controlled trials and quasi-experimental studies, where interventions were assigned by investigators or randomisation software.23
Exclusion criteria included: non-clinical trial study designs; adult only participants; clinical trials still recruiting; clinical trials with no published results; clinical trials without Australian sites; non-English publications; abstracts, without available full texts.
Data extraction and presentation
Data extraction was completed in Air Table24 by three independent authors and analysis was completed in SPSS V29.0.0.0. All categorisation by Indigenous status, cultural background, country of birth and language are in accordance with the authors reporting. Results were presented in tabular format as counts and percentages. A dendrogram was generated using RAWGraphs 2.0.
Results
The search yielded 2717 studies, upon removal of 592 duplicates in Endnote V.20.2.0.17373 and 45 duplicates in Covidence, 2080 studies progressed to screening, as illustrated in Supplementary material Fig. S1. A total of 830 studies progressed to full text screening, and overall, 262 studies were eligible for inclusion and proceeded to data extraction in Air Table.
As demonstrated in Table 1, studies included in this review primarily consisted of RCTs (n = 158; 60.3%). Most studies only had Australian sites (n = 209; 80.2%), but of those that had international sites (n = 53; 19.8%), sites were predominately within Australia and New Zealand (n = 53; 15.9%), Europe (n = 28; 8.4%) and North America (n = 27; 8.1%). There was significant heterogeneity in the diseases and interventions examined in the included studies, however, most studies considered diseases from medicine and clinical science research disciplines (n = 211; 80.5%), encompassing medical or health interventions in clinical settings focused on therapeutic outcomes.
| Characteristics | Total | % | ||
|---|---|---|---|---|
| Year of publication | 2018 | 56 | 21.4 | |
| 2019 | 46 | 17.6 | ||
| 2020 | 54 | 20.6 | ||
| 2021 | 60 | 22.9 | ||
| 2022 | 46 | 17.6 | ||
| Clinical trial design | RCT | 158 | 60.3 | |
| Cluster RCT | 63 | 24 | ||
| Non-RCT | 18 | 6.9 | ||
| Pilot RCT | 17 | 6.5 | ||
| Quasi-experimental | 6 | 2.3 | ||
| Clinical trial phase | I | 4 | 1.5 | |
| II | 3 | 1.1 | ||
| III | 19 | 7.3 | ||
| IV | 1 | 0.4 | ||
| Not applicable | 216 | 82.4 | ||
| Not reported | 19 | 7.3 | ||
| Number of trial arms | 1 | 13 | 5.0 | |
| 2 | 187 | 71.4 | ||
| 3 | 36 | 13.7 | ||
| 4 | 24 | 9.2 | ||
| >5 | 2 | 0.8 | ||
| Blinding | No blinding | 87 | 33.2 | |
| Single blinded | 76 | 29 | ||
| Double blinded | 53 | 20.2 | ||
| Not reported | 46 | 17.6 | ||
| Randomisation | Yes | 237 | 90.5 | |
| Clinical trial purpose | Treatment | 148 | 56.5 | |
| Prevention | 110 | 42.0 | ||
| Diagnostic | 3 | 1.1 | ||
| Screening | 1 | 0.4 | ||
| Clinical trial status | Completed | 257 | 98.1 | |
| FundingA (N = 378) | Government | 162 | 42.9 | |
| Other (including not for profit organisations and self-funded) | 113 | 29.9 | ||
| Industry | 48 | 12.7 | ||
| University | 39 | 10.3 | ||
| Not reported | 12 | 3.2 | ||
| None | 4 | 1.1 | ||
| Centre type | Multi-centre | 187 | 71.4 | |
| Single centre | 75 | 28.6 | ||
| Location | Australia only | 209 | 79.8 | |
| International | 53 | 20.2 | ||
| ContinentA (N = 333) | Australia only | 209 | 62.8 | |
| Australia and New Zealand | 53 | 15.9 | ||
| Asia | 14 | 4.2 | ||
