Geographic frequency and ecological correlates of juvenile colour polymorphism in green pythons (Morelia azurea and Morelia viridis)
Daniel J. D. Natusch A B C and Jessica A. Lyons B
A B C and Jessica A. Lyons B
A Department of Biological Sciences, Macquarie University, Sydney, NSW 2109, Australia.
B EPIC Biodiversity, Frogs Hollow, NSW 2550, Australia.
C Corresponding author. Email: d.natusch@epicbiodiversity.com
Australian Journal of Zoology 68(2) 62-67 https://doi.org/10.1071/ZO21002
Submitted: 2 February 2021 Accepted: 9 April 2021 Published: 10 May 2021
Journal Compilation © CSIRO 2020 Open Access CC BY-NC
Abstract
Colour polymorphisms are common in nature, but their evolutionary significance and the mechanisms maintaining them sometimes remain poorly understood. Polymorphic green pythons (Morelia azurea and Morelia viridis) are born either red or yellow. Several processes are proposed to maintain such polymorphisms, and the assumption that colour is adaptive predicts that it may be correlated with a series of life-history and/or ecological traits. We examined 1090 green pythons from northern Australia and New Guinea and reveal strong geographic variation in the frequency of juvenile polymorphism. Some variation is explained by known genetic structure among populations, while stochastic processes (e.g. bottlenecks, founder effects) likely explain remaining variation. The yellow juvenile morph occurs in all populations of M. azurea and M. viridis, whereas the red morph occurs only in some populations of M. azurea and at varying frequencies. Yellow and red juveniles did not differ in morph-specific survival, sex ratios, morphology (tail length, head shape and mass) or diet. We discuss our results in relation to several hypotheses relating to maintenance of colour polymorphisms in nature. Although inconclusive, we are reluctant to suggest that colour is non-adaptive, and encourage additional experimental field research on the significance of polymorphism in these taxa.
Keywords: Australia, Biak Island, geographic variation, green python, natural selection, Morelia azurea, Morelia viridis, New Guinea, niche divergence, snake
Introduction
The adaptive significance of animal colour polymorphism has long been of interest to biologists (Darwin 1859; Cott 1940; Huxley 1955). For many taxa, the evolutionary forces that give rise to polymorphisms are well known, whereas in others polymorphisms may simply arise through non-selective processes (e.g. migration, genetic drift and bottlenecks: see Reillo and Wise 1988; Brakefield 1990; King and Lawson 1995). Of equal importance to the development of polymorphisms are the mechanisms that maintain them (Galeotti et al. 2003; Roulin 2004; Gray and McKinnon 2007). The most common mechanisms include: (1) frequency-dependant selection, where fitness is higher in the rarer colour morph because predators select for more abundant morphs (Allen 1988; Olendorf et al. 2006); (2) disruptive selection, where several different optima are possible within a population, thus selecting for extreme phenotypes that exploit different niches (Endler 1986; Roulin 2004); (3) sexual selection, where preferential mate choice selects for a particular morph (Kingston et al. 2003; Roulin 2004); and (4) neutral selection, where neither colour morph receives a fitness advantage and the population is large enough so that alleles are not eliminated through genetic drift (Galeotti et al. 2003). Although these general processes are well understood, identifying their role in maintaining specific cases of polymorphism remains challenging, particularly in vertebrates (Hoffman and Blouin 2000; Wilson et al. 2007).
Arguably one of the most outstanding vertebrate colour polymorphisms is that exhibited by the tropical arboreal green pythons, Morelia azurea and Morelia viridis (Natusch et al. 2020). Green pythons are medium-sized (<2 m) ambush predators widely distributed in mainland New Guinea, several offshore islands, and a small area of Australia (O’Shea 1996; Natusch and Natusch 2011). Juvenile green pythons are born either banana yellow or brick red before changing colour to green; both morphs can occur in a single clutch (Maxwell 2005; Natusch and Lyons 2012). Colouration in this species appears to be adaptive: ontogenetic colour changes coincide with changes in head shape, diet, and habitat use, and the yellow juvenile and green adult morphs have been shown to be optimally cryptic to avian predators in their preferred habitats (Wilson et al. 2006, 2007; Natusch and Lyons 2012).
