General practitioner management of chronic diseases in adults with severe mental illness: a community intervention trial
Cate M. Cameron A B F , Jose Cumsille Nazar B , Carolyn Ehrlich A B , Elizabeth Kendall A B , David Crompton B C D , Ann Maree Liddy E and Steve Kisely C DA Menzies Health Institute Queensland, Griffith University, Meadowbrook, Qld 4131, Australia. Email: c.ehrlich@griffith.edu.au; e.kendall@griffith.edu.au
B School of Health Services and Social Work, Griffith University, Meadowbrook, Qld 4131, Australia. Email: j.cumsillenazar@griffith.edu.au
C School of Medicine, The University of Queensland, Princess Alexandra Hospital, Woolloongabba, Qld 4102, Australia. Email: s.kisely@uq.edu.au
D Metro South Addiction and Mental Health Service, PO Box 6046, Upper Mt Gravatt, Qld 4122, Australia. Email: David.Crompton@health.qld.gov.au
E General Practice Queensland t/a CheckUP Australia, PO Box 3205, South Brisbane, Qld 4101, Australia. Email: aliddy@checkup.org.au
F Corresponding author. Email: cate.cameron@griffith.edu.au
Australian Health Review 41(6) 665-671 https://doi.org/10.1071/AH16151
Submitted: 8 July 2016 Accepted: 11 November 2016 Published: 16 December 2016
Journal compilation © AHHA 2017 Open Access CC BY-NC-ND
Abstract
Objective The aim of the present study was to assess the effects of a community intervention aimed at general practitioners (GPs) by comparing Medicare claims data from patients with severe mental illness (SMI) of GPs exposed to the intervention and controls that were not.
Methods A comparison was made of primary care consultation and pathology data of people with SMI from intervention and control areas. Negative binomial regression models were used to compare the frequency and length of GP consultations, as well as the number and type of pathology examinations.
Results Records of 103 people from intervention area and 98 controls were obtained. Intervention and control areas were not different at baseline in terms of age and claims data, but females had higher consultation rates. After adjusting for gender, people from intervention areas had more GP consultations, especially long consultations (adjusted incidence rate ratio 1.56; 95% confidence interval 1.28–1.91). They also had more pathology screening for chronic diseases, in accordance with implemented guideline recommendations. These benefits persisted after the end of the intervention.
Conclusion These findings suggest that the ACTIVATE program aimed at training GPs to screen and better manage chronic diseases in adults with SMI had a positive effect up to 6 months after the trial, with demonstrated desired changes in medical management practices by GPs in the intervention area during that time.
What is known about the topic? People with an SMI have higher mortality and poorer physical health than the general population.
What does this paper add? The community intervention had a significant and sustained effect, with demonstrated desired changes in screening and medical management by GPs for adults with SMI in the intervention area.
What are the implications for practitioners? GPs are ideally placed to assist in the prevention and better management of health conditions, thereby reducing avoidable illness and deaths in vulnerable populations, such as adults with SMI. Ongoing professional training and dissemination of clinical guidelines are critical for raising awareness about the physical and oral health care needs of people with SMI.
Additional keyword: health services research.
Introduction
People with a severe mental illness (SMI) have greater morbidity and mortality from chronic physical illnesses, such as metabolic, cardiovascular and musculoskeletal conditions, than the general population.1–5 Higher mortality is frequently associated with ‘lifestyle choices’, such as smoking, poor diet, physical inactivity, substance misuse and abuse and unsafe sexual practices.3,6 However, even after adjusting for behavioural risk factors, mortality remains high for this population.7,8 There are many contributory factors, the majority of which point to the complex interactions between SMI, the social support systems in which these people live and the health care they receive.9–11
For example, physical ill health goes unrecognised and is under-diagnosed among those with SMI by both psychiatrists and general practitioners (GPs).12–15 This is because GPs do not feel well equipped to deal with people with SMI, whereas psychiatrists tend to underestimate physical health complaints and nurses in mental health settings tend to undervalue clinical physical care.16 Even if physical health problems are diagnosed, this population may be less likely to receive or adhere to adequate treatment.17,18 For example, people with SMI are less likely to receive appropriate medications, such as beta-blockers and statins following myocardial infarction,17 even though their physician consultation rates are generally high.14,19,20 Although patient-based factors may be a partial explanation for these findings, it is also likely that the attitudes of health professionals, inefficient organisation of health services and social stigma are also contributing factors.21 Thus, poor access, an absence of integration between elements of the system and poor quality care all contribute to the poor physical health of this population.22
The implementation of guidelines to improve care can only work if health professionals take responsibility for monitoring the health of this population.16,23 In the present study, we investigated whether a community intervention aimed at GPs (ACTIVATE), changed the medical management and screening of chronic diseases in adults with an SMI.