| Europe | 28 | 8.4 | ||
| North America | 27 | 8.1 | ||
| South America | 2 | 0.6 | ||
| Population sample size | <50 | 39 | 14.3 | |
| 50–100 | 48 | 17.6 | ||
| 101–150 | 30 | 11.0 | ||
| 151–200 | 26 | 9.6 | ||
| 201–250 | 16 | 5.9 | ||
| >250 | 103 | 37.9 | ||
| Research discipline | Medicine/clinical sciences | 211 | 80.5 | |
| Psychosocial research | 41 | 15.6 | ||
| Dentistry/oral health | 10 | 3.8 | ||
| Disease category | Neurology | 47 | 17.9 | |
| Lifestyle | 43 | 16.4 | ||
| Mental health | 43 | 16.4 | ||
| Respiratory | 28 | 10.7 | ||
| Immunology and infectious disease | 21 | 8.0 | ||
| Neonatology | 19 | 7.3 | ||
| Dentistry | 10 | 3.8 | ||
| Endocrinology | 8 | 3.1 | ||
| Oncology | 8 | 3.1 | ||
| Vascular access | 8 | 3.1 | ||
| Cardiac | 6 | 2.3 | ||
| Speech pathology | 5 | 1.9 | ||
| Plastics and burns | 3 | 1.1 | ||
| Dermatology | 2 | 0.8 | ||
| Optometry | 2 | 0.8 | ||
| Orthopaedics | 2 | 0.8 | ||
| Audiology | 1 | 0.4 | ||
| Chronic disease | 1 | 0.4 | ||
| Gastroenterology | 1 | 0.4 | ||
| General practice | 1 | 0.4 | ||
| Musculoskeletal | 1 | 0.4 | ||
| Renal | 1 | 0.4 | ||
| Vascular | 1 | 0.4 | ||
| Location (regionality)A (N = 306) | Not reported | 141 | 46.1 | |
| Metropolitan | 97 | 31.7 | ||
| Regional | 30 | 9.8 | ||
| Rural | 19 | 6.2 | ||
| Remote | 19 | 6.2 | ||
Overall, 154 (58.8%) clinical trials did not report Indigenous status or any of the diversity indicators of interest. For studies reporting these variables, ‘Indigenous status’ was most reported (n = 40, 15.3%), followed by ‘ethnicity’ (n = 30, 11.5%), ‘language’ (n = 27, 10.3%), ‘country of birth’ (n = 25, 9.5%), ‘race’ (n = 19, 7.3%), ‘race and ethnicity’ (n = 9, 3.4%) and ‘natural skin colour’ (n = 1, 0.4%). Table 2 demonstrates the reporting of Indigenous status, race, ethnicity, language used at home and country of birth as per author definitions. No study reported a triple-phased descriptor (i.e. reported 1: a cultural indicator such as Indigenous status/race/ethnicity; 2: language and 3: country of birth).
| Australian clinical trials (N = 209) | International clinical trials with Australian sites (N = 262) | ||||
|---|---|---|---|---|---|
| Reporting items | N | % | N | % | |
| Indigenous status | 37 | 17.7 | 40 | 15.3 | |
| Ethnicity/cultural background | 15 | 7.2 | 30 | 11.5 | |
| Language used at home | 27 | 12.9 | 27 | 10.3 | |
| Country of birth | 23 | 11.0 | 25 | 9.5 | |
| Race | 1 | 0.5 | 19 | 7.3 | |
| Race and ethnicity (grouped together) | 4 | 1.9 | 9 | 3.4 | |
| Natural skin colour | 1 | 0.5 | 1 | 0.4 | |
‘N’ refers to number of responses. Studies could report more than one category.
Table 2 also illustrates the differences observed in capturing the variables of interest for Australian only clinical trials and international clinical trials with Australian sites. While Indigenous status (n = 37, 17.7%), language (n = 27, 12.9%) and country of birth (n = 23, 11.0%) were the three most commonly reported variables in Australian only clinical trials, it was apparent that Indigenous status (n = 40, 15.3%), ethnicity (n = 30, 11.5%) and language (n = 27, 10.3%) were the most commonly reported variables in international clinical trials with Australian sites. Race was also reported in higher rates within the international clinical trials with Australian sites (n = 19, 7.3%), in comparison to Australian clinical trials (n = 1, 0.5%).
