Pedipalp anatomy of the Australian black rock scorpion, Urodacus manicatus, with implications for functional morphology
Russell D. C. Bicknell A B * , Gregory D. Edgecombe C , Christopher H. R. Goatley A D E , Glen Charlton F and John R. Paterson A
A B * , Gregory D. Edgecombe C , Christopher H. R. Goatley A D E , Glen Charlton F and John R. Paterson A
A
B
C
D
E
F
Abstract
Pedipalps – chelate ‘pincers’ as the second pair of prosomal appendages – are a striking feature of scorpions and are employed in varied biological functions. Despite the distinctive morphology and ecological importance of these appendages, their anatomy remains underexplored. To rectify this, we examined the pedipalps of the Australian black rock scorpion, Urodacus manicatus, using a multifaceted approach consisting of microcomputed tomography, scanning electron microscopy, energy dispersive X-ray spectroscopy, and live pinch force measurements. In doing so, we document the following aspects of the pedipalps: (1) the musculature in three dimensions; (2) the cuticular microstructure, focusing on the chelae (tibial and tarsal podomeres); (3) the elemental construction of the chelae teeth; and (4) the chelae pinch force. We recognise 25 muscle groups in U. manicatus pedipalps, substantially more than previously documented in scorpions. The cuticular microstructure – endo-, meso-, and exocuticle – of U. manicatus pedipalps is shown to be similar to other scorpions and that mesocuticle reinforces the chelae for predation and burrowing. Elemental mapping of the chelae teeth highlights enrichment in calcium, chlorine, nickel, phosphorus, potassium, sodium, vanadium, and zinc, with a marked lack of carbon. These elements reinforce the teeth, increasing robustness to better enable prey capture and incapacitation. Finally, the pinch force data demonstrate that U. manicatus can exert high pinch forces (4.1 N), further highlighting the application of chelae in subduing prey, as opposed to holding prey for envenomation. We demonstrate that U. manicatus has an array of adaptions for functioning as a sit-and-wait predator that primarily uses highly reinforced chelae to process prey.
Keywords: Australia, energy dispersive X-ray spectroscopy, micro-computed tomography, microstructure, morphology, musculature, scanning electron microscopy, scorpions, Urodacus manicatus.
Introduction
Chelate pedipalps are one of the most striking structures displayed by scorpions. These modified prosomal appendages serve disparate functions, including predation (Lamoral 1971; Simone and van der Meijden 2018; Leeming 2019; Cunha et al. 2022), defence (van der Meijden et al. 2013), and burrowing (Harington 1977; Abdel-Nabi et al. 2004). As such, the mechanics (van der Meijden et al. 2012a; Bicknell et al. 2022), structure (Zhao et al. 2016; Zhang et al. 2023), and general biology (Snodgrass 1952) of pedipalps have been discussed at length.
The pedipalp chelae are one of few regions of the scorpion body to have been subjected to three dimensional (3D) documentation (van der Meijden et al. 2012a; Simone and van der Meijden 2018; Kellersztein et al. 2019). This information is supplemented by 3D data for the stinger (Zhao et al. 2016; van der Meijden and Kleinteich 2017; Simone and van der Meijden 2021), metasomal muscles (Günther et al. 2021a, 2021b), and the vascular system (Wirkner and Prendini 2007). Limited examination of scorpion appendages using 3D methods contrasts with the current state of research into other arachnids, such as camel spiders (van der Meijden et al. 2012b; Runge and Wirkner 2020), pseudoscorpions (Michalski et al. 2022), whip scorpions (Grams et al. 2018), and whip spiders (Schmidt et al. 2022). However, scorpion appendage musculature has a long history of documentation in two dimensions (2D) (Lankester 1885; Snodgrass 1948; Alexander 1967; Durale and Vyas 1968; Manton 1977; Bowerman and Root 1978; Shultz 1989, 1992, 2007; Wolf and Harzsch 2002), providing a precedent for presenting such data within a more interactive and tangible framework.
Other aspects of the pedipalps that require further study are the microstructure and chemical composition of the cuticle. Scorpion diversity has inspired extensive documentation of many exoskeletal structures, and, as a result, the group has the most comprehensive information on cuticle among chelicerates (Krishnan 1953; Kennaugh 1959; Malek 1964; Mutvei 1978; Filshie and Hadley 1979; Dalingwater 1987; Kellersztein et al. 2019, 2021). However, the pedipalps, particularly the chelate sections, have not been subject to detailed study (see Mutvei 1978; Kellersztein et al. 2019, 2021), and there are limited data on the chemical composition of chelae (Schofield et al. 2003).
To better understand the morphofunctionality of the pedipalps as a key adaptation of scorpions, we present an integrative, multifaceted investigation of an exemplar species framed around four core scientific questions:
How is the morphology of scorpion pedipalp musculature expressed, how many muscles can be identified, and to what extent can we homologise these muscles with other scorpion species?
What is the cuticular microstructure of pedipalps and does microstructure vary in different regions and cross-sectional orientations?
What are the elemental constituents of scorpion pedipalp appendages and how can the elemental composition be used to inform functional interpretations?
How much force can scorpion pedipalps exert while pinching and what does this mean for species ecology?
Beyond these scientific questions, we aim to illustrate how a synthesis of methodologies can build impactful frameworks to thoroughly document arthropod predatory morphologies. Using this objective, we highlight how multiple topics in animal ecology and morphology can be explored.
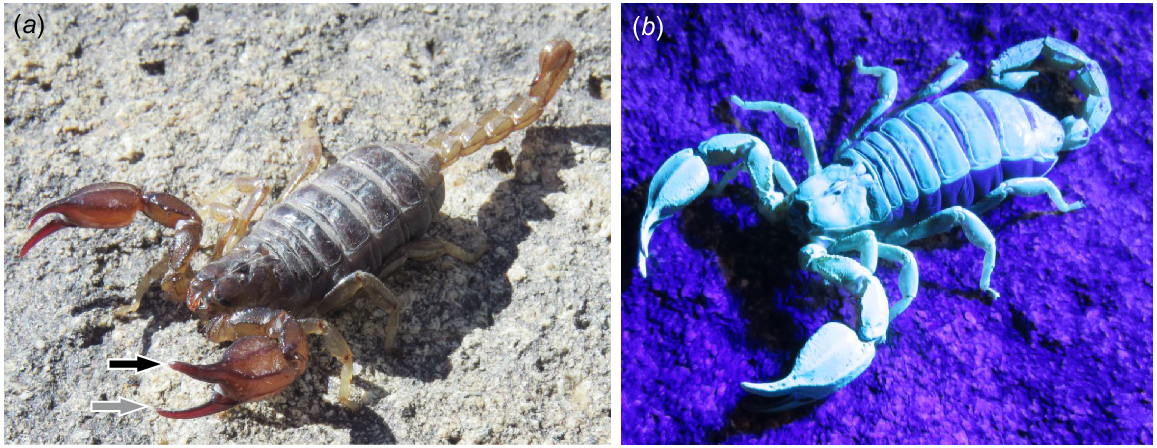
To address these questions, we examine a well-known Australian scorpion species, Urodacus manicatus (Thorell 1876) (Fig. 1), as a case study. This species was chosen because its distribution (Pocock 1888; Southcott 1955; Koch 1978; Harvey and Volschenk 2002), ecology (Southcott 1955; Smith 1966; Shorthouse and Marples 1982; Warburg and Rosenberg 1994; Holden 1997; Woodman 2008), toxicology (Lowe and Farrell 2011; Luna-Ramirez et al. 2017), taxonomy (Pocock 1898; Harvey and Volschenk 2002; Soleglad et al. 2005), and phylogenetic position (Soleglad et al. 2005) are well documented. In addressing our four core research questions, we present a 3D anatomical atlas of the pedipalp muscles, and document the cuticular microstructure, elemental composition, and pinch force of the chelae of U. manicatus.
Materials and methods
Specimen collection
Five individuals of Urodacus manicatus were collected from a private property in Invergowrie, New South Wales, Australia (coordinates: 30°31′29”S, 151°30′20”E). Individuals were sampled at night, located using an ultraviolet (UV) light source during the Australian summer of February 2020. Based on the enlarged mesosoma, relatively small pectines, and lack of genital papillae, the specimens are identified as females; U. manicatus does not exhibit notable pedipalp sexual dimorphism. Two complete specimens, in addition to three pedipalps extracted post-mortem from two individuals are housed in the arthropod collection of the Natural History Museum (NENH-AR) at the University of New England (UNE), Armidale, Australia; specimen numbers are NENH-AR-00015, NEHM-AR-00016, NENH-AR-00017, NEHM-AR-00018, and NENH-AR-00019. The two largest specimens (NENH-AR-00015, NEHM-AR-00016) were initially kept alive in captivity, fed crickets, and used to determine pinch force values. The other two individuals from which pedipalps were removed (represented by NENH-AR-00017, NEHM-AR-00018, NENH-AR-00019) were frozen soon after collection, while NEHM-AR-00015 and NEHM-AR-00016 were frozen after pinch force data were gathered. The largest individual (NEHM-AR-00015) was selected for microcomputed tomography (micro-CT) scanning and reconstruction. The fifth (unregistered) specimen was released after being photographed under sunlight and UV light using a Canon PowerShot SX60 HS camera.
Micro-CT scanning and 3D reconstruction
Specimen NEHM-AR-00015 was micro-CT scanned to document the pedipalp muscles. Prior to scanning, it was submerged in ethanol to dehydrate muscles for more effective identification in the micro-CT scan. A scan with just dehydration through ethanol was conducted. Contrast-enhancement using phosphotungstic acid (PTA) (Metscher 2009; Gignac et al. 2016) was subsequently undertaken with NEHM-AR-00015. In this case, the specimen was immersed in a 70 mL mixture of 70% ethanol and PTA, with a PTA concentration of 0.5%. To examine possible increase in density difference due to the PTA, scans were conducted 12, 36, 48, 72, 168, and 192 hours after submergence. However, these scans were abandoned as they generated lower contrast images compared to ethanol fixation. While this might reflect slow diffusion of the PTA through the specimen, we did not pursue this approach further as the scan of the ethanol-only condition was sufficient. NEHM-AR-00015 was scanned in a GE-Phoenix v|tome|xs micro-CT scanner using the ‘Direct’ tube at UNE, under optimised X-ray tube settings (200 kV, 100 A, 1000 × 1000 pixels capture conditions, an isotropic voxel side length of 22.5 μm, and a rotation step of 0.1125°). The scan focused on the pedipalp and prosoma. Scan data were captured and reconstructed using datos|x software ver. 2.2.1 (phoenix, Wunstorf, Germany).
The scan was imported into Mimics ver. 23.0 (Materialise, Leuven, Belgium) and segmented using the ‘Segmenting’ tool. The prosoma, anterior walking legs, pedipalp podomeres, and pedipalp muscles were segmented using the ‘Segmenting’ tool (Supplemental Fig. 1). Muscle descriptions presented here are based on the scans, muscle fibre directions in scans, 3D reconstructions, and publications previously detailing scorpion pedipalp muscles (Vyas 1970; Shultz 2007). To identify a muscle as an independent unit, two criteria were considered. Firstly, segmented muscles were reconstructed in 3D in Mimics and correspondences to muscles in published examples were identified (Vyas 1970; Shultz 2007). Secondly, other muscle groups were defined by matching morphologies and common attachment points, muscle bands and fibre orientation, and positions in both pedipalps. In taking this approach, we consider the identified muscles to be functionally and morphologically independent. Muscles were described following the terminology in Shultz (2007). This includes the origin location, insertion location, and the morphology of the muscles themselves. Muscles are numbered proximal to distal along the appendage. Once the digital dissection was complete, 3D models of the exoskeleton and individual muscles were exported as .STL files from Mimics and imported into Geomagic Studio (3D Systems, North Carolina, USA). All reconstructions were smoothed in Geomagic Studio. Smoothed .STL files were exported from Geomagic Studio, and a 3D PDF was generated using Tetra4D. Distinct muscles were grouped together and distinctly coloured to delineate the different muscle groups. Supplemental Fig. 1 is available from the following link: 10.17605/OSF.IO/GD7TV. All scan data are available as unedited .TIFF stacks on MorphoSource.org at ark:/87602/m4/495251.
Cuticle microstructure and elemental mapping
The three extracted pedipalps were embedded in epoxy blocks for sectioning in transverse, sagittal, and coronal planes. Embedded appendages were ground through to section the pedipalps. Sectioned surfaces were polished, gold coated and imaged using the JEOL JSM-6010LA Scanning Electron Microscope (SEM) at UNE. Accelerating voltages of 10 kV (NEHM-AR-00019) and 20 kV (NEHM-AR-00017, NEHM-AR-00018) were used to acquire the secondary electron (SE) images of the appendages.
The elemental composition of the chelae teeth and proximal cuticle for NEHM-AR-00018 and NEHM-AR-00019 were derived with SEM Energy Dispersive X-Ray Spectroscopy (EDS). The specimens were gold coated and analysed under 20 kV for 6 h in the JEOL JSM-6010LA SEM to produce a series of elemental maps. Coating arthropod cuticle with inert metals follows previously developed methodologies (Tadayon et al. 2020; Bentov et al. 2021). This approach avoids build-up of charge while specimens are in the SEM. We did not carbon coat the specimens as this would have rendered carbon measurements unreliable. No distinction regarding elemental distributions in EDS has been reported when different coating materials have been used (Bentov et al. 2021). As such, we are confident our approach did not impact the distribution and relative abundance of elements within the examined sections. Additionally, backscatter images of mapped regions were made under these conditions. Preliminary maps were made to reveal the most informative elements for mapping. Elements, such as iron, were therefore not included as they showed no notable abundance in the preliminary maps.