Methods
Study design
The present study was a non-randomised, community intervention.
Study population and setting
The sampling frame included all people with SMIs from two adjacent health districts, Metro South Area Health Service and West Moreton Area Health Service, in South East Qld, Australia. Metro South covers an area of 3586 km2 with a total population of approximately 1 million.24 West Moreton covers an area more than 13 591 km2 with a total population of approximately 260 000.25 Both areas are culturally diverse, with 28.5% of the population in Metro South and 16.9% in West Moreton born overseas.
Community intervention
The ACTIVATE: Mind and Body program was designed to raise awareness in the public mental health, primary care and non-governmental sectors about the physical and oral health care needs of people with SMI.26 ACTIVATE was a joint initiative of Queensland Health and General Practice Queensland that was introduced in December 2011 and included the following: (1) distribution of care guidelines for managing physical comorbidities to GP clinics and public mental health facilities, including the use of brief interventions and motivational interviewing;27 (2) the development of a website for both health professionals and community members; and (3) the development of strategies so that mental health clinicians actively linked people with SMI to general practices (e.g. key performance indicators of the number of people for whom GP details were recorded). General Practice Queensland undertook the introduction of the guidelines across primary care, including academic detailing to a total 41 of 249 practices in the district. In parallel, guidelines for mental health clinicians were implemented across Metro South, and people with SMI were actively connected to GPs. Implementation largely occurred in late 2011 and the beginning of 2012. West Moreton was chosen as the comparison area because it had a similar population and healthcare delivery structures and was geographically well located to undertake data collection.
Participants
Participants were adults with SMI who attended community-based public mental health services for regular administration of either depot medication or clozapine, in either district. Clinicians identified people they believed had the cognitive capacity to understand and to consent to inclusion in the study. Researchers explained the research to potential participants and provided participants with information and consent forms. All participants were offered an A$20.00 shopping voucher in recognition of the time associated with engagement in the research.
Ethics approval
Ethics approvals were received from the three relevant human research ethics committees: Griffith University, University of Queensland and Metro South Health Service District.
Data sources
Participants consented to retrospective and prospective data linkage for extraction of data from Medicare Australia, including the Medical Benefits Schedule (MBS) and Pharmaceutical Benefits Schedule (PBS). The pre-trial and trial period was from 1 December 2009 to 31 December 2012, whereas the post-trial period was from 1 January 2013 to 30 June 2013, inclusive.
Variables
Demographic factors
Participant age and gender were the available demographic factors. Dates of birth were used to calculate age in years at the time of the retrospective pre-trial period.
