Phylogenetic relationships in the Eugongylini (Squamata: Scincidae): generic limits and biogeography
David G. Chapple A B C * , Stephanie N. J. Chapple B C , Sarah A. Smith D , Glenn M. Shea E F , Ian G. Brennan G and Ross A. Sadlier
A B C * , Stephanie N. J. Chapple B C , Sarah A. Smith D , Glenn M. Shea E F , Ian G. Brennan G and Ross A. Sadlier  F
F
A School of Biological Sciences, Monash University, Clayton, Vic. 3800, Australia.
B Museums Victoria, Division of Sciences, GPO Box 666, Melbourne, Vic. 3001, Australia.
C Allan Wilson Centre for Molecular Ecology and Evolution, School of Biological Sciences, Victoria University of Wellington, PO Box 600, Wellington 6140, New Zealand.
D Department of Biology, Villanova University, 800 Lancaster Avenue, Villanova, PA 19085-1699, USA.
E Sydney School of Veterinary Science B01, University of Sydney, Camperdown, NSW 2006, Australia.
F Australian Museum Research Institute, Australian Museum, 1 William Street, Sydney, NSW 2010, Australia.
G Division of Ecology & Evolution, Australian National University, Canberra, ACT 2601, Australia.
Australian Journal of Zoology 70(6) 165-203 https://doi.org/10.1071/ZO23007
Submitted: 27 February 2023 Accepted: 28 April 2023 Published: 25 May 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Skinks (Family Scincidae) are the most diverse family of lizards (~1745 described species worldwide), and the Australasian region (Australia, New Caledonia, New Zealand) is a recognised global hotspot (>600 species) for skinks. Here we focus on determining the phylogenetic relationships and biogeography within the tribe Eugongylini, one of three lineages in the region. We used mtDNA (ND2) and nuclear (RAG-1, c-mos) DNA sequences and phylogenetic analyses to reveal the presence of three well-supported lineages of Australian Eugongylini. We found a sister relationship between the monotypic genera Eroticoscincus and Harrisoniascincus, and that the monotypic Anepischetosia has close affinities with Carinascincus coventryi and Pseudemoia. C. coventryi represents a separate lineage from the main Carinascincus radiation. Emoia was not found to be monophyletic, with Emoia s.s. part of an Australian lineage, and the remainder of the genus representing an older divergence within the tribe. The widespread and speciose Cryptoblepharus represented a well-supported lineage within an Australian lineage. Our analyses confirm previous suggestions that four Sphenomorphus species (louisiadensis, minutus, bignelli, and aignanus) are misplaced, and are part of the Eugongylini. Our phylogenetic analyses support the hypothesis that the origin of the tribe lies in Asia, with dispersal events to Africa, Australasia, and Oceania.
Keywords: Australia, biogeography, lizard, mitochondrial DNA, New Caledonia, New Zealand, nuclear DNA, Zealandia.
Introduction
The Australian skink fauna is widely recognised as a being exceptionally rich and diverse, with ~460 taxa (in 46 genera), representing about ~26% of the world’s ~1745 described species, most of which are endemic to the continent (Uetz et al. 2022). The origin and relationships of the Australian taxa have been the subject of a number of morphological and genetic studies (e.g. Greer 1979; Skinner et al. 2011). To date these studies have largely agreed upon there being three major lineages represented in Australia, the Sphenomorphini (~267 of the world’s 610 species), the Eugongylini (~140 of the world’s 461 species), and the Tiliquini (~49 of the world’s 62 species) (number of taxa from Uetz et al. (2022); suprageneric taxonomy follows Shea 2021). While species richness in the Australian Sphenomorphini is nearly double that of the Australian Eugongylini, this lies largely within two genera, Ctenotus (107 species) and Lerista (97 species) which account for ~80% of the Australian taxa in that group (Wilson and Swan 2021; Uetz et al. 2022). When genera are compared, the two tribes are of similar diversity in Australia, with 21 genera in each.
Outside of Australia, the Sphenomorphini is distributed throughout the Indo-Pacific region (including New Guinea and the Solomon Islands) and Asia, with one species in the genus Ornithuroscincus also present on some outer Melanesian islands via human-mediated dispersal (Austin 1999). Genetic studies (Skinner et al. 2011) have inferred a relatively recent (mid Cenozoic) ~36–25 mya (i.e. between the Late Eocene and Late Oligocene) origin for the Australian Sphenomorphini from Asia via the Papuan region. The Tiliquini is primarily an Australo-Papuan radiation with the greatest diversity within Australia, but with highly divergent taxa present in New Guinea and the Solomon Islands. The origin of the Tiliquini is less clear, but genetic and palaeontological evidence are consistent with the hypothesis that Tiliquini group lygosomines originated in Sahul prior to its detachment from Antarctica (Skinner et al. 2011). The Eugongylini has been estimated to have arisen 31 mya (Skinner et al. 2011), with a suggested origin from south-east Asia proposed. It is a highly diverse lineage of 50 genera with the majority of taxa centred on Australia and the adjacent Pacific islands of New Caledonia and New Zealand, and with a moderately diverse African radiation (Uetz et al. 2022).
The concept of the subgroup of skinks within the Lygosominae which now comprises the Eugongylini was first informally recognised by Greer and Parker (1968) as an unnamed assemblage, then more formally by Greer (1974) as his Groups II and III in reviewing of ‘Leiolopisma’ and its relatives. Later it was more extensively diagnosed as the ‘Eugongylus Group’ of the Lygosominae by Greer (1979) in defining subdivisions within Australian lygosomine skinks. It is now formally recognised as the tribe Eugongylini (Welch 1982; Shea 2021), or as a subfamily (Uetz et al. 2022), within Scincidae. Monophyly of the Eugongylini is supported by multiple genetic (Honda et al. 2000, 2003; Whiting et al. 2003; Schmitz et al. 2005; Austin and Arnold 2006; Smith et al. 2007; Chapple et al. 2009; Skinner et al. 2011), karyological (Donnellan 1985) and immunological (Hutchinson 1981; Hutchinson et al. 1990) studies. However, Pyron et al. (2013) in a reanalysis of previously published DNA sequence data, placed two species of Emoia outside the tribe and Zheng and Wiens (2016) also recovered Eugongylus itself as nested within the Sphenomorphini, a result at odds with previous analyses and with only low support (see Shea (2021) for a review).
Despite consistent support for the Eugongylini, resolution of relationships within the tribe has proved problematic. This is primarily due to the often regionally based partitioning over time of taxa formerly in the genus ‘Leiolopisma’, which once represented the window-eyed skinks within the group. Greer (1974) removed the window-eyed species that were diagnosably outside the Eugongylini, and there has since been a progressive dismantling of ‘Leiolopisma’ by various workers. Revisionary studies on the species assigned to ‘Leiolopisma’ from New Caledonia (Sadlier 1987; Bauer and Sadlier 1993; Sadlier et al. 2006a) and New Zealand (Hardy 1977; Patterson and Daugherty 1995; Chapple et al. 2009) have resulted in the generic separation of taxa in these regions to largely island-endemic genera. The Australian ‘Leiolopisma’ have similarly been subject to revision and generic reassignment, although the various studies have not always produced congruent results.
Since the establishment of the current diagnosis of the Eugongylini, various subgroups of genera have been proposed on morphological and immunological criteria. Initial recognition of presumptive monophyletic groupings of genera based on morphological character states was presented by Greer over a 30-year period. Greer and Parker (1968) and Greer (1974, 1979) progressively used the presence of a ‘beta’ secondary palate to refine a subgroup of genera within the Eugongylini (variously as Group III of Greer (1974), and the Lampropholis Subgroup of Greer (1979)). In its earliest form, this group consisted of several Australian genera (Lampropholis, Carlia, Menetia, Notoscincus – Lampropholis now further subdivided into Saproscincus, and Carlia into Libernascincus and Lygisaurus), one from New Guinea (Geomyersia), and several African genera (Afroablepharus, Cophoscincopus and Panaspis – the former now synonymised into Panaspis, and the latter now further divided into Lacertaspis and Leptosiaphos – see Schmitz et al. 2005; Medina et al. 2016). Greer (1989) later used a different, newly-recognised osteological character, the fusion of the hemilaminae to the intercentrum of the atlas, to recognise a new grouping of eugongylin genera, the Pseudemoia Group. This character transected each of his two previous subgroups within the Eugongylus Group.
Hutchinson et al. (1990) tested Greer’s (1974, 1980, 1982a) earlier proposals of relationships within the Eugongylus Group with immunological comparisons of serum albumins, using microcomplement fixation techniques, and found that while the Australian Eugongylini sampled formed a monophyletic group, the Australian ‘Leiolopisma’ were paraphyletic within this group. Further, the results of these early immunological studies were not fully congruent with the grouping of genera created by the atlantal arch character of Greer (1989). Baverstock and Donnellan (1990) obtained comparable results to the immunological studies by Hutchinson using the same techniques. To remove the paraphyly inherent in Greer’s concept of Leiolopisma, Hutchinson et al. (1990) recognised those Australian taxa remaining in the genus as comprising several genera, and effectively restricted Leiolopisma to three species (only one extant) from the Mascarene Islands (Hutchinson et al. 1990; Arnold and Bour 2008), although a single species of skink from a remote island in Fiji whose affinities are yet to be determined, Leiolopisma alazon Zug, 1995, remains in that genus by default.
Two regionally focused studies on the eugongylin taxa endemic to New Caledonia (Smith et al. 2007) and New Zealand (Chapple et al. 2009) identified these as comprising a monophyletic group, collectively referred to as the Tasmantis Lineage, initially by Smith et al. (2007) and later as the Zealandia Lineage by Chapple et al. (2009) to reflect the ancient geographical origins of these landmasses in the south-east Pacific. While both studies were taxon-rich for each of their focal regions of study, their representation of other eugongylin taxa was limited, particularly with regard to Australia, and this obscured any meaningful interpretation of relationships outside the Tasmantis/Zealandia Lineage.
The relationships of five Australian eugongylin species have remained particularly problematic, and there is a variety of hypotheses of relationship based on often fragmentary evidence. These are: the small fossorial species Lygosoma graciloides Lönnberg & Andersson, 1913 and Siaphos maccoyi Lucas & Frost, 1894, initially placed in Anotis by Mittleman (1952) and Greer (1974), but then transferred by default to the genera Eroticoscincus Wells & Wellington, 1984 and Anepischetosia Wells & Wellington, 1985 respectively; the species described as Leiolopisma zia Ingram & Ehmann, 1981 and Leiolopisma jigurru Covacevich, 1984 for which the monotypic genera Cautula and Bartleia were erected by Hutchinson et al. (1990) (although earlier names, Harrisoniascincus Wells & Wellington, 1985 and Techmarscincus Wells & Wellington, 1985, were available, and have subsequently been resurrected for these species); and Leiolopisma coventryi Rawlinson, 1975, whose relationships have been confounded by a lack of congruence in morphological characters with related taxa assigned to genera on immunological and genetic data.
The affinities of the two largest genera in the Eugongylini, Emoia (78 species) and Cryptoblepharus (53 species) (Uetz et al. 2022), both with geographic distributions covering much of the distribution of the Eugongylini overall, and extending widely across the south-east Pacific islands, have also been problematic. Cryptoblepharus is a morphologically homogeneous group with minimal differences among the many species. For many years most taxa within the genus were considered to be subspecies of a single cosmopolitan species (Mertens 1931, 1964). However, a recent revision of the genus (Horner 2007) elevated most to species status, and found a considerably higher species richness than previously thought. The relationship of Cryptoblepharus to other eugongylin genera is confounded by conflicting hypotheses based on morphology (Fuhn 1969; Greer 1974, 1983 versus Greer 1989), and those based on genetic data (Austin and Arnold 2006; Smith et al. 2007; Pyron et al. 2013; Zheng and Wiens 2016). By contrast, Emoia shows a high degree of intrageneric heterogeneity in morphology. The genus was most recently defined by Brown (1991), but the diagnosis included characters now understood to be plesiomorphic, either at the level of the Lygosominae generally or within the larger part of the Eugongylini. Brown (1991) proposed eight species groups within the genus, noting that: five of these (the adspersa, atrocostata, baudini, physicae and cyanogaster Groups) shared a presumed morphological synapomorphy and hypothesised a common ancestor for all five groups. Two of the remaining three species groups (the cyanura and ponapea Groups) showed one or more unique apomorphic states within the genus. The remaining group (the samoensis Group), was identified largely on the basis of plesiomorphic features. Most immunological and genetic studies (Hutchinson et al. 1990; Austin and Arnold 2006; Smith et al. 2007; Skinner et al. 2011) have generally included only one or two species of Emoia, apparently assuming monophyly for the genus, and these studies showed little congruence in the relationships retrieved among the representatives of the species groups sampled, or with their relationships to other genera in the Eugongylini (Pyron et al. 2013; Zheng and Wiens 2016).
Four species placed in the genus Sphenomorphus by Mittleman (1952), Sphenomorphus aignanus (Boulenger, 1898a), Sphenomorphus louisiadensis (Boulenger, 1903), Sphenomorphus minutus (Meyer, 1874) and Sphenomorphus bignelli Schmidt, 1932, have repeatedly been suggested to be members of the Eugongylini (Greer and Parker 1968; Greer 1977, 1979). None of these taxa have been included in recent genetic studies, and their relationships remain a largely unresolved anomaly that has persisted since first identified half a century ago. If genetic studies confirm their placement in the Eugongylini, they would represent an additional lineage outside the Australian/Zealandia (Zealandia = New Caledonia, New Zealand, Lord Howe Island, Norfolk Island) region (additional to Leiolopisma, Eugongylus, Panaspis and its relatives, Geomyersia, and one or more species groups of Emoia), and potentially be important in determining the biogeographic origins of the tribe.
Here we provide a new analysis of relationships within the Eugongylini using mtDNA (ND2) and nuclear (RAG-1, c-mos) DNA sequences. We emphasise a broader sampling of the diversity within the Australian genera and problematic species, and add in new sequences for some non-Australian taxa, to try to better resolve the affinities of the problematic taxa. In particular, we evaluate the following questions:
Do the Australian Eugongylini form a monophyletic group, sister to the Zealandia Lineage, and does the Zealandia Lineage of eugongylin genera remain monophyletic with more complete sampling of Australian species?
Does the expanded sampling of Australian Eugongylini provide further resolution of the content, generic limits and relationships of genera whose species were historically assigned to ‘Leiolopisma’ and related genera?
What are the relationships of the monotypic genera currently recognised among the Australian Eugongylini?
Is Emoia monophyletic, and where does it, or do its constituent parts, lie in relation to other eugongylin genera?
Does Cryptoblepharus form part of the core Australian eugongylin radiation, or does it represent a separate migration to Australia from elsewhere?
Are the four Sphenomorphus species, long considered part of the Eugongylini, a part of that tribe, and, if so, what are their affinities?
Are the geographic origins of the Eugongylini in the Australian region, south-east Asia, or Africa, and what are the ages of the various lineages and groups?
Materials and methods
Taxon sampling
We obtained new squences for 139 samples, representing 76 species from 20 eugongylin genera, plus sequences from 80 species which we had previously used in two related studies (Smith et al. 2007; Chapple et al. 2009) from the Australian, Papua New Guinean, New Caledonian, New Zealand, Indian Ocean and Pacific Ocean region, which we combined with sequences from 66 species obtained from Genbank from these regions (Table 1). Our sampling, totalling 321 specimens from 198 species representing 39 eugongylin genera, encompasses all genera from this region except Geomyersia (two species from Papua New Guinea and the Solomon Islands), Geoscincus (a monotypic genus from New Caledonia not observed since the 1970s), Kuniesaurus (a monotypic genus from New Caledonia), Pygmaeascincus (three species from Queensland, two with very restricted distributions), Tachygyia (a monotypic genus from Tonga, presumed extinct) and Techmarscincus (a monotypic genus from Mt Bartle Frere in north Queensland, Australia) (Table 1). We also obtained sequences from Genbank representing two of the four African eugongylin genera (Leptosiaphos and Panaspis). We included samples representing as many as possible of the identified infrageneric lineages. For genera with multiple species, we used at least two species. Where there were nomenclatural issues, we obtained samples from the type species of nominal genera whenever possible. Where a number of species were represented within a lineage, we selected species that encompassed the extremes of morphological variation, or those that had been identified by previous studies as being the most genetically divergent. We included three of the Sphenomorphus species (bignelli, louisiadensis, minutus) that, based on morphological evidence, appear to be members of the Eugongylini rather than the Sphenomorphini. For a majority of species (118/198 eugongylin species), we obtained sequences from at least two individuals, as a check for errors in sequencing or identification.
