Muscular anatomy of the tail of the western grey kangaroo, Macropus fuliginosus
Rebekah Dawson A C , Nick Milne A and Natalie M. Warburton BA School of Anatomy, Physiology and Human Biology, University of Western Australia, Nedlands, WA 6009, Australia.
B School of Veterinary and Life Sciences, Murdoch University, Murdoch, WA 6150, Australia.
C Corresponding author. Email: rebekah.dawson@uwa.edu.au
Australian Journal of Zoology 62(2) 166-174 https://doi.org/10.1071/ZO13085
Submitted: 17 October 2013 Accepted: 26 March 2014 Published: 17 April 2014
Abstract
The western grey kangaroo, Macropus fuliginosus, is a large-bodied kangaroo that engages in pentapedal locomotion at low speeds and bipedal hopping at high speeds. The tail is thought to have functional roles in both of these modes of locomotion. In pentapedal locomotion the tail acts as a ‘fifth limb’ to support the body weight together with the forelimbs while the hind limbs are drawn forward. The tail has also been suggested to have a role as a counterbalance during bipedal hopping. On the basis of these functional roles for the tail in locomotion, the caudal musculature of the western grey kangaroo was dissected and described in this study. The arrangement of the caudal musculature showed particular adaptations for the role of the tail in both pentapedal locomotion and bipedal hopping.
Introduction
The heavy tail of a kangaroo is often referred to as the fifth limb, and appears to have important roles during both slow and fast gaits. The most striking use of the tail in locomotion in the kangaroo is during slow progression, where the tail is employed together with the forelimbs to form a tripod, to support the body weight while the two hind limbs are drawn forward simultaneously (Frith and Calaby 1969; Windsor and Dagg 1971; Dawson and Taylor 1973). The term pentapedal locomotion was coined by Dawson and Taylor (1973) when they described the large-bodied red kangaroo, Macropus rufus, engaging in this form of slow progression and noting that the animal was ‘supporting itself on front limbs and tail … while swinging its [hind]limbs forward’ (p. 313). The weight-bearing role of the tail has been associated with the development of chevron bones, which provide attachment sites for flexor musculature in the kangaroo (Owen 1848, 1876; Flower 1885; Parsons 1896). The relationship between chevron bone development and ventral flexor musculature has been reported in other mammal groups such as aardvarks (Endo et al. 2012), prehensile-tailed New World monkeys (Chang and Ruch 1947; Organ 2010), cetaceans (Murie 1870; Howell 1971) and ground sloths (Flower 1885).
During bipedal hopping the role of the tail is less well defined in the kangaroo. Hopwood and Butterfield (1990) described the changing role of the tail during the different parts of the hopping cycle, in an eastern grey kangaroo, M. giganteus. While the kangaroo is hopping, the tail was noted to be curved upwards and being actively moved up and down. The tail is at its lowest point in the take-off stage of the hop, when it is positioned just caudal to the extended hind limb. The tail is at its highest point towards the end of the floating stage of the hop, as the kangaroo is about to land. This description highlights the role of the kangaroo tail in fast locomotion as an active and engaged organ, rather than as a passive appendage (Hopwood and Butterfield 1976). Baudinette (1994) also found that the large, heavy tail, along with the enlarged hind limbs and the conical shape of the body, serves to place the centre of mass in a more posterior position in macropodids, in order to facilitate balance in a bipedal posture. Further, that downward rotation of the tail whilst the limbs move backward provides a counteractive force during bipedal hopping. In Baudinette’s (1994) study it was also postulated that the heavy tail in the kangaroo prevents forward pitching upon landing during the bipedal hopping cycle. Alexander and Vernon (1975) and Baudinette (1994) have both suggested that the action of the tail moving up and down works as a counterbalance during locomotion. The movement of the tail, which is counter to the movement of the hind limbs, is suggested to counteract the tendency of the body to pitch forward while hopping at high speeds. Grand (1990) also suggested that the heaviness of the tail may minimise the tendency of the body to pitch upon landing and take-off. More recently, Usherwood and Hubel (2012) report that energetic savings during bipedal hopping are also achieved through the long tail and head of kangaroos.
Despite its potentially multifaceted role, the detailed muscular anatomy of the tail has not yet been documented. Hopwood and Butterfield (1990) emphasise the active role of the tail in both pentapedal locomotion and bipedal hopping in eastern grey kangaroos and report that these actions are caused by the contraction of the sacrocaudal musculature rather than by a passive dragging of the tail. Comparative studies of the body composition of macropodines show the tail to be highly muscular and comprise a relatively large proportion of the total body weight, especially in large-bodied macropods, suggesting specialisation of the caudal muscles for locomotion (Tribe and Peel 1963; Alexander and Vernon 1975; Grand 1990).
Here we report the detailed muscular anatomy of the western grey kangaroo, M. fuliginosus. This species is one of the largest extant kangaroos – males stand up to 2 m tall. Adults show high sexual dimorphism in body mass and males usually weigh up to 54 kg, and occasionally up to 80 kg, while females usually weigh up to 28 kg (Hume et al. 1989; Nowak 1991; Strahan 1995). Western grey kangaroos are strong bipedal hoppers and inhabit grasslands, woodlands, open forests, shrubland and heathland (Coulson 1990, 1993). It is expected that the caudal musculature of the western grey kangaroo will show functional specialisations, which may reflect the unique role of the tail in pentapedal locomotion and bipedal hopping.
