Health service utilisation by people living with chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study
Suzanne Nielsen A B G , Gabrielle Campbell A , Amy Peacock C , Kimberly Smith A , Raimondo Bruno A C , Wayne Hall D , Milton Cohen E and Louisa Degenhardt A FA National Drug and Alcohol Research Centre, UNSW, 22–32 King Street, Randwick, NSW 2031, Australia. Email: g.campbell@unsw.edu.au; kimberley.smith@unsw.edu.au
B South East Sydney Local Health District (SESLHD) Drug and Alcohol Services, 591–623 S Dowling Street, Surry Hills, NSW 2010, Australia.
C School of Medicine, University of Tasmania, Private Bag 30, Hobart, Tas. 7001, Australia. Email: Amy.Peacock@utas.edu.au; Raimondo.Bruno@utas.edu.au
D Centre for Youth Substance Abuse Research, University of Queensland, Australia. Email: w.hall@uq.edu.au
E St Vincent’s Clinical School, UNSW Medicine, Level 5 deLacy Building, St Vincent’s Hospital, Victoria Street, Darlinghurst, NSW 2010, Australia. Email: M.Cohen@unsw.edu.au
F School of Population and Global Health, Level 4, 207 Bouverie Street, The University of Melbourne, Vic. 3010, Australia. Email: l.degenhardt@unsw.edu.au
G Corresponding author. Email: suzanne.nielsen@unsw.edu.au
Australian Health Review 40(5) 490-499 https://doi.org/10.1071/AH15047
Submitted: 9 March 2015 Accepted: 26 October 2015 Published: 25 November 2015
Journal Compilation © AHHA 2016
Abstract
Objective The aims of the present study were to describe the use, and barriers to the use, of non-medication pain therapies and to identify the demographic and clinical correlates of different non-opioid pain treatments.
Methods The study was performed on a cohort (n = 1514) of people prescribed pharmaceutical opioids for chronic non-cancer pain (CNCP). Participants reported lifetime and past month use of healthcare services, mental and physical health, pain characteristics, current oral morphine equivalent daily doses and financial and access barriers to healthcare services.
Results Participants reported the use of non-opioid pain treatments, both before and after commencing opioid therapy. Services accessed most in the past month were complementary and alternative medicines (CAMs; 41%), physiotherapy (16%) and medical and/or pain specialists (15%). Higher opioid dose was associated with increased financial and access barriers to non-opioid treatment. Multivariate analyses indicated being younger, female and having private health insurance were the factors most commonly associated with accessing non-opioid treatments.
Conclusions Patients on long-term opioid therapy report using multiple types of pain treatments. High rates of CAM use are concerning given limited evidence of efficacy for some therapies and the low-income status of most people with CNCP. Financial and insurance barriers highlight the importance of considering how different types of treatments are paid for and subsidised.
What is known about the topic? Given concerns regarding long-term efficacy, adverse side-effects and risk of misuse and dependence, prescribing guidelines recommend caution in prescribing pharmaceutical opioids in cases of CNCP, typically advising a multidisciplinary approach to treatment. There is a range of evidence supporting different (non-drug) treatment approaches for CNCP to reduce pain severity and increase functioning. However, little is known about the non-opioid treatments used among those with CNCP and the demographic and clinical characteristics that may be associated with the use of different types of treatments. Understanding the use of non-drug therapy among people with CNCP is crucial given the potential to improve pain control for these patients.
What does this paper add? The present study found that a wide range of non-opioid treatments was accessed by the study sample, both before and after commencing opioids, indicating that in this sample opioids were not the sole strategy used for pain management. The most common treatment (other than opioids) was CAM, reported by two-fifths of the sample. Having private health insurance was associated with increased use of non-opioid treatments for pain, highlighting the importance of considering how treatments are paid for and potential financial barriers to effective treatments.
What are the implications for practitioners? Patients’ beliefs and financial barriers may affect the uptake of different treatments. Many patients may be using complementary and alternative approaches with limited evidence to support their use, highlighting the need for clinicians to discuss with patients the range of prescribed and non-prescribed treatments they are accessing and to help them understand the benefits and risks of treatments that have not been tested sufficiently, or have inconsistent evidence, as to their efficacy in improving pain outcomes.
Additional keywords: acupuncture, barriers, chiropractor, complementary and alternative medicines.
