Effect of post-hospital discharge telephonic intervention on hospital readmissions in a privately insured population in Australia
G. Brent Hamar A E , Carter Coberley B , James E. Pope A , Andrew Cottrill C , Scott Verrall C , Shaun Larkin C and Elizabeth Y. Rula DA Healthways Inc., 701 Cool Springs Boulevard, Franklin, TN 37067, USA.
B 4080 Oxford Glen Drive, Franklin, TN 37067, USA. Email: carter@coberley.com
C Hospitals Contribution Fund of Australia (HCF), Level 6, 403 George Street, Sydney, NSW 2000, Australia. Email: acottrill@hcf.com.au; sverrall@hcf.com.au; slarkin@hcf.com.au
D Tivity Health, 701 Cool Springs Boulevard, Franklin, TN 37067, USA. Email: elizabeth.rula@tivityhealth.com
E Corresponding author. Email: brent.hamar@healthways.com
Australian Health Review 42(3) 241-247 https://doi.org/10.1071/AH16059
Submitted: 3 March 2016 Accepted: 2 February 2017 Published: 10 April 2017
Journal compilation © AHHA 2018 Open Access CC BY-NC-ND
Abstract
Objective The aim of the present study was to evaluate the effect of telephone support after hospital discharge to reduce early hospital readmission among members of the disease management program My Health Guardian (MHG) offered by the Hospitals Contribution Fund of Australia (HCF).
Methods A quasi-experimental retrospective design compared 28-day readmissions of patients with chronic disease between two groups: (1) a treatment group, consisting of MHG program members who participated in a hospital discharge (HODI) call; and (2) a comparison group of non-participating MHG members. Study groups were matched for age, gender, length of stay, index admission diagnoses and prior MHG program exposure. Adjusted incidence rate ratios (IRR) and odds ratios (OR) were estimated using zero-inflated negative binomial and logistic regression models respectively.
Results The treatment group exhibited a 29% lower incidence of 28-day readmissions than the comparison group (adjusted IRR 0.71; 95% confidence interval (CI) 0.59–0.86). The odds of treatment group members being readmitted at least once within 28 days of discharge were 25% lower than the odds for comparison members (adjusted OR 0.75; 95% CI 0.63–0.89). Reduction in readmission incidence was estimated to avoid A$713 730 in cost.
Conclusions The HODI program post-discharge telephonic support to patients recently discharged from a hospital effectively reduced the incidence and odds of hospital 28-day readmission in a diseased population.
What is known about the topic? High readmission rates are a recognised problem in Australia and contribute to the over 600 000 potentially preventable hospitalisations per year.
What does this paper add? The present study is the first study of a scalable intervention delivered to an Australian population with a wide variety of conditions for the purpose of reducing readmissions. The intervention reduced 28-day readmission incidence by 29%.
What are the implications for practitioners? The significant and sizable effect of the intervention support the delivery of telephonic support after hospital discharge as a scalable approach to reduce readmissions.
Introduction
Hospital readmissions are a major burden to healthcare systems in countries around the world, negatively affecting the financial viability of the individual systems, as well as the health and well-being of patients. Readmission rates have not been broadly studied in Australia; however, a recent US study found that 17.9% of recently discharged hospitalised patients had at least one hospital admission and/or emergency department (ED) visit within 30 days.1 In the US Medicare population, approximately 20% return to the hospital within 30 days. Estimates were that only one-tenth of these readmissions were likely planned, whereas the total costs of these unplanned readmissions were over US$17 billion.2 Higher-risk Medicare heart failure, myocardial infarction and pneumonia patients have reported 30-day readmission rates of 24.8%, 19.9% and 18.3% respectively.3
Although there is a dearth of broad Australian-based studies that fully describe the occurrence of readmissions in the Australian delivery system, it is a recognised problem and gap. In commenting on three recent US studies of readmissions, Professor David Ben-Tovim, Director of Flinders Medical Centre’s Clinical Epidemiology and Redesigning Care department, stated that hospital readmission rates are ‘a legitimate source of concern’ and deserve closer examination to minimise their occurrence and the associated burden on hospitals.4 Likewise, Professor Debora Picone, Chief Executive Officer of the Australian Commission on Safety and Quality in Health Care, has added that Australian readmission rates are too high and should be the subject of research.4
Available Australian studies of specific populations have shown that early readmission is common, costly and can be life-threatening. One study of a tertiary-level Australian hospital found that 25% of hospitalised older, acutely ill patients suffered 30-day readmissions and that suboptimal quality of care indicated a potential for prevention.5 Another study of discharged intensive care unit (ICU) patients concluded that early ICU readmission was significantly associated with in-hospital mortality.6 A study of atherothrombotic disease in Western Australia found that 32% of patients experienced a readmission within 24 months of the index admission.7 Further, these readmissions were costly (A$30 million) and accounted for 42% of what the original index admissions cost (A$71 million); more than three-quarters of readmission costs occurred in the first 12 months.7