| Species | Sample code | Tissue voucher | Specimen voucher | Location | GenBank accession number | |||
|---|---|---|---|---|---|---|---|---|
| ND2 | RAG-1 | c-mos | ||||||
| Australia | ||||||||
| Acritoscincus (3 of 3 species) | ||||||||
| Acritoscincus duperreyi (T) | SAS01 | NR393 | AMR133016 | Chappell Island, TAS | JQ610275 | JQ610387 | JQ610522 | |
| Acritoscincus duperreyi | SAS02 | NR394 | AMR133017 | Chappell Island, TAS | JQ610276 | JQ610388 | JQ610523 | |
| Acritoscincus platynotus | SAS03 | NR5005 | AMR151840 | Riamukka SF, Grundy Fire Tower Area, NSW | JQ610277 | JQ610389 | JQ610524 | |
| Acritoscincus platynotus | SAS04 | NR5008 | AMR155841 | Riamukka SF, Grundy Fire Tower Area, NSW | JQ610278 | JQ610390 | JQ610525 | |
| Acritoscincus trilineatus | – | – | SAMAR50093 | Wanna Dunes, Lincoln National Park, SA | – | – | HQ655198 | |
| Anepischetosia (1 of 1 species) | ||||||||
| Anepischetosia maccoyi (T) | EUG58 | NR10502 | AMR167329 | Bondi SF, NSW | JQ610242 | JQ610352 | JQ610467 | |
| Anepischetosia maccoyi | EUG59 | NR10503 | AMR167330 | Bondi SF, NSW | JQ610243 | JQ610353 | JQ610468 | |
| Austroablepharus (1 of 3 species) | ||||||||
| Austroablepharus kinghorni (T) | SAS32 | NR5064 | AMR151602 | Sturt NP, NSW | JQ610300 | JQ610412 | JQ610548 | |
| Austroablepharus kinghorni | SAS33 | NR5065 | AMR151601 | Sturt NP, NSW | JQ610301 | JQ610413 | JQ610549 | |
| Carinascincus (6 of 8 species) | ||||||||
| Carinascincus coventryi | SAS29 | NR7280 | AMR157297 | 10 km N Wombeyan Caves, NSW | JQ610297 | JQ610409 | JQ610545 | |
| Carinascincus coventryi | SAS30 | NR7281 | AMR157298 | 10 km N Wombeyan Caves, NSW | JQ610298 | JQ610410 | JQ610546 | |
| Carinascincus greeni (T) | EUG75 | ABTC23415 | TMHC545 | Mt Rufus, TAS | JQ610259 | JQ610369 | JQ610484 | |
| Carinascincus greeni | EUG76 | ABTC23429 | TMHC559 | Mt Rufus, TAS | JQ610260 | JQ610370 | JQ610485 | |
| Carinascincus metallicus | LDA197 | – | – | Greens Beach Coastal Trail, Tamar Valley, TAS | JQ610273 | JQ610385 | JQ610500 | |
| Carinascincus metallicus | LDA212 | – | – | Cascade Gardens, South Hobart, TAS | JQ610274 | JQ610386 | JQ610501 | |
| Carinascincus microlepidotus | EUG77 | ABTC23447 | TMHC577 | Mt Rufus, TAS | JQ610261 | JQ610371 | JQ610486 | |
| Carinascincus microlepidotus | EUG78 | ABTC23450 | TMHC580 | Mt Rufus, TAS | JQ610262 | JQ610372 | JQ610487 | |
| Carinascincus palfreymani | EUG72 | ABTC1079 | AMR122005 | Pedra Branca Rock, TAS | JQ610256 | JQ610366 | JQ610481 | |
| Carinascincus pretiosus | EUG09 | NR391 | AMR133014 | Chappell Island, TAS | DQ675234 | DQ675314 | DQ675374 | |
| Carinascincus pretiosus | SAS31 | NR392 | AMR133015 | Chappell Island, TAS | JQ610299 | JQ610411 | JQ610547 | |
| Carlia (10 of 26 Australian species) | ||||||||
| Carlia amax | SAS05 | NR293 | AMR135971 | Gemco Mining Lease, Groote Eylandt, NT | JQ610279 | JQ610391 | JQ610526 | |
| Carlia amax | SAS06 | NR294 | AMR135972 | Gemco Mining Lease, Groote Eylandt, NT | JQ610280 | JQ610392 | JQ610527 | |
| Carlia dogare | EUG12 | NR218 | AMR133216 | Lizard Island, QLD | JQ610206 | JQ610317 | JQ610431 | |
| Carlia dogare | EUG13 | NR219 | AMR133217 | Lizard Island, QLD | JQ610207 | JQ610318 | JQ610432 | |
| Carlia jarnoldae | EUG33 | NR2115 | AMR142515 | Lamb Range, QLD | JQ610226 | JQ610337 | JQ610451 | |
| Carlia jarnoldae | EUG34 | NR2116 | AMR142516 | Lamb Range, QLD | JQ610227 | JQ610338 | JQ610452 | |
| Carlia johnstonei | EUG16 | NR458 | AMR136130 | Tributary of Mitchell R, Mitchell Plateau, WA | JQ610210 | JQ610321 | JQ610435 | |
| Carlia johnstonei | EUG17 | NR459 | AMR136131 | Tributary of Mitchell R, Mitchell Plateau, WA | JQ610211 | JQ610322 | JQ610436 | |
| Carlia longipes | EUG14 | NR298 | AMR135921 | Groote Eylandt, Gemco Mining Lease, NT | JQ610208 | JQ610319 | JQ610433 | |
| Carlia longipes | EUG15 | NR299 | AMR135922 | Groote Eylandt, Gemco Mining Lease, NT | JQ610209 | JQ610320 | JQ610434 | |
| Carlia munda (T) | EUG23 | NR2050 | AMR142535 | Lamb Range, QLD | JQ610216 | JQ610327 | JQ610441 | |
| Carlia munda | EUG24 | NR2051 | AMR142536 | Lamb Range, QLD | JQ610217 | JQ610328 | JQ610442 | |
| Carlia rostralis | EUG27 | NR2064 | AMR142687 | Lamb Range, QLD | JQ610220 | JQ610331 | JQ610445 | |
| Carlia rostralis | EUG28 | NR2076 | AMR142600 | Lamb Range, QLD | JQ610221 | JQ610332 | JQ610446 | |
| Carlia rubrigularis | EUG29 | NR2083 | AMR142704 | Lamb Range, QLD | JQ610222 | JQ610333 | JQ610447 | |
| Carlia rubrigularis | EUG30 | NR2084 | AMR142705 | Lamb Range, QLD | JQ610223 | JQ610334 | JQ610448 | |
| Carlia tetradactyla | EUG21 | NR2040 | AMR142757 | 4.2 km NE Mandurama, on Mid Western Hwy, NSW | JQ610214 | JQ610325 | JQ610439 | |
| Carlia tetradactyla | EUG22 | NR2041 | AMR142758 | 4.2 km NE Mandurama, on Mid Western Hwy, NSW | JQ610215 | JQ610326 | JQ610440 | |
| Carlia vivax | EUG25 | NR2052 | AMR142538 | Lamb Range, QLD | JQ610218 | JQ610329 | JQ610443 | |
| Carlia vivax | EUG26 | NR2053 | AMR142539 | Lamb Range, QLD | JQ610219 | JQ610330 | JQ610444 | |
| Cryptoblepharus (13 of 24 Australian species) | ||||||||
| Cryptoblepharus adamsi | – | ABTC16251 | QMJ48420 | Townsville, QLD | MH216007 | – | – | |
| Cryptoblepharus daedalos | – | CCM0619 | – | Victoria River region, Joe Creek, NT | MH216021 | – | – | |
| Cryptoblepharus juno | – | BP02478 | WAMR174930 | Berkeley SR, NT | MH216017 | – | – | |
| Cryptoblepharus juno | – | CCM2973 | – | East Baines Camp, NT | MH216019 | – | – | |
| Cryptoblepharus megastictus | EUG19 | NR1018 | AMR140118 | 1 km S McGowens Beach, Kalumburu area, WA | JQ610212 | JQ610323 | JQ610437 | |
| Cryptoblepharus megastictus | EUG20 | NR1019 | AMR140119 | 1 km S McGowens Beach, Kalumburu area, WA | JQ610213 | JQ610324 | JQ610438 | |
| Cryptoblepharus mertensi | – | – | NTMR35690 | Roper Bar, NT | MH216009 | – | – | |
| Cryptoblepharus aff. metallicus | – | WAMTR1025 | – | Balgo Hill, WA | MW026389 | – | – | |
| Cryptoblepharus pannosus | SAS13 | NR8874 | AMR156831 | Yarra, 35 km from Mt Hope on Euabalong Rd, NSW | JQ610285 | JQ610397 | JQ610532 | |
| Cryptoblepharus pannosus | – | CCM0071 | Lornevale, QLD | MH216008 | – | – | ||
| Cryptoblepharus pulcher | SAS16 | NR5758 | AMR159896 | Ashford Caves, NSW | JQ610288 | JQ610400 | JQ610535 | |
| Cryptoblepharus ruber | – | CCM1451 | WAMR176241 – | Chamberlain Valley Camp, WA | MH216012 | – | – | |
| Cryptoblepharus tytthos | – | – | NMVZ29018 | Wilare Bridge, WA | MH216023 | – | – | |
| Cryptoblepharus virgatus | – | CCM05274 | QMJ94651 | West of Watsonville, 8.6 km E of Irvine Bank, QLD | MH216011 | – | – | |
| Cryptoblepharus zoticus | – | CCM0421 | – | Massacre Hill camp site, QLD | MH216010 | – | – | |
| Eroticoscincus (1 of 1 species) | ||||||||
| Eroticoscincus graciloides (T) | SAS17 | – | QMJ76831 | Lake Weyba, QLD | JQ610289 | JQ610401 | JQ610536 | |
| Harrisoniascincus (1 of 1 species) | ||||||||
| Harrisoniascincus zia (T) | SAS11 | NR4972 | AMR151811 | Tweed Valley Lookout, Border Ranges NP, NSW | JQ610283 | JQ610395 | JQ610530 | |
| Harrisoniascincus zia | SAS12 | NR4973 | AMR151812 | Tweed Valley Lookout, Border Ranges NP, NSW | JQ610284 | JQ610396 | JQ610531 | |
| Lampropholis (5 of 14 species) | ||||||||
| Lampropholis amicula | SAS18 | NR1944 | AMR142814 | Ellenborough, 5 km up Tom’s Creek Rd, NSW | JQ610290 | JQ610402 | JQ610537 | |
| Lampropholis amicula | SAS19 | NR1945 | AMR142815 | Ellenborough, 5 km up Tom’s Creek Rd, NSW | JQ610291 | JQ610403 | JQ610538 | |
| Lampropholis coggeri | EUG31 | NR2087 | AMR142720 | Lamb Range, QLD | JQ610224 | JQ610335 | JQ610449 | |
| Lampropholis coggeri | EUG32 | NR2088 | AMR142710 | Lamb Range, QLD | JQ610225 | JQ610336 | JQ610450 | |
| Lampropholis delicata | LDA65 | ABTC77179 | SAMR55858 | 6 km N The Crater turnoff on Kennedy, QLD | JF438213 | JQ610382 | JQ610497 | |
| Lampropholis delicata | LDA187 | NMVZ6222 | NMVD73624 | Junction Princes Hwy and Murrungowar Rd, VIC | JF438107 | JQ610384 | JQ610499 | |
| Lampropholis elongata | EUG36 | NR3748 | AMR148192 | Riamukka SF, Grundy Fire Tower Area, NSW | JQ610229 | JQ610340 | JQ610454 | |
| Lampropholis elongata | EUG37 | NR3749 | AMR148193 | Riamukka SF, Grundy Fire Tower Area, NSW | JQ610230 | JQ610341 | JQ610455 | |
| Lampropholis guichenoti (T) | EUG07 | NR2639 | AMR145994 | near Penrith, NSW | DQ675212 | DQ572292 | DQ675352 | |
| Lampropholis guichenoti | LDA181 | NMVZ6231 | NMVD73633 | Lilydale Lake, Lilydale, Melbourne, VIC | JQ610272 | JQ610383 | JQ610498 | |
| Liburnascincus (3 of 4 species) | ||||||||
| Liburnascincus coensis (T) | EUG71 | – | A004536 | – | JQ610255 | JQ610365 | JQ610480 | |
| Liburnascincus mundivensis | SAS07 | NR2060 | AMR142641 | Lamb Range, QLD | JQ610281 | JQ610393 | JQ610528 | |
| Liburnascincus mundivensis | SAS08 | NR2061 | AMR142642 | Lamb Range, QLD | JQ610282 | JQ610394 | JQ610529 | |
| Liburnascincus scirtetis | EUG70 | – | A002000 | – | JQ610254 | JQ610364 | JQ610479 | |
| Lygisaurus (2 of 12 species) | ||||||||
| Lygisaurus foliorum | SAS20 | NR4878 | AMR151327 | Lake Burragorang area, NSW | DQ675222 | DQ675302 | DQ675362 | |
| Lygisaurus foliorum | SAS21 | NR4879 | AMR151328 | Lake Burragorang area, NSW | JQ610292 | JQ610404 | JQ610539 | |
| Lygisaurus laevis | SAS22 | NR2085 | AMR142708 | Lamb Range, QLD | JQ610293 | JQ610405 | JQ610540 | |
| Lygisaurus laevis | SAS23 | NR2086 | AMR142709 | Lamb Range, QLD | JQ610294 | JQ610406 | JQ610541 | |
| Menetia (3 of 6 species) | ||||||||
| Menetia alanae | EUG85 | ABTC79077 | SAMR57117 | Wildman Reserve, NT | JQ610268 | JQ610378 | JQ610493 | |
| Menetia alanae | EUG86 | ABTC79080 | SAMR57119 | Wildman Reserve, NT | JQ610269 | JQ610379 | JQ610494 | |
| Menetia surda cresswelli | EUG81 | ABTC63309 | WAMR125960 | East Yuna Nature Reserve, WA | JQ610264 | JQ610374 | JQ610489 | |
| Menetia greyii (T) | EUG57 | NR8712 | AMR155233 | Sturt NP, NSW | JQ610241 | JQ610351 | JQ610466 | |
| Menetia greyii | SAS24 | NR5185 | AMR151714 | Sturt NP, NSW | DQ675266 | DQ675346 | JQ610542 | |
| Morethia (5 of 8 species) | ||||||||
| Morethia adelaidensis | EUG10 | NR8560 | AMR155359 | Sturt NP, NSW | DQ675228 | DQ675308 | DQ675368 | |
| Morethia boulengeri | SAS26 | NR8605 | AMR155384 | Sturt NP, NSW | JQ610295 | JQ610407 | JQ610543 | |
| Morethia obscura | EUG38 | NR7169 | AMR153213 | Warrakoo Station, NSW | JQ610231 | JQ610342 | JQ610456 | |
| Morethia obscura | EUG39 | NR7702 | AMR154151 | Belmore Station, NSW | JQ610232 | JQ610343 | JQ610457 | |
| Morethia ruficauda | EUG35 | NR3441 | AMR147246 | 19.9 km N Wauchope on Stuart Hwy, NT | JQ610228 | JQ610339 | JQ610453 | |
| Morethia taeniopleura | SAS27 | NR2062 | AMR142646 | Lamb Range, QLD | JQ610296 | JQ610408 | JQ610544 | |
| Proablepharus (1 of 2 species) | ||||||||
| Proablepharus tenuis (T) | EUG74 | ABTC77012 | SAMR55701 | Kennedy Developmental Rd to Porcupine Gorge, QLD | JQ610258 | JQ610368 | JQ610483 | |
| Proablepharus tenuis | EUG82 | ABTC76972 | SAMR55664 | Bullock Creek crossing on Shirley HS Rd, QLD | JQ610265 | JQ610375 | JQ610490 | |
| Pseudemoia (3 of 6 species) | ||||||||
| Pseudemoia entrecasteauxii | EUG11 | NR4922 | AMR51776 | Picadilly Circus, Brindabella Ranges, ACT | EU837084 | EU837131 | JQ610430 | |
| Pseudemoia entrecasteauxii | SAS34 | NR4923 | AMR51777 | Picadilly Circus, Brindabella Ranges, ACT | JQ610302 | JQ610414 | JQ610550 | |
| Pseudemoia pagenstecheri | SAS35 | NR2790 | AMR148177 | Riamukka SF, Grundy Fire Tower Area, NSW | DQ675267 | DQ675347 | JQ610551 | |
| Pseudemoia pagenstecheri | SAS36 | NR2791 | AMR148178 | Riamukka SF, Grundy Fire Tower Area, NSW | JQ610303 | JQ610415 | JQ610552 | |
| Pseudemoia spenceri (T) | EUG73 | ABTC14201 | AMR122952 | Jenolan Caves, NSW | JQ610257 | JQ610367 | JQ610482 | |
| Pseudemoia spenceri | EUG79 | ABTC40884 | NMVD60988 | Mt Baw Baw, VIC | JQ610263 | JQ610373 | JQ610488 | |
| Saproscincus (6 of 12 species) | ||||||||
| Saproscincus basiliscus | SAS37 | NR2191 | AMR143188 | Lamb Range, QLD | JQ610304 | JQ610416 | JQ610553 | |
| Saproscincus basiliscus | SAS38 | NR2192 | AMR143193 | Lamb Range, QLD | JQ610305 | JQ610417 | JQ610554 | |
| Saproscincus challengeri | SAS39 | NR232 | AMR133457 | Mt Warning NP, NSW | JQ610306 | JQ610418 | JQ610555 | |
| Saproscincus challengeri | SAS40 | NR233 | AMR133458 | Mt Warning NP, NSW | JQ610307 | JQ610419 | JQ610556 | |
| Saproscincus czechurai | SAS41 | NR2137 | AMR142688 | Lamb Range, QLD | JQ610308 | JQ610420 | JQ610557 | |
| Saproscincus czechurai | SAS42 | NR2187 | AMR143161 | Lamb Range, QLD | JQ610309 | JQ610421 | JQ610558 | |
| Saproscincus spectabilis | SAS43 | NR618 | AMR138035 | Brindle Creek Rest Area, Border Ranges NP, NSW | JQ610310 | JQ610422 | JQ610559 | |
| Saproscincus spectabilis | SAS44 | NR619 | AMR138036 | Brindle Creek Rest Area, Border Ranges NP, NSW | JQ610311 | JQ610423 | JQ610560 | |
| Saproscincus mustelinus (T) | EUG08 | NR3782 | AMR148227 | Riamukka SF, Grundy Fire Tower Area, NSW | EF567305 | EU837130 | JQ610429 | |
| Saproscincus rosei | SAS46 | NR6107 | AMR152292 | Williams River, near Barrington Guest House, NSW | DQ675248 | DQ675328 | JQ610561 | |
| Saproscincus rosei | SAS47 | NR6108 | AMR152293 | Williams River, near Barrington Guest House, NSW | JQ610312 | JQ610424 | – | |
| New Caledonia | ||||||||
| Caesoris (1 of 1 species) | ||||||||
| Caesoris novaecaledoniae (T) | SAS102 | EBU 11487 | AMR166364 | Bourail | DQ675251 | DQ675331 | – | |
| Caesoris novaecaledoniae | SAS103 | EBU 11490 | AMR166367 | Bourail | DQ675252 | DQ675332 | – | |
| Caledoniscincus (15 of 15 species) | ||||||||
| Caledoniscincus aquilonius | SAS48 | EBU 15127 | AMR161247 | Dôme de Tiébaghi | DQ675194 | DQ675274 | DQ675399 | |
| Caledoniscincus atropunctatus | SAS49 | – | CAS231910 | NW of Moindoi | DQ675195 | DQ675275 | DQ675400 | |
| Caledoniscincus atropunctatus | SAS50 | EBU 15208 | AMR161083 | Ⓘle Art, Ⓘles Belep | DQ675196 | DQ675276 | DQ675401 | |
| Caledoniscincus auratus | SAS51 | EBU 33768 | AMR157911 | Tia | DQ675197 | DQ675277 | DQ675402 | |
| Caledoniscincus austrocaledonicus (T) | SAS52 | EBU 15310 | AMR161186 | Creek Hervouët | DQ675198 | DQ675278 | DQ675403 | |
| Caledoniscincus bodoi | SAS53 | – | CAS2317888 | Ⓘle des Pins | DQ675199 | DQ675279 | DQ675404 | |
| Caledoniscincus bodoi | – | – | AMR163236 | Ⓘle Moro, Ⓘle des Pins | MG456917 | – | – | |
| Caledoniscincus bodoi | – | – | AMR163262 | Ⓘle Koomo, Ⓘle des Pins | MG456918 | – | – | |
| Caledoniscincus chazeaui | – | – | AMR138515 | Koulnoué, Hienghène Region | DQ675272 | – | – | |
| Caledoniscincus constellatus | – | – | AMR171470 | Vavouto Peninsula | JQ743845 | – | – | |
| Caledoniscincus constellatus | – | – | AMR171496 | Vavouto Peninsula | JQ743846 | – | – | |
| Caledoniscincus cryptos | – | – | AMR135141 | 8.3 km from Kouaoua/Canala Road intersection on La Foa Rd | JQ743852 | – | – | |
| Caledoniscincus festivus | SAS54 | EBU 35279 | AMR161882 | Monts Kwa Né Mwa | DQ675200 | DQ675280 | DQ675405 | |
| Caledoniscincus festivus | – | – | AMR161881 | Monts Kwa Né Mwa | KF130791 | – | – | |
| Caledoniscincus haplorhinus | SAS55 | EBU15039 | – | Maa Bwén, Ile Baaba | DQ675201 | DQ675281 | DQ675406 | |
| Caledoniscincus notialis | – | – | AMSR172617 | Massif du Humbolt | MG456923 | – | – | |
| Caledoniscincus notialis | – | – | AMSR172622 | Massif du Humbolt | MG456924 | – | – | |
| Caledoniscincus orestes | SAS56 | EBU 6552 | AMR149926 | Néoua area, Mé Adeo | DQ675202 | DQ675282 | DQ675407 | |
| Caledoniscincus orestes | – | – | AMR149983 | Mt. Panié | JQ743856 | – | – | |
| Caledoniscincus pelletieri | – | – | AMR174994 | Dôme de Tiébaghi | JX988522 | – | – | |
| Caledoniscincus pelletieri | – | – | AMR174984 | Dôme de Tiébaghi, | JX988521 | – | – | |
| Caledoniscincus renevieri | – | – | AMR165851 | Mt Aoupinié, | DQ675268 | – | – | |
