Comparative echolocation and foraging ecology of horseshoe bats (Rhinolophidae) and Old World leaf-nosed bats (Hipposideridae)1
Chris R. Pavey
CSIRO Land and Water, Private Mail Bag 44, Winnellie, NT 0822, Australia. Email: chris.pavey@csiro.au
Australian Journal of Zoology - https://doi.org/10.1071/ZO20047
Submitted: 11 June 2020 Accepted: 14 December 2020 Published online: 22 January 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Horseshoe (Rhinolphidae) and Old World leaf-nosed (Hipposideridae) bats are high duty cycle (HDC) echolocators sharing a suite of adaptations including long duration signals relative to their signal periods, peak energy concentrated in a narrow spectral band dominated by a constant frequency (CF) component, ‘auditory fovea’ (over-representation and sharp tuning of neurons responsible for frequencies at or around the CF) and ability to compensate for Doppler shifts in echoes. HDC bats separate signals from returning echoes in the frequency domain. Rhinolophids are more specialised neurobiologically than hipposiderids, producing longer duration signals at higher duty cycles, and have narrowly tuned auditory fovea and almost full Doppler shift compensation. Here, I examine whether these differences have produced ecological divergence between the families by testing predictions of differences in prey perception, prey capture behaviour, foraging habitat and diet. I found no discernible differences in these variables between the two families. Rhinolophids and hipposiderids both forage close to vegetation, capture prey by aerial hawking and gleaning from surfaces, and consume mostly flying insects with spiders and terrestrial, flightless arthropods taken occasionally. The data presented here show that the two families are similar in foraging ecology despite differences in echolocation and audition.
Keywords: aerial hawking, auditory fovea, constant frequency, Doppler shift compensation, duty cycle, echolocation, gleaning, insectivore.
Introduction
Echolocation is a sensory system involving the tight coupling of signal production and echo reception that is used by species in four orders of mammals and two orders of birds. Echolocating animals orient by collecting information from the difference between signal pulses and returning echoes to form an acoustic image of their environment (Fenton et al. 2012). While all echolocators use the system for orientation, echolocation is also used for prey detection by bats and odontocete whales (Fenton et al. 2012).
Among bats (Chiroptera) several distinct approaches to echolocation have evolved, with each approach differing in the structure of the echolocation calls and in how the calls are separated from echoes. Each approach has resulted in the evolution of a unique set of auditory adaptations that enable signals to be received and processed by the brain (Neuweiler 1990; Fenton et al. 2012). Most echolocating bats avoid forward masking, the process by which louder outgoing signals mask or reduce the sensitivity of the animal to the weaker returning echoes, by separating pulse and echo in the time domain (Fenton et al. 1995). An alternative strategy is found in high duty cycle (HDC) echolocators that separate pulse and echo in the frequency domain (Schuller 1974). The duty cycle (DC) is the percentage of time that a bat is producing sound (Fawcett et al. 2015). Duty cycles of HDC bats range between 25 and 70%, whereas those of other echolocating bats typically range between 5 and 20% (Fenton et al. 1995).
Fenton et al. (2012) state that HDC echolocators share a unique combination of four adaptations. First, they emit echolocation signals with long durations relative to their signal periods. Second, the peak energy of each signal is concentrated in a narrow spectral band dominated by a constant frequency (CF) component. These signals typically consist of two or more harmonics each composed of a long constant frequency component terminated by a brief frequency modulation (FM) (Neuweiler 2003). The most intense harmonic is the second harmonic (CF2). The resting frequency of CF2 is very stable and shows little deviation from the mean value. Third, they possess an ‘auditory fovea’, a spatial over-representation and sharp tuning of the neurons responsible for frequencies at or around the second harmonic of the CF call component, both on the basilar membrane within the cochlear of the inner ear and in the neurons of the centres of the ascending auditory pathway of the brain. Narrow tuning is achieved via several pronounced anatomical adaptations within the fovea, including structural specialisation of the basilar and tectorial membrane of the inner ear (Neuweiler 2003). Last, they show Doppler shift compensation (DSC), an adaptation to overcome Doppler shifts, the change in sound frequency associated with the movement of the sound source (nostril of the bat) relative to the receiver (ears of the bat). Typically, Doppler shifts result in the frequency of returning echoes being higher than the narrow frequency range to which the auditory fovea is tuned, therefore, DSC involves lowering the frequency of the next outgoing signal to compensate for the Doppler-shifted increase in frequency of the echo from the previous signal resulting from the bat’s flight (Neuweiler 2000, 2003). DSC is a precise behavioural mechanism that operates at exceptional speed (Grinnell 1989).
HDC echolocation, as defined above, is known from ~200 species of echolocating bats. All except one species are in three families in the suborder Yinpterochiroptera: Rhinolophidae (horseshoe bats, 103 species in a single genus), Hipposideridae (leaf-nosed bats, 88 species in seven genera) and Rhinonycteridae (trident-nosed bats, nine species in four genera) (Mammal Diversity Database as at May 2020: American Society of Mammalogists 2020). The only other known HDC echolocator, Pteronotus parnellii (Mormoopidae) is within the suborder Yangochiroptera.
The features of the echolocation system of HDC bats are clearly interconnected and appear to have evolved in response to the same selective pressures, thus representing a complex adaptation (sensu Walter 2003). The auditory fovea could not function effectively without the ability to compensate for Doppler shifts. In turn, these auditory adaptations enable bats to call at high duty cycles because they can cope with temporal overlap between signals and echoes. Although HDC echolocators share the four characters outlined above, there are differences in these characters among the families of HDC echolocators. The functioning of the HDC echolocation system has been examined in most detail in the Rhinolophidae where a close link has been established between the use of HDC echolocation and foraging within dense vegetation, which has high levels of acoustic clutter (i.e. echoes from background objects that interfere with the perception of echoes from the target). This work indicates that the rhinolophid echolocation system is adapted for coping with clutter while enabling horseshoe bats to detect and capture flying insects (e.g. Neuweiler et al. 1987). Because of this understanding the rhinolophid approach to echolocation is described as a flutter detection and clutter rejection system (Schnitzler and Denzinger 2011).
Multiple studies suggest that rhinolophids are the most specialised HDC echolocators in terms of neurobiological characters, with the hipposiderids and rhinonycterids the least specialised and Pteronotus parnellii in between (Fenton et al. 2012, and references therein). Jacobs et al. (2007) expanded this perspective and questioned whether the neurobiological differences meant that rhinolophid and hipposiderid bats had diverged in foraging ecology and, therefore, should not be regarded as belonging to the same foraging guild. The purpose of this review is to examine the proposition of Jacobs et al. (2007) that the differences in neurobiological adaptations among the two families has resulted in differences in foraging ecology, i.e. to address whether Rhinolophidae and Hipposideridae belong to the same foraging guild. I have excluded Rhinonycteridae from this analysis. Formerly included within the Hipposideridae, Rhinonycteridae was recently separated into its own family (Foley et al. 2015) and little information is available for most species. The Rhinolophidae and Hipposideridae both possess an ornate noseleaf and have broad mobile ears (Hall 1989a, 1989b). The two families broadly overlap in wing morphology, having wing designs suited for slow and manoeuvrable flight (Norberg and Rayner 1987).
The review first provides a summary of HDC echolocation in rhinolophid bats focussing on explanations of how neurobiological adaptations influence important aspects of foraging, including prey perception, prey capture behaviour, habitat and diet. Next, I summarise differences in echolocation signals and neurobiological structures between rhinolophids and hipposiderids and make specific, testable predictions of how these differences could influence foraging ecology. I then test the predictions by summarising published information on prey perception, prey capture behaviour, habitat and diet of the two families.
The HDC echolocation system in rhinolophid bats
Rhinolophid bats frequently forage around vegetation (e.g. Neuweiler et al. 1987; Jones and Rayner 1989; Pavey 1998a). While foraging around vegetation, rhinolophid bats are able to reject acoustic clutter and detect the wingbeats of fluttering insects (Schnitzler et al. 1985; Neuweiler et al. 1987; Neuweiler 1990). The pure tone echoes from the calls of these bats are highly noise resistant, being able to maintain their structure despite the movement of foliage (Neuweiler 1989, 1990). Rübsamen et al. (1988) proposed that the requirement for clutter rejection led to bats, which already had pure tone signals, developing narrow auditory foveae. A pure tone signal is more resistant than any other type of signal provided the receiver is tuned to the frequency of the signal. The fovea provides such auditory tuning. The use of such a narrow receiving filter requires a long duration signal. Therefore, a byproduct of the evolution of a narrow auditory fovea was the use of long duration pure tone signals. Thus the narrowly tuned auditory filter can be seen as an evolutionary adaptation for clutter resistance (Neuweiler 1989).