The first step towards understanding the maintenance of juvenile polymorphism in green pythons is to test the null hypothesis that colour variation has no major biological correlates. For example, if polymorphism is maintained by frequency-dependent selection we might expect one morph to be less abundant and have higher survivorship than the other. Alternatively, if disruptive selection maintains polymorphism, we might expect morphs to utilise different habitats, select different prey types, and display subtle differences in morphology (Shine et al. 1998). Here, we examine morph frequencies of green pythons from throughout their range and test for divergence in ecological traits between juvenile colour morphs that may explain how polymorphism is maintained in these taxa.
Materials and methods
Polymorphism frequency
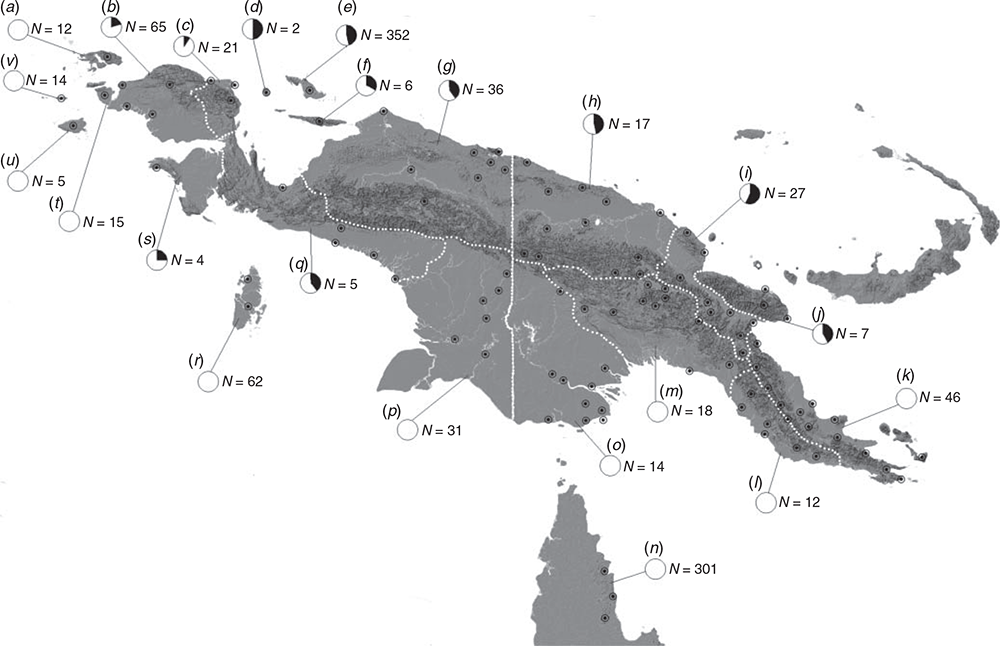
We examined 1090 juvenile and adult green pythons from throughout their range and recorded juvenile colouration as either red or yellow. Records came from snakes collected in New Guinea by local villagers (see Lyons and Natusch 2011; Natusch and Lyons 2014) or through fieldwork in Australia (see Natusch and Natusch 2011). We also supplemented our sample with green pythons from the collections of the following specimen repositories: American Museum of Natural History, New York (AMNH); Australian Museum, Sydney (AMS); Australian National Wildlife Collection, Canberra (ANWC); British Museum of Natural History, London (BMNH); Bernice P. Bishop Museum, Honolulu (BPBM); California Academy of Sciences, San Francisco (CAS); Louisiana Museum of Natural History, Baton Rouge (LSUMZ); National Museum of Natural History, Paris (MNHN); Museum Victoria, Melbourne (MV); Museum Zoologicum Bogoriense, Bogor (MZB); Queensland Museum, Brisbane (QM); National Museum of Natural History, Washington DC (USNM); and the University of Papua, Manokwari (Natusch et al. 2020). We determined the original juvenile colour of adult green specimens using the residual juvenile pattern present on the snake’s dorsal surface. The pattern of red juveniles consists of prominent triangular saddles, whereas the pattern of yellow juveniles comprises thin lines or rosettes alternating either side of the vertebral ridge (Natusch et al. 2020). We confirmed the retention of juvenile patterning into adulthood by following captive specimens as they grew (Natusch, unpubl. data 2021). Although this was straightforward for most specimens, we excluded several older and heavily scarred individuals, and some museum specimens in poor state of preservation, because of difficulties in accurately determining juvenile colouration. Nevertheless, this is unlikely to bias our results because such difficulties are not colour dependant. Finally, we examined colour morph frequencies in seven wild clutches of eggs from New Guinean green pythons. We divided specimens of green pythons a priori into regional samples based on known biogeographical barriers to gene flow and known genetic structure (see Beehler 2007; Deiner et al. 2011; Natusch et al. 2020). Regional groupings are depicted in Fig. 1.