Inadequate hospital bed capacity and resulting delays in admitting ED patients,8–10 to which readmissions are a contributing factor, are indicative of the increasing burden being placed on the Australian healthcare system. One estimate of bed capacity states that a 62% increase in hospital beds will be required to meet the projected demand of hospital care.11 Numerous studies show that access block and the resulting ED overcrowding and increased wait times are associated with decreased quality and processes of patient care, increased length of hospital stay and increased patient mortality.5,12–15
Reducing the rates of potentially avoidable hospitalisations is a key objective in several current Australian Government healthcare agreements, with the goal of improving patient outcomes, reducing the pressure on Australian hospitals and increasing the health delivery system’s quality and efficiency. These agreements include Australia’s National Health Performance Framework,16 the National Strategic Framework for Aboriginal and Torres Strait Islander Health17 and the Council of Australian Governments (COAG) National Healthcare Agreement.18 Finding effective approaches to avoid readmissions is a logical target of such initiatives given that nearly one-quarter of 30-day readmissions are considered preventable.19
Identified shortcomings that can lead to increased risk of adverse health events and early hospital readmission include deficiencies in the hospital discharge process,20 lack of patient and family education on patient condition and management,21 medication-related issues,22,23 lack of patient discharge readiness24 and discontinuity of care and communication gaps between hospital staff and primary care physicians.23,25,26 Improving upon patient discharge processes, providing better patient and/or caregiver education and promotion of self-management, as well as enhanced transitioning support and coordination of care as the patient moves from the hospital to the home, are recommended approaches to reduce preventable readmissions.27,28
In order to address the recognised problem of readmissions, the Hospitals Contribution Fund of Australia (HCF), Australia’s largest for-purpose (not-for-profit) health insurer, instituted an intervention to provide post-discharge support for members admitted to hospital. The intervention is part of the broader My Health Guardian (MHG) program, a chronic disease management program initiated in 2009 as a long-term strategy to improve the health and well-being of covered members. The purpose of the present study was to evaluate the efficacy of post-discharge telephone support to recently discharged MHG program members in reducing their risk of early hospital readmission. This study is a continuation of a series of outcome studies that have been conducted to evaluate the effectiveness of HCF’s MHG program in improving upon the health outcomes of covered program members.29,30
Methods
Hospital discharge intervention and the MHG program
The MHG program includes a hospital discharge (HODI) intervention to support recently discharged MHG members. The MHG program is a population health and well-being program available free of charge to HCF members with a qualifying chronic condition and provides tailored assistance in managing existing conditions and adopting healthier behaviours. The MHG program provides higher severity members with telephonic support from program clinicians (registered nurses).
MHG members admitted to a hospital are eligible for the HODI intervention, in which a telephone call is attempted as soon as possible after hospital discharge with the goal of providing support during the critical transition period where there is increased risk of avoidable readmission. Discharged members are identified and called using hospital pre-authorisation records, which are requests for HCF coverage information before or during the admission process. These records represent the timeliest data available for identification. Program nurses are trained to use standardised guidelines, along with all available member data and their own clinical judgment, to ensure the member understands discharge instructions from the hospital and schedules follow-up care needed, as well as to help the member with any problems associated with their medical condition and recent admission.
Study design and data overview
A quasi-experimental retrospective cohort study was conducted to evaluate the effect of HODI calls on readmission risk, testing the hypothesis that MHG programs members who receive timely discharge follow-up calls have reduced risk of readmissions within 28 days after discharge compared with MHG program members who did not participate in the HODI program. The HODI intervention commenced operations in January 2010. The evaluation time frame was from 1 January 2010 through 31 August 2014.
Study population
The study population included all MHG members with a pre-authorised admission with exclusion of some International Classifications of Diseases 10th Revision (ICD-10; http://apps.who.int/classifications/icd10/browse/2015/en, accessed 8 April 2015) categories, detailed below. Attempts were made to make HODI calls to everyone in the study population; however, not all calls were successful. Of the study population of admitted patients, the treatment group was drawn from those members who received an HODI intervention call within 14 days of discharge, whereas the comparison group was drawn from those members who were not successfully contacted for an HODI call within 90 days of discharge.