The Doppler-shifted long pure tone signals of rhinolophids allow the detection of glints from fluttering insects, thus being effective in the detection of fluttering targets but not targets in other situations. Rhinolophids are able to use both positive and negative DSC and therefore are able to deal with increases and decreases in the frequency of returning echoes (Metzner et al. 2002). The echoes carry distinct ‘acoustical glints’ from fluttering targets such as flying insects. The glints are brief frequency and amplitude modulations superimposed on the CF component of the echo by reflections from the moving wings of an insect. The glints are generated by the rhythmic motion of the insect’s wings relative to the direction of sound propagation from the bat (Schnitzler 1987; Kober and Schnitzler 1990). Amplitude glints are produced by changes in the reflective area of the fluttering insect; when the insect’s wings are perpendicular to the direction of sound propagation it presents a larger reflective surface compared with the body of the insect than when the wings are horizontal. Frequency glints are spectral broadenings in the CF component of the echo produced by the movement of the insect’s wings towards or away from the bat. Frequency glints provide information on the direction of travel of the insect and are superimposed on the overall CF echo from the insect’s body (Fenton et al. 2012, and references therein). In comparison to the high level of detail obtained from an insect moving its wings, the Doppler-shifted echoes do not carry glints from insects that do not move their wings.
The information on wingbeats of insects available to rhinolophid bats from amplitude and frequency glints in returning Doppler-shifted echoes raises the possibility that the bats can recognise particular types of insects (Schnitzler 1987; von der Emde and Schnitzler 1990; Kober and Schnitzler 1990). Rhinolophid bats in the laboratory are able to classify insects on the basis of their wingbeat frequency and, consequently, may be able to actively select prey in the wild (von der Emde and Menne 1989). A higher duty cycle increases the probability that a glint is contained in an echo coming from fluttering prey and only bats with CF durations >40 ms will frequently receive multiple acoustic glints over several wingbeat cycles of a fluttering insect in a single echo (von der Emde and Schnitzler 1986; Fenton et al. 2012).
The ability of rhinolophids to detect fluttering targets is not confined to areas with high levels of acoustic clutter. However, because flutter detection is advantageous in a highly cluttered environment, rhinolophid bats are at a competitive advantage in areas of dense vegetation. Insects flying around vegetation at night are a rich, underexploited food resource, particularly in the Old World where a small proportion of bat species forage in cluttered settings (Fenton et al. 1995). Therefore, rhinolophid species are expected to favour such foraging habitat.
Comparison of HDC echolocation in rhinolophids and hipposiderids
Here I examine differences in echolocation signals and neurobiological structures between rhinolophids and hipposiderids. I summarise information on signal duration and duty cycle, Doppler shift compensation and the degree of tuning of the auditory fovea.
Signal duration and duty cycle
Data on signal duration and duty cycle were collated for those studies that recorded resting frequency of hand-held bats, to avoid Doppler-shift effects (e.g. Jacobs and Bastian 2018), or search phase signals of flying bats (i.e. feeding buzzes were excluded). Data on signal duration are available for a sample of 25 rhinolophid and 10 hipposiderid species (Fig. 1). No overlap in signal duration occurs between the two families. Rhinolophid species have longer duration calls (range of 20.8 to 53.5 ms) than hipposiderids (range of 5.4 to 12.0 ms) (Fig. 1).

|
Data on duty cycle are available for 24 rhinolophid and 6 hipposiderid species (Fig. 2). The data summarised in the figure disagree with a previous summary (see table 1 of Jones 1999), which showed that the duty cycles of the two families did not overlap and variation within families was not great. After Jones (1999), a wider sample of rhinolophid species has been assessed. Evidence has emerged of overlap in duty cycles across the two families and of intraspecific variation in signal frequency across geographic ranges and depending on the task being undertaken and the habitat (e.g. Pavey et al. 2001a). Intrafamily variation in duty cycles can be noticeable. For example, hand-held individuals of R. macrotis (forearm length, 41.8 mm) and R. lepidus (forearm length, 41.5 mm) that roosted in the same cave had duty cycles of 39.23% and 54.70%, respectively, when measured using identical methods (Shi et al. 2009).

|
In general, duty cycles of rhinolophids are greater than those of hipposiderids. Typical duty cycles of rhinolophid bats in flight are over 50%, whereas those of hipposiderid species are 35% or less (Fig. 2). Fullard et al. (2008) recorded the highest duty cycle for a hipposiderid bat so far. In their study, H. ater recorded flying inside a roost had a duty cycle of 54.2% with an interpulse interval of 4.6 ms and a signal duration of 5.0 ms. In the same study, R. megaphyllus had a duty cycle of 51.7% with an interpulse interval of 55.4 ms and a signal duration of 52.9 ms. In this case the duty cycles of the two species were similar despite the much longer duration signals of R. megaphyllus. Hipposideros ater had a high duty cycle as a result of a shorter interpulse interval and higher pulse reputation rate.
Doppler shift compensation
Accurate measurement of DSC requires experimentation on captive animals under controlled conditions. Therefore, data are available only for a small sample: two rhinolophids, and three hipposiderid species. Both R. ferrumequinum and R. rouxi (Schuller 1980) exhibit close to full compensation (100%) of Doppler-shifts in returning echoes. In comparison, H. speoris and H. bicolor exhibit 55–56% compensation (Habersetzer et al. 1984), whereas H. lankadiva had a higher capacity to compensate at 77% (Pillat and Schmidt 1998).
Auditory fovea and stability of the resting frequency
The degree of tuning of the auditory fovea has been measured with respect to tonotopic organisation of brain centres. Audiograms of rhinolophid and hipposiderid species allow comparison of the auditory fovea. A finely tuned auditory fovea was first demonstrated in R. ferrumequinum (Schuller and Pollak 1979). In this species the auditory fovea is considered to consist of a 1.5 kHz band from 83.0 to 84.5 kHz. A total of 16% (96 neurons) of all neurons had best frequencies within this band (Schuller and Pollak 1979). A similarly very sharply tuned fovea has been shown in R. rouxi (Schuller 1980). By comparison, audiograms of three hipposiderid species are not so narrowly tuned and less sensitive to emitted frequencies. The species assessed have been H. speoris and H. bicolor (Neuweiler et al. 1984) and H. lankadiva (Peters 1987 cited in Foeller and Kössl (2000)). Further, in H. speoris and H. bicolor the neurons that process pure tone frequencies occur in a smaller and more confined part of the inferior colliculus (Neuweiler et al. 1984).
Measurements of otoacoustic emissions of the bat cochlea also provide comparative data on the hearing abilities of bats. Otoacoustic emissions are sound waves generated by the cochlea that can be measured in the outer ear canal. If stimulated with two tones (f1 and f2) the cochlea generates distortion-product otoacoustic emissions (DPOAEs). The threshold curves for these DPOAEs, the so-called distortion-product audiogram, gives a close approximation to the neuronal audiogram for a given species (Vater 1998). Using this approach Foeller and Kössl (2000) demonstrated that H. lankadiva has a broader cochlear fovea than R. rouxi. The DPOAE threshold curves showed the threshold increase to CF2 in H. lankadiva amounted to ~20 dB compared with 40 dB in R. rouxi. Further, H. lankadiva lacked a sharply tuned threshold minimum slightly above CF2 (Foeller and Kössl 2000).
Stability of the resting frequency of calls has been measured in several species of rhinolophid and hipposiderid. Bats at rest do not experience Doppler shift and emit calls at the resting frequency, which can be used as an indirect measure of the auditory fovea (Jacobs et al. 2007). The resting frequency of rhinolophid bats is kept with high accuracy; standard deviation of calls around the resting frequency was 0.20% in R. rouxi (Schuller 1980) and 0.113% in R. ferrumequinum (Zhang et al. 2019). By comparison, resting frequency of hipposiderid species shows more variation. Standard deviation of the resting frequency was 0.50% for H. speoris and 0.75% for H. bicolor (Schuller 1980). However, Zhang et al. (2019) reported a high precision in H. armiger of 0.165%, similar to the results of Schoeppler et al. (2018) for the same species (0.17%).