Outcome measures
GP consultations were obtained using MBS item numbers 3–51, 193, 195, 197, 199, 597, 599, 2497–2559 and 5000–5067, as well as GP Mental Health Treatment item codes 2700–2717.28 Consultations were then dichotomised as short visits (Levels A and B: consultation <20 min) or long visits (Levels C and D: consultation ≥20 min, including after hours and home visits).28 Total cost to the patient (out-of-pocket) and cost to the Federal Government (benefits paid) were calculated for the intervention and control areas. MBS data on pathology tests, with the potential for chronic disease screening or monitoring outlined in the ACTIVATE program,26 were included for analysis. Items were categorised into six groups: cardiac enzymes or marker (Items 66518, 66519); electrolytes (Item 66509); full blood examination (FBE) or coagulation (COAG) studies (Items 65070, 65129); liver function tests (LFTs) and urea, electrolytes, creatinine (Item 66512); lipid studies (Item 66503); and syphilis serology (Item 69387).28
Statistical analysis
The significance of differences in demographic characteristics (age and gender) between intervention and control areas was assessed using the Chi-squared test for categorical data and t-tests for continuous data. Claims data were collapsed into monthly counts and rates calculated as the mean number of claims per 100 people, per month. Mean monthly rates and type of pathology claims were compared between the intervention and control areas. Costs to the patient and Federal Government were based on the mean monthly sum of benefits paid and out-of-pocket patient costs for GP consultations and pathology. Regression models were used to compare the frequency and length of GP consultations and, where cell sizes permitted, types of pathology claims. Based on the likelihood ratio test and the goodness of fit criteria, negative binomial regression was the best model for the MBS and pathology count data. All tests were two-sided with a 5% level of significance.
Results
The intervention area (Metro South) enrolled 103 participants and the control area (West Moreton) enrolled 98 participants. Medicare data for participants from both the intervention and control areas included a total of 24 075 claims between 1 December 2009 and 30 June 2013, inclusive, with 53.3% of the claims derived from the intervention area and 46.7% from the control area.
Demographic characteristics and baseline claims data
The proportion of males and females in the intervention and control areas differed significantly (P < 0.05). The intervention area contained fewer males than the control area (63.1% vs 78.6% respectively). However, there was no significant difference in the age of participants in either area (P > 0.05; range 14–65 years). Using a 3-month baseline pre-trial period, there was no difference in the mean monthly rate of MBS claims between the intervention and control areas, for total claims or GP-specific claims (overall, short or long consultations; P > 0.05; Table 1).
Follow-up
Data were examined to confirm completeness of participant follow-up during the total period. With the exception of one participant (0.5%), all participants were confirmed as present and active in the dataset with at least one MBS or PBS claim in the post-trial period, or after.
GP claims
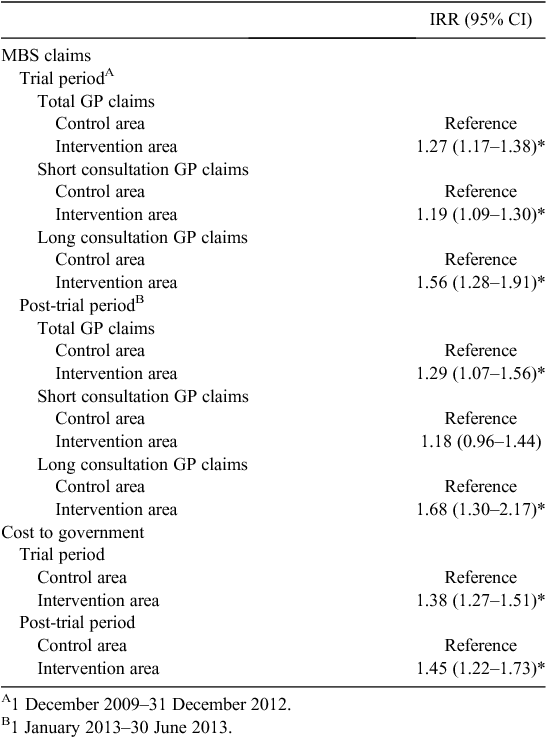
During the trial period there was a total of 4901 GP claims from both the intervention and control areas, with 85.1% regular or short consultations and 10.6% long consultations or home visits. For 214 GP claims (4.3%), the consultation length was unable to be determined. The intervention area had greater average monthly rates of claims for short, long and total GP consultations than the control area during the trial period (P < 0.05; Table 2). This was reflected in higher mean monthly costs to government for benefits paid for all GP claims during the trial period (Table 2). All participants were eligible for concession cards, with 99.1% of GP claims bulk billed; therefore the out-of-pocket expenses for participants were negligible.

