Are we missing opportunities to detect acute rheumatic fever and rheumatic heart disease in hospital care? A multijurisdictional cohort study
John A. Woods A * , Nita Sodhi-Berry
A * , Nita Sodhi-Berry  B , Bradley R. MacDonald
B , Bradley R. MacDonald  B C D , Anna P. Ralph
B C D , Anna P. Ralph  E , Carl Francia
E , Carl Francia  F , Ingrid Stacey
F , Ingrid Stacey  B and Judith M. Katzenellenbogen
B and Judith M. Katzenellenbogen  B C
B C
A
B
C
D
E
F
Abstract
This study aimed to investigate potential missed diagnoses of acute rheumatic fever and rheumatic heart disease during hospital-based care among persons subsequently identified with these conditions.
This retrospective cohort study used linked emergency department and inpatient administrative records from Queensland, Northern Territory, South Australia, and New South Wales during 2003–2018 (varying between jurisdictions by completeness of data) of all persons first identified with acute rheumatic fever or rheumatic heart disease while aged 8–24 years. Using coded discharge diagnoses from the preceding 3 years, we identified presentations (e.g. joint pains or heart murmur without specific identified cause) that potentially mimic and thereby represent a missed opportunity to detect acute rheumatic fever or rheumatic heart disease. Sociodemographic factors associated with experiencing ≥1 mimic diagnoses were investigated using multivariable logistic regression models.
Among 1855 persons, 65 (3.5%) (using narrow diagnostic inclusions) and 146 (7.9%) (with broad inclusions) experienced ≥1 mimic diagnosis. Joint disorders predominated. Mimics categorised as ‘high-likelihood’ (most specific) were more frequent among persons subsequently diagnosed as young adults (18–24 years) than as children (8–12 years) (odds ratio [OR] 2.45, 95% confidence interval [CI] 1.34–4.47), and those from low-risk ethnic groups (including Australian-born non-Indigenous persons) compared with Aboriginal and Torres Strait Islander peoples (OR 2.44, 95% CI 1.02–5.85).
Missed opportunities to detect acute rheumatic fever and rheumatic heart disease continue to occur in Australian hospitals, and present disproportionately among persons from demographic groups considered to be at low risk, suggesting the need for enhanced clinical suspicion in these groups.
Keywords: Australia, cohort studies, delayed diagnosis, hospital medicine, missed diagnosis, rheumatic fever, rheumatic heart disease, routinely collected health data.
Introduction
Acute rheumatic fever (ARF) is an autoimmune response to group A Streptococcus infection.1 Cardiac valve damage arising from a single severe episode or recurrent episodes of ARF leads to rheumatic heart disease (RHD), which may progress to heart failure and/or death.1 These conditions are uncommon in the general populations of high-income countries,2 but in Australia occur frequently among some Aboriginal and Torres Strait Islander peoples’ communities.3 ARF is chiefly a condition of children and young adults,4–6 with antecedent ARF typically having occurred during the youth of persons whose RHD is first diagnosed later in life.
Both ARF and RHD have non-specific clinical features and pose diagnostic challenges. There is considerable potential for under-recognition and misdiagnosis of ARF, given the transient symptoms of and absence of a diagnostic test for this condition.5,6 RHD typically has a long asymptomatic phase and often presents at an advanced stage with complications of advanced valvular lesions (e.g. breathlessness and fatigue), requiring echocardiography for confirmation.7
Many occurrences of ARF continue to pass undetected in Australia, as is evident from the high proportion (~75%)8 of RHD cases diagnosed in persons without recognised antecedent ARF. Although some people with ARF may never present to health services with symptoms, many missed cases can likely be attributed to incorrect diagnoses. A low index of suspicion among many clinicians for these conditions due to their rarity in the general population, along with cultural and communication barriers faced by Aboriginal and Torres Strait Islander peoples in health care9 possibly contributes to under-diagnosis.10 Since adverse outcomes of these conditions are often amenable to early intervention through secondary prophylaxis,11 it is crucial to elucidate the frequency and determinants of delayed/missed diagnoses. However, current evidence is limited to single-institution case series12,13 and case report10 data on delayed recognition of ARF, and RHD cases that were detected only at autopsy.14
This study used routinely collected data to investigate missed opportunities for the diagnosis of ARF and/or RHD (ARF/RHD) among young people in Australian emergency department (ED) and inpatient settings. We aimed to: (i) quantify the frequency of presentations prior to ARF/RHD recognition with diagnoses reflecting conditions that potentially indicate missed ARF or RHD, and (ii) determine the sociodemographic risk factors for these putative missed opportunities.