| Caledoniscincus renevieri | – | NR2899 | – | Mt Aoupinié | KF130793 | – | – | |
| Caledoniscincus terma | – | – | CAS198680 | Mt Mandjélia | DQ675271 | – | – | |
| Celatiscincus (2 of 2 species) | ||||||||
| Celatiscincus euryotis (T) | SAS57 | EBU 22564 | AMR138574 | Ⓘle des Pins | DQ675204 | DQ675284 | DQ675409 | |
| Celatiscincus similis | SAS58 | EBU 22552 | AMR153504 | Tsiba | DQ675203 | DQ675283 | DQ675408 | |
| Cryptoblepharus (1 of 1 NC species) | ||||||||
| Cryptoblepharus novocaledonicus | SAS59 | – | AMR165930 | Ⓘle des Pins | DQ675205 | DQ675285 | DQ675410 | |
| Cryptoblepharus novocaledonicus | SAS14 | EBU15083 | AMR161202 | Ⓘle Yande | JQ610286 | JQ610398 | JQ610533 | |
| Cryptoblepharus novocaledonicus | SAS15 | EBU15106 | AMR161226 | Sommet Poum | JQ610287 | JQ610399 | JQ610534 | |
| Epibator (1 of 3 species) | ||||||||
| Epibator nigrofasciolatus (T) | SAS69 | EBU 6389 | AMR149334 | Mt Panié | DQ675215 | DQ675295 | DQ675355 | |
| Epibator nigrofasciolatus | SAS70 | EBU 3519 | AMR138624 | Ⓘle des Pins | DQ675216 | DQ675296 | DQ675356 | |
| Emoia (2 of 2 NC species) | ||||||||
| Emoia cyanura | SAS100 | – | AMR163421 | Maré, Ⓘles Loyauté | DQ675263 | DQ675343 | – | |
| Emoia loyaltiensis | SAS60 | – | AMR163417 | Maré, Ⓘles Loyauté | DQ675206 | DQ675286 | DQ675411 | |
| Graciliscincus (1 of 1 species) | ||||||||
| Graciliscincus shonae (T) | SAS61 | – | AMR147856 | Mt Koghis | DQ675254 | DQ675334 | DQ675391 | |
| Graciliscincus shonae | SAS62 | – | AMR165813 | Mt Ouin | DQ675207 | DQ675287 | DQ675412 | |
| Kanakysaurus (1 of 2 species) | ||||||||
| Kanakysaurus viviparus (T) | SAS63 | EBU15112 | AMR161232 | Dome de Tiébaghi | DQ675208 | DQ675288 | DQ675413 | |
| Kanakysaurus viviparus | SAS64 | EBU15180 | AMR161299 | Ⓘle Pott, Ⓘles Belep | DQ675209 | DQ675289 | DQ675349 | |
| Lacertoides (1 of 1 species) | ||||||||
| Lacertoides pardalis (T) | SAS65 | – | CAS20583 | Kwa Néie | DQ675210 | DQ675290 | DQ675350 | |
| Lacertoides pardalis | SAS66 | EBU5551 | AMR148051 | Kwa Néie | DQ675211 | DQ675291 | DQ675351 | |
| Lioscincus (2 of 2 species) | ||||||||
| Lioscincus steindachneri (T) | SAS71 | EBU6487 | AMR149418 | Mt Aoupinié | DQ675217 | DQ675297 | DQ675357 | |
| Lioscincus steindachneri | SAS72 | EBU6543 | AMR149890 | Mé Adéo | DQ675218 | DQ675298 | DQ675358 | |
| Lioscincus vivae | SAS75 | – | CAS22663 | Massif de Kopéto | DQ675221 | DQ675301 | DQ675361 | |
| Marmorosphax (5 of 5 species) | ||||||||
| Marmorosphax boulinda | SAS79 | EBU15596 | AMR163197 | Massif du Boulinda | DQ675225 | DQ675305 | DQ675365 | |
| Marmorosphax boulinda | – | – | AMR167263 | Massif du Tchingou | KF176385 | – | – | |
| Marmorosphax boulinda | – | – | AMR163196 | Massif du Boulinda | KF176383 | – | – | |
| Marmorosphax kaala | – | – | AMR161091 | Mt Kaala, | KF130804 | – | – | |
| Marmorosphax montana | SAS76 | EBU15779 | AMR165922 | Mt Ouin | DQ675226 | DQ675306 | DQ675366 | |
| Marmorosphax montana | SAS77 | – | AMR165802 | Mt Ouin | DQ675255 | DQ675335 | DQ675392 | |
| Marmorosphax taom | SAS78 | EBU16935 | AMR165973 | Mt Taom | DQ675224 | DQ675304 | DQ675364 | |
| Marmorosphax taom | – | – | AMR168011 | Dôme de Tièbaghi | KF176392 | – | – | |
| Marmorosphax tricolor (T) | SAS80 | – | CAS214451 | Mt Koghis | DQ675227 | DQ675307 | DQ675367 | |
| Marmorosphax tricolor | SAS81 | EBU15577 | AMR163178 | Massif du Boulinda | DQ675223 | DQ675303 | DQ675363 | |
| Marmorosphax cf. tricolor | – | – | AMR167158 | Mt Menazi | KF176409 | – | – | |
| Marmorosphax cf. tricolor | – | – | AMR167263 | Massif du Tchingou | KF176411 | – | – | |
| Nannoscincus (8 of 12 species) | ||||||||
| Nannoscincus exos | – | – | AMR174663 | Roches de la Ouaïème | JX015441 | – | – | |
| Nannoscincus garrulus | – | – | AMR163453 | Pic Ningua | DQ675261 | – | – | |
| Nannoscincus garrulus | – | – | CAS226166 | Pic Ningua | DQ675262 | – | – | |
| Nannoscincus gracilis | SAS82 | EBU5247 | AMR144351 | Sarraméa | DQ675229 | DQ675309 | DQ675369 | |
| Nannoscincus gracilis | SAS83 | EBU6538 | AMR149892 | Mé Adéo | DQ675233 | DQ675313 | DQ675373 | |
| Nannoscincus greeri | SAS84 | – | CAS231942 | Nèmèrétina | DQ675230 | DQ675310 | DQ675370 | |
| Nannoscincus hanchisteus | – | – | AMR149355 | Pindaï | DQ675270 | – | – | |
| Nannoscincus hanchisteus | – | – | AMR149356 | Pindaï | JX015464 | – | – | |
| Nannoscincus humectus | – | – | AMR149498 | Forêt Plate | DQ675269 | – | – | |
| Nannoscincus manautei | – | – | AMR163123 | Massif de Kopéto | JX015465 | – | – | |
| Nannoscincus manautei | – | – | MNHN2003.1001 | Massif de Kopéto | JX015466 | – | – | |
| Nannoscincus mariei | SAS85 | EBU2863 | AMR135111 | Mt Koghis | DQ675231 | DQ675311 | DQ675371 | |
| Nannoscincus mariei | SAS86 | EBU3609 | AMR146484 | Mt Mou | DQ675232 | DQ675312 | DQ675372 | |
| Phaeoscincus (1 of 2 species) | ||||||||
| Phaeoscincus taomensis (T) | SAS95 | EBU15306 | AMR161182 | Mt Taom | DQ675193 | DQ675273 | DQ675398 | |
| Phasmasaurus (2 of 2 species) | ||||||||
| Phasmasaurus maruia | SAS67 | EBU15564 | AMR163164 | Plateau de Tia | DQ675213 | DQ675293 | DQ675353 | |
| Phasmasaurus maruia | SAS68 | EBU6542 | AMR149897 | Mé Adéo | DQ675214 | DQ675294 | DQ675354 | |
| Phasmasaurus tilleri (T) | SAS73 | EBU5537 | AMR148013 | Mt Mou | DQ675219 | DQ675299 | DQ675359 | |
| Phasmasaurus tilleri | SAS74 | EBU5577 | AMR148037 | Mt Vulcain | DQ675220 | DQ675300 | DQ675360 | |
| Phoboscincus (2 of 2 species) | ||||||||
| Phoboscincus bocourti (T) | – | – | – | Ⓘle des Pins | KF130806 | – | – | |
| Phoboscincus garnieri | SAS87 | EBU4441 | AMR146293 | Ⓘle des Pins | DQ675236 | DQ675316 | DQ675376 | |
| Phoboscincus garnieri | SAS88 | EBU7545 | AMR151964 | Mt Dore | DQ675237 | DQ675317 | DQ675377 | |
| Sigaloseps (6 of 6 species) | ||||||||
| Sigaloseps balios | – | – | AMR172620 | Massif du Humbolt | KC164635 | – | – | |
| Sigaloseps balios | – | – | AMR172621 | Massif du Humbolt | KC164636 | – | – | |
| Sigaloseps conditus | – | – | AMR147916 | Rivière Bleue | KC164616 | – | – | |
| Sigaloseps conditus | – | – | AMR147952 | Rivière Bleue | KC164617 | – | – | |
| Sigaloseps deplanchei (T) | SAS89 | EBU5556 | AMR148065 | Plaines des Lacs | DQ675238 | DQ675318 | DQ675378 | |
| Sigaloseps ferrugiacauda | – | – | AMR162964 | Mt Ouin | KC164634 | – | – | |
| Sigaloseps ferrugiacauda | – | – | AMR171611 | Mt Humbolt | KC164633 | – | – | |
| Sigaloseps pisinnus | – | – | AMR171249 | Pic Ningua | KC164626 | – | – | |
| Sigaloseps pisinnus | – | – | AMR171250 | Pic Ningua | KC164627 | – | – | |
| Sigaloseps ruficauda | SAS90 | EBU4019 | AMR146482 | Mt Mou | DQ675239 | DQ675319 | DQ675379 | |
| Simiscincus (1 of 1 species) | ||||||||
| Simiscincus aurantiacus (T) | SAS91 | EBU5255 | AMR144356 | Mt Koghis | DQ675250 | JQ610425 | DQ675389 | |
| Simiscincus aurantiacus | SAS104 | EBU44755 | AMR171364 | Kwé Nord, Goro Plateau | JQ610313 | JQ610426 | JQ610562 | |
| Tropidoscincus (3 of 3 species) | ||||||||
| Tropidoscincus aubrianus (T) | – | – | CAS198661 | Ⓘle des Pins | DQ675260 | – | – | |
| Tropidoscincus boreus | SAS92 | EBU 15584 | AMR163185 | Massif du Boulinda | DQ675241 | DQ675321 | DQ675381 | |
| Tropidoscincus variabilis | SAS93 | EBU 35275 | AMR161879 | Monts Kwa Né Mwa | DQ675242 | DQ675322 | DQ675382 | |
| Tropidoscincus variabilis | SAS94 | EBU 6715 | AMR150734 | Mt Ouin | DQ675243 | DQ675323 | DQ675383 | |
| Oligosoma (43 of 52 species) | ||||||||
| Lord Howe Island/Norfolk Island | ||||||||
| Oligosoma lichenigerum | LIC02 | ABTC58889 | – | Blackburn Island, Lord Howe Island Group | EU567704 | EU568108 | JQ610510 | |
| New Zealand | ||||||||
| Oligosoma acrinasum | OAC1 | CD826 | RE6179 | Fiordland | EF033046 | EU568098 | JQ610512 | |
| Oligosoma aeneum | CAE2 | FT5253 | RE5201 | Pukerua Bay | DQ675244 | DQ675324 | DQ675384 | |
| Oligosoma alani | CAL1 | FT145 | RE5471 | Green Island, Mercury Islands | EF043106 | EU568065 | JQ610502 | |
| Oligosoma alani | CAL2 | FT3016 | – | Matapia Island | EF567170 | EU568070 | JQ610503 | |
| Oligosoma auroraense | – | – | – | Cape Kidnappers | MH756037 | – | – | |
| Oligosoma auroraense | – | – | – | Cape Kidnappers | MH756039 | – | – | |
| Oligosoma awakopaka | – | – | – | Homer Saddle, Fiordland | MH756050 | – | – | |
| Oligosoma chloronoton | OCH1 | FT555 | RE5452 | Codfish Island, Stewart Island | EF103955 | EU568051 | JQ610513 | |
| Oligosoma elium | – | – | – | Jack Taylor’s Farm, Ward, South Island | EF103970 | EU568054 | – | |
| Oligosoma fallai | OFA1 | FT597 | RE6102 | Great Island, Three Kings Islands | EU567722 | EU568061 | JQ610514 | |
| Oligosoma fallai | OFA2 | FT598 | RE6103 | Great Island, Three Kings Islands | EU567723 | EU568062 | JQ610515 | |
| Oligosoma grande | OGR1 | CD1055 | RE6915 | Central Otago | EU567720 | EU568031 | JQ610516 | |
| Oligosoma hardyi | PKS1 | CD1036 | RE5442 | Aorangi Island, Poor Knights Islands | EF567122 | EU568059 | JQ610520 | |
| Oligosoma hardyi | PKS2 | CD1037 | RE5443 | Aorangi Island, Poor Knights Islands | EF567125 | EU568060 | JQ610521 | |
| Oligosoma homalonotum | OHO1 | FT6290 | RE6099 | Shoal Bay, Great Barrier Island | EF447146 | EU568085 | JQ610517 | |
| Oligosoma homalonotum | OHO2 | FT6291 | RE6100 | Tryphena, Great Barrier Island | EU567724 | EU568086 | JQ610518 | |
| Oligosoma hoparatea | – | – | – | Mt Somers | MF458303 | – | – | |
| Oligosoma hoparatea | – | – | – | Mt Somers | MF458304 | – | – | |
| Oligosoma hoparatea | – | – | – | Mt Harper | EU567714 | EU568044 | – | |
| Oligosoma hoparatea | – | – | – | Mt Harper | EU567714 | EU568045 | – | |
| Oligosoma inconspicuum | – | FT3783 | – | Awarua Point, Big Bay, Westland | EU567705 | EU568025 | – | |
| Oligosoma inconspicuum | – | FT3786 | – | Mouth of Mackenzie River, Big Bay, Westland | EU567706 | EU568026 | – | |
| Oligosoma judgei | – | – | – | Takitimu Mountains | JQ995168 | – | – | |
| Oligosoma judgei | – | – | – | Takitimu Mountains | JQ995169 | – | – | |
| Oligosoma kakerakau | – | Bream Head_1 | – | Bream Head Scenic Reserve | MT661457 | – | – | |
| Oligosoma kakerakau | – | Bream Head_3 | – | Bream Head Scenic Reserve | MT661458 | – | – | |
| Oligosoma kahurangi | – | – | – | Lonely Lake, Nelson | MT498325 | – | – | |
| Oligosoma levidensum | – | FT3729 | – | Kauri Bush, Pandora Track, Northland | EF567121 | – | – | |
| Oligosoma levidensum | – | – | RE4749 | Mt Unuwhao, Spirits Bay, Northland | EF567120 | – | – | |
| Oligosoma longipes | OLO1 | FT161 | RE6891 | Clarence River, Lake Tennyson | EU567717 | EU568042 | – | |
| Oligosoma maccanni | OMA1 | CD930 | RE5987 | Nevis Range, central Otago | EF081195 | EU568032 | – | |
| Oligosoma macgregori | CMA2 | FT1095 | RE5469 | Mana Island | EF567174 | EU568064 | JQ610504 | |
| Oligosoma microlepis | OMI1 | CD1299 | RE6200 | Taihape | DQ675235 | DQ675315 | DQ675375 | |
| Oligosoma moco | OMO1 | FT156 | RE6234 | Stanley Island, Mercury Islands | EF567286 | EU568071 | – | |
| Oligosoma moco | OMO2 | CD1031 | RE6232 | Aorangi Island, Poor Knights Islands | EF567287 | EU568072 | – | |
| Oligosoma newmani | OIF1 | CD545 | RE5407 | Stephens Island | EF033050 | EU568104 | – | |
| Oligosoma newmani | – | – | – | Waimea Creek | MH756041 | – | – | |
| Oligosoma newmani | – | – | – | Mt Arthur | MH756048 | – | – | |
| Oligosoma nigriplantare | – | CD1061 | – | South East Island, Chatham Islands | EF043108 | EU568029 | – | |
| Oligosoma nigriplantare | – | FT3615 | – | Mangere Island, Chatham Islands | EF043119 | EU568030 | – | |
| Oligosoma oliveri | COL1 | CD1034 | RE6648 | Aorangi Island, Poor Knights Islands | EF033045 | EU568074 | JQ610505 | |
| Oligosoma oliveri | COL3 | FT137 | RE6650 | Green Island, Mercury Islands | EF081176 | EU568075 | JQ610506 | |
| Oligosoma ornatum | – | FT188 | – | Devonport, Auckland | EF103954 | EU568079 | – | |
| Oligosoma ornatum | – | FT3733 | – | Botanic Gardens, Wellington | EF567196 | EU568078 | – | |
| Oligosoma otagense | OOT1 | CD1053 | RE6188 | Central Otago | EF033053 | EU568096 | – | |
| Oligosoma pikitanga | SVS1 | FT7648 | RE5315 | Sinbad Gully, Milford Sound, Fiordland | EU567713 | EU568097 | – | |
| Oligosoma polychroma | ONP1 | FT5252 | RE5200 | Pukerua Bay | EF033052 | EU568038 | – | |
| Oligosoma prasinum | – | – | – | Lake Tekapo | EF103971 | EU568052 | – | |
| Oligosoma repens | – | Eyres1 | – | Eyre Mountains, Southland | HQ113371 | – | – | |
| Oligosoma repens | – | Eyres2 | – | Eyre Mountains, Southland | HQ113372 | – | – | |
| Oligosoma smithi | OSM2 | FT193 | RE6274 | Ocean Beach, Whangarei | DQ675246 | DQ675326 | JQ610519 | |
| Oligosoma stenotis | – | FT2 | – | Mt Anglem, Stewart Island | EU567718 | – | – | |
| Oligosoma stenotis | – | FT289 | – | Table Hill, Stewart Island | EU567719 | – | – | |
| Oligosoma striatum | OSR2 | FT3301 | – | Waipuku, Taranaki | EF447147 | EU568055 | – | |
| Oligosoma suteri | OSU1 | FT148 | RE6244 | Green Island, Mercury Islands | DQ675247 | DQ675327 | DQ675387 | |
| Oligosoma suteri | OSU2 | FT602 | RE6258 | Great Island, Three Kings Island | EF567282 | EU568106 | – | |
| Oligosoma taumakae | – | – | – | Barn Islands | JQ995167 | – | – | |
| Oligosoma taumakae | – | – | RE5237 | Open Bay Islands | EF033048 | EU568091 | – | |
| Oligosoma tekakahu | – | FT7650 | – | Chalky Island | EU728655 | – | – | |
| Oligosoma toka | – | Nevis1 | – | Eyre Mountains, Southland | HQ113373 | – | – | |
| Oligosoma townsi | COL14 | – | – | Ahuriri Stream, Great Barrier Island | EF081173 | EU568068 | JQ610507 | |
| Oligosoma townsi | MOS1 | FT182 | RE5917 | Mokohinau Island | EF081184 | EU568080 | JQ610511 | |
| Oligosoma waimatense | – | CD1209 | – | Wairau River, Malborough | EF033056 | EU568095 | – | |
| Oligosoma waimatense | – | – | – | Rag and Famish Stream Valley, Marlborough | JN999945 | – | – | |
| Oligosoma whitakeri | CWH1 | CD949 | RE5931 | Pukerua Bay | EF081182 | EU568083 | JQ610508 | |
| Oligosoma whitakeri | CWH2 | FT294 | – | Middle Island, Mercury Islands | EF081183 | EU568084 | JQ610509 | |
| Oligosoma zelandicum (T) | OZE2 | FT6516 | – | Pukerua Bay | EF447181 | EU568081 | – | |
| Papua New Guinea | ||||||||
| Cryptoblepharus (1 of 5 New Guinea species) | ||||||||
| Cryptoblepharus yulensis | – | – | ABTC101989 | Juha survey | MH216014 | – | – | |
| Emoia (5 of 40 New Guinea species) | ||||||||
| Emoia atrocostata (T) | EUG65 | NR9864 EBU 43930 | BPBM19978 | Araeda, Sudest Island, Louisiade Archipelago, Milne Bay Province | JQ610249 | JQ610359 | JQ610474 | |
| Emoia atrocostata | EUG66 | NR9843 EBU 43909 | BPBM19979/80 | Nimowa Island, Louisiade Archipelago, Milne Bay Province | JQ610250 | JQ610360 | JQ610475 | |
| Emoia caerulecauda | – | – | LSUMZ93869 | Togarau Two Village, Central Bougainville, SE slope of Mt. Balbi, Bougainville Province | KU851260 | – | – | |
| Emoia jakati | EUG53 | NR1682 EBU 4310 | AMR129849 | Guleguleu Village, Normanby Island, Milne Bay Province | JQ610239 | JQ610349 | JQ610464 | |
| Emoia jakati | EUG54 | NR1683 EBU 4311 | AMR129851 | Guleguleu Village, Normanby Island, Milne Bay Province | JQ610240 | JQ610350 | JQ610465 | |
| Emoia jakati | EUG63 | EBU50108 | BPBM16875 | Sibonai, Normanby Island, Louisiade Archipelago, Milne Bay Province | JQ610247 | JQ610357 | JQ610472 | |
| Emoia jakati | EUG64 | EBU50109 | BPBM16876 | Sibonai, Normanby Island, Louisiade Archipelago, Milne Bay Province | JQ610248 | JQ610358 | JQ610473 | |
| Emoia longicauda | EUG67 | NR9893 | BPBM20747 | Cheme, Rossel Island, Louisiade Archipelago, Milne Bay Province | JQ610251 | JQ610361 | JQ610476 | |
| Emoia longicauda | EUG62 | EBU50107 | BPBM16803 | Sibonai, Normanby Island, Louisiade Archipelago, Milne Bay Province | JQ610246 | JQ610356 | JQ610471 | |
| Emoia tetrataenia | EUG60 | EBU50104 | BPBM16713 | 200 m NE Point 1, Normanby Island, Louisiade Archipelago, Milne Bay Province | JQ610244 | JQ610354 | JQ610469 | |