|
Regardless of area, females were found to have the greatest service use with higher average monthly GP claims rates compared with males. The greatest difference was found for long consultations, with females having almost twice the male ‘long consultation’ claims rates, in both areas. Males in the intervention area also had more GP claims and long consultations per month compared with the control area, but there was no difference in the rate of short consultations during the trial period (P > 0.05). After the trial, females continued to account for the greatest difference in claims for all GP visits, short and long, as well as costs to government when comparing the areas (P < 0.05; Table 2). By contrast, there was no longer a significant difference in the claims rates for males for any of the GP claim types or cost to government.
After adjusting for gender, the intervention area had 1.27-fold the rate of GP claims when comparing mean monthly claims rates over the trial period (95% confidence interval (CI) 1.17–1.38; Table 3). The intervention area had 1.19-fold the adjusted mean rate of short consultations (95% CI 1.09–1.30) and 1.56-fold the adjusted mean rate of long consultations (95% CI 1.28–1.91) during the community trial period. The total cost to the Federal Government for GP claims in the intervention area was 1.38-fold that of the control area (95% CI 1.27–1.51).
|
After the trial period, the adjusted claims data demonstrated ongoing differences, with the intervention area showing continued increased mean monthly rates for total GP claims, particularly for long consultations, and total costs to government (P < 0.05; Table 3). However, there was no difference in the mean monthly short consultations. By 30 June 2013, 6 months after the end of the community trial and evaluation period, there was no longer any significant difference between the intervention and control areas on any of the claims measures (P > 0.05; data not shown).
Pathology claims
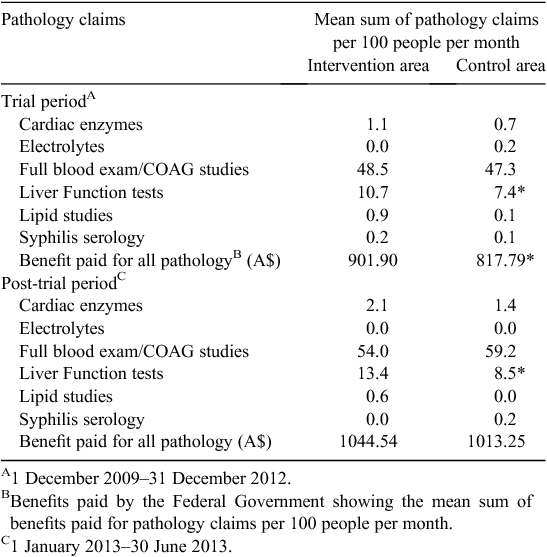
There was a total of 5206 pathology claims during the total study period. Two pathology claims groups, namely FBE or COAG studies and LFTs, accounted for the majority of pathology claims in the present study (81.6% and 15.5% respectively). The remaining 2.9% of pathology tests were scattered across the other four categories.
Although the intervention area had had higher mean monthly claims for most of the pathology subgroups and for total claims during the community trial period, only the two largest subgroups had sufficient numbers for regression analyses (Table 4). After adjusting for gender, there was no significant difference in the rates of claims for FBE or COAG studies between the intervention and control areas for either the trial period or the post-trial period (data not shown). However, the intervention area had significantly more claims submitted for LFTs during both the trial and post-trial periods compared with the control area (trial adjusted incidence rate ratio (IRR) = 1.45 (95% CI 1.22–1.72); post-trial adjusted IRR = 1.61 (95% CI 1.03–2.52)). More LFTs and FBE or COAG studies were conducted on females overall (P < 0.05). During the trial period, the intervention area had significantly higher pathology costs per month compared with the control area (P < 0.05), but this cost difference was not sustained in the post-trial period (Table 4).
|
Discussion
GPs are ideally placed to assist in the prevention and better management of health conditions, thereby reducing avoidable illness and deaths in patients with SMI. However, communication difficulties, social distance and the overall poor quality of interactions between healthcare providers can act as barriers to healthcare for people with SMI. In addition, diagnostic overshadowing, being the over-attribution of symptoms to an underlying psychiatric condition, results in missed diagnoses and the improper management of physical comorbidities.29
The aim of the present study was to assess whether the implementation of clinical guidelines for GPs as part of a wider community intervention changed the management and screening of chronic diseases in adults with an SMI. The project consisted of several coordinated activities for GPs with a focus on prevention, early detection and intervention for physical and oral health conditions in this population. Resources included care and clinical guidelines, information handbooks, brief intervention training and website materials. Two particular geographic areas, one with intensive, focused intervention activities and a second area with more limited activities, were evaluated to assess differences in GP service use and pathology claims by patients with SMI before, during and after the trial.