Materials and methods
Six female western grey kangaroos were dissected for this study. The specimens were thawed and skinned. The most ventral part of the pubis was cut through in five of the specimens. This allowed a clear view of the ventral surface of the proximal tail and sacrum to observe the sacrocaudal musculature of the ventral compartment. The other specimen was cut transversely into 2-cm slices for muscle identification. Specimens were then fixed in 10% formalin for six weeks before dissection. Muscles were identified and reflected to confirm points of origin, from the cranial end to the insertion at the caudal end. Radiographs of two of the specimens were used to complement the dissection and confirm bony structures, particularly of the first caudal vertebrae. Additional loose and articulated lumbar, sacral and caudal vertebrae and os coxae were used to confirm landmarks and structures. Published descriptions of the proximal hind limb (Hopwood and Butterfield’s (1976) and an illustration of epaxial muscles (Dawson et al. 2004) were consulted during dissection. Illustrations of the musculature were produced by tracing or sketching high-quality photographs of the dissected specimens and the cross-sectioned tail. The muscles were named based on the guidelines set out in the Nomina Anatomica Veterinaria (International Committee on Veterinary Gross Anatomical Nomenclature 2012) and, where necessary, functional synonyms are given, based on caudal muscle descriptions in the literature (Barbour 1963; Lemelin 1995).
Results
Tendon tracks, functional groups and basic osteology in the tail of the western grey kangaroo
The tail is covered in deep caudal fascia, which is a continuation of the thick and fibrous thoracolumbar fascia. Across the dorsum the fascia provides an area for attachment of fibres from the large caudal extensor groups (see below). The deep caudal fascia along with bony processes divides the tail into six osteofascial compartments in the tail: two dorsal, two lateral and two ventral compartments.The dorsal compartments lie between the mammillary processes and the midline, the lateral compartments between the mammillary processes and the transverse processes and the ventral compartments between the transverse processes and the chevron bones, which attach to the haemal processes (Fig. 1). These six compartments are enclosed in deep caudal fascia. Embedded in the deep caudal fascia are six tracks of tendons that run along the length of the tail: the dorsal, lateral and ventral tracks. The dorsal tracks comprise tendons that insert on the mammillary processes (Fig. 1), and the muscles that send these tendons comprise the extensor group. These muscles lie in the dorsal and lateral compartments of the tail and are supplied by dorsal rami only. The lateral tracks comprise tendons that insert on the transverse processes, and the muscles that send these tendons comprise the lateral flexor group (Fig. 1). These muscles lie in the lateral and ventral compartments of the tail and are supplied by both dorsal and ventral rami (the precise supply is indicated later in the text). The ventral tracks comprise tendons that insert on the chevron bones, which attach to haemal processes ‘B’ in Fig. 1, and the muscles that send these tendons comprise the ventral flexor group. The flexors are located only in the ventral compartment of the tail and are supplied by exclusively ventral rami. The tail is divided into two regions, the proximal region, which includes the first four caudal vertebrae, and the distal region, which contains the last 20 or so caudal vertebrae. The transitional caudal vertebra (Ca5 in M. fuliginosus) divides these two regions and is characterised by the presence of cranial zygapophyses but not caudal zygapophyses (beyond the transitional vertebrae). The distal caudal vertebrae have no zygapophyses, though they retain mammillary processes and transverse processes (German 1982). Observations of articulated skeletons of M. fuliginosus suggest that caudal vertebrae 11–13 are likely to contact the ground during pentapedal locomotion. The proximal and transitional caudal vertebrae possess only a distal transverse process, which is quite large. Through the distal caudal series, a proximal transverse process (Fig. 1) appears and gradually increases in size while the distal transverse process diminishes towards the end of the tail.
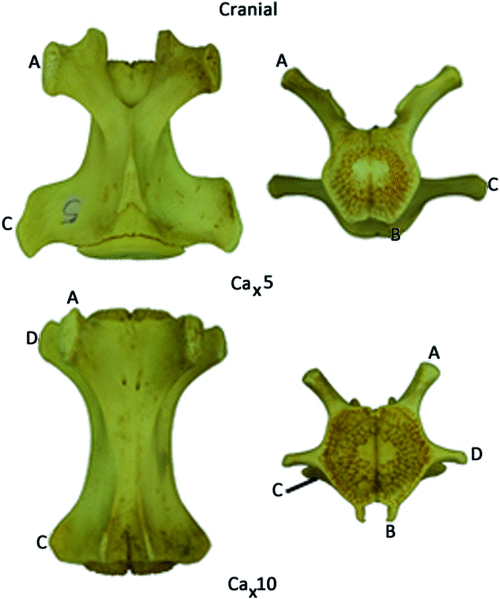
|
Extensor group
Mm. sacrocaudalis dorsalis lateralis (extensor caudae lateralis)
Most of mm. sacrocaudalis dorsalis lateralis (SDL) is restricted to the lumbar and sacral regions, where it occupies the space between the ilia, and is divided into medial and lateral bellies. Although the bellies are restricted to the base of the tail, their tendons extend further into the tail where they are embedded in deep caudal fascia. This muscle lies deep to the thoracolumbar fascia, which is continuous with the deep caudal fascia that insheathes the tail. Each belly comprises multisegmental fascicles, which form a strong rounded muscle. The long tendons from the lateral belly and the short tendons from the medial belly insert on mammillary processes and form the proximal portion of the dorsal track of tendons (Figs 2a, b and 4).
