A comparison of ecomorphology between introduced and native Australian dung beetles
Alexander Harvey A and Emma Sherratt
A and Emma Sherratt  A B *
A B *
A School of Biological Sciences, The University of Adelaide, Adelaide, SA 5005, Australia.
B South Australian Museum, North Terrace, Adelaide, SA 5000, Australia.
Australian Journal of Zoology 70(4) 115-125 https://doi.org/10.1071/ZO22044
Submitted: 29 November 2022 Accepted: 23 February 2023 Published: 27 April 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Among the many catastrophic introductions of exotic species to Australia, the Australian Dung Beetle Project stands apart as a success story. From 1965 dung beetles (Coleoptera: Scarabaeinae) were introduced for biological control purposes, and 23 species survived to become integrated into the environment with apparently little-to-no competition with native species. To understand this, we investigated ecomorphological diversity in the Australian dung beetle fauna, examining variation in functional traits among rolling and tunnelling species that are native to Australia and introduced. We found that introduced species are, on average, larger than native species of the same nidification strategy, but the size ranges overlap. Native and introduced tunnellers are convergent in body shape, whereas introduced rollers have distinct body shape compared with native species. Rollers and tunnellers also have distinct allometric patterns, where shape variation predicted by size aligns along two diverging allometric trajectories between nidification strategies. Our results suggest that ecomorphological differences do not explain the apparent lack of competition between tunnellers, but this may be the factor for rollers. Also, these results indicate that body size and associated allometric scaling is an important aspect of the ecomorphology of dung beetles that should be considered in future studies.
Keywords: convergence, diversity, dung beetles, ecomorphology, functional morphology, morphometrics, niche partitioning, Scarabaeinae.
Introduction
Australia is often presented as an example of what devastating ecological effects occur after introducing alien species into an environment. Yet there have been some success stories (Dodd 1940; Briese 2004): dung beetles (Coleoptera: Scarabaeinae) were introduced into Australia for biological control purposes by the Australian Dung Beetle Project, which ran from 1965 to 1985 (Bornemissza 1976), and research suggests there has been little competition between the introduced and native species (Edwards 2007; Ridsdill-Smith and Edwards 2011). Introductions were made to reduce the adverse effects of dung from introduced livestock, primarily that of cattle and sheep. Native dung beetles appear to favour consuming and brooding in the dry, pellet-like dung of Australia’s native mammal fauna (e.g. Hill 1993, 1996; Wright 1997; Vernes et al. 2005; Ebert et al. 2019; Carvalho et al. 2020). Therefore, when cattle and sheep were introduced with colonial settlers, the native beetles left the soft, moist dung of livestock untouched (e.g. Ferrar 1975), resulting in an excess that had the effect of vegetation spoilage, blow fly propagation and cattle parasitism (Losey and Vaughan 2006). Introducing dung beetles from geographical regions that naturally have cattle and sheep was proposed to mitigate these issues. Overall, 43 species of dung beetles were initially chosen for release, generally from Mediterranean and African regions, after stringent quarantining procedures (Bornemissza 1976). Of these, 23 species survived and are thriving in every state and territory of Australia (Edwards 2007; Doube et al. 2014; Edwards et al. 2015). Since then, further research and introduction events have occurred, with two more species (Onthophagus vacca and Bubalus bubalis) being approved for release in the early 2000s, resulting in 25 introduced species thriving in Australia (Edwards et al. 2015).
Ecological community assemblages and interspecific competition can be inferred indirectly via the study of ecomorphology, how functional morphological traits such as form (shape and size) relate to an organism’s environment (Williams 1972; Karr and James 1975). Dung beetles can be ecologically partitioned by their approach to dung manipulation and brood formation, called nidification, with the main types being tunnelling, rolling, or dwelling (Halffter and Matthews 1966). Their body form is well known to correlate with nidification strategy, particularly with respect to different body and limb proportions (e.g. Inward et al. 2011; Raine et al. 2018; Alves and Hernández 2019). Therefore, morphometric data are a good proxy for ecological diversity in dung beetles (Alves and Hernández 2019).