Chelae force measurements
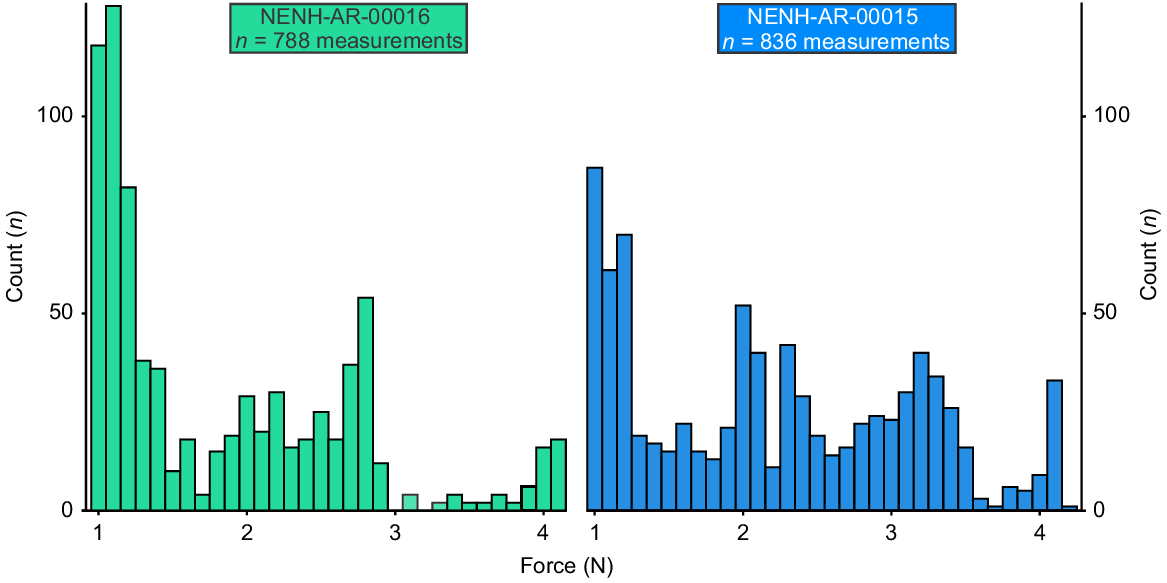
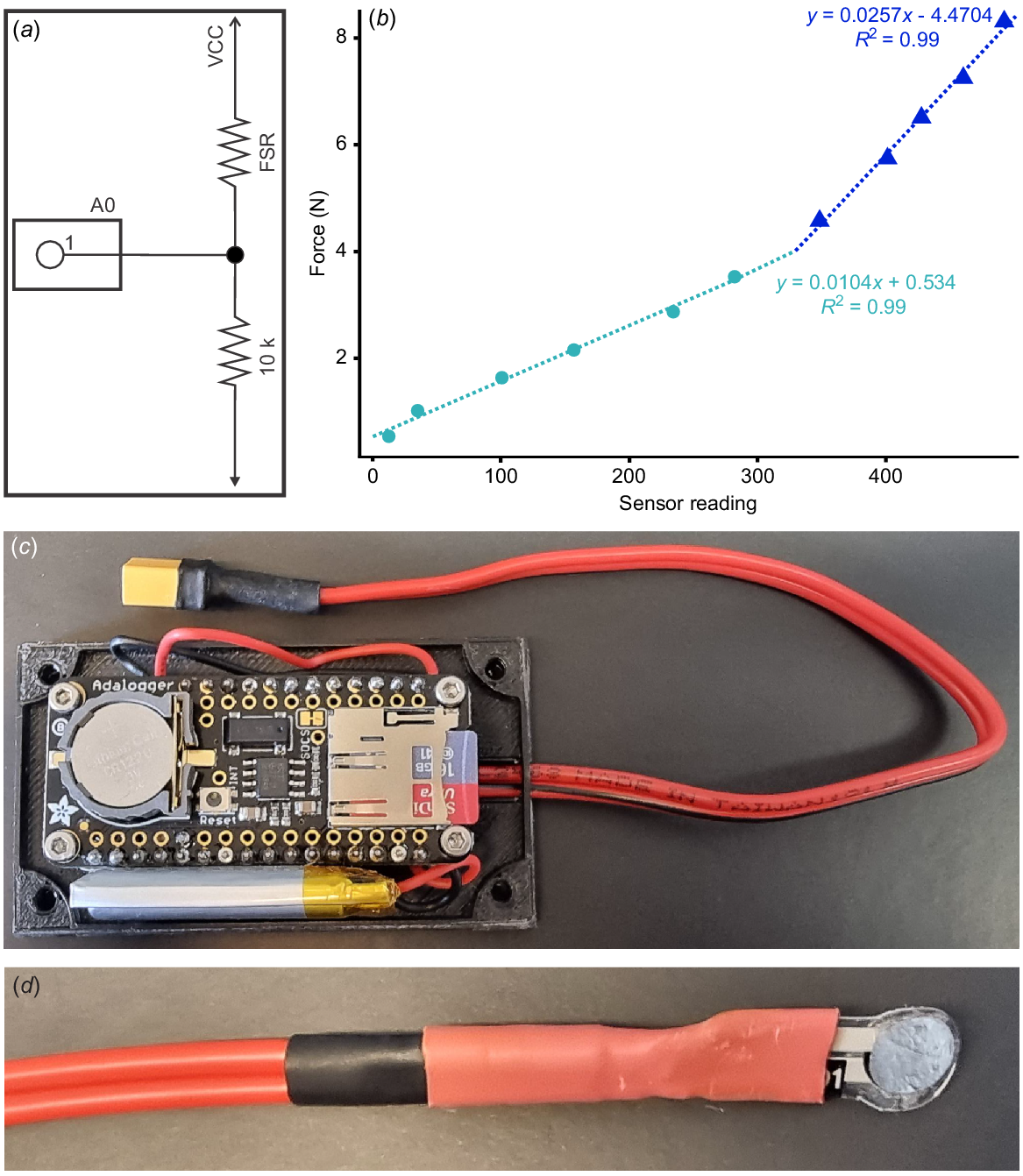
To measure the pinch force, a force-sensitive resistor (FlexiForce A101 Sensor; Tekscan Inc., Massachusetts, United States) was constructed using a voltage divider circuit (Fig. 2a). The A101 sensor was used as its small size (3.8 mm diameter; Fig. 2d) was the most appropriate for measuring pinch force of U. manicatus pedipalps, and helped to avoid unequal loading during pinching. A 50 Hz frequency was used with an analogue input pin and an Adafruit Feather ATSAMD21 Cortex M0 Adafruit (Adafruit Industries, New York, United States) prototyping board. This approach to building a force transducer had not previously been conducted and was novel for this project (Fig. 2c). Sensor calibrations were needed to calculate pinch force. These calibrations were derived by adding weight to the sensor, increasing the force registered through gravity. Two linear equations representing the logarithmic relationship between resistance and force were derived using Microsoft Excel and used to calculate force based on the measured values, while allowing the closest fit of data to calibration values (Fig. 2b; Supplemental Data 1). This approach allowed force data to be gathered at a frequency of 1000 Hz. However, to limit the dataset to a manageable number of observations (<1000), we gathered data at a frequency of 50–100 Hz. To derive pinch force values, NENH-AR-00015 and NEHM-AR-00016 were agitated and prompted to pinch down on the resistor. The outer edge of the force transducer was positioned within the pincer, close to the moveable and fixed finger articulation to induce pinching and avoid unequal loading. This experiment was conducted between 20 and 25°C. This resulted in a dataset of 836 distinct force data points from seven distinct pinch events (NEHM-AR-00015) and 788 distinct force data points from four distinct pinch events (NEHM-AR-00016). Data were plotted as histograms to examine pinch force distribution.
Details of force transducer for pedipalp pinch force tests. (a) Schematic of the circuit used for the transducer. (b) Calibration data used to derive equations for determining force output from the transducer. (c) An image of the force transducer. (d) Example of force plate used. Abbreviations: A0, Analogue measuring point; FSR, Force sensitive resistor; N, Newtons; VCC, Power source (5 V); 10k, Voltage dividing resistor.
Results
Pedipalp musculature
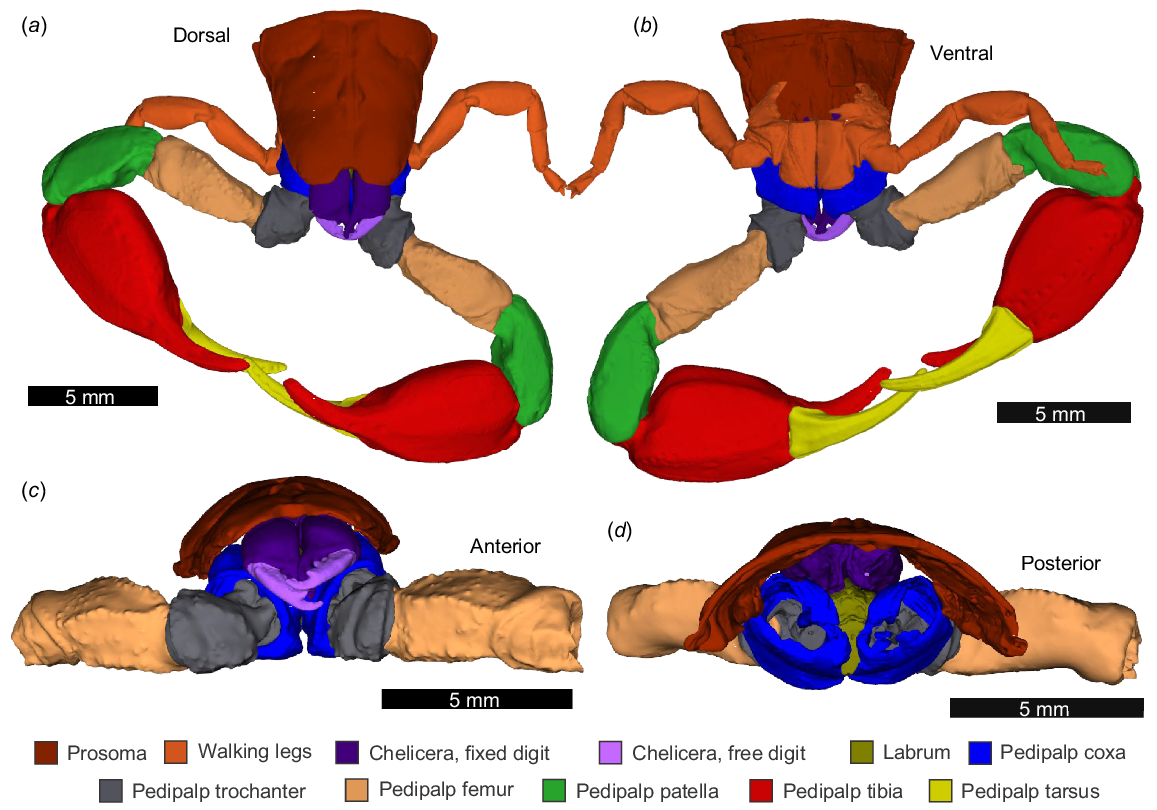
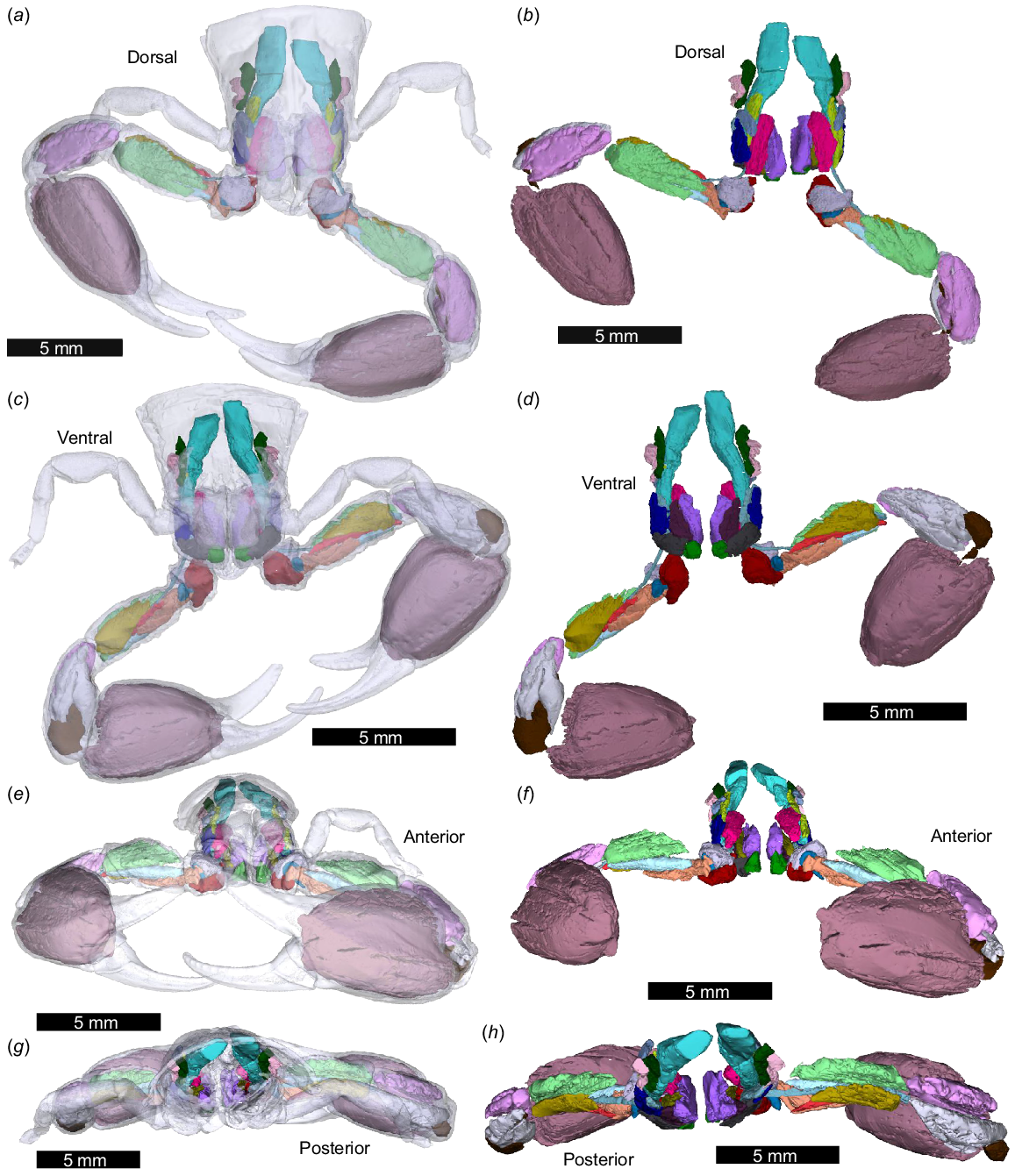
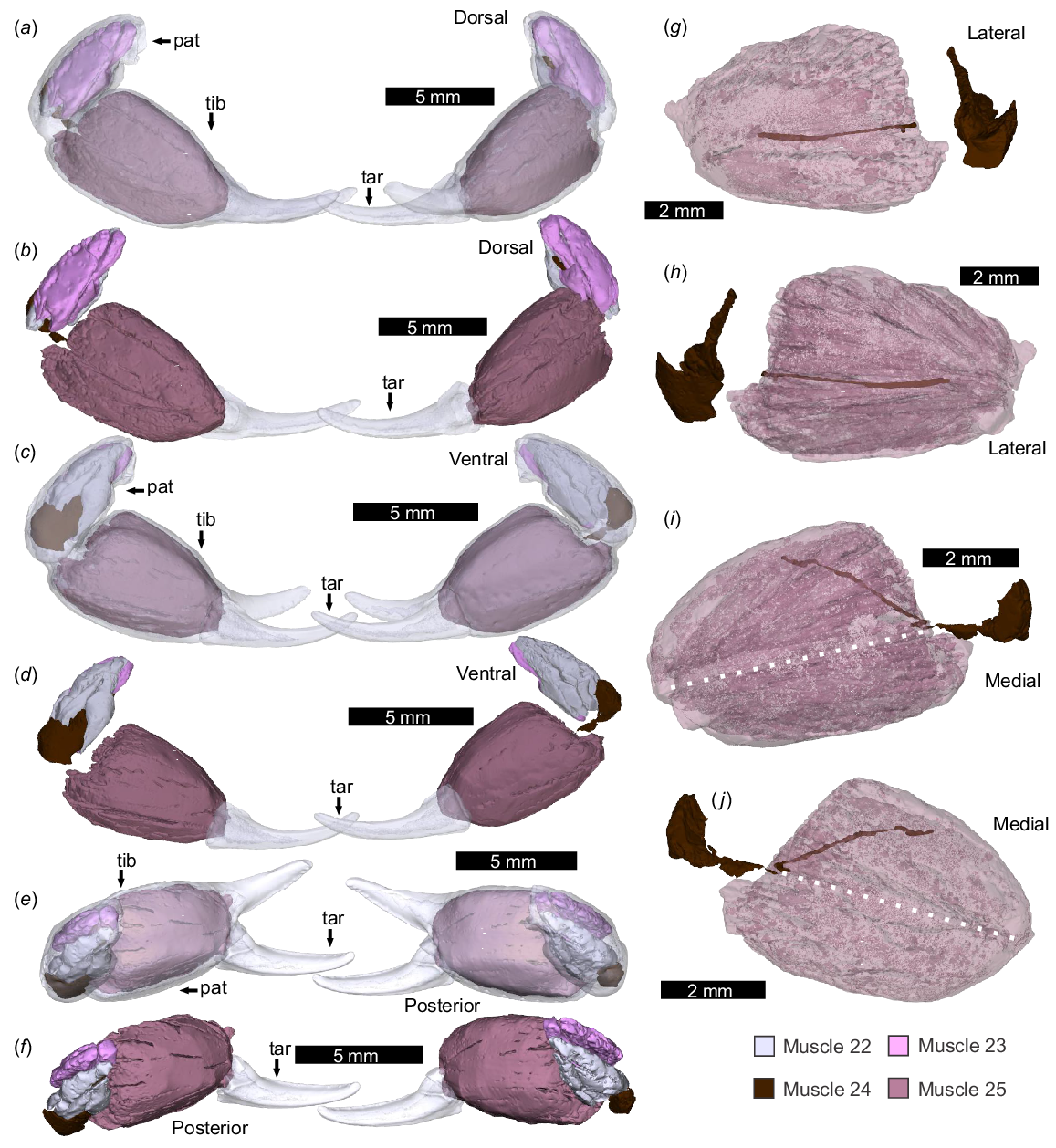
The anteriormost walking legs, fixed and movable cheliceral segments, labrum, prosoma, and pedipalp podomeres (coxa, trochanter, femur, patella, tibia, and tarsus) were resolved (Fig. 3). The pedipalp morphology consists of reduced, stout coxae and trochanters, elongated femurs and patellae, bulbus manus regions in the tibia, with elongate and robust tibial and tarsal fingers. In total, 25 muscle groups associated with the pedipalps were identified (Table 1; Figs 4, 5, 6, 7 and 8), expanding on the muscles presented by Vyas (1970) and Shultz (2007) (Table 1). These additional muscles are primarily located within the trochanter (Muscles 8–13) and are generally smaller and located proximally to larger muscle groups, so may have been missed or grouped together in previous studies (Table 1). The 25 muscles include groups with a prosomal origin (Muscles 1–5; see depiction of Muscles P1, P2, P3, P8, P9 in Shultz 2007; Table 1) and groups located wholly within the pedipalps (Muscles 6–25). Most pedipalp muscles have an origin proximally within their respective appendage segments (Figs 4, 5, 6, 7 and 8) and insert within or proximal to the next distal podomere (Table 1). There are two muscles with large tendons that extend to more distal podomeres: Muscles 9 and 24. In the micro-CT scan, several longer muscle groups show breakage or truncation. In particular, we note that Muscles 1, 2, and 21 and the tendons of Muscles 9 and 24 show this condition. These breakages reflect fixing from ethanol and muscle damage during dehydration.