Introduction
Chronic non-cancer pain (CNCP) is prevalent worldwide. It is estimated that, in 2010, 21% of the global population experienced tension-type headache, 15% suffered from migraines, 9% experienced lower back pain and 5% experienced neck pain.1 CNCP is responsible for considerable social, economic and healthcare burden,2–4 and is predicted to increase with an aging population.5 As such, there is a public health imperative to better understand the use of evidence-based treatments by people with CNCP.
Currently, evidence to support the long-term use of opioid medication for CNCP is lacking.6,7 Chronic pain is most common in older adults, who are most at risk from adverse effects from medications, such cognitive effects of medications and drug interactions.8 Research indicates that several non-drug treatment alternatives may be effective for CNCP. These include cognitive behavioural therapy,9,10 pain education and functional rehabilitation therapy.11 Recent systematic reviews highlight the efficacy of multidisciplinary biopsychosocial approaches that coordinate physical and psychological approaches over unimodal approaches (e.g. pharmacological treatment only)12,13 in terms of improved mood and a greater likelihood of return to work.12 Together, these studies provide a strong argument for examining the potential role of non-drug treatments in CNCP.
Not all non-drug therapies are equally supported by empirical evidence; for example, there is mixed support for exercise therapy.14 The clinical efficacy of some complementary and alternative medicines (CAMs), such as osteopathy, chiropractic, homeopathy, acupuncture, aromatherapy, biofeedback, hypnosis, herbalism, vitamins, spiritual healing, massage, relaxation and yoga, are not well established.15–17 Research has shown that between one- and two-fifths of people living with chronic pain in the US and UK reported the use of CAM in the previous 12 months, with the majority of these individuals also reporting opioid analgesic use within the same period.15,18
Given that reductions in pain from medication alone are often modest,19 there is considerable scope to improve pain control by using non-pharmacological treatments. Potential barriers to accessing healthcare options may include perceptions that they are ineffective, time and effort commitments, limited availability and costs (including lack of government subsidies or health insurance coverage).20 There is a need to understand the current uptake of traditional and non-traditional health services and to identify barriers to using non-medication treatments for CNCP.
Thus, the aims of the present study were to: (1) describe the use of non-medication pain therapies by a group of people with CNCP who were prescribed opioids; (2) examine barriers to accessing non-medication-based treatments, including financial constraints and beliefs; and (3) examine associations with past month use of different non-medication-based pain treatments.
Methods
Study design and setting
The Pain and Opioids IN Treatment (POINT) study is a prospective cohort study of 1514 people across Australia who have been prescribed opioids for CNCP (a full description of the cohort methodology has been published elsewhere21). Data were collected via telephone interview with a researcher. In addition, a self-completed survey and medication diary were completed at home in the week following the telephone interview.
The study was approved by the Human Research Ethics Committee of the University of New South Wales (HREC reference #HC12149).
Eligibility criteria
Eligibility criteria included being ≥18 years of age, competent in English, mentally and physically able to complete telephone interviews and self-completed questionnaires, without apparent serious cognitive impairments, living with CNCP (defined as pain present for a minimum of 3 months) and having been prescribed a Schedule 8 (S8) opioid (e.g. morphine, oxycodone or fentanyl) for more than 6 weeks before baseline (all opioids that were S8 in the Standard for the Uniform Scheduling of Medicines and Poisons22 were included). Exclusion criteria included use of S8 opioids for cancer pain or solely for the treatment of heroin dependence.
Participants and procedure
Participants were recruited through 1868 pharmacies (35% of pharmacies in Australia). Of those who were referred (n = 2725), 1873 were eligible and 1514 chose to participate in the study. Participants were included in these analyses if they completed both the baseline telephone interview and the self-completed measures, including the medication diary (n = 1243).
Selection of measures
The measures were chosen based on Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT).23 IMMPACT is an international multidisciplinary group with representatives from health, academia and regulatory agencies that have conducted consensus reviews and provide recommendations for improving the design, execution and interpretation of clinical trials of treatments for pain.
Demographic characteristics
Basic demographics of age, gender, employment (included in the analyses as a dichotomous variable of employed or not) and postcode were collected at baseline. Participants were coded as living in major cities, inner or outer regional, remote or very remote locations by postcode in accordance with the 2006 edition of the Australian Standard Geographical Classification.24 A binary variable was formed for the subsequent analyses based on metropolitan compared with inner regional, outer regional, remote and very remote locations.
