Brushtail possums (Trichosurus vulpecula) dominate dama wallabies (Notamacropus eugenii) and Bennett’s wallabies (Notamacropus rufogriseus) at bait feeders: implications for invasive species management
Graham J. Hickling A * and Tim D. Day B
A * and Tim D. Day B
A
B
Abstract
Dama wallaby (Notamacropus eugenii) and Bennett’s wallaby (N. rufogriseus) are invasive pests on the New Zealand mainland and consequently are subject to ongoing control measures that include deployment of toxic baits in feeding stations.
We investigated whether behavioural interactions between wallabies and introduced brushtail possums (Trichosurus vulpecula) at bait feeders are likely to reduce the efficacy of this method for wallaby control.
Wallaby and possum visits and encounters at cereal bait feeders were monitored with trail cameras for several months in dama wallaby habitat near Rotorua, and in Bennett’s wallaby habitat in South Canterbury. The response of Bennett’s wallabies to possum carcases placed at feeders was also assessed.
The diurnal activity of wallabies and possums at the feeders overlapped extensively, although Bennett’s wallabies exhibited more daytime activity than the other two species. Thousands of visits by wallabies and possums were recorded but close encounters between the species at feeders were infrequent (N = 251). When encounters did occur, the wallaby was usually excluded from the feeder (72% of 229 encounters at Titoki Estate, near Rotorua, and 95% of 22 encounters at Blue Cliffs, South Canterbury) regardless of which species arrived at the feeder first. Zero instances of a wallaby excluding a possum from a feeder were recorded. When possum carcases were placed beside feeders, visitations by Bennett’s wallabies reduced significantly, by 86% during the first week when a possum carcase was present. This effect was short lived, however, because the carcase soon decayed or was scavenged by other wildlife.
Despite their smaller body size, possums are strongly behaviourally dominant over both wallaby species. Consequently, possums may empty feeders of bait before visiting wallabies have an opportunity to feed. Furthermore, interruption and exclusion of feeding wallabies by possums will increase the risk of sublethal toxic dosing and consequent bait shyness.
Since possums are common throughout most wallaby habitat in New Zealand, their dominance behaviour is likely to reduce the efficacy of bait feeder control of wallabies at many sites. Possum population suppression is, therefore, likely to increase the effectiveness of wallaby bait feeder programs but will be costly to achieve.
Keywords: aggression, bait stations, behavioural interaction, Bennett’s wallaby, brushtail possum, dama wallaby, invasive species, social dominance, vertebrate pest control.
Introduction
Dama (or tammar) wallaby (Notamacropus eugenii) and Bennett’s (or red-necked) wallaby (N. rufogriseus) are invasive pests on the New Zealand mainland. Dama wallabies were liberated in the Rotorua district in 1912 and again in 1940 and have since expanded in abundance and geographic range throughout that region (Latham et al. 2019). Bennett’s wallabies were liberated near Waimate, South Canterbury in 1874; their initial spread was limited but low-density populations have since emerged over a large area of Canterbury and parts of Otago (Latham et al. 2019). Brushtail possums (Trichosurus vulpecula, hereafter ‘possums’) were repeatedly introduced from Australia in the mid to late 1800s and are now widespread across the New Zealand mainland (Cowan and Glen 2021), including throughout most habitats occupied by wallabies.
Both wallaby species are primarily grazers, supplementing their diet with browse when in scrub and forest habitats. Dama wallabies are a potential pasture pest and damage new production forestry plantings, although their largest impacts are on indigenous vegetation (Latham and Warburton 2021). In 2016, their annual impact on agriculture and the environment was estimated as being as high as NZ$4 million (Latham et al. 2016). Bennett’s wallabies have been recognised as a significant pest of pasture, crops and production forestry since the 1940s (Warburton 1986). In 2016, their annual impact was estimated as being as high as NZ$24 million (Latham et al. 2016).
Coordinated efforts to control dama wallabies in the Rotorua district using aerial 1080 operations began in 1962, with various methods and intensities of control applied in subsequent decades. Regional pest management plans for the dama population, which overlaps the boundaries of three administrative regions, have variously aimed for containment or elimination of the species (Latham and Warburton 2021) but have been unsuccessful in preventing spread.