Body size is well known to define an animal’s ecological niche (Peters and Peters 1986) and usually influences body shape as a result of allometric scaling (Huxley and Teissier 1936). Allometric shape variation is important to consider because this is the component that relates to physical scaling laws (i.e. more robust limbs in larger bodied animals) or changes due to growth (in the case of ontogenetic allometry, see Klingenberg 2016). Furthermore, it is postulated that speciation along this allometric axis can be a line of least resistance for evolution (Marroig and Cheverud 2005). However, previous studies of ecomorphology in dung beetles have mostly overlooked the contribution of allometric scaling to morphological diversity. Raine et al. (2018) showed how morphological traits predict nidification strategy but did not dissociate proportional shape changes (allometric variation) from magnitudes of size. In a study across assemblages of dung beetles in different biogeographic regions, Inward et al. (2011) employed a regression residuals procedure in an effort to remove effects of scale (body size) among species, but which actually explicitly removes morphological variation due to allometry. A common approach to ‘correct for size’ in a morphometric study is to perform linear regressions of each variable on a measure of size (e.g. body length), and take the residuals from those regressions as size-corrected variables. While this method does adjust the variables for overall magnitudes of size differences among observations, it also removes the allometric component of shape (the variation in shape that changes proportionally with size). The residual approach thus has two main problems: it assumes a unified scaling pattern (estimated regression slope) across all observations, so when there is group structure in the data and groups have different scaling patterns, it incorrectly estimates the slope and resulting residuals for all observations; secondly, it assumes that allometric variation is not important, yet some clades exhibit most of their morphological diversity because of allometry (e.g. Marcy et al. 2020). By comparison, the log-shape ratio approach (sensu Mosimann 1970; Claude 2013) only performs the scaling step to standardise for magnitude of size difference among observations, thus leaving allometric shape variation to be examined explicitly using a linear regression, which can then be used to remove the allometric component of shape via residuals if required. This approach has been shown to be the most robust for size correction in morphometric data (Jungers et al. 1995).
This paper aims to address whether the apparent lack of competition between assemblages of dung beetles in Australia is due to their different ecology, as interpreted from different morphology (body shape and size). In measuring body shape, we use a transformation approach that standardises linear morphometric variables for size (scale) but retains the allometric shape component of variance, in order to separate shape and size (Bookstein 1989), and explicitly study patterns of allometric and non-allometric shape variation. We measured morphological traits on representative dung beetle taxa of every Australian genus and all introduced species to address two questions: (1) are there differences in body size and shape between introduced and native species of dung beetles of different nidification strategies? and (2) how much does allometry contribute to morphological diversity across species, and do the nidification strategies have different allometric scaling patterns?
Materials and methods
Strategies for nidification
Dung beetles differ in their approach to dung manipulation and brood formation (nidification), with the main types being tunnelling, rolling, or dwelling (Halffter and Matthews 1966). The latter is uncommon and not easily morphologically distinguished so we do not consider it further. A hierarchy of competition has been suggested to explain the evolution of nidification strategies (Hanski and Cambefort 2014). Rolling (telecoprid) beetles form compressed balls of dung that are either buried near the dung pile or are rolled a distance away from the pile before being buried. Commonly, it is the male beetle that rolls the dung away before pheremonally attracting a female, which then proceeds to lay an egg inside the brood ball (Halffter and Edmonds 1982). Tunnelling (paracoprid) beetles burrow into the dung pile, taking a small amount of the waste material with them as they do so. They then pack the material into a brood ball and the female lays a single egg inside each ball (Halffter and Edmonds 1982). This is a display of mass provisioning, a parental investment strategy in which the adult insect stockpiles food and supplies in an area before producing an egg. Australian native and introduced taxa demonstrate both strategies.
Samples and phylogenetic affinities of species
We sampled all genera of native Australian dung beetles (n = 128) and every species of introduced dung beetle (n = 25), with both data groups including both rolling and tunnelling taxa (Matthews 1971, 1974, 1976; Edwards et al. 2015). There are more rolling than tunnelling native species (73 and 55 respectively). Of the introduced species, almost all are tunnellers (23) compared with just two rollers. Specimens were sampled from two invertebrate museum collections: the South Australian Museum (SAM) in Adelaide, South Australia, and the Australian National Insect Collection (ANIC) in Canberra, Australian Capital Territory. Across these two collections, a total of 262 specimens were measured (Table S1). Where possible, only males were selected for each species; however, this was not always an option, as sometimes only females had been collected, or the specimen was preserved in a way that did not allow for sexing of the beetle. In total, 212 males were selected, 81% of the data, alongside 35 females (13%) and 14 of unknown sex (5%).
The taxonomic classification of native Australian dung beetles has historically comprised three extant tribes: Deltochilini, Coprini, and Onthophagini (Matthews 1971, 1974, 1976). A recently published multigene phylogeny (Tarasov and Dimitrov 2016) found that the Australian genera are paraphyletic with respect to other neotropical genera, thus rendering most of the genera incertae sedis (only Onthophagini remains valid). Their study is the most comprehensive with respect to number of taxa, and includes 21 of the 26 genera sampled here (missing the native rolling genera Aulacopris, Labroma, Mentophilus, Tesserodon and native tunnelling genus Thyregis) (Fig. 1). Gunter et al. (2019) published an Australian-focussed study, revealing a congruent topology, and showing Tesserodon to be sister to Coproecus, but positions of the other genera have yet to be resolved. Limited phylogenetic sampling for the species studied here precludes the use of phylogenetic comparative methods for this study. However, the topology in Fig. 1 suggests that each nidification strategy has evolved more than once in the native and introduced samples.