Pain and pain-related measures
Participants were asked about lifetime (i.e. ever experienced) and current experience (i.e. past 12 months) of a range of pain conditions (back or neck problems, arthritis or rheumatism, frequent or severe headaches, visceral pain, fibromyalgia, complex regional pain syndrome, shingles-related pain), in addition to being asking about other pain conditions not on the list. Current pain severity and pain interference were measured using the brief pain inventory (BPI).25 The BPI consists of four items assessing current pain severity and seven items assessing how pain interferes with daily functioning rated on an 11-point scale (from 0 (no pain) to 10 (pain as bad as you can imagine) for pain severity and from 0 (does not interfere) to 10 (completely interferes) for daily functioning). The arithmetic mean was calculated for the severity and interference subscales. Binary variables were created with a cut-off score ≥5 on each scale indicative of moderate to severe pain severity or interference, respectively. The pain self-efficacy questionnaire (PSEQ),26,27 a 10-item scale, was used to measure patients’ confidence in a range of activities and situations when they are in pain, rated on a seven-point scale (from 0 (not at all confident) to 6 (completely confident)). A total score over the range 0–60 was calculated, with higher scores indicating stronger feelings of self-efficacy. Scores <30 have been shown to be indicative of less sustainable treatment gains and predictive of a lower rate of return to work and/or maintenance of treatment gains.28
Physical and mental health (SF-12)
The 12-item short form health survey (SF-12) is a set of questions measuring self-reported function and quality of life. Physical and mental health component scores were calculated from the SF-12.29,30 The SF-12 has been validated in Australian populations where normative data are available.30
Health service utilisation and barriers
Barriers to treatment were based on those reported in previous research.31 Participants were asked whether they had ever experienced particular barriers, and responses were collected as a binary variable (yes/no). Barriers examined included being unable to get to a pharmacy or doctor, being unable to access specialist advice, being unable to afford other types of medication and being unable to afford other treatments (e.g. counselling, physiotherapy, chiropractor, yoga).
Participants were asked whether they had private health insurance (collected as a binary ‘yes/no’ variable) and about their lifetime and past month use of health services specifically for pain treatment, including physiotherapy, chiropractor, acupuncture, psychiatrist, psychologist, counsellor and/or social worker, medical specialist (i.e. pain specialist), support groups, vitamins and minerals, surgery and other. Subjects were asked how helpful they found the service and whether they commenced accessing this service before, at the same time or after starting opioid medications. A binary variable was created for the rating of helpfulness of service as helpful or very helpful compared with neutral, unhelpful or very unhelpful. Health services were grouped into ‘physical therapies’ (physiotherapy or ‘other physical therapies,’ which included massage, osteopathy, yoga, tai chi, Feldenkrais, Pilates, supervised exercise, tens machine and Bowen therapy, or CAMs (chiropractor, acupuncture, Chinese medicine, naturopath, support groups, hypnosis, meditation, homeopathy, vitamins and minerals, spiritual healing and reflexology) according to available definitions.32
Medications
Oral morphine equivalent (OME) daily doses were estimated using available references33 based on self-reported opioid use in a 1-week medication diary. Four groups were formed (1–20, 21–90, 91–199 and ≥200 mg OME) based on previously determined cut-offs of opioid dose associated with different levels of risk.34 OME groups were used in between-groups analyses to compare medication beliefs.
Medication beliefs were assessed through the beliefs about medications questionnaire (BMQ).35 Two subscales from the BMQ were included: (1) the Specific Necessity subscale, which assesses the participants’ beliefs about the necessity of their current medication; and (2) the Specific Concerns subscale, which measures concerns about prescribed medication. The score range for each subscale is 0–25, with higher scores reflecting more strongly held beliefs.36 The variables were analysed as continuous variables.
The prescribed opioids difficulty scale (PODS) is a patient-centred scale that was used to measure participants’ current problems and concerns around using prescribed opioids, such as problematic side effects, loss of control over use and concerns around addiction.36 A cut-off score of ≥8 was considered a medium score for which participants would have endorsed at least two problems relating to their opioid use.36
Data analysis
Analyses were conducted using IBM SPSS Statistics for Windows, version 21.0 (Armonk, NY, USA) or Stata version 13.0 (Stata Corp., College Station, TX, USA). Normally distributed continuous data are presented as the mean ± s.d. and were compared using t-tests. For continuous variables that were not normally distributed, data are presented as median values with the interquartile range (IQR), with comparisons made using non-parametric statistics (Mann–Whitney U-test). Percentages with 95% confidence intervals (95% CI) were calculated for categorical outcomes.
We tested whether factors such as age, gender, income, private health insurance, living in metropolitan areas vs regional/remote and OME were associated with certain barriers to accessing other health services by conducting multivariate logistic regression, with the lowest opioid dose (0–20 mg OME) category serving as the reference category.