Government-subsidised hunting to suppress the Bennett’s wallaby population was first implemented in 1947. In late 1989, government subsidies ended and thereafter the full cost of control has been met by landowners (Latham and Warburton 2021). A regional pest management plan aimed at preventing further geographic spread of Bennett’s wallabies has been unsuccessful, in part because culling was insufficient but also because of deliberate relocations of wallabies (Latham et al. 2019).
In July 2020, the Tipu Mātoro National Wallaby Eradication Programme – a partnership between central and regional government, landowners, and other stakeholders – was initiated. The Programme’s stated goal is to push mainland wallaby populations back into containment areas designated in regional pest management plans, as a first step towards the longer term goal of eradicating wallabies from New Zealand (MPI 2022).
In addition to contracted and private hunting, deployment of toxic 1080-impregnated cereal baits from aircraft has proven an effective method for large-area wallaby pest control (Latham and Warburton 2021). There are; however, significant limitations to where aerial 1080 can be deployed because of potential risk to non-target species and other social concerns. Consequently, there have been ongoing efforts to develop effective ground-based wallaby baiting methods, including the development of alternative toxins (e.g. Shapiro et al. 2011), foliage baiting techniques (Warburton 1990) and the use of bait feeder stations (Williams 1997).
Bait feeders deploying cereal baits are an attractive alternative to aerially-sown or hand-laid distribution of baits because they facilitate removal of uneaten toxic bait at the end of an operation, thereby eliminating ongoing risk to livestock and other non-target species. Some feeder designs also provide weather protection for the cereal bait. Bait feeders deploying an initial pulse of non-toxic pre-feed followed by a toxic baiting phase are a well-established control method in New Zealand for possums (Warburton and Thomson 2002) but are of unproven efficacy for wallaby control (Williams 1997).
The observations reported here arose from a field trial funded by the Tipu Mātoro National Wallaby Eradication Programme with the aim of developing more effective bait feeder designs to assist wallaby control efforts. During set-up of the trial, we were advised by local field staff (D. Williams, Bay of Plenty Regional Council, pers. comm.) that possum interference with the feeders was likely. Consequently, it became an additional aim of the study to investigate whether possum activity at the feeders might reduce the efficacy of the feeders for wallaby control. Here, we report and discuss only our wallaby–possum behavioural findings; our feeder-design results will be reported elsewhere.
Methods
Field sites
Bait feeder observations were carried out on a 300 ha area of Titoki Estate (lat −38.066, lon 176.389) north-east of Rotorua in the North Island (for dama wallabies) and on a 320 ha block at Blue Cliffs Station (lat −44.517, lon 170.876) in inland South Canterbury in the South Island (for Bennett’s wallabies). Titoki Estate is a privately owned low-intensity livestock operation, with substantial areas of native forest, pine forest, scrubland, pasture, restoration plantings and wetlands, all of which provide suitable habitat for dama wallabies. Aside from limited recreational shooting, the wallaby population on the farm had been relatively undisturbed before this trial.
Blue Cliffs Station is a livestock operation on hilly farmland pasture, short and tall tussock, scrub, and remnant forest in gullies dominated by māhoe (Melicytus lanceolatus) and broadleaf/kapuka (Griselinia littoralis). Bennett’s wallabies are periodically hunted at the farm by contract shooters and occasionally by recreational hunters. Possum populations at the site had been reduced in previous years as part of a large-area bovine tuberculosis control programme.
No possum density estimates were available at either site prior to our field trials; however, possum numbers were expected to be lower at Blue Cliffs because the habitat was less forested and there had been large-scale possum control in the area in recent years. We were advised by local field staff that possums were likely to interfere with the Titoki feeders (D. Williams, Bay of Plenty Regional Council, pers. comm.) so to reduce that possibility, possum numbers at Titoki were lowered over a 2-week period prior to the trial using methods that did not affect wallaby numbers (i.e. a combination of leg-hold traps and night-shooting). The efficacy of this possum control was not formally assessed but our field staff reported that nightly possum encounters at Titoki had been reduced by >90% in the vicinity of the feeders by the end of the control work.