Methods
We conducted a multijurisdictional retrospective cohort study (as per STROBE guidelines;12 Supplementary Table S1) of persons identified with ARF/RHD, based on various sources (enumerated below). To identify possible missed diagnoses, we used their ED and inpatient administrative records from the 3 years preceding first identification.
Data sources and study population
The cohort for this study was a subset of the End RHD in Australia: Study of Epidemiology (ERASE) database.13 In brief, ERASE includes harmonised data from five Australian jurisdictions: New South Wales (NSW), Northern Territory (NT), Queensland (Qld), South Australia (SA), and Western Australia (WA), with cross-jurisdictional linkages between SA and NT. The database comprises routinely collected and de-identified, person-linked records from ARF/RHD registers, inpatient separations, ED presentations, a surgery registry, and death registries.13 ARF/RHD ascertainment and first-ever diagnosis dates were established from RHD register records, surgery data, and/or diagnosis codes from hospital separations through a validated algorithm.13 Our cohort comprised persons whose initial diagnosis of ARF/RHD had occurred when they were aged 8–24 years, and for whom jurisdictional ED and hospital inpatient data were available for a 3-year lookback period prior to diagnosis. A high proportion (44.2%) of missing diagnosis codes in ED records throughout the study period precluded use of WA data. The usable date range of ED data from different jurisdictions varied widely, i.e. NSW (1 July 2005–30 June 2018), NT (1 January 2000–30 June 2018), Qld (1 July 2008–31 December 2017), and SA (1 July 2003–31 December 2017). We excluded 74 persons (2.1%) who, in addition to their ARF/RHD diagnosis, had any recorded diagnosis of a congenital cardiovascular disorder and/or a specified heritable disorder known to be associated with these.
Care episodes
To account for inter- and intra-hospital transfers, a hospital admission occurring on the same day or day following a discharge was considered part of the previous episode. Similarly, an ED presentation on the same day as or day prior to an inpatient admission was considered part of the admission episode, and instances of multiple same-day ED presentations were considered a single episode. If first identification of ARF/RHD was nested within an episode, the identification date was assigned to the episode commencement date. Discharges within 7 days before ARF/RHD identification were excluded from the analysis, as mimic diagnoses within this interval were considered potentially to reflect provisional rather than final diagnostic assessments.
Mimic diagnoses
Diagnosis codes were harmonised to International Classification of Disease (ICD) Version 10 Australian Modification (ICD-10-AM) codes14 by mapping where necessary from ICD-9-Clinical Modification codes (used prior to July 1999 in all included jurisdictions) and SnoMED-CT codes15 (used solely in NSW ED data). SnoMED-CT to ICD-10-AM mapping was conducted using the SnoMAP® tool.16
An exhaustive list of all differential diagnoses (and corresponding ICD-10-AM codes) of ARF and/or (chronic) RHD was compiled manually, and complemented with a published list of differential diagnoses.17 Subsequently, authors APR (an infectious diseases physician with expertise in ARF) and BRM (a paediatrician with expertise in RHD) categorised the codes into mutually exclusive groups (Supplementary Table S2) as those representing disorders with: (1) a high likelihood of mimicking only ARF, (2) a high likelihood of mimicking either ARF or RHD (i.e. relevant cardiac disorders other than pericarditis), (3) a medium likelihood of mimicking ARF only or either condition, or (4) (excluded from further consideration) a low likelihood of mimicking ARF only or either condition. Decision-making to assign these categories was guided by consideration of the extent to which the original diagnosis could have represented true ARF or RHD in someone later diagnosed with either or both conditions. High likelihood mimics excluded codes indicating a specific alternative aetiology (e.g. gonococcal infection, drug-induced chorea). To allow for within-episode diagnostic refinement, only diagnosis codes from the final record of an episode were considered. As the study was based on de-identified administrative data, validation of mimic diagnoses through chart review of cases with relevant codes was not undertaken.
Covariates
Sociodemographic attributes including date of birth, sex, population group (categorised as Aboriginal and Torres Strait Islander peoples, immigrant from low- or middle-income country, or other Australians) and residential remoteness were derived from the broader ERASE project.13 Age at first ARF/RHD diagnosis was categorised as 8–12, 13–17, and 18–25 years. In order to investigate secular trends, calendar period was collapsed into three categories: 2003–2007, 2008–2012, and 2013–2018.