Ecological and morphological comparisons
To test for ecological and morphological divergence between green python colour morphs we examined a sample of 120 red and 154 yellow juvenile M. azurea azurea from Biak Island in northern Papua. We analysed ecological differences between colour morphs in a single taxon only to reduce the potential effect of interpopulation trait divergence, and because sample sizes for both morphs were sufficiently large for this population only. We measured all snakes captured to determine: (1) snout-to-vent length (SVL), by measuring the snake against a steel ruler to the nearest 0.5 cm, (2) tail length, from the cloaca to the tip of the tail, (3) head length, from the tip of the snout along the dorsal midline to the base of the skull, (4) head width, at the widest point of the skull, (5) mass, to the nearest 1 g using Pesola spring scales (Pesola AG, Baar, Switzerland), and (6) sex, by inserting a blunt probe into the cloacal bursae and recording depth. We did not sex exceptionally small juveniles to avoid injury to the snakes. Prey types captured by red and yellow juveniles were determined by microscopy of snake scats.
Statistical analysis
If colour morphs differ in survival, we would expect morph frequency to vary between size classes. However, because green pythons change colour with age, differential survival between morphs is relevant only for juvenile snakes. We thus divided juveniles into two size classes (30–49 cm and 50–70 cm) and used the proportion of the two colour morphs in each class as a proxy for morph-specific survival. Although a small number of the larger juveniles had begun to change colour, each retained the majority of their juvenile colouration, making them useful for our analysis (Natusch and Lyons 2012). We used contingency table analysis to test for differences in morph survival between size classes, differences in morph frequencies between geographic regions, and for morph divergence from an equal sex ratio. Because morphological traits in snakes vary with increasing body size, we used analysis of covariance (ANCOVA) to examine morphological divergence between the two colour morphs. We used tail length, body mass, head length and head width as dependent variables, SVL as the covariate, and colour as the factor. We log-transformed all data to meet assumptions of normality and homogeneity of variance. All analyses were conducted in JMP Pro 14 (SAS Institute, Cary, NC).
Results
Polymorphism frequency
The relative frequency of red and yellow juvenile morphs varied significantly between taxa and between populations within taxa (Fig. 1). The red juvenile morph was never recorded in populations of M. viridis. The red morph was also notably absent from island populations in the Raja Ampat Archipelago (Fig. 1). Relative numbers of the red and yellow morphs in most other populations were approximately equal (Fig. 1). However, populations from the Vogelkop and Bomberai Peninsula were skewed towards a greater proportion of yellow juveniles (although sample sizes for the Bomberai population are small) (Fig. 1). Geographic variation in the frequency of the two morphs was corroborated further via examination of juveniles from seven wild clutches of eggs (Table 1).