Bait feeder deployment
In early summer (October – December) 2022, we recorded wallaby and possum visits to four designs of bait feeders stocked with non-toxic bait and to a pile of cereal pellets on the ground, which served as an experimental control. Two of the feeder designs consisted of plastic containers mounted on stakes, each containing 1 kg of cereal pellets (e.g. Fig. 1). The other two designs consisted of 8 × 18 g Ferafeed ‘strikers’ (https://www.connovation.co.nz/products/ferafeed-striker-18g-213-paste-backing-card) stapled to wooden boards (one laid on the ground and the other mounted on a stake). In this report we focus on behavioural interactions between wallabies and possums seen at these various baited sites. We refer to all sites as ‘feeders’ but emphasise that this term encompasses all five treatments, including cereal pellets on the ground.
Example of a bait feeder deployed for dama wallabies at Titoki; the feeder contains 1 kg of non-toxic cereal pellets and is being monitored by a pair of infrared trail cameras.

At both field sites, replicates of these five treatments were deployed at 200 m intervals along a transect that ran through habitat with abundant recent wallaby signs (i.e. runs, pads and faecal pellets). At Titoki, six replicates of each treatment were observed for 10 weeks. At Blue Cliffs, where deployment of stations was delayed by livestock operations, eight replicates of the treatments were observed for 4 weeks. At weekly intervals, any missing bait was replaced.
During the field study we received an anecdotal report that wallabies tended to avoid possum carcases lying close to feeders (R. Harland, Greater Wellington Regional Council, pers. comm.). To investigate this possibility, for an additional 2 weeks at Blue Cliffs, a single possum carcase (freshly kill-trapped by a local pest control contractor) was placed beside three to four replicates of each treatment for either 1 week (n = 12 feeders) or 2 weeks (n = 8). Notes were kept on the rate of carcase decomposition. The field trial at Titoki was near completion when this extension to the study was approved, so we did not have an opportunity to deploy carcases at that site.
In winter (July – September) 2023 we ran a second field trial to evaluate the effectiveness of a pulsed non-toxic/toxic baiting strategy aimed at reducing possum and wallaby numbers at each treatment. Feeders were deployed in the same general areas as the summer trial but at locations where wallabies would not have encountered the previous feeders. At Titoki, the new transect was in similar habitat as before. At Blue Cliffs, a change in livestock grazing meant that the new transect was located in high-elevation tussock grasslands distant from possums’ preferred habitat. Feeders were monitored for 8 weeks at both sites. At Titoki, 30 feeders were pre-fed with non-toxic bait in Weeks 1, 2, 5 and 7, and with toxic bait (Pindone plus either Feratox or Cyanara50 assigned randomly) in Weeks 3, 4, 6 and 8. At Blue Cliffs, the toxic bait was deployed in Weeks 2, 6, 7 and 8.
Behavioural observations
All treatments were monitored by deploying pairs of Reconyx HyperFire 2 Covert IR infrared trail cameras (http://reconyx.com) at each feeder. Cameras were placed 2.0 m and 3.5 m from the feeder, with the cameras’ angles of view perpendicular to each other (e.g. Fig. 1). Analysis of continuous video from 140 cameras was impractical, so cameras were set to record bursts of five images per triggering event, with ‘no delay’ between images and between successive triggering events (the actual delay was approximately 0.4 s). This provided image sequences of wallabies interacting with the feeders, conspecifics and occasionally other wildlife. During each trip to replenish the bait feeders, the previous week’s images were retrieved and camera batteries were checked and replaced if necessary.
The cameras recorded 2.3 million and 1.8 million images from Titoki and Blue Cliffs, respectively, so to reduce processing time, images from the 2.0 m cameras were reviewed only if the 3.5 m camera’s view of visiting animals was obscured. Once all bait had been removed from a feeder, subsequent images for that week from that site were disregarded. Images without animals (e.g. triggered by wind-blown vegetation) were excluded using the AI image recognition model MegaDetector v5 (Microsoft AI for Earth 2020). Date and time metadata were extracted from the remaining images using an Excel macro to assign each image to a unique ‘visit’, defined as a sequence of images separated by intervals of no more than 5 min of one or several individuals of a single species (identified manually by a researcher). Individuals making repeat visits to stations were not identifiable, so visitation rate is an index of animal activity rather than of population abundance. Mean visitation rates before and during the deployment of carcases were compared using ANOVA with post hoc comparison of means, using R (R Core Team 2023).