Statistical analysis
Patient characteristics were summarised descriptively within age strata. The proportion of persons who experienced at least one mimic diagnosis was calculated. Determinants of experiencing one or more care episodes with a mimic diagnosis were investigated, using several univariate and multivariable logistic regression models based on different sets of diagnostic codes, with associations expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Firstly, odds were estimated of experiencing ≥1 combined ARF/RHD high- or medium-likelihood mimic diagnosis. Secondly, odds were estimated with restriction of outcomes to high-likelihood combined ARF/RHD diagnostic mimics only. Thirdly, the models were restricted to diagnostic mimics representing ARF only, (a) for the full lookback period (7 days–3 years), and (b) for a lookback period restricted to 90 days–3 years before first ARF/RHD identification, in order to capture only completely missed (rather than delayed) ARF diagnoses. Residential remoteness data (Accessibility/Remoteness Index of Australia Plus [ARIA+])18 were excluded from the models presented because of separation problems and constraints on interpretability due to a substantial proportion of missing values, however, in preliminary analyses this covariate did not materially alter overall results. All analyses were conducted using Stata® version 16.1 (Stata Corporation, College Station, TX, USA).
Results
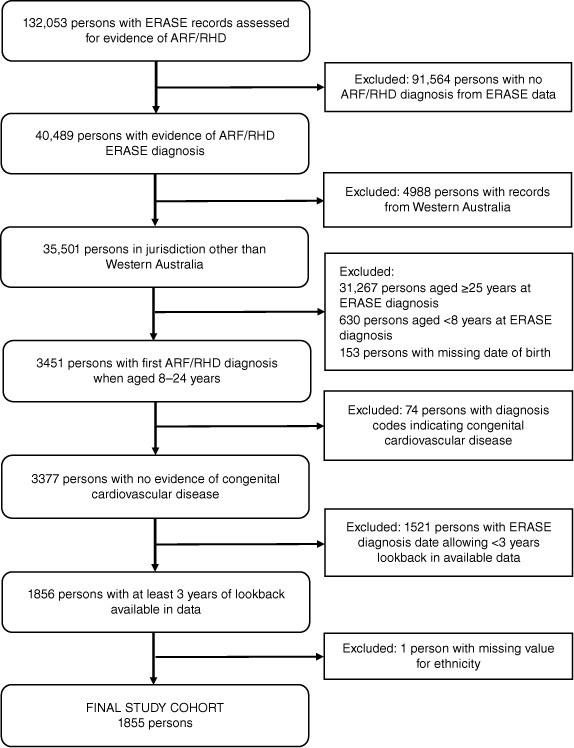
The cohort eligible for analysis (Fig. 1) comprised 1855 persons identified with ARF/RHD when aged 8–24 years, among whom nearly half (45.3%) were diagnosed in childhood (<13 years) (Table 1). Aboriginal and Torres Strait Islander peoples (83.4%) and females (56.9%) predominated. About half (53.0%) lived in the NT and 58.0% in remote or very remote areas.
| Age-group at ARF/RHD diagnosis | |||||
|---|---|---|---|---|---|
| 8–12 years | 13–17 years | 18–24 years | Total | ||
| n (% within age group) | n (% within age group) | n (% within age group) | n (% of total cohort) | ||
| Total | 840 (45.3) | 528 (28.5) | 487 (26.3) | 1855 (100%) | |
| Sex | |||||
| Male | 399 (47.5) | 243 (46.0) | 157 (32.2) | 799 (43.1) | |
| Female | 441 (52.5) | 285 (54.0) | 330 (67.8) | 1056 (56.9) | |
| Ethnicity risk status | |||||
| Aboriginal and Torres Strait Islander peoples | 725 (86.3) | 455 (86.2) | 367 (75.4) | 1547 (83.4) | |
| Immigrant high risk | 60 (7.1) | 37 (7.0) | 50 (10.3) | 147 (7.9) | |
| Other | 55 (6.6) | 36 (6.8) | 70 (14.4) | 161 (8.7) | |
| Jurisdiction | |||||
| Queensland | 265 (31.6) | 186 (35.2) | 118 (24.2) | 569 (30.7) | |
| Northern Territory | 478 (56.9) | 264 (50.0) | 242 (49.7) | 984 (53.0) | |
| South Australia | 32 (3.8) | 28 (5.3) | 45 (9.2) | 105 (5.7) | |
| New South Wales | 65 (7.7) | 50 (9.5) | 82 (16.8) | 197 (10.6) | |
| Calendar period of diagnosis | |||||
| 2003–2007 | 55 (6.6) | 26 (4.9) | 36 (7.4) | 117 (6.3) | |
| 2008–2012 | 297 (35.4) | 183 (34.7) | 203 (41.7) | 683 (36.8) | |
| 2013–2018 | 488 (58.1) | 319 (60.4) | 248 (50.9) | 1055 (56.9) | |
| ARIA+ of residence | |||||
| Major cities/inner regional | 102 (12.1) | 74 (14.0) | 97 (19.9) | 273 (14.7) | |