3D reconstructions of the anterior region of Urodacus manicatus, as modelled by micro-CT scanning. NEHM-AR-00015. (a) Dorsal view. (b) Ventral view. (c) Anterior view excluding patella, tibia, and tarsus. (d) Posterior view excluding patella, tibia, and tarsus. The 3D PDF is found in Supplemental Fig. 1.
| Muscle number | Origin | Insertion | Muscle morphology | Muscle name in Vyas (1970) using Heterometrus fulvipes (Koch 1837) | Muscle name in Shultz (2007) using Heterometrus spinifer (Ehrenberg, 1828) | Figure | |
|---|---|---|---|---|---|---|---|
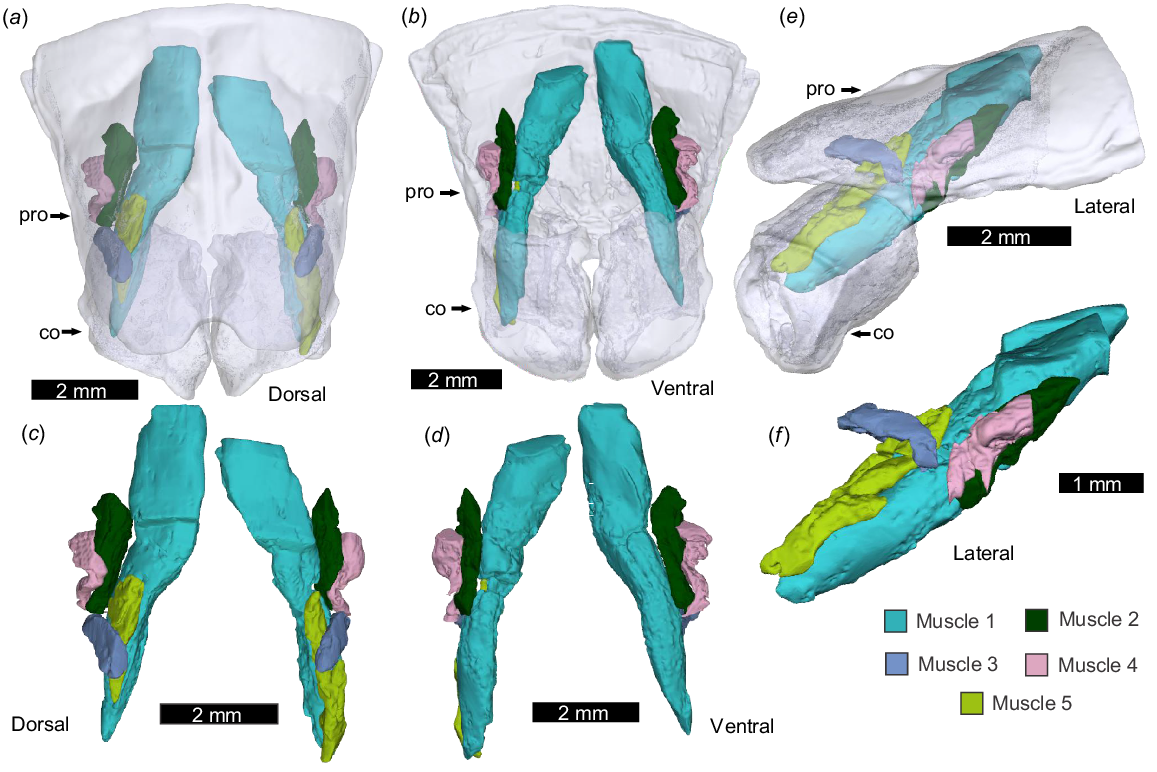
| Muscle 1 | Medial, ventral cuticle prosoma | Distolateral coxa | Large, elongate, tapering to insertion | ? Muscle depressor trochanteris posterioris | Pedipalp 9 | Fig. 5 | |
| Muscle 2 | Mediolateral, ventral cuticle prosoma | Proximolateral margin coxa | Elongate, limited tapering to insertion | – | Pedipalp 8 | Fig. 5 | |
| Muscle 3 | Anterolateral, ventral cuticle prosoma | Proximodorsal margin coxa | Slender, tapering to insertion | Muscle abductor coxalis | Pedipalp 2 | Fig. 5 | |
| Muscle 4 | Mediolateral, ventral cuticle prosoma, more lateral than Muscle 3 | Proximoventral margin coxa | Elongate, marked tapering to insertion | Muscle protractor coxalis | Pedipalp 3 | Fig. 5 | |
| Muscle 5 | Anterolateral, ventral cuticle prosoma | Distolateral coxa | Elongate, limited tapering to insertion | ? Muscle promotor coxalis | Pedipalp 1 | Fig. 5 | |
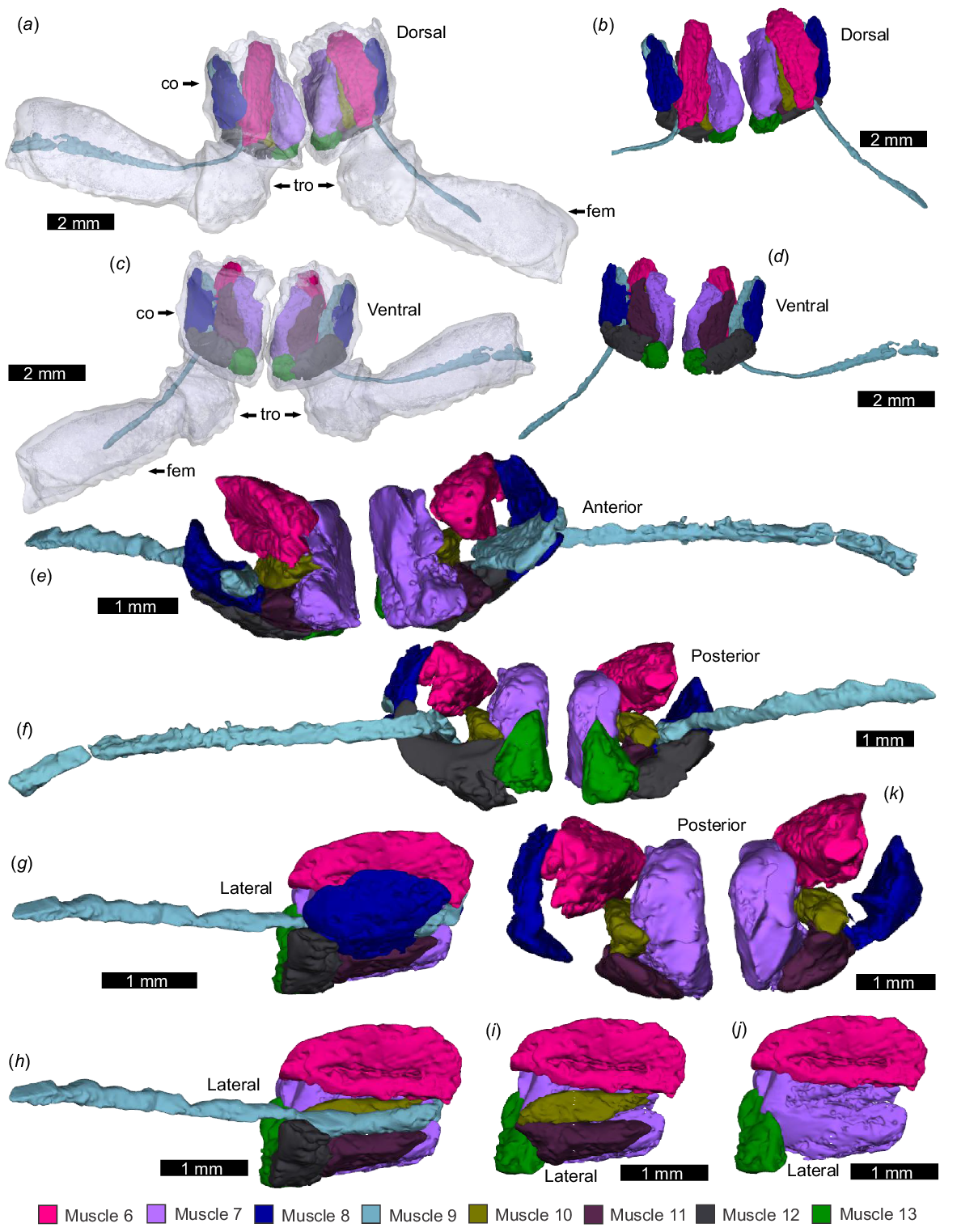
| Muscle 6 | Proximomedial coxa | Proximolateral margin trochanter | Large, no tapering to insertion | Muscle levator trochanteris obliquus | – | Fig. 6 | |
| Muscle 7 | Proximoventral coxa | Proximomedial margin trochanter | Large, ovate, no tapering to insertion | ?Muscle depressor trochanteris anterioris | – | Fig. 6 | |
| Muscle 8 | Proximolateral to proximoventral coxa | Proximolateral margin trochanter | Large, reniform, limited tapering to insertion | ?Muscle levator trochanteris ventralis | – | Fig. 6 | |
| Muscle 9* | Proximolateral to proximoventral coxa, nested within Muscle 8 | Tendon extending to proximomedial patella | Round, extending into elongate tendon | – | – | Figs 6 and 7 | |
| Muscle 10* | Proximomedial coxa, nested within Muscle 7 | Proximoventral margin trochanter | Elongate, limited tapering to insertion | – | – | Fig. 6 | |
| Muscle 11* | Proximoventral through to ventromedial coxa | Lateral, proximoventral margin trochanter | Fan shape, increasing in size towards insertion | – | – | Fig. 6 | |
| Muscle 12* | Lateral, distoventral coxa | Medial, proximoventral margin trochanter | Subrectangular, limited tapering | – | Fig. 6 | ||
| Muscle 13* | Distomedial coxa | Medial, proximoventral margin trochanter | Triangular | – | Fig. 6 | ||
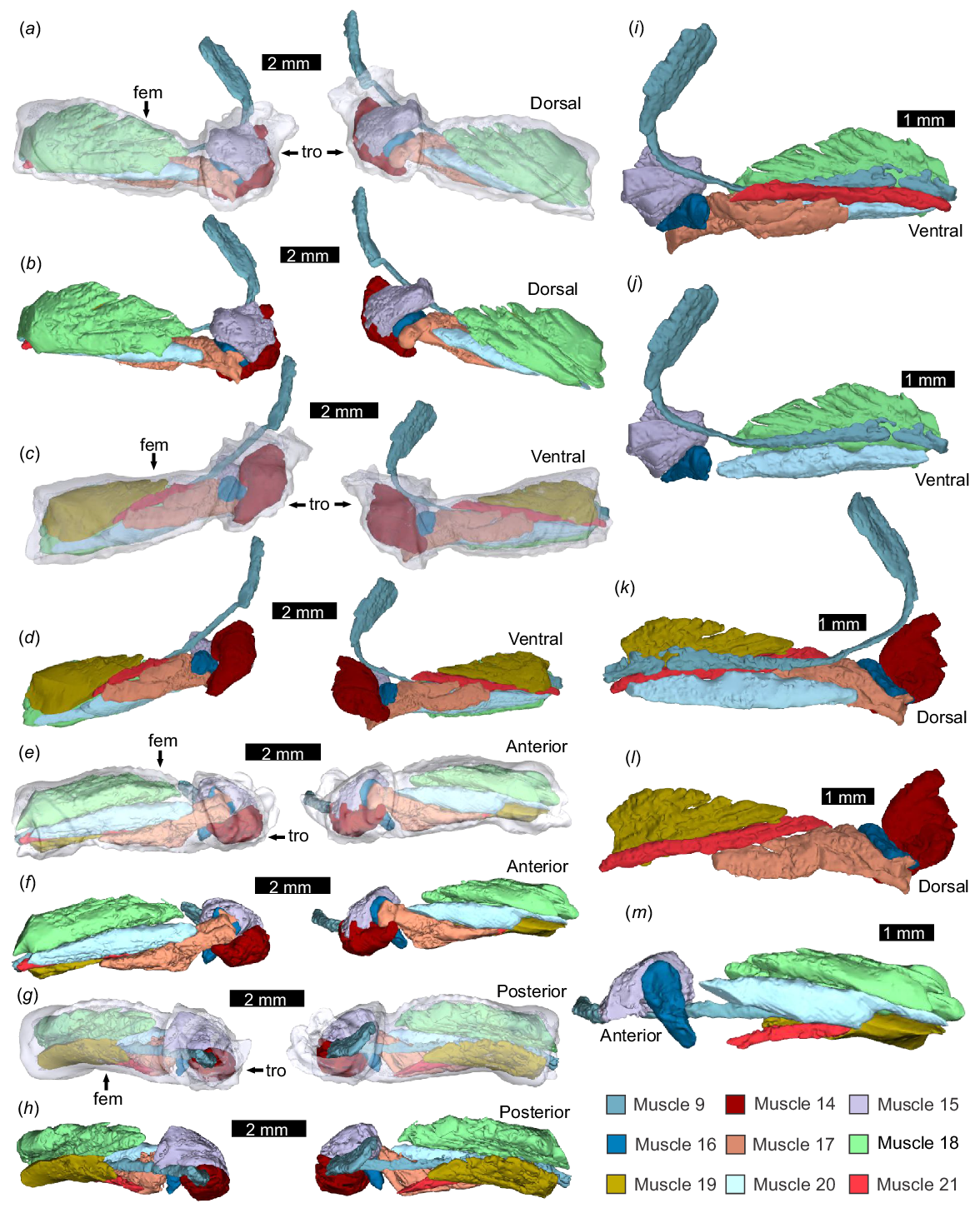
| Muscle 14* | Proximomedial though to ventral region trochanter | Medial, proximoventral margin femur | Round, tapering into elongate shape towards insertion | – | Fig. 7 | ||
| Muscle 15 | Dorsomedial trochanter | Dorsodistal trochanter and proximodorsal margin femur | Fan shaped, tapering to insertion | ? Muscle levator femoralis | – | Fig. 7 | |
| Muscle 16 | Dorsodistal trochanter | Proximoventral femur | Fan shaped, tapering to insertion | ? Muscle depressor femoralis posterioris | – | Fig. 7 | |
| Muscle 17 | Distal trochanter | Ventral femur, half way along femur | Elongate, marked tapering to insertion | ? Muscle depressor femoralis anterioris | – | Fig. 7 | |
| Muscle 18 | Proximo-dorsolateral to disto-dorsolateral femur | Proximodorsal margin patella | Large, fan shaped, tapering to insertion | Muscle depressor patellae anterioris, Muscle depressor patellae posterioris | – | Fig. 7 | |
| Muscle 19 | Proximo-ventrolateral femur | Proximoventral margin patella | Fan shaped, tapering to insertion | Muscle depressor patallae ventralis | – | Fig. 7 | |
| Muscle 20 | Proximomedial at trochanter-femur articulation | Proximomedial margin patella | Elongate, no tapering | Muscle depressor patellae internus | – | Fig. 7 | |