For each visit, we manually recorded: the number of animals seen; which species were present; whether an encounter between wallabies and possums occurred – and if so which individual arrived first, what behaviour was exhibited by each individual, and what outcome resulted. Here, we report on these encounters between wallabies and possums, defined as instances where the timing of visits to the feeder by wallabies and possums overlapped. ‘Behaviours’ and ‘outcomes’ resulting from encounters were assigned using the categories listed in Table 1. As there was no indication that feeder design affected encounter outcomes, these data were pooled for all feeders. Wallaby responses to the individual feeder designs will be reported elsewhere.
| Behaviours | ||
| Ignore | No visible reaction to the other animal | |
| Caution/vigilance | Alert posture, motionless or cautious movement, and/or gaze directed at the other animal | |
| Aggression | Raised posture, raised forelimbs, gaping mouth, vocalisations, pouncing, and/or chasing | |
| Submission/retreat | Submissive posture, averted gaze, and/or avoidance movements | |
| Outcomes | ||
| Gains/retains access to feeder | Animal accesses bait (either directly from the feeder or from spillage on the ground beneath it) | |
| Waits near feeder | Animal remains visible on-camera but is unable to access bait | |
| Departs feeder | Animal moves out of the cameras’ field of view | |
Results
Over 10 weeks in October – December 2022, we recorded 1228 visits by dama wallabies at the 30 Titoki feeders, with multiple wallabies present during 613 visits (49.9%). We also recorded 4476 possum visits to the feeders, for an average visitation rate of 12.1 wallabies and 22.1 possums per feeder per week. Over 4 weeks in November 2022 at Blue Cliffs, we recorded 2660 visits by Bennett’s wallabies at the 40 feeders, with multiple wallabies present during 604 visits (22.3%). We also recorded 2668 possum visits, for an average visitation rate of 26.1 wallabies and 16.7 possums per feeder per week. We also recorded several hundred visits by European hedgehogs (Erinaceus europaeus) at both sites and by ship rats (Rattus rattus) at Titoki, and infrequent visits by various other birds and introduced mammals; all of these animals were essentially ignored by the wallabies and possums.