|
Ecological and morphological comparisons
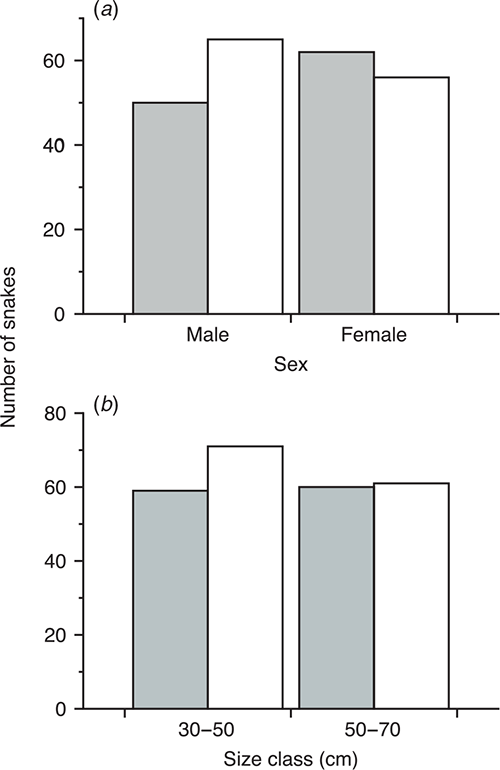
The relative numbers of each colour morph from Biak Island differed slightly between the sexes, with more yellow males and more red females, but this difference was not great enough to be statistically significant (χ2 = 1.92, d.f. = 1, P = 0.16) (Fig. 2). Similarly, survival was not morph specific (χ2 = 0.42, d.f. = 1, P = 0.52) (Fig. 2). Red and yellow morphs did not differ significantly in any of the traits that we measured; ANCOVA revealed P > 0.05 for relative tail length, head length, head width and body mass. Red and yellow juvenile morphs did not differ in food habits; all preyed almost exclusively upon scincid lizards. In total, 41 juvenile scats contained the scales, feet, tail remnants and undigested jawbones of small, mostly unidentified, scincid lizards. Thirteen prey items were representatives of the scincid genus Emoia (very common lizards on Biak Island); however, it was not possible to identify these to species level. The only non-reptilian remains were recovered from a 69 cm SVL yellow juvenile that had eaten a small, unidentified bird.
|
Discussion
Geographic variation in morph frequencies
Our results reveal strong geographic variation in the occurrence and frequency of juvenile colour polymorphism in green pythons. The most notable divergence is between the two species, M. azurea and M. viridis (two versus one juvenile morph, respectively). Whether the red juvenile morph was lost in M. viridis after the two species diverged, or whether it evolved independently within M. azurea is unclear. However, the extensive geographic range occupied by M. viridis supports the latter interpretation. The apparent absence of the red morph in M. azurea from the islands of Raja Ampat is also noteworthy because of their proximity to the Vogelkop Peninsula where the red juvenile morph is present (Fig. 1). The islands within the Raja Ampat archipelago were most recently connected to mainland New Guinea approximately 8000 years ago (Voris 2000). The red morph may have been lost due to inevitably small population sizes and random genetic processes taking place on these islands as sea levels rose and fell (e.g. genetic drift, founder effects, bottlenecks: see Roulin 2004). In agreement, the yellow morph was the more common morph on the neighbouring west Vogelkop Peninsula, potentially facilitating the loss of the red morph from these islands (Fig. 1).
We also corroborated colour morph frequencies from wild hatchlings (Table 1). The two wild clutches from M. viridis consisted entirely of yellow individuals and captive breeding records for green pythons from the Aru Islands, Australia, and southern New Guinea have similarly never hatched red juveniles (M. Cermak and V. Odinchenko, pers. comm.). Despite the genetic basis of juvenile colour in green pythons being poorly understood, our limited data suggest that, like other polymorphic snake species, parental colouration may act on offspring colour through simple Mendelian inheritance (Zweifel 1981; King 2003). However, captive breeding efforts to date have been unable to unequivocally prove either colour to be recessive. Further study is needed to clarify this.