|
|
Measurements
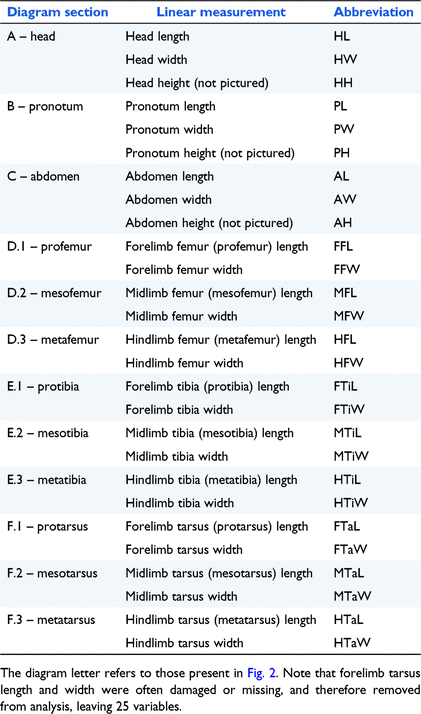
Macrographs and measurements were acquired on a Nikon SMZ1270 stereo microscope and with a Plan Apo 0.5×/WF lens, using the software NIS-Elements (Nikon Corporation; Tokyo, Japan) at the SAM. At the ANIC, macrographs and measurements were acquired on a Leica M205C stereomicroscope and with a Leica DF500 camera, using the software Leica Application Suite 3.4 (Leica Camera; Wetzlar, Germany). A total of 27 linear measurements (measured in micrometres) (Table 1, Fig. 2) were recorded from the live camera view for each specimen, as well as their sex (male, female, or unknown) and preservation orientation (dorsal, ventral, or both). The complete dataset can be found as supplementary material (Table S1). The order in which measurements were taken varied between specimens, as each was pinned in a unique manner, but generally began with dorsal measurements of the abdomen, pronotum, and head, followed by the limbs ventrally, and finally the depth of the tagmata (body segments). It was assumed that the left and right sides of all beetle bodies were symmetrical and therefore produced identical measurements.
|
|
|
Some measurements had to be adapted for select genera as follows: Head: The head height and length excluded any horns present, as these tended to be quite large and may have caused distortion of the data. Pronotum: The pronotum height and length included any ridges or horns present, as they were valid height markers and when compared to the head horns, had far less impact on the data. Abdomen: The abdomen height excluded the femora, trochanters, or coxae. Femur: The forelimb femora included the attached trochanter; mid and hindlimb femora excluded it. To keep the width consistent when measuring the femur, it was always measured at a 90° angle to the length measurement, and then the measurement repositioned to encompass the widest section of the limb. Spurs located on the hindlimb femora were exclude from the hindlimb width measurement (example, Onitis caffer). This was achieved by measuring the width of the hindlimb at a 90° angle from the base of the spur to the corresponding side of the hindlimb. Tibia: The forelimb tibiae sometimes displayed signs of tibial wear, where the tibial teeth have been worn away from use, thus producing a smaller measurement of width. This was avoided by selecting young beetles that had more pronounced tibial teeth. All tibiae were measured at their widest point, which was generally near the distal end. As with the femur, the tibia width was measured at a 90° angle to the tibia length. Spurs or hooks located on the hind or midlimb tibiae were also excluded from the measurement (example, Coptodactyla ducalis). Tarsus: The tarsus width was generally measured across the first tarsomere, as this was the widest; however, this was not always true. The tarsus length was not included if there were any tarsomeres missing. The tarsus length excluded the claws, or ungues, at the distal end of the tarsus.
Morphometric analysis
Data analysis was performed using the R Statistical Environment v.4.2.1 (R Development Core Team 2022). The following packages were used: geomorph v.4.0.4 (Adams et al. 2022), stats v.4.2.1 (R Development Core Team 2022), and missForest v.1.5 (Stekhoven and Stekhoven 2013).
Many specimens had missing data points, typically owing to the loss of limbs, or features obscured from preservation orientation. As such, missing values were imputed using a non-parametric random forest approach to imputation, implemented with the ‘missForest’ function in missForest (maximum iterations = 10; number of trees = 100). Forelimb tarsus length and width were missing in nearly 40% of all specimens, and therefore removed completely from the dataset prior to missing data imputation (leaving 25 variables). Species averages were calculated prior to analysis.
To standardise the morphometric variables for body size, the data were scaled prior to analysis using the log-shape ratio approach (LSR); each variable is divided by the geometric mean of all variables and log-transformed to produce new shape variables (sensu Mosimann 1970; Claude 2013). This method was used instead of a linear regression–based approach (e.g. Inward et al. 2011) to remove size but retain the allometric shape variation.
To test for body size differences between nidification strategies among the native and introduced species we used the geometric mean of the 25 measurements. An analysis of variance (ANOVA) model was evaluated (size ~ origin × nidification strategy) implemented with the stats R package, and statistical significance was assessed at ɑ = 0.05.