The intervention area showed a significant increase in the number and length of GP consultations for patients with an SMI compared with the control area, even after controlling for gender differences. More frequent and longer consultations suggested GPs were engaging more often and for longer periods with their patients with SMI. Although information on the content of the consultations was not available, the longer consultation length combined with the increase in pathology screening for chronic diseases, in accordance with the implemented guideline recommendations and the ACTIVATE interventions, suggest that the program had the desired changes in the medical practice of GPs in the intervention area. For example, we were able to show a significant increase in the use of LFTs. This an important investigation because atypical antipsychotics are associated with increased liver enzymes in up to one-third of patients after 6 months of treatment.30
Females had higher overall GP claims and, following ACTIVATE, females also had a more sustained increase in claims in the intervention area. Gender-based differences in health seeking are well known, with females consistently using health services more, regardless of age (over and above obstetric reasons), than males.31–33 Combined with an SMI, males are at particular risk of increased morbidity and mortality. Although initial results suggested increased GP contact by males during the trial period, this was not maintained after the trial period as it was with female patients with SMI. Therefore, strategies for engaging with males and fostering ongoing primary care contact are critical for improving health outcomes in this patient group.
Approximately 6 months after the trial, claims in the two areas converged. This either indicated that changes in practice were not sustained long-term, or more likely that as this was a defined cohort of participants, they did not need ongoing pathology and longer consultations once screening and increased GP management had occurred. Although increased consultation and investigation rates resulted in greater initial costs, it is possible that the early detection and management of chronic diseases has the potential to reduce the longer-term costs of untreated and preventable chronic diseases in this vulnerable population.
Study limitations include a lack of more detailed demographic data, for which we could control in the regression analyses. Although none of the baseline GP claims rates, overall or for short or long consultations, demonstrated a difference by group, suggesting that any baseline claims bias in this study is likely to be minimal, caution is warranted. We were only able to measure changes in billing, not health improvement. However, claims data are objective and not subject to recall or other common biases. Checks of the post-trial claims data available demonstrated that only one of 201 participants was unaccounted for in the datasets. This person may have either moved out of Australia or have died.
In conclusion, the findings of the present study suggest that the ACTIVATE program that encouraged GPs to screen and better manage chronic diseases in adults with an SMI had a positive effect on medical management in the intervention area. Early detection and management of chronic diseases has the potential to reduce long-term illness and associated healthcare costs in vulnerable populations, such as adults with SMI.
Competing interests
None declared.
Acknowledgements
This research was supported by an Australian Research Council Linkage grant (LP110200261). Cate M. Cameron was supported by a Public Health Fellowship (ID 428254) from the National Health and Medical Research Council of Australia.
References
[1] Kisely S, Smith M, Lawrence D, Maaten S. Mortality in individuals who have had psychiatric treatment: population-based study in Nova Scotia. Br J Psychiatry 2005; 187 552–8.| Mortality in individuals who have had psychiatric treatment: population-based study in Nova Scotia.Crossref | GoogleScholarGoogle Scholar |
[2] Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, Lieberman JA. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86 15–22.
| Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline.Crossref | GoogleScholarGoogle Scholar |
[3] De Hert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, Detraux J, Gautam S, Möller H-J, Ndetei DM, Newcomer JW, Uwakwe R, Leucht S. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011; 10 52–77.
| Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care.Crossref | GoogleScholarGoogle Scholar |
[4] Lawrence D, Kisely S. Inequalities in healthcare provision for people with severe mental illness. J Psychopharmacol 2010; 24 61–8.