Pattern of variation in HDC echolocation between rhinolophids and hipposiderids
The data summarised here show differences in signal and auditory characters between the two families. Rhinolophid bats typically call at high duty cycles by giving long duration signals with low pulse repetition rates, have narrowly tuned auditory fovea, and almost full DSC. In contrast, hipposiderid bats typically call at lower duty cycles by producing moderate duration signals with high pulse repetition rates. The auditory fovea of hipposiderids is more broadly tuned than that of rhinolophids and they perform less well at DSC.
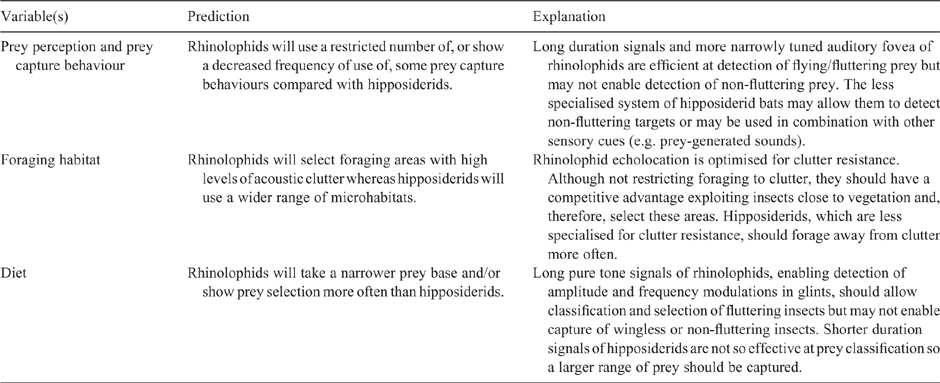
Prediction of ecological consequences of differences in HDC echolocation
The evidence presented in the previous section indicates that the HDC echolocation system of rhinolophids is adapted to enable them to exploit insects flying within areas of dense vegetation, a resource that is underexploited. Long duration pure tone signals produced at high duty cycles and a very narrowly tuned auditory fovea facilitated by DSC allow these bats to capture fluttering insects while overcoming environmental clutter. Following the logic for the functioning of the specialised HDC system in rhinolophid bats, the shorter CF signals, more broadly tuned auditory filter, individual variation in CF2 frequency and only partial compensation for Doppler-shifts demonstrate that hipposiderids are not restricted to focus the CF2 echo in a very narrowly tuned auditory fovea (Foeller and Kössl 2000). As a result, if the interpretation of the consequences for foraging of HDC echolocation in rhinolophids is correct, hipposiderids should not be restricted to hunting fluttering insects in dense vegetation. Based this understanding, predictions of expected differences in aspects of foraging ecology between the two families are outlined in Table 1.
|
Hipposiderids may have an entirely unique approach to foraging. Recently, Zhang et al. (2019) suggested that the broader auditory fovea and DSC of hipposiderids could provide higher tolerance to changes in Doppler shifts. This, in turn, may enable them to more effectively capture fast evasive prey in relatively open habitats without needing to significantly adjust their vocalisations to overcome Doppler shift changes in echoes (Zhang et al. 2019).
Comparison of prey capture behaviour, foraging habitat and diet in rhinolophids and hipposiderids
Most research on HDC echolocators has occurred in controlled environments, including the laboratory and flight tents/rooms (e.g. Fawcett et al. 2015; Schoeppler et al. 2018; Zhang et al. 2019). Field-based research has been comparatively limited, with most data coming from studies of bat assemblages (e.g. Aldridge and Rautenbach 1987; Rakotoarivelo et al. 2007). Relatively few single-species studies have been carried out on rhinolophid and hipposiderid bats foraging in the wild. However, sufficient information is available to provide a preliminary assessment of the predictions given in Table 1.
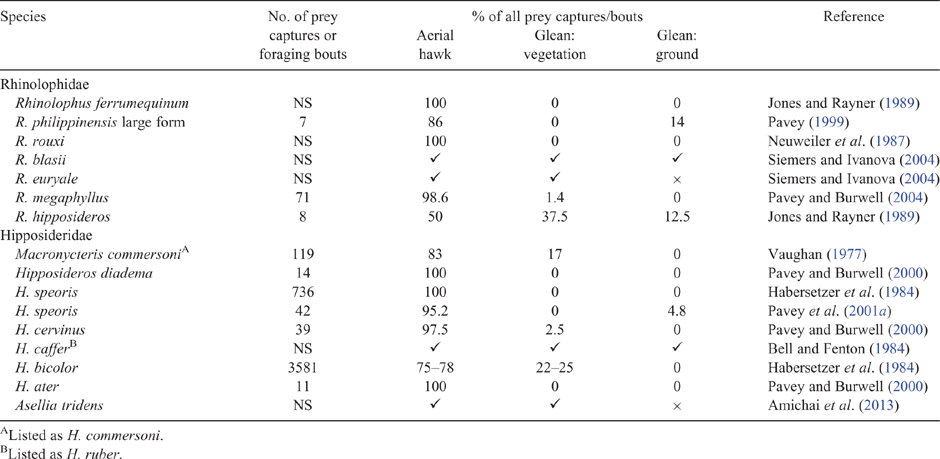
Prey perception
The sensory cues involved in prey capture have been assessed for three species each of Rhinolophidae and Hipposideridae (Table 2). Observations were carried out in the laboratory (Link et al. 1986), a field tent (Siemers and Ivanova 2004) or on free-flying bats (Bell and Fenton 1984). Each species was able to detect flying and fluttering insects; however, only H. bicolor captured walking insects (Table 2). Cockroach nymphs were gleaned from the ground by H. bicolor during 24 of 33 trials (Link et al. 1986). These observations support the prediction that hipposiderid bats can use a wider range of sensory cues than rhinolophid bats, including the capture of non-flying prey.

|
Prey capture behaviour
Quantified observations of prey capture behaviour in wild bats are available for seven species of Rhinolophus and eight hipposiderid species (Table 3). The majority of studies have used radio-telemetry as the method to track bats; however, light tagging and repeated observation of known individuals at foraging sites have also been used.
Rhinolophids and hipposiderids both forage using continuous flight and/or perch hunting with continuous flight being the dominant behaviour of each species, except for the two largest hipposiderid species (forearm length >78 mm). Rhinolophids and hipposiderids captured prey by aerial hawking or by gleaning from surfaces, including the ground (Table 3). All species, except for Asellia tridens, captured prey mostly by aerial hawking. Asellia tridens hunted mostly by gleaning from vegetation with occasional aerial hawking (Amichai et al. 2013). Four species from each family captured insects by gleaning from vegetation. Three rhinolophid species and two hipposiderid species captured prey from the ground. In addition, ground gleaning has been observed or inferred from dietary data for R. ferrumequinum (Ransome 1990; Ahmim and Moali 2013), R. euryale (Ahmim and Moali 2013) and R. blasii (Ahmim and Moali 2013). No other method of prey capture has been recorded for these families.
In summary, the available data on prey capture behaviour provide no support for the prediction that hipposiderid bats use a larger range of behaviours than rhinolophids. Although the experimental data presented in Table 2 indicated that only H. bicolor could capture walking prey, species of Rhinolophus are clearly able to glean prey from the ground.
Foraging habitat
Research on foraging by HDC bats in the wild enables a comparison of foraging habitat on the basis of the amount of clutter within foraging habitat (Table 4). For those studies where foraging areas were based on microhabitat use, as defined by Aldridge and Rautenbach (1987), areas within 2 m of vegetation or water surfaces were considered to be high in clutter. These included the following microhabitats of Aldridge and Rautenbach (1987); Zone 2, within 0.5 m of water surface; Zone 4, within stands of vegetation but >0.5 m from foliage; Zone 5, within stands of vegetation but ≤0.5 m from vegetation; Zone 6, surfaces of foliage; and Zone 7, within foliage. The classification of ‘open space’ used by Pavey and Burwell (2000) referred to foraging areas within stands of vegetation that were >0.5 m but <2.0 m from vegetation; therefore, such areas are classified as being high in clutter for the purposes of the current assessment.