In early summer, possum activity at the feeders was highest from 20:00 to 05:00 h at Titoki and 21:00 to 05:00 h at Blue Cliffs (Fig. 2a, b). The 1-h difference between sites consistent with sunset being about 40 min earlier at Titoki in the north (at c. 20:20 h) relative to Blue Cliffs in the South Island (at c. 21:00 h). This timing of brushtail possum activity matches past reports that possums typically become active about 30 min after sunset both in their native Australia (e.g. MacLennan 1984) and in New Zealand (e.g. Ward 1978).
Wallaby and brushtail possum diurnal activity at bait feeders: (a) at 30 stations monitored for 10 weeks at Titoki (Rotorua district, October – December 2022); (b) at 40 stations monitored for 4 weeks at Blue Cliffs (South Canterbury; November 2022). Shaded blocks indicate the hours between sunset and sunrise in November at the two sites.
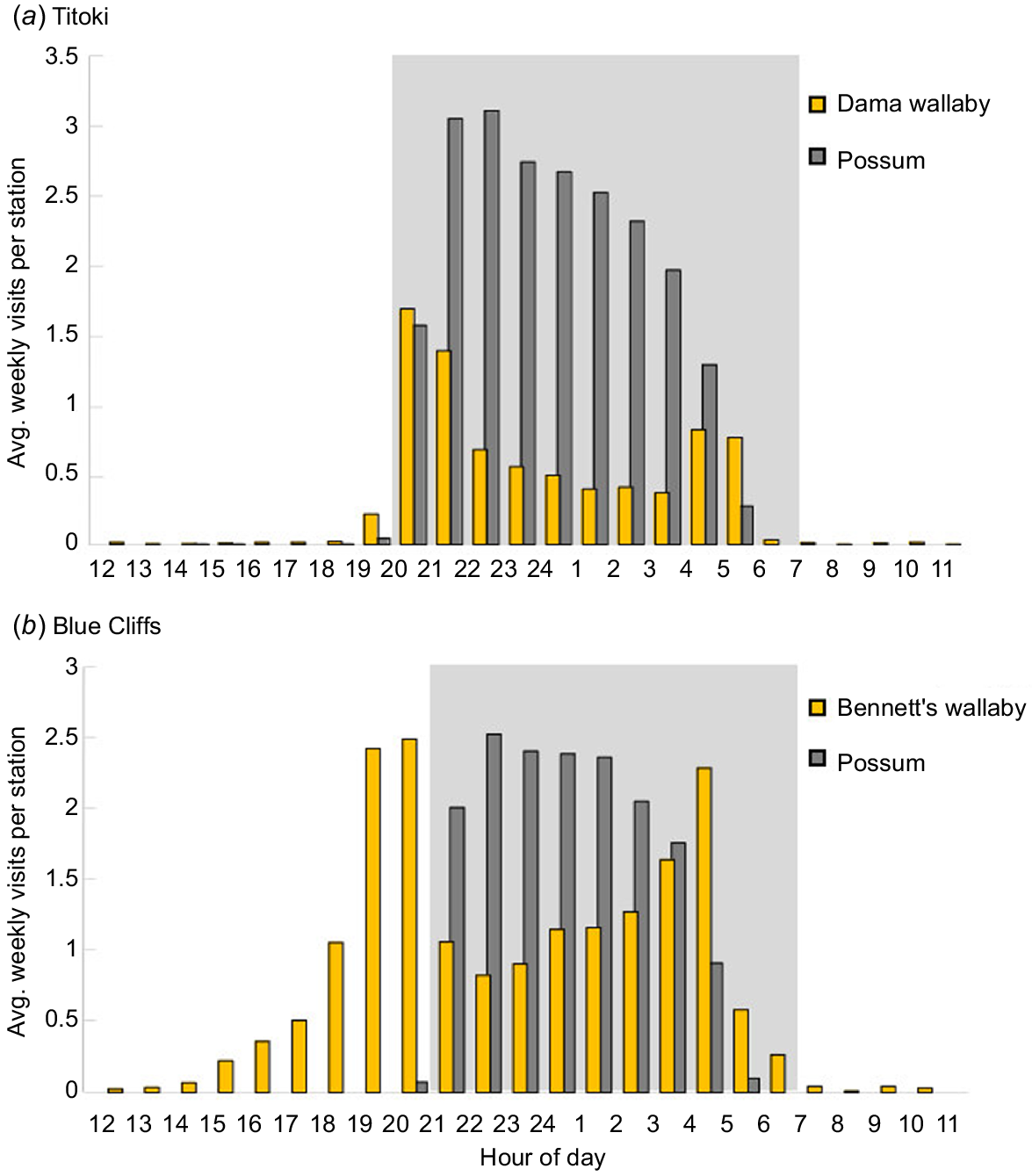
Dama wallabies’ activity at feeders occurred almost entirely between sunset and sunrise, coinciding with possums’ active period (Fig. 2a). Bennett’s wallabies visited throughout the night but were also often seen at feeders in daylight hours when possums were inactive, particularly in the late afternoon (Fig. 2b). Both wallaby species’ activity levels peaked near sunset, declined as possums become active, and then increased again when possum activity dropped off as dawn approached.
Despite >10,000 visits by wallabies and possums recorded across all treatments, it was uncommon for wallabies and possums to be seen together at a feeder. During our 2022 trial, possums were seen during 134 of 1228 visits by dama wallaby and during 22 of 2660 visits by Bennett’s wallaby (10.9% and 0.8% of wallaby visits, respectively; Fisher Exact test, P < 0.0001). In winter 2023, possums were seen during a further 95 of 1826 dama wallaby visits but were never seen during 1530 Bennett’s wallaby visits (5.2% and 0% of wallaby visits, respectively).