| Inequalities in healthcare provision for people with severe mental illness.Crossref | GoogleScholarGoogle Scholar |
[5] Kisely S, Baghaie H, Lalloo R, Siskind D, Johnson NW. A systematic review and meta-analysis of the association between poor oral health and severe mental illness. Psychosom Med 2015; 77 83–92.
| A systematic review and meta-analysis of the association between poor oral health and severe mental illness.Crossref | GoogleScholarGoogle Scholar |
[6] Robson D, Gray R. Serious mental illness and physical health problems: a discussion paper. Int J Nurs Stud 2007; 44 457–66.
| Serious mental illness and physical health problems: a discussion paper.Crossref | GoogleScholarGoogle Scholar |
[7] Hamer M, Stamatakis E, Steptoe A. Psychiatric hospital admissions, behavioral risk factors, and all-cause mortality: the Scottish health survey. Arch Intern Med 2008; 168 2474–9.
| Psychiatric hospital admissions, behavioral risk factors, and all-cause mortality: the Scottish health survey.Crossref | GoogleScholarGoogle Scholar |
[8] Happell B, Gaskin CJ, Stanton R. Addressing the physical health of people with serious mental illness: a potential solution for an enduring problem. Int J Soc Psychiatry 2016; 62 201–2.
| Addressing the physical health of people with serious mental illness: a potential solution for an enduring problem.Crossref | GoogleScholarGoogle Scholar |
[9] Black DW. Iowa record-linkage study: death rates in psychiatric patients. J Affect Disord 1998; 50 277–82.
| Iowa record-linkage study: death rates in psychiatric patients.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1M%2FntlOqug%3D%3D&md5=f756b1008f6e1581b9faee519d6aee0aCAS |
[10] Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000; 177 212–7.
| Causes of the excess mortality of schizophrenia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3cvptVOqsQ%3D%3D&md5=7736e1a2069be75d78194e0f4382b082CAS |
[11] Crisanti AS, Love EJ. Mortality among involuntarily admitted psychiatric patients: a survival analysis. Soc Psychiatry Psychiatr Epidemiol 1999; 34 627–33.
| Mortality among involuntarily admitted psychiatric patients: a survival analysis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7msl2itQ%3D%3D&md5=ddc07160e001af73e1a31c523b7b4539CAS |
[12] Bernardo M, Canas F, Banegas JR, Casademont J, Risego Y, Varela C, on behalf of the RICAVA Study Group Prevalence and awareness of cardiovascular risk factors in patients with schizophrenia: a cross-sectional study in a low cardiovascular disease risk geographical area. Eur Psychiatry 2009; 24 431–41.
| Prevalence and awareness of cardiovascular risk factors in patients with schizophrenia: a cross-sectional study in a low cardiovascular disease risk geographical area.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mjnt1ygsA%3D%3D&md5=dd1612231231afb277f3eb9e92d6bb69CAS |
[13] Muir-Cochrane E. Medical co-morbidity risk factors and barriers to care for people with schizophrenia. J Psychiatr Ment Health Nurs 2006; 13 447–52.
| Medical co-morbidity risk factors and barriers to care for people with schizophrenia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28vksFSitA%3D%3D&md5=c442e5630c9c4e8d210884c22d725103CAS |
[14] Phelan M, Stradins L, Morrison S. Physical health of people with severe mental illness. BMJ 2001; 322 443–4.
| Physical health of people with severe mental illness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7otlylsg%3D%3D&md5=ad02df555db9fb0bf7ff687b220ce3e2CAS |
[15] Osborn DP. The poor physical health of people with mental illness. West J Med 2001; 175 329–32.
| The poor physical health of people with mental illness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3Mnkt1Sltg%3D%3D&md5=cfacaa04b32fc7847ea5e6402db915bcCAS |
[16] Nitkin DI, Gastaldo D. Addressing physical health problems experienced by people with schizophrenia in Canada: a critical literature review. Can J Nurs Res 2010; 42 124–40.