Study eligibility required availability of demographic information and continuous HCF coverage of at least 1 year before the patient’s index admission, the month of their index admission and the 3 months following their index admission. Members under 20 or over 89 years of age were excluded because demographic information was not made available to ensure the anonymity in these lower-frequency age bands. Additional eligibility requirements and matching methodology are detailed below.
Study definitions and data
Data used in conducting this study included HCF plan coverage and demographic records, HCF hospital claims records with associated primary and secondary diagnosis records, HCF pre-authorisation records, MHG program enrolment data and MHG call records. No identifiable member information was included in any data file; data were linked between files using a unique member identification number.
Index admissions were defined at the member level as the first identified hospital claim with an associated pre-authorisation record (‘enquiry date’ occurring within a 10-day window before the admission date) and that occurred between January 2010 and August 2014, with a discharge date no later than 31 August 2014. This allowed for a 3-month claims run-out period in the evaluation of 28-day readmissions. Index admissions were limited to hospital records with a primary diagnosis in one of the following six ICD-10 categories: endocrine, circulatory, respiratory, digestive, musculoskeletal and skin. Index admissions without an associated ICD-10 code or with a primary ICD-10 category considered to be not applicable or less amenable to prevention or mitigation (e.g. special purpose codes, signs and symptoms, pregnancy, injury and poisoning) were excluded from use.
HODI program calls were defined as post-discharge calls that successfully reached the member within 14 days of the discharge date associated with the member’s index admission. These calls were coded as HODI calls in MHG call records.
Readmissions were defined as hospital claims with an admission date occurring between 1 and 28 days of an index admission. Hospital admissions occurring on the same date as the index admission discharge date were excluded because such cases are typically transfers, as opposed to avoidable readmissions. Readmissions were not limited to specific ICD-10 codes.
In addition to using ICD-10 categories to exclude a subset of index admissions from the analysis, all available standardised healthcare coding systems used to more broadly identify diagnoses associated with the index hospital admission record (ICD-9, ICD-10, Commonwealth Medicare Benefits Schedule procedure coding, diagnosis-related groups and hospital payment coding) were used for the purpose of matching and adjusting for disease burden. An additional diagnosis identifier generated by HCF was used on a limited basis when standardised codes were not available on a claim.
Because the present study was a retrospective analysis of a health promotion initiative conducted anonymously on deidentified data, it was outside the scope of requiring ethics board review or informed consent according to Australia’s National Statement on Ethical Conduct in Human Research (2007; https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/e72_ national_statement_may_2015_150514_a.pdf, accessed 1 September 2015), chapter 5.1.22, and the principles outlined in the Declaration of Helsinki.
Study group matching
Propensity score matching (PSM) is a commonly used method to reduce potential confounder bias while developing a matched comparison study group. This two-step procedure first attempts to reduce the multivariate dataset to a scalar ‘propensity score’ measure, followed by using this measure to match potential comparison member candidates to treatment group members. A problem with this method is that a great deal of information can be lost by collapsing all the covariates in the initial step, before treatment and comparison group members are matched. More simply stated, the same propensity score can be achieved with very different sets of covariate values, which can result in dissimilarity in the match with regard to risk of the outcome of interest.
In attempt to address these challenges the present study used a more recent matching method coming into use in quasi-experimental design studies, namely coarsened exact matching (CEM).31–34 CEM enables more comparable evaluation of study groups by creating proportionality among characteristics and factors contributing to the outcome of study through blocking members into distinct strata based on these contributing factors. Evaluation studies conducted by King et al.31 comparing PSM to CEM found CEM to yield estimates of the causal effect with the lowest variance and bias for any sample size.
From the entire eligible population of treatment and comparison index admission records, comparable study groups were created using CEM. Members eligible for the treatment and comparison groups were matched exactly within a non-parametric framework into distinct strata, or subgroups matched with regard to a set of shared characteristics and factors (coarsened variables) associated with the outcome of interest. This matching process is designed to optimally reduce selection bias and variance between study groups while excluding as few cases as possible. This optimal balance minimises bias in the final estimate of the studied treatment effect while allowing for a more generalisable study result.32,33
Matching variables used to create strata in CEM included: (1) age group, categorised into 10-year increments (20–29 through 80–89 years); (2) gender; (3) length of stay for the index admission (0, 1, 2–5, 6–10, 11+ days); (4) a count of the following conditions documented on the index admission (score 0–7): diabetes, coronary artery disease (CAD), heart failure (HF), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), asthma and hypertension; and (5) the number of MHG program calls in the year before the index admission (0, 1, 2, 3–5, 6+).