Of a sample of seven species of Rhinolophus, each species favoured foraging areas that were classified as having high levels of clutter (Table 4). Only three of these species were observed foraging in areas classified as having low levels of clutter. Rhinolophus ferrumequinum showed seasonal variation in use of clutter, avoiding low clutter foraging areas in spring but using them frequently in autumn (Jones and Morton 1992) or summer/autumn (Bontadina et al. 1995).
The same pattern of foraging in areas with high levels of clutter is shown in studies that do not quantify habitat use and in studies that measure activity using bat detectors. Cluttered areas are not necessarily restricted to stands of continuous vegetation, with some Rhinolophus species exploiting edge habitat such as hedgerows, edges of stands of forest and internal forest edges (e.g. Bontadina et al. 2002; Law and Chidel 2002; Russo et al. 2002, 2005; Goiti et al. 2008; Jiang et al. 2008; Lee et al. 2012, 2020; Law et al. 2020).
As with rhinolophid bats, each species in the sample of hipposiderid bats favoured foraging areas that were classified as having high levels of clutter (Table 4). Similar to Rhinolophus, three species were also observed foraging in areas classified as having low levels of clutter.
The preference for areas with high levels of clutter by hipposiderid species is demonstrated by assessments of habitat preference. For example, Hipposideros aff. ruber foraging in a mosaic of agricultural land and remnant forest in Ghana, West Africa, selected seminatural habitats and wooded savannah while avoiding cocoa farms and grass savannah (Nkrumah et al. 2016). The preference for high levels of clutter is also shown by the large species of Macronycteris and Hipposideros that forage solely or mostly by perch hunting (e.g. Pavey 1998b; Razafimanahaka et al. 2016). Macronycteris commersoni foraging in a forest–agricultural mosaic in eastern Madagascar selected sheltered littoral forest over agricultural land and sea-inundated forest (Razafimanahaka et al. 2016).
In summary, the information collected to date does not show a difference in use of habitat by the two families based on the levels of clutter. Hipposiderid bats, including the larger species, which have no similar sized equivalents among Rhinolophus, show a preference for foraging in areas of high clutter as do rhinolophid species. Although Pavey et al. (2001a) suggested that hipposiderid bats may spend more time foraging in edge habitats, at a microhabitat scale they select areas of high clutter within habitat mosaics.
Diet
Studies that assessed the diet of rhinolophid and hipposiderid bats were collated to compare the diets of the two families. I used published research that reported a sample size of at least 20 faecal pellets (or at least five stomachs of individual bats) per species, providing a dietary sample for 15 Rhinolophus species and 12 hipposiderid bats. Some species were covered by multiple studies. For example, I found eight papers on the diet of Rhinolophus hipposideros that matched my criteria (Supplementary Material).
All species assessed captured predominantly flying insects (Fig. 3 and Supplementary Material). Apart from flying insects, several other prey groups were captured by both families but always in low numbers (Fig. 3). Rhinolophus species captured spiders, centipedes and isopods. Four species (R. ferrumequinum, R. euryale, R. blasii, R. hipposideros) captured centipedes, all in the Great Kabylia region of north-east Algeria (Ahmim and Moali 2013). Rhinolophus hipposideros also captured spiders and isopods. Isopod predation was restricted to one study in Britain and Ireland (Williams et al. 2011), whereas spider predation took place in four of eight studies (Supplementary Material) and an additional three studies listed by Mitschunas and Wagner (2015). The other species recorded taking spiders was R. ferrumequinum (Ahmim and Moali 2013).

|
Hipposiderid species were recorded consuming insect larvae, spiders and scorpions. Insect larvae were prey of H. caffer in a single study (Whitaker and Black 1976). Likewise, scorpion predation was noted only for Macronycteris commersoni in Madagascar (Rakotoarivelo et al. 2009). However, spider predation was noted for seven of the 12 hipposiderid species (Fig. 3): H. diadema (Pavey and Burwell 1997), H. khasiana (Thabah et al. 2006), H. speoris (Pavey et al. 2001a, 2001b), H. cervinus (Pavey and Burwell 2000), H. caffer (Bowie et al. 1999), H. ater (Milne et al. 2016) and Asellia tridens (Loumassine et al. 2019). One species of Hipposideros also captured birds (Pavey and Burwell 1997).
The dietary information summarised here provides the unexpected result that species of Rhinolophus are not restricted to capturing winged insects. While spiders may be captured aerially while they are ballooning this behaviour is mostly restricted to small individuals (Sutter 1999) and bats are most likely to glean them from webs, foliage or the ground. Centipedes and isopods are terrestrial and flightless and must be gleaned from the ground. There appears to be no alternative situation in which Rhinolophus could encounter these two groups of invertebrates.
Although further investigation is needed, the dietary data summarised here do not indicate that rhinolophid bats have a restricted prey base. Species from both families captured flying and flightless invertebrates. While this range of prey is lower than the extreme diversity shown by, for example, the terrestrial foraging New Zealand lesser short-tailed bat (Mystacina tuberculata) in wet forests (Czenze et al. 2018), both families captured a range of arthropods.
Summary
An important premise in interpretations of the evolutionary ecology of insectivorous bats is that both their echolocation signals and auditory capacities are adapted to the acoustical constraints of their foraging environment (Neuweiler 2000). Based on this relationship, it is expected that the echolocation and neurobiological differences between rhinolophid and hipposiderid bats, identified above, should result in differences in their prey capture behaviour, foraging habitat and diet. The major finding from this review is that there is no discernible difference in prey capture behaviour, foraging habitat and diet based on available information. Rather, the data summarised here indicate that rhinolophids and hipposiderids occupy similar foraging habitat and exploit the same prey base, but each family uses a distinct approach to echolocation to enable it to do so. Further insights on this issue will require more field-based investigations that combine assessment of echolocation and foraging ecology (prey capture, foraging habitat and diet) of species from both families. An understanding of the echolocation and foraging ecology of the little known and enigmatic species in the Rhinonycteridae may also prove fruitful. There is a need to seek further clarification on how laboratory assessments of audition translate into foraging performance in the wild. Evidence of predation on centipedes by four species of rhinolophid demonstrates that these bats do capture non-fluttering prey (Ahmim and Moali 2013) and challenges explanations of the importance of fluttering target detection in prey capture by this family.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
I am grateful for time spent at various stages of my career in the laboratories of Professor Gerhard Neuweiler (LMU, Munich) and Professor Fritz Geiser (University of New England) while developing the ideas presented in this manuscript. I thank many colleagues for discussing components of this research with me, particularly Gimme Walter, James Fullard, Manfred Kössl, Elisabeth Foeller, Jan Grunwald, Gerhard Neuweiler, David Jacobs, and Mark Brigham. I am grateful for input on this manuscript received from Eric Vanderduys, Damian Milne, Brock Fenton and Kyle Armstrong. Funding was received from the Humboldt Foundation.
References
Ahmim, M., and Moali, A. (2013). The diet of four species of horseshoe bat (Chiroptera: Rhinolophidae) in a mountainous region of Algeria: evidence for gleaning. Hystrix, the Italian Journal of Mammalogy 24, 174–176.Aldridge, H. D. J. N., and Rautenbach, I. L. (1987). Morphology, echolocation and resource partitioning in insectivorous bats. Journal of Animal Ecology 56, 763–778.
| Morphology, echolocation and resource partitioning in insectivorous bats.Crossref | GoogleScholarGoogle Scholar |
American Society of Mammalogists (2020). Mammal diversity database. Available at www.mammaldiversity.org [accessed 11 May 2020].