As illustrated in Table 3, the reporting of ethnicity and/or cultural background increased over the 5 years captured. No trends in the reporting of Indigenous status, language used at home or country of birth were observed over time.
| Year | N | Reporting of Indigenous status | Reporting of ethnicity/cultural background | Reporting of language used at home | Reporting of country of birth | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| 2018 | 56 | 6 (10.7) | 50 (89.3) | 2 (3.6) | 54 (96.4) | 2 (3.6) | 54 (96.4) | 5 (8.9) | 51 (91.1) | |
| 2019 | 46 | 8 (17.4) | 38 (82.6) | 4 (8.7) | 42 (91.3) | 6 (13.0) | 40 (87.0) | 3 (6.5) | 43 (93.5) | |
| 2020 | 54 | 7 (13.0) | 47 (87.0) | 8 (14.8) | 46 (85.2) | 6 (11.1) | 48 (88.9) | 8 (14.8) | 46 (85.2) | |
| 2021 | 60 | 11 (18.3) | 49 (81.7) | 5 (8.3) | 55 (91.7) | 7 (11.7) | 53 (88.3) | 4 (6.7) | 56 (93.3) | |
| 2022 | 46 | 8 (17.4) | 38 (82.6) | 11 (23.9) | 35 (76.1) | 6 (13.0) | 40 (87.0) | 5 (10.9) | 41 (89.1) | |
‘N’ refers to number of responses.
Methods used to capture the variables of interest were predominately self-reported for Indigenous status (n = 14, 35.0%), ethnicity (n = 14, 46.7%), language (n = 10, 37.0%), race (n = 13, 68.4%) and race and ethnicity combined (n = 4, 44.4%). Other common methods of determining the variables of interest included parents and other methods, such as school-identified, carer-identified, educator-identified, Elder-identified or identification based on school or medical records. Failure to disclose the methods used to determine the variables of interest was common as demonstrated in Table 4.
| Reported items | N | Method used | |||||
|---|---|---|---|---|---|---|---|
| Self-identified | Researcher assigned | Parent-identified | OtherA | Not reported | |||
| N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Indigenous status | 40 | 14 (35.0) | 3 (7.5) | 9 (22.5) | 4 (10.0) | 10 (25.0) | |
| Ethnicity | 30 | 14 (46.7) | 0 (0.0) | 10 (33.3) | 1 (3.3) | 5 (16.7) | |
| Language used at home | 27 | 10 (37.0) | 0 (0.0) | 5 (18.5) | 3 (11.1) | 9 (33.3) | |
| Country of birth | 25 | 16 (64.0) | 0 (0.0) | 2 (8.0) | 3 (12.0) | 4 (16.0) | |
| Race | 19 | 13 (68.4) | 0 (0.0) | 3 (15.8) | 0 (0.0) | 3 (15.8) | |
| Race and ethnicity combined | 9 | 4 (44.4) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 3 (33.3) | |
| Natural skin colour | 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
Of the studies that reported Indigenous status (n = 40), 14 (35.0%) included only Indigenous participants. Two studies (5.0%) completed a formal subgroup analysis specific to Indigenous participants, with results specific to the Indigenous participants and intervention under investigation. In these studies (n = 2), the mean percentage of Indigenous participants were 8.3% and 8.85% respectively. Two studies (5.0%) made comments about the results of interventions in relation to the included Indigenous participants but did not report a formal subgroup analysis in the publication. In these studies (n = 2), the mean proportion of Indigenous participants were 7.4% and 14.0%. Twenty-two (55%) studies that reported Indigenous status did not complete a subgroup analysis specific to Indigenous participants and the intervention under investigation. In these studies, the percentage of Indigenous participants ranged from 0.39% to 66.7%.