Because no direct measure of severity was available, the length of stay and condition count variables were used as proxies to balance the two groups on severity. The prior MHG program calls count variable balances the groups on program exposure as well as any selection bias with regard to the likelihood to take program calls.
The matching process results in matched members assigned a weight that is specific to the stratum they are in and representative of the proportion of all study members in the respective stratum. Strata without at least one treatment member and one comparison member were excluded. After strata assignment, CEM-generated weights that account for study group differences with regard to the matching variables were used as covariates in statistical models.34 Study group comparability after CEM was assessed objectively using the L1 metric, a non-parametric measure that quantifies intergroup imbalance by comparing relative frequencies of the two groups across each of the strata.35 Values of L1 close to zero indicate a higher fidelity match with minimal imbalance, whereas an L1 value of 1 indicates complete dissimilarity or disproportionality between the groups.
Statistical analysis
A comparison of study groups with regard to demographic and medical condition characteristics was conducted using independent sample t-tests, Chi-squared tests and Fisher’s exact statistical testing. Zero inflated negative binomial (ZINB) multivariate models were used to estimate intervention effect on 28-day readmissions while adjusting for potential confounders. Independent variables used in all models included a study group indicator (treatment or comparison), CEM weights and condition status (yes/no) for diabetes, CAD, HF, COPD, CKD, asthma, pneumonia, acute myocardial infarction, stroke, hypertension, hip or knee replacement, cancer and depression. Adjusted incidence rate ratios (IRR; relative risk) were produced from ZINB models by taking the exponential of the intervention variable coefficient, using the comparison group as the reference. Multivariate logistic regression models using the same covariates were used to estimate adjusted odds ratios (OR) and 95% confidence intervals (CI) of the intervention effect on the likelihood of the treatment group having at least one 28-day readmission, with the comparison group as the reference.
Unadjusted cost avoidance attributed to the program was estimated by multiplying the number of avoided readmissions in the treatment group by the unadjusted average cost of a readmission for the respective study period. The number of avoided readmissions was estimated by first multiplying the count of treatment members by the unadjusted readmission rate of the comparison group, the product being the estimated number of readmissions in the treatment group in the absence of intervention. This product was then multiplied by the estimated reduction in relative risk in the treatment group relative to the comparison group to estimate avoided readmissions in the 28-day evaluation period. Average readmission cost was estimated by taking the mean cost of all study readmission claims (both study groups). All data manipulation and analyses were performed using SAS version 9.2 (SAS Institute Inc.).
Results
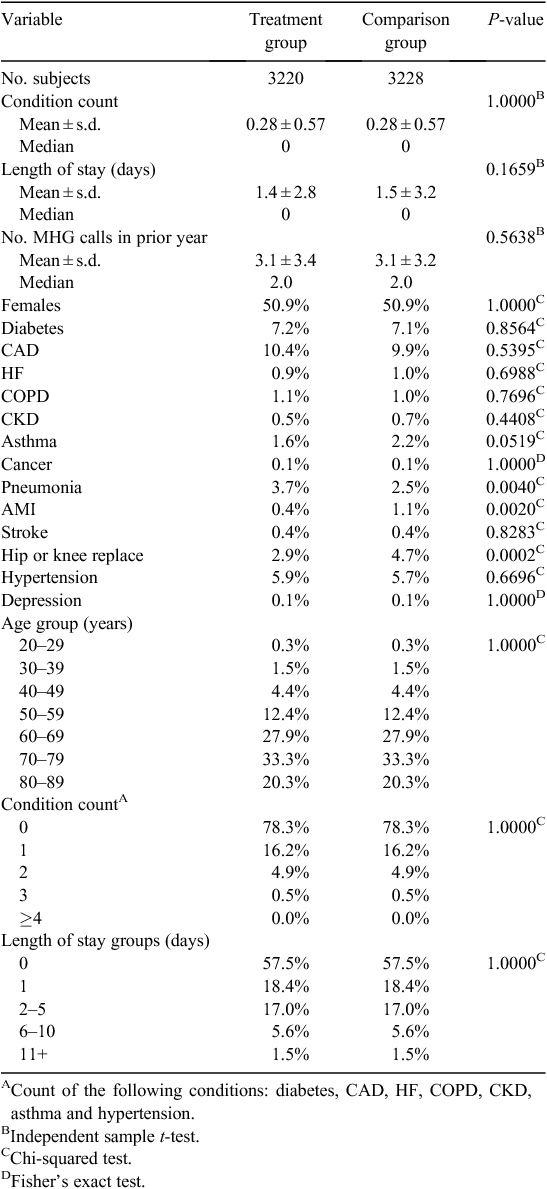
Table 1 lists the size of study groups before and after CEM matching. The matching process resulted in pruning 146 (4.3%) treatment group members and 305 (8.6%) comparison group members. An L1 post-match statistic of 1.83 × 10–16 compared with the pre-match value of 0.192 is indicative of a high-quality match with minimal imbalance between final study groups. A comparison of descriptive characteristics of the final matched treatment and comparison study groups is given in Table 2; values have been adjusted using CEM-generated weights. After CEM weighting, a statistically significant difference (P < 0.05) was noted with regard to three medical conditions: pneumonia, acute myocardial infarction and hip or knee replacement. These remaining differences observed between study groups were adjusted for in subsequent multivariate modelling.