Maintenance of polymorphism
Our data allow us to make some preliminary conclusions about the mechanisms maintaining juvenile colour polymorphism in green pythons. Non-random mating is unlikely to maintain juvenile polymorphism in this species because both juvenile morphs become monomorphic before reaching sexual maturity (Natusch and Lyons 2012). Similarly, frequency-dependant selection is unlikely to maintain polymorphism because our results suggest that the two morphs are found in similar frequencies in most populations in which they occur (Fig. 1) and survival, sex and other ecological traits are not colour dependant (Fig. 2). Juvenile colour polymorphism in green pythons could be explained via disruptive selection, where we might expect differences in ecological traits between morphs utilising different niches. In agreement, yellow juvenile and green adult morphs are known to partition their niche (Wilson et al. 2007; Natusch and Lyons 2012). However, contrary to other studies of colour in snakes (Brodie 1992; Shine et al. 1998) we found no association between ecological attributes and colour. We have located both colour morphs hunting near the ground during the day in the same places (Natusch, pers. obs.). In these microhabitats both morphs are equally cryptic, and background matching tests found that both morphs preferred dark rather than light backgrounds (Garrett and Smith 1994; Wilson et al. 2007). That being said, such niche divergences may be subtle, and may be mediated in other ways (e.g. thermal advantages or constraints) untested in our study.
In summary, despite our inconclusive results we are reluctant to suggest that juvenile polymorphism in green pythons is non-adaptive (i.e. neutral) because (1) intuition suggests that the colouration of highly cryptic ambush predators would affect their fitness, and (2) because many species of unrelated snakes exhibit similar life-history traits (e.g. heavy bodied, colour polymorphic, ambush predators: Garrett and Smith 1994; Johnston 1996; Shine et al. 1998), suggesting an adaptive role for colour. Our study offers preliminary insights into potential avenues of future research. Experimental studies could use model snakes of different colours to understand habitat and morph-specific predation frequency. Likewise, captive breeding studies are needed to resolve the mode of genetic inheritance and clarify the underlying mechanisms affecting morph frequencies in this species.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was funded in part by the Australian Geographic Society, the Linnean Society of New South Wales, and SeaSwift Far North Queensland.
Acknowledgements
We thank B. Koutsamanis, F. Maltzahn, M. McCloskey, L. McIntyre, R. McIntyre, D. Natusch, B. Osborne and S. Spence for their help in the field. N. Hobson, T. Jaffer, J. and J. Mulholland, S. Templeton, Y. Lukin, and V. Odinchenko provided generous logistic support. We also thank the numerous museum curatorial staff who allowed us to examine specimens in their care. Research was conducted under permits WISP04847807 and WITK04847707. All work was conducted in accordance with University of New South Wales Animal Ethics Protocol (Permit nos 07/60A and 10/90A). Thanks to two anonymous reviewers for comments that improved an earlier draft of this manuscript.
References
Allen, J. A. (1988). Frequency-dependent selection by predators. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 319, 485–503.| 2905488PubMed |
Beehler, B. M. (2007). Papuan terrestrial biogeography, with special reference to birds. In ‘The Ecology of Papua’. (Eds A. J. Marshall, and B. M. Beehler.) pp. 196–206. (Periplus Editions: Singapore.)
Brakefield, P. M. (1990). Genetic drift and patterns of diversity among colour-polymorphic populations of the homopteran Philaenus spumarius in an island archipelago. Biological Journal of the Linnean Society 39, 219–237.
| Genetic drift and patterns of diversity among colour-polymorphic populations of the homopteran Philaenus spumarius in an island archipelago.Crossref | GoogleScholarGoogle Scholar |
Brodie, E. D. (1992). Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution 46, 1284–1298.
| Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides.Crossref | GoogleScholarGoogle Scholar | 28568995PubMed |
Cott, H. B. (1940). ‘Adaptive Coloration in Animals.’ (Methuen and Co: United Kingdom.)
Darwin, C. (1859). ‘On the Origin of Species by Means of Natural Selection.’ (Murray: United Kingdom.)