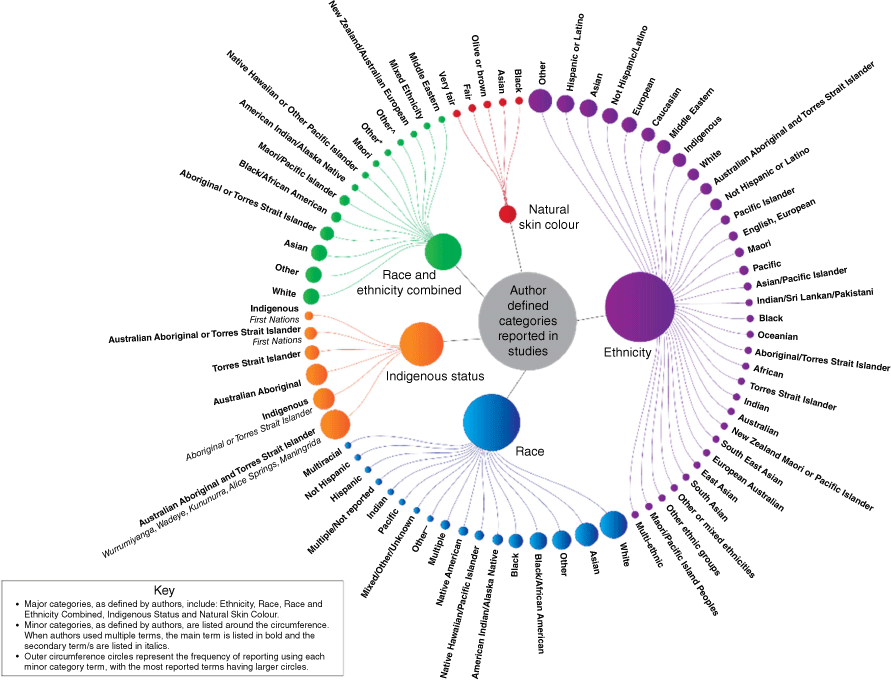
When reported, terms used by study authors varied extensively with authors primarily categorising cultural indicators as ‘Indigenous status’, ‘race’, ‘race and ethnicity’, ‘ethnicity’ or ‘natural skin colour’. As shown in Fig. 1, while these terms formed the main categories used for analysis, there was significant heterogeneity in the terms reported by authors. Overall, more than 60 terms were used to categorise study participants in relation to ‘Indigenous status’, ‘race’, ‘race and ethnicity’, ‘ethnicity’ or ‘natural skin colour’.
Discussion
This scoping review has demonstrated low levels of reporting of Indigenous status, race, ethnicity, language and country of birth for children participating in international clinical trials with Australian sites. It is well established that the effectiveness and safety of clinical interventions may be influenced by ethnicity and Indigeneity indicators. Subsequently, many of these clinical trials are not generalisable and do not adequately reflect the appropriateness of interventions for an entire population.10,12,14,15,25 This review has provided a comprehensive and contemporaneous aggregation of clinical trial diversity reporting. The level of reporting captured in the clinical trial is lower than previously reported in prior studies evaluating other regions of the world.15 Our review demonstrated that 41.2% of clinical trials captured reported at least one diversity indicator. Marginally better reporting was observed when results considered international clinical trials with Australian study sites (n = 108/262; 41.2%), in comparison to clinical trials with only Australian sites (n = 78/209; 37.3%). This may indicate that Australia’s diversity reporting is lagging behind global benchmarks.