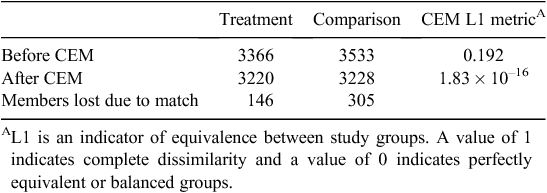
|
|
Adjusted IRRs (relative risk), which take recurrent readmissions into account, showed significantly lower rates of 28-day readmissions in the treatment group. The incidence of 28-day readmission was 29% less for the treatment group than the comparison group (adjusted IRR 0.71; 95% CI 0.59–0.86).
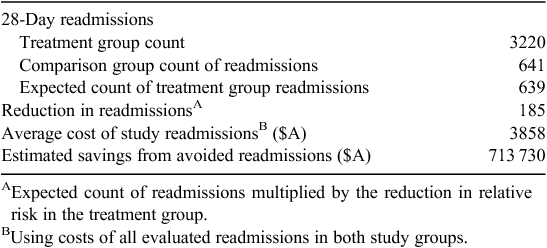
Logistic analysis indicated that the adjusted odds of treatment group members having one or more readmissions within 28 days was significantly lower (adjusted OR 0.75; 95% CI 0.63–0.89) relative to comparison group members. Switching the reference group, the adjusted odds of having one or more readmission was 1.34-fold higher for the comparison group relative to the treatment group (adjusted OR 1.34; 95% CI 1.13–1.59). Estimates of the number and cost of avoided readmissions attributed to the treatment effect are presented in Table 3.
|
Discussion
The present evaluation of the HODI program showed that it was effective at reducing readmissions. Participation in the HODI program was associated with a significantly lower incidence of 28-day readmissions. In addition, MHG program members who did not participate in the HODI intervention after hospital discharge have significantly increased odds of returning to hospital within 28 days of initial discharge. The estimated avoided costs from the reduced incidence of 28-day readmissions were substantial. Because both study groups were enrolled in the broader MHG program and thus had exposure to regular disease management support, the results indicate an incremental benefit of the focused post-discharge HODI intervention in avoiding readmission.
The effectiveness of HODI calls was assessed with regard to readmission risk using two measures, the IRR and OR, which answer different questions. OR results provide a person-level evaluation, whereas the IRR results take into account the total number of readmission events occurring in the designated period, taking into account recurrent readmission. OR results have the advantage of being an easy to interpret treatment effect; however, the IRR provides a more direct link between IRR and readmission-related costs. The slightly higher effect size evaluating the incidence versus odds of readmission, a 29% and 25% reduction respectively, indicates that the HODI program is effective not only at avoiding an initial readmission, but also in preventing recurring readmissions within the evaluation window.
The present study represents the first evaluation, of which we are aware, of an Australian program that focuses on avoiding readmissions across a wide variety of conditions that may be associated with preventable readmissions. The results are consistent with an Australian meta-review of controlled trials that concluded that telephonic follow up was an effective strategy for specific high-risk populations, such as older patients and those with heart failure,36 but also indicate that a scalable program can be effective in more than just these specific high-risk populations. The results of the present study are also consistent with results from a previous study of telephonic-only post-discharge support delivered by Healthways that exhibited a significant effect on hospital readmissions.37 The advantage of a scalable intervention is that it can affect a broad population in a cost-effective manner.
