Complementary medicines advertising policy Part I: unethical conduct in the Australian market before July 2018
Malcolm Vickers A and Ken Harvey
A and Ken Harvey  A B
A B
A Public Health and Preventive Medicine, Monash University, Level 4, 553 St Kilda Road, Melbourne, Vic. 3004, Australia. Email: mvic0003@student.monash.edu
B Corresponding author. Email: kenneth.harvey@monash.edu
Australian Health Review - https://doi.org/10.1071/AH19267
Submitted: 3 December 2019 Accepted: 31 March 2020 Published online: 19 October 2020
Journal Compilation © AHHA 2020 Open Access CC BY-NC-ND
Abstract
Objective This study assessed the effects of complementary medicines advertising policy before major changes in 2018.
Methods The study consisted of an analysis of Complaints Resolution Panel determinations from 1999 to 2018, Therapeutic Goods Administration (TGA) post-marketing surveillance data of listed products from 2014 to 2018 and a 2018 consumer survey.
Results Over 1999–2018, one company, Pharmacare Laboratories (with its acquisition, Cat Media), repeatedly breached the Therapeutic Goods Advertising Code at a level threefold higher than that of any other company. Determinations of the Panel were ineffective at reducing code breaches. When the Panel referred problems to the TGA, usually no action resulted. Over 2014–18, on average there were 763 breaches of the Therapeutic Goods Advertising Code per year, most commonly because claims were misleading, unverifiable or exaggerated efficacy. Over the same period, TGA post-marketing surveillance reviewed, on average, 289 listed products each year; 77% were found to be non-compliant, primarily because of an inability to substantiate the claims made. Only 15% of 684 knowledgeable consumers surveyed agreed that complementary medicines were appropriately regulated.
Conclusions Numerous complementary medicines (and medical devices) that were extensively advertised failed to meet real health needs, diverted consumers from more evidence-based treatment and wasted their money. The laws to protect consumers were adequate: the problem was lack of enforcement.
What is known about the topic? The previous co-regulatory system for complementary medicines has been the subject of long-standing criticism; however, definitive data about the problems were largely unanalysed or disregarded.
What does this paper add? This is the first analysis of the Complaints Resolution Panel’s determinations over its entire life (1999–June 2018). The paper provides a baseline from which the outcomes of the new complaint system (after July 2018) can be assessed. At that time, the Panel was abolished and the TGA took over the complaint system, with enhanced investigative and enforcement powers. The analysis shows that most complaints received were upheld by the Panel and a small number of sponsors repeatedly breached the Code. TGA post-marketing data from 2014 to 2018 revealed a high level of regulatory non-compliance by listed products, and a 2018 consumer survey showed low levels of trust in the regulatory system.
What are the implications for practitioners? The failure of the TGA to ensure regulatory compliance by advertisers of complementary medicines (and medical devices) meant that health practitioners and consumers were unlikely to recognise the extent of misleading and deceptive claims in the marketplace. Practitioners rarely have the time or resources to investigate claims themselves. The consequence is that consumers will waste their money on useless products and be diverted from seeking more evidence-based remedies. It remains to be seen whether the new regulatory system will address these problems.
Introduction
In Australia, medicines, including vitamin and minerals, fish oil, Western herbal medicine, Chinese traditional medicine, Ayurveda (Indian) medicines, Indigenous medicines, homeopathic medicines, probiotics and aromatherapy products are referred to as ‘complementary medicines’ (called ‘dietary supplements’ in the US).1 Most complementary medicines are listed products.
With few exceptions, therapeutic goods for sale in Australia must first be listed, registered or included on the Australian Register of Therapeutic Goods (ARTG).2 The relevant legislative instruments are the Therapeutic Goods Act 1989 (the Act), regulations and orders.3 The Therapeutic Goods Administration (TGA) is the responsible authority.4
Listed medicines, labelled ‘AUST L’, may contain only ‘low-risk’ ingredients, which require approval by the TGA and are not subject to an entry in the Poisons Standard. These medicines must be manufactured by a facility with a Good Manufacturing Practice (GMP) licence to ensure product quality. These medicines can make claims (for which the sponsor must hold evidence) only for health maintenance, health enhancement or the alleviation of non-serious, self-limiting conditions. The sponsor must certify that the goods meet all the above requirements, but there is no premarket evaluation by the TGA.5
A similar process applies to ‘low-risk’ medical devices, such as ear candles, detox foot pads and bioresonance machines: the sponsor certifies that the device fulfils its intended purpose. The TGA accepts company assurances that the devices comply with the law.