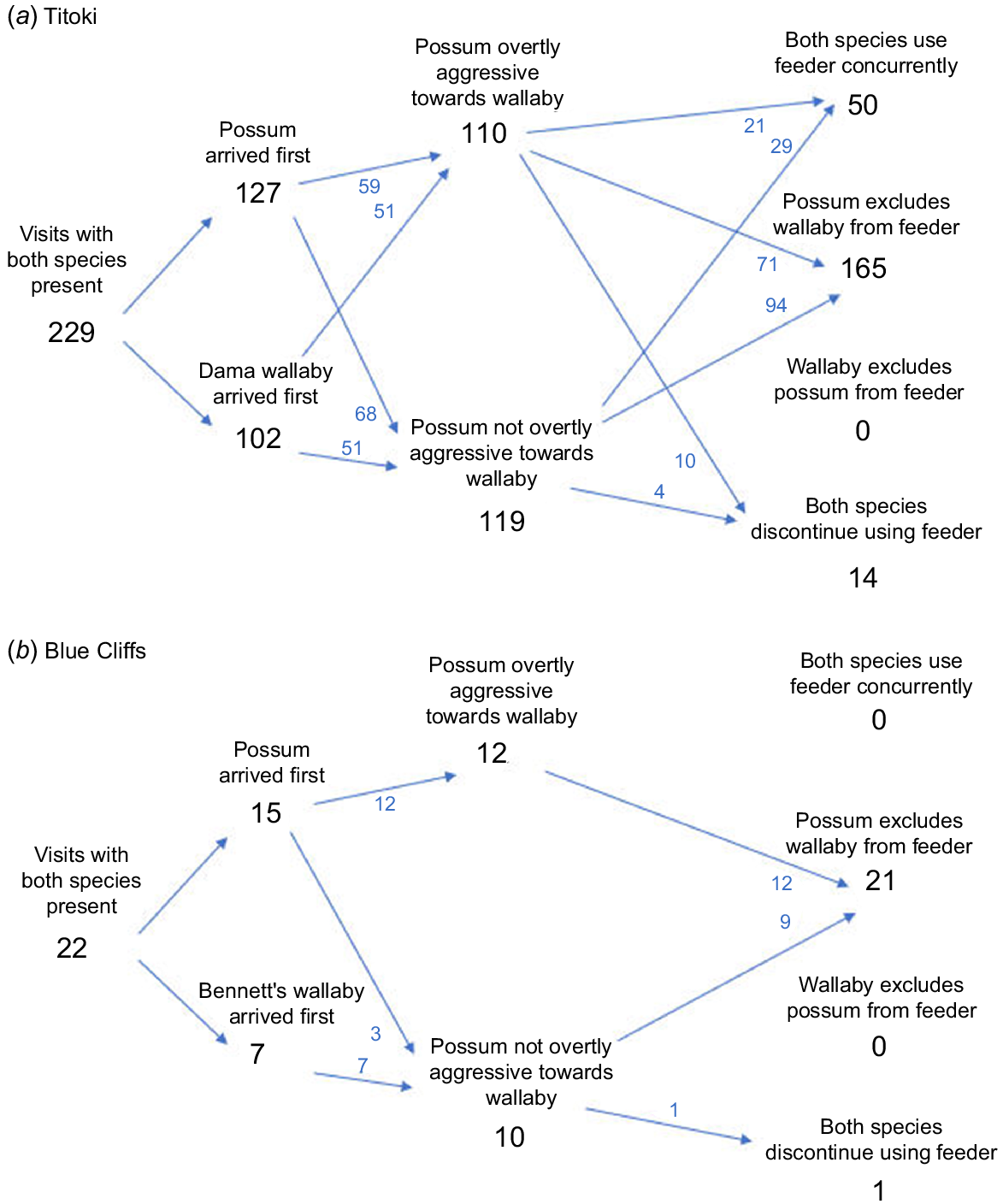
Outcomes of the 251 encounters recorded between wallabies and possums are summarised in Fig. 3. When encounters occurred, possums tended to be first at the feeder (55% of encounters at Titoki, 68% of encounters at Blue Cliffs; Fig. 3a, b). At both sites, the most frequent outcome of the encounter was that the possum excluded the wallaby from the feeder (72% of encounters at Titoki and 95% of encounters at Blue Cliffs; Fig. 3a, b). Exclusion could result simply from the presence of the possum; aggressive behaviours by the possum were exhibited in only around half of encounters that resulted in wallaby exclusion (48% of encounters at Titoki, 55% of encounters at Blue Cliffs; Fig. 3a, b). Possums’ aggressive behaviours included vocalisations, bipedal threat displays, pouncing and chasing (e.g. Fig. 4a–c). During the remaining encounters, the possum either ignored the wallaby or simply exhibited increased vigilance – the wallaby retreated from the feeder nevertheless (e.g. Fig. 4d). We recorded only three encounters, all at Blue Cliffs, where a wallaby responded aggressively towards the possum. All three efforts were unsuccessful, each ending with the wallaby retreating from the feeder.