Given the opportunity in Australia, where it is reported that there are over 600 000 potentially preventable hospitalisations per year,38 a scalable post-discharge intervention could decrease that number by reducing the likelihood of hospitalisations occurring in close proximity to a prior discharge. The data in the present study indicate a base readmission rate of nearly 20%, a rate that is comparable to rates observed in various studies of US populations.1–3 Thus, the 29% reduction on such a high base rate translates to a significant decrease in admissions. Of note, the HODI intervention was not tested in isolation, so the effect is incremental over all other initiatives in place to reduce preventable admissions and readmissions.
For the case of HCF, the present study evaluated only 3220 index admissions, approximately 11.7% of all hospital admissions (index and non-index) that occurred during the study period among the chronic diseased members of the MHG program, but avoided over A$700 000 in estimated readmission costs for these index admissions alone. These numbers emphasise the sizeable opportunity to deliver the HODI intervention more broadly in Australia to avoid the burden and cost associated with preventable readmissions.
The present study was conducted with specific limitations. Only claims submitted to HCF were available, limiting visibility of admissions and readmissions in the Australian public healthcare system. Admissions of MHG program members were identified indirectly using hospital pre-authorisation requests because direct data feeds from hospitals were not available. Evaluated index admissions in both study groups were limited based on pre-authorisation records to avoid potential bias that may come from including non-preauthorised admissions in index admission identification. Selection bias may have been introduced because readmitted or sicker patients may have been less available to take an HODI call. Matching was used to balance the groups on severity and other characteristics that may affect readmission risk using the available data; however, omitted variable bias is possible due to the limitations of available data. There were no direct measures of medical condition severity or frailty, or psychosocial factors available for member matching.
We also recognise that an analysis of program costs in conducting the study intervention compared with hospital utilisation savings would have been a valuable addition to the study. However, the intervention was part of an overall larger program, and pricing for these specific program operations were not calculated at this granular a level. Inclusion of intervention costs is planned as part of further studies being undertaken with regard to the HCF program.
A quasi-experimental study design was used due to the retrospective nature of the analysis, as opposed to prospective randomisation. However, robust quasi-experimental studies can often provide realistic and more generalisable results than highly controlled experimental designed studies.39
Conclusions
The results of the present study demonstrate that HODI post-discharge telephone calls effectively reduced hospital readmissions, above and beyond regular MHG disease management support. In addition, the reduced incidence of readmissions translated to meaningful cost avoidance. The MHG HODI intervention offers a scalable approach to improve the quality of and reduce the costs associated with the transition from the hospital to the home by supporting patients.
Competing interests
G. Brent Hamar, Elizabeth Y. Rula, Carter Coberley and James E. Pope were employees and shareholders of Healthways at the time this present study was conducted. Healthways provided the services evaluated in this study. Currently, G. Brent Hamar works at Healthways.
References
[1] Vashi AA, Fox JP, Carr BG, D’Onofrio G, Pines JM, Ross JS, Gross CP. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA 2013; 309 364–71.| Use of hospital-based acute care among patients recently discharged from the hospital.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFahtbc%3D&md5=52d0444f4f8f2ea2804de70b9f6f02c6CAS |
[2] Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360 1418–28.
| Rehospitalizations among patients in the Medicare fee-for-service program.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvFGju7c%3D&md5=132d8b98c2ae98728971a3ebd263fb24CAS |
[3] Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309 355–63.
| Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFWrsrc%3D&md5=59d3c68f74d19396f85c48f8633374eaCAS |
[4] Swannell C. Readmission rates too high. MJA InSight 2013; 2. Available at: https://www.mja.com.au/insight/2013/2/readmission-rates-too-high [verified 31 July 2015].
[5] Scott I, Shohag H, Ahmed M. Quality of care factors associated with unplanned readmissions of older medical patients: a case-control study. Intern Med J 2014; 44 161–70.
| Quality of care factors associated with unplanned readmissions of older medical patients: a case-control study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2c3kslGnsA%3D%3D&md5=23c2269bc37a2a363b209d992334d6b1CAS |
[6] Renton J, Pilcher D, Santamaria J, Stow P, Bailey M, Hart G, Duke G. Factors associated with increased risk of readmission to intensive care in Australia. Intensive Care Med 2011; 37 1800–8.
| Factors associated with increased risk of readmission to intensive care in Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbhslKrtg%3D%3D&md5=84bcdd6b700bf77530ca109d71595c42CAS |
[7] Atkins ER, Geelhoed EA, Knuiman M, Briffa TG. One third of hospital costs for atherothrombotic disease are attributable to readmissions: a linked data analysis. BMC Health Serv Res 2014; 14 338–46.
| One third of hospital costs for atherothrombotic disease are attributable to readmissions: a linked data analysis.Crossref | GoogleScholarGoogle Scholar |
[8] Australasian College for Emergency Medicine. Policy on standard terminology. Document no. P02. 2014. Available at: https://www.acem.org.au/getattachment/3907984e-2a6c-4789-9f11-5d1d75f0e837/Policy-on-Standard-Terminology.aspx [verified 11 August 2015].