In contrast, ‘higher-risk’ medicines, including pharmacy-only and prescription medicines, labelled ‘AUST R’, undergo full premarket assessment of quality, safety and efficacy, as do high-risk implantable medical devices.
Advertising of registered prescription medicines to the public is not allowed. Listed medicines, some registered medicines (e.g. over-the-counter (OTC) products) and some medical devices may be advertised, but their promotion must comply with the Therapeutic Goods Advertising Code (the Code), including all its amendments over time.6
The aim of the Code is to ensure that promotion is conducted in a manner that promotes safe and effective use, is socially responsible and does not mislead or deceive the consumer. The target audience for these products is often vulnerable because of limited health literacy and concerns about their health. This can affect a person’s ability to critically evaluate advertising (including labels) and to assess whether a medicine or medical device is appropriate for them.7
The Therapeutic Goods Advertising Complaints Resolution Panel (the Panel) first appeared in legislation in 1997(Therapeutic Goods Regulations Amendment 1997 No. 400 - REG 17 Div3). The Panel was independent from the TGA, with members representing diverse stakeholder organisations: general practitioners, pharmacists, the therapeutic goods industry and consumers. Food Standards Australia New Zealand (FSANZ) and the TGA had observer status. From 1 January 1999 to 30 June 2018, the Panel invited complaints from anyone concerned about advertisements for therapeutic goods in Australia.8
When a complaint was received by the Panel, the adjudication process included contacting the responsible company and requesting a response to the complaint. The complaint and the response were then considered by the Panel, and a determination was published online. In the present study, the authors used this publicly available resource for analysis (https://www.tgacrp.com.au/complaint-register/, accessed 3 August 2020). The Panel also published annual complaint summary documents from 2007 to 2018 (https://www.tgacrp.com.au/, accessed 3 August 2020), as well as decision highlights.9
Instead of making a determination, from 2017 to June 2018 the Panel published the outcome of some complaints as ‘Treated as withdrawn’ or ‘Better dealt with by another authority (referred to TGA)’ (https://www.tgacrp.com.au/withdrawn-complaints/, accessed 3 August 2020). Most of these complaints were referred to the TGA because they were repetitious and involved systemic regulatory issues. Advertisements for foot detox patches and pads were one example. From 2012 onwards the Panel received many complaints about these devices, most of which were then referred to the TGA without a Panel determination being made.
Because the Panel had no power to enforce its own determinations, they made a ‘Recommendation to Secretary’ (for a Regulation 9 order) when an advertiser declined to correct a serious Code breach. These orders provided a mechanism by which the TGA could exercise an array of powers under the Therapeutic Goods Regulations. The outcome of some of these referrals to the TGA has been published.10–12 A glossary of technical terms relating to regulations, regulatory pathways and types of listings is provided in Appendix 1.
The TGA published annual performance statistics from July 2014 to June 2019, including limited post-marketing surveillance data of listed products (https://www.tga.gov.au/annual-performance-statistics-reports, accessed 3 August 2020).
In June–July 2018, the TGA conducted a baseline survey of Australian adults about their awareness of the TGA, its roles and perceived effectiveness.13
In 2018, significant changes were made to the regulation of complementary medicines and the advertising system. These included a new, legally enforceable Code and stronger investigative and compliance powers for the TGA. The changes culminated on 1 July 2018, when the TGA Advertising Compliance Section took over the advertising complaint system and the Panel was abolished.14
This paper provides an analysis of Panel determinations from 1999 to June 2018, the TGA’s post-marketing surveillance data of listed products from 2014 to 2018 and the TGA’s 2018 consumer survey. The aim of the study was to obtain objective data on the effect of pre-June 2018 policy settings highlighting unethical conduct in the complementary medicines market and extracting lessons that may be useful for the new regulatory system.
Methods
The outcome of complaints dealt with by the Panel were published as text fields on its website (https://www.tgacrp.com.au/, accessed 3 August 2020). All complaints upheld by the Panel had the following fields available for analysis: complaint number allocated, date received, product involved, complainant (who could be anonymous), respondent (company advertising the product or other advertiser), Panel finding (justified or not justified) and action recommended (e.g. withdrawal of the advertisement).
Many changes occurred over the 19-year life of the Panel. There were changes to the amount of detail published in determinations, as well as amendments to the Code against which complaints were assessed. In addition, we uncovered inconsistencies in terms used in determinations, such as company names. For this study, we standardised the terms used, including company names. Where a Panel determination named more than one company, the sponsor name was used or, if this was unclear, the name of the first company named in the determination was used.