We examined relationships between medication beliefs and OME groups using multinomial logistic regression. Odds ratios (ORs) and 95% CI were used for proportions, with the lowest opioid dose (0–20 mg OME) category serving as the reference category.
Logistic regression was used to compare those who did and did not report past month use of physiotherapy, mental health treatment (in association with pain condition), pain or medical specialists, complementary and alternative therapies and physical therapies. To identify variables that were independently associated with past month use of these five treatment types, all variables were entered into a multivariate analyses.
Results
Sample characteristics
The median age of the sample (n = 1243) was 59 years (IQR 49–68 years), and over half (57%) were female. Nearly one-third of the sample had retired (31%), and half were unemployed (49%). Participants reported being in pain for a median of 10 years (IQR 5–21 years) and had been taking pharmaceutical opioids for CNCP for a median of 4 years (IQR 1.5–10 years). Most reported at least one pain condition in the past 12 months, with chronic back or neck problems (76%) and arthritis or rheumatism (62%) being the most commonly reported conditions. Private health insurance was reported by 37% (95% CI 34%–40%). The sample reported mean BPI severity and interference scores of 5.0 ± 1.8 and 5.6 ± 2.3, respectively, indicating moderate pain severity and interference. Median OME was 73 mg (IQR 36–145 mg), and 13% of the sample (95% CI 11%–15%) reported OME >200 mg per day.
Healthcare service access
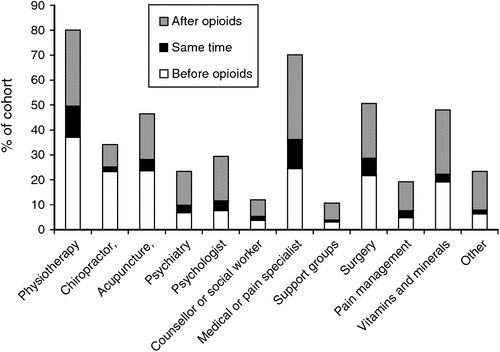
Participants reported accessing multiple different types of pain treatments over their lifetime. Physiotherapy (80%) and pain specialists (71%) were the most common services accessed, with participants accessing a median of 4 (IQR 2–5) healthcare services in their lifetime (Fig. 1 and Table 1). Past month access was reported by far fewer participants; approximately one in seven had used physiotherapy (16%) and pain specialists (15%), with participants accessing a median of 1 (IQR 1–2) healthcare service in the past month. The most common treatment for pain (excluding opioids) in the past month was taking vitamins and minerals (Table 1), reported by 37% of the sample. A median of 1 (IQR 0–3) healthcare service was used before the prescription of opioids, with a median of 2 (IQR 0–3) healthcare services used after commencing opioid treatment (Table 1).
|

|
Barriers to healthcare service access
Variables including age, gender, income below A$400 per week, having private health insurance, living in a metropolitan area (vs regional or remote) and OME were entered into multivariate regressions to examine factors that are independently associated with barriers to treatment (Table 2). Younger people were more likely to experience all barriers to treatment (unable to access pharmacist or doctor, unable to access specialist advice, unable to afford other types of medication and other treatments). Females were less likely to experience barriers to treatment. Those with private health insurance were less likely to experience barriers to treatment (i.e. unable to access specialist advice, unable to afford other types of medication and other treatments). A higher OME was associated with being unable to get to a pharmacy or doctor, and being unable to access specialist advice. There were no differences in barriers to treatment between those living metropolitan versus regional or remote areas, nor did income have an impact on barriers to treatment.

|
Those currently receiving a daily OME opioid dose ≥200 mg reported stronger beliefs in the necessity of their medications compared with those on 0–20 mg OME (Table 3).
Correlates of past month health service use
Past month physiotherapy was reported by 16% of the sample. Participants who had recently accessed physiotherapy treatment were more likely to have private health insurance and were less likely to be male than those who had not received physiotherapy treatment (Table 4). In a multivariate model, having poorer mental health and having private health insurance were associated with a greater past month use of physiotherapy treatment (after controlling for age, gender and other variables reported in Table 4, i.e. age, gender, employment, metropolitan location, mental and physical health, and pain factors).
Past month use of mental health services (e.g. counselling, psychologist or psychiatrist) for pain was reported by 11% of the sample. Use of mental health services was predicted by younger age, being unemployed, poorer mental health, moderate to severe pain interference, poorer pain self-efficacy, being unable to afford other pain treatments and reporting greater problems with prescription opioids (as measured with the PODS). After controlling for age, gender and other variables (employment, metropolitan location, mental and physical health, and pain factors), younger age, being female, poorer mental health, lower pain self-efficacy and having private health insurance were associated with past month use of mental health services for pain.