[9] Australasian College for Emergency Medicine. Access block and overcrowding in emergency departments. 2004. Available at: https://www.acem.org.au/getattachment/56688d18-4f4c-467a-bba3-704d994d9f2d/Access-Block-2004-literature-review.aspx [verified 11 August 2015].
[10] Forero R, McCarthy S, Hillman K. Access block and emergency department overcrowding. Crit Care 2011; 15(2) 1–6.
| Access block and emergency department overcrowding.Crossref | GoogleScholarGoogle Scholar |
[11] Scott IA. Public hospital bed crisis: too few or too misused? Aust Health Rev 2010; 34 317–24.
| Public hospital bed crisis: too few or too misused?Crossref | GoogleScholarGoogle Scholar |
[12] Bernstein SL, Aronsky D, Duseja R, Epstein S, Handel D, Hwang U, McCarthy M, McConnell KJ, Pines JM, Rathlev N, Schafermeyer R, Zwemer F, Schull M, Asplin BR. The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Med 2009; 16 1–10.
| The effect of emergency department crowding on clinically oriented outcomes.Crossref | GoogleScholarGoogle Scholar |
[13] Richardson DB. Increase in patient mortality at 10 days associated with emergency department overcrowding. Med J Aust 2006; 184 213–6.
[14] Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP, DELAY-ED Study Group Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007; 35 1477–83.
| Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit.Crossref | GoogleScholarGoogle Scholar |
[15] Sprivulis PC, Da Silva J, Jacobs IG, Frazer AR, Jelinek GA. The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments. Med J Aust 2006; 184 208–12.
[16] Australian Institute of Health and Welfare. National Healthcare Agreement: PI 18 – selected potentially preventable hospitalisations, 2015. 2016. Available at: http://meteor.aihw.gov.au/content/index.phtml/itemId/559032 [verified 2 March 2016].
[17] Australian Health Ministers’ Advisory Council (AHMAC). Aboriginal and Torres Strait Islander health performance framework 2014 report. Canberra: AHMAC; 2015. Available at: https://www.dpmc.gov.au/sites/default/files/publications/Aboriginal_and_Torres_Strait_Islander_HPF_2014%20-%20edited%2016%20June2015.pdf [verified 11 February 2017].
[18] Australian Institute of Health and Welfare. National healthcare agreement 2015. 2017. Available at: http://meteor.aihw.gov.au/content/index.phtml/itemId/629963 [verified 11 February 2017].
[19] van Walraven C, Jennings A, Forster AJ. A meta‐analysis of hospital 30‐day avoidable readmission rates. J Eval Clin Pract 2012; 18 1211–8.
| A meta‐analysis of hospital 30‐day avoidable readmission rates.Crossref | GoogleScholarGoogle Scholar |
[20] Greenwald JL, Denham CR, Jack BW. The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process. J Patient Saf 2007; 3 97–106.
| The hospital discharge: a review of a high risk care transition with highlights of a reengineered discharge process.Crossref | GoogleScholarGoogle Scholar |
[21] Marcantonio ER, McKean S, Goldfinger M, Kleefield S, Yurkofsky M, Brennan TA. Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan. Am J Med 1999; 107 13–7.
| Factors associated with unplanned hospital readmission among patients 65 years of age and older in a Medicare managed care plan.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1MzjtVWnsw%3D%3D&md5=73ad778bc4e6743794900179e3896a49CAS |
[22] Snyderman D, Salzman B, Mills G, Hersh L, Parks S. Strategies to help reduce hospital readmissions. J Fam Pract 2014; 63 430–8.