Amichai, E., Levin, E., Kronfeld-Schor, N., Roll, U., and Yom-Tov, Y. (2013). Natural history, physiology and energetic strategies of Asellia tridens (Chiroptera). Mammalian Biology 78, 94–103.
| Natural history, physiology and energetic strategies of Asellia tridens (Chiroptera).Crossref | GoogleScholarGoogle Scholar |
Arlettaz, R., Godat, S., and Meyer, H. (2000). Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros). Biological Conservation 93, 55–60.
| Competition for food by expanding pipistrelle bat populations (Pipistrellus pipistrellus) might contribute to the decline of lesser horseshoe bats (Rhinolophus hipposideros).Crossref | GoogleScholarGoogle Scholar |
Beck, A., Stutz, H.-P. B., and Ziswiler, V. (1989). Das Beutespecktrum der kleinen Hufeisennase Rhinolophus hipposideros (Bechstein, 1800) (Mammalia, Chiroptera). Revue Suisse de Zoologie 96, 643–650.
| Das Beutespecktrum der kleinen Hufeisennase Rhinolophus hipposideros (Bechstein, 1800) (Mammalia, Chiroptera).Crossref | GoogleScholarGoogle Scholar |
Bell, G. P., and Fenton, M. B. (1984). The use of Doppler-shifted echoes as a flutter detection and clutter rejection system: the echolocation and feeding behavior of Hipposideros ruber (Chiroptera: Hipposideridae). Behavioral Ecology and Sociobiology 15, 109–114.
| The use of Doppler-shifted echoes as a flutter detection and clutter rejection system: the echolocation and feeding behavior of Hipposideros ruber (Chiroptera: Hipposideridae).Crossref | GoogleScholarGoogle Scholar |
Bontadina, F., Beck, A., Gloor, S., Hotz, T., Lutz, M., and Mühlethaler, E. (1995). Jagt die Grosse Hufeisennase Rhinolophus ferrumequinum im wald? – Grundlagen zum Schutz von Jagdgebieten der letzten grösseren Kolonie in der Schweiz. Der Ornithologische Beobachter 92, 325–327.
Bontadina, F., Schofield, H., and Naef-Denzer, B. (2002). Radio-tracking reveals that lesser horseshoe bats (Rhinolophus hipposideros) forage in woodland. Journal of Zoology 258, 281–290.
| Radio-tracking reveals that lesser horseshoe bats (Rhinolophus hipposideros) forage in woodland.Crossref | GoogleScholarGoogle Scholar |
Bontadina, F., Schmied, S. F., Beck, A., and Arlettaz, R. (2008). Changes in prey abundance unlikely to explain the demography of a critically endangered Central European bat. Journal of Applied Ecology 45, 641–648.
| Changes in prey abundance unlikely to explain the demography of a critically endangered Central European bat.Crossref | GoogleScholarGoogle Scholar |
Bowie, R. C. K., Jacobs, D. S., and Taylor, P. J. (1999). Resource use by two morphologically similar insectivorous bats (Nycteris thebaica and Hipposideros caffer). South African Journal of Zoology 34, 27–33.
| Resource use by two morphologically similar insectivorous bats (Nycteris thebaica and Hipposideros caffer).Crossref | GoogleScholarGoogle Scholar |
Czenze, Z. J., Tucker, J. L., Clare, E. L., Littlefair, J. E., Hemprich-Bennett, D., Oliveira, H. F. M., Brigham, R. M., Hickey, A. J. R., and Parsons, S. (2018). Spatiotemporal and demographic variation in the diet of New Zealand lesser short-tailed bats (Mystacina tuberculata) Ecology and Evolution 8, 7599–7610.
| Spatiotemporal and demographic variation in the diet of New Zealand lesser short-tailed bats (Mystacina tuberculata)Crossref | GoogleScholarGoogle Scholar | 30151174PubMed |
Eckrich, M., and Neuweiler, G. (1988). Food habits of sympatric insectivorous bats Rhinolophus rouxi and Hipposideros lankadiva from Sri Lanka. Journal of Zoology 215, 729–737.
| Food habits of sympatric insectivorous bats Rhinolophus rouxi and Hipposideros lankadiva from Sri Lanka.Crossref | GoogleScholarGoogle Scholar |
Fawcett, K., Jacobs, D. S., Surlykke, A., and Ratcliffe, J. M. (2015). Echolocation in the bat, Rhinolophus capensis: the influence of clutter, conspecifics and prey on call design and intensity. Biology Open 4, 693–701.
| Echolocation in the bat, Rhinolophus capensis: the influence of clutter, conspecifics and prey on call design and intensity.Crossref | GoogleScholarGoogle Scholar | 25987587PubMed |
Fenton, M. B., and Rautenbach, I. L. (1986). A comparison of the roosting and foraging behaviour of three species of African insectivorous bats (Rhinolophidae, Vespertilionidae, and Molossidae). Canadian Journal of Zoology 64, 2860–2867.
| A comparison of the roosting and foraging behaviour of three species of African insectivorous bats (Rhinolophidae, Vespertilionidae, and Molossidae).Crossref | GoogleScholarGoogle Scholar |
Fenton, M. B., Audet, D., Obrist, M. K., and Rydell, J. (1995). Signal strength, timing, and self-deafening: the evolution of echolocation in bats. Paleobiology 21, 229–242.
| Signal strength, timing, and self-deafening: the evolution of echolocation in bats.Crossref | GoogleScholarGoogle Scholar |
Fenton, M. B., Faure, P. A., and Ratcliffe, J. M. (2012). Evolution of high duty cycle echolocation in bats. The Journal of Experimental Biology 215, 2935–2944.
| Evolution of high duty cycle echolocation in bats.Crossref | GoogleScholarGoogle Scholar | 22875762PubMed |
Foeller, E., and Kössl, M. (2000). Mechanical adaptations for echolocation in the cochlea of the bat Hipposideros lankadiva. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 186, 859–870.
| Mechanical adaptations for echolocation in the cochlea of the bat Hipposideros lankadiva.Crossref | GoogleScholarGoogle Scholar |
Foley, N. M., Thong, V. D., Soisook, P., Goodman, S. M., Armstrong, K. N., Jacobs, D. S., Puechmaille, S. J., and Teeling, E. C. (2015). How and why overcome the impediments to resolution: lessons from rhinolophid and hipposiderid bats. Molecular Biology and Evolution 32, 313–333.
| How and why overcome the impediments to resolution: lessons from rhinolophid and hipposiderid bats.Crossref | GoogleScholarGoogle Scholar | 25433366PubMed |
Fullard, J. H., Jackson, M. E., Jacobs, D. S., Pavey, C. R., and Burwell, C. J. (2008). Surviving cave bats: auditory and behavioural defences in the Australian noctuid moth, Speiredonia spectans. The Journal of Experimental Biology 211, 3808–3815.
| Surviving cave bats: auditory and behavioural defences in the Australian noctuid moth, Speiredonia spectans.Crossref | GoogleScholarGoogle Scholar | 19043053PubMed |
Goiti, U., Aihartza, J. R., Garin, I., and Zabala, J. (2003). Influence of habitat on the foraging behaviour of the Mediterranean horseshoe bat, Rhinolophus euryale. Acta Chiropterologica 5, 75–84.
| Influence of habitat on the foraging behaviour of the Mediterranean horseshoe bat, Rhinolophus euryale.Crossref | GoogleScholarGoogle Scholar |
Goiti, U., Aihartza, J. R., and Garin, I. (2004). Diet and prey selection in the Mediterranean horseshoe bat Rhinolophus euryale (Chiroptera: Rhinolophidae) during the pre-breeding season. Mammalia 68, 397–402.
| Diet and prey selection in the Mediterranean horseshoe bat Rhinolophus euryale (Chiroptera: Rhinolophidae) during the pre-breeding season.Crossref | GoogleScholarGoogle Scholar |
Goiti, U., Garin, I., Almenar, D., Salsamendi, E., and Aihartza, J. (2008). Foraging by Mediterranean horseshoe bats (Rhinolophus euryale) in relation to prey distribution and edge habitat. Journal of Mammalogy 89, 493–502.
| Foraging by Mediterranean horseshoe bats (Rhinolophus euryale) in relation to prey distribution and edge habitat.Crossref | GoogleScholarGoogle Scholar |
Grinnell, A. D. (1989). Listening to the voice within. Nature 341, 488–489.
| Listening to the voice within.Crossref | GoogleScholarGoogle Scholar | 2797176PubMed |
Habersetzer, J., Schuller, G., and Neuweiler, G. (1984). Foraging behaviour and Doppler shift compensation in echolocating hipposiderid bats, Hipposideros bicolor and Hipposideros speoris. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 155, 559–567.
| Foraging behaviour and Doppler shift compensation in echolocating hipposiderid bats, Hipposideros bicolor and Hipposideros speoris.Crossref | GoogleScholarGoogle Scholar |
Hall, L. S. (1989a). Rhinolophidae. In ‘Fauna of Australia: Mammalia’. (Eds D. W. Walton, and B. J. Richardson.) pp. 857–863. (Australian Government Publishing Service: Canberra.)