Clinical trial participant diversity is a socio-political, moral and ethical issue that requires prompt rectification to minimise harm and improve patient outcomes.10,26 The low level of reporting demonstrated in this review raises questions as to the diversity of participants in those trials that did not report any diversity indicators. Thus, concerns remain surrounding access to these trials for children from Indigenous, racially and ethnically diverse backgrounds. This review demonstrated that the frequency of reporting diversity indicators was 41.2% in international clinical trials with Australian sites in paediatric or mixed paediatric/adult populations over the preceding 5 years. Similar levels of reporting were observed by Turner et al.15 who reported that overall, 43% of United States (US) clinical trials conducted between 2000 and 2020 reported race/ethnicity data, with 45% of trials reporting race/ethnicity data since 2007.15 Turner et al.15 reported that the reporting of any race/ethnicity enrolment data had increased from 26% in 2008 to 91% in 2018. Turner et al.15 also reported an increase in reporting over time, with an annual growth rate of 13.5%, which was not observed in this review. This may suggest that clinical trials with Australian sites are not accelerating in the reporting domain at the same pace as international counterparts. Despite similarities between this review and this US study, reviews conducted on United Kingdom (UK) RCTs from 2016 to 2021 have yielded far higher rates of reporting for ethnicity at 67.3%.27 Currently, global interventions including the United Nation’s Sustainable Development Goals and Leave No One Behind Agenda are leading the charge to ensure adequate access to health care for everyone.19,20 The increasing global movement to address racism has expedited reporting requirements in some countries, resulting in a global urgency to improve the transparency in the diversity of data collection and reporting for clinical trials, particularly as it pertains to Indigenous status and ethnicity.1,6,18,26 Subsequently, the inclusivity and reporting of clinical trial population characteristics have modestly improved over the past decade internationally, such as in the UK and the US.15,28 In particular, the Children’s Oncology Group supported by the National Cancer Institute has placed significant emphasis on ensuring diversity in paediatric cancer clinical trials, resulting in improved diversity reporting and improved access to clinical trials for diverse populations.17
Improvements to diversity reporting in clinical trials are multifaceted. Ensuring that Indigenous and ethnicity measures are appropriate to the study and study population and reported transparently with justification for the decisions made as per international guidelines should be employed by all clinical trialists. Indigenous status, race, ethnicity and cultural background are self-defined attributes and constitute social rather than biological constructs.8,29 There is no one size fits all. It is common for a person to identify with more than one ethnicity or cultural background and subsequently it is paramount that categories are not viewed in isolation with a single absolute option.8 Subsequently, it is essential that clinical trialists ensure participants or parents are identifying their Indigenous status and ethnicity indicators, rather than researchers using other methods to determine these factors. The heterogeneity in terminology associated with the diversity attributes of interest reported in this scoping review reflects the diversity of clinical trial participants and forms a basis to advocate for self-identification. Furthermore, adequate reporting includes capturing multiple variables.8 Through capturing multiple demographic variables, participants or parents can ensure that their or their child’s representation is an authentic reflection. Engaging with consumers across all geographic clinical trial sites to co-create clinical trial diversity data collection tools may offer a simple solution to ensure diversity information is adequately captured and reported.30 Ensuring transparency in research encapsulates a clear notation of how demographic variables are determined. When reporting clinical trials, methods used to capture diversity variables must be adequately defined.31 Clearer reporting guidelines, with consideration for socio-geographical differences across clinical trial sites and mandating the capturing of participant diversity information, are urgently required.
Limitations
The limitations of this review must be acknowledged. First, this review only examined clinical trials and subsequently, diversity reporting captured in cohort studies may have been inadvertently excluded. Furthermore, this review only considered clinical trials from the preceding 5 years and such trends in change over time may have been present and subsequently not captured in this review due to the contemporaneous nature of included trials. This scoping review was limited to clinical trials published in English. Due to the international nature of this scoping review, there may have been studies published in languages other than English that were excluded. The clinical trial timeline is long, with years between when clinical trials are designed, conducted and then reported. Subsequently, there may be current clinical trials yet to report results that may have employed more comprehensive diversity reporting methods that were not captured. The nature of paediatric clinical trials and rarity of the diseases treated leads to small recruitment numbers, which may limit generalisability. Furthermore, the quality of this review relies on the quality of the included studies. A quality assessment was not conducted and as such this review may have included low quality studies. However, this review captured paediatric clinical trials with Australian sites published in the past 5 years and the high quantity of included publications enables a generalised overview of diversity reporting.