Text data were extracted and filled into a Microsoft (Bellevue, WA, USA) Excel spreadsheet, maintaining the fields listed above. Some complaint determinations were found to be missing in relation to the published index information. The Panel executive administrator subsequently provided these missing determinations so that a complete record was available for the present analysis.
An analysis of justified complaints by company was conducted over the entire 19-year life of the Panel. An analysis of common code breaches and the products involved was conducted for a restricted period (July 2014–June 2018), during which amendments to the Code were minimal.
The Panel produced annual reports from 2007 to 2018 that classified complaints into various product categories. These data were extracted and averaged.
The ARTG was searched to establish the total number of listed products. TGA post-marketing surveillance data of listed products published from July 2015 to June 2019 were extracted and tabulated.
Data relevant to the regulation of complementary medicines were extracted from the 2018 TGA consumer survey.
Results
Analysis of Panel data
Complaints handled
Over its 19-year life, the Panel received 3185 complaints and made 2303 determinations, the difference being due primarily to multiple complainants highlighting the same issue. The 19-year average was 167 complaints received per year. Fewer than 2% of determinations ruled the complaint ‘not justified’. Of the complaints received, 755 were referred by the Panel to the TGA.
Justified complaints by sponsor name
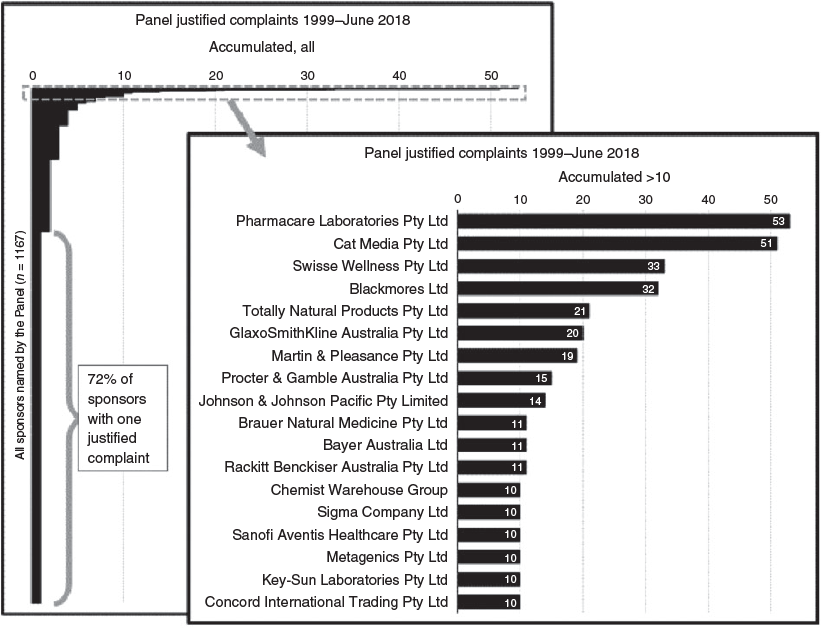
Of the 2303 determinations, 2078 noted a breach of the Code or Regulations; these were termed ‘justified complaints’. The companies acting for the production and advertising of therapeutic goods were described as ‘respondents’ and product ‘sponsors’ by the Panel. In all, 1167 sponsor names were found listed in the justified complaints; 72% of names had one justified complaint. The remaining 28% of sponsors had two or more justified complaints against their name.
Figure 1 ranks 18 sponsor names accumulating more than 10 justified complaints over the life of the Panel. Sponsor ranking could be affected by company name changes, mergers, demergers and acquisitions. An example of inconsistency with the current marketplace was the company Totally Natural Products, currently trading as Caruso’s Natural Health; the company name change occurred in 2012.15 The new company name accumulated seven further justified complaints after the change, although an insufficient number for the new name to appear in Fig. 1.