| Muscle 21* | Proximo-ventrolateral femur | Proximomedial margin patella | Elongate, no tapering | – | Fig. 7 | ||
| Muscle 22 | Proximo-ventrolateral and proximo-ventromedial patella | Proximolateral margin tibia | Fan shaped, two major sections, tapering to insertion | Muscle extensor tibialis | – | Fig. 8 | |
| Muscle 23 | Proximo-dorsolateral and proximo-dorsomedial patella | Proximomedial margin tibia | Fan shaped, two major sections, tapering to insertion | Muscle flexor tibialis | – | Fig. 8 | |
| Muscle 24 | Distoventral patella | Tendon extending to tarsus | Fan shaped tapering rapidly into elongate tendon passing through Muscle 25 | Muscle depressor taraslis posterioris | – | Fig. 8 | |
| Muscle 25 | All surfaces of tibia | Proximal tarsus | Large, ovate to fan shaped | Muscle depressor taraslis anterioris | – | Fig. 8 |
Proposed homologies with the pedipalp muscles in Vyas (1970) and Shultz (2007) are included. ‘*’ indicates new muscles identified here. ‘–‘ indicates that this muscle was not examined in the comparative publications. ‘?’ indicates an uncertain homology based on comparing our reconstructions with other publications. See Figs 5–8 and Supplemental Fig. 1.
3D reconstructions of all identified prosomal and pedipalp muscles in NEHM-AR-00015, as modelled from micro-CT scanning. No key is included as some groups are not easily visualised when all muscles are illustrated. Separate muscles are illustrated in Figs 5–8. (a, c, e, g) Muscles with the exoskeleton as transparent. (b, d, f, h) Muscles without the exoskeleton. (a, b) Dorsal view. (c, d) Ventral view. (e, f) Anterior view. (g, h) Posterior view. All muscles are coloured separately. The 3D PDF associated with this reconstruction is found in Supplemental Fig. 1.
Pedipalp muscles with prosomal origins, as modelled by micro-CT scanning. NEHM-AR-00015. (a, b, e) Prosoma and coxa as transparent. (c, d, f) Muscles without the exoskeleton. (a, c) Dorsal view. (b, d) Ventral view. (e, f) Left lateral view. Abbreviations: co, coxa; pro, prosoma. The 3D PDF associated with this reconstruction is found in Supplemental Fig. 1.
Pedipalp muscles with coxal origins, as modelled from micro-CT scanning. NEHM-AR-00015. (a, c) Coxa, trochanter, and femur transparent with muscles. (b, d–j) Muscles without the exoskeleton. (a, b) Dorsal view. (c, d) Ventral view. (e) Anterior view. (f) Posterior view. (g–j) Left lateral view showing digital dissection of superimposed muscles. (k) Posterior view owing digital dissection of superimposed muscles. Abbreviations: co: coxa; fem: femur; tro: trochanter. The 3D PDF associated with this reconstruction is found in Supplemental Fig. 1.
Pedipalp muscles of the trochanter and femur, as modelled from micro-CT scanning. NEHM-AR-00015. (a, c, e, g) Trochanter and femur transparent with muscles. (b, d, f, h–m) Muscles without the exoskeleton. (a, b) Dorsal view. (c, d) Ventral view. (e, f) Anterior view. (g, h) Posterior view. (i, j) Ventral view of right appendage showing digital dissection of superimposed muscles. (k, l) Dorsal view of right appendage showing digital dissection of superimposed muscles. (m) Anterior view of left appendage showing digital removal of Muscles 14 and 17. Abbreviations: fem: femur; tro: trochanter. The 3D PDF associated with this reconstruction is found in Supplemental Fig. 1.
Pedipalp muscles from the patella, tibia, and tarsus, as modelled from micro-CT scanning. NEHM-AR-00015. (a, c, e) Patella, tibia, and tarsus transparent with muscles. (b, d, f) Patella, tibia, and tarsus muscles with transparent tarsus. (a, b) Dorsal view. (c, d) Ventral view. (e, f) Posterior view. (g–j) Muscles 24 and 25 without the exoskeleton showing Muscle 25 as transparent. Dashed white line indicates the direction of Muscle 24. (g, h) Left appendage. (i, j) Right appendage. (g, i) Lateral view. (h, j) Medial view. Abbreviations: pat: patella; tar: tarsus; tib: tibia. The 3D PDF associated with this reconstruction is found in Supplemental Fig. 1.