To examine the effects of allometry and nidification strategy on body shape we used a non-parametric multivariate analysis of variance (np-MANOVA), which assesses across multiple dependent variables simultaneously via a distance matrix (Anderson 2001), implemented with the ‘procD.lm’ function in geomorph. The model evaluated (shape ~ log(size) × nidification strategy) performs a multivariate regression of all 25 log-shape ratios against the log-transformed geometric mean, and tests whether the slope and intercept of each strategy are different (the interaction term). Statistical significance was evaluated using a permutational approach (1000 iterations) and assessed at ɑ = 0.05. To visualise the allometric trajectories of each group, the regression score approach (Drake and Klingenberg 2010) and PC1 of predicted values from the regression (Adams and Nistri 2010) were used. These methods reduce the dimensionality of the data to provide a univariate output that can be plotted against log-transformed size (equivalent to a regular regression scatterplot).
A principal component analysis (PCA) was performed on the log-shape ratio data to visualise the shape variation among species. This was implemented with the ‘prcomp’ function in stats R package (with centre and scale settings as TRUE). Variable loadings of the PC axes were plotted as bar plots. Scatterplots of the significant PC axes were plotted with points (representing species) scaled to body size, such that shape and size variation can be visualised together.
Results
There is a significant difference in body size between native and introduced species (ANOVA, F1,149 = 37.6, P < 0.001) (Fig. 3), where introduced species are, on average, larger than native species. Species that use the rolling nidification strategy are, on average, smaller than tunnelling species, but this is not statistically significant (F1,149 = 1.11, P = 0.295). The interaction term of origin and nidification strategy was not significant (F1,149 = 0.98, P = 0.324), indicating that introduced species are, on average, larger than native species for both strategies (Fig. 3). The range of body sizes between introduced and native species overlaps in both nidification strategies.
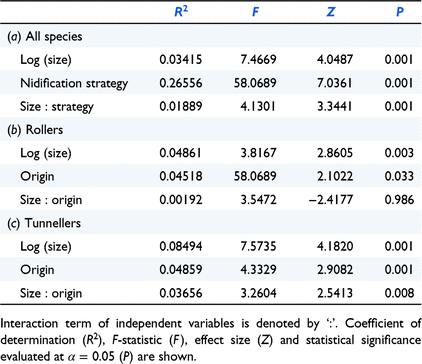
Across all species, there is a small (R2 = 0.034) but significant effect of body size on body shape, a large (R2 = 0.266) effect of nidification strategy, as well as a small but significant interaction between body size and nidification strategy (R2 = 0.019), indicating that allometric scaling of traits among rollers is different from that of tunnellers (Table 2a, Fig. 4). Separate np-MANOVA analyses for rolling and tunnelling taxa revealed that in rolling species, body size accounts for 4.8% of the shape variation, and native and introduced species are significantly different in shape, but the interaction term is not significant, indicating that the two groups share a common allometric trajectory (Table 2b). However, the number of roller species introduced is only two compared with 73 native species. Although body size accounts for 8.5% of the shape variation in tumblers, native and introduced species are significantly different in shape, and the interaction term is significant, indicating that the two groups have different allometric trajectories (Table 2c).
|
Principal components analysis of the log-shape ratios representing body shape produced three axes representing 51.4% of the total variation (Fig. 5a), and five axes each representing more than 5% of the variation (67.3%). PC1 variation is associated with the strong inverse correlation between mid- and hindlimb widths and lengths (Fig. 5b), where rollers have long and slender mid- and hindlimbs. PC2 variation is associated with a correlation between the tagma widths and overall forelimb size, while reducing overall mid- and hindlimb size, producing beetles with wider bodies, larger forelimbs, and smaller hindlimbs at the positive end of the axis. PC3 accounts for ~12% of the total variance and indicates an inverse correlation between the pronotum length relative to head width and abdomen length.
|
|
Rollers and tunnellers occupy different positions along the PC1 axis, with rollers having mostly positive PC1 scores, and tunnellers with negative scores. The two species of introduced rollers (Sisyphus rubrus and Sisyphus spinipes) occupy a distinct position along PC2 and PC3 (Fig. 5a) compared with native rollers. Tunnellers occupy a smaller region of the total shape space defined by the first three axes, and native and introduced species are strongly overlapping, indicating many shared shape features. Average body shape and size for each nidification strategy between native and introduced species is shown in Fig. 6.
Discussion
We investigated whether introduced species of dung beetle differ in their body shape and size compared with native species, comparing the two dominant nidification strategies in Australia. This was done to address whether the apparent lack of competition between assemblages of dung beetles in Australia (Edwards 2007) is due to their different ecologies, inferred from ecomorphological principles.
Ecomorphology of dung beetles has been well characterised, where distinct morphological features distinguish the different nidification strategies (Inward et al. 2011; Raine et al. 2018; Alves and Hernández 2019). It has been shown that these strategies have convergently evolved on different continents (Inward et al. 2011). Some of our results support this: Australian native and introduced tunnelling species have converged on a similar body shape and occupy a very restricted range in morphospace. They also have overlapping body sizes, although introduced species are, on average, larger. Fig. 1 confirms that these taxa are not morphologically similar because of shared ancestry. These results support the ‘consistent tunnelling morphospace’ observation of Inward et al. (2011). Their close similarity in shape and overlapping size range suggests that it is not ecomorphological differences contributing to the lack of competition between introduced and native species.