| Emoia tetrataenia | EUG61 | EBU50106 | BPBM16717 | Sibonai, Normanby Island, Louisiade Archipelago, Milne Bay Province | JQ610245 | JQ610355 | JQ610470 | |
| Eugongylus (2 of 5 species) | ||||||||
| Eugongylus albofasciolatus | EUG83 | ABTC98502 | SAMR69478 | North Dump Rainforest, Misima Island, Milne Bay Province | JQ610266 | JQ610376 | JQ610491 | |
| Eugongylus albofasciolatus | EUG84 | ABTC98524 | SAMR69479 | Lagua Camp, Misima Island, Milne Bay Province | JQ610267 | JQ610377 | JQ610492 | |
| Eugongylus rufescens (T) | SAS97 | – | AMR122480 | Bobole, Southern Highland Province | DQ675253 | DQ675333 | DQ675390 | |
| Sphenomorphus? | ||||||||
| Sphenomorphus louisiadensis | EUG88 | NR9851 EBU 43917 | AMR.181087.001 | Rossel Island, Louisiade Archipelago, Papua New Guinea | JQ610270 | JQ610380 | JQ610495 | |
| Sphenomorphus louisiadensis | EUG89 | NR9865 EBU 43931 | AMR.181101.001 | Rossel Island, Louisiade Archipelago, Papua New Guinea | JQ610271 | JQ610381 | JQ610496 | |
| Sphenomorphus minutus | EUG69 | – | BPBM18918 | Mt Shungol, Morobe Province, Papua New Guinea | JQ610253 | JQ610363 | JQ610478 | |
| Indonesia | ||||||||
| Carlia (1 of 8 Indonesian species) | ||||||||
| Carlia beccarii | – | – | TNHC59553 | Propinsi Maluku, Kai Kecil Island, Kota Tual | JQ610702 | – | – | |
| Cryptoblepharus (4 of 9 Indonesian species) | ||||||||
| Cryptoblepharus burdeni | – | – | WAMR104772 | Pasir Island | MH216005 | – | – | |
| Cryptoblepharus keiensis | – | – | JAM2259 | Propinsi Maluku, Seram Island, Kecematan Kairata | JQ610706 | – | – | |
| Cryptoblepharus leschenault | – | – | WAMR105231 | Merdeka | MH216004 | – | – | |
| Cryptoblepharus novaeguineae | – | ABTC90118 | – | Raja Ampat, Indonesia | MH216006 | – | – | |
| Christmas Island | ||||||||
| Cryptoblepharus (1 of 1 CI species) | ||||||||
| Cryptoblepharus egeriae | – | – | AMR152685 | Old Mine Fields 25 and Ml139 N Murray Hill | MH216022 | – | – | |
| Emoia (1 of 2 CI species) | ||||||||
| Emoia nativitatis | – | – | FS01 | Egeria Point | MH124072 | – | – | |
| Solomon Islands | ||||||||
| Emoia (8 of 14 Solomons species) | ||||||||
| Emoia cyanogaster | – | – | KU307235 | Poroi village, Ranongga Island | JF498111 | – | – | |
| Emoia cyanura | EUG47 | NR1340 EBU 3988 | AMR137200 | Su’U Bay, Malaita Island | JQ610233 | JQ610344 | JQ610458 | |
| Emoia cyanura | EUG48 | NR1425 EBU 4066 | AMR137225 | Bsurata village, Malaita Island | JQ610234 | JQ610345 | JQ610459 | |
| Emoia impar | – | – | KUH307191 | Solomon Islands | KU851342 | – | – | |
| Emoia impar | – | – | KUH307192 | Poroi village, Ranongga Island, Solomon Islands | KU851349 | – | – | |
| Emoia isolata | – | – | KU851480, KU851481 | Bellona Island, Solomon Islands | KU851365 | – | – | |
| Emoia pseudeocyanura | – | – | KUH307335 | Honiara Botanical Gardens, Guadalcanal Island | KU851370 | – | – | |
| Emoia pseudeocyanura | – | – | KUH307336 | Honiara Botanical Gardens, Guadalcanal Island | KU851372 | – | – | |
| Emoia rufilabialis | – | – | USNM533508 | 1–2 km S of Lata, along Graciosa Bay rd, Santa Cruz Islands, | KU851373 | – | – | |
| Emoia rufilabialis | – | – | USNM533509 | 1–2 km S of Lata, along Graciosa Bay rd, Santa Cruz Islands | KU851374 | – | – | |
| Emoia schmidti | – | – | KUH307097 | Zipolo Habu Resort, Lola Island, Western Province, Solomon Islands | KU851375 | – | – | |
| Emoia schmidti | – | – | KUH307098 | Zipolo Habu Resort, Lola Island, Western Province, Solomon Islands | KU851376 | – | – | |
| Emoia taumakoensis | – | – | USNM533523 | Village at Mahele Point, Taumako, Duff Islands | KU851379 | – | – | |
| Emoia taumakoensis | – | – | USNM533524 | Village at Mahele Point, Taumako, Duff Islands | KU851380 | – | – | |
| Sphenomorphus? | ||||||||
| Sphenomorphus bignelli | EUG68 | NR1457 | AMR134955 | Mt Javi, New Georgia, Solomon Islands | JQ610252 | JQ610362 | JQ610477 | |
| Vanuatu | ||||||||
| Emoia (2 of 10.Vanuatu species) | ||||||||
| Emoia nigra | EUG49 | NR1646 | AMR138856 | Plantation near Namasa River, Gaua Island | JQ610235 | – | JQ610460 | |
| Emoia nigra | EUG50 | NR1648 | AMR138857 | Plantation near Namasa River, Gaua Island | JQ610236 | JQ610346 | JQ610461 | |
| Emoia sanfordi | EUG51 | NR1656 | AMR138861 | Plantation near Namasa River, Gaua Island | JQ610237 | JQ610347 | JQ610462 | |
| Emoia sanfordi | EUG52 | NR1657 EBU 4286 | AMR138862 | Plantation near Namasa River, Gaua Island | JQ610238 | JQ610348 | JQ610463 | |
| Micronesia | ||||||||
| Emoia (2 of 8 Micronesian species) | ||||||||
| Emoia boettgeri | – | – | USNM576213 | Kolonia, Pohnpei Island, Caroline Islands, Federated States of Micronesia, | MH124071 | – | – | |
| Emoia ponapea | – | – | – | Micronesia | KU851366 | – | – | |
| Emoia ponapea | – | – | – | Micronesia | KU851367 | – | – | |
| Japan | ||||||||
| Cryptoblepharus nigropunctatus | – | 13–5130 | – | Ogasawara Gunto | MH216020 | – | – | |
| French Polynesia | ||||||||
| Cryptoblepharus poecilopleurus (T) | – | MS082 | – | Fakarava – Tetamanu, French Polynesia | MH216013 | – | – | |
| Mauritius | ||||||||
| Leiolopisma telfairii (T) | SAS101 | – | – | Round Island, Mauritius | DQ675259 | – | DQ675396 | |
| Africa | ||||||||
| Leptosiaphos (mixed sample: blochmanni RAG-1; vigintiserierum c-mos) | – | – | UTEP21177/ZFMK69429 | Bichaka, South Kivu, Democratic Republic of the Congo (blochmanni); Mt Nlonako, above Nguengue, Cameroon (vigintiserierum) | – | KU298722 | EU164504 | |
| Panaspis africanus | – | – | CAS218730 | Principe Island, Sao Tome and Principe | DQ675257 | DQ675337 | EU164499 | |
| Panaspis breviceps | – | – | CAS249945 | Wawne, Cameroon | KY696706 | KU298714 | – | |
| Panaspis togoensis | – | – | KU290440 | Lajuma Wilderness Camp, Limpopo Province, South Africa | JF498127 | KU298679 | – | |
| Outgroup- Acontiinae | ||||||||
| Acontias meleagris | – | – | – | South Africa | AY662553 | AY662639 | DQ249056 | |
| Outgroup- Lygosomini | ||||||||
| Lamprolepis smaragdina | EUG43 | NR503 EBU 3201 | AMR129523 | Amelei Village, West New Britain Province, Papua New Guinea | JQ610315 | JQ610427 | JQ610564 | |
| Lamprolepis smaragdina | EUG44 | NR506 EBU 3204 | AMR129538 | Amelei Village, West New Britain Province, Papua New Guinea | JQ610316 | JQ610428 | JQ610565 | |
| Riopa punctata | – | – | – | India | DQ675265 | MK409549 | MK409397 | |
| Outgroup- Mabuyini | ||||||||
| Toenayar novemcarinata | – | – | CAS216022 | Na Htoe Gyi Township, Minsontaung Wildlife Sanctuary, Htan Taw Village, Mandalay Division, Myanmar | KX365035 | KX365042 | KX364989 | |
| Outgroup- Scincinae | ||||||||
| Feylinia polylepis | – | – | – | – | AY662556 | – | – | |
| Scincus scincus | – | – | – | – | AB738956 | HM161238 | AY217873 | |
| Outgroup- Sphenomorphini | ||||||||
| Ablepharus kitaibelii | – | – | ZMMUR16815 | Kardamali, Greece | MZ820275 | – | MN418804 | |
| Ablepharus kitaibelii | – | – | NHMC80.3 | Olympos Mt, Greece | MZ848097 | – | MN418806 | |
| Ablepharus pannonicus | – | – | R14554 | Khasyanata, Uzbekistan | MZ820287 | – | AF039466 | |
| Alpinoscincus alpinus | – | – | BPBM44217 | Mt Yule, north lookout, Papua New Guinea | MZ516539 | – | – | |
| Alpinoscincus sp. | – | – | BPBM47913 | Randon Ridge, Papua New Guinea | MZ516567 | – | – | |
| Ctenotus (mixed robustus ND2/RAG-1, taeniolatus c-mos) | – | – | – | Australia | AY662548 | AY662630 | AY818792 | |
| Lobulia brongersmai | – | – | BPBM34733 | Imuk, East Sepik Province, Papua New Guinea | MZ516593 | – | – | |
| Lobulia elegans | – | – | BPBM18692 | Fane, Central Province, Papua New Guinea | MZ516588 | – | – | |
| Nubeoscincus glacialis | – | – | BPBM14705 | Lake Wanagong, ~9 km (by air) N of Tembagapura town centre, Fakfak Division, Indonesia | MZ516528 | – | – | |
| Nubeoscincus glacialis | – | – | BPBM14708 | Lake Wanagong, ~9 km (by air) N of Tembagapura town centre, Fakfak Division, Indonesia | MZ516529 | – | – | |
| Sphenomorphus fasciatus | SAS99 | – | CCA1251 | Mindanao Camp, Philippines | DQ675240 | DQ675320 | DQ675380 | |
| Outgroup- Tiliquini | ||||||||
| Tiliqua scincoides | SAS98 | EBU 32767 | AMR154684 | Yathong NR, NSW, Australia | DQ675249 | DQ675329 | DQ675388 | |
| Tribolonotus schmidti | EUG41 | NR486 EBU 3184 | AMR127360 | Mt Austen, Guadalcanal, Solomon Islands | JQ610314 | – | JQ610563 | |
Taxonomic type species (on current taxonomy) for genera indicated by (T) against the species name. Museum acronyms: ABTC, Australian Biological Tissue Collection (housed at the South Australian Museum); AM, Australian Museum, Sydney; BPBM, Bishop Museum, Honolulu, Hawaii; CAS, California Academy of Sciences; CD or FT, National Frozen Tissue Collection (NFTC) housed at Victoria University of Wellington, New Zealand; EBU or NR, Evolutionary Biology Unit, Australian Museum; NMV, Museum Victoria, Melbourne; QM, Queensland Museum, Brisbane; RE, National Museum of New Zealand, Te Papa Tongarewa, Wellington, New Zealand; SAM, South Australian Museum, Adelaide; TMH, Tasmanian Museum and Art Gallery, Hobart; WAM, Western Australian Museum, Perth; LSUMZ, Louisiana Museum of Natural History, Baton Rouge, Louisiana; KU, University of Kansas Biodiversity Institute, Lawrence, Kansas; USNM, National Museum of Natural History, Washington DC; TNHC, Texas Natural History Collection, Austin, Texas; CCM, Craig Moritz tissue collect at the Australian National University; MNHN, Muséum national d’Histoire naturelle, Paris; ZFMK, Zoologisches Museum Alexander Koenig, Bonn; ZMMUR, Zoological Museum of Mikhail V. Lomonosov, Moscow State University, Moscow. Australian state abbreviations: NSW, New South Wales; NT, Northern Territory; QLD, Queensland; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
Our tissue samples were obtained from museum collections in Australia (Australian Museum; Museum Victoria; Queensland Museum; South Australian Museum), New Zealand (Victoria University of Wellington; National Museum of New Zealand, Te Papa Tongarewa), and North America (Californian Academy of Sciences; Bishop Museum, Honolulu) (Table 1). To root our tree, we included 21 outgroup samples representing 19 species across 14 genera from other skink subfamilies and tribes covering the Acontiinae, Scincinae, Sphenomorphini, Lygosomini, Mabuyini, Sphenomorphini and Tiliquini (Table 1). Sequences from the remaining two tribes in the Lygosominae, the Ristellini (two genera) and Ateuchosaurini (one genus) were unavailable for the loci we used. For two genera (Ctenotus and Leptosiaphos), due to the lack of sequence data from the same species, we used composites of two species to create a single taxon for analysis (see Table 1).
DNA extraction, amplification and sequencing
Total genomic DNA was extracted from liver, muscle, toe or tail-tip samples using a Qiagen DNeasy Blood and Tissue Extraction Kit (Qiagen, Hilden, Germany). For each sample we sequenced portions of the ND2 mitochondrial gene (~600 bp) and two nuclear genes: recombination activating gene 1 (RAG-1, ~900 bp) and oocyte maturation factor (c-mos, ~550 bp). These regions were targeted because previous work on the Eugongylini has indicated useful levels of variability (Smith et al. 2007; Chapple et al. 2009). The primers used to amplify and sequence the three genes are provided in Table S1. PCR was conducted as outlined in Chapple et al. (2009). PCR products were purified using ExoSAP-IT (USB Corporation, Cleveland, Ohio USA). The purified product was sequenced directly using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and then analysed on an ABI 3730XL capillary sequencer.
Sequence data were edited using Geneious v5.4 (Drummond et al. 2011), roughly aligned by eye, and alignments optimised using MUSCLE (Edgar 2004). We translated all sequences to confirm that none contained premature stop codons. Sequence data were submitted to GenBank under the accession numbers provided in Table 1.
Among ingroup taxa, sequences for all three loci were available for 190 individuals (59%), for two loci for 32 individuals (10%) and for one locus for 99 individuals (31%). We obtained 777 of a possible 1026 sequences across all 342 individuals, for 76% coverage.
Phylogenetic and divergence time analyses
Our phylogenetic approach was designed to optimise the topological and temporal resolution of our mito-nuclear dataset given recent phylogenomic datasets of skinks. We started by downloading exon capture data focusing on Australian eugongylins (Brandley et al. 2015) and squamates (Burbrink et al. 2020) to build a consistent set of nuclear alignments and gene trees, and ultimately generate a time-calibrated backbone of the Scincidae. Data from different Anchored Hybrid Enrichment projects were combined using custom scripts which relied on metablastr to identify orthologous loci (blast_best_reciprocal_hit) (Benoit and Drost 2021), mafft to align them (--add, --keeplength) (Katoh et al. 2002), and AMAS to manipulate alignments (Borowiec 2016). We reconstructed individual genealogies for our exon-capture data (n = 272) under maximum-likelihood in IQTREE (Nguyen et al. 2015), allowing the program to assign the best-fitting model of molecular evolution using PartitionFinder, then perform 1000 ultrafast bootstraps (Hoang et al. 2018). We then estimated a species tree using the shortcut coalescent method ASTRAL III (Zhang et al. 2017) with IQTREE gene trees as input (Supplementary Fig. S1). To estimate divergence times among taxa we applied a series of primary and secondary calibrations (Table S2) and used the Bayesian divergence time software MCMCtre (Rannala and Yang 2007). We started by concatenating all loci and partitioning them into two partitions, first and second codons together, and third codons separately. We then used baseml to estimate approximate likelihoods and branch lengths before running mcmctree on the gradient and Hessian (in.BV file) for 10 replicate analyses (Reis and Yang 2011). We inspected mcmc files for stationarity and compared for convergence, then combined them using logCombiner (Bouckaert et al. 2019) and used this combined mcmc file to summarise divergence times on our tree (print = −1 in.ctl file).
We then turned our attention to the mito-nuclear dataset. We started by constraining a handful of nodes to match the topology of our ASTRAL exon capture species tree. We estimated the topology from our locus-partitioned concatenated mito-nuclear alignment using IQTREE, allowing PartitionFinder to choose and apply the preferred evolutionary model for each partition, and approximated topological support with 1000 ultrafast bootstraps (Supplementary Fig. S2). Following the methodology above, we then used this mito-nuclear species tree as input for MCMCtree, and split our alignment into four partitions (first and second mtDNA codons, third mtDNA codons, first and second nDNA codons, third nDNA codons). We applied a series of secondary calibrations (Table S2) and followed the procedure described above to estimate divergences between samples in our mito-nuclear dataset. This tree is presented twice: in Fig. 1 to highlight the intergeneric divergence times, and as a fully sampled phylogeny in Fig. 2.
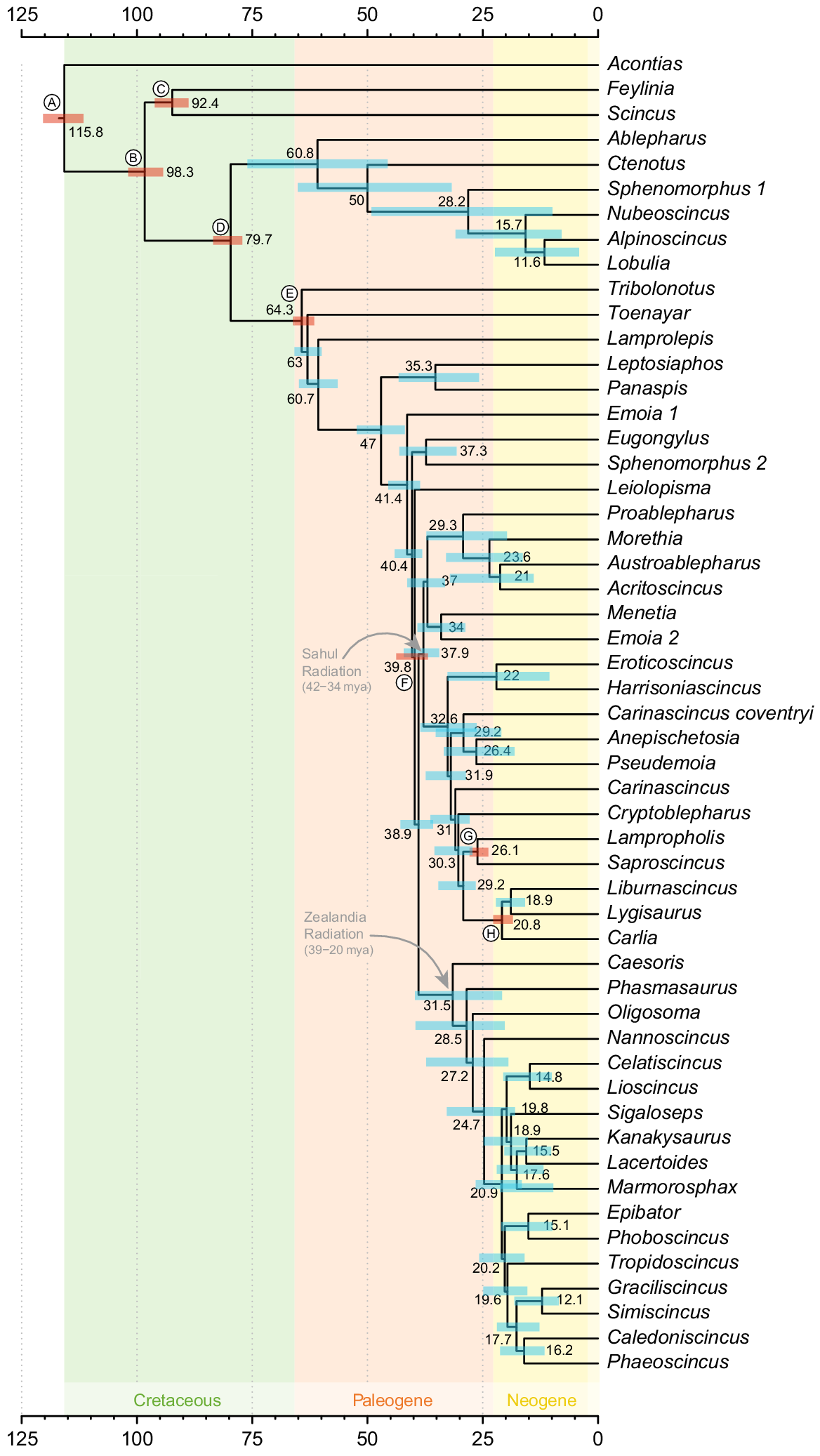
Time-calibrated phylogeny of skinks with focus on topology and divergence times among the subfamily Eugongylini. This tree is based on the three-locus mito-nuclear dataset (ND2, RAG-1, c-mos) estimated using MCMCtree and shows the genus-level relationships within the subfamily as a subset of Fig. 2. Numbers at nodes indicate mean divergence times. Coloured bars at nodes indicate the 95% confidence estimates of divergence times. Nodes with red bars and a circle containing a letter show the placement of secondary calibrations used in the divergence time analysis (Table S2). Topological support values are shown on the tree in Fig. 2.