Hall, L. S. (1989b). Hipposideridae. In ‘Fauna of Australia: Mammalia’. (Eds D. W. Walton, and B. J. Richardson.) pp. 864–870. (Australian Government Publishing Service: Canberra.)
Jacobs, D. S. (2000). Community level support for the allotonic frequency hypothesis. Acta Chiropterologica 2, 197–207.
Jacobs, D. S., and Bastian, A. (2018). High duty cycle echolocation may constrain the evolution of diversity within horseshoe bats (Family: Rhinolophidae). Diversity 10, 85.
| High duty cycle echolocation may constrain the evolution of diversity within horseshoe bats (Family: Rhinolophidae).Crossref | GoogleScholarGoogle Scholar |
Jacobs, D. S., Barclay, R. M. R., and Walker, M. H. (2007). The allometry of echolocation call frequencies of bats: why does Rhinolophus clivosus have a higher than expected frequency? Oecologia 152, 583–594.
| The allometry of echolocation call frequencies of bats: why does Rhinolophus clivosus have a higher than expected frequency?Crossref | GoogleScholarGoogle Scholar | 17345101PubMed |
Jiang, T., Feng, J., Sun, K., and Wang, J. (2008). Coexistence of two sympatric and morphologically similar bat species Rhinolophus affinis and Rhinolophus pearsoni. Progress in Natural Science 18, 523–532.
| Coexistence of two sympatric and morphologically similar bat species Rhinolophus affinis and Rhinolophus pearsoni.Crossref | GoogleScholarGoogle Scholar |
Jones, G. (1990). Prey selection by the greater horseshoe bat (Rhinolophus ferrumequinum): optimal foraging by echolocation. Journal of Animal Ecology 59, 587–602.
| Prey selection by the greater horseshoe bat (Rhinolophus ferrumequinum): optimal foraging by echolocation.Crossref | GoogleScholarGoogle Scholar |
Jones, G. (1999). Scaling of echolocation call parameters in bats. The Journal of Experimental Biology 202, 3359–3367.
| 10562518PubMed |
Jones, G., and Morton, M. (1992). Radio-tracking studies on habitat use by greater horseshoe bats (Rhinolophus ferrumequinum). In ‘Wildlife Telemetry. Remote Monitoring and Tracking of Animals’. (Eds I. G. Priede, and S. M. Swift.) pp. 521–537. (Ellis Horwood: Chichester, UK.)
Jones, G., and Rayner, J. M. V. (1989). Foraging behavior and echolocation of wild horseshoe bats Rhinolophus ferrumequinum and R. hipposideros (Chiroptera, Rhinolophidae). Behavioral Ecology and Sociobiology 25, 183–191.
| Foraging behavior and echolocation of wild horseshoe bats Rhinolophus ferrumequinum and R. hipposideros (Chiroptera, Rhinolophidae).Crossref | GoogleScholarGoogle Scholar |
Jones, G., Morton, M., Hughes, P. M., and Budden, R. M. (1993). Echolocation, flight morphology and foraging strategies of some West African hipposiderid bats. Journal of Zoology 230, 385–400.
| Echolocation, flight morphology and foraging strategies of some West African hipposiderid bats.Crossref | GoogleScholarGoogle Scholar |
Kober, R., and Schnitzler, H. U. (1990). Information in sonar echoes of fluttering insects available for echolocating bats. The Journal of the Acoustical Society of America 87, 882–896.
| Information in sonar echoes of fluttering insects available for echolocating bats.Crossref | GoogleScholarGoogle Scholar |
Law, B., and Chidel, M. (2002). Tracks and riparian zones facilitate the use of Australian regrowth forest by insectivorous bats. Journal of Applied Ecology 39, 605–617.
| Tracks and riparian zones facilitate the use of Australian regrowth forest by insectivorous bats.Crossref | GoogleScholarGoogle Scholar |
Law, B., Chidel, M., Brassil, M. T., and Potter, T. (2020). Changes in bat activity over 10 years in silviculturally treated wet sclerophyll forest. Australian Mammalogy , .
| Changes in bat activity over 10 years in silviculturally treated wet sclerophyll forest.Crossref | GoogleScholarGoogle Scholar |
Lee, Y.-F., Kuo, Y.-M., Chu, W.-C., Lin, Y.-H., Chang, H.-Y., and Chen, W.-M. (2012). Ecomorphology, differential habitat use, and nocturnal activities of Rhinolophus and Hipposideros species in East Asian tropical forests. Zoology 115, 22–29.
| Ecomorphology, differential habitat use, and nocturnal activities of Rhinolophus and Hipposideros species in East Asian tropical forests.Crossref | GoogleScholarGoogle Scholar | 22230387PubMed |
Lee, Y.-F., Kuo, Y.-M., Chu, W.-C., and Lin, Y.-H. (2020). Perch use by flycatching Rhinolophus formosae in relation to vegetation structure. Journal of Mammalogy 101, 455–463.
| Perch use by flycatching Rhinolophus formosae in relation to vegetation structure.Crossref | GoogleScholarGoogle Scholar |
Li, G., Liang, B., Wang, Y., Zhao, H., Helgen, K. M., Lin, L., Jones, G., and Zhang, S. (2007). Echolocation calls, diet, and phylogenetic relationships of Stoliczka’s trident bat, Aselliscus stoliczkanus (Hipposideridae). Journal of Mammalogy 88, 736–744.
| Echolocation calls, diet, and phylogenetic relationships of Stoliczka’s trident bat, Aselliscus stoliczkanus (Hipposideridae).Crossref | GoogleScholarGoogle Scholar |
Link, A., Marimuthu, G., and Neuweiler, G. (1986). Movement as a specific stimulus for prey catching behaviour in rhinolophid and hipposiderid bats. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 159, 403–413.
| Movement as a specific stimulus for prey catching behaviour in rhinolophid and hipposiderid bats.Crossref | GoogleScholarGoogle Scholar |
Lino, A., Fonseca, C., Goiti, U., and Pereira, M. J. R. (2014). Prey selection by Rhinolophus hipposideros (Chiroptera: Rhinolophidae) in a modified forest in southwest Europe. Acta Chiropterologica 16, 75–83.
| Prey selection by Rhinolophus hipposideros (Chiroptera: Rhinolophidae) in a modified forest in southwest Europe.Crossref | GoogleScholarGoogle Scholar |
Loumassine, H. E., Marniche, F., Bounaceur, F., and Aulagnier, S. (2019). Seasonal diet of Asellia tridens (Chiroptera: Hipposideridae) in north-western Africa. The European Zoological Journal 86, 354–362.
| Seasonal diet of Asellia tridens (Chiroptera: Hipposideridae) in north-western Africa.Crossref | GoogleScholarGoogle Scholar |
Ma, J., Liang, B., Zhang, S., and Metzner, W. (2008). Dietary composition and echolocation call design of three sympatric insectivorous bats from China. Ecological Research 23, 113–119.
| Dietary composition and echolocation call design of three sympatric insectivorous bats from China.Crossref | GoogleScholarGoogle Scholar |
McAney, C. M., and Fairley, J. S. (1989). Analysis of the diet of the lesser horseshoe bat Rhinolophus hipposideros in the west of Ireland. Journal of Zoology 217, 491–498.
| Analysis of the diet of the lesser horseshoe bat Rhinolophus hipposideros in the west of Ireland.Crossref | GoogleScholarGoogle Scholar |
McDonald, J. T., Rautenbach, I. L., and Nel, J. A. J. (1990). Foraging ecology of bats observed at De Hoop Provincial Nature Reserve, southern Cape Province. South African Journal of Wildlife Research 20, 133–145.