Pedipalp microstructure
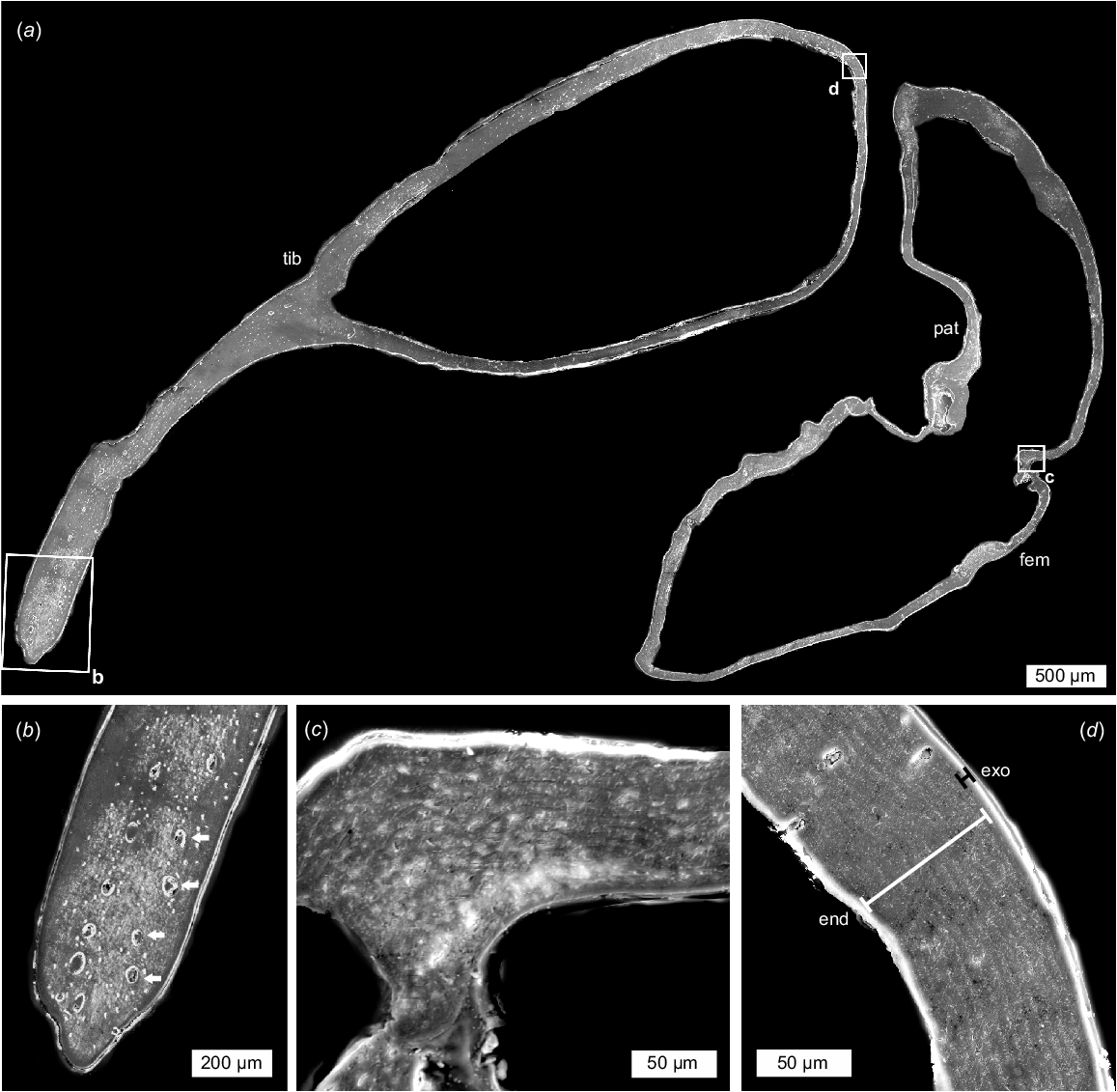
Under the SEM, cuticle structures (sensuRichards 1951; Dalingwater 1987) were observed along the sectioned appendages (Fig. 9). In the sagittal section (NENH-AR-00017), laminate endocuticle is ~70 μm thick, with single lamina ~5 μm thick, and exocuticle 8 μm thick (Fig. 9d). There is a notable lack of laminae in the tibial fixed finger, but 14–60 μm wide pore canals are observed here (Fig. 9b).
SEM-SE images of a sagittal section of the pedipalp. NEHM-AR-00017. (a) Section through the femor, patella, and tibia regions; boxes outline the three areas illustrated in b–d. (b) Section of fixed tibia finger. Note limited laminate cuticle and extensive pore canals (white arrows). (c) Close-up of articulation between patella and femur showing laminate endocuticle. (d) Close-up of the proximal tibia edge showing laminate endocuticle (white line) and thin exocuticle (black line). The white outermost layer is epoxy resin, not cuticle. Abbreviations: end: endocuticle; exo: exocuticle; fem: femur; pat: patella; tib: tibia.
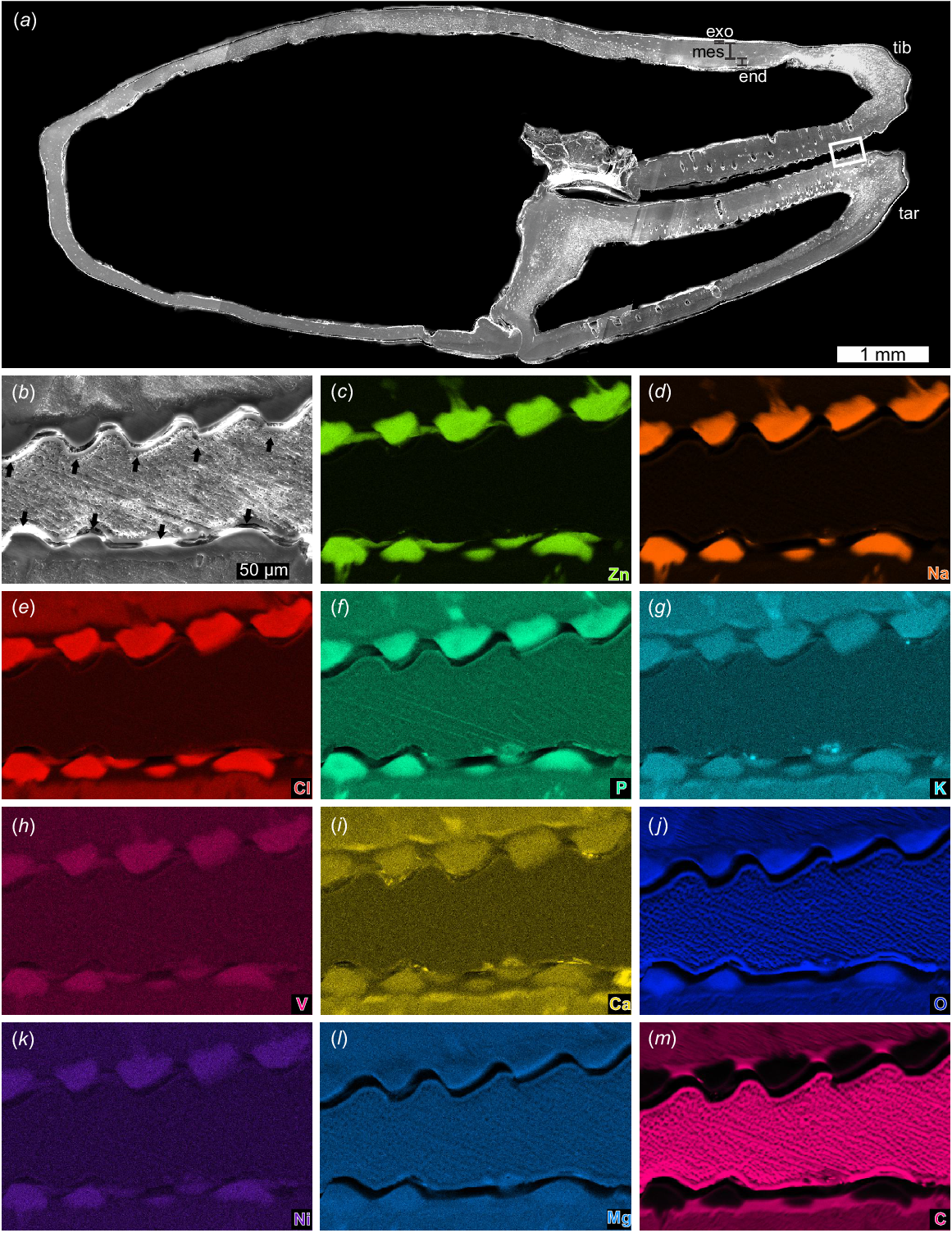
Cuticle morphology along both fingers is visible in the coronal section (Fig. 10a; NENH-AR-00018). Laminate endocuticle (~80–200 μm, single lamina ~5 μm thick) is overlain by 70–130 μm-thick mesocuticle (sensuZhang et al. 2023) that lacks any structure when viewed coronally. Above the mesocuticle is thin exocuticle (12–17 μm) with 2–5 laminae (~4 μm thick) (Fig. 10a). Pore canals up to 180 μm wide are present in the finger and proximal tarsal regions. Finger teeth are darker than other cuticular areas in backscatter imagery and show tooth roots extending into the fingers (Fig. 10b).
SEM images and EDS elemental maps of coronal section of chela. NEHM-AR-00018. (a) SEM image of sectioned tibia and tarsus showing pore canals in the moveable and fixed fingers. Lines show cuticle section thickness. Box outlines close up in (b–m). (b) Backscatter image of box in (a) showing teeth (black arrows). (c–m) Elemental maps of zinc, sodium, chlorine, phosphorous, potassium, vanadium, calcium, oxygen, nickel, magnesium, and carbon, respectively. Note the teeth roots in (c–g). Scale bar in (b) is the same for (c–m). Abbreviations: end: endocuticle; exo: exocuticle; mes: mesocuticle; tib: tibia; tar: tarsus.
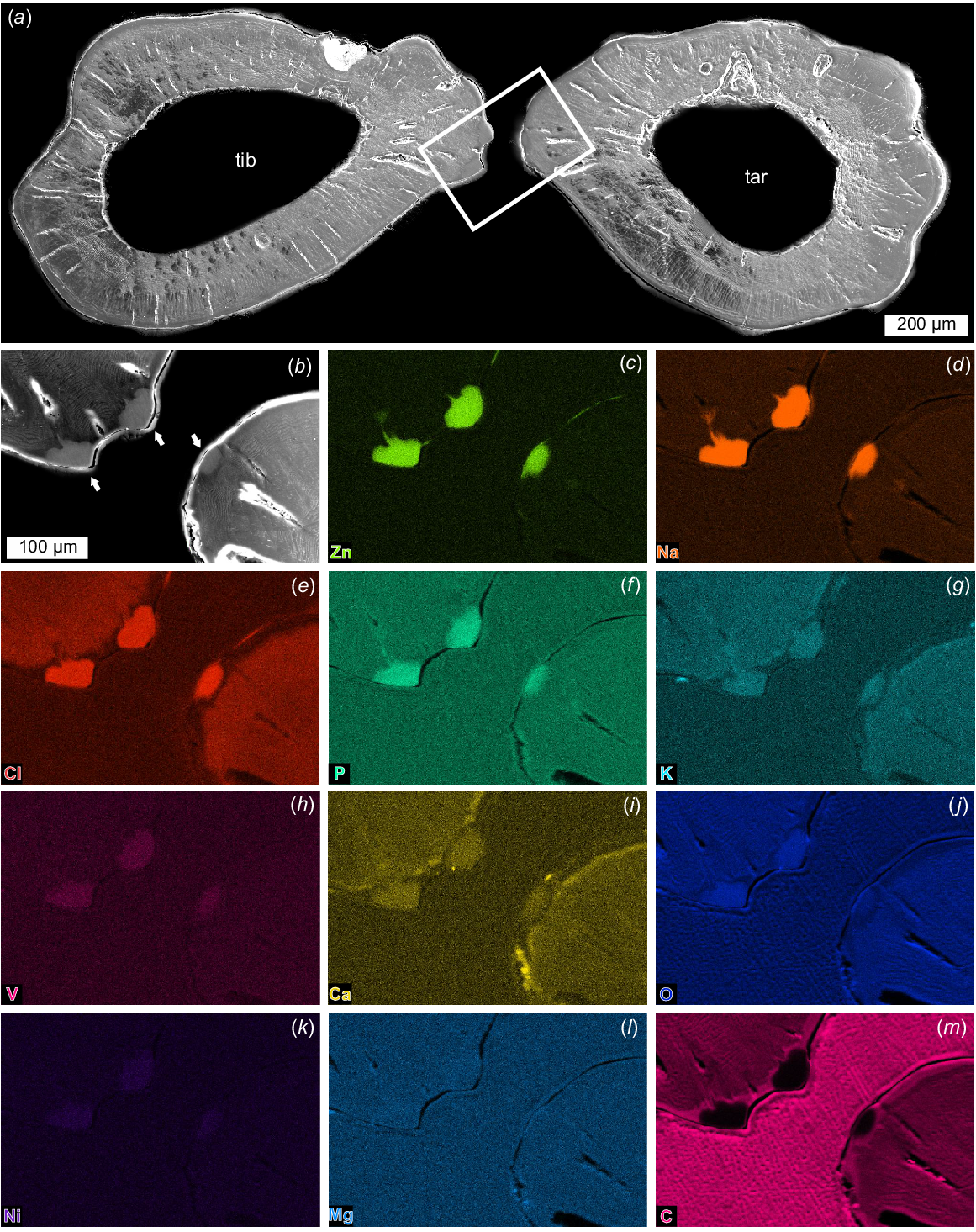
In the transverse section (Fig. 11; NENH-AR-00019), the laminate endocuticle is ~90 μm thick, with single lamina ~5 μm thick. Regions where the endocuticle extends to the teeth are observed, with a thickness of ~290 μm (Fig. 11a). Where the endocuticle does not extend to the teeth, the endocuticle is overlain by mesocuticle, consisting of vertically stacked cuticle that is 60–70 μm thick. The mesocuticle is overlain by exocuticle that is 20–30 μm thick, with ~4 μm thick laminae. Pore canals reaching 165 μm in length and cross-cut endo-, meso-, and exocuticle layers. Teeth are lighter than other cuticle in backscatter imagery (Fig. 11b).