Lateral belly
The lateral belly of SDL appears to be a continuation of the m. longissimus thoracis. Fibres of the lateral belly run between the mammillary processes in the lumbar region to those in the base of tail (Figs 2a and 3). The lateral belly shares the mammillary processes with mm. longissimus and mm. multifidus in the lumbar region. Long thin tendons from each segment of the muscle stretch into the tail and attach to the mammillary processes of the fourth to the ninth caudal vertebra (Fig. 2a). The 12 tendons (six on each side) of the lateral belly insert laterally on their respective mammillary process (Figs 2a and 3). Each of these long tendons arise from intermuscular tendons within the belly itself. Fig. 4 shows the long tendons of the lateral belly inserting on mammillary processes.
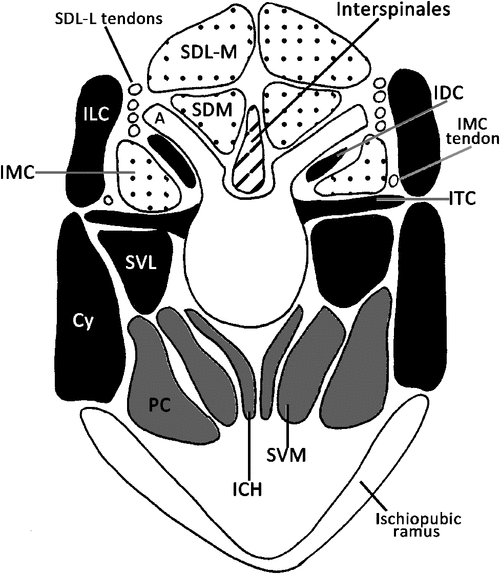
|
Medial belly
The medial belly is much larger and extends farther caudally than the lateral belly, and appears to be homologous with the m. semispinalis thoracis. The thoracolumbar fascia over the medial belly of SDL remains attached to the dorsal surface of the belly, even when freed from the surrounding muscle. The thoracolumbar fascia gives rise to an intermuscular septa, which divides the left and right sides of the medial belly and attaches to the spinous processes of the lower thoracic and lumbar vertebrae. The deep fascia covering this gives rise to the cranial head of m. caudofemoralis. M. caudofemoralis is a thick muscle with two heads, which extends laterally from its origin from this fascia, the ilium and the transverse processes of the first two caudal vertebrae, over the medial belly of SDL, to insert its tendon on the patelloid (Hopwood and Butterfield 1976). At its medial edge, m. caudofemoralis and the medial belly of SDL share some muscular fibres (Hopwood and Butterfield 1976). The segments of the medial belly run from the spinous processes of the lumbar and sacral vertebrae to the mammillary processes of the caudal vertebrae, crossing five or six segments. In the lumbar region, the fibres arise from the lateral surface of the spinous processes. In this region the medial belly occupies the space between the mammillary processes and the spinous processes (Fig. 3). At the caudal end, short tendons of the medial belly attach to the lateral sides of the first seven mammillary processes. The medial belly is the most superficial muscle on the dorsal surface before it terminates on the mammillary processes of the seventh caudal vertebra. Fig. 2 shows the dorsal and lateral aspects of SDL in the proximal region of the tail. In Figs 3 and 4 the relative reduction of SDL can be seen along the tail.
M. sacrocaudalis dorsalis medialis (extensor caudae medialis)
M. sacrocaudalis dorsalis medialis (SDM) is a small multisegmental muscle that is the caudal continuation of the m. multifidus lumborum. The fibres of SDM run from spinous processes of the sacrum to the medial side of the mammillary processes, spanning two or three segments. The three segments at the cranial part of the muscle are quite fleshy and distinct from each other, whilst those more caudal are less distinct. At the cranial end of the tail, this muscle lies deep to SDL (Figs 2a, 3 and 4). The deeper parts of SDM have shorter fibres that span only one or two segments (Fig. 2) similar to the mm. rotatores in the presacral vertebral column (Jüschke 1972; Evans and Christensen 1979).
M. intertransversarius medialis caudalis
M. intertransversarius medialis caudalis (IMC) lies in the medial part of the space between the mammillary process and the transverse process (Figs 3 and 4) and is supplied by dorsal rami. At the cranial end, IMC arises from a tendinous arch that is continuous with the m. longissimus lumborum. The tendinous arch extends caudally, attaching to the transverse processes of the sacrum and the first three caudal vertebrae. At the cranial border of the fourth caudal vertebra the tendinous arch gives rise to muscle fibres. These fibres form a small belly that generally spans three vertebrae, with a long tendon that inserts on the medial surface of the mammillary process of the 13th caudal vertebra. In the remainder of the tail this muscle forms nine more bellies. Each of these bellies arise from the cranial border of the transverse process, span three vertebrae and send a long tendon that inserts on the medial surface of the mammillary process of the caudal vertebra, which is 10 segments caudal from the transverse process of origin. Each tendon runs more laterally than the preceding tendon, and they are embedded in deep caudal fascia. These tendons form the distal part of the dorsal track of tendons.