Metzner, W., Zhang, S., and Smotherman, M. (2002). Doppler-shift compensation behavior in horseshoe bats revisited: auditory feedback controls both a decrease and an increase in call frequency. The Journal of Experimental Biology 205, 1607–1616.
| 12000805PubMed |
Milne, D. J., Burwell, C. J., and Pavey, C. R. (2016). Dietary composition of insectivorous bats of the Top End of Australia. Australian Mammalogy 38, 213–220.
| Dietary composition of insectivorous bats of the Top End of Australia.Crossref | GoogleScholarGoogle Scholar |
Mitschunas, N., and Wagner, M. (2015). Diet of the lesser horseshoe bat (Rhinolophus hipposideros) in central Germany and its seasonal and site-specific variation. Acta Chiropterologica 17, 379–392.
| Diet of the lesser horseshoe bat (Rhinolophus hipposideros) in central Germany and its seasonal and site-specific variation.Crossref | GoogleScholarGoogle Scholar |
Neuweiler, G. (1989). Foraging ecology and audition in bats. Trends in Ecology & Evolution 4, 160–166.
| Foraging ecology and audition in bats.Crossref | GoogleScholarGoogle Scholar |
Neuweiler, G. (1990). Auditory adaptations for prey capture in echolocating bats. Physiological Reviews 70, 615–641.
| Auditory adaptations for prey capture in echolocating bats.Crossref | GoogleScholarGoogle Scholar | 2194220PubMed |
Neuweiler, G. (2000). ‘The Biology of Bats.’ (Oxford University Press: Oxford.)
Neuweiler, G. (2003). Evolutionary aspects of bat echolocation. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 189, 245–256.
| Evolutionary aspects of bat echolocation.Crossref | GoogleScholarGoogle Scholar | 12743729PubMed |
Neuweiler, G., Singh, S., and Sripathi, K. (1984). Audiograms of a south Indian bat community. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 154, 133–142.
| Audiograms of a south Indian bat community.Crossref | GoogleScholarGoogle Scholar |
Neuweiler, G., Metzner, W., Heilmann, U., Rübsamen, R., Eckrich, M., and Costa, H. H. (1987). Foraging behaviour and echolocation in the rufous horseshoe bat (Rhinolophus rouxi) of Sri Lanka. Behavioral Ecology and Sociobiology 20, 53–67.
| Foraging behaviour and echolocation in the rufous horseshoe bat (Rhinolophus rouxi) of Sri Lanka.Crossref | GoogleScholarGoogle Scholar |
Nkrumah, E. E., Vallo, P., Klose, S. M., et al. (2016). Foraging behaviour and habitat selection of Noack’s round-leaf bat (Hipposideros aff. ruber) and conservation implications. Tropical Conservation Science 2016, 1–11.
Norberg, U. M., and Rayner, J. M. V. (1987). Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 316, 335–427.
Pavey, C. R. (1998a). Habitat use by the eastern horseshoe bat, Rhinolophus megaphyllus, in a fragmented woodland mosaic. Wildlife Research 25, 489–498.
| Habitat use by the eastern horseshoe bat, Rhinolophus megaphyllus, in a fragmented woodland mosaic.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R. (1998b). Colony size, roost use and foraging ecology of Hipposideros diadema reginae, a rare bat from tropical Australia. Pacific Conservation Biology 4, 232–239.
| Colony size, roost use and foraging ecology of Hipposideros diadema reginae, a rare bat from tropical Australia.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R. (1999). Foraging ecology of the two taxa of large-eared horseshoe bat, Rhinolophus philippinensis, on Cape York Peninsula. Australian Mammalogy 21, 135–138.
Pavey, C. R., and Burwell, C. J. (1997). The diet of the diadem leafnosed-bat Hipposideros diadema: confirmation of a morphologically-based prediction of carnivory. Journal of Zoology 243, 295–303.
| The diet of the diadem leafnosed-bat Hipposideros diadema: confirmation of a morphologically-based prediction of carnivory.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., and Burwell, C. J. (2000). Foraging ecology of three species of hipposiderid bats in tropical rainforest in north-east Australia. Wildlife Research 27, 283–287.
| Foraging ecology of three species of hipposiderid bats in tropical rainforest in north-east Australia.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., and Burwell, C. J. (2004). Foraging ecology of the horseshoe bat, Rhinolophus megaphyllus (Rhinolophidae), in eastern Australia. Wildlife Research 31, 403–413.
| Foraging ecology of the horseshoe bat, Rhinolophus megaphyllus (Rhinolophidae), in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., Grunwald, J.-E., and Neuweiler, G. (2001a). Foraging habitat and echolocation behaviour of Schneider’s leafnosed bat, Hipposideros speoris in a habitat mosaic in Sri Lanka. Behavioral Ecology and Sociobiology 50, 209–218.
| Foraging habitat and echolocation behaviour of Schneider’s leafnosed bat, Hipposideros speoris in a habitat mosaic in Sri Lanka.Crossref | GoogleScholarGoogle Scholar |
Pavey, C. R., Burwell, C. J., Grunwald, J.-E., Marshall, C. J., and Neuweiler, G. (2001b). Dietary benefits of twilight foraging by the insectivorous bat, Hipposideros speoris. Biotropica 33, 670–681.
| Dietary benefits of twilight foraging by the insectivorous bat, Hipposideros speoris.Crossref | GoogleScholarGoogle Scholar |
Pillat, J., and Schmidt, S. (1998). A comparative study of Doppler shift compensation behaviour in the bat Hipposideros lankadiva. In ‘Proceedings of the 26th Göttingen Neurobiology Conference 1998: Vol. 2. Thieme, Stuttgart’. (Eds N. Elsner, and R. Wehner.) Abstract no. 324.
Rakotoarivelo, A. A., Ranaivoson, N., Ramilijaona, O. R., Kofoky, A. F., Racey, P. A., and Jenkins, R. K. B. (2007). Seasonal food habits of five sympatric forest microchiropterans in western Madagascar. Journal of Mammalogy 88, 959–966.
| Seasonal food habits of five sympatric forest microchiropterans in western Madagascar.Crossref | GoogleScholarGoogle Scholar |
Rakotoarivelo, A. A., Ralisata, M., Ravoahangimalala, O. R., Rakotomalala, M. R., Racey, P. A., and Jenkins, R. K. B. (2009). The food habits of a Malagasy giant: Hipposideros commersoni (E. Geoffroy, 1813). African Journal of Ecology 47, 283–288.
| The food habits of a Malagasy giant: Hipposideros commersoni (E. Geoffroy, 1813).Crossref | GoogleScholarGoogle Scholar |
Ransome, R. D. (1990). ‘The Natural History of Hibernating Bats.’ (Christopher Helm: London.)
Razafimanahaka, J. H., Ralisata, M., Randrianandrianina, F., Jenkins, R. K. B., Ratsirarson, J., and Racey, P. A. (2016). Habitat use by the endemic Malagasy bat Hipposideros commersoni in a littoral forest. Acta Chiropterologica 18, 423–431.
| Habitat use by the endemic Malagasy bat Hipposideros commersoni in a littoral forest.Crossref | GoogleScholarGoogle Scholar |
Rübsamen, R., Neuweiler, G., and Sripathi, K. (1988). Comparative collicular tonotopy in two bat species adapted to movement detection, Hipposideros speoris and Megaderma lyra. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 163, 271–285.
| Comparative collicular tonotopy in two bat species adapted to movement detection, Hipposideros speoris and Megaderma lyra.Crossref | GoogleScholarGoogle Scholar |
Russo, D., Jones, G., and Migliozzi, A. (2002). Habitat selection by the Mediterranean horseshoe bat, Rhinolophus euryale (Chiroptera: Rhinolophidae) in a rural area of southern Italy and implications for conservation. Biological Conservation 107, 71–81.
| Habitat selection by the Mediterranean horseshoe bat, Rhinolophus euryale (Chiroptera: Rhinolophidae) in a rural area of southern Italy and implications for conservation.Crossref | GoogleScholarGoogle Scholar |
Russo, D., Almenar, D., Aihartza, J., Goiti, U., Salsamendi, E., and Garin, I. (2005). Habitat selection in sympatric Rhinolophus mehelyi and R. euryale (Chiroptera: Rhinolophidae). Journal of Zoology 266, 327–332.
| Habitat selection in sympatric Rhinolophus mehelyi and R. euryale (Chiroptera: Rhinolophidae).Crossref | GoogleScholarGoogle Scholar |
Salsamendi, E., Garin, I., Almenar, D., Goiti, U., Napal, M., and Aihartza, J. (2008). Diet and prey selection in Mehelyi’s horseshoe bat Rhinolophus mehelyi (Chiroptera, Rhinolophidae) in the south-western Iberian Peninsula. Acta Chiropterologica 10, 279–286.
| Diet and prey selection in Mehelyi’s horseshoe bat Rhinolophus mehelyi (Chiroptera, Rhinolophidae) in the south-western Iberian Peninsula.Crossref | GoogleScholarGoogle Scholar |
Salsamendi, E., Garin, I., Arostegui, I., Goiti, U., and Aihartza, J. (2012). What mechanism of niche segregation allows the coexistence of sympatric sibling rhinolophid bats? Frontiers in Zoology 9, 30.
| What mechanism of niche segregation allows the coexistence of sympatric sibling rhinolophid bats?Crossref | GoogleScholarGoogle Scholar | 23148596PubMed |
Schnitzler, H. U. (1987). Echoes of fluttering insects: information for echolocating bats. In ‘Recent Advances in the Study of Bats’. (Eds M. B. Fenton, P. A. Racey, and J. M. V. Rayner.) pp. 226–243. (Cambridge University Press: Cambridge.)