Outcomes of encounters between wallabies and brushtail possums at bait feeders at: (a) Titoki, Rotorua district; (b) Blue Cliffs, South Canterbury. Numbers, which are pooled for 2022 and 2023, represent tallies of the total number of encounters in each category. Numbers are low at Blue Cliffs in part because the 2023 transects at that site were distant from possum habitat.
Examples of encounters at bait feeders between brushtail possums and: (a, b) dama wallabies; (b, c) Bennett’s wallabies. Aggressive behaviours by possums included bipedal threat displays (a-frame3, b-frame2, c-frame3) and pounces (b-frame 3). Wallabies often retreated from the feeder as soon as they detected an approaching possum (visible by its eye-shine in the upper-right of d-frame4).

The majority of the excluded wallabies moved away from the feeder site (116 of 165 at Titoki; 15 of 21 at Blue Cliffs); the remainder waited near the feeder until the possum departed and then began feeding at it (49 of 165 at Titoki; 6 of 21 at Blue Cliffs). Regardless of which species arrived first, wallabies were never successful in excluding possums from a feeder, although occasionally both species departed (6% of encounters at Titoki, 5% of encounters at Blue Cliffs; Fig. 3a, b), usually because the possum chased after the retreating wallaby.
At Titoki, we recorded 50 examples (22% of encounters) of possums and dama wallabies both accessing bait during the same visit. These possums and dama wallabies never fed side by side from the feeder itself; concurrent feeding only occurred when one or both animals were feeding from the ground on bait spilled from the feeder. When dama wallabies investigated feeders in the absence of possums, 32% of their visits resulted in feeding – this was a significantly higher rate than the 22% seen feeding when possums were present (Fisher Exact test, P < 0.0001).
No concurrent feeding by Bennett’s wallabies and possums was observed at the feeders, whereas 54% of Bennett’s wallabies investigating feeders in the absence of possums fed at them (again a highly significant difference; Fisher Exact test, P = 0.004).
At Titoki in 2022, where 1.8 times more possums than wallabies visited the feeders (average visitation rate of 12.1 wallabies and 22.1 possums per feeder per week), 100% of bait was removed from every feeder during every week during the 2022 trial; we suspect mostly by the possums. Wallabies visiting these depleted feeders consequently had no opportunity to consume bait until the feeders were replenished the following week. At Blue Cliffs in 2022, where 1.6 times more wallabies than possums visited (average visitation rate of 26.1 wallabies and 16.7 possums per feeder per week), the raised feeders were rarely emptied although bait on the ground was typically consumed within 2 or 3 days, mostly by the possums.
Wallaby response to possum carcases
During the Spring 2022 Blue Cliffs trial, placing a possum carcase close to a feeder initially deterred wallabies from interacting with that feeder (Fig. 5), with their visitation rate falling by 86% during the first week the carcase was in place (Fig. 6; P < 0.01). The ‘missing’ wallabies presumably came close enough to detect the carcase but not close enough to be photographed.
Progressive decay of a brushtail possum carcase at a feeder: (a) on placement day; (b) after 1 week; (c) after 2 weeks. In the first week all baits were removed by possums, which were not deterred by the carcase. Wallabies resumed feeding on baits during the second week.

The effect of brushtail possum carcase placement on the average number of weekly visits of wallabies with bait stations. (Vertical lines show means ± s.e.m.; n = 20 sites before placement and for Week 1 of placement; n = 8 for Week 2 of placement. **, significant difference (P < 0.01) compared to mean weekly visits when no carcase was present; NS, no significant difference (P ≥ 0.05).