Past month use of pain specialists was reported by 15% of the sample. This was predicted by younger age, poorer physical health, greater pain interference (as measured with the BPI), lower pain self-efficacy, higher opioid dose, more opioid-related problems (as measured with the PODS) and having private health insurance. After controlling for age, gender and other variables (employment, metropolitan location, mental and physical health, and pain factors), younger age, poorer physical health and having private health insurance were associated with recent access of pain specialist services. Participants with private health insurance had twice the odds of recently accessing pain specialist treatment.
Past month use of CAMs was reported by 41% of the sample, making this the most commonly reported treatment approach. Being female and having private health insurance were associated with CAM use both at a univariate level and in multivariate analyses after controlling for age, gender and other variables (employment, metropolitan location, mental and physical health, and pain factors).
Past month use of massage and other physical therapies was reported by 8% of the sample and was associated with living in a rural/regional setting, although the model itself explained very little of the total variance in the likelihood of the use of massage and other physical therapies.
Discussion
The present study was a unique examination of use of non-medication-based treatments and barriers to these treatments in a community-based sample of people with CNCP who had been prescribed opioids. A wide range of non-opioid treatments was accessed by this sample, both before and after commencing opioids, indicating that in this sample opioids were not the sole strategy used for pain management. A median of 1 treatment was tried before commencing opioids. This may be related to affordability of non-medication-based treatments or beliefs around the efficacy of other treatments. It is a concern that few treatments were tried before opioids, given the potential adverse known effects. Two-fifths of the sample reported past month use of CAM, with 8% reporting the use of massage and other physical therapies. These rates are similar to, or higher than, rates recorded in US and UK studies of chronic pain patients.15,18
There are considerable health and economic implications of the findings of the present study. Prior research indicates that the primary motivations for CAM use include the belief that CAM will reduce pain and improve functioning.37 However, the evidence base for the efficacy of some of these treatments is either lacking or insufficient to support efficacy.15–17 This, coupled with indications of exponential increases in expenditure on CAM among the general population,38,39 suggests that people with CNCP may be spending substantial proportions of their (often limited) income on treatments of uncertain value. Of note, less than half the participants who reported using CAM described these treatments as helpful, despite their frequent use. In contrast, although lower numbers reported accessing psychological therapies and support groups, these were rated by participants as some of the most helpful treatments.
Younger people, males and those without private health insurance reported the most barriers to treatment. A higher OME was associated with being unable to get to a pharmacy or doctor and unable to access specialist advice. Work in veteran populations has identified that both access to and beliefs about non-pharmacological treatments can act as barriers to multimodal pain treatments.40 In light of concerns regarding the risk of overdose with higher opioid doses41 and the greater complexity of younger participants observed in the present sample,42 further work to understand barriers for young people with chronic pain is critical.
The association between health insurance and treatment access highlights the importance of considering how different types of treatments are paid for and subsidised. Those with private health insurance reported fewer barriers to treatment, in addition to higher utilisation of non-medication-based treatments. Currently, certain evidence-based treatments (e.g. psychological therapies and multimodal treatment programs) for CNCP may be unaffordable for some, yet may represent treatments that are safe and effective (both in terms of the literature and participants’ perceptions of how helpful they are) for chronic pain. Those taking the highest doses of opioids reported greater beliefs in the importance of medication for pain, further suggesting that beliefs about efficacy and affordability may affect the uptake of non-opioid strategies for pain.
The strongest predictor of increased use for four of the five non-opioid treatment types examined was having private health insurance. It should be noted that in Australia there is a universal basic healthcare system, although it does not cover a range of adjunctive pain treatments. Because many private health insurers subsidise a range of other treatments, this may be an expected finding. It does raise the important issues of ensuring that subsidised treatments include those that are evidence based and that financial barriers to evidence-based treatments are examined. Gender was the other variable that was associated with reduced uptake of both mental health services for pain and CAM, consistent with previous work finding that men are more likely to report barriers of ‘acceptability’ to seek help for mental health.43
There are study limitations to consider. First, most of the models were relatively poor at explaining most of the variance in accessing pain services, suggesting that there are other factors that may explain why people are not accessing non-opioid treatments that were not assessed in the present study. Future studies may explore patients’ beliefs about different treatment approaches, as well as barriers that were not captured in the present study. In addition, the study relied on self-report of health service access, which may be subject to recall and social desirability bias. Although an important variable, data on medication beliefs were collected from only approximately half the sample to minimise data burden on participants. As such, it was not able to be included in the multivariate models. Finally, because of only small numbers reporting the use of some types of treatments (e.g. physical therapies), there may have been low power to examine predictors.