|
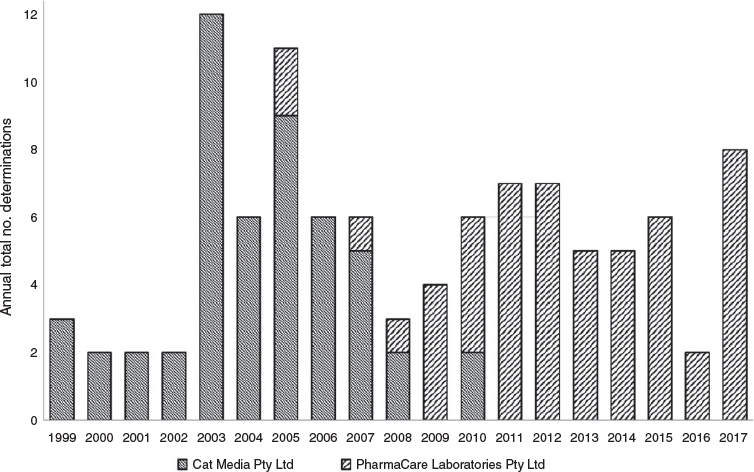
The top two sponsor names (Fig. 1) are currently trading as a single entity, Pharmacare Laboratories Pty Ltd. Pharmacare acquired Cat Media Pty Ltd in 2006.16 Pharmacare now markets products such as FatBlaster Max, FatBlaster Magnet, and Horney Goat Weed, which were previously sponsored by Cat Media before the acquisition.17–19 Together, these companies repeatedly breached the Therapeutic Goods Advertising Code, at a rate threefold higher the level of any other company. Pharmacare currently markets products under 23 brand names,20 including Naturopathica, Nature’s Way, Bioglan, Kids Smart, Sambucol, Promensil and FatBlaster. Fig. 2 shows complaints attributed to Cat Media and Pharmacare by calendar year before and after the acquisition.
Referrals to the TGA
Over the life of the Panel, 755 complaints were found that the Panel referred to the TGA. Of these, 543 were referred as ‘Treated as withdrawn’ or ‘Better dealt with by another authority (referred to TGA)’ because of previous upheld complaints, and 212 were referred as ‘Recommendation to the Secretary’ for a Regulation 9 order.
To quantify the TGA’s actioning of all Panel referrals, TGA outcomes arising from all referrals were investigated. The TGA published 66 such outcomes.10,11 Each included the Panel determination identification number, allowing comparison with Panel determinations. The 66 TGA outcomes consisted of 36 ‘Regulation 9 Orders’ published by the TGA within a 3-year time period (2011–14).10,11 An additional 30 complaints referred for ‘Regulation 9 Orders’ between 2015 and 2016 were closed by other means, such as negotiation with the advertiser or sponsor-initiated removal of the ARTG listing.12 We could not find any published TGA outcomes arising from Panel ‘Treated as withdrawn’ or ‘Better dealt with by another authority (referred to TGA)’. The TGA was contacted regarding the paucity of published outcomes, but no further information was provided.
In summary, the level of enforcement action arising from Panel to TGA referrals could not be accurately determined. However, the overall response rate appeared to be around 9% (755 Panel referrals; 66 TGA outcomes).
Common code breaches
Data used in this analysis were limited to the final 4 years of operation of the Panel (1 July 2014–30 June 2018). This was because the 2015 Code was operational over most of this time and there were substantial changes between the 2015 Code and its 2007 predecessor.
Of 454 justified complaint determinations made within the 4-year period, 86% included a breach of Code 4(2)(c), which means the advertisement was found to be misleading. The next most common breach was of Code 4(1)(b), the advertisement was unverifiable, found in 81% of determinations. Further, 78% of determinations contained exaggeration of efficacy (breach of Code 4(2)(a)) and 47% included a claim to treat a serious disease (breach of Code 5(2)). Each justified complaint often included multiple code breaches. The rate of individual code breaches found by the Panel averaged 763 per year over this 4-year period.
Product types associated with justified complaints
The Panel published 11 annual reports from 2007 to 2017–18 (https://www.tgacrp.com.au/, accessed 3 August 2020). These annual reports listed complaints by product category (one complaint could include multiple products recorded in more than one category). Over this time, complementary medicines, on average, accounted for 50% of the complaints, followed by medical devices (32%), Schedule 4 (prescription only) medicines (9%) and OTC medicines (4%). Food, cosmetics and other products not specified by the Panel accounted for the remaining 5%.
From the Panel determinations data, product name fields were analysed to identify common product types complained about. Weight loss, detox products and ear candles were the most common products complained about.