SEM images and EDS elemental maps of transverse section of chela. NEHM-AR-00019. (a) SEM image of sectioned tibia and tarsus fingers showing endocuticle, mesocuticle, and pore canals. Box outlines close up in (b–m). (b) Backscatter image of box in (a) showing teeth (white arrows). (c–m) Elemental maps of zinc, sodium, chlorine, phosphorous, potassium, vanadium, calcium, oxygen, nickel, magnesium, and carbon, respectively. Scale bar in (b) is the same for (c–m). Abbreviations: end, endocuticle; exo, exocuticle; mes, mesocuticle; tib, tibia; tar, tarsus.
Elemental construction of chelae cuticle and teeth
Elemental maps of the coronal and transverse sections focusing on the teeth show similar elemental distributions (Figs 10, 11; NENH-AR-00018, NENH-AR-00019). There is a pervasive enrichment of chlorine (Cl), nickel (Ni), phosphorus (P), potassium (K), sodium (Na), vanadium (V), zinc (Zn), and oxygen (O) in the teeth and roots (Figs 10, 11) and a notable lack of carbon (C) in the teeth. Calcium (Ca) has limited enrichment along the chelae edges and teeth roots. Magnesium (Mg) has a consistent distribution across teeth and cuticle. The general pedipalp cuticle is distinctly lacking in Na, Zn, V, and Ni, has a limited abundance of Cl, P, K, Ca, and O, and an enrichment of C relative to teeth.
Pedipalp pinch force
Pinch force values from specimens NENH-AR-00015 (n = 836 measurements) and NENH-AR-00016 (n = 788 measurements) have similar distributions. Both distributions are right skewed, with major peaks at about 1 N (Fig. 12). NENH-AR-00016 has two smaller peaks at 2.8 N and 4.2 N, and a mean pinch force of 1.84 N. NENH-AR-00015 has three smaller peaks at 2.0 N, 3.2 N, and 4.2 N, and a mean pinch force of 2.22 N. NENH-AR-00015, the larger of the two studied individuals, tended to produce higher pinch forces.
Discussion
Using micro-CT to inform on scorpion anatomy
Our documentation of pedipalp muscles expands on previous research by Vyas (1970, on Heterometrus fulvipes) and Shultz (2007, on Heterometrus spinifer) by presenting 20 muscle groups located solely within pedipalp podomeres and five muscle groups with prosomal origins. Based on the origin, insertion, and muscle shape, we can suggest homologies between 18 of these muscles across these studies (Vyas 1970; Shultz 2007). This demonstrates that at least seven muscles documented herein are novel. These data illustrate how pedipalp muscles engage distinct podomeres. For example, the long tendon of Muscle 9 illustrates that pedipalp coxal muscles engage more distal pedipalp regions than previously realised. These muscle reconstructions have the potential to support arguments about possible homologies between the pedipalp and walking leg muscles of scorpions (see Shultz 2007 for a detailed discussion). In comparing muscles reconstructed here with previous depictions of walking leg muscles (Snodgrass 1952; Shultz 1989; Wolf and Harzsch 2002), similarities in muscle arrangements in the femur and patella can be drawn. In particular, we propose that pedipalp Muscles 17 and 18 of Urodacus manicatus (Fig. 7) are homologous with leg Muscles 19 and 16, respectively, of Pandinus sp. illustrated by Snodgrass (1952, fig. 19G), and Muscles 22 and 23 (Fig. 7) correspond to Muscles 21 and 20 (cf. Snodgrass 1952, fig. 19G). A detailed examination of these muscles using similar tools is needed to document these proposed homologies more thoroughly and confidently.
Functional interpretations for musculature can be drawn using muscle origins, insertions, and size. Muscles 1–5 have prosomal origins, inserting into the coxae. As they are extrinsic muscles (Beck 1885; Lankester 1885; Shultz 2007), they reinforce and maintain the coxal position. Muscles 6–8 and 10–13 are positioned within the coxae, with insertion into the trochanter, likely permitting the large range of motion for the articulation, similar to muscles in the walking legs (Snodgrass 1952). Muscle 9, extending from the coxa to the patella as a tendon, may function to rapidly retract the more distal pedipalp podomeres, possibly during or after capturing prey. This muscle apparently does not have an immediate analogue for comparison within other scorpion appendages. However, Muscle 9 is morphologically comparable to Muscle 10 of horseshoe crab walking legs (Snodgrass 1952, fig. 11a; Bicknell et al. 2018a, fig. 6) which is a flexor that moves the patella and other distal podomeres of the xiphosurid appendage towards more proximal podomeres and the body (Shultz 1989; 2001). A similar tendon extends across multiple appendage podomeres in the scorpion pedipalp. This morphological similarity suggests a comparable function – as a flexor muscle that moves the appendage towards the body, after attacking, or in defence. Such an explanation is also possible for Muscle 21 that could be used to retract the patella. Muscles 14–16 inserting proximal to the patella from the femur also permit a large range of motion for the patella–femur articulation. Muscles 17–20 are likely used to rotate and position the pedipalps relative to the prosoma. Developing on the homology with the walking leg muscles documented by Snodgrass (1952), Muscles 22 and 23 are flexor and extensor muscles, respectively. Among other possible uses, such as mating (Kellersztein et al. 2019), the large size of these muscles can be used to pull prey to the mouth for processing. Finally, Muscles 24 and 25 are well documented in other taxa as the muscles used in the closing and opening of the tarsal podomere (Snodgrass 1952; van der Meijden et al. 2012a; Simone and van der Meijden 2021). More confident determination of the muscle roles discussed here requires in vitro examination of muscle movement (see Walker et al. 2014).
Identifying and reconstructing muscle clusters in 3D represents an important approach for testing hypotheses on the synapomorphies in related groups. One possible direction for this application of 3D anatomy is the grouping of scorpions and pseudoscorpions into Panscorpiones based on phylogenomics and rare genomic changes (Ontano et al. 2021); the traditional sister group hypothesis between pseudoscorpions and solifuges is challenged by the latter not sharing a whole genome duplication characteristic of arachnopulmonate arachnids (Gainett et al. 2024). In this case, one could determine whether the muscles groups in scorpion pedipalps are also observed in pseudoscorpion taxa. While pseudoscorpion coxal muscles have been documented in 3D (Michalski et al. 2022), a detailed consideration of muscles in their pedipalps has not yet been undertaken in a 3D context.
Documenting pedipalp muscles of other scorpion species in 3D to build on our data may be useful for uncovering possible anatomical conservation in scorpions. Examining metasomal muscles of different scorpion genera in this framework has shown how modifying metasomal segment morphologies impacts muscle shape, but conserves muscle groups (Günther et al. 2021a, 2021b). A similar situation is expected for pedipalp muscles. However, as scorpion chelae are extremely morphologically diverse (van der Meijden et al. 2010, 2012a; Simone and van der Meijden 2018; Bicknell et al. 2022), modifications to distal pedipalp muscles are likely. Such muscle data represent as-yet unexplored ecological and phylogenetic signals.
Microstructure of the chelae
Our observations of the cuticular microstructure align with previous research (Mutvei 1978; Kellersztein et al. 2019; Zhang et al. 2023), demonstrating common cuticle construction across scorpion chelae. Among the microstructures we observed, the dorsoventrally oriented mesocuticle laminae has been noted only within the chelae and walking legs (Zhang et al. 2023). This modified cuticle provides reinforcement by orienting cuticle in the same direction as the maximum force, similar to the vertically oriented cuticle in Limulus polyphemus (Linnaeus 1758) gnathobases (Bicknell et al. 2018b). This increases chelae robustness overall.
Mechanical and functional interpretations can be derived by considering the three main cuticle layers. The presence of these layers in the coronal and transverse orientations demonstrates how the Urodacus manicatus chela cuticle is constructed to experience higher stresses and strains (Kellersztein et al. 2019). The marked thickness of the endo- and mesocuticle, compared to the exocuticle, permits more plastic deformation within the structure, allowing for higher loads to be tolerated (Zhang et al. 2023). The thick endocuticle in the sagittal orientation illustrates that chelae fingers are reinforced to limit development of cracks within the cuticle (Zhang et al. 2023). These observations illustrate that U. manicatus chelae are well adapted for handling prey (Farley 1999; Simone and van der Meijden 2018; Kellersztein et al. 2019).
Examination of cuticle microstructure here failed to differentiate exocuticle into hyaline and inner exocuticle regions – the two main divisions of scorpion exocuticle (Filshie and Hadley 1979; Dalingwater 1987; Rubin et al. 2017). This likely results from our study of unstained specimens. Staining, such as Mallory’s Triple Stain technique (Mallory 1900) involves the use of heavy-metal acids (e.g. phosphotungstic acid). We avoided this technique, as it would have impacted the results of the elemental mapping. However, the florescence of Urodacus manicatus under UV light (Fig. 1b) confirms the expected presence of hyaline exocuticle (Rubin et al. 2017).
Elemental composition of the chelae
Elemental mapping revealed several heavy elements in the cuticle. Many arthropods utilise heavy elements to reinforce cuticular structures used in food processing, such as mandibles and raptorial appendages (Hillerton et al. 1984; Fontaine et al. 1991; Schofield et al. 2002, 2021; Lichtenegger et al. 2003; Morgan et al. 2003; Cribb et al. 2008; Gallant and Hochberg 2017; Bentov et al. 2021). Zinc functions as a cross-linker of different proteins to increase hardness and provide reinforcement (Lichtenegger et al. 2003; Cribb et al. 2008, 2010; Gallant et al. 2016), allowing for higher mechanical loads (Tadayon et al. 2020). Increased zinc abundance in arachnids is thus far limited to chelicerae and pedipalp regions (Schofield et al. 1989; Schofield 2001; Cutler and McCutchen 2006; Politi et al. 2012; Gallant et al. 2016; Gallant and Hochberg 2017; Radosavljevic et al. 2021) and is associated with cuticle hardening for handling prey and interacting with substrate (Radosavljevic et al. 2021). Here, the increased zinc in Urodacus manicatus chelate teeth demonstrates similarly robust cuticle. A comparable abundance of chlorine in teeth reflects the presence of chlorides bonded to zinc (Hillerton and Vincent 1982; Schofield 2001; Politi et al. 2012) – a condition already known in scorpions (Schofield et al. 2003; Radosavljevic et al. 2021). The lack of iron within the pedipalps, as observed in the initial scans, reflects a similar pattern of enriched zinc and paucity of iron in non-buthid scorpions (Schofield 2001). The other very abundant element was sodium. However, as NaK and ZnL shell energies overlap (Radosavljevic et al. 2021), the presence of sodium records the overlap of energy shells, not a true abundance. Finally, nickel, potassium, and vanadium may reflect similar reinforcement as zinc in the teeth (Gallant et al. 2016), but their lower abundances likely demonstrate limited involvement in the hardening process.
Phosphorus is uncommon within terrestrial chelicerate cuticle (Cutler and McCutchen 2006; Gallant et al. 2016). When present, phosphorus is incorporated as calcium phosphate [Ca3(PO4)2] (Currey et al. 1982; Becker et al. 2005; Gallant et al. 2016). As we observed phosphorus and calcium in the teeth and roots (Fig. 10f, i), the presence of calcium phosphate seems likely. An alternative form of calcium in the cuticle is calcium oxalate (CaC2O4) (Norton and Behan-Pelletier 1991; Gallant and Hochberg 2017). However, the lack of carbon in roots and teeth (Figs 10m, 11m) excludes this possibility. Calcium has been documented only in spider and whip scorpion exoskeletons (Schofield et al. 2003; Radosavljevic et al. 2021). The record here suggests that calcium is likely more abundant in the chelicerate exoskeleton than previously realised.
The pathway for incorporating additional elements into exoskeletal structures is worth considering, as these elements are very uncommon within other exoskeletal regions. Heavier and otherwise uncommon elements are obtained through diet (Vohland et al. 2003; Gallant and Hochberg 2017) or the environment (Schofield 2001) and incorporated into the exocuticle either during sclerotisation (Cribb et al. 2010) or, in the case of zinc, after sclerotisation (Schofield et al. 2002). The process of incorporating these elements into the teeth is likely related to the roots – these structures are defined by an abundance of zinc, sodium, phosphorus, and potassium (Figs 10c, d, f, g and 11c, d, f, g), with limited vanadium and calcium (Fig. 10h, i). It seems likely that tooth roots allow zinc and other elements to move through the cuticle after sclerotisation (Schofield et al. 2003).
The elemental distributions demonstrate how teeth of Urodacus manicatus chelae are reinforced with different elements and compounds. Metal elements such as zinc, nickel, potassium, and vanadium within the teeth illustrate that they are present to decrease wear and increase the resistance to localised force. This aligns with the three main cuticle layers oriented for more plastic deformation to limit cracks developing within the cuticle during use. Taken together, the teeth and surrounding cuticular region on the chelae are reinforced for functions such as burrowing, mating, or prey capture (Kellersztein et al. 2021).
Most research considering the elemental construction of arthropod cuticle has employed spectrograms of specified regions (e.g. Cribb et al. 2008; Gallant et al. 2016; Gallant and Hochberg 2017; Radosavljevic et al. 2021). This approach uncovers an overall chemical signal but fails to demonstrate elemental distribution. By mapping elements using EDS, a thorough depiction of elemental concentrations in different regions can be determined, and structures such as the teeth roots can be detected (see also Schofield et al. 1989, 2002; Fawke et al. 1997; Politi et al. 2012; Tadayon et al. 2020; Bentov et al. 2021). While EDS mapping is more time-consuming than spectrograms, it presents a more detailed illustration of element location.