Notwithstanding these limitations, the present study is one of the first to describe the use of a wide range of services for pain. Lack of private health insurance was associated with lower uptake of many treatments. A concerning finding is that some of the most common treatments accessed were those that potentially have the least evidence demonstrating their efficacy and were rated to be least helpful by participants. Identifying ways to increase access to effective treatment is a worthy area for future work.
Competing interests
Some of the authors have received untied educational grants from Reckitt Benckiser (LD, RB, GC and SN); and MundiPharma for post-marketing surveillance studies of a tamper-resistant formulation of oxycodone (BL, LD and RB), and for development and presentation of educational material (MC). These bodies had no involvement in or knowledge of the current study.
Acknowledgements
The authors acknowledge study investigators not involved in the direct writing of this paper: Michael Farrell, Nicholas Lintzeris, Richard Mattick, Fiona Shand and Briony Larance. Thanks to Jessica Belcher, Sarah Freckleton, Kimberley Smith, Ranira Moodley, Rachel Urquhart-Secord and Anika Martin for their contribution to data collection. Thanks also to the POINT advisory committee for their advice on the design and conduct of the study. This study was funded by the Australian National Health and Medical Research Council (NHMRC; #1022522). LD, BL, SN, WH and RPM are supported by NHMRC research fellowships (#1013803, #1041472, and #569738). The National Drug and Alcohol Research Centre at UNSW Australia is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grant Fund. The funders had no role in the preparation or decision to submit this manuscript.
References
[1] Hoy D, March L, Brooks P, Blyth F, Woolf A, Bain C, Williams G, Smith E, Vos T, Barendregt J, Murray C, Burstein R, Buchbinder R. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014; 73 968–74.| The global burden of low back pain: estimates from the Global Burden of Disease 2010 study.Crossref | GoogleScholarGoogle Scholar | 24665116PubMed |
[2] Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380 2163–96.
| Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010.Crossref | GoogleScholarGoogle Scholar | 23245607PubMed |
[3] Beubler E, Jaksch W, Devulder J, Le Poloin B, Bo Honsen O, Meynadier J, Muller-Schwefe G, Zenz M, Mac Sullivan R, O’Brien T, Eisenberg E, Mercodante S, Ventafriddo V, Varrossi G, Vielvoye-Kerkmeer A, Zylicz B, Breivik H, Krajnik M, Lopez JC, Puig M, Rhodin A, Borgeat A, Collett B, Hanna M, Hunt T, Simpson K. The white paper on opioids and pain: a pan-European challenge. The European white paper on the use of opioids in chronic pain management. J Pain Palliat Care Pharmacother 2006; 20 79–87.
[4] Access Economics. The high price of pain: the economic impact of persistent pain in Australia. Sydney: MBF Foundation; 2007.
[5] Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012; 64 2028–37.
| A systematic review of the global prevalence of low back pain.Crossref | GoogleScholarGoogle Scholar | 22231424PubMed |
[6] Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010; 1 CD006605
| 20091598PubMed |
[7] Michna E, Cheng WY, Korves C, Birnbaum H, Andrews R, Zhou Z, Joshi AV, Schaaf D, Mardekian J, Sheng M. Systematic literature review and meta-analysis of the efficacy and safety of prescription opioids, including abuse-deterrent formulations, in non-cancer pain management. Pain Med 2014; 15 79–92.
| Systematic literature review and meta-analysis of the efficacy and safety of prescription opioids, including abuse-deterrent formulations, in non-cancer pain management.Crossref | GoogleScholarGoogle Scholar | 24112715PubMed |
[8] Gomez C, Vega-Quiroga S, Bermejo-Pareja F, Medrano MJ, Louis ED, Benito-Leon J. Polypharmacy in the elderly: a marker of increased risk of mortality in a population-based prospective study (NEDICES). Gerontology 2015; 61 301–9.