M. intermammillary
Deep in the extensor group lies the unisegmental m. intermammillary, which comprises fascicles that run from mammillary process to mammillary process, similar to the m. intermammillary (intermammilares in Slijper 1946) described in the lumbar region of the kangaroo (Lickley 1904). Due to the arrangement of these fibres, and the nature of the sections being in line with the mammillary processes, this muscle is not depicted in Figs 3 and 4.
Lateral flexor group
M. coccygeus
M. coccygeus is a multisegmental muscle that fans out medially from the coccygeal fossa of the ischium towards the distal transverse processes of the proximal eight caudal vertebrae (Fig. 2b). At the base of the tail the belly of m. coccygeus lies on the lateral aspect of the tail until the level of the seventh/eighth caudal vertebra where it forms a broad aponeurotic attachment to the deep caudal fascia; note its absence from the distal segments of the tail (Fig. 2b, towards the caudal end, and Fig. 4). M. coccygeus is a triangular-shaped muscle, with an elongated base and a short, stout apex. Although it is a relatively thin muscle in the sacrocaudal region, as the muscle passes the tuber ischia on the caudal end of the pelvis it becomes very thick, but becomes thinner upon its caudal attachment. At the caudal end of its belly m. coccygeus is crossed cranially by m. piriformis, and both of these muscles lie deep to m. caudofemoralis, as it passes the pelvis laterally. M. coccygeus is supplied by ventral rami.
M. intertransversarius lateralis caudalis
M. intertransversarius lateralis caudalis (ILC) is multisegmental and lies laterally. Typically, fibres pass from distal transverse process to distal transverse process and span five segments. At the base of the tail, the cranial attachment is formed by two thin tendinous slips, which arise close to the attachment of m. longissiumus thoracis on the iliac crest. ILC also receives fibres from IMC and the lateral part of the dorsal surface of the distal transverse process (note its relations in Figs 3 and 4). The belly of ILC tapers from its cranial attachment until it is eventually represented by a thin aponeurosis (Fig. 2b); note its absence at the cross-section at the level of the 14th caudal vertebra in Fig. 4. This aponeurosis lies ventral to the dorsal track of tendons and becomes continuous with the deep caudal fascia (9th caudal vertebral level, Fig. 4). ILC lies laterally to the lateral belly of SDM over the sacrum, and is deep to the cranial head of m. caudofemoralis, and is supplied by ventral rami.
M. intertransversarii dorsalis caudalis (IDC)
This muscle is unisegmental and fascicles run from the mammillary processes to the root of the distal transverse processes of the first six caudal vertebrae and the proximal transverse process of the remainder of the caudal vertebrae. Its close relation to the mammillary process can be seen in Figs 3 and 4. The bundles of fibres are relatively small in the proximal part of the tail. The IDC becomes larger towards its caudal end, as the proximal transverse processes become more pronounced (14th caudal vertebral level, Fig. 4). The IDC is supplied by dorsal rami.
M. intertransversarii ventralis caudalis
M. intertransversarii ventralis caudalis (IVC) is a unisegmental muscle supplied by ventral rami. This muscle comprises short fibres that run between consecutive distal transverse processes throughout the tail and in the distal portion of the tail also comprises fibres that run from proximal transverse process to proximal transverse process (Figs 3 and 4).
M. sacrocaudalis ventralis lateralis (flexor caudae longus/lateralis)
M. sacrocaudalis ventralis lateralis (SVL) is the most dorsal bundle of fibres in the ventral compartment (Figs 2b, c, 3 and 4). The fibres of SVL arise from the ventral surfaces of the transverse processes and the lateral part of the ventral side of the sacrum. A few fibres also blend with the ILC. The most cranial segment of SVL inserts on the tip of the transverse process of the fifth caudal vertebra, and subsequent tendons continue to the end of the tail. This muscle group forms the lateral tendon track, which merges into the ventral tendon track in the distal tail.
Ventral flexor group
M. pubococcygeus
M. pubococcygeus (PC) is the most superficial of the ventral musculature in the tail (Fig. 2). M. pubococcygeus arises from the cranial end of the sacrum, the ventral surfaces of the pubis and the ischium. The belly of m. pubococcygeus does not extend farther into the tail than the fourth caudal vertebra; note that its belly is present at level 4, but not at 9 or 14 in Fig. 4. The tendon of the most cranial segment of m. pubococcygeus attaches to the chevron bone of the intervertebral joint between the 12th and 13th caudal vertebrae. Nine more consecutive tendons are inserted; the tendons lie laterally to the preceding tendon. The medial tendons of the ventral track initially comprise tendons from the m. pubococcygeus. The adjacent tendons extend from muscle bellies that arise from the segments of m. sacrocaudalis ventralis medialis.