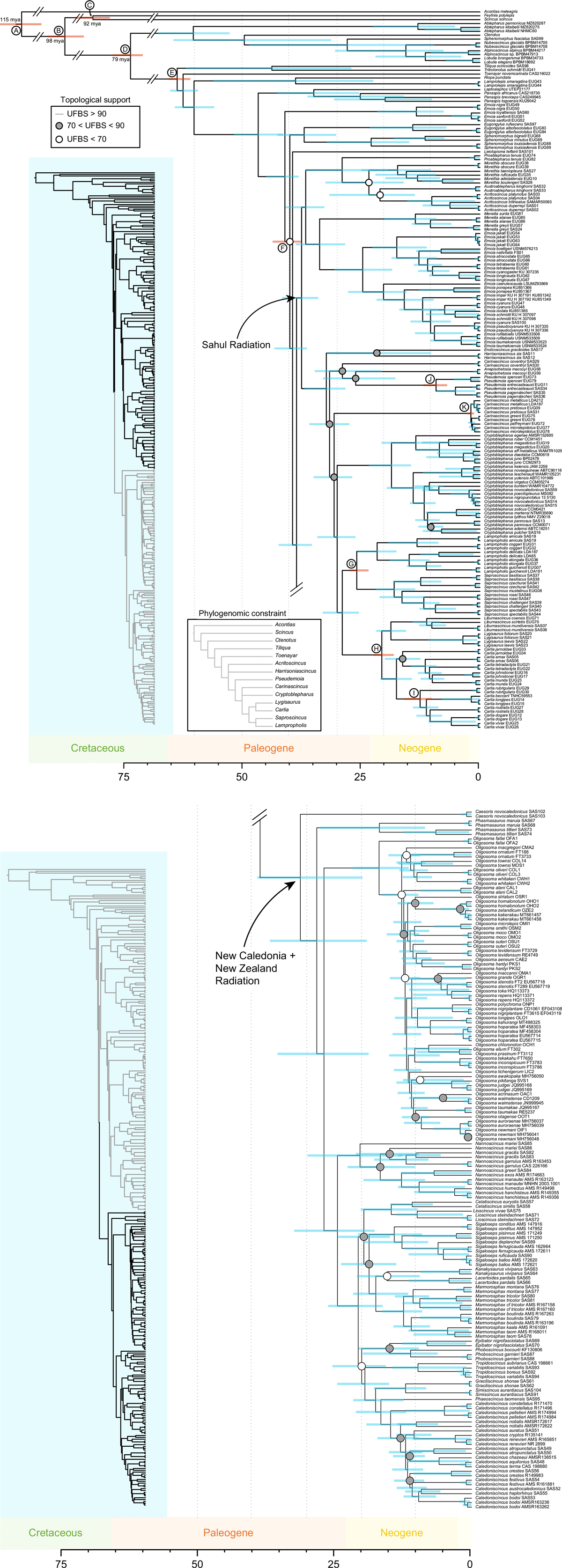
Fully sampled time-calibrated phylogeny of the tribe Eugongylini. The tree is split across two images---an inset on the left shows the corresponding portion of the tree. This tree is based on the three-locus mito-nuclear dataset (ND2, RAG-1, c-mos) estimated using MCMCtree and shows the species-level relationships within the subfamily. The topology of this tree was first estimated using IQTREE using a constrained guide tree based on a reliable phylogenomic hypothesis (see inset Phylogenomic Constraint). Topological support is indicated by the absence, presence, and colour of circles at nodes. Nodes with ultrafast bootstrap support values of >90 indicating strong topological support are not noted on the tree to simplify the visualisation. Nodes with moderate support (ultrafast bootstrap values of >70 < 90 are indicated by a grey circle, and poorly supported or equivocal nodes (ufbs <70) are noted by a white circle.
Results
The edited alignment comprised 1907 bp of sequence data (523 bp ND2, 834 bp RAG-1, 550 bp c-mos). The number of variable sites, number of parsimony-informative sites, and nucleotide frequencies are provided in Table 2.
| Mitochondrial DNA | Nuclear DNA | ||
|---|---|---|---|
| ND2 | RAG-1 | c-mos | |
| Sequence length (bp) | 523 | 834 | 550 |
| No. variable sites (%) | |||
| All taxa | 404 (77.2) | 572 (68.5) | 273 (49.6) |
| Ingroup only | 390 (74.6) | 495 (59.4) | 221 (40.2) |
| No. PI sites (%) | |||
| All taxa | 361 (69.0) | 487 (58.4) | 219 (39.8) |
| Ingroup only | 350 (66.9) | 435 (52.2) | 169 (30.7) |
| Mean nucleotide frequency | |||
| A | 0.327 | 0.312 | 0.244 |
| C | 0.308 | 0.212 | 0.206 |
| G | 0.132 | 0.257 | 0.239 |
| T | 0.233 | 0.219 | 0.311 |
PI, parsimony informative.
There was strong support (Figs 1 and 2) for the monophyly of the Eugongylini, and we estimated the subfamily to have originated ~48 mya (95% confidence estimate 55–44 mya) (Figs 1 and 2). Three well-supported lineages of Australian Eugongylini were evident: (A) Eroticoscincus–Harrisoniascincus–(Carinascincus)coventryi–Anepischetosia–Pseudemoia–Carinascincus–Cryptoblepharus–Lampropholis–Saproscincus–Liburnascincus–Lygisaurus–Carlia (Figs 1 and 2), (B) Proablepharus–Morethia–Austroablepharus–Acritoscincus, and (C) Menetia–Emoia s.s. (Figs 1 and 2). Each of these lineages form part of the same well supported eugongylin radiation of primarily Australian species (Sahul Radiation; Figs 1 and 2), which is estimated to have arisen ~38 (42–34) mya (Fig. 2). Monophyly of this radiation received strong support (Fig. 2). The Group B and C lineages form a well-supported sister relationship (Fig. 2), and the relationships among genera within each of these lineages is generally well resolved (Fig. 2). The Group A lineage is well-supported as the sister to the Group B + C lineages (Fig. 2). Intergeneric relationships among the Group A genera are well resolved at the higher nodes (Fig. 2).
The results strongly supported the monophyly (~31 (39–20) mya) of the eugongylin species of the Zealandia region (Figs 1 and 2). There is strong support for Caesoris and Phasmasaurus being the earliest divergences within the Zealandia Lineage (Figs 1 and 2). There is also strong support for the New Zealand (+ Lord Howe Island) Oligosoma as the sister to the New Caledonia genera (exclusive of Caesoris and Phasmasaurus) (Figs 1 and 2). The main New Caledonian radiation is also well-supported, with Nannoscincus as the sister lineage to a well-supported lineage that includes all endemic New Caledonian genera apart from Caesoris and Phasmasaurus, its diversification commencing ~25 mya (Figs 1 and 2). The New Zealand skink radiation is also strongly supported, its diversification commencing ~18 mya (Figs 1 and 2).
Our phylogenetic analyses recovered four to five well-supported basal eugongylin lineages (Figs 1 and 2). These lineages included: (1) a group of African taxa represented by Leptosaiphos and Panaspis, (2) a lineage of taxa currently included in Emoia (loyaltiensis (Roux, 1913), nigra (Duméril & Duméril, 1851) and sanfordi Schmidt & Burt, 1930), all members of the Emoia samoensis Group of Brown (1991), (3) a lineage of Melanesian taxa that includes Eugongylus and three misplaced Sphenomorphus species (bignelli, louisadensis, minutus), and that forms a well-supported group, and (4) the Indian Ocean genus Leiolopisma represented by the extant species telfairii (Desjardin, 1831) (Mascarene Islands) (Figs 1 and 2).
A primary Emoia lineage (represented by atrocostata (Lesson, 1830), boettgeri (Sternfeld, 1918), caeruleocauda (De Vis, 1892), cyanura (Lesson, 1830), impar (Werner, 1898), isolata Brown, 1991, jakati (Kopstein, 1926), longicauda (Macleay, 1877), nativitatis (Boulenger, 1887a), ponapea Kiester, 1982, pseudocyanura Brown, 1991, rufilabialis McCoy & Webber, 1984, schmidti Brown, 1954, taumakoensis McCoy & Webber, 1984 and tetrataenia (Boulenger, 1895)) that included the type species for the genus was found to be part of the Australian lineage, with Menetia as its sister lineage (Figs 1 and 2).
Within the Australian eugongylin radiation (Sahul Radiation), our analyses resolved several phylogenetic relationships. There was strong support for the monophyly of the Tasmanian Carinascincus species, to the exclusion of coventryi (Fig. 2). The species coventryi is not part of any described genus, but rather represents its own divergent lineage, with affinities to Anepischetosia maccoyi and Pseudemoia (Figs 1 and 2). Cryptoblepharus was found to be monophyletic, and part of the Sahul Radiation, and nested well within the main group of the Australian eugongylins (Figs 1 and 2). There was clear evidence for A. maccoyi being part of the Sahul eugongylin radiation, rather than the New Caledonian lineage containing Nannoscincus (Figs 1 and 2). There was evidence for a close phylogenetic relationship (origin ~21 mya) between the monotypic genera Harrisoniascincus and Eroticoscincus (Figs 1 and 2).
Our results support the monophyly of both Morethia and Acritoscincus, and indicate that Proablepharus and Austroablepharus are not sister groups (Figs 1 and 2). Similarly, our results strongly support the reciprocal monophyly of Lampropholis and Saproscincus, and the recognition of Liburnascincus, Lygisaurus, and Carlia as three distinct, but closely related, genera (Figs 1 and 2).
Discussion
We have generated the most complete molecular phylogeny for the Eugongylini ever produced in terms of coverage of both genera and species. Our phylogenetic analyses strongly support the monophyly of the Eugongylini. This result is consistent with previous morphological (Fuhn 1969; Greer 1967, 1974), genetic (Honda et al. 2000, 2003; Whiting et al. 2003; Schmitz et al. 2005; Austin and Arnold 2006; Smith et al. 2007; Chapple et al. 2009; Skinner et al. 2011; Pyron et al. 2013), karyological (Donnellan 1985), and immunological (Hutchinson 1981; Hutchinson et al. 1990) studies of the group. However, it is inconsistent with the heterodox result of Zheng and Wiens (2016) that placed Eugongylus itself in the Sphenomorphini. Our phylogeny resolves several long-standing questions and issues regarding the taxonomy and evolutionary history of the group; however, several older relationships within the group remain unresolved.
Monophyly of the Australian Eugongylini and its relationship to the Zealandia Lineage
Our study is evidence for a monophyletic Australian radiation, inclusive of Emoia s.s., within the Eugongylini (Figs 1 and 2) – this is the first time clear support has been provided for this relationship. The Zealandia Lineage is retrieved as the sister to the Sahul Radiation within the Eugongylini. Previous molecular studies on the New Caledonia (Smith et al. 2007) and New Zealand (Hickson et al. 2000; Chapple et al. 2009) eugongylin fauna hinted at this relationship, but limited sampling of Australian genera clouded demonstration of their affinity. A broader study of Australian skink phylogenetic relationships (Skinner et al. 2011) also recovered this relationship between the Australian and Zealandia eugongylin faunas, but that study included only a single New Caledonian and New Zealand species, and only six Australian species. These previous studies had shown the Zealandia Lineage to be monophyletic (Smith et al. 2007; Chapple et al. 2009; Skinner et al. 2011; Pyron et al. 2013), and we found comparable results with the broader taxonomic sampling employed in our study. Our results also retrieved some of the key phylogenetic relationships previously identified within Zealandia, including monophyly of the major New Caledonia radiation of endemic genera, apart from the genera Caesoris and Phasmasaurus as identified by Smith et al. (2007); and the New Zealand fauna as a sister lineage to the New Caledonia radiation as identified by Smith et al. (2007) and Chapple et al. (2009). However, our study placed Caesoris and Phasmasaurus progressively as the sister lineages to the New Caledonian + New Zealand radiation with high support (>0.90), rather than as the sister to the New Zealand species as in the study by Smith et al. (2007) with low support (<0.50 bootstrap), or with Phasmascincus nested within the New Caledonian radiation as recovered by Chapple et al. (2009) with high support (0.97) – Chapple et al. (2009) did not include Caesoris in their sampling. We found Oligosoma lichenigerum (O’Shaughnessy 1874), which lies between New Caledonia and New Zealand on the Norfolk Ridge and Lord Howe Rise, to be nested within a monophyletic New Zealand Oligosoma (though with low support for several earliest divergences for the genus), rather than as the sister to it, as found by Smith et al. (2007) with weak support (<0.50 bootstrap support), and in the more extensively sampled study of the New Zealand species by Chapple et al. (2009) with much higher support (0.96).
Relationships of the former Australian ‘Leiolopisma’
Early attempts by Greer (1974) at refining Leiolopisma Duméril & Bibron, 1839 resulted in the removal of window-eyed species that were outside the early concept of the Eugongylini (Scincella, Prasinohaema, Lipinia and Lobulia were erected or resurrected, and placed in what would become the Sphenomorphini), and provision of generic status for some obvious morphological clusters of window-eyed skinks within the Eugongylini (Carlia, Lampropholis, Anotis, Emoia, Panaspis), but Greer (1974, 1979) still retained ‘Leiolopisma’ as a speciose genus including taxa from Mauritius, Australia, New Caledonia and New Zealand. Hardy (1977) subdivided the New Zealand ‘Leiolopisma’ in resurrecting Cyclodina for a subset of species while still retaining ‘Leiolopisma’ for the remaining taxa, and proposed a detailed biogeographic history and dispersal routes for the New Zealand skink fauna. The concepts of ‘Leiolopisma’ as presented by Greer (1974) and Hardy (1977) were that it was paraphyletic, as they proposed the evolution of multiple genera and origins from within that genus.
Although Greer (1982a) continued to use Leiolopisma for the Australian species, he did suggest (following the lead of Rawlinson 1974, 1975) that the species known at the time fell into two groups on morphology, a small-scaled group with long limbs, low litter size, arboreal or saxicoline habits, and generally lacking chromatic hues (the ‘spenceri Species Group’), and a large-scaled group with short limbs, larger clutches/litters, terrestrial habits, and with chromatic hues usually present (the ‘baudini Species Group’). Subsequently, Greer (1989) used a single newly recognised osteological character, the fusion of the hemilaminae to the intercentrum of the atlas, to recognise a new grouping of eugongylin genera, the ‘Pseudemoia Group’. This character transected each of his previous spenceri and baudini Species Groups. The members of the former baudini Group were separated into two genera, Claireascincus for the cluster of species around entrecasteauxii (Duméril & Bibron, 1839), which possessed atlantal arch fusion and was hence grouped with a number of other Australian Eugongylini that comprised the Pseudemoia Group, and Eulepis (now Acritoscincus) for the cluster of species around trilineata (Gray, 1838) which lacked atlantal arch fusion. His former spenceri Group was also divided by this character, with Pseudemoia restored for the two species that showed atlantal arch fusion (spenceri (Lucas & Frost, 1894) and palfreymani (Rawlinson, 1974), thus restoring Rawlinson’s earlier concept of the genus but on different grounds), and Carinascincus for the Tasmanian snow skinks, reportedly lacking fusion.
Hutchinson et al. (1990) tested Greer’s (1974, 1979, 1980, 1982a) proposals with immunological comparisons of serum albumins, using microcomplement fixation techniques. They used four Australian ‘Leiolopisma’ species, entrecasteauxii, duperreyi (Gray, 1838), pretiosum (O’Shaughnessy, 1874) and palfreymani. The first two of these represented members of Greer’s (1982a) baudini Species Group, and the latter two were members of the spenceri Species Group. For these species they made two-way comparisons with three other genera, Emoia (represented by Emoia longicauda), Morethia (represented by M. adelaidensis (Peters, 1874)) and Lampropholis (represented by Lampropholis guichenoti (Duméril & Bibron, 1839) and Lampropholis challengeri (Boulenger, 1887b); it is worth noting that, based on the localities provided for their serum samples, their sample of the latter species represents what is now Saproscincus spectabilis (De Vis, 1888)). They found that the Australian Eugongylini sampled formed a monophyletic group, but the Australian ‘Leiolopisma’ were paraphyletic within this group, with Morethia and Lampropholis nested within it, and trilineatum (then as duperreyi) closely related to Morethia, supporting Greer’s (1980) early concept of affinities between these taxa. However, Greer’s (1982a) later hypotheses of the baudini and spenceri Species Groups were less congruent with the immunological results, as was the division created by the atlantal arch character of Greer (1989). In effect, the immunological study split the content of both Greer’s baudini and spenceri species groups, and was similarly not congruent with the taxa assigned to the Pseudemoia group on the atlantal arch character. To resolve the paraphyly inherent in the Australian Leiolopisma, Hutchinson et al. (1990) erected the genus Niveoscincus for the Tasmanian snow skinks (greeni (Rawlinson, 1974), microlepidotus (O’Shaughnessy, 1874), ocellatus (Gray, 1845), orocryptum (Hutchinson et al., 1988), pretiosus (O’Shaughnessy, 1874) and Leiolopisma palfreymani (in content Greer’s spenceri Group but without Leiolopisma spenceri itself), and also included Leiolopisma metallicum and L. coventryi (both formerly of the baudini Group). They resurrected the name Pseudemoia for several species in the baudini Group1 (baudini (Greer, 1982a), entrecasteauxii, rawlinsoni (Hutchinson & Donnellan, 1988)) plus L. spenceri, and created the genus Bassiana for the remaining species that formerly comprised part of the baudini Group (duperreyi, platynota (Peters, 1881), trilineata). Complicating the nomenclature of this arrangement, Wells and Wellington (1985) had previously created the new generic name Acritoscincus for the species included in Bassiana by Hutchinson et al. (1990), and Carinascincus, Litotescincus, and Tasmascincus for the majority of species placed by Hutchinson et al. (1990) within Niveoscincus. There has been a gradual replacement of Bassiana with Acritoscincus in the herpetological literature, and a more recent transition from Niveoscincus to Carinascincus for the Tasmanian snow skinks.
For Pseudemoia, we included three of the six species, including the type species (Pseudemoia spenceri) and the type species of the synonym Claireascincus (P. entrecasteauxii). For Carinascincus we sampled six of the eight species, including the type species (C. greeni), which is also the type species of its synonym Niveoscincus, and also including the type species of two other synonyms, Litoteoscincus (Carinascincus metallicus), and Tasmascincus (Carinascincus palfreymani). We find high support for the species palfreymani as part of the group of snow skinks included in Carinascincus, and for Pseudemoia to include the type species spenceri and those taxa grouping around entrecasteauxii, rejecting earlier suggestions by Rawlinson (1974) and Greer (1989) that spenceri and palfreymani are congeneric. Further, these two genera are not sister-taxa within the Australian eugongylin radiation. This is congruent with phylogenies reconstructed by Brandley et al. (2015).
A close relationship of Acritoscincus (as Bassiana) with Morethia had been proposed on morphological evidence by Greer (1980, 1983), and on immunological evidence by Hutchinson et al. (1990) and Baverstock and Donnellan (1990). Greer (1980, 1983) further suggested that Proablepharus was also part of this lineage, as the sister taxon to Morethia (see also Fuhn 1969). Further, he later suggested that an unpublished cladistic analysis of morphological characters raised the possibility that the two genera were not reciprocally monophyletic (Greer et al. 2004). Early genetic studies either omitted representation of Proablepharus (Smith et al. 2007; Zheng and Wiens 2016), or were limited to inclusion of a single species (Skinner et al. 2011; Pyron et al. 2013). Couper et al. (2010) noted that character state incompatibility within Proablepharus rendered cladistic analysis of relationships difficult, and later (Couper et al. 2018) presented morphological (all described species) and genetic data (tenuis (Broom, 1896), reginae (Glauert, 1960) and kinghorni (Copland, 1947)) that identified paraphyly in the genus. As a result, Proablepharus was redefined to include only tenuis (type species of the genus) and reginae, and the genus Austroablepharus was created to accommodate kinghorni (type species for genus), and two recently described taxa, barrylyoni (Couper et al., 2010) and naranjicaudus (Greer et al., 2004; Couper et al. 2018).
Our results indicate that Acritoscincus, Morethia and Austroablepharus form a well-supported lineage within the Australian eugongylin radiation, with Proablepharus strongly supported as the sister, a relationship broadly in agreement with proposals initially based on morphological (Greer 1980, 1983) and immunological (Baverstock and Donnellan 1990; Hutchinson et al. 1990) evidence, and which has previously received some molecular support (Skinner et al. 2011; Ivan et al. 2022). We demonstrate that both Morethia and Acritoscincus are monophyletic, and support the results found by Couper et al. (2018) for polyphyly within earlier concepts of Proablepharus. However, our results offer only weak support for relationships among the three genera Australoblepharus, Morethia and Acritoscincus.
The Australian species (Carinascincus) coventryi was regarded by Greer (1979) as a member of Leiolopisma within the Eugongylus Subgroup, and later (Greer 1982a) as a member of the baudini Species Group (see above). One-way immunological comparisons by Hutchinson et al. (1990) found it to lie closest to the group of species now in Carinascincus, although it was outside the core of that group. It shared a live-bearing (viviparous) mode of reproduction with species in that group, but had morphological traits inconsistent with being placed with that group (it lacked the fused frontoparietal scales diagnostic of the group, and had lost the upper temporal opening on the skull, which was otherwise present in the group). Hence, Hutchinson et al. (1990) were uncertain of its relationship to this group of species, but tentatively placed it in the genus erected for them (then Niveoscincus). Their findings agreed in part with the earlier suggestion of Rawlinson (1975), who suggested affinities of coventryi with metallicus, and with placement of coventryi by Wells and Wellington (1985) in their genus Litotescincus. Greer later (1989) treated coventryi as congeneric with Harrisoniascincus zia, presumably following the earlier suggestion of Ingram and Ehmann (1981), which was largely based on colouration and ecological similarities. Stewart and Thompson (1998) found that the species did not possess the unique placental morphology of the species now assigned to Carinascincus, and this difference in placental morphology is mirrored by differences in placental physiology between coventryi and these taxa (Thompson et al. 2001). A sister relationship of coventryi with Carinascincus was also recovered by Brandley et al. (2015) and Ivan et al. (2022). Our results found that coventryi is not part of any described genus, but rather comes out as its own divergent lineage, most closely related to Anepischetosia and Pseudemoia, though with only moderate support, and as such warrants recognition as its own monotypic genus (see taxonomic implications section below).