| Outer regional | 147 (17.5) | 79 (15.0) | 76 (15.6) | 302 (16.3) | |
| Remote/very remote | 507 (60.4) | 316 (59.9) | 253 (52.0) | 1076 (58.0) | |
| Missing | 84 (10.0) | 59 (11.2) | 61 (12.5) | 204 (11.0) | |
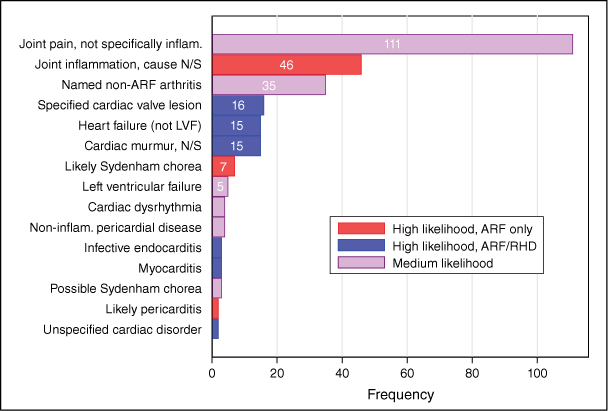
Most of the mimic diagnoses identified were categorised as disorders of joints (Fig. 2, Supplementary Table S3). The proportions of the cohort with one or more ARF/RHD mimic diagnoses during the 3-year lookback period were 7.9% (146 persons) for combined high- or medium-likelihood mimics, and 3.5% (n = 65) for high-likelihood mimics (Table 2). ARF-only mimics occurred in 1.8% (n = 34), among whom 1.2% (n = 22) experienced ≥1 mimics more than 90 days before identification, suggesting a completely missed ARF episode (Table 3).
Frequency of emergency department/inpatient mimic diagnosis presentations in the 3-year period prior to first identification of ARF/RHD. Numbers of mimic diagnosis categories with fewer than five occurrences have been withheld to protect anonymity. inflam., inflammatory; LVF, left ventricular failure; N/S, not specified.
| Model 1: 7 days–3 years lookback; Combined high- and medium-likelihood mimic diagnoses | Model 2: 7 days–3 years lookback; High-likelihood mimic diagnoses only | ||||||
|---|---|---|---|---|---|---|---|
| N = 1855 persons, including 146 with ≥1 ARF/RHD mimic | N = 1855 persons, including 65 with ≥1 ARF/RHD mimic | ||||||
| ≥1 mimic (n) | Crude OR (95% CI) | Adjusted OR (95% CI) | ≥1 mimic (n) | Crude OR (95% CI) | Adjusted OR (95% CI) | ||
| Sex | |||||||
| Male | 57 | Reference | Reference | 24 | Reference | Reference | |
| Female | 89 | 1.20 (0.85–1.69) | 1.11 (0.78–1.58) | 41 | 1.30 (0.78–2.18) | 1.25 (0.74–2.11) | |
| Age group at ERASE diagnosis | |||||||
| 8–12 years | 51 | Reference | Reference | 19 | Reference | Reference | |
| 13–17 years | 32 | 1.00 (0.63–1.57) | 1.05 (0.66–1.66) | 16 | 1.35 (0.69–2.65) | 1.37 (0.70–2.70) | |
| 18–24 years | 63 | 2.30 (1.56–3.39) | 2.21 (1.48–3.29) | 30 | 2.84 (1.58–5.10) | 2.45 (1.34–4.47) | |
| Ethnicity risk status | |||||||
| Aboriginal and Torres Strait Islander peoples | 117 | Reference | Reference | 44 | Reference | Reference | |
| Immigrant high risk | 11 | 0.99 (0.52–1.88) | 1.33 (0.64–2.77) | 8 | 1.97 (0.91–4.26) | 1.81 (0.75–4.37) | |
| Other Australians | 18 | 1.54 (0.91–2.60) | 1.67 (0.82–3.37) | 13 | 3.00 (1.58–5.70) | 2.44 (1.02–5.85) | |
| Jurisdiction | |||||||
| Queensland | 25 | Reference | Reference | 18 | Reference | Reference | |
| Northern Territory | 94 | 2.30 (1.46–3.62) | 2.34 (1.43–3.84) | 30 | 0.96 (0.53–1.74) | 0.95 (0.49–1.87) | |
| South Australia | 7 | 1.55 (0.65–3.69) | 1.28 (0.53–3.11) | <5 | 0.90 (0.26–3.11) | 0.68 (0.19–2.43) | |
| New South Wales | 20 | 2.46 (1.33–4.53) | 1.64 (0.79–3.38) | 14 | 2.34 (1.14–4.80) | 1.22 (0.51–2.92) | |
| Calendar period | |||||||
| 2003–2007 | 15 | Reference | Reference | 7 | Reference | Reference | |
| 2008–2012 | 53 | 0.57 (0.31–1.05) | 0.65 (0.35–1.21) | 25 | 0.60 (0.25–1.41) | 0.45 (0.18–1.12) | |
| 2013–2018 | 78 | 0.54 (0.30–0.98) | 0.72 (0.39–1.32) | 33 | 0.51 (0.22–1.17) | 0.41 (0.17–1.02) | |
Bold indicates 95% CI not including 1.