M. sacrocaudalis ventralis medialis (flexor caudae brevis/medialis)
M. sacrocaudalis ventralis medialis (SVM) is a multisegmental muscle that arises from along the ventral midline of the sacral and caudal vertebrae, deep to m. pubococcygeus, and lateral to the m. interchevronii muscle groups (Figs 2c, 3 and 4). The most cranial segment of SVM attaches to the tip of the chevron bone between the 13th and 14th caudal vertebrae, the next segment attaches on the same place at the consecutive intervertebral joint, this continues throughout the tail (Fig. 4). The first three segments of SVM, those that lie under the sacrum and the proximal caudal vertebrae are distinct segments, are relatively uniform in size and shape. Beyond this point the segments of SVM become less distinct, but retain approximately the same size. These tendons span up to 10 caudal vertebrae before inserting. Nervous supply from the ventral rami is visible to SVM. Deeply, fibres of SVM blend with the m. interchevronii muscles.
M. interchevronii
The m. interchevronii (ICH) muscle group comprises superficial and deep fibres. The superficial fibres arise from the ventral aspect of the sacrocaudal joint and continue between bony segments to the tip of the tail. The superficial fibres of m. interchevronii span two segments and attach on the tip of the chevron bone of the next intervertebral joint. The deep fibres of m. interchevronii are short, unisegmental fibres that run between adjacent chevron bones (Figs 2c, 3 and 4).
Discussion
The role of the tail in kangaroo locomotion is that of an active organ, which provides support as a fifth limb during pentapedal locomotion, and contributes to the efficiency of bipedal hopping. In this study, we have revealed the anatomy of the caudal musculature, which has shed new light about the potential role of the caudal musculature in both bipedal hopping and pentapedal locomotion.
During the floating phase of bipedal hopping, the tail is thought to be actively raised (Hopwood and Butterfield 1990). Such action would require powerful extension of the tail and lumbar spine. It is likely that the relatively large mm. sacrocaudalis dorsalis lateralis, which passes from the lower lumbar region and the sacrum, and attaches to mammillary processes in the proximal tail would be responsible for this action. The large mm. sacrocaudalis dorsalis lateralis are also likely to be responsible for the action of the tail moving counter to the hind limbs, which may act to prevent pitching of the body (Baudinette 1994). The caudal extensors (mm. sacrocaudalis dorsalis lateralis and medialis) run continuously with the extensors of the back (mm. longissimus and mm. multifidus systems). The connection forms a functional chain of muscles from the presacral spine to the tail, and may act to maintain posture and stability of the body-axis during the floating phase of hopping. This function is analogous to that seen in quadrupedal mammals during running (Schilling 2011). The extensive thoracolumbar fasciae of the lumbar and caudal regions are also likely to contribute to support the body-axis during the hopping cycle.
Upon landing, the tail is at its highest (most extended) point of the bipedal hopping cycle (Hopwood and Butterfield 1990). The large proximal extensor mass, mainly the mm. sacrocaudalis dorsalis lateralis and medialis, may act to extend the tail and the trunk before landing. On landing, active extension of the tail and trunk may prevent the tail from hitting the ground in reaction to vertical ground reaction forces (Usherwood and Hubel 2012), which would tend to flex both the trunk and tail; the mm. sacrocaudalis dorsalis lateralis and medialis may act eccentrically to resist such flexion of the tail, and to prevent forward pitching of the body (Dawson and Taylor 1973; Bennett 1987; Baudinette 1994). As such, the tail may function as a dynamic cantilever in conjunction with the presacral spine to prevent forward pitching during the contact and floating phases of bipedal hopping.
The function of the tail as a counterbalance may be emphasised by the sharing of fibres between the medial belly of the mm. sacrocaudalis dorsalis lateralis with the cranial head of m. caudofemoralis, together with the extensive dense thoracolumbar fascia over the lumbar region (Hopwood and Butterfield 1976). This appears to create a second functional chain between the extensors of the tail and an extensor of the hip and knee joints.
During pentapedal locomotion the kangaroo alternates between using the hind limbs, forelimb and tail to support its body weight (Windsor and Dagg 1971; Dawson and Taylor 1973). Before the body weight can be supported by the tail, the proximal tail is flexed and drawn under the pelvis (close to the centre of mass), while the mid-distal tail (Ca8–13) is extended, creating the characteristic ‘S-shape’ of the tail (Frith and Calaby 1969; Windsor and Dagg 1971; Dawson and Taylor 1973; Alexander and Vernon 1975; Dawson 1977; Bennett 1999) (Fig. 5b). The relatively large ventral flexors, the m. pubococcygeus and the m. sacrocaudalis ventralis lateralis, which extend from the sacrum to the chevron bones and transverse processes of the mid-distal region, would appear to be largely responsible for the repositioning of the distal tail. The lateral flexors, the m. coccygeus from the ischium to the proximal caudal vertebrae, and the intertransversarius lateralis caudalis and the m. sacrocaudalis ventralis lateralis, which connect the ilium to the caudal vertebrae, are also likely to be involved in the repositioning of the proximal tail under the kangaroo’s centre of mass (Fig. 5a, b). The strong action of m. coccygeus is evident on the ischium, where a marked fossa indicates the origin of this muscle in M. fuliginosus. Beyond the sacral region, the flexor musculature is relatively larger than the other functional groups in the tail of M. fuliginosus, in comparison to other terrestrial mammals that have relatively equal dorsal and ventral caudal musculature, highlighting the functional importance of these muscles (Lemelin 1995; Endo et al. 2012). The role of the flexor caudal musculature for weight-bearing in large kangaroos is further evidenced by the large ‘hatchet-shaped’ chevron bones that provide large areas for insertion of the caudal flexors (Owen 1876; Flower 1885).