Urodacus manicatus pinch force and ecology
Scorpion chelae have disparate functions, the most common of which are burrowing, mating, prey capture, and occasional prey termination (van der Meijden et al. 2012a; Kellersztein et al. 2021; Bicknell et al. 2022). The strongest Urodacus manicatus chelae pinch force values (4.16 N, Supplemental Data 2) are comparable to those of the African fat-tail scorpion [Androctonus amoreuxi (Audouin 1826)] (4.4 N) and the yellow forest scorpion [Opistophthalmus boehmi (Kraepelin 1896)] (4.4 N) (Bicknell et al. 2022; Supplemental Table 3), both of which have chelae used for defence and predation (van der Meijden et al. 2010, 2013), although we acknowledge that comparison is complicated by differences in animal size. Combined with the robust morphology and the array of adaptions within the cuticular microstructure and elemental composition, this high pinch force suggests that U. manicatus likely uses its chelae to attack, capture, and crush prey. This contrasts with forms that display lower force values (<1 N) and rapid chelae closing speeds, using the chelae to hold prey for incapacitation by the stinger (Casper 1985; van der Meijden et al. 2013; Simone and van der Meijden 2018). This observation also aligns with the life mode of U. manicatus as a predator that waits at burrow entrances for prey (Holden 1997) and uses pedipalps, rather than the stinger, to initially subdue prey (Simone and van der Meijden 2018).
A major complication in attempting to more thoroughly understand scorpion feeding ecology is the infrequent consumption of prey (Simone and van der Meijden 2021). Urodacus manicatus, in particular, can survive for up to eight months without food (Southcott 1955). As such, our morphofunctional interpretations of predation are not supported by behavioural observations. However, the application of chelae to subdue prey aligns with the substantial reinforcement of chelate teeth with metals, the additional mesocuticle, and a large tarsal muscle. Field studies of this and other scorpion species, supplemented by metabarcoding of gut contents (Simone et al. 2022), are required to validate these proposed ecological hypotheses.
Synthesis of methodologies
The examination of one biological structure using four methods has given insight into the morphofunctionality of Urodacus manicatus and, more broadly, offers an integrated framework for exploring a key predatory adaptation. Pedipalp musculature is more elaborate than previously noted, including multiple muscles used in prey capture and manipulation. The microstructural data show how thick chelae endo- and mesocuticle allow for plastic deformation, while promoting robustness for enduring increased force during predation. This is supported by the high concentration of metals within the chelae teeth that likely reduce the wear these regions experience. Finally, the force data allow us to contextualise the mechanical performance compared to other species, while ground-truthing the microstructural and skeletomuscular data. Together, these adaptions and morphologies result in a biological structure employed in prey capture and processing.
Future directions
The synthetic approach to understanding Urodacus manicatus presented here forms a base for developing research on this species, and for scorpions more broadly. During this research, we identified three main future directions that are important for expanding on this line of enquiry:
Homology and variation of scorpion appendage muscles: To better understand scorpion pedipalp muscles more broadly, detailed examination of other scorpion species is needed. Building on Günther et al. (2021b), micro-CT scans and segmentation of species from other families will uncover the location and morphology of muscles that could inform on patterns in muscle evolution. Documentation of walking leg muscles would provide the data needed to test homology statements tentatively outlined here.
Metals in chelae teeth: It is interesting to consider how uncommon elements, such as vanadium, have been sequestered into the scorpion exoskeleton and, furthermore, to examine if this condition is common across Scorpiones. We propose two approaches to explore these ideas: (a) Modification of diet under controlled conditions to see if consumption of different prey items affects elemental concentrations in the chelae teeth, building on Schofield (2001); (b) More comprehensive documentation of elemental concentrations within chelae teeth across Scorpiones to explore phylogenetic trends associated with composition (Schofield 2001).
Black Rock Scorpion ecology: Most research focusing on Urodacus manicatus biology has considered burrowing (Woodman 2008), reproduction (Southcott 1955; Warburg and Rosenberg 1994), toxicology (Lowe and Farrell 2011; Luna-Ramirez et al. 2017), and overall physiology (Holden 1997). There is little information on how the species subdues prey. To build on the pinch force analyses presented here, we propose that continued examination into U. manicatus predatory behaviours will further contextualise the force data and, more broadly, the morphofunctional synthesis presented here.
Data availability
The data that support this study are available as supplemental data files associated with this work at OSF (Supplemental Fig. 1: 10.17605/OSF.IO/GD7TV) and MorphoSource.org (ark:/87602/m4/495251).
Declaration of funding
This research was supported by funding from an Australian Research Council Discovery Project grant (DP200102005 to JRP and GDE), UNE Postdoctoral Research Fellowships (to RDCB and CHRG), and an MAT Postdoctoral Fellowship (to RDCB).
Acknowledgements
We thank Jeffrey Shultz for discussions on muscle groups and homologies, as well as four anonymous reviewers, Carolin Haug, and Paul Cooper for the constructive feedback that has improved the manuscript.
References
Abdel-Nabi IM, McVean A, Abdel-Rahman MA, Omran M (2004) Intraspecific diversity of morphological characters of the burrowing scorpion Scorpio maurus palmatus (Ehrenberg, 1828) in Egypt (Arachnida: Scorpionida: Scorpionidae). Serket 9(2), 41-67.
| Google Scholar |
Alexander AJ (1967) Problems of limb extension in the scorpion, Opisthophthalmus latimanus Koch. Transactions of the Royal Society of South Africa 37(3), 165-181.
| Crossref | Google Scholar |
Beck EJ (1885) Description of the muscular and endoskeletal systems of Scorpio. Transactions of the Zoological Society of London 11, 339-360.
| Google Scholar |
Becker A, Ziegler A, Epple M (2005) The mineral phase in the cuticles of two species of Crustacea consists of magnesium calcite, amorphous calcium carbonate, and amorphous calcium phosphate. Dalton Transactions 10, 1814-1820.
| Crossref | Google Scholar |
Bentov S, Palmer BA, Bar-On B, Shelef Y, Aflalo ED, Sagi A (2021) Reinforcement of bio-apatite by zinc substitution in the incisor tooth of a prawn. Acta Biomaterialia 120, 116-123.
| Crossref | Google Scholar |
Bicknell RDC, Klinkhamer AJ, Flavel RJ, Wroe S, Paterson JR (2018a) A 3D anatomical atlas of appendage musculature in the chelicerate arthropod Limulus polyphemus. PLoS ONE 13(2), e0191400.
| Crossref | Google Scholar |
Bicknell RDC, Paterson JR, Caron J-B, Skovsted CB (2018b) The gnathobasic spine microstructure of recent and Silurian chelicerates and the Cambrian artiopodan Sidneyia: functional and evolutionary implications. Arthropod Structure & Development 47(1), 12-24.
| Crossref | Google Scholar |
Bicknell RDC, Simone Y, van der Meijden A, Wroe S, Edgecombe GD, Paterson JR (2022) Biomechanical analyses of pterygotid sea scorpion chelicerae uncover predatory specialisation within eurypterids. PeerJ 10, e14515.
| Crossref | Google Scholar |
Bowerman RF, Root TM (1978) External anatomy and muscle morphology of the walking legs of the scorpion Hadrurus arizonensis. Comparative Biochemistry and Physiology Part A: Physiology 59(1), 57-63.
| Crossref | Google Scholar |
Casper GS (1985) Prey capture and stinging behavior in the emperor scorpion, Pandinus imperator (Koch) (Scorpiones, Scorpionidae). The Journal of Arachnology 13, 277-283.
| Google Scholar |
Cribb BW, Stewart A, Huang H, Truss R, Noller B, Rasch R, Zalucki MP (2008) Unique zinc mass in mandibles separates drywood termites from other groups of termites. Naturwissenschaften 95(5), 433-441.
| Crossref | Google Scholar |
Cribb BW, Lin C-L, Rintoul L, Rasch R, Hasenpusch J, Huang H (2010) Hardness in arthropod exoskeletons in the absence of transition metals. Acta Biomaterialia 6(8), 3152-3156.
| Crossref | Google Scholar |
Cunha HP, Santos AB, Foerster SÍA, Moura GJB, Lira AFA (2022) Can contrasting habitats influence predatory behavior in tropical forest scorpions? Acta Ethologica 25(2), 107-113.
| Crossref | Google Scholar |
Currey JD, Nash A, Bonfield W (1982) Calcified cuticle in the stomatopod smashing limb. Journal of Materials Science 17(7), 1939-1944.
| Crossref | Google Scholar |
Cutler B, McCutchen L (2006) Heavy metals in cuticular structures of Palpigradi, Ricinulei, and Schizomida (Arachnida). The Journal of Arachnology 34(3), 653-656.
| Crossref | Google Scholar |
Durale MS, Vyas AB (1968) The structure of the chela of Heterometrus sp. and its mode of operation. Bulletin of the Southern California Academy of Sciences 67(4), 240-244.
| Google Scholar |
Ehrenberg CG (1828) Phytozoa turbellaria Africana et Asiatica in Phytozoorum Tabula IV et V delineata. In ‘Symbolae physicae, seu icones et descriptiones corporum naturalium novorum aut minus cognitorum quae ex itineribus per Libyam, Aegyptium, Nubiam, Dongalam Syriam, Arabiam et Habessiniam, pars zoologica II, animalia evertebrata exclusis insectis’. (Eds FG Hemprich, CG Ehrenberg) pp. 53–67. (Officina Academica: Berolina)
Fawke JD, Mcclements JG, Wyeth P (1997) Cuticular metals: quantification and mapping by complementary techniques. Cell Biology International 21(10), 675-678.
| Crossref | Google Scholar |
Filshie BK, Hadley NF (1979) Fine structure of the cuticle of the desert scorpion, Hadrurus arizonensis. Tissue and Cell 11(2), 249-262.
| Crossref | Google Scholar |
Fontaine AR, Olsen N, Ring RA, Singla CL (1991) Cuticular metal hardening of mouthparts and claws of some forest insects of British Columbia. Journal of the Entomological Society of British Columbia 88, 45-55.
| Google Scholar |
Gainett G, Klementz BC, Setton EVW, Simian C, Iuri H, Edgecombe GD, Peretti AV, Sharma PP (2024) A plurality of morphological characters need not equate with phylogenetic accuracy: a rare genomic change refutes the placement of Solifugae and Pseudoscorpiones in Haplocnemata. Evolution and Development e12467.
| Crossref | Google Scholar |
Gallant J, Hochberg R (2017) Elemental characterization of the exoskeleton in the whipscorpions Mastigoproctus giganteus and Typopeltis dalyi (Arachnida: Thelyphonida). Invertebrate Biology 136(3), 345-359.
| Crossref | Google Scholar |
Gallant J, Hochberg R, Ada E (2016) Elemental characterization of the cuticle in the marine intertidal pseudoscorpion, Halobisium occidentale. Invertebrate Biology 135(2), 127-137.
| Crossref | Google Scholar |
Gignac PM, Kley NJ, Clarke JA, Colbert MW, Morhardt AC, Cerio D, Cost IN, Cox PG, Daza JD, Early CM, Echols MS, Henkelman RM, Herdina AN, Holliday CM, Li Z, Mahlow K, Merchant S, Müller J, Orsbon CP, Paluh DJ, Thies ML, Tsai HP, Witmer LM (2016) Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. Journal of Anatomy 228, 889-909.
| Crossref | Google Scholar |
Grams M, Wirkner CS, Runge J (2018) Serial and special: comparison of podomeres and muscles in tactile vs walking legs of whip scorpions (Arachnida, Uropygi). Zoologischer Anzeiger 273, 75-101.
| Crossref | Google Scholar |
Günther A, Drack M, Monod L, Wirkner CS (2021a) A unique yet technically simple type of joint allows for the high mobility of scorpion tails. Journal of the Royal Society Interface 18(182), 20210388.
| Crossref | Google Scholar |
Günther A, Monod L, Wirkner CS (2021b) Comparative morphology of scorpion metasomata: Muscles and cuticle. Arthropod Structure & Development 60, 101003.
| Crossref | Google Scholar |
Harington A (1977) Burrowing biology of the scorpion Cheloctonus jonesii Pocock (Arachnida: Scorpionida: Scorpionidae). Journal of Arachnology 5, 243-249.
| Google Scholar |
Harvey MS, Volschenk ES (2002) A forgotten scorpion: the identity of Buthus flavicruris Rainbow, 1896 (Scorpiones), with notes on Urodacus manicatus (Thorell). Records of the Western Australian Museum 21(1), 105-106.
| Crossref | Google Scholar |
Hillerton JE, Vincent JFV (1982) The specific location of zinc in insect mandibles. Journal of Experimental Biology 101, 333-336.
| Crossref | Google Scholar |
Hillerton JE, Robertson B, Vincent JFV (1984) The presence of zinc or manganese as the predominant metal in the mandibles of adult, stored-product beetles. Journal of Stored Products Research 20(3), 133-137.
| Crossref | Google Scholar |
Kellersztein I, Cohen SR, Bar-On B, Wagner HD (2019) The exoskeleton of scorpions’ pincers: structure and micro-mechanical properties. Acta Biomaterialia 94, 565-573.