Relationships of the ‘beta Group’ skinks
Greer (1974, 1979) proposed a subgroup of genera within the Eugongylini (variously as Group III of Greer (1974), and the Lampropholis Subgroup of Greer 1979) on the basis of a single morphological character, the development of a hook on the pterygoid bone to extend the secondary palate (the beta palate). Initially as Group III it consisted of the Australian genera Lampropholis, Carlia, Menetia, Notoscincus, and the Solomon Island genus Geomyersia, together with the African genera Afroablepharus, Cophoscincopus and Panaspis (the latter now further divided into Lacertaspis and Leptosiaphos – see Schmitz et al. (2005), and with Afroablepharus returned to synonymy of Panaspis – see Medina et al. 2016). Greer (1979) later removed Notoscincus from the Lampropholis Subgroup, transferring it to the Sphenomorphini (a placement that has received consistent support from later genetic studies: Reeder 2003; Rabosky et al. 2007; Skinner 2007; Skinner et al. 2011; Pyron et al. 2013; Zheng and Wiens 2016). Inclusion of the African genera by Greer in his Group III, or Lampropholis Subgroup, was not supported by the genetic studies of Pyron et al. (2013) and Zheng and Wiens (2016), although they did find these lie within a monophyletic group within the Eugongylini, but outside the Sahul Radiation. Our results similarly recover the African genera as basal within the Eugongylini.
Greer and Kluge (1980) recognised two lineages within Lampropholis (the delicata Group and the challengeri Group), of which the challengeri Group was later generically separated as Saproscincus (Wells and Wellington 1984; Greer 1989). Three lineages were later recognised in Saproscincus (Greer and Kluge 1980; Sadlier et al. 1993, 2005; Moussalli et al. 2005). Some studies have hypothesised (Schuster 1981) or provided evidence for recognition of Lampropholis and Saproscincus as sister taxa (Hutchinson et al. 1990 using microcomplement fixation), but other studies have not recovered such a close relationship exclusive of other taxa (Stuart-Fox et al. 2002; Moussalli et al. 2005; Smith et al. 2007; Pyron et al. 2013), and two-way immunological comparisons by Baverstock and Donnellan (1990) did not find any closer relationships between Lampropholis and Saproscincus species than to Niveoscincus, Pseudemoia or Carlia. We have been able to demonstrate monophyly of both Lampropholis and Saproscincus, consistent with previous studies (Greer 1989; Sadlier et al. 1993, 2005; Moussalli et al. 2005; Brandley et al. 2015; Ivan et al. 2022). As suggested by Schuster (1981), Hutchinson et al. (1990), Brandley et al. (2015) and Ivan et al. (2022), we found a well-supported sister-taxa relationship between Lampropholis and Saproscincus, contradicting the conclusions of several previous studies (Baverstock and Donnellan 1990; Moussalli et al. 2005; Smith et al. 2007; Pyron et al. 2013; Zheng and Wiens 2016).
Carlia has similarly been subject to generic division (Greer 1974; Ingram and Covacevich 1988; Stuart-Fox et al. 2002; Zug 2004, 2010; Dolman and Hugall 2008). The expanded concept of this genus created by Greer (1974) included a small species, burnetti Oudemans, 1894 (now foliorum De Vis, 1884, following reallocation of an earlier name of uncertain identity) with an ablepharine eye in a genus otherwise with a ‘spectacled’ lower eyelid. This species, which had for many years been placed in Ablepharus (Oudemans 1894; Copland 1949), had later been considered to be of uncertain affinity (Greer and Parker 1968; Fuhn 1969), but its transfer to Carlia placed it with a number of small species of similar phenotype, which were later grouped and formally recognised as the genus Lygisaurus (Ingram & Covacevich, 1988) on morphological criteria. However, Stuart-Fox et al. (2002), in a genetic analysis of relationships among Carlia species using a single mitochondrial gene (ND4), were unable to identify a monophyletic Carlia as sister to Lygisaurus. While the species assigned to Lygisaurus formed a discrete lineage, which also included one species that had recently been described in Carlia (Carlia parrhasius Couper et al., 1994) due to its lack of several of the purported diagnostic morphological characters of Lygisaurus, the Lygisaurus lineage was nested in Carlia, and there was little resolution of relationships among the remaining Carlia species. Hence, they returned Lygisaurus to the synonymy of Carlia. Zug (2004) presented a cladistic analysis of a limited number of morphological characters for this expanded Carlia, and was able to recover a clade representing 28 of the 30 species of Carlia (including parrhasius), but this clade formed an unresolved polytomy with the other two species of Carlia and the two Lygisaurus species, although Zug’s analysis did not include one internal character (number of premaxillary teeth) that had been identified as a putative synapomorphy of Lygisaurus by Ingram and Covacevich (1988), and his analysis found very low support for many of the clades recovered within Carlia. Indeed, removal of two characters that showed some intraspecific variation resulted in nearly complete collapse of the cladogram into a large polytomy (including the outgroups) with only two small clades identified among species assigned to Carlia. A later genetic analysis, incorporating wider species sampling and more genes (two mitochondrial, two nuclear introns: Dolman and Hugall 2008) provided much more resolution, with three groups recognised among Carlia sensu lato. Although their analyses were unable to unequivocally determine the relationships among these three lineages, the high support for each lineage led to them recognising each as a distinct genus: Carlia, Liburnascincus (for several saxicoline species that had formerly been placed in Carlia), and Lygisaurus (including parrhasius). Similar results were obtained by Ivan et al. (2022), though with a lesser range of species. While Dolman and Hugall (2008) identified lower support for relationships within Carlia, Zug (2010) mapped morphological characters onto their gene tree to diagnose nine species groups, one of which (the Carlia peronii Species Group) was unsampled in the earlier genetic analyses. Subsequently, Pyron et al. (2013), while using mostly the same sequences as Dolman and Hugall, were not able to replicate the results of that study. Instead, they found that Lygisaurus, while still monophyletic, was nested within a broader paraphyletic Carlia, with Liburnascincus remaining outside this, a result also recovered by Zheng and Wiens (2016). Pyron et al. (2013) also found the species timlowi Ingram, 1977, initially described in Menetia, later transferred to Lygisaurus by Ingram and Covacevich (1988), then back to Menetia by Greer (1991), to be nested among the Carlia group genera, but not within Lygisaurus. This finding prompted Couper and Hoskin (2014) to create the genus Pygmaeascincus, with Menetia timlowi as type species for the genus, and also including the species Pygmaeascincus koshlandae and Pygmaeascincus sadlieri described by Greer (1991) which had formerly resided in Menetia, but identified as close to timlowi by Greer. Pygmaeascincus (represented by two of the three species) was recovered as sister to the other genera within the Carlia group by Ivan et al. (2022), with high (>95) bootstrap support.
Our study provides strong support for the reciprocal monophyly of the Carlia group, comprising Carlia, Lygisaurus and Liburnascincus, with the relationships among the genera well resolved. This result confirms previous suggestions, from Ingram and Covacevich (1988) and Dolman and Hugall (2008), for the recognition of multiple genera within the Carlia group.
The relationships of Menetia have been particularly problematic, with a lack of concordance between its assignment to beta palate group of skinks of Greer on morphological criteria (Group III of Greer (1974), and the Lampropholis Subgroup of Greer (1979)), and its affinities to other genera in genetic studies. The genus was resurrected from within Ablepharus by Fuhn (1969), and was suggested by Greer (1974, 1979) to be derived from Carlia, and later (Greer 1991) as closely related to Carlia. At the time of these initial studies, the genus was monotypic, with the sole species, Menetia greyii Gray, 1845, having a unique and very distinctive head scalation. However, the discovery of additional species (Storr 1976; Ingram 1977; Rankin 1979; Sadlier 1984) required a broadening of the morphological diagnosis and the loss of some of these apomorphies. Immunological studies by Baverstock and Donnellan (1990) found M. greyii to be closest to Acritoscincus and Morethia, and distant to the other beta-palate skinks sampled (Carlia, Saproscincus, Lampropholis). Smith et al. (2007) did not recover a relationship of M. greyii to the other Australian beta palate genera sampled (Lampropholis, Saproscincus and Lygisaurus) but did not include Carlia in their analysis. Instead, they found it was more closely allied to a species of Emoia, Emoia cyanura. Pyron et al. (2013) added a second species, Menetia alanae Rankin, 1979, and similarly identified a sister-group relationship of Menetia s.s. to a more extended group of Emoia, Their findings were replicated by Zheng and Wiens (2016), but with much lower support. However, those analyses were limited to a single shared gene (ND4) for the Menetia species included in that study. Most recently, Ivan et al. (2022) have included four species of Menetia. Their main published tree (their Fig. 1) recovered Menetia as sister to Cryptoblepharus. However, although the tree provided in their Accessory fig. 3 found strong (>95% bootstrap) support for a relationship of Menetia with the genera Acritoscincus, Austroablepharus, Morethia and Proablepharus, they did not include Emoia in their analysis.
Our study, which included three species, M. greyi, M. alanae and M. surda Storr, 1976, placed these as the sister to a broadly sampled Emoia s.s. within the Sahul Radiation, similar to the affinities suggested by Smith et al. (2007), Pyron et al. (2013) and Zheng and Wiens (2016), and not as having any relationship to previously suggested genera (e.g. Saproscincus, Lampropholis, Carlia, Lygisaurus, Liburnascincus, Acritoscincus, Austroablepharus, Morethia, Proablepharus or Cryptoblepharus). These results again indicate two independent evolutions of the beta-palate within the Australian radiation of eugongylin skinks.
Relationships of the monotypic genera currently recognised among the Australian Eugongylini
Our phylogenetic analyses resolve the taxonomic uncertainties related to the monotypic genera Anepischetosia Wells & Wellington, 1985 (type species S. maccoyi Lucas & Frost, 1894), Eroticoscincus Wells & Wellington, 1984 (type species L. graciloides Lönnberg & Andersson, 1913) and Harrisoniascincus Wells & Wellington, 1985 (type species Leiolopisma zia Ingram & Ehmann, 1981). Mittleman (1952) and Greer (1974) on morphological criteria placed the species graciloides and maccoyi in Anotis, a genus at that time otherwise restricted to New Caledonia. Schuster (1981) suggested there were undescribed members of this genus in north Queensland, but his statements have not been subsequently corroborated. Czechura (1981), in reviewing graciloides, noted the homonymy of Anotis with an insect taxon, and resurrected Nannoscincus for this genus. Sadlier (1987) included maccoyi as part of Nannoscincus, but excluded graciloides from that genus, noting that it was ‘only distantly related’. Further explanation was provided by Sadlier (1990) with a rediagnosis of Nannoscincus, noting that graciloides showed a different pattern of phalangeal reduction to Nannoscincus, although its further affinities were left unresolved. The affinities of graciloides have since been largely unexplored. Wells and Wellington (1984) proposed the generic name Eroticoscincus for it, and Hutchinson (1993) suggested that it was phenetically similar to Saproscincus czechurai, though lacking the beta-palate of the species of that genus, and tentatively transferred it to Saproscincus. In contrast, Greer (1991) considered graciloides to form a lineage with Carlia, Lygisaurus and Menetia, based on a shared phalangeal formula of a loss of the first digit of the manus. The continued retention of maccoyi in Nannoscincus by Sadlier (1990), albeit in a different subgenus (Nannoseps) to the other species, created a biogeographic anomaly, this being the only New Caledonian genus with Australian representatives. Smith et al. (2007) confirmed that Nannoscincus was part of an otherwise endemic New Caledonian clade, but did not include Nannoscincus maccoyi in their study. Sadlier et al. (2006b) resurrected the Wells and Wellington (1985) genus Anepischetosia (itself a replacement name for the preoccupied Anepischetos Wells & Wellington, 1984) to replace his subgenus Nannoseps for the Australian species maccoyi, but did not otherwise offer any argument in support of their action in raising Anepischetosia to generic status
Hutchinson et al. (1990), with only one-way immunological comparisons possible for this part of their analysis, found that ‘Nannoscincus’ maccoyi, together with two other problematic species, Leiolopisma zia Ingram & Ehmann, 1981 and L. jigurru Covacevich, 1984, represented relatively early offshoots from a group also including Carinascincus (as Niveoscincus) and the beta-palate skinks Lampropholis and Saproscincus, but were uncertain about their placement within this group. They erected monotypic genera, Cautula for zia and Bartleia for jigurru, although earlier names, Harrisoniascincus and Techmarscincus, were available respectively for these species (Wells and Wellington 1985; Couper et al. 2006) and have subsequently been applied. The comparisons made by Hutchinson et al. (1990) did not include taxa within other Australian eugongylin genera that were a part of the Lampropholis Subgroup (Carlia, Lygisaurus, Liburnascincus and Menetia), nor did they include graciloides, nor any of the New Caledonian Nannoscincus, and hence were limited in hypothesising affinities, although they did suggest that maccoyi and zia might be related. Subsequent molecular studies have variously reported a close relationship of Harrisoniascincus zia to Lampropholis (Moussalli et al. 2005), or the Carlia group (Smith et al. 2007; Pyron et al. 2013; Zheng and Wiens 2016), but these studies had only sparse sampling of other Australian eugongylin genera. Schuster (1981) suggested that zia was closer to Lampropholis and Saproscincus than to maccoyi. Brandley et al. (2015) recovered zia as either basal (along with Acritoscincus) to other Australian eugongylins sampled, or sister to Pseudemoia at the base of the tree in different analyses.
Our study clearly demonstrates that A. maccoyi is not part of the New Caledonian Nannoscincus genus, but rather represents a divergent lineage within the Sahul Radiation. Our analyses place it within a moderately supported group that also includes (Carinascincus) coventryi and Pseudemoia, with a divergence time to its nearest relative (Pseudemoia) of ~28 mya, an age over twice that of the species within Pseudemoia, and with a morphology and physiology very different to Pseudemoia. Our results support the retention of Anepischetosia as a monotypic genus within the tribe. We also found Eroticoscincus graciloides and Harrisoniascincus zia each represent a highly divergent lineage within the Sahul eugongylin radiation, with a moderately supported sister-taxa relationship. Given the extent of genetic divergence between the two taxa (dating back ~22 mya) and the extent of morphological differentiation, we here retain them as monotypic genera.
Monophyly of Emoia
The intra- and interspecific relationships of Emoia Gray, 1845 have received only limited morphological and genetic investigation. Greer (1974) was uncertain of the affinities of Emoia, although he treated it as a single unit, and suggested an origin either from a Eugongylus-like stock or a L. spenceri-like stock. Thereafter the genus received only cursory mention by Greer (1979) as a member of the Eugongylus Subgroup of the Eugongylus Group. The genus was defined by Brown (1991) on morphological criteria that included a suite of plesiomorphic characters, either at the level of the Lygosominae generally or within the larger part of the Eugongylini. Within Emoia, Brown (1991) proposed eight species groups. Five of these (the adspersa, atrocostata, baudini, physicae and cyanogaster Groups) shared a common synapomorphy in osteology, two of the remaining three species groups (the cyanura and monotypic ponapea Groups) showed one or more unique apomorphic states in osteology within the genus, and the remaining group (samoensis Group) was identified largely on the basis of plesiomorphic features, and as being the only species group within the genus that does not have a fixed clutch size of two for its species (Greer 1968; Schwaner 1980; Brown 1991). However, the robustness of these species groups is confounded by Brown (1991) not indicating which species he had examined osteologically, and it is likely that not all species were examined for the diagnostic criteria to be sure of correct group allocation. Regardless, the work by Brown remains the only comprehensive overview of the genus.
Early immunological (Hutchinson et al. 1990) and genetic studies (Austin and Arnold 2006; Smith et al. 2007; Skinner et al. 2011) were limited in their utility to interpret the intra- and intergeneric relationships of Emoia by consequence of including only one or two species in their sampling, apparently assuming monophyly for the genus and/or as a legacy of the unavailability of samples for analysis. Hutchinson et al. (1990) found the Emoia cyanogaster Group (represented by E. longicauda) to lie well outside the group of Australian genera sampled. Austin and Arnold (2006) similarly found the E. cyanura Group (represented by Emoia impar) and the Emoia physicae Group (represented by the nominotypic species) to be weakly supported as sister to Mauritian Leiolopisma outside the Australian and New Zealand endemic eugongylin genera. Smith et al. (2007) did not retrieve a monophyletic Australian lineage, and found the E. samoensis Group (represented by Emoia loyaltiensis) to be sister to Leiolopisma s.s., and the E. cyanura Group (represented by E. cyanura) to be sister to Menetia (represented by M. greyii), but with only moderate Bayesian posterior probabilities (0.75 and 0.70 respectively) and bootstrap support (<50%). However, these groupings each belonged to a branch in a polytomy of several across which the non-Zealandia skink genera sampled were distributed. While Smith et al. (2007) gave no evidence for any close relationship of the E. cyanura Group or E. samoensis Group to each other, their analysis did suggest a relationship between the E. cyanura Group and Menetia which is reflected in the later studies by Pyron et al. (2013), Zheng and Wiens (2016) and our study, and for the affinities of the E. samoensis Group to lie outside the endemic Australian and Zealandia Lineage genera.
The study by Pyron et al. (2013) provided a more extensive coverage of Emoia taxa than earlier genetic studies, and included 13 species across six species groups. They found a relationship between the samoensis Group species E. loyaltiensis (using the sequences from Smith et al. 2007) and a group that included Leiolopisma s.s. and several African region genera (Panaspis, Lacertaspis, Leptosaiphos). However, two species of the samoensis Species Group not previously sampled, E. concolor (Duméril & Duméril, 1851) and E. tongana (Werner, 1899), were external to the Eugongylini, instead being sister to the Lygosomini. A similar placement, this time with these two species embedded within the Lygosomini, was identified by Zheng and Wiens (2016). This unexpected result may be an artefact of sampling, as the latter two species were represented in the analysis by only a single gene, which was not one of the three available for E. loyaltiensis. Both studies also found the other species of Emoia included, representing those previously studied by Austin and Arnold (2006), Smith et al. (2007) and Skinner et al. (2011), together with three other species in the cyanura Group (Emoia schmidti, Emoia pseudocyanura and Emoia isolata), and representatives of the Emoia baudini Group (Emoia jakati) and Emoia atrocostata Group (nominotypic species), to form a monophyletic group. This group clustered with Cryptoblepharus and Menetia as sister to the Australian group of genera, with the New Zealand and New Caledonian radiations external to this, albeit with relatively low support for these higher relationships.
A more recent genetic sudy focusing on the intra- and interspecific relationships of E. atrocostata (Richmond et al. 2021) included two species in the E. atrocostata Species Group not included in previous studies, boettgeri and nativitatis. It also included members of Brown’s (1991) adspersa Species Group, adspersa (Steindachner, 1870) and lawesii (Günther, 1874), also not represented in previous studies, and as an outgroup utilised several taxa in the cyanogaster Species Group, cyanogaster (Lesson, 1829), kordoana (Meyer, 1874), and longicauda (the latter two of which had not been included in previous studies). This study found E. atrocostata to be paraphyletic with respect to other members of the species group sampled, and the atrocostata Species Group to be polyphyletic with respect to inclusion of species from the adspersa Species Group. It also found the three outgroup species included from the cyanogaster Species Group to form a well supported lineage sister to the lineage in which the atrocostata and adspersa Group species resided. While this study lacked representation of taxa from the other five species groups within Emoia identified by Brown, or other taxa in the Eugongylini, its relevance here lies in the extent of apparent genetic affinity between the atrocostata Species Group and the adspersa Species Group, the latter of which has been regarded as unusual within the genus in having much smaller body scales than species in other groups within Emoia.
Within Emoia, we sampled 16 species representing six of the eight putative species groups of Brown (1991), including the type species of Emoia (atrocostata), and three species in the E. samoensis Group (E. loyaltiensis, Emoia nigra and Emoia sanfordi), the nominotypic species of the Emoia ponapea Group, and two species in the E. cyanura Group (Emoia rufilabialis and Emoia taumakoensis), not previously reported. We found clear evidence that the widespread and diverse Emoia genus is not monophyletic, but rather represents two major lineages within the Eugongylini. One lineage, represented in our study by three (of 13+) species in the E. samoensis Group (E. loyaltiensis, E. nigra, E. sanfordi), represents a basal lineage within the tribe outside the Australian radiation. For E. loyaltiensis, this result is consistent with the results of previous molecular studies by Smith et al. (2007), Pyron et al. (2013) and Zheng and Wiens (2016), although as noted above the latter two studies found two other taxa in the species group to be placed within the Lygosomini.