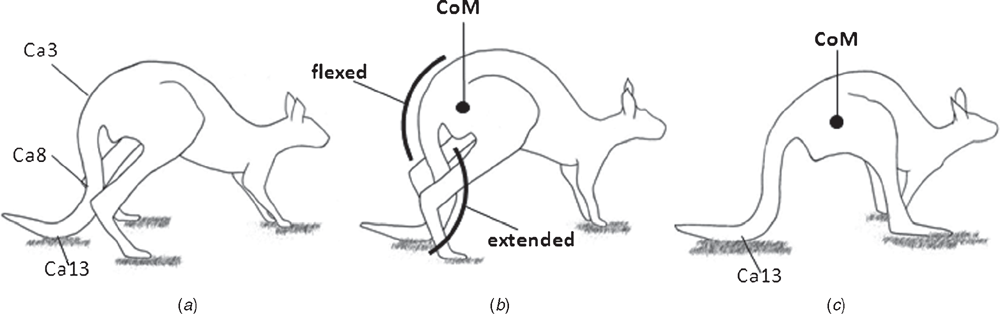
|
After the tail has been repositioned under the pelvis, it then provides a rigid column of support for the body weight in conjunction with the forelimbs, while the hind limbs are swung forward (Dawson and Taylor 1973) (Fig. 5b, c). During this movement, it is likely that the proximal extensor mass (m. sacrocaudalis dorsalis lateralis and m. sacrocaudalis dorsalis medialis) acts eccentrically to prevent the proximal tail from flexing further under load bearing. Similarly, the flexors, which send long tendons to the mid-distal tail (m. pubococcygeus and m. sacrocaudalis dorsalis ventralis) may also act eccentrically to prevent further extension of the mid-distal tail under load. The maintenance of the relatively stable pelvis by the tail during this weight-bearing phase of pentapedal locomotion is likely to be the action of lateral flexors that provide a muscular link between the pelvis and the proximal caudal vertebrae. Intersegmental muscles (m. interchevronii, m. intermamillary, m. intertransversarius medialis caudalis, m. intertransversarius lateralis caudalis and m. intertransversarius dorsalis caudalis) are all likely to provide additional support, in a similar manner to the short postural muscles of the lumbar spine in humans (Lickley 1904; Basmajian and Slonecker 1989). Not considered here are the caudal intervertebral discs, which could contribute to the weight-bearing role of the tail during pentapedal locomotion.
While the tail is supporting the body weight, the centre of mass moves forwards as the hind limbs are swung forward, and the tail straightens from the characteristic ‘S-shape’ (Fig. 5b, c). The extensors may act concentrically to straighten (or ‘un-flex’) the proximal tail, while the flexors may act to ‘un-extend’ the mid-distal tail. The action of these muscles likely contribute to the propulsive force that causes the forward movement of the centre of mass while the tail is supporting the body weight during pentapedal locomotion (Fig. 5) (Dawson and Taylor 1973).
The inclusion of only female specimens is a potential limitation of this study, and certainly this will need to be considered in future studies. M. fuliginosus displays significant sexual dimorphism in body size: mature males may attain double the body mass of breeding females (Jarman 1991). Warburton et al. (2013) demonstrated in M. fuliginosus that the extreme forelimb development of males, with significant positive allometry of muscle mass and disproportionate development of muscles specifically involved in male–male fighting, are under sexual selection. That study highlighted the potential infraspecific implications on muscle development where there are differences in behaviour by males and females. In terms of tail use, male kangaroos utilise their tails as a supporting limb during male–male fighting, and thus sexual dimorphism in the muscular anatomy of the tail is likely (Jarman 1991). As the focus of the current study was the functional morphology of the tail for locomotion, the study was restricted to females in order to remove the possibly confounding effects of male-specific fighting behaviour and growth traits.
This study has shown that the extensor muscles dominate in the sacral and most proximal parts of the tail and that the flexors dominate in the proximal half of the tail, and how this relates to the tail’s role in pentapedal locomotion and bipedal hopping. However, the arrangement of the long tendons and short muscles appears to continue in a uniform, segmented pattern along the entire length of the tail, emphasising its unitary structure in continuity with the presacral spinal musculature.
Acknowledgements
The authors thank Dianna Nottle, Joe Hong and Lance Boston from the School of Veterinary and Life Sciences at Murdoch University for their time and effort in preparing the specimens for dissection. We also acknowledge the editor, Dr Paul Cooper, the anonymous reviewer and Dr Vanessa Hayes from the School of Anatomy, Physiology and Human Biology at UWA for their helpful suggestions and comments. This project was funded by the School of Anatomy, Physiology and Human Biology at the University of Western Australia.