| Polypharmacy in the elderly: a marker of increased risk of mortality in a population-based prospective study (NEDICES).Crossref | GoogleScholarGoogle Scholar | 25502492PubMed |
[9] Williams ACDC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012; 11 CD007407
| Psychological therapies for the management of chronic pain (excluding headache) in adults.Crossref | GoogleScholarGoogle Scholar |
[10] Bernardy K, Klose P, Busch Angela J, Choy Ernest HS, Häuser W. Cognitive behavioural therapies for fibromyalgia. Cochrane Database Syst Rev 2013; 9 CD009796
| Cognitive behavioural therapies for fibromyalgia.Crossref | GoogleScholarGoogle Scholar | 24018611PubMed |
[11] Schonstein E, Kenny DT, Keating J, Koes BW. Work conditioning, work hardening and functional restoration for workers with back and neck pain. Cochrane Database Syst Rev 2003; 1 CD001822
| 12535416PubMed |
[12] Flor H, Fydrich T, Turk DC. Efficacy of multidisciplinary pain treatment centers: a meta-analytic review. Pain 1992; 49 221–30.
| Efficacy of multidisciplinary pain treatment centers: a meta-analytic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK38zgtFyhsQ%3D%3D&md5=47d6281338208aed600f264a12aecc2cCAS | 1535122PubMed |
[13] Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary rehabilitation for chronic low back pain: systematic review. BMJ 2001; 322 1511–16.
| Multidisciplinary rehabilitation for chronic low back pain: systematic review.Crossref | GoogleScholarGoogle Scholar | 11420271PubMed |
[14] van Tulder M, Malmivaara A, Esmail R, Koes B. Exercise therapy for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2000; 25 2784–96.
| Exercise therapy for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FkvVKgsQ%3D%3D&md5=737f7efd552db6893184a1f93ef40aefCAS | 11064524PubMed |
[15] Haetzman M, Elliott A, Smith B, Hannaford P, Chambers W. Chronic pain and the use of conventional and alternative therapy. Fam Pract 2003; 20 147–54.
| Chronic pain and the use of conventional and alternative therapy.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s3gsVOgsw%3D%3D&md5=0db106d9a6f8976341e9478b85ba84d0CAS | 12651788PubMed |
[16] Ezzo J, Berman B, Hadhazy VA, Jadad AR, Lao L, Singh BB. Is acupuncture effective for the treatment of chronic pain? A systematic review. Pain 2000; 86 217–25.
| Is acupuncture effective for the treatment of chronic pain? A systematic review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3nsVSlsA%3D%3D&md5=5e3f7ec1116da22d9bcc6f94b75b4254CAS | 10812251PubMed |
[17] McEachrane-Gross F, Liebschutz J, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med 2006; 6 34
| Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey.Crossref | GoogleScholarGoogle Scholar | 17026768PubMed |
[18] Fleming S, Rabago D, Mundt M, Fleming M. CAM therapies among primary care patients using opioid therapy for chronic pain. BMC Complement Altern Med 2007; 7 15
| CAM therapies among primary care patients using opioid therapy for chronic pain.Crossref | GoogleScholarGoogle Scholar | 17506893PubMed |
[19] Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 2006; 174 1589–94.
| Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects.Crossref | GoogleScholarGoogle Scholar | 16717269PubMed |
[20] Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O’Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10 113–30.
| Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlCgsb0%3D&md5=0fbb5bd1022ee6834126614151d88a3fCAS | 19187889PubMed |
[21] Campbell G, Mattick R, Bruno R, Larance B, Nielsen S, Cohen M, Lintzeris N, Shand F, Hall WD, Hoban B, Kehler C, Farrell M, Degenhardt L. Cohort protocol: the Pain and Opioids IN Treatment (POINT) study. BMC Pharmacol Toxicol 2014; 15
| Cohort protocol: the Pain and Opioids IN Treatment (POINT) study.Crossref | GoogleScholarGoogle Scholar | 24646721PubMed |
[22] Therapeutic Goods Administration. Standard for the uniform scheduling of medicines and poisons (SUSMP). Canberra: Australia Government; 2013.
[23] Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113 9–19.
| Core outcome measures for chronic pain clinical trials: IMMPACT recommendations.Crossref | GoogleScholarGoogle Scholar | 15621359PubMed |
[24] Australian Bureau Statistics (ABS). Statistical geography volume 1: Australian standard geographical classification (ASGC). Canberra: ABS; 2006.
[25] Cleeland C. The brief pain inventory (BPI). Houston; 1991.