ARF, acute rheumatic fever; CI, confidence interval; OR, odds ratio; RHD, rheumatic heart disease.
| Model 1: 7 days–3 years lookbackA (delayed or missed diagnosis) | Model 2: 90 days–3 years lookbackA,B (episode completely missed) | ||||||
|---|---|---|---|---|---|---|---|
| N = 1855 persons, including 34 with ≥1 ARF mimic | N = 1855 persons, including 22 with ≥1 ARF mimic | ||||||
| ≥1 mimic (n) | Crude OR (95% CI) | Adjusted OR (95% CI) | ≥1 mimic (n) | Crude OR (95% CI) | Adjusted OR (95% CI) | ||
| Sex | |||||||
| Male | 12 | Reference | Reference | 8 | Reference | Reference | |
| Female | 22 | 1.40 (0.69–2.84) | 1.40 (0.69–2.87) | 14 | 1.33 (0.55–3.18) | 1.34 (0.56–3.25) | |
| Age group at ERASE diagnosis | |||||||
| 8–12 years | 12 | Reference | Reference | 7 | Reference | Reference | |
| 13–17 years | 11 | 1.47 (0.64–3.35) | 1.52 (0.66–3.50) | 7 | 1.60 (0.56–4.58) | 1.60 (0.56–4.62) | |
| 18–24 years | 11 | 1.59 (0.70–3.64) | 1.35 (0.58–3.14) | 8 | 1.99 (0.72–5.51) | 1.60 (0.56–4.56) | |
| Ethnicity risk status | |||||||
| Aboriginal and Torres Strait Islander peoples | 23 | Reference | Reference | 13 | Reference | Reference | |
| Immigrant high risk | <5 | 1.85 (0.63–5.43) | 2.48 (0.73–8.41) | <5 | 2.46 (0.69–8.73) | 2.54 (0.61–10.51) | |
| Other Australians | 7 | 3.01 (1.27–7.13) | 4.21 (1.30–13.64) | 6 | 4.57 (1.71–12.19) | 4.86 (1.29–18.24) | |
| Jurisdiction | |||||||
| Queensland | 9 | Reference | Reference | 7 | Reference | Reference | |
| Northern Territory | 18 | 1.16 (0.52–2.6) | 1.32 (0.52–3.32) | 9 | 0.74 (0.27–2.00) | 0.97 (0.32–2.98) | |
| South Australia | <5 | 0.60 (0.08–4.77) | 0.50 (0.06–4.15) | <5 | 0.77 (0.09–6.34) | 0.68 (0.08–5.85) | |
| New South Wales | 6 | 1.95 (0.69–5.56) | 0.78 (0.23–2.72) | 5 | 2.09 (0.66–6.66) | 0.81 (0.20–3.22) | |
| Calendar period | |||||||
| 2003–2007 | <5 | Reference | Reference | <5 | Reference | Reference | |
| 2008–2012 | 17 | 0.97 (0.28–3.36) | 0.82 (0.23–2.99) | 10 | 1.72 (0.22–13.59) | 1.19 (0.14–9.94) | |
| 2013–2018 | 14 | 0.51 (0.14–1.80) | 0.44 (0.12–1.67) | 11 | 1.22 (0.16–9.55) | 0.86 (0.10–7.26) | |
Persons first identified with ARF/RHD as a young adult (18–24 years) experienced ≥1 ARF/RHD mimic diagnoses more often than those diagnosed in childhood (<13 years) (adjusted OR for combined high- and medium-likelihood mimics 2.20, 95% CI 1.46–3.31; for high-likelihood mimics alone 2.45 95% CI 1.34–4.47 (Table 2)). This association was not evident for ARF-only mimics. Non-Indigenous Australian residents (excluding immigrants from low- and middle-income countries) compared with Aboriginal and Torres Strait Islander peoples had higher odds of high-likelihood combined ARF/RHD mimics (adjusted OR 4.21, 95% CI 1.30–13.46, Table 2) and mimics of ARF modelled (Table 3) both with 7 days–3 years lookback (adjusted OR 4.21, 95% CI 1.30–13.46) and 90 days–3 years lookback (OR 4.86, 95% CI 1.29–18.24), but not combined high- and medium-likelihood mimic diagnoses (Table 2). No significant associations were evident in relation to sex, jurisdiction, or calendar period.