Conversely, no Australian rolling species have evolved the same extreme shape of the two introduced rolling species (S. rubrus and S. spinipes). Native rolling species display a wide range of body shapes, but this region of morphospace is not overlapping with the introduced species. Although introduced species are similar in size to some larger Australian rolling species, they have a very distinct shape (with distinctive elongated tarsi). It is not currently known why Sisyphus has one of the largest body to hindlimb ratios of all dung beetle genera (Davis et al. 2002). Therefore, for rolling species, the lack of competition may be due to different rolling techniques resulting from the different morphologies between native species and the introduced Sisyphus.
Our results show that rollers and tunnellers have distinct and divergent allometric trajectories, indicating a change in shape relative to size happens by very different proportions if one is a tunneller or a roller. Although introduced species share some similar allometric shape variation to the Australian native species (Fig. 4), statistical analysis suggests that introduced tunnelling species also have a distinct allometric relationship compared with native tunnelling species (Table 2c). Size may only contribute a small proportion of the shape variation in each group (~5–9%), but this is similar to the results of other studies of evolutionary allometry in diverse invertebrate taxa (e.g. Klingenberg and Zimmermann 1992; Benítez et al. 2022). This observation was made possible because we used a morphometric standardisation approach that allows allometric variation to be examined separately from isometric size variation. While Hernández et al. (2011) did not explicitly identify different nidification strategies, they presented a similar divergent regression plot in their study of dung beetle body shape characterised using a geometric morphometrics approach (landmark coordinates that characterise shape variation are adjusted for size using Procrustes superimposition, see Zelditch et al. 2012). These results indicate that fitting a single linear regression model to all species would inaccurately capture the scaling relationships, and the residuals from this regression would not accurately represent ‘size free’ shape variables. Therefore we recommend that researchers use the log-shape ratio or geometric morphometrics approaches to adjust for size (see Claude (2013) for explanations of equivalence), and a subsequent linear regression to examine allometric variation. If there is no group structure, or groups with parallel slopes, then allometric variation can be removed by using the residuals approach. Caution with regression residuals must be taken if there is group structure in the data and the groups have non-parallel allometric slopes.
We recognise that geographic location and niche specificity play an important role in whether there is competition between species, and that this aspect has not been investigated here. Previous studies have documented the dung or habitat preferences of Australian dung beetles in particular bioregions (e.g. Doube and Macqueen 1991; Hill 1993, 1996; Wright 1997; Vernes et al. 2005; Ebert et al. 2019; Carvalho et al. 2020). Some experimental research into interspecific competition between dung beetles has been done in other countries (e.g. Giller and Doube 1989; Finn and Gittings 2003; Horgan 2005), but here in Australia there is patchy knowledge (Vernes et al. 2005). Climatic variables do not predict species distributions of introduced species across the continent (Duncan et al. 2009), thus the factors involved in their partitioning across the landscape require further investigation.
Another factor not considered in this study is time of day the beetle is active – there are known morphological differences between diurnal and nocturnal species of the same nidification strategy (Hernández et al. 2011; Raine et al. 2018), indicating that this can be a proxy for ecological differences when activity pattern is not known. Doube (1990) classified dung beetle assemblages and recognised this characteristic, but found it was a subcategory of the nidification strategy. It was not considered in other studies of habitat specificity (e.g. Davis 1996; Vernes et al. 2005) but trapping methods can bias data collection of beetles to a particular time of day (e.g. Hill 1996). This aspect of the dung beetle behaviour needs further study to understand if there is a difference between Australian and introduced species.
Although not a direct comparison of native and introduced species, the comprehensive study by Davis (1996) is an important and perhaps overlooked contribution to the Australia-wide assemblage of dung beetles. He assigned species into functional groups modified from Doube (1990), recognising not only rollers and tunnellers, but the size of the dung mass they can move. Soil type (clay, sand), soil depth, and surrounding vegetation were all found to be important environmental factors. Body size was an important morphological trait in his analyses, and species partitioned by body size across these different environments. Together with our in-depth ecomorphological analysis of Australian and introduced dung beetles, these findings suggest there is great scope for employing modern spatial mapping methods to examine functional diversity, in order to understand how the modern dung beetle assemblage has partitioned the Australian continent.
Supplementary material
Supplementary material is available online.
Data availability
Morphometric data associated with this study are available on Figshare (doi:10.25909/21638591).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by the University of Adelaide Student Support Fund to AH, and a University of Adelaide Research Fellowship to ES.
Acknowledgements
We thank Chris Watts and Matt Shaw at South Australian Museum for training, access to collections and valuable discussions. For access to the Australian National Insect Collection, we thank Cate Lemman, Debbie Jennings, and Patrick Gleeson.