| Crossref | Google Scholar |
Kellersztein I, Greenfeld I, Wagner HD (2021) Structural analysis across length scales of the scorpion pincer cuticle. Bioinspiration & Biomimetics 16(2), 026013.
| Crossref | Google Scholar |
Kennaugh J (1959) An examination of the cuticles of two scorpions, Pandinus imperator and Scorpiops hardwickii. Journal of Cell Science S3-100(49), 41-50.
| Crossref | Google Scholar |
Koch LE (1978) A comparative study of the structure, function and adaptation to different habitats of burrows in the scorpion genus Urodacus (Scorpionida, Scorpionidae). Records of the Western Australian Museum 6(2), 119-146.
| Google Scholar |
Kraepelin K (1896) Neue und weniger bekannte Scorpione. Mitteilungen aus dem Naturhistorischen Museum (Mitteilungen aus dem Naturhistorischen Museum (Beiheft zum Jahrbuch der Hamburgischen Wissenschaftlichen Anstalten) 13, 119-146.
| Google Scholar |
Krishnan G (1953) On the cuticle of the scorpion Palamneus swammerdami. Journal of Cell Science S3-94(25), 11-22.
| Crossref | Google Scholar |
Lamoral BH (1971) Predation on terrestrial molluscs by scorpions in the Kalahari Desert. Annals of the Natal Museum 21(1), 17-20.
| Google Scholar |
Lankester ER (1885) On the muscular and endoskeletal systems of Limulus and Scorpio; with some notes on the anatomy and generic characters of scorpions. The Transactions of the Zoological Society of London 11(10), 311-384.
| Crossref | Google Scholar |
Lichtenegger HC, Schöberl T, Ruokolainen JT, Cross JO, Heald SM, Birkedal H, Waite JH, Stucky GD (2003) Zinc and mechanical prowess in the jaws of Nereis, a marine worm. Proceedings of the National Academy of Sciences 100(16), 9144-9149.
| Google Scholar |
Lowe RM, Farrell PM (2011) A portable device for the electrical extraction of scorpion venom. Toxicon 57(2), 244-247.
| Crossref | Google Scholar |
Luna-Ramirez K, Tonk M, Rahnamaeian M, Vilcinskas A (2017) Bioactivity of natural and engineered antimicrobial peptides from venom of the scorpions Urodacus yaschenkoi and U. manicatus. Toxins 9(1), 22.
| Crossref | Google Scholar |
Mallory FB (1900) A contribution to staining methods: I. A differential stain for connective-tissue fibrillae and reticulum. II. Chloride of iron haematoxylin for nuclei and fibrin. III. Phosphotungstic acid haematoxylin for neuroglia fibres. Journal of Experimental Medicine 5(1), 15-20.
| Crossref | Google Scholar |
Metscher BD (2009) MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology 9(1), 11.
| Crossref | Google Scholar |
Michalski H, Harms D, Runge J, Wirkner CS (2022) Evolutionary morphology of coxal musculature in Pseudoscorpiones (Arachnida). Arthropod Structure & Development 69, 101165.
| Crossref | Google Scholar |
Morgan TD, Baker P, Kramer KJ, Basibuyuk HH, Quicke DLJ (2003) Metals in mandibles of stored product insects: do zinc and manganese enhance the ability of larvae to infest seeds? Journal of Stored Products Research 39(1), 65-75.
| Crossref | Google Scholar |
Mutvei H (1978) SEM studies on arthropod exoskeletons II. Horseshoe crab Limulus polyphemus (L.) in comparison with extinct eurypterids and recent scorpions. Zoologica Scripta 6(3), 203-213.
| Crossref | Google Scholar |
Norton RA, Behan-Pelletier VM (1991) Calcium carbonate and calcium oxalate as cuticular hardening agents in oribatid mites (Acari: Oribatida). Canadian Journal of Zoology 69(6), 1504-1511.
| Crossref | Google Scholar |
Ontano AZ, Gainett G, Aharon S, Ballesteros JA, Benavides LR, Corbett KF, Gavish-Regev E, Harvey MS, Monsma S, Santibáñez-López CE, Setton EVW, Zehms JT, Zeh JA, Zeh DW, Sharma PP, Pupko T (2021) Taxonomic sampling and rare genomic changes overcome long-branch attraction in the phylogenetic placement of pseudoscorpions. Molecular Biology and Evolution 38(6), 2446-2467.
| Crossref | Google Scholar |
Pocock RI (1888) XX. – The species of the genus Urodacus contained in the collection of the British (Natural-History) Museum. Annals and Magazine of Natural History 2(8), 169-175.
| Crossref | Google Scholar |
Pocock RI (1898) VIII. – The Australian scorpions of the genus Urodacus, Pet. Annals and Magazine of Natural History 2(7), 59-67.
| Crossref | Google Scholar |
Politi Y, Priewasser M, Pippel E, Zaslansky P, Hartmann J, Siegel S, Li C, Barth FG, Fratzl P (2012) A spider’s fang: how to design an injection needle using chitin-based composite material. Advanced Functional Materials 22(12), 2519-2528.
| Crossref | Google Scholar |
Radosavljevic D, Ada E, Hochberg R (2021) Elemental enrichment of the exoskeleton of the whip spider Phrynus marginemaculatus (Arachnida: Amblypygi). The Journal of Arachnology 49(2), 235-249.
| Crossref | Google Scholar |
Rubin M, Lamsdell JC, Prendini L, Hopkins MJ (2017) Exocuticular hyaline layer of sea scorpions and horseshoe crabs suggests cuticular fluorescence is plesiomorphic in chelicerates. Journal of Zoology 303(4), 245-253.
| Crossref | Google Scholar |
Runge J, Wirkner CS (2020) Evolutionary and functional substitution of extrinsic musculature in Solifugae (Arachnida). Journal of Morphology 281(12), 1524-1533.
| Crossref | Google Scholar |
Schmidt M, Melzer RR, Bicknell RDC (2022) Kinematics of whip spider pedipalps: a 3D comparative morpho-functional approach. Integrative Zoology 17(1), 156-167.
| Crossref | Google Scholar |
Schofield R, Lefevre H, Shaffer M (1989) Complementary microanalysis of Zn, Mn and Fe in the chelicera of spiders and scorpions using scanning MeV-ion and electron microprobes. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 40, 698-701.
| Crossref | Google Scholar |
Schofield RMS, Nesson MH, Richardson KA (2002) Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 89, 579-583.
| Crossref | Google Scholar |
Schofield RMS, Nesson MH, Richardson KA, Wyeth P (2003) Zinc is incorporated into cuticular “tools” after ecdysis: The time course of the zinc distribution in “tools” and whole bodies of an ant and a scorpion. Journal of Insect Physiology 49(1), 31-44.
| Crossref | Google Scholar |
Schofield RMS, Bailey J, Coon JJ, Devaraj A, Garrett RW, Goggans MS, Hebner MG, Lee BS, Lee D, Lovern N, Ober-Singleton S, Saephan N, Seagal VR, Silver DM, Som HE, Twitchell J, Wang X, Zima JS, Nesson MH (2021) The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Scientific Reports 11(1), 17481.
| Crossref | Google Scholar |
Shorthouse DJ, Marples TG (1982) The life stages and population dynamics of an arid zone scorpion Urodacus yaschenkoi (Birula 1903). Australian Journal of Ecology 7(2), 109-118.
| Crossref | Google Scholar |
Shultz JW (1989) Morphology of locomotor appendages in Arachnida: evolutionary trends and phylogenetic implications. Zoological Journal of the Linnean Society 97(1), 1-55.
| Crossref | Google Scholar |
Shultz JW (1992) Muscle firing patterns in two arachnids using different methods of propulsive leg extension. Journal of Experimental Biology 162(1), 313-329.
| Crossref | Google Scholar |
Shultz JW (2001) Gross muscular anatomy of Limulus polyphemus (Xiphosura, Chelicerata) and its bearing on evolution in the Arachnida. Journal of Arachnology 29(3), 283-303.
| Crossref | Google Scholar |
Shultz JW (2007) Morphology of the prosomal endoskeleton of Scorpiones (Arachnida) and a new hypothesis for the evolution of cuticular cephalic endoskeletons in arthropods. Arthropod Structure & Development 36(1), 77-102.
| Crossref | Google Scholar |
Simone Y, van der Meijden A (2018) Fast and fine versus strong and stout: a trade-off between chela closing force and speed across nine scorpion species. Biological Journal of the Linnean Society 123(1), 208-217.
| Crossref | Google Scholar |
Simone Y, van der Meijden A (2021) Armed stem to stinger: a review of the ecological roles of scorpion weapons. Journal of Venomous Animals and Toxins including Tropical Diseases 27, e20210002.
| Crossref | Google Scholar |
Simone Y, Chaves C, van der Meijden A, Egeter B (2022) Metabarcoding analysis of different portions of the digestive tract of scorpions (Scorpiones, Arachnida) following a controlled diet regime shows long prey DNA half-life. Environmental DNA 4(5), 1176-1186.
| Crossref | Google Scholar |
Smith GT (1966) Observations on the life history of the scorpion Urodacus abruptus (Scorpionidae), and the analysis of its home sites. Australian Journal of Zoology 14(3), 383-398.
| Crossref | Google Scholar |
Snodgrass RE (1948) The feeding organs of Arachnida, including mites and ticks. Smithsonian Miscellaneous Collections 110(10), 1-93.
| Google Scholar |
Soleglad ME, Fet V, Kovařík F (2005) The systematic position of the scorpion genera Heteroscorpion Birula, 1903 and Urodacus Peters, 1861 (Scorpiones: Scorpionoidea). Euscorpius 2005(20), 1-37.
| Crossref | Google Scholar |
Southcott RV (1955) Some observations on the biology, including mating and other behavior, of the Australian scorpion Urodacus abruptus Pocock. Transactions of the Royal Society of South Australia 78, 145-154.
| Google Scholar |
Tadayon M, Younes-Metzler O, Shelef Y, Zaslansky P, Rechels A, Berner A, Zolotoyabko E, Barth FG, Fratzl P, Bar-On B (2020) Adaptations for wear resistance and damage resilience: micromechanics of spider cuticular “tools”. Advanced Functional Materials 30(32), 2000400.
| Crossref | Google Scholar |
Thorell T (1876) I. – On the classification of scorpions. Annals and Magazine of Natural History 17(97), 1-15.
| Crossref | Google Scholar |
van der Meijden A, Kleinteich T (2017) A biomechanical view on stinger diversity in scorpions. Journal of Anatomy 230(4), 497-509.
| Crossref | Google Scholar |
van der Meijden A, Herrel A, Summers A (2010) Comparison of chela size and pincer force in scorpions; getting a first grip. Journal of Zoology 280(4), 319-325.
| Crossref | Google Scholar |
van der Meijden A, Kleinteich T, Coelho P (2012a) Packing a pinch: functional implications of chela shapes in scorpions using finite element analysis. Journal of Anatomy 220(5), 423-434.
| Crossref | Google Scholar |
van der Meijden A, Langer F, Boistel R, Vagovic P, Heethoff M (2012b) Functional morphology and bite performance of raptorial chelicerae of camel spiders (Solifugae). Journal of Experimental Biology 215(19), 3411-3418.
| Crossref | Google Scholar |
van der Meijden A, Lobo Coelho P, Sousa P, Herrel A (2013) Choose your weapon: defensive behavior is associated with morphology and performance in scorpions. PLoS ONE 8(11), e78955.
| Crossref | Google Scholar |
Vohland K, Furch K, Adis J (2003) Contrasting central Amazonian rainforests and their influence on chemical properties of the cuticle of two millipede species – a first study. Tropical Ecology 44(2), 235-241.
| Google Scholar |
Walker SM, Schwyn DA, Mokso R, Wicklein M, Müller T, Doube M, Stampanoni M, Krapp HG, Taylor GK (2014) In vivo time-resolved microtomography reveals the mechanics of the blowfly flight motor. PLoS Biology 12(3), e1001823.
| Crossref | Google Scholar | PubMed |
Warburg MR, Rosenberg M (1994) The female reproductive system of the eastern Australian scorpion. Tissue and Cell 26(5), 779-783.
| Crossref | Google Scholar |
Wirkner CS, Prendini L (2007) Comparative morphology of the hemolymph vascular system in scorpions – A survey using corrosion casting, MicroCT, and 3D-reconstruction. Journal of Morphology 268(5), 401-413.
| Crossref | Google Scholar |
Wolf H, Harzsch S (2002) Evolution of the arthropod neuromuscular system. 1. Arrangement of muscles and innervation in the walking legs of a scorpion: Vaejovis spinigerus (Wood, 1863) Vaejovidae, Scorpiones, Arachnida. Arthropod Structure & Development 31(3), 185-202.
| Crossref | Google Scholar |
Woodman JD (2008) Living in a shallow burrow under a rock: gas exchange and water loss in an Australian scorpion. Journal of Thermal Biology 33(5), 280-286.
| Crossref | Google Scholar |
Zhang H, Kellersztein I, Freychet G, Zhernenkov M, Wagner HD, Greer JR (2023) Chemo-mechanical-microstructural coupling in the tarsus exoskeleton of the scorpion Scorpio palmatus. Acta Biomaterialia 160, 176-186.
| Crossref | Google Scholar |
Zhao Z-L, Shu T, Feng X-Q (2016) Study of biomechanical, anatomical, and physiological properties of scorpion stingers for developing biomimetic materials. Materials Science and Engineering: C 58, 1112-1121.
| Crossref | Google Scholar |