References
Alexander, R. M., and Vernon, A. (1975). The mechanics of hopping by kangaroos (Macropodidae). Journal of Zoology 177, 265–303.| The mechanics of hopping by kangaroos (Macropodidae).Crossref | GoogleScholarGoogle Scholar |
Barbour, R. A. (1963). The musculature and limb plexuses of Trichosurus vulpecula. Australian Journal of Zoology 11, 488–610.
| The musculature and limb plexuses of Trichosurus vulpecula.Crossref | GoogleScholarGoogle Scholar |
Basmajian, J. V., and Slonecker, C. E. (1989). ‘Grant’s Method of Anatomy. A Clinical Problem-solving Approach.’ (Williams & Wilkins: Baltimore.)
Baudinette, R. V. (1994). Locomotion in macropodoid marsupials: gaits, energetics and heat balance. Australian Journal of Zoology 42, 103–123.
| Locomotion in macropodoid marsupials: gaits, energetics and heat balance.Crossref | GoogleScholarGoogle Scholar |
Bennett, M. B. (1987). Fast locomotion of some kangaroos. Journal of Zoology 212, 457–464.
| Fast locomotion of some kangaroos.Crossref | GoogleScholarGoogle Scholar |
Bennett, M. B. (1999). Foot areas, ground reaction forces and pressure beneath the feet of kangaroos, wallabies and rat-kangaroos (Marsupialia: Macropodoidea). Journal of Zoology (London) 247, 365–369.
Chang, H., and Ruch, T. C. (1947). Morphology of the spinal cord, spinal nerves, caudal plexus, tail segmentation, and caudal musculature of the spider monkey. The Yale Journal of Biology and Medicine 19, 345–377.
| 1:STN:280:DyaH2s%2FksFyjsg%3D%3D&md5=654c07b743b8896b191b2385ed35d87aCAS | 20284286PubMed |
Coulson, G. (1990). Habitat separation in the grey kangaroos, Macropus giganteus (Shaw) and M. fuliginosus (Desmarest) (Marsupialia: Macropodidae) in Grampians National Park, western Victoria. Australian Mammalogy 13, 33–40.
Coulson, G. (1993). Use of heterogeneous habitat by the western grey kangaroo, Macropus fuliginosus. Wildlife Research 20, 137–139.
| Use of heterogeneous habitat by the western grey kangaroo, Macropus fuliginosus.Crossref | GoogleScholarGoogle Scholar |
Dawson, T. J. (1977). Kangaroos. Scientific American 237, 78–89.
Dawson, T. J., and Taylor, C. R. (1973). Energetic cost of locomotion in kangaroos. Nature 246, 313–314.
| Energetic cost of locomotion in kangaroos.Crossref | GoogleScholarGoogle Scholar |
Dawson, T. J., Mifsud, B., Raad, M. C., and Webster, K. N. (2004). Aerobic characteristics of red kangaroo skeletal muscles: is a high capacity matched by muscle mitochondrial and capillary morphology as in placental mammals? Journal of Experimental Biology 207, 2811–2821.
| Aerobic characteristics of red kangaroo skeletal muscles: is a high capacity matched by muscle mitochondrial and capillary morphology as in placental mammals?Crossref | GoogleScholarGoogle Scholar | 15235010PubMed |
Endo, H., Mori, K., Koyabu, D., Kawada, S., Komiya, T., Itou, T., Koie, H., Kitagawa, M., and Sakai, T. (2012). Functional morphology of the aardvark tail. Anatomia, Histologia, Embryologia , .
| Functional morphology of the aardvark tail.Crossref | GoogleScholarGoogle Scholar | 22713114PubMed |
Evans, H. E., and Christensen, G. C. (1979). ‘Miller’s Anatomy of the Dog.’ 2nd edn. (W.B. Saunders Co.: Philadelphia.)
Flower, W. H. (1885). ‘An Introduction to the Osteology of the Mammalia.’ (Macmillan and Co.: London.)
Frith, H.J., and Calaby, J.H. (1969). ‘Kangaroos.’ (Wilke and Co. Ltd: Melbourne.)
German, R. Z. (1982). The functional morphology of caudal vertebrae in New World monkeys. American Journal of Physical Anthropology 58, 453–459.
| The functional morphology of caudal vertebrae in New World monkeys.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s%2FitVShsQ%3D%3D&md5=ef4e6d84de5bcc21022ff64092d9964eCAS | 7124940PubMed |
Grand, T. I. (1990). Body composition and the evolution of the Macropodidae (Potorous, Dendrolagus and Macropus). Anatomy and Embryology 182, 85–92.
| Body composition and the evolution of the Macropodidae (Potorous, Dendrolagus and Macropus).Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M%2Flt1Citg%3D%3D&md5=8290554690fe3a595ef760e855e92ce6CAS | 2240596PubMed |
Hopwood, P. R., and Butterfield, R. M. (1976). The musculature of the proximal pelvic limb of the eastern grey kangaroo Macropus major (Shaw) and Macropus giganteus (Zimm). Journal of Anatomy 121, 259–277.
| 1:STN:280:DyaE283ht1Ohuw%3D%3D&md5=5f1d147ca6565af25a2a514be96eb74bCAS | 931780PubMed |
Hopwood, P. R., and Butterfield, R. M. (1990). The locomotor apparatus of the crus and pes of the eastern grey kangaroo, Macropus giganteus. Australian Journal of Zoology 38, 397–413.
| The locomotor apparatus of the crus and pes of the eastern grey kangaroo, Macropus giganteus.Crossref | GoogleScholarGoogle Scholar |
Howell, A. B. (1971). ‘Aquatic Mammals and their Adaptations to Life in the Water.’ (Dover Publications Inc.: New York.)