References
Adams, DC, and Nistri, A (2010). Ontogenetic convergence and evolution of foot morphology in European cave salamanders (family: Plethodontidae). BMC Evolutionary Biology 10, 216.| Ontogenetic convergence and evolution of foot morphology in European cave salamanders (family: Plethodontidae).Crossref | GoogleScholarGoogle Scholar |
Adams DC, Collyer ML, Kaliontzopoulou A, Baken EK (2022) geomorph: software for geometric morphometric analyses. R package version 4.0. Available at https://cran.r-project.org/package=geomorph
Alves, VM, and Hernández, MIM (2019). Local extinctions may be evidenced by the holes of the morphometric hypervolume in dung beetle communities. Austral Ecology 44, 827–837.
| Local extinctions may be evidenced by the holes of the morphometric hypervolume in dung beetle communities.Crossref | GoogleScholarGoogle Scholar |
Anderson, MJ (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology 26, 32–46.
| A new method for non-parametric multivariate analysis of variance.Crossref | GoogleScholarGoogle Scholar |
Benítez, HA, Püschel, TA, and Suazo, MJ (2022). Drosophila wing integration and modularity: a multi-level approach to understand the history of morphological structures. Biology 11, 567.
| Drosophila wing integration and modularity: a multi-level approach to understand the history of morphological structures.Crossref | GoogleScholarGoogle Scholar |
Bookstein, FL (1989). “Size and shape”: a comment on semantics. Systematic Zoology 38, 173–180.
| “Size and shape”: a comment on semantics.Crossref | GoogleScholarGoogle Scholar |
Bornemissza, GF (1976). Australian dung beetle project, 1965–1975. AMRC Review. Australian Meat Research Committee 30, 1–30.
Briese, DT (2004). Weed biological control: applying science to solve seemingly intractable problems. Australian Journal of Entomology 43, 304–317.
| Weed biological control: applying science to solve seemingly intractable problems.Crossref | GoogleScholarGoogle Scholar |
Carvalho, RL, Weir, T, Vasconcelos, HL, and Andersen, AN (2020). Dung beetles of an Australian tropical savanna: species composition, food preferences and responses to experimental fire regimes. Austral Ecology 45, 958–967.
| Dung beetles of an Australian tropical savanna: species composition, food preferences and responses to experimental fire regimes.Crossref | GoogleScholarGoogle Scholar |
Claude, J (2013). Log-shape ratios, Procrustes superimposition, elliptic Fourier analysis: three worked examples in R. Hystrix – Italian Journal of Mammalogy 24, 94–102.
| Log-shape ratios, Procrustes superimposition, elliptic Fourier analysis: three worked examples in R.Crossref | GoogleScholarGoogle Scholar |
Davis, ALV (1996). Community organization of dung beetles (Coleoptera: Scarabaeidae): differences in body size and functional group structure between habitats. African Journal of Ecology 34, 258–275.
| Community organization of dung beetles (Coleoptera: Scarabaeidae): differences in body size and functional group structure between habitats.Crossref | GoogleScholarGoogle Scholar |
Davis, ALV, Scholtz, CH, and Philips, TK (2002). Historical biogeography of scarabaeine dung beetles. Journal of Biogeography 29, 1217–1256.
| Historical biogeography of scarabaeine dung beetles.Crossref | GoogleScholarGoogle Scholar |
Dodd AP (1940) ‘The Biological Campaign against Prickly-pear.’ (AH Tucker, Government Printer: Brisbane, Qld, Australia)
Doube, BM (1990). A functional classification for analysis of the structure of dung beetle assemblages. Ecological Entomology 15, 371–383.
| A functional classification for analysis of the structure of dung beetle assemblages.Crossref | GoogleScholarGoogle Scholar |
Doube, BM, and Macqueen, A (1991). Establishment of exotic dung beetles in Queensland: the role of habitat specificity. Entomophaga 36, 353–360.
| Establishment of exotic dung beetles in Queensland: the role of habitat specificity.Crossref | GoogleScholarGoogle Scholar |
Doube B, Macqueen A, Ridsdill-Smith T, Weir T (2014) Native and introduced dung beetles in Australia. In ‘Dung Beetle Ecology’. (Eds I Hanski, Y Cambefort) pp. 255–278. (Princeton University Press: Princeton, NJ, USA)
Drake, AG, and Klingenberg, CP (2010). Large-scale diversification of skull shape in domestic dogs: disparity and modularity. The American Naturalist 175, 289–301.
| Large-scale diversification of skull shape in domestic dogs: disparity and modularity.Crossref | GoogleScholarGoogle Scholar |
Duncan, RP, Cassey, P, and Blackburn, TM (2009). Do climate envelope models transfer? A manipulative test using dung beetle introductions. Proceedings of the Royal Society B: Biological Sciences 276, 1449–1457.
| Do climate envelope models transfer? A manipulative test using dung beetle introductions.Crossref | GoogleScholarGoogle Scholar |
Ebert, KM, Monteith, GB, Menéndez, R, and Merritt, DJ (2019). Bait preferences of Australian dung beetles (Coleoptera: Scarabaeidae) in tropical and subtropical Queensland forests. Austral Entomology 58, 772–782.
| Bait preferences of Australian dung beetles (Coleoptera: Scarabaeidae) in tropical and subtropical Queensland forests.Crossref | GoogleScholarGoogle Scholar |
Edwards PB (2007) Introduced dung beetles in Australia 1967–2007: current status and future directions. Landcare Australia, Sydney, NSW, Australia. p. 66.