[23] Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med 2007; 2 314–23.
| Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.Crossref | GoogleScholarGoogle Scholar |
[24] Weiss ME, Piacentine LB, Lokken L, Ancona J, Archer J, Gresser S, Holmes SB, Toman S, Toy A, Vega-Stromberg T. Perceived readiness for hospital discharge in adult medical-surgical patients. Clin Nurse Spec 2007; 21 31–42.
| Perceived readiness for hospital discharge in adult medical-surgical patients.Crossref | GoogleScholarGoogle Scholar |
[25] van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med 2002; 17 186–92.
| Effect of discharge summary availability during post‐discharge visits on hospital readmission.Crossref | GoogleScholarGoogle Scholar |
[26] Roy CL, Poon EG, Karson AS, Ladak-Merchant Z, Johnson RE, Maviglia SM, Gandhi TK. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005; 143 121–8.
| Patient safety concerns arising from test results that return after hospital discharge.Crossref | GoogleScholarGoogle Scholar |
[27] Mudge AM, Shakhovskoy R, Karrasch A. Quality of transitions in older medical patients with frequent readmissions: opportunities for improvement. Eur J Intern Med 2013; 24 779–83.
| Quality of transitions in older medical patients with frequent readmissions: opportunities for improvement.Crossref | GoogleScholarGoogle Scholar |
[28] Tricco AC, Antony J, Ivers NM, Ashoor HM, Khan PA, Blondal E, Ghassemi M, MacDonald H, Chen MH, Ezer LK, Straus SE. Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: a systematic review and meta-analysis. CMAJ 2014; 186 E568–78.
| Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: a systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[29] Hamar GB, Rula EY, Wells A, Coberley C, Pope JE, Larkin S. Impact of a chronic disease management program on hospital admissions and readmissions in an Australian population with heart disease or diabetes. Popul Health Manag 2013; 16 125–31.
| Impact of a chronic disease management program on hospital admissions and readmissions in an Australian population with heart disease or diabetes.Crossref | GoogleScholarGoogle Scholar |
[30] Hamar GB, Rula EY, Coberley C, Pope JE, Larkin S. Long-term impact of a chronic disease management program on hospital utilization and cost in an Australian population with heart disease or diabetes. BMC Health Serv Res 2015; 15 174–82.
| Long-term impact of a chronic disease management program on hospital utilization and cost in an Australian population with heart disease or diabetes.Crossref | GoogleScholarGoogle Scholar |
[31] King G, Nielsen R, Coberley C, Pope E, Wells A. Comparative effectiveness of matching methods for causal inference. Unpublished white paper. 2011. Available at: http://gking.harvard.edu/files/psparadox.pdf [verified 2 February 2017].
[32] Wells AR, Hamar B, Bradley C, Gandy WM, Harrison PL, Sidney JA, Coberley CR, Rula EY, Pope JE. Exploring robust methods for evaluating treatment and comparison groups in chronic care management programs. Popul Health Manag 2013; 16 35–45.
| Exploring robust methods for evaluating treatment and comparison groups in chronic care management programs.Crossref | GoogleScholarGoogle Scholar |
[33] Blackwell M, Iacus S, King G, Porro G. CEM: coarsened exact matching in Stata. Stata J 2009; 9 524–46.
[34] King G, Iacus SM. How coarsening simplifies matching-based causal inference theory. Cambridge, MA: Harvard University; 2012. Available online at: https://www.semanticscholar.org/paper/How-Coarsening-Simplifies-Matching-Based-Causal-Iacus-King/6838125d944b31a1f958f86c08940b37a2e6047c [verified 23 February 2017].
[35] Iacus SM, King G, Porro G. CEM: software for coarsened exact matching. J Stat Softw 2009; 30 1–27.
| CEM: software for coarsened exact matching.Crossref | GoogleScholarGoogle Scholar |
[36] Scott IA. Preventing the rebound: improving care transition in hospital discharge processes. Aust Health Rev 2010; 34 445–51.
| Preventing the rebound: improving care transition in hospital discharge processes.Crossref | GoogleScholarGoogle Scholar |
[37] Harrison PL, Hara PA, Pope JE, Young MC, Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag 2011; 14 27–32.
| The impact of postdischarge telephonic follow-up on hospital readmissions.Crossref | GoogleScholarGoogle Scholar |
[38] Australian Institute of Health and Welfare (AIHW). Admitted patient care 2013–2014: Australian hospital statistics. Health Services Series no. 60, Catalogue no. HSE 156. Canberra: AIHW; 2015.
[39] Velengtas P, Mohr P, Messner DA. Making informed decisions: assessing the strengths and weaknesses of study designs and analytic methods for comparative effectiveness research, a briefing document for stakeholders. Washington, DC: National Pharmaceutical Council; 2012.