Schnitzler, H. U., and Denzinger, A. (2011). Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 197, 541–559.
| Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals.Crossref | GoogleScholarGoogle Scholar | 20857119PubMed |
Schnitzler, H. U., Hackbarth, H., Heilmann, U., and Herbert, H. (1985). Echolocation behavior of rufous horseshoe bats hunting for insects in the flycatcher-style. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 157, 39–46.
| Echolocation behavior of rufous horseshoe bats hunting for insects in the flycatcher-style.Crossref | GoogleScholarGoogle Scholar |
Schoeppler, D., Schnitzler, H.-U., and Denzinger, A. (2018). Precise Doppler shift compensation in the hipposiderid bat, Hipposideros armiger. Scientific Reports 8, 4598.
| Precise Doppler shift compensation in the hipposiderid bat, Hipposideros armiger.Crossref | GoogleScholarGoogle Scholar | 29545520PubMed |
Schofield, H. W. (1996). The ecology and conservation of Rhinolophus hipposideros, the lesser horseshoe bat. Ph.D. Thesis, University of Aberdeen.
Schuller, G. (1974). The role of overlap of echo with outgoing echolocation sound in the bat Rhinolophus ferrumequinum. Naturwissenschaften 61, 171–172.
| The role of overlap of echo with outgoing echolocation sound in the bat Rhinolophus ferrumequinum.Crossref | GoogleScholarGoogle Scholar |
Schuller, G. (1980). Hearing charactersitics and Doppler shift compensation in south Indian CF-FM bats. Journal of Comparative Physiology 139, 349–356.
| Hearing charactersitics and Doppler shift compensation in south Indian CF-FM bats.Crossref | GoogleScholarGoogle Scholar |
Schuller, G., and Pollak, G. (1979). Disproportionate frequency representation in the inferior colliculus of Doppler-compensating greater horseshoe bats: evidence for an acoustic foveationate frequency representation in the inferior colliculus. Journal of Comparative Physiology 132, 47–54.
| Disproportionate frequency representation in the inferior colliculus of Doppler-compensating greater horseshoe bats: evidence for an acoustic foveationate frequency representation in the inferior colliculus.Crossref | GoogleScholarGoogle Scholar |
Shi, L., Feng, J., Liu, Y., Ye, G., and Zhu, X. (2009). Is food resource partitioning responsible for deviation of echolocation call frequencies from allometry in Rhinolophus macrotis? Acta Theriologica 54, 371–382.
| Is food resource partitioning responsible for deviation of echolocation call frequencies from allometry in Rhinolophus macrotis?Crossref | GoogleScholarGoogle Scholar |
Siemers, B. M., and Ivanova, T. (2004). Ground gleaning in horseshoe bats: comparative evidence from Rhinolophus blasii, R. euryale and R. mehelyi. Behavioral Ecology and Sociobiology 56, 464–471.
| Ground gleaning in horseshoe bats: comparative evidence from Rhinolophus blasii, R. euryale and R. mehelyi.Crossref | GoogleScholarGoogle Scholar |
Sutter, R. B. (1999). An aerial lottery: the physics of ballooning in a chaotic atmosphere. The Journal of Arachnology 27, 281–293.
Thabah, A., Rossiter, S. J., Kingston, T., et al. (2006). Genetic divergence and echolocation call frequency in cryptic species of Hipposideros larvatus s.l. (Chiroptera: Hipposideridae) from the Indo-Malayan region. Biological Journal of the Linnean Society 88, 119–130.
| Genetic divergence and echolocation call frequency in cryptic species of Hipposideros larvatus s.l. (Chiroptera: Hipposideridae) from the Indo-Malayan region.Crossref | GoogleScholarGoogle Scholar |
Vater, M. (1998). Adaptation of the auditory periphery of bats for echolocation. In ‘Bat Biology and Conservation’. (Eds T. H. Kunz, and P. A. Racey.) pp. 231–245. (Smithsonian Institution Press: Washington, DC.)
Vaughan, T. A. (1977). Foraging behaviour of the giant leaf-nosed bat (Hipposideros commersoni). East African Wildlife Journal 15, 237–249.
| Foraging behaviour of the giant leaf-nosed bat (Hipposideros commersoni).Crossref | GoogleScholarGoogle Scholar |
Vestjens, W. J. M., and Hall, L. S. (1977). Stomach contents of forty-two species of bats from the Australasian region. Australian Wildlife Research 4, 25–35.
| Stomach contents of forty-two species of bats from the Australasian region.Crossref | GoogleScholarGoogle Scholar |
von der Emde, G., and Menne, D. (1989). Discrimination of insect wingbeat-frequencies by the bat Rhinolophus ferrumequinum. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 164, 663–671.
| Discrimination of insect wingbeat-frequencies by the bat Rhinolophus ferrumequinum.Crossref | GoogleScholarGoogle Scholar |
von der Emde, G., and Schnitzler, H. U. (1986). Fluttering target detection in hipposiderid bats. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 159, 765–772.
| Fluttering target detection in hipposiderid bats.Crossref | GoogleScholarGoogle Scholar |
von der Emde, G., and Schnitzler, H. U. (1990). Classification of insects by echolocating greater horseshoe bats. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology 167, 423–430.
| Classification of insects by echolocating greater horseshoe bats.Crossref | GoogleScholarGoogle Scholar |
Walter, G. H. (2003). ‘Insect Pest Management and Ecological Research.’ (Cambridge University Press: Cambridge.)
Weterings, R., Wardenaar, J., Dunn, S., and Umponistira, C. (2015). Dietary analysis of five insectivorous bat species from Kamphaeng Phet, Thailand. The Raffles Bulletin of Zoology 63, 91–96.
Whitaker, J. O., and Black, H. (1976). Food habits of cave bats from Zambia, Africa. Journal of Mammalogy 57, 199–204.
| Food habits of cave bats from Zambia, Africa.Crossref | GoogleScholarGoogle Scholar |
Williams, C., Salter, L., and Jones, G. (2011). The winter diet of the lesser horseshoe bat (Rhinolophus hipposideros) in Britain and Ireland. Hystrix, the Italian Journal of Mammalogy 22, 159–166.
Zhang, Y., Lin, A., Ding, J., Yang, X., Jiang, T., and Liu, Y. (2019). Performance of Doppler shift compensation in bats varies with species rather than with environmental clutter. Animal Behaviour 158, 109–120.
| Performance of Doppler shift compensation in bats varies with species rather than with environmental clutter.Crossref | GoogleScholarGoogle Scholar |
1 This paper is dedicated to Les Hall, who was one of my doctoral supervisors and the person who introduced me to the study of bats. To me, the standout contribution that Les made to bat ecology and conservation in Australia was his ability and motivation as an educator. Les was an excellent university lecturer and had a wonderful way of enthusing the general public about bats. One fond memory of Les is when we stayed at a pub in Glen Innes for a night on our way to the 1991 Mammal Society Conference in Armidale. Within half an hour of arriving at the front bar Les was sharing stories of bats with the patrons, informing them of fascinating aspects of their biology and eliciting information on locations of bat roosts in return. Les was very interested in horseshoe and leaf-nosed bats. I have tried to write this manuscript in the way that he would have done it.