The other lineage found in our study, represented here by three (of six) taxa from the E. atrocostata Group (E. atrocostata, Emoia boettgeri and Emoia nativitatis), one (of 21) species from the E. baudini Group (E. jakati), two (of six) species from the E. cyanogaster Group (E. longicauda and Emoia tetrataenia), the sole species in the E. ponapea Group, and eight (of 13) species from the E. cyanura Group (Emoia caeruleocauda, E. cyanura, E. impar, E. isolata, E. pseudocyanura, E. rufilabialis, E. schmidti and E. taumakoensis), forms part of the Sahul Radiation of Eugongylini. For some taxa, their inclusion in this lineage and within the Australian eugongylin radiation has previously been suggested (Austin and Arnold 2006; Smith et al. 2007; Skinner et al. 2011; Pyron et al. 2013; Zheng and Wiens 2016), but their affinitites within this radiation have varied. Our study has provided strong support for these species as a monophyletic lineage within the Sahul Radiation, and by virture of inclusion of the type species, as representing Emoia s.s. Within this redefined Emoia s.s. two well-supported sublineages were retrieved, one comprising taxa from the E. cyanura + E. ponapea Groups, and the other taxa from the E. atrocostata + E. baudini + E. cyanogaster Species Groups. However, the content of this redefined Emoia s.s., and of the sublineages within, may change with the inclusion of other taxa from species groups not (Emoia adspersa Species Group, E. physicae Species Group) or poorly (E. baudini Species Group) represented in our study.
Our study places Menetia as the sister genus to the Emoia s.s. lineage, a relationship consistent with the previous findings of Smith et al. (2007), Pyron et al. (2013) and Zheng and Wiens (2016), but with broader taxon sampling and strong support.
Cryptoblepharus as part of the Sahul Radiation
Cryptoblepharus Wiegmann, 1834 was suggested by Fuhn (1969) to be related to Emoia, and Greer (1974) suggested it evolved from within Emoia, particularly from the E. cyanura Species Group (Greer 1983). However, Greer (1989) later considered Cryptoblepharus to be part of his Pseudemoia Group, along with most of the other Australian eugongylins, with Emoia outside that lineage, a hypothesis incompatible with his previous view. Neither of the genetic studies by Austin and Arnold (2006), or Smith et al. (2007) found any evidence for a relationship of Cryptoblepharus with Emoia species in their analyses. Horner (2007) provided the most comprehensive modern review of the genus, based primarily on morphology, but also utilising genetic data from allozyme electrophoresis (Horner and Adams 2007). Horner did not speculate on the relationships of Cryptoblepharus, but suggested an origin of the genus in south-east Asia. Pyron et al. (2013) and Zheng and Wiens (2016) did recover a sister-taxon relationship between Cryptoblepharus and Emoia s.s. + Menetia. Blom et al. (2016) provided an assessment of genetic relationships of 24 Australian species to elucidate the mechanisms driving evolution across the continent, and extended the study more broadly in taxonomic and regional representation (Blom et al. 2019) with regard to trans-oceanic dispersal. Both studies appear to have assumed monophyly of Cryptoblepharus, and did not extend beyond exploring the intrageneric relationships of the genus.
Our study provides strong evidence for the monophyly of Cryptoblepharus as sampled (although we did not include any of the Indian Ocean taxa) and strong support for this genus being part of the Sahul eugongylin radiation. The genus was found to be part of a group that includes the beta-palate genera (Saproscincus/Lampropholis/Lygisaurus/Liburnascincus/Carlia), diverging from them ~30 mya. This result suggests that the genus originated in Australia before spreading out across the Pacific.
Affinities of the Sphenomorphus species S. aignanus, S. louisiadensis, S. minutus and S. bignelli to the Eugongylini
These four species, by virtue of their generic placement within Sphenomorphus, are currently part of the Sphenomorphini, despite having been recognised as members of the Eugongylini for more than half a century (Greer and Parker 1968; Greer 1974, 1977, 1979). Our study provides confirmation that three ‘Sphenomorphus’ species (S. louisiadensis, S. minutus and S. bignelli) are indeed part of the Eugongylini, a result consistent with previous morphological evidence (Greer and Parker 1968; Greer 1977, 1979). Our unpublished ND2 data indicate that S. aignanus is also part of this Sphenomorphus clade within the Eugongylini. Our study found these four Sphenomorphus species were the sister to Eugongylus, and provides evidence for this group as one of several basal lineages within the tribe.
Geographic origins of the Eugongylini and the ages of the various lineages
The Eugongylini is one of seven tribes (Lygosomini, Ateuchosaurini, Eugongylini, Mabuyini, Ristellini, Sphenomorphini, and Tiliquini) in the subfamily Lygosominae, as recently refined by Shea (2021). While monophyly of the Lygosominae, and of most tribes, is well supported in the taxon-rich study of genetic relationships within the Scincidae by Pyron et al. (2013)
Acknowledgements
We thank Charles Daugherty, Peter Ritchie, Colin Miskelly, and Gillian Stone for their assistance during the project. We also thank the museum collection staff that provided the tissue samples for this project (Terry Bertozzi [South Australian Museum], Addison Wynn [Smithsonian]).
References
Arnold EN, Bour R (2008) A new Nactus gecko (Gekkonidae) and a new Leiolopisma skink (Scincidae) from La Reunion, Indian Ocean, based on recent fossil remains and ancient DNA sequence. Zootaxa 1705, 40-50.
| Crossref | Google Scholar |
Austin CC (1999) Lizards took express train to Polynesia. Nature 397, 113-114.
| Crossref | Google Scholar |
Austin JJ, Arnold EN (2006) Using ancient and recent DNA to explore relationships of extinct and endangered Leiolopisma skinks (Reptilia: Scincidae) in the Mascarene islands. Molecular Phylogenetics and Evolution 39, 503-511.
| Crossref | Google Scholar |
Bauer AM, Sadlier RA (1993) Systematics, biogeography and conservation of the lizards of New Caledonia. Biodiversity Letters 1, 107-122.
| Crossref | Google Scholar |
Bavay A (1869) Catalogue des Reptiles de la Nouvelle-Caledonie et description d’especes nouvelles. Memoires Societe Linniene de Normandie 15, 1-37.
| Google Scholar |
Baverstock PR, Donnellan SC (1990) Molecular evolution in Australian dragons and skinks: a progress report. Memoirs of the Queensland Museum 29, 323-331.
| Google Scholar |
Benoit M, Drost H-G (2021) A predictive approach to infer the activity and natural variation of retrotransposon families in plants. In ‘Plant transposable elements. Methods in molecular biology, Vol. 2250’. (Ed. J Cho) pp. 1–14. (Humana: New York, NY). doi:10.1007/978-1-0716-1134-0_1
Blom MPK, Horner P, Moritz C (2016) Convergence across a continent: adaptive diversification in a recent radiation of Australian lizards. Proceedings of the Royal Society B: Biological Sciences 283, 20160181.
| Crossref | Google Scholar |
Blom MPK, Matzke NJ, Bragg JG, Arida E, Austin CC, Backlin AR, Carretero MA, Fisher RN, Glaw F, Hathaway SA, Iskandar DT, McGuire JA, Karin BR, Reilly SB, Rittmeyer EN, Rocha S, Sanchez M, Stubbs AL, Vences M, Moritz C (2019) Habitat preference modulates trans-oceanic dispersal in a terrestrial vertebrate. Proceedings of the Royal Society B: Biological Sciences 286, 20182575.
| Crossref | Google Scholar |
Böhme W (1976) Uber die Gattung Eugongylus Fitzinger, mit Beschreibung einer neuen Art (Reptilia: Scincidae). Bonner Zoologische Beiträge 27, 245-251.
| Google Scholar |
Böhme W, Schmitz A, Ziegler T (2000) A review of the West African skink genus Cophoscincopus Mertens (Reptilia: Scincidae: Lygosominae): resurrection of C. simulans (Vaillant, 1884) and description of a new species. Revue Suisse de Zoologie 107, 777-791.
| Crossref | Google Scholar |
Borowiec ML (2016) AMAS: a fast tool for alignment manipulation and computing of summary statistics. PeerJ 4, e1660.
| Crossref | Google Scholar |
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, du Plessis L, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu C-H, Xie D, Zhang C, Stadler T, Drummond AJ (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15, e1006650.
| Crossref | Google Scholar |
Boulenger GA (1887a) Report on a zoological collection made by the officers of H.M.S. Flying Fish at Christmas Island, Indian Ocean. Proceedings of the Zoological Society London 1887, 516-517.
| Google Scholar |
Boulenger GA (1895) III. – On a collection of reptiles and batrachians from Ferguson Island, D’Entrecasteaux group, British New Guinea. Annals and Magazine of Natural History Ser.6 16, 28-32.
| Crossref | Google Scholar |
Boulenger GA (1898a) Third report on additions to the lizard collection in the Natural-History Museum. Proceedings of the Zoological Society London 1898, 912-923.
| Google Scholar |
Boulenger GA (1903) XLI.– Descriptions of new lizards in the collection of the British Museum. Annals and Magazine of Natural History Ser. 7 12, 429-435.
| Crossref | Google Scholar |
Brandley MC, Bragg JG, Singhal S, Chapple DG, Jennings CK, Lemmon AR, Lemmon EM, Thompson MB, Moritz C (2015) Evaluating the performance of anchored hybrid enrichment at the tips of the tree of life: a phylogenetic analysis of Australian Eugongylus group scincid lizards. BMC Evolutionary Biology 15, 62.
| Crossref | Google Scholar |
Brocchi P (1876) Sur un Scincoiden nouveau appartenant au genre Eumeces. Bulletin de la Société Philomathique de Paris Ser. 6 12, 95-97.
| Google Scholar |
Broom R (1896) On two new species of Ablepharus from north Queensland. Annals and Magazine of Natural History Ser. 6 18, 342-343.
| Google Scholar |
Brown WC (1954) Notes on several lizards of the genus Emoia, with descriptions of new species from the Solomon Islands. Fieldiana (Zoology) 34, 263-276.
| Crossref | Google Scholar |
Brown WC (1991) Lizards of the genus Emoia (Scincidae) with observations on their evolution and biogeography. Memoirs of the California Academy of Sciences 15, 1-94.
| Google Scholar |
Burbrink FT, Grazziotin FG, Pyron RA, Cundall D, Donnellan S, Irish F, Keogh JS, Kraus F, Murphy RW, Noonan B, Raxworthy CJ, Ruane S, Lemmon AR, Lemmon EM, Zaher H (2020) Interrogating genomic-scale data for Squamata (lizards, snakes, and amphisbaenians) shows no support for key traditional morphological relationships. Systematic Biology 69, 502-520.
| Crossref | Google Scholar |
Chapple DG, Ritchie PA, Daugherty CH (2009) Origin, diversification, and systematics of the New Zealand skink fauna (Reptilia: Scincidae). Molecular Phylogenetics and Evolution 52, 470-487.
| Crossref | Google Scholar |
Copland SJ (1947) Taxonomic notes on the genus Ablepharus (Sauria: Scincidae). 1. A new species from the Darling River. Proceedings of the Linnean Society New South Wales 71, 282-286.
| Google Scholar |
Copland SJ (1949) Taxonomic notes on the genus Ablepharus (Sauria: Scincidae). II. The races of Ablepharus burnetti Oudemans. Proceedings of the Linnean Society of New South Wales 73, 362-371.
| Google Scholar |
Couper PJ, Hoskin CJ (2014) A new genus to accommodate three skinks currently assigned to Menetia (Lacertilia: Scincidae). Zootaxa 3884, 597-599.
| Crossref | Google Scholar |
Couper PJ, Covacevich JA, Lethbridge PJ (1994) Carlia parrhasius, a new Queensland skink. Memoirs of the Queensland Museum 35, 31-33.
| Google Scholar |
Couper PJ, Covacevich JA, Amey AP, Baker AM (2006) The genera of skinks (Family Scincidae) of Australia and its island territories: diversity, distribution and identification. In ‘Evolution and biogeography of Australasian vertebrates’. (Eds JR Merrick, M Archer, GM Hickey, MSY Lee) pp. 367–383. (Auscipub: Oatlands)
Couper PJ, Limpus CJ, McDonald KR, Amey AP (2010) A new species of Proablepharus (Scincidae: Lygosominae) from Mt Surprise, north-eastern Queensland, Australia. Zootaxa 2433, 62-68.
| Crossref | Google Scholar |
Couper PJ, Hoskin CJ, Potter S, Bragg JG, Moritz C (2018) A new genus to accommodate three skinks currently assigned to Proablepharus (Lacertilia: Scincidae). Memoirs of the Queensland Museum – Nature 60, 227-231.
| Crossref | Google Scholar |
Covacevich J (1984) A biogeographically significant new species of Leiolopisma (Scincidae) from north eastern Queensland. Memoirs of the Queensland Museum 21, 401-411.
| Google Scholar |
Czechura GV (1981) The rare scincid lizard, Nannoscincus graciloides: a reappraisal. Journal of Herpetology 15, 315-320.
| Crossref | Google Scholar |
Datta-Roy A, Singh M, Karanth KP (2014) Phylogeny of endemic skinks of the genus Lygosoma (Squamata: Scincidae) from India suggests an in situ radiation. Journal of Genetics 93, 163-167.
| Crossref | Google Scholar |
Desjardin J (1831) Sur trois espèces de lézard du genre scinque, qui habitent l’île Maurice (Ile-de-France). Annales des Sciences Naturelles Paris 22, 292-299.
| Google Scholar |
De Vis CW (1884) New Queensland lizards. The Proceedings of the Royal Society of Queensland 1, 77-78.
| Crossref | Google Scholar |
De Vis CW (1888) A contribution to the herpetology of Queensland. Proceedings of the Linnean Society of New South Wales Ser. 2 2, 811-826.
| Crossref | Google Scholar |
De Vis CW (1892) Zoology of British New Guinea. Part 1.–Vertebrata. Annals of the Queensland Museum 2, 3-12.
| Google Scholar |
Dolman G, Hugall AF (2008) Combined mitochondrial and nuclear data enhance resolution of a rapid radiation of Australian rainbow skinks (Scincidae: Carlia). Molecular Phylogenetics and Evolution 49, 782-794.
| Crossref | Google Scholar |
Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S, Moir R (2011) Geneious v5.4. Available at http://www.geneious.com
Dubey S, Shine R (2010) Evolutionary diversification of the lizard genus Bassiana (Scincidae) across southern Australia. PLoS ONE 5, e12982.
| Crossref | Google Scholar |
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792-1797.
| Crossref | Google Scholar |
Fisher R, Hamilton A, Allison A, Tallowin O (2013) Leiolopisma alazon. IUCN Red List Threatened Spec. 2013, e.T196628A2468178. Available at http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T196628A2468178.en
Freitas ES, Datta-Roy A, Karanth P, Grismer LL, Siler CD (2019) Multilocus phylogeny and a new classification for African, Asian and Indian supple and writhing skinks (Scincidae: Lygosominae). Zoological Journal of the Linnean Society 186, 1067-1096.
| Crossref | Google Scholar |
Fuhn IE (1969) The “polyphyletic” origin of the genus Ablepharus (Reptilia: Scincidae): a case of parallel evolution. Journal of Zoological Systematics and Evolutionary Research 7, 67-76.
| Crossref | Google Scholar |
Giribet G, Baker CM (2019) Further discussion on the Eocene drowning of New Caledonia: discordances from the point of view of zoology. Journal of Biogeography 46, 1912-1918.
| Crossref | Google Scholar |
Glauert L (1960) Herpetological miscellanea. XII. The family Scincidae in Western Australia. Pt. 3. The genus Ablepharus. Western Australian Naturalist 7, 115-122.
| Google Scholar |
Gray JE (1838) XXX.–Catalogue of the slender-tongued saurians, with descriptions of many new genera and species. Annals and Magazine of Natural History 1, 274-283.
| Crossref | Google Scholar |
Greer AE (1967) A new generic arrangement for some Australian scincid lizards. Breviora 267, 1-19.
| Google Scholar |
Greer AE (1968) Clutch size in the scincid genus Emoia. Copeia 1968, 417-418.
| Crossref | Google Scholar |
Greer AE (1974) The genetic relationships of the scincid lizard genus Leiolopisma and its relatives. Australian Journal of Zoology Supplementary Series 22(31), 1-67.
| Crossref | Google Scholar |
Greer AE (1977) On the adaptive significance of the loss of an oviduct in reptiles. Proceedings of the Linnean Society of New South Wales 101, 242-249.
| Google Scholar |
Greer AE (1979) A phylogenetic subdivision of Australian skinks. Records of the Australian Museum 32, 339-371.
| Crossref | Google Scholar |
Greer AE (1980) A new species of Morethia (Lacertilia: Scincidae) from northern Australia, with comments on the biology and relationships of the genus. Records of the Australian Museum 33, 89-122.
| Crossref | Google Scholar |
Greer AE (1982a) A new species of Leiolopisma (Lacertilia: Scincidae) from Western Australia. Records of the Australian Museum 34, 549-573.
| Crossref | Google Scholar |
Greer AE (1982b) A new species of Geomyersia (Scincidae) from the Admiralty Islands, with a summary of the genus. Journal of Herpetology 16, 61-66.
| Crossref | Google Scholar |
Greer AE (1983) On the adaptive significance of the reptilian spectacle: the evidence from scincid, teiid, and lacertid lizards. In ‘Advances in herpetology and evolutionary biology. Essays in honor of Ernest E. Williams.’ (Eds AGJ Rhodin, K Miyata) pp. 213–221. (Museum of Comparative Zoology: Cambridge)
Greer AE (1991) Two new species of Menetia from northeastern Queensland, with comments on the generic diagnoses of Lygisaurus and Menetia. Journal of Herpetology 25, 268-272.
| Crossref | Google Scholar |
Greer AE, Kluge AG (1980) A new species of Lampropholis (Lacertilia: Scincidae) from the rainforests of northeastern Queensland. Occasional Papers of the Museum of Zoology, University of Michigan 691, 1-12.
| Google Scholar |
Greer AE, Parker F (1968) Geomyersia glabra, a new genus and species of scincid lizard from Bougainville, Solomon Islands, with comments on the relationships of some lygosomine genera. Breviora 302, 1-17.
| Google Scholar |
Greer AE, Shea GM (2000) A major new head scale character in non-lygosomine scincid lizards. Journal of Herpetology 34, 629-634.
| Crossref | Google Scholar |
Greer AE, Fisher A, Horner P (2004) A new species of Proablepharus (Squamata: Scincidae) from the Northern Territory of Australia. The Beagle: Records of the Museums and Art Galleries of the Northern Territory 20, 199-205.
| Crossref | Google Scholar |
Günther A (1874) A contribution to the fauna of Savage Island. Proceedings of the Zoological Society of London 1874, 295-297.
| Google Scholar |
Hardy GS (1977) The New Zealand Scincidae (Reptilia: Lacertilia); a taxonomic and zoogeographic study. New Zealand Journal of Zoology 4, 221-325.
| Crossref | Google Scholar |
Hickson RE, Slack KE, Lockhart P (2000) Phylogeny recapitulates geography, or why New Zealand has so many species of skinks. Biological Journal of the Linnean Society 70, 415-433.
| Crossref | Google Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518-522.
| Crossref | Google Scholar |
Honda M, Ota H, Kobayashi M, Nabhitabhata J, Yong H.-S, Hikida T (2000) Phylogenetic relationships, character evolution, and biogeography of the subfamily Lygosominae (Reptilia: Scincidae) inferred from mitochondrial DNA sequences. Molecular Phylogenetics and Evolution 15, 452-461.
| Crossref | Google Scholar |
Honda M, Ota H, Köhler G, Ineich I, Chirio L, Chen S-L, Hikida T (2003) Phylogeny of the lizard subfamily Lygosominae (Reptilia: Scincidae), with special reference to the origin of the New World taxa. Genes & Genetic Systems 78, 71-80.
| Crossref | Google Scholar |
Horner P (2007) Systematics of the snake-eyed skinks, Cryptoblepharus Wiegmann (Reptilia: Squamata: Scincidae) – an Australian-based review. The Beagle, Records of the Museum and Art Gallery Northern Territory Supplement 3, 21-198.
| Google Scholar |
Horner P, Adams M (2007) A molecular systematic assessment of species boundaries in Australian Cryptoblepharus (Reptilia: Squamata: Scincidae) – a case study for the combined use of allozymes and morphology to explore cryptic biodiversity. The Beagle, Records of the Museum and Art Gallery Northern Territory Supplement 3, 1-19.