Hume, I. D., Jarman, P. J., Renfree, M. B., and Temple-Smith, P. D. (1989). Macropodidae. In ‘Fauna of Australia. Vol. 1B. Mammalia’. (Eds D. W. Walton and B. J. Richardson.) pp. 1–70. (AGPS: Canberra)
International Committee on Veterinary Gross Anatomical Nomenclature (2012). ‘Nomina Anatomica Veterinaria.’ (World Association of Veterinary Anatomists: New York.)
Jarman, P. J. (1991). Social behaviour and organization in the Macropodoidea. In ‘Advances in the Study of Behavior’. (Eds P. J. B. Slater, J. S. Rosenblatt and C. Beer.) pp. 1–47. (Academic Press: San Diego, CA.)
Jüschke, S. (1972). Untersuchungen zur funktionellen Anpassung der Rückernmuskulatur und der Wirbelsäule quadrupeder Affen und Känguruhs. Zeitschrift für Anatomie und Entwicklungsgeschichte 137, 47–85.
| Untersuchungen zur funktionellen Anpassung der Rückernmuskulatur und der Wirbelsäule quadrupeder Affen und Känguruhs.Crossref | GoogleScholarGoogle Scholar | 4627009PubMed |
Lemelin, P. (1995). Comparative and functional myology of the prehensile tail in New World monkeys. Journal of Morphology 224, 351–368.
| Comparative and functional myology of the prehensile tail in New World monkeys.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2MzhvV2rsg%3D%3D&md5=9d4ca41d562449beeb0828702c6279d4CAS | 7595958PubMed |
Lickley, J. D. (1904). Morphology of human intertransverse muscles. Journal of Anatomy and Physiology 39, 90–98.
| 1:STN:280:DC%2BD2s%2FosFKltw%3D%3D&md5=b12e61a4a7d16564c36f7017e60266f5CAS | 17232627PubMed |
Murie, J. (1870). Notes on the white-beaked bottlenose, Lagenorhynchus albirostris, Gray. Journal of the Linnean Society of London, Zoology 11, 141–153.
Nowak, R. M. (Ed.) (1991). ‘Walker’s Mammals of the World.’ 5th edn. (The Johns Hopkins University Press: Baltimore, MD.)
Organ, J. M. (2010). Structure and function of plattyrrhine caudal vertebrae. Anatomical Record 293, 730–745.
| Structure and function of plattyrrhine caudal vertebrae.Crossref | GoogleScholarGoogle Scholar |
Owen, R. (1848). ‘On the Archetype and Homologies of the Vertebrate Skeleton.’ (John Van Voorst, Paternoster Row: London.)
Owen, R. (1876). IX. On the osteology of the Marsupialia. (Part V.) Fam. Poephaga, Genus Macropus. Transactions of the Zoological Society of London 9, 417–446.
Parsons, F. G. (1896). On the anatomy of Petrogale xanthopus, compared with that of other kangaroos. Proceedings of the Zoological Society of London 64, 683–714.
| On the anatomy of Petrogale xanthopus, compared with that of other kangaroos.Crossref | GoogleScholarGoogle Scholar |
Schilling, N. (2011). Evolution of the axial system in craniates: morphology and function of the perivertebral musculature. Frontiers in Zoology 8, 1–19.
Slijper, E. J. (1946). ‘Comparative Biologic–Anatomical Investigations on the Vertebral Column and Spinal Muscles of Mammals.’ (N.V. Noord-Hollandsche Uitgevers Maatschappij: Amsterdam.)
Strahan, R. (1995). ‘The Mammals of Australia.’ 2nd edn. (Reed New Holland: Sydney.)
Tribe, D. E., and Peel, L. (1963). Body composition of the kangaroo (Macropus sp). Australian Journal of Zoology 11, 273–289.
| Body composition of the kangaroo (Macropus sp).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXktVOquw%3D%3D&md5=7f255e6c1e4f9ed25cea04a2d1c7eebeCAS |
Usherwood, J. R., and Hubel, T. Y. (2012). Energetically optimal running requires torques about the centre of mass. Journal of the Royal Society, Interface 9, .
| Energetically optimal running requires torques about the centre of mass.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38nhvVelug%3D%3D&md5=ddd687e1da80722bb11091ccc512fa36CAS | 22572024PubMed |
Warburton, N. M., Bateman, P. W., and Fleming, P. A. (2013). Sexual selection on forelimb muscles of western grey kangaroos (Skippy was clearly a female). Biological Journal of the Linnean Society 109, 923–931.
| Sexual selection on forelimb muscles of western grey kangaroos (Skippy was clearly a female).Crossref | GoogleScholarGoogle Scholar |
Windsor, D. E., and Dagg, A. I. (1971). The gaits of the Macropodinae (Marsupialia). Journal of Zoology 163, 165–175.
| The gaits of the Macropodinae (Marsupialia).Crossref | GoogleScholarGoogle Scholar |