Edwards P, Wilson P, Wright J (2015) ‘Introduced Dung beetles in Australia: a Pocket Field Guide.’ (CSIRO Publishing: Melbourne, Vic., Australia)
Ferrar, P (1975). Disintegration of dung pads in north Queensland before the introduction of exotic dung beetles. Australian Journal of Experimental Agriculture 15, 325–329.
| Disintegration of dung pads in north Queensland before the introduction of exotic dung beetles.Crossref | GoogleScholarGoogle Scholar |
Finn, JA, and Gittings, T (2003). A review of competition in north temperate dung beetle communities. Ecological Entomology 28, 1–13.
| A review of competition in north temperate dung beetle communities.Crossref | GoogleScholarGoogle Scholar |
Giller, PS, and Doube, BM (1989). Experimental analysis of inter- and intraspecific competition in dung beetle communities. Journal of Animal Ecology 58, 129–142.
| Experimental analysis of inter- and intraspecific competition in dung beetle communities.Crossref | GoogleScholarGoogle Scholar |
Gunter, NL, Monteith, GB, Cameron, SL, and Weir, TA (2019). Evidence from Australian mesic zone dung beetles supports their Gondwanan origin and Mesozoic diversification of the Scarabaeinae. Insect Systematics & Evolution 50, 162–188.
| Evidence from Australian mesic zone dung beetles supports their Gondwanan origin and Mesozoic diversification of the Scarabaeinae.Crossref | GoogleScholarGoogle Scholar |
Halffter G, Edmonds WD (1982) ‘The Nesting Behavior of Dung Beetles (Scarabaeinae). An Ecological and Evolutive Approach.’ (Instituto de Ecologia: Mexico)
Halffter, G, and Matthews, EG (1966). The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomologica Mexicana 12–14, 1–312.
Hanski I, Cambefort Y (2014) ‘Dung Beetle Ecology.’ (Princeton University Press: Princeton, NJ, USA)
Hernández, MIM, Monteiro, LR, and Favila, ME (2011). The role of body size and shape in understanding competitive interactions within a community of Neotropical dung beetles. Journal of Insect Science 11, 13.
| The role of body size and shape in understanding competitive interactions within a community of Neotropical dung beetles.Crossref | GoogleScholarGoogle Scholar |
Hill, C (1993). The species composition and seasonality of an assemblage of tropical Australian dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). The Australian Entomologist 20, 121–126.
Hill, CJ (1996). Habitat specificity and food preferences of an assemblage of tropical Australian dung beetles. Journal of Tropical Ecology 12, 449–460.
| Habitat specificity and food preferences of an assemblage of tropical Australian dung beetles.Crossref | GoogleScholarGoogle Scholar |
Horgan, FG (2005). Aggregated distribution of resources creates competition refuges for rainforest dung beetles. Ecography 28, 603–618.
| Aggregated distribution of resources creates competition refuges for rainforest dung beetles.Crossref | GoogleScholarGoogle Scholar |
Huxley, JS, and Teissier, G (1936). Terminology of relative growth. Nature 137, 780–781.
| Terminology of relative growth.Crossref | GoogleScholarGoogle Scholar |
Inward, DJG, Davies, RG, Pergande, C, Denham, AJ, and Vogler, AP (2011). Local and regional ecological morphology of dung beetle assemblages across four biogeographic regions. Journal of Biogeography 38, 1668–1682.
| Local and regional ecological morphology of dung beetle assemblages across four biogeographic regions.Crossref | GoogleScholarGoogle Scholar |
Jungers, WL, Falsetti, AB, and Wall, CE (1995). Shape, relative size, and size-adjustments in morphometrics. American Journal of Physical Anthropology 38, 137–161.
| Shape, relative size, and size-adjustments in morphometrics.Crossref | GoogleScholarGoogle Scholar |
Karr JR, James FC (1975) Eco-morphological configurations and convergent evolution of species and communities. In ‘Ecology and Evolution of Communities’. (Eds ML Cody, JM Diamond) pp. 258–291. (Belknap: Cambridge, MA, USA)
Klingenberg, CP (2016). Size, shape, and form: concepts of allometry in geometric morphometrics. Development Genes and Evolution 226, 113–137.
| Size, shape, and form: concepts of allometry in geometric morphometrics.Crossref | GoogleScholarGoogle Scholar |
Klingenberg, CP, and Zimmermann, M (1992). Static, ontogenetic, and evolutionary allometry: a multivariate comparison in nine species of water striders. The American Naturalist 140, 601–620.