| Google Scholar |
Hutchinson MN, Donnellan SC (1988) A new species of scincid lizard related to Leiolopisma entrecasteauxii, from southeastern Australia. Transactions of the Royal Society of South Australia 112, 143-151.
| Google Scholar |
Hutchinson MN, Schwaner TD, Medlock K (1988) A new species of scincid lizard (Lygosominae: Leiolopisma) from the highlands of Tasmania. Proceedings of the Royal Society Victoria 100, 67-73.
| Google Scholar |
Hutchinson MN, Donnellan SC, Baverstock PR, Krieg M, Simms S, Burgin S (1990) Immunological relationships and generic revision of the Australian lizards assigned to the genus Leiolopisma (Scincidae, Lygosominae). Australian Journal of Zoology 38, 535-554.
| Crossref | Google Scholar |
Ineich I, Zug GR (1993–1996) Tachygyia, the gaint Tongan skink: extinct or extant? Cryptozoology 12, 30-35.
| Google Scholar |
Ineich I, Sadlier RA, Bauer AM, Jackman TR, Smith SA (2014) Bocourt’s Terrific Skink, Phoboscincus bocourti (Brocchi, 1876), and the monophyly of the genus Phoboscincus Greer, 1974. In ‘Zoologia Neocaledonica 8. Biodiversity studies in New Caledonia, Vol. 206’. (Eds E Guilbert, T Robillard, H Jourdan, P Grandcolas) pp. 69–78. (Mémoires du Muséum National d’Histoire Naturelle: Paris)
Ingram GJ (1977) Three species of small lizards – two of them new – genus Menetia (Lacertilia, Scincidae) in Queensland. Victorian Naturalist 94, 184-187.
| Google Scholar |
Ingram GJ, Covacevich J (1988) Revision of the genus Lygisaurus De Vis (Scincidae: Reptilia) in Australia. Memoirs of the Queensland Museum 25, 335-354.
| Google Scholar |
Ingram GJ, Ehmann H (1981) A new species of scincid lizard of the genus Leiolopisma (Scincidae: Lygosominae) from south-eastern Queensland and north-eastern New South Wales. Memoirs of the Queensland Museum 20, 311-317.
| Google Scholar |
Ivan J, Moritz C, Potter S, Bragg J, Turakulov R, Hua X (2022) Temperature predicts the rate of molecular evolution in Australian Eugongylinae skinks. Evolution 76, 252-261.
| Crossref | Google Scholar |
Karin BR, Metallinou M, Weinell JL, Jackman TR, Bauer AM (2016) Resolving the higher-order phylogenetic relationships of the circumtropical Mabuya group (Squamata: Scincidae): an out-of-Asia diversification. Molecular Phylogenetics and Evolution 102, 220-232.
| Crossref | Google Scholar |
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059-3066.
| Crossref | Google Scholar |
Kiester AR (1982) A new forest skink from Ponape. Breviora 468, 1-10.
| Google Scholar |
Kopstein PF (1926) Reptilien von den Molukken und den benachbarten Inseln. Zoologische Mededelingen 9, 71-112.
| Google Scholar |
Lee MSY, Hutchinson MN, Worthy TH, Archer M, Tennyson AJD, Worthy JP, Scofield RP (2009) Miocene skinks and geckos reveal long-term conservatism of New Zealand’s lizard fauna. Biology Letters 5, 833-837.
| Crossref | Google Scholar |
Le Houedec S, Meynadier L, Cogné J-P, Allègre CJ, Gourlan AT (2012) Oceanwide imprint of large tectonic and oceanic events on seawater Nd isotope composition in the Indian Ocean from 90 to 40 Ma. Geochemistry, Geophysics, Geosystems 13, Q06008.
| Crossref | Google Scholar |
Lesson R-P (1829) Reptiles Planche 3. In ‘Lesson R.-P., Voyage autour du monde, exécuté par ordre du Roi, sur la Corvette de Sa Majesté, la Coquille, pendant les années 1822, 1823, 1824 et 1825, sous le Ministère de S.E.M. le Marquis de Clermont-Tonnerre, et publié sous les auspices de Son Excellence Mgr. le Cte. de Chabrol, Ministre de la Marine et des Colonies, par L.I. Duperrey, Chevalier de Saint-Louis et de la Légion d’Honneur, capitaine de frégate, commandant de l’expédition. Histoire Naturelle. Zoologie. Atlas’. (Arthus Bertrand: Paris)
Lesson R-P (1830) Reptiles Planche 4. In ‘Lesson R.-P., Voyage autour du monde, exécuté par ordre du Roi, sur la Corvette de Sa Majesté, la Coquille, pendant les années 1822, 1823, 1824 et 1825, sous le Ministère de S.E.M. le Marquis de Clermont-Tonnerre, et publié sous les auspices de Son Excellence Mgr. le Cte. de Chabrol, Ministre de la Marine et des Colonies, par L.I. Duperrey, Chevalier de Saint-Louis et de la Légion d’Honneur, capitaine de frégate, commandant de l’expédition. Histoire Naturelle. Zoologie. Atlas’. (Arthus Bertrand: Paris)
Lönnberg E, Andersson LG (1913) Results of Dr. E. Mjöberg’s Swedish Scientific Expeditions to Australia 1910-1913. III. Reptiles. Kongliga Svenska Vetenskaps Academiens Nya Handlingar 52, 1-173.
| Google Scholar |
Lucas AHS, Frost C (1894) The lizards indigenous to Victoria. Proceedings of the Royal Society Victoria (ns) 6, 24-92.
| Google Scholar |
Macleay W (1877) The lizards of the Chevert Expedition. Proceedings of the Linnean Society of New South Wales 2, 60-69.
| Google Scholar |
Maurizot P, Campbell HJ (2020) Paleobiogeography of New Caledonia. In ‘New Caledonia: geology, geodynamic evolution and mineral resources, Vol. 51’. (Eds P Maurizot, N Mortimer) pp. 189–213. (Geological Society, London, Memoirs) 10.1144/M51.0
McCoy M, Webber P (1984) Two new species of scincid lizards of the Genus Emoia from Santa Cruz and Duff Islands, Solomon Islands. Copeia 1984, 571-578.
| Crossref | Google Scholar |
Medina MF, Bauer AM, Branch WR, Schmitz A, Conradie W, Nagy ZT, Hibbitts TJ, Ernst R, Portik DM, Nielsen SV, Colston TJ, Kusamba C, Behangana M, Rödel M-O, Greenbaum E (2016) Molecular phylogeny of Panaspis and Afroablepharus skinks (Squamata: Scincidae) in the savannas of sub-Saharan Africa. Molecular Phylogenetics and Evolution 100, 409-423.
| Crossref | Google Scholar |
Melville J, Swain R (2000) Mitochondrial DNA-sequence based phylogeny and biogeography of the snow skinks (Squamata: Scincidae: Niveoscincus) of Tasmania. Herpetologica 56, 196-208.
| Google Scholar |
Mertens R (1931) Ablepharus boutonii (Desjardins) und seine geographische variation. Zoologische Jahrbücher Abteilung für Systematik, Geographie und Biologie der Tiere 61, 63-210.
| Google Scholar |
Mertens R (1934) Die Scinciden-Gattung Cophoscincopus Vaillant. Zoologischer Anzeiger 102, 188-190.
| Google Scholar |
Mertens R (1964) Weitere Mitteilungen über die Rassen von Ablepharus boutonii (Desjardin), III. Zoologischer Anzeiger 173, 100-110.
| Google Scholar |
Meyer AB (1874) Eine Mittheilung von Hrn. Dr. Adolf Bernhard Meyer über die von ihm auf Neu-Guinea und den Inseln Jobi, Mysore und Mafoor im Jahre 1873 gesammelten Amphibien. Monatsberichte der königlich Akademie der Wissenschaften zu Berlin 1874, 128-140.
| Google Scholar |
Mittleman MB (1952) A generic synopsis of the lizards of the subfamily Lygosominae. Smithsonian Miscellaneous Collection 117(4069), 1-35.
| Google Scholar |
Moussalli A, Hugall AF, Moritz C (2005) A mitochondrial phylogeny of the rainforest skink genus Saproscincus, Wells and Wellington (1984). Molecular Phylogenetics and Evolution 34, 190-202.
| Crossref | Google Scholar |
Nattier R, Pellens R, Robillard T, Jourdan H, Legendre F, Caesar M, Nel A, Grandcolas P (2017) Updating the phylogenetic dating of New Caledonian biodiversity with a meta-analysis of the available evidence. Scientific Reports 7, 3705.
| Crossref | Google Scholar |
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268-274.
| Crossref | Google Scholar |
O’Shaughnessy AWE (1874) XL.– descriptions of new species of Scincidæ in the collection of the British Museum. Annals and Magazine of Nature History Ser. 4 13, 298-301.
| Crossref | Google Scholar |
Patterson GB, Daugherty CH (1995) Reinstatement of the genus Oligosoma (Reptilia, Lacertilia, Scincidae). Journal of the Royal Society of New Zealand 25, 327-331.
| Crossref | Google Scholar |
Perret J-L (1973) Contribution à l’étude des Panaspis (Reptilia, Scincidae) d’Afrique occidentale avec la description de deux espèces nouvelles. Revue suisse de Zoologie 80, 595-630.
| Crossref | Google Scholar |
Perret J-L (1975) La différenciation dans le genre Panaspis Cope (Reptilia, Scincidae). Bulletin de la Société des Sciences Naturelles de Neuchâtel 98, 5-16.
| Google Scholar |
Perret J-L (1982) Le sous-genre Leptosiaphos (Lacertilia, Scincidae) et ses implications. Bulletin de la Société des Sciences Naturelles de Neuchâtel 105, 107-121.
| Google Scholar |
Peters W (1874) Über einige neue Reptilien (Lacerta, Eremias, Diploglossus, Euprepes, Lygosoma, Sepsina, Ablepharus, Simotes, Onychocephalus). Monatsberichte der Königlichen Preussische Akademie des Wissenschaften zu Berlin 1874, 368-377.
| Google Scholar |
Peters W (1881) Drei neue Eidechsen, zu der Familie der Scincoiden gehörig, eine Lipinia (mit geckonenuahnlicher Bildung der Zehen!) aus Neu-Guinea und wie Mocoa aus Neuholland. Sitzungsber. Sitzungsberichte der Gesellschaft Naturforschender Freunde zu Berlin 1881, 81-85.
| Google Scholar |
Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13, 93.
| Crossref | Google Scholar |
Rabosky DL, Donnellan SC, Talaba AL, Lovette IJ (2007) Exceptional among-lineage variation in diversification rates during the radiation of Australia’s most diverse vertebrate clade. Proceedings of the Royal Society of London B: Biological Sciences 274, 2915-2923.
| Crossref | Google Scholar |
Rankin PR (1979) A taxonomic revision of the genus Menetia (Lacertilia, Scincidae) in the Northern Territory. Records of the Australian Museum 32, 491-499.
| Crossref | Google Scholar |
Rannala B, Yang Z (2007) Inferring speciation times under an episodic molecular clock. Systematic Biology 56, 453-466.
| Crossref | Google Scholar |
Rawlinson PA (1974) Revision of the endemic southeastern Australian lizard genus Pseudemoia (Scincidae: Lygosominae). Memoirs of the National Museum of Victoria 35, 87-96.
| Crossref | Google Scholar |
Rawlinson PA (1975) Two new lizard species from the genus Leiolopisma (Scincidae: Lygosominae) in south-eastern Australia and Tasmania. Memoirs of the National Museum of Victoria 36, 1-15.
| Crossref | Google Scholar |
Reeder TW (2003) A phylogeny of the Australian Sphenomorphus group (Scincidae: Squamata) and the phylogenetic placement of the crocodile skinks (Tribolonotus): Bayesian approaches to assessing congruence and obtaining confidence in maximum likelihood inferred relationships. Molecular Phylogenetics and Evolution 27, 384-397.
| Crossref | Google Scholar |
Reis Md, Yang Z (2011) Approximate likelihood calculation on a phylogeny for Bayesian estimation of divergence times. Molecular Biology and Evolution 28, 2161-2172.
| Crossref | Google Scholar |
Richmond JQ, Ota H, Grismer LL, Fisher RN (2021) Influence of niche breadth and position on the historical biogeography of seafaring scincid lizards. Biological Journal of the Linnean Society 132, 74-92.
| Crossref | Google Scholar |
Sadlier RA (1984) A new Australian scincid lizard, Menetia concinna, from the Alligator Rivers region, Northern Territory. Records of the Australian Museum 36, 45-49.
| Crossref | Google Scholar |
Sadlier RA (1987) A review of the scincid lizards of New Caledonia. Records of the Australian Museum 39, 1-66.
| Crossref | Google Scholar |
Sadlier RA (1990) The scincid lizard genus Nannoscincus Günther: a revaluation. Memoirs of the Queensland Museum 29, 487-494.
| Google Scholar |
Sadlier RA, Colgan DJ, Shea GM (1993) Taxonomy and distribution of the scincid lizard Saproscincus challengeri and related species in southeastern Australia. Memoirs of the Queensland Museum 34, 139-158.
| Google Scholar |
Sadlier RA, Couper PJ, Colgan DJ, Vanderduys E, Rickard E (2005) A new species of scincid lizard, Saproscincus eungellensis, from mid-eastern Queensland. Memoirs of the Queensland Museum 51, 559-571.
| Google Scholar |
Sadlier RA, Smith SA, Bauer AM (2006a) A new genus for the New Caledonian scincid lizard Lygosoma euryotis Werner, 1909, and the description of a new species. Records of the Australian Museum 58, 19-28.
| Crossref | Google Scholar |
Sadlier RA, Bauer AM, Smith SA (2006b) A new species of Nannoscincus Günther (Squamata: Scincidae) from high elevation forest in southern New Caledonia. Records of the Australian Museum 58, 29-36.
| Crossref | Google Scholar |
Sadlier RA, Deuss M, Bauer AM, Jourdan H (2019) Kuniesaurus albiauris, a new genus and species of scincid lizard from the Ⓘle des Pins, New Caledonia, with comments on the diversity and affinities of the region’s lizard fauna. Pacific Science 73, 123-141.
| Crossref | Google Scholar |
Schmidt KP (1932) Reptiles and amphibians from the Solomon Islands. Field Museum of Natural History Zoology Series 18, 175-190.
| Google Scholar |
Schmidt KP, Burt CE (1930) Herpetological results of the Whitney South Sea Expedition V. Description of Emoia sanfordi, a new lizard from islands of the Western Pacific (Scincidæ). American Museum Novitates 436, 1-3.
| Google Scholar |
Schmitz A, Ineich I, Chirio L (2005) Molecular review of the genus Panaspis sensu lato (Reptilia: Scincidae) in Cameroon, with special reference to the status of the proposed subgenera. Zootaxa 863, 1-28.
| Crossref | Google Scholar |
Schwaner TD (1980) Reproductive biology of lizards on the American Samoan islands. Occasional Papers of the Museum of Natural History, University of Kansas 86, 1-53.
| Google Scholar |
Shea GM (2021) Nomenclature of supra-generic units within the Family Scincidae (Squamata). Zootaxa 5067(3), 301-351.
| Crossref | Google Scholar |
Skinner A (2007) Phylogenetic relationships and rate of early diversification of Australian Sphenomorphus group scincids (Scincoidea, Squamata). Biological Journal of the Linnean Society 92, 347-366.
| Crossref | Google Scholar |
Skinner A, Hugall AF, Hutchinson MN (2011) Lygosomine phylogeny and the origins of Australian scincid lizards. Journal of Biogeography 38, 1044-1058.
| Crossref | Google Scholar |
Smith SA, Sadlier RA, Bauer AM, Austin CC, Jackman T (2007) Molecular phylogeny of the scincid lizards of New Caledonia and adjacent areas: evidence for a single origin of the endemic skinks of Tasmantis. Molecular Phylogenetics and Evolution 43, 1151-1166.
| Crossref | Google Scholar |
Steindachner F (1870) Herpetologische Notizen (II). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften in Wien 62, 326-348.
| Google Scholar |
Sternfeld R (1918) Zur Tiergeographie Papuasiens und der pazifischen Inselwelt. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft (Frankfurt) 36, 375-436.
| Google Scholar |
Stewart JR, Thompson MB (1998) Placental ontogeny of the Australian scincid lizards Niveoscincus coventryi and Pseudemoia spenceri. Journal of Experimental Zoology 282, 535-559.
| Crossref | Google Scholar |
Storr GM (1976) The genus Menetia (Lacertilia, Scincidae) in Western Australia. Records of the Western Australian Museum 4, 189-200.
| Google Scholar |
Stuart-Fox DM, Hugall AF, Moritz C (2002) A molecular phylogeny of rainbow skinks (Scincidae: Carlia): taxonomic and biogeographic implications. Australian Journal of Zoology 50, 39-51.
| Crossref | Google Scholar |
Thompson MB, Stewart JR, Speake BK, Russell KJ, McCartney RJ (2001) Utilisation of nutrients by embryos of the enigmatic Australian viviparous skink Niveoscincus coventryi. Journal of Experimental Zoology 290, 291-298.
| Crossref | Google Scholar |
Towns DR, Daugherty CH, Newman DG (1985) An overview of the ecological biogeography of the New Zealand lizards (Gekkonidae, Scincidae). In ‘Biology of Australasian frogs and reptiles’. (Eds G Grigg, R Shine, H Ehmann) pp. 107–115. (Surrey Beatty and Sons, Royal Zoological Society of New South Wales: Chipping Norton, Sydney)
Uetz P, Freed P, Aguilar R, Hošek J (Eds) (2022) The reptile database. Available at http://www.reptile-database.org. [Accessed 2 May 2022]
Welch KRG (1982) Herpetology of the Old World 2. Preliminary comments on the classification of skinks (family Scincidae) with specific reference to those genera found in Africa, Europe and southwest Asia. Herptile 7(4), 25-27.
| Google Scholar |
Wells RW, Wellington CR (1984) A synopsis of the Class Reptilia in Australia. Australian Journal of Herpetology 1(3-4), 73-129.
| Google Scholar |
Wells RW, Wellington CR (1985) A classification of the amphibia and reptilia of Australia. Australian Journal of Herpetology Supplementary Series 1, 1-61.
| Google Scholar |
Werner F (1898) Vorläufige Mitteilung über die von Herrn Prof. F. Dahl im Bismarck-Archipel gesammelten Reptilien und Batrachier. Zoologischer Anzeiger 21, 552-556.
| Google Scholar |
Werner F (1899) Beiträge zur Herpetologie der pacifischen Inselwelt und von Kleinasien. Zoologischer Anzeiger 22, 371-378.
| Google Scholar |
Whiting AS, Bauer AM, Sites JW (2003) Phylogenetic relationships and limb loss in sub-Saharan African scincine lizards (Squamata: Scincidae). Molecular Phylogenetics and Evolution 29, 582-598.
| Crossref | Google Scholar |
Wiegmann AFA (1834) In: Dr. F. J. F. Meyen: Beiträge zur Zoologie gesammelt auf einer Reise um die Erde. Siebente Abhandlung. Amphibien. Nova Acta Physico-Medica Academia Caesarea Leopoldino-Carolina (Halle) 17, 185-268.
| Google Scholar |
Zhang C, Sayyari E, Mirarab S (2017) ASTRAL-III: increased scalability and impacts of contracting low support branches. In ‘Comparative genomics. RECOMB-CG 2017. Vol. 10562’. Lecture Notes in Computer Science. (Eds J Meidanis, L Nakhleh) pp. 53–75. (Springer: Cham). doi:10.1007/978-3-319-67979-2_4
Zheng Y, Wiens JJ (2016) Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Molecular Phylogenetics and Evolution 94(B), 537-547.
| Crossref | Google Scholar |
Zug GR (1995) A new skink (Reptilia: Sauria: Leiolopisma) from Fiji. Proceedings of the Biological Society of Washington 98(1), 221-231.
| Google Scholar |
Zug GR (2004) Systematics of the Carlia “fusca” lizards (Squamata: Scincidae) of New Guinea and nearby islands. Bishop Museum Bulletin of Zoology 5, 1-84.
| Google Scholar |
Zug GR (2010) An outlying Carlia population from Java and comments on species groups within the genus Carlia (Reptilia: Squamata: Scincidae). Proceedings of the Californian Academy of Sciences 61(8), 389-408.
| Google Scholar |
Footnotes
1 The name Pseudemoia has been therefore used for multiple concepts: spenceri alone (Fuhn 1969; Wells and Wellington 1985); spenceri + palfreymani (fide Rawlinson 1974; Greer 1989); spenceri + the entrecasteauxii complex (Hutchinson et al. 1990), all Australian skinks formerly placed in Leiolopisma (Cogger 1992, as a temporary nomenclatural solution to the issue created by the recognition of other genera for all non-Australian taxa, and the Pseudemoia group (Greer 1989) as a suprageneric group consisting of various Australian, New Caledonian and New Guinean genera, but not all genera formerly placed in Leiolopisma in each region.