[26] Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007; 11 153–63.
| The pain self-efficacy questionnaire: taking pain into account.Crossref | GoogleScholarGoogle Scholar | 16446108PubMed |
[27] Nicholas MK, Asghari A, Blyth FM. What do the numbers mean? Normative data in chronic pain measures. Pain 2008; 134 158–73.
| What do the numbers mean? Normative data in chronic pain measures.Crossref | GoogleScholarGoogle Scholar | 17532138PubMed |
[28] Coughlan GM, Ridout KL, Williams AC, Richardson PH. Attrition from a pain management programme. Br J Clin Psychol 1995; 34 471–9.
| Attrition from a pain management programme.Crossref | GoogleScholarGoogle Scholar | 8845785PubMed |
[29] Ware JE, Kosinksi MMA, Keller SD. How to score the SF-12 physical and mental health summary scales. 2nd edn. Boston: The Health Institute, New England Medical Centre; 1995.
[30] Sanderson K,, Andrews G. The SF-12 in the Australian population: cross-validation of item selection. Aust N Z J Public Health 2002; 26 343–5.
| The SF-12 in the Australian population: cross-validation of item selection.Crossref | GoogleScholarGoogle Scholar |
[31] Glajchen M. Chronic pain: treatment barriers and strategies for clinical practice. J Am Board Fam Pract 2001; 14 211–18.
| 1:STN:280:DC%2BD3M3lsFOksg%3D%3D&md5=dcb6e6f039aa2fa5e23967e92dd76fb6CAS | 11355054PubMed |
[32] Innovation New South Wales Agency for Clinical Innovation. Complementary and alternative medicine (CAM). 2014. Available from: http://www.aci.health.nsw.gov.au/__data/assets/pdf_file/0018/212823/Pain-and-CAM-Therapy.pdf [verified 15 December 2014].
[33] Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. Technical Report No. 329. Sydney: National Drug and Alcohol Research Centre, UNSW Australia; 2014.
[34] Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, et al American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2 – guidance. Pain Physician 2012; 15 S67–116.
| 22786449PubMed |
[35] Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14 1–24.
| The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication.Crossref | GoogleScholarGoogle Scholar |
[36] Sullivan MD, Von Korff M, Banta-Green C, Merrill JO, Saunders K. Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain. Pain 2010; 149 345–53.
| Problems and concerns of patients receiving chronic opioid therapy for chronic non-cancer pain.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXlt1Smtrg%3D&md5=2db08576623b09f34f78fff6453e43eeCAS | 20334974PubMed |
[37] Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data 2004; 343 1–19.
| Complementary and alternative medicine use among adults: United States, 2002.Crossref | GoogleScholarGoogle Scholar | 15188733PubMed |
[38] Xue CC, Zhang AL, Lin V, Da Costa C, Story DF. Complementary and alternative medicine use in Australia: a national population-based survey. J Altern Complement Med 2007; 13 643–50.
| Complementary and alternative medicine use in Australia: a national population-based survey.Crossref | GoogleScholarGoogle Scholar | 17718647PubMed |
[39] Thomas KJ, Nicholl JP, Coleman P. Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med 2001; 9 2–11.
| Use and expenditure on complementary medicine in England: a population based survey.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M7msFGmtQ%3D%3D&md5=7e7e2c4d5667215fa5f9992fd3526a72CAS | 11264963PubMed |
[40] Simmonds MJ, Finley EP, Vale S, Pugh MJ, Turner BJ. A qualitative study of veterans on long-term opioid analgesics: barriers and facilitators to multimodality pain management. Pain Med 2015; 16 726–32.
| A qualitative study of veterans on long-term opioid analgesics: barriers and facilitators to multimodality pain management.Crossref | GoogleScholarGoogle Scholar | 25528887PubMed |
[41] Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F, Rajan P, Baser O, Murrelle L. Risk factors for serious prescription opioid-related toxicity or overdose among veterans health administration patients. Pain Med 2014; 15 1911–29.
| Risk factors for serious prescription opioid-related toxicity or overdose among veterans health administration patients.Crossref | GoogleScholarGoogle Scholar | 24931395PubMed |
[42] Campbell G, Nielsen S, Bruno R, Lintzeris N, Cohen M, Hall W, Larance B, Mattick RP, Degenhardt L. The Pain and Opioids IN Treatment (POINT) study: characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain 2015; 156 231–42.
| The Pain and Opioids IN Treatment (POINT) study: characteristics of a cohort using opioids to manage chronic non-cancer pain.Crossref | GoogleScholarGoogle Scholar | 25599444PubMed |
[43] Slaunwhite AK. The role of gender and income in predicting barriers to mental health care in Canada. Community Ment Health J 2015; 51 621–7.
| The role of gender and income in predicting barriers to mental health care in Canada.Crossref | GoogleScholarGoogle Scholar | 25563485PubMed |