Deiner, K., Lemmon, A. R., Mack, A. L., Fleischer, R. C., and Dumbacher, J. P. (2011). A passerine bird’s evolution corroborates the geologic history of the island of New Guinea. PLoS One 6, e19479.
| A passerine bird’s evolution corroborates the geologic history of the island of New Guinea.Crossref | GoogleScholarGoogle Scholar | 21573115PubMed |
Endler, J. A. (1986). ‘Natural Selection in the Wild.’ (Princeton University Press: United States of America.)
Galeotti, P., Rubolini, D., Dunn, P. O., and Fasola, M. (2003). Colour polymorphism in birds: causes and functions. Journal of Evolutionary Biology 16, 635–646.
| Colour polymorphism in birds: causes and functions.Crossref | GoogleScholarGoogle Scholar | 14632227PubMed |
Garrett, C. M., and Smith, B. E. (1994). Perch color preference in juvenile green tree pythons, Chondropython viridis. Zoo Biology 13, 45–50.
| Perch color preference in juvenile green tree pythons, Chondropython viridis.Crossref | GoogleScholarGoogle Scholar |
Gray, S. M., and McKinnon, J. S. (2007). Linking color polymorphism maintenance and speciation. Trends in Ecology & Evolution 22, 71–79.
| Linking color polymorphism maintenance and speciation.Crossref | GoogleScholarGoogle Scholar |
Hoffman, E. A., and Blouin, M. S. (2000). A review of colour and pattern polymorphisms in anurans. Biological Journal of the Linnean Society 70, 633–665.
| A review of colour and pattern polymorphisms in anurans.Crossref | GoogleScholarGoogle Scholar |
Huxley, J. (1955). Morphism in birds. Acta International Congress in Ornithology 11, 309–328.
Johnston, G. R. (1996). Genetic and seasonal variation in body colour of the Australian death adder, Acanthophis antarcticus (Squamata: Elapidae). Journal of Zoology 239, 187–196.
| Genetic and seasonal variation in body colour of the Australian death adder, Acanthophis antarcticus (Squamata: Elapidae).Crossref | GoogleScholarGoogle Scholar |
King, R. B. (2003). Mendelian inheritance of melanism in the garter snake Thamnophis sirtalis. Herpetologica 59, 484–489.
| Mendelian inheritance of melanism in the garter snake Thamnophis sirtalis.Crossref | GoogleScholarGoogle Scholar |
King, R. B., and Lawson, R. (1995). Color pattern variation in Lake Erie water snakes: the role of gene flow. Evolution 49, 885–896.
| Color pattern variation in Lake Erie water snakes: the role of gene flow.Crossref | GoogleScholarGoogle Scholar | 28564875PubMed |
Kingston, J. J., Rosenthal, G. G., and Ryan, M. J. (2003). The role of sexual selection in maintaining a colour polymorphism in the pygmy swordtail, Xiphophorus pygmaeus. Animal Behaviour 65, 735–743.
| The role of sexual selection in maintaining a colour polymorphism in the pygmy swordtail, Xiphophorus pygmaeus.Crossref | GoogleScholarGoogle Scholar |
Lyons, J. A., and Natusch, D. J. D. (2011). Wildlife laundering through breeding farms: illegal harvest, population declines and a means of regulating the trade of green pythons (Morelia viridis) from Indonesia. Biological Conservation 144, 3073–3081.
| Wildlife laundering through breeding farms: illegal harvest, population declines and a means of regulating the trade of green pythons (Morelia viridis) from Indonesia.Crossref | GoogleScholarGoogle Scholar |
Maxwell, G. (2005). ‘The More Complete Chondro.’ (Eco Publishing: United States of America.)