[17] Kisely S, Campbell LA, Wang Y. Treatment of ischaemic heart disease and stroke in individuals with psychosis under universal healthcare. Br J Psychiatry 2009; 195 545–50.
| Treatment of ischaemic heart disease and stroke in individuals with psychosis under universal healthcare.Crossref | GoogleScholarGoogle Scholar |
[18] Kisely S, Smith M, Lawrence D, Cox M, Campbell LA, Maaten S. Inequitable access for mentally ill patients to some medically necessary procedures. CMAJ 2007; 176 779–84.
| Inequitable access for mentally ill patients to some medically necessary procedures.Crossref | GoogleScholarGoogle Scholar |
[19] Mai Q, Holman CD, Sanfilippo FM, Emery JD, Stewart LM. Do users of mental health services lack access to general practitioner services? Med J Aust 2010; 192 501–6.
[20] Morgan VA, Waterreus A, Jablensky A, et al People living with psychotic illness in 2010: the second Australian national survey of psychosis. Aust N Z J Psychiatry 2012; 46 735–52.
| People living with psychotic illness in 2010: the second Australian national survey of psychosis.Crossref | GoogleScholarGoogle Scholar |
[21] Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand 2007; 116 317–33.
| Physical illness and schizophrenia: a review of the literature.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2srpvFWrsQ%3D%3D&md5=3fb63f3d4b19c1cca7a1187b44562340CAS |
[22] Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics 2010; 51 458–65.
| The impact of serious mental illness on health and healthcare.Crossref | GoogleScholarGoogle Scholar |
[23] Goff DC, Cather C, Evins AE, et al Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry 2005; 66 183–94.
| Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists.Crossref | GoogleScholarGoogle Scholar |
[24] Queensland Health Metro South Hospital and Health Service: 2013–14 Annual Report. Brisbane: Queensland Health; 2014.
[25] Queensland Health . West Moreton Hospital and Health Service Annual Report 2014–2015. Brisbane: Queensland Health; 2015.
[26] Health General Practice Queensland and Queensland Health ACTIVATE: mind and body handbook for the physical and oral health management of people with severe mental illness. 2010. Available from: www.activatemindandbody.com.au [verified 25 November 2016].
[27] Dosh SA, Holtrop JS, Torres T, Arnold AK, Baumann J, White LL. Changing organizational constructs into functional tools: an assessment of the 5 A’s in primary care practices. Ann Fam Med 2005; 3 S50–2.
| Changing organizational constructs into functional tools: an assessment of the 5 A’s in primary care practices.Crossref | GoogleScholarGoogle Scholar |
[28] Australian Government, Department of Health. MBS online: Medicare benefits schedule. The July 2014 Medicare Benefits Schedule. 2014. Available at: http://www.health.gov.au/internet/mbsonline/publishing.nsf/Content/Downloads-201407 [verified 9 April 2015].
[29] Pelletier JF, Lesage A, Boisvert C, Denis F, Bonin JP, Kisely S. Feasibility and acceptability of patient partnership to improve access to primary care for the physical health of patients with severe mental illnesses: an interactive guide. Int J Equity Health 2015; 14 78
| Feasibility and acceptability of patient partnership to improve access to primary care for the physical health of patients with severe mental illnesses: an interactive guide.Crossref | GoogleScholarGoogle Scholar |
[30] Stanley SH, Laugharne JD. Physical health algorithms for mental health care. Aust N Z J Psychiatry 2014; 48 889–94.
| Physical health algorithms for mental health care.Crossref | GoogleScholarGoogle Scholar |
[31] Courtenay WH, Keeling RP. Men, gender, and health: toward an interdisciplinary approach. J Am Coll Health 2000; 48 243–6.
| Men, gender, and health: toward an interdisciplinary approach.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3czit1ajtA%3D%3D&md5=1bd0143af40f883acbe16fbe75caa9c8CAS |
[32] Santosh J, Crampton P. Gender differences in general practice utilisation in New Zealand. J Prim Health Care 2009; 1 261–9.
[33] Smith JA, Braunack-Mayer A, Wittert G. What do we know about men’s help-seeking and health service use? Med J Aust 2006; 184 81–3.