TGA post-marketing review data
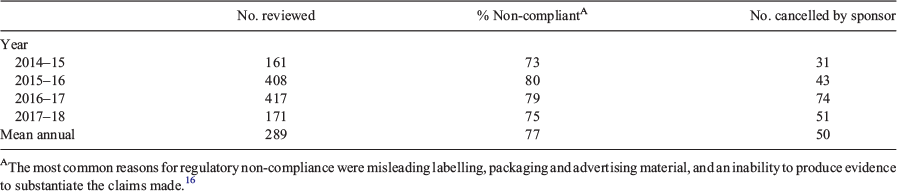
At the time of writing (November 2019), 11 126 listed medicines were found on the ARTG. Table 1 shows the number reviewed by the TGA from 2015 to 2018, the percentage non-compliant with regulatory requirements and the number cancelled by the sponsor when informed the product had been selected for a review.
|
TGA 2018 consumer survey
The survey involved a dual sampling methodology resulting in two separate samples.13 In all, 1729 responses to the survey were recorded during the June–July 2018 survey period. The results are summarised in Table 2, they broadly indicate low-level trust in the TGA’s activities. For example, of the opt-in (expert and consumer group) sample, 15% of respondents agreed that complementary medicines were appropriately regulated, most respondents chose to disagree with the TGA statement or had no opinion.

|
Discussion
The present analysis was limited by significant amendments to the Code, changing procedures and understandable human error that occurred over the 19-year history of the Panel. To determine justified complaints by company and referrals to the TGA, we were able to use the entire dataset. To determine common code breaches, we used a recent time period (July 2014–June 2018), during which amendments to the Code were minimal and detailed complaint determinations were published by the Panel. We identified and corrected many variations in the names assigned to products and companies, and the Panel secretariat helped us retrieve clearly missing data. However, we cannot assert that all these problems were eliminated.
The large number of complaints received by the Panel, and the finding that almost all were upheld, showed that consumers were subjected to a high level of unethical advertising of therapeutic goods, primarily complementary medicines. The most common problems found by the Panel were misleading, unverifiable and exaggerated advertising claims.
A small number of companies breached the Code (and the law) repeatedly and were likely to have gained undeserved market advantage by doing so. Together, one company (Cat Media) and the company that acquired it (Pharmacare) accumulated more than threefold the number of justified complaints of any other repeat offender.
It was apparent that the educational approach taken by the Panel (i.e. the publication of determinations and decision highlights) was ineffective in changing the behaviour of recalcitrant companies. Furthermore, there was little evidence that the TGA enforced determinations referred from the Panel. This may be a contributing factor to the overall poor performance shown in the post-market reviews.
Consumers motivated to lose weight have been the target of many advertisements that breach the law. These products exploit vulnerable, overweight consumers. This problem was highlighted 11 years ago;21 it (and many other problems) have yet to be addressed by the TGA.
The failure of the TGA to ensure regulatory compliance by advertisers of complementary medicines (and medical devices) has resulted in loss of trust of the TGA as a regulator, as shown by the 2018 consumer survey. It also means that health professionals and consumers are unlikely to recognise the extent of misleading and deceptive claims in the marketplace. Practitioners, let alone consumers, rarely have the time or resources to investigate advertising claims themselves. The consequence is that consumers will waste their money on useless products and be diverted from seeking more evidence-based remedies, to the detriment of individual and overall public health.
The 2018 changes to the regulation of complementary medicines and the advertising system included a new legally enforceable Code and stronger investigative and compliance powers for the TGA. A companion paper evaluates the effect of the first 12 months of the new therapeutic goods advertising system.22
Competing interests
Mal Vickers is a member and Ken Harvey is President of Friends of Science in Medicine. Ken Harvey represented Choice (Australian Consumers Association) on the old Therapeutic Goods Advertising Code Council and Complaint Resolution Panel and continues to represent Choice on the new Therapeutic Goods Advertising Consultative Committee.
Acknowledgements
The authors thank Judith Brimer (Executive Officer), Josie Barisic (Executive Administrator) and Allan Asher (Chair) of the Complaints Resolution Panel (CRP) for their encouragement and assistance. In addition, without the voluntary assistance of Dane Lewis and Mathew Bishop (IT experts who converted data on the CRP website into Excel format), this analysis would not have been possible. Some of the complaints submitted and analysed are based on research by Monash University students. This research did not receive any specific funding.
References
[1] Therapeutics Goods Administration. An overview of the regulation of complementary medicines in Australia. Therapeutic Goods Administration. 2013. Available at: https://www.tga.gov.au/overview-regulation-complementary-medicines-australia [verified 17 November 2019].[2] Therapeutics Goods Administration. Guidance on conducting clinical trials in Australia using ‘unapproved’ therapeutic goods. 2018. Available at: https://www.tga.gov.au/book-page/advertising-unapproved-therapeutic-goods [verified 2 August 2020].