| Static, ontogenetic, and evolutionary allometry: a multivariate comparison in nine species of water striders.Crossref | GoogleScholarGoogle Scholar |
Losey, JE, and Vaughan, M (2006). The economic value of ecological services provided by insects. BioScience 56, 311–323.
| The economic value of ecological services provided by insects.Crossref | GoogleScholarGoogle Scholar |
Marcy, AE, Guillerme, T, Sherratt, E, Rowe, KC, Phillips, MJ, and Weisbecker, V (2020). Australian rodents reveal conserved cranial evolutionary allometry across 10 million years of murid evolution. The American Naturalist 196, 755–768.
| Australian rodents reveal conserved cranial evolutionary allometry across 10 million years of murid evolution.Crossref | GoogleScholarGoogle Scholar |
Marroig, G, and Cheverud, JM (2005). Size as a line of least evolutionary resistance: diet and adaptive morphological radiation in New World monkeys. Evolution 59, 1128–1142.
| Size as a line of least evolutionary resistance: diet and adaptive morphological radiation in New World monkeys.Crossref | GoogleScholarGoogle Scholar |
Matthews, EG (1971). A revision of the Scarabaeine dung beetles of Australia. I. Tribe Onthophagini. Australian Journal of Zoology Supplementary Series 19, 3–330.
| A revision of the Scarabaeine dung beetles of Australia. I. Tribe Onthophagini.Crossref | GoogleScholarGoogle Scholar |
Matthews, EG (1974). A revision of the Scarabaeine dung beetles of Australia. II. Tribe Scarabaeini*. Australian Journal of Zoology Supplementary Series 22, 1–211.
| A revision of the Scarabaeine dung beetles of Australia. II. Tribe Scarabaeini*.Crossref | GoogleScholarGoogle Scholar |
Matthews, EG (1976). A revision of ths Scarabaeine dung beetles of Australia. III. Tribe Coprini*. Australian Journal of Zoology Supplementary Series 24, 1–52.
| A revision of ths Scarabaeine dung beetles of Australia. III. Tribe Coprini*.Crossref | GoogleScholarGoogle Scholar |
Mosimann, JE (1970). Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. Journal of the American Statistical Association 65, 930–945.
| Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions.Crossref | GoogleScholarGoogle Scholar |
Nemes, SN, and Price, DL (2015). Illustrated keys to the Scarabaeinae (Coleoptera: Scarabaeidae) of Maryland. Northeastern Naturalist 22, 318–344.
| Illustrated keys to the Scarabaeinae (Coleoptera: Scarabaeidae) of Maryland.Crossref | GoogleScholarGoogle Scholar |
Peters RH, Peters RH (1986) ‘The Ecological Implications of Body Size.’ (Cambridge University press: Cambridge, UK)
Raine, EH, Gray, CL, Mann, DJ, and Slade, EM (2018). Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses. Ecology and Evolution 8, 8686–8696.
| Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses.Crossref | GoogleScholarGoogle Scholar |
R Development Core Team (2022) R: a language and environment for statistical computing (CRAN). Available at http://www.R-project.org
Ridsdill-Smith TJ, Edwards PB (2011) Biological control: ecosystem functions provided by dung beetles. In ‘Ecology and Evolution of Dung Beetles’. (Eds LW Simmons, TJ Ridsdill-Smith) pp. 243–263. (John Wiley & Sons: West Sussex, UK)
Stekhoven DJ, Stekhoven MDJ (2013) R Package “missForest”. Available at https://cran.r-project.org/web/packages/missForest
Tarasov, S, and Dimitrov, D (2016). Multigene phylogenetic analysis redefines dung beetles relationships and classification (Coleoptera: Scarabaeidae: Scarabaeinae). BMC Evolutionary Biology 16, 257.
| Multigene phylogenetic analysis redefines dung beetles relationships and classification (Coleoptera: Scarabaeidae: Scarabaeinae).Crossref | GoogleScholarGoogle Scholar |
Vernes, K, Pope, LC, Hill, CJ, and Bärlocher, F (2005). Seasonality, dung specificity and competition in dung beetle assemblages in the Australian Wet Tropics, north-eastern Australia. Journal of Tropical Ecology 21, 1–8.
| Seasonality, dung specificity and competition in dung beetle assemblages in the Australian Wet Tropics, north-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Williams EE (1972) The origin of faunas. Evolution of lizard congeners in a complex island fauna: a trial analysis. In ‘Evolutionary Biology, Vol. 6’. (Eds T Dobzhansky, MK Hecht, WC Steere) pp. 47–89. (Springer: New York, NY, USA)
Wright KL (1997) An examination of the commensal interaction between the Australian native dung beetle, Onthophagus peramelinus and the rufous bettong, Aepyprymnus rufescens. BSc Honours Thesis, James Cook University, Townsville, Qld, Australia.
Zelditch ML, Swiderski DL, Sheets HD (2012) ‘Geometric Morphometrics for Bologists: a Primer.’ (Elsevier: Amsterdam, The Netherlands)