Discussion
In this study, 3.5–7.9% of young people with ARF and/or RHD (depending on whether the analytical model incorporated a narrow or broader set of diagnostic codes) presented to hospital with a mimicking condition in the 3 years prior to their diagnosis being made. With the benefit of hindsight, these prior presentations represent potential missed opportunities for earlier detection of ARF/RHD. Joint disorders (i.e. with a differential diagnosis specifically of ARF rather than RHD) predominated among these mimics. Evidence linking demographic characteristics to ED/inpatient encounters resulting in a diagnosis mimicking ARF/RHD was not consistent across all the models that we investigated. These encounters were more frequent among persons whose ARF/RHD was made at a relatively older age, and also in a population group among whom ARF/RHD is especially uncommon, i.e. non-Indigenous Australian-born persons.
While there is a growing body of literature on the utility of screening for RHD in Australia and elsewhere,19 we believe that our study is the first systematic investigation of missed opportunities for hospital-based diagnosis of symptomatic ARF and RHD. Likely barriers to clinical recognition of ARF/RHD include the inherent nature of both conditions along with the absence of a definitive diagnostic test for ARF20 and a low index of suspicion among clinicians trained in settings where occurrence is rare.21 In our study, the higher odds of being diagnosed with an illness mimicking both ARF/RHD among persons aged ≥18 years at identification compared with those identified in childhood plausibly reflects a lower index of suspicion for these conditions among older patients, among whom incident disease is less frequent.3 Similarly, diminished diagnostic suspicion due to disease rarity22 may account for our finding of higher odds of experiencing missed opportunities among persons of population groups in which ARF/RHD is rare compared with occurrence in Aboriginal and Torres Strait Islander peoples, who continue to experience very high risk.3 This is especially notable considering anecdotal evidence of clinicians failing to heed suggestions from Aboriginal and Torres Strait Islander patients’ parents who are familiar with the signs and symptoms of ARF/RHD through personal or family history.23
Strengths and limitations
The principal strength of this study is its unique examination at scale of precursor diagnoses among hospital presentations. The use of multijurisdictional data provided the study with a representative sample, although the rarity of new ARF/RHD cases constrained numbers. Missed opportunities to detect ARF/RHD occur in contexts that are beyond the scope of our study design. A key limitation is that we lacked data from primary care, which likely represents an important setting for diagnostic delay.24 Moreover, there is recent evidence based on echocardiographic screening investigation that a proportion of ARF cases characterised by subclinical carditis present with fever but are otherwise clinically silent, and that detection of ARF can be substantially augmented in high-risk settings by echocardiographic screening of febrile children.25 These findings, along with the inevitable shortcomings of symptom recall, generate uncertainty in relation to the proportion of persons detected with latent RHD through echocardiographic screening programs whose antecedent ARF was genuinely asymptomatic, even when previous joint symptoms are denied. We excluded the code indicating fever per se from our analyses, based on our judgement that the positive predictive value of this symptom for ARF would be low, even in our study cohort defined by a future diagnosis of ARF/RHD. Considering the foregoing, together with the restriction of our study cohort to individuals identified with ARF/RHD (i.e. excluding those not yet or never diagnosed), our findings possibly constitute the ‘iceberg tip’ of under-diagnosis of these conditions. However, the true total extent of missed diagnoses remains speculative. Reliance on diagnosis codes for case ascertainment is a limitation of all studies based on administrative data. In this study, it was not possible to validate cases identified as mimic diagnoses by means of chart review. Noting that the potential gain from early diagnosis is greatest among persons presenting acutely with ARF, it was not possible in our study to determine the total proportion of presentations that potentially represented a missed diagnosis of ARF specifically, because most of the cardiac disorder-related codes in our analysis were compatible with either ARF or RHD. The combination of sparse outcome data and inconsistent results for subgroups across different models mandates additional caution in the interpretation of our findings. Our selection of disease codes that represent mimics of ARF/RHD was developed with the input of expert clinicians (authors APR and BRM) but has not been independently validated. Our approach aimed to be specific rather than highly sensitive, by excluding diagnoses indicating a convincing alternative cause. In so doing, we may have under-counted mimic diagnoses. Our estimates from 3-year lookback (chosen, for consistency, as the maximum possible across all included jurisdictions) inevitably underestimated lifelong pre-diagnosis missed detection, which was not feasible to investigate with the available data. We could not determine with certainty whether an individual mimic diagnosis indicated a genuine missed ARF/RHD case or an unrecognised alternative condition (e.g. infective arthritis).26
Conclusion
ED and inpatient diagnoses that potentially represent missed opportunities for detection of ARF/RHD continue to occur in Australia. Our frequency estimates are possibly very conservative. Missed opportunities are possibly most common among persons from demographic groups in whom these conditions are perceived to be least frequent, suggesting that training to enhance clinicians’ index of suspicion for ARF/RHD is needed, especially in relation to very low-risk patients. Similar studies encompassing primary care attendances are warranted to investigate missed opportunities more broadly. The ARF & RHD Guideline App27 and efforts to develop a diagnostic test for ARF28 can be anticipated to reduce missed diagnoses further.