[3] Therapeutic Goods Administration. Legislation & legislative instruments. 2019. Available at: https://www.tga.gov.au/legislation-legislative-instruments [verified 17 November 2019].
[4] Therapeutic Goods Administration. About the TGA. 2019. Available at: https://www.tga.gov.au/about-tga [verified 17 November 2019].
[5] Therapeutics Goods Administration. Complementary medicine regulation basics. 2019. Available at: https://www.tga.gov.au/complementary-medicine-regulation-basics [verified 17 November 2019].
[6] Therapeutics Goods Administration. Therapeutic goods advertising code. 2019. Available at: https://www.tga.gov.au/publication/therapeutic-goods-advertising-code [verified 17 November 2019].
[7] Therapeutics Goods Administration. Why and how advertising of therapeutic goods is regulated. 2018. Available at: https://www.tga.gov.au/why-and-how-advertising-therapeutic-goods-regulated [verified 17 November 2019].
[8] Therapeutic Goods Advertising Complaints Resolution Panel. Complaints to CRP. 2018. Available at: http://www.tgacrp.com.au/complaints-to-crp/ [verified 17 November 2019].
[9] Therapeutic Goods Advertising Complaint Resolution Panel. Highlighted points from recent panel determinations. 2018. Available at: http://www.tgacrp.com.au/decision-highlights/ [verified 17 November 2019].
[10] Therapeutic Goods Administration. Archived decisions in relation to complaints about advertisements. 2018. Available at: https://www.tga.gov.au/archived-decisions-relation-complaints-about-advertisements [verified 17 November 2019].
[11] Therapeutic Goods Administration. Decisions in relation to complaints about advertisements. 2019. Available at: https://www.tga.gov.au/decisions-relation-complaints-about-advertisements [verified 17 November 2019].
[12] Therapeutic Goods Administration. Outcomes of advertising complaints – compliance following TGA intervention. 2018. Available at: https://www.tga.gov.au/ws-rec2sec-index [verified 17 November 2019].
[13] Therapeutic Goods Administration. TGA consumer survey 2018. 2018. Available at: https://www.tga.gov.au/tga-consumer-survey-2018 [verified 17 November 2019].
[14] Therapeutic Goods Administration. Advertising hub. 2019. Available at: https://www.tga.gov.au/advertising-hub [verified 17 November 2019].
[15] Therapeutic Goods Advertising Complaint Resolution Panel. Determination 2012-10-013 FATaway. 2012. Available at: https://www.tgacrp.com.au/complaint-register/?_id=2190 [verified 7 February 2020].
[16] Pharmacare Laboratories Pty Ltd. While the world changed, we change too! 2018. Available at: https://www.pharmacare.com.au/history/ [verified 17 November 2019].
[17] Therapeutic Goods Advertising Complaint Resolution Panel. Determination 6-0503 FatBlaster/FatBlaster Max. 2008. Available at: https://www.tgacrp.com.au/complaint-register/?_id=244 [verified 6 February 2020].
[18] Therapeutic Goods Advertising Complaint Resolution Panel. Determination 2008-05-002 and 2008-08-006 Naturopathica FatBlaster FatMagnet. 2008. Available at: https://www.tgacrp.com.au/complaint-register/?_id=1141 [verified 6 February 2020].
[19] Therapeutic Goods Advertising Complaint Resolution Panel. Determination 2-0602 Horny Goat Weed. 2008. Available at: https://www.tgacrp.com.au/complaint-register/?_id=436 [verified 6 February 2020].
[20] Pharmacare Laboratories Pty Ltd. Our brands. 2018. Available at: https://www.pharmacare.com.au/our-brands/ [verified 17 November 2019].
[21] Harvey KJ, Korczak VS, Marron LJ, Newgreen DB. Commercialism, choice and consumer protection: regulation of complementary medicines in Australia. Med J Aust 2008; 188 21–5.
| 18205557PubMed |
[22] Harvey KJ, Vickers M, Arnold BB. Complementary medicines advertising policy Part II: unethical conduct in the Australian market after July 2018. Aust Health Rev 2020;
| Complementary medicines advertising policy Part II: unethical conduct in the Australian market after July 2018.Crossref | GoogleScholarGoogle Scholar |
Appendix 1. Glossary of technical terms relating to regulatory bodies, regulations, regulatory actions, medicines and types of listings

|