Natusch, D. J. D., and Lyons, J. A. (2012). Relationships between ontogenetic changes in prey selection, trophic structure, sexual maturity and colour in an Australasian python (Morelia viridis). Biological Journal of the Linnean Society 107, 269–276.
| Relationships between ontogenetic changes in prey selection, trophic structure, sexual maturity and colour in an Australasian python (Morelia viridis).Crossref | GoogleScholarGoogle Scholar |
Natusch, D. J. D., and Lyons, J. A. (2014). Geographic and sexual variations in body size, morphology and diet among five populations of green pythons (Morelia viridis). Journal of Herpetology 48, 317–323.
| Geographic and sexual variations in body size, morphology and diet among five populations of green pythons (Morelia viridis).Crossref | GoogleScholarGoogle Scholar |
Natusch, D. J. D., and Natusch, D. F. S. (2011). Distribution, abundance and demography of green pythons (Morelia viridis) in Cape York Peninsula, Australia. Australian Journal of Zoology 59, 145–155.
| Distribution, abundance and demography of green pythons (Morelia viridis) in Cape York Peninsula, Australia.Crossref | GoogleScholarGoogle Scholar |
Natusch, D. J. D., Esquerré, D., Lyons, J. A., Hamidy, A., Lemmon, A. R., Moriarty-Lemmon, E., Riyanto, A., Scott Keogh, J., and Donnellan, S. (2020). Species delimitation and systematics of the green pythons (Morelia viridis complex) of Melanesia and Australia. Molecular Phylogenetics and Evolution 142, 106640.
| Species delimitation and systematics of the green pythons (Morelia viridis complex) of Melanesia and Australia.Crossref | GoogleScholarGoogle Scholar |
O’Shea, M. (1996). ‘A Guide to the Snakes of Papua New Guinea.’ (Independent Publishing Group: Papua New Guinea.)
Olendorf, R., Rodd, F. H., Punzalan, D., Houde, A. E., Hurt, C., Reznick, D. N., and Hughes, K. A. (2006). Frequency-dependent survival in natural guppy populations. Nature 441, 633–636.
| Frequency-dependent survival in natural guppy populations.Crossref | GoogleScholarGoogle Scholar | 16738659PubMed |
Reillo, P. R., and Wise, D. H. (1988). An experimental evaluation of selection on color morphs of the polymorphic spider Enoplognatha ovata (Araneae: Theridiidae). Evolution 42, 1172–1189.
| An experimental evaluation of selection on color morphs of the polymorphic spider Enoplognatha ovata (Araneae: Theridiidae).Crossref | GoogleScholarGoogle Scholar | 28581089PubMed |
Roulin, A. (2004). The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biological Reviews of the Cambridge Philosophical Society 79, 815–848.
| The evolution, maintenance and adaptive function of genetic colour polymorphism in birds.Crossref | GoogleScholarGoogle Scholar | 15682872PubMed |
Shine, R., Ambariyanto, , Harlow, P. S., and Mumpuni, (1998). Ecological divergence among sympatric colour morphs in blood pythons, Python brogersmai. Oecologia 116, 113–119.
| Ecological divergence among sympatric colour morphs in blood pythons, Python brogersmai.Crossref | GoogleScholarGoogle Scholar | 28308515PubMed |
Voris, H. K. (2000). Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. Journal of Biogeography 27, 1153–1167.
| Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations.Crossref | GoogleScholarGoogle Scholar |
Wilson, D., Heinsohn, R., and Legge, S. (2006). Age and sex-related differences in the spatial ecology of a dichromatic tropical python (Morelia viridis). Austral Ecology 31, 577–587.
| Age and sex-related differences in the spatial ecology of a dichromatic tropical python (Morelia viridis).Crossref | GoogleScholarGoogle Scholar |
Wilson, D., Heinsohn, R., and Endler, J. (2007). The adaptive significance of ontogenetic colour change in a tropical python. Biology Letters 3, 40–43.
| The adaptive significance of ontogenetic colour change in a tropical python.Crossref | GoogleScholarGoogle Scholar | 17443961PubMed |
Zweifel, R. G. (1981). Genetics of color pattern polymorphism in the California kingsnake. The Journal of Heredity 72, 238–244.
| Genetics of color pattern polymorphism in the California kingsnake.Crossref | GoogleScholarGoogle Scholar |