Data availability
The ERASE database cannot be shared publicly. Australian-based researchers can apply to the ERASE Project team with a proposal to analyse a pertinent research question using the ERASE Project data, subject to internal and ethics approval of the investigator and their research plans.
Conflicts of interest
All authors declare that they have no conflicts of interest. The supporting sources had no role in the study design, data collection, analysis or interpretation, or in writing of the article, and did not control or influence the decision to submit the final manuscript for publication.
Declaration of funding
This project received funding from the National Health and Medical Research Council through a project grant (#1146525) and seed funds from the End-RHD Centre for Research Excellence (National Health and Medical Research Council #1080401) and HeartKids. JMK is supported by a Heart Foundation of Australia Future Leader Fellowship (#102043). APR has been supported by a National Health and Medical Research Council Career Development Fellowship (#1142011).
Acknowledgements
The authors thank the staff of the data linkage units of the state and territory governments (WA, SA-NT, NSW, Qld) for linkage of the ERASE project data, and also the state and territory Registries of Births, Deaths and Marriages, the State and Territory Coroners, and the National Coronial Information System for enabling Cause of Death Unit Record File data to be used for this project. We also thank the data custodians and data managers for the provision of the following data: (1) inpatient hospital and emergency department data (five states and territories); (2) RHD registers; (3) The Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database (single registry covering all states and territories); (4) Royal Melbourne Children’s Hospital Paediatric Cardiac Surgery database (single data source for RHD paediatric patients from SA and NT receiving surgical intervention in Melbourne); and (5) primary healthcare data from NT Department of Health. The authors value the support/endorsement provided to the ERASE project by the following peak bodies representing the Aboriginal Community Controlled Health sector: Aboriginal Medical Services Alliance Northern Territory, Kimberley Aboriginal Medical Service (the health service serving the high-burden region of WA), Aboriginal Health Council of South Australia, and Aboriginal Health and Medical Research Council (NSW). We also received support from the Aboriginal divisions of Queensland and WA Health Departments. Finally, we are grateful to Dr Michael Lawley, Kylynn Loi, and Robyn Richards of the Australian e-Health Research Centre at CSIRO for their expert advice and assistance in SnoMED-CT to ICD-10-AM code mapping.
References
1 Carapetis JR, Beaton A, Cunningham MW, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers 2016; 2: 15084.
| Crossref | Google Scholar | PubMed |
2 Zühlke LJ, Steer AC. Estimates of the global burden of rheumatic heart disease. Glob Heart 2013; 8(3): 189-95.
| Crossref | Google Scholar | PubMed |
3 Katzenellenbogen JM, Bond-Smith D, Seth RJ, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: The case for policy change. J Am Heart Assoc 2020; 9(19): e016851.
| Crossref | Google Scholar | PubMed |
4 Hanna JN, Heazlewood RJ. The epidemiology of acute rheumatic fever in Indigenous people in north Queensland. Aust N Z J Public Health 2005; 29(4): 313-7.
| Crossref | Google Scholar | PubMed |
5 Ralph A, Jacups S, McGough K, McDonald M, Currie BJ. The challenge of acute rheumatic fever diagnosis in a high-incidence population: a prospective study and proposed guidelines for diagnosis in Australia’s Northern Territory. Heart Lung Circ 2006; 15(2): 113-8.
| Crossref | Google Scholar | PubMed |
6 Noonan S, Zurynski YA, Currie BJ, et al. A national prospective surveillance study of acute rheumatic fever in Australian children. Pediatr Infect Dis J 2013; 32(1): e26-32.
| Crossref | Google Scholar | PubMed |
7 Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: Incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation 2013; 128(5): 492-501.
| Crossref | Google Scholar | PubMed |
8 Hardie K, Ralph AP, de Dassel JL. RHD elimination: action needed beyond secondary prophylaxis. Aust N Z J Public Health 2020; 44(5): 427.
| Crossref | Google Scholar | PubMed |
9 Haynes E, Walker R, Mitchell AG, Katzenellenbogen J, D’Antoine H, Bessarab D. Decolonizing Indigenous health: Generating a productive dialogue to eliminate Rheumatic Heart Disease in Australia. Soc Sci Med 2021; 277: 113829.