Future research and practice
This review has demonstrated areas in which questions remain, and therefore it is imperative that future research is conducted. While research demonstrates the importance of collecting and reporting demographic data to ensure transparency and generalisability of clinical trial results, there is limited evidence on the viewpoints of clinical trialists. Future research encompassing qualitative research on clinical trialists opinions on the collection and reporting of demographic variables may be useful to develop strategies and pathways to ensure better reporting in future clinical trials. Furthermore, it may be beneficial for multi-disciplinary communication between Indigenous peoples, people from ethnically, linguistically and geographically diverse backgrounds, communities, social scientists, biologists and geneticists to determine how collected information, social self-identity versus biologic identity, can be captured and accounted for in future research. This review has demonstrated that while some studies included Aboriginal and Torres Strait Islander peoples and ethnically and linguistically diverse backgrounds, using Indigenous status as an example, there were very few sub-group analyses and studies were underpowered to analyse and report findings specific to Indigenous participants. Therefore, it is essential that future research is designed to be powered to analyse and report findings for all participants. Clinical trials are subject to thorough ethics approvals to ensure safety of clinical trial participants. Ethics applications commonly require additional approvals to collect and report data provided by Indigenous peoples; this is especially the case for clinical trials conducted within Australia.32 Therefore, the ethics process may provide an important mark in the clinical trial journey to ensure participants from diverse backgrounds have adequate access to clinical trials and trials are powered to analyse and report findings for all populations.
Conclusion
This review has highlighted low rates of reporting of diversity variables in international paediatric clinical trials with Australian sites. Clearer reporting standards, partnering with consumers to co-design trials and self-identification during data collection are required. Ensuring adequate access to clinical trials for Indigenous children, and children from ethnically, linguistically and geographically diverse backgrounds, is essential in building health equity and ensuring patient safety.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Acknowledgements
The authors of this scoping review acknowledge and thank the University of Queensland Librarian for their assistance in refining the search criteria. They also thank Naomi Priest for her contributions throughout the design and review phase of this project.
References
1 Cunningham J, Garvey G. Are there systematic barriers to participation in cancer treatment trials by Aboriginal and Torres Strait Islander cancer patients in Australia? Aust N Z J Public Health 2021; 45(1): 39-45.
| Crossref | Google Scholar | PubMed |
2 Greenberg RG, McCune S, Attar S, Hovinga C, Stewart B, Lacaze-Masmonteil T. Pediatric Clinical Research Networks: Role in Accelerating Development of Therapeutics in Children. Ther Innov Regul Sci 2022; 56(6): 934-47.
| Crossref | Google Scholar | PubMed |
3 Joseph PD, Craig JC, Caldwell PHY. Clinical trials in children. Br J Clin Pharmacol 2015; 79(3): 357-69.
| Crossref | Google Scholar | PubMed |
4 Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community 2004; 12(5): 382-8.
| Crossref | Google Scholar | PubMed |
5 Gross AS, Harry AC, Clifton CS, Della Pasqua O. Clinical trial diversity: An opportunity for improved insight into the determinants of variability in drug response. Br J Clin Pharmacol 2022; 88(6): 2700-17.
| Crossref | Google Scholar | PubMed |
6 Oh S, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med 2015; 12(12): e1001918.
| Crossref | Google Scholar | PubMed |
7 Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, et al. Race and Genetic Ancestry in Medicine — A Time for Reckoning with Racism. N Engl J Med 2021; 384(5): 474-80.
| Crossref | Google Scholar | PubMed |
8 Flanagin A, Frey T, Christiansen SL,, AMA Manual of Style Committee. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021; 326(7): 621-7.
| Crossref | Google Scholar | PubMed |
9 Eneanya ND, Boulware LE, Tsai J, Bruce MA, Ford CL, Harris C, et al. Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol 2022; 18(2): 84-94.
| Crossref | Google Scholar | PubMed |
10 Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, Gorham M, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol 2019; 44(5): 148-72.
| Crossref | Google Scholar | PubMed |
11 Fawzy A, Wu TD, Wang K, Robinson ML, Farha J, Bradke A, et al. Racial and Ethnic Discrepancy in Pulse Oximetry and Delayed Identification of Treatment Eligibility Among Patients With COVID-19. JAMA Intern Med 2022; 182(7): 730-8.
| Crossref | Google Scholar | PubMed |
12 Sharif MZ, Maghbouleh N, Baback Boozary AS. COVID-19 Disparities Among Arab, Middle Eastern, and West Asian Populations in Toronto: Implications for Improving Health Equity Among Middle Eastern and North African Communities in the United States. Health Promot Pract 2024; 25(4): 531-36.
| Crossref | Google Scholar | PubMed |
13 Bhavani SV, Wiley Z, Verhoef PA, Coopersmith CM, Ofotokun I. Racial Differences in Detection of Fever Using Temporal vs Oral Temperature Measurements in Hospitalized Patients. JAMA 2022; 328(9): 885-6.
| Crossref | Google Scholar | PubMed |
14 Corbie-Smith G, St. George DMM, Moody-Ayers S, Ransohoff DF. Adequacy of reporting race/ethnicity in clinical trials in areas of health disparities. J Clin Epidemiol 2003; 56(5): 416-20.
| Crossref | Google Scholar | PubMed |
15 Turner BE, Steinberg JR, Weeks BT, Rodriguez F, Cullen MR. Race/ethnicity reporting and representation in US clinical trials: A cohort study. Lancet Reg Health - Americas 2022; 11: 100252.
| Google Scholar |
16 Durant RW, Wenzel JA, Scarinci IC, Paterniti DA, Fouad MN, Hurd TC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer 2014; 120: 1097-105.
| Crossref | Google Scholar | PubMed |
17 Lund MJ, Eliason MT, Haight AE, Ward KC, Young JL, Pentz RD. Racial/ethnic diversity in children’s oncology clinical trials. Cancer 2009; 115(16): 3808-16.
| Crossref | Google Scholar | PubMed |
18 Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book 2019; Vol. 39: 105-14.
| Google Scholar |
19 United Nations. Transforming our World: The 2030 Agenda for Sustainable Development: United Nations. 2015. Available at https://sdgs.un.org/2030agenda
20 United Nations. Universal Values Principle Two: Leave No One Behind: United Nations. 2023. Available at https://unsdg.un.org/2030-agenda/universal-values/leave-no-one-behind#:~:text=Universal%20Values&text=It%20represents%20the%20unequivocal%20commitment,of%20humanity%20as%20a%20whole
21 Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; 62(10): e1-34.
| Crossref | Google Scholar | PubMed |
22 Veritas Health Innovation. Covidence systematic review software Melbourne, Australia; 2019. Available at www.covidence.org.
24 Airtable. Digital operations for the AI era. 2012. Available at https://www.airtable.com/
25 Saatci D, Ranger TA, Garriga C, Clift AK, Zaccardi F, Tan PS, et al. Association Between Race and COVID-19 Outcomes Among 2.6 Million Children in England. JAMA Pediatr. 2021; 175(9): 928-38.
| Crossref | Google Scholar | PubMed |
26 Priest N, Thurber KA, Maddox R, Jones R, Truong M. COVID-19 racism is making kids sick. Insight+; 2020. Available at https://insightplus.mja.com.au/2020/18/covid-19-racism-is-making-kids-sick/
27 Wallace N, O’Keeffe S, Gardner H, Shiely F. Underrecording and underreporting of participant ethnicity in clinical trials is persistent and is a threat to inclusivity and generalizability. J Clin Epidemiol 2023; 162: 81-9.
| Crossref | Google Scholar | PubMed |
28 Hirano SA, Murray SB, Harvey VM. Reporting, Representation, and Subgroup Analysis of Race and Ethnicity in Published Clinical Trials of Atopic Dermatitis in the United States Between 2000 and 2009. Pediatr Dermatol 2012; 29(6): 749-55.
| Crossref | Google Scholar | PubMed |
29 Lu C, Ahmed R, Lamri A, Anand SS. Use of race, ethnicity, and ancestry data in health research. PLoS Global Public Health 2022; 2(9): e0001060.
| Crossref | Google Scholar | PubMed |
30 Vale CL, Thompson LC, Murphy C, Forcat S, Hanley B. Involvement of consumers in studies run by the Medical Research Council Clinical Trials Unit: Results of a survey. Trials 2012; 13(1): 9.
| Crossref | Google Scholar | PubMed |
31 Schulz K, Altman D, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8: 18.
| Google Scholar | PubMed |
32 National Health and Medical Research Council. Ethical conduct in research with Aboriginal and Torres Strait Islander Peoples and communities. 2018. Available at https://www.nhmrc.gov.au/about-us/resources/ethical-conduct-research-aboriginal-and-torres-strait-islander-peoples-and-communities