| Crossref | Google Scholar | PubMed |
10 Kaminecki I, Verma R, Brunetto J, Rivera LI. Delayed Diagnosis of Acute Rheumatic Fever in a Patient with Multiple Emergency Department Visits. Case Rep Pediatr 2018; 2018: 9467131.
| Crossref | Google Scholar | PubMed |
11 Stacey I, Ralph AP, de Dassel JL, et al. The evidence that rheumatic heart disease control programs in Australia are making an impact. Aust N Z J Public Health 2023; 47(4): 100071.
| Crossref | Google Scholar | PubMed |
12 von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE initiative.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370(9596): 1453-7.
| Crossref | Google Scholar | PubMed |
13 Katzenellenbogen JM, Bond-Smith D, Seth RJ, et al. The End Rheumatic Heart Disease in Australia Study of Epidemiology (ERASE) project: Data sources, case ascertainment and cohort profile. Clin Epidemiol 2019; 11: 997-1010.
| Crossref | Google Scholar | PubMed |
14 Australian Consortium for Classification Development. International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification Darlinghurst: Independent Hospital Pricing Authority. 2017. Available at https://www.ihacpa.gov.au/resources/icd-10-amachiacs-tenth-edition (accessed 17 July 2024)
15 Donnelly K. SNOMED-CT: The advanced terminology and coding system for ehealth. Stud Health Technol Inform 2006; 121: 279-90.
| Google Scholar | PubMed |
16 Lawley M, Truran D, Hansen D, Good N, Staib A, Sullivan C. SnoMAP: Pioneering the path for clinical coding to improve patient care. Stud Health Technol Inform 2017; 239: 55-62.
| Google Scholar | PubMed |
17 Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet 2005; 366(9480): 155-68.
| Crossref | Google Scholar | PubMed |
18 Australian Bureau of Statistics. The Australian Statistical Geography Standard (ASGS) remoteness structure. 2016. Available at https://www.abs.gov.au/websitedbs/D3310114.nsf/home/remoteness+structure [accessed 17 December 2020].
19 Francis JR, Whalley GA, Kaethner A, et al. Single-View Echocardiography by Nonexpert Practitioners to Detect Rheumatic Heart Disease: A Prospective Study of Diagnostic Accuracy. Circ Cardiovasc Imaging 2021; 14(8): e011790.
| Crossref | Google Scholar | PubMed |
20 Karthikeyan G, Guilherme L. Acute rheumatic fever. Lancet 2018; 392(10142): 161-74.
| Crossref | Google Scholar | PubMed |
21 Khanna K, Liu DR. Acute Rheumatic Fever: An Evidence-Based Approach To Diagnosis And Initial Management. Pediatr Emerg Med Pract 2016; 13(8): 1-23.
| Google Scholar | PubMed |
22 Blöß S, Klemann C, Rother AK, et al. Diagnostic needs for rare diseases and shared prediagnostic phenomena: Results of a German-wide expert Delphi survey. PLoS One 2017; 12: e0172532.
| Crossref | Google Scholar | PubMed |
23 Haynes E, Marawili M, Marika MB, et al. Living with Rheumatic Heart Disease at the Intersection of Biomedical and Aboriginal Worldviews. Int J Environ Res Public Health 2022; 19(8): 4650.
| Crossref | Google Scholar | PubMed |
24 Murdoch J, Davis S, Forrester J, Masuda L, Reeve C. Acute rheumatic fever and rheumatic heart disease in the Kimberley: using hospitalisation data to find cases and describe trends. Aust N Z J Public Health 2015; 39(1): 38-43.
| Crossref | Google Scholar | PubMed |
25 Ali S, Beaton A, Ndagire E, Alhag L. Silent acute rheumatic fever unmasked by using handheld echocardiography for febrile children presenting in a rheumatic heart disease-endemic area. J Pediatr 2024; 268: 113954.
| Crossref | Google Scholar | PubMed |
26 Tuttle CSL, Van Dantzig T, Brady S, Ward J, Maguire G. The epidemiology of gonococcal arthritis in an Indigenous Australian population. Sex Transm Infect 2015; 91(7): 497-501.
| Crossref | Google Scholar | PubMed |
27 RHDAustralia. ARF & RHD Guideline App - with ARF diagnosis calculator. 2012. Available at https://www.rhdaustralia.org.au/resources/arf-rhd-guideline-app-arf-diagnosis-calculator-android (accessed 17 July 2024)
28 Ralph AP, Webb R, Moreland NJ, et al. Searching for a technology-driven acute rheumatic fever test: the START study protocol. BMJ Open 2021; 11(9): e053720.
| Crossref | Google Scholar | PubMed |