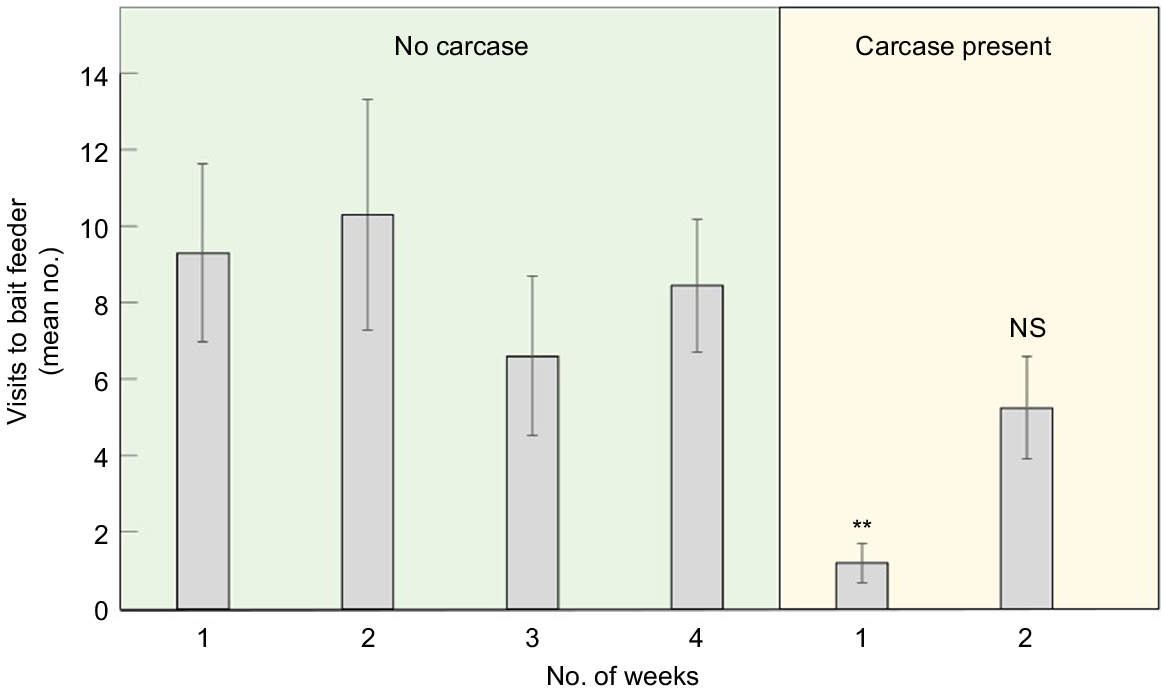
This deterrent effect was relatively short-lived. The carcase placement trial was undertaken during warm summer weather and by the second week of placement all carcases had either decomposed (Fig. 5c) or had been dragged away by feral pigs or feral cats. The wallaby visitation rate during the second week was only 38% lower than the pre-carcase average, which was no longer a statistically significant reduction (Fig. 6; P = 0.52).
Discussion
Our field study, which was primarily aimed at developing more effective designs of bait feeder for wallaby pest control, also provided an opportunity to investigate whether possums’ behavioural interference reduces the efficacy of bait feeders targeting wallabies. Here we present our findings on this latter objective. We acknowledge that had our focus only been on wallaby–possum interactions, we would have adopted a more straightforward study design than is reported here.
Two weeks of pre-trial possum control were undertaken at Titoki in an attempt to reduce possum interference; nevertheless, thousands of possum visits were recorded both at Titoki and at Blue Cliffs. Similarly, in 2023, pulses of toxic bait were deployed in the feeders with the expectation that this might reduce possum interference as well as wallaby numbers – but again we recorded many possum visits to the Titoki feeders. In contrast to the possums’ unimodal activity pattern, both wallaby species exhibited bimodal activity with peaks near dawn and dusk (Fig. 2a, b). Nevertheless, the times of day during which most wallaby and possum feeder visits occurred overlapped extensively, although wallaby activity declined as possums became more active and then rebounded once possums stopped visiting the feeders. The extent to which the wallabies’ bimodal activity pattern might imply proximate avoidance of possums at our sites – rather than simply an intrinsic diurnal pattern of the species – is unclear, as dama wallabies and particularly Bennett’s wallabies are described in some studies as being crepuscular (e.g. Havlin et al. 2018; Dickman and Calver 2023; Latham et al. 2023). However, we note that on Kawau Island (off the north-eastern coast of the North Island), where possum numbers were low, dama wallaby exhibited a unimodal activity pattern that peaked around midnight, with no indication of bimodality (Lentle et al. 2005). Relative to dama wallaby, a much higher proportion of Bennett’s wallaby activity was during daylight hours, during which time they were able to forage without encountering possums.
We observed that possums were consistently able to dominate both wallaby species, despite the Bennett’s wallabies’ size advantage. Based on studies reviewed by Cowan and Glen (2021), the body weight of adult possums at our sites probably averaged c. 2.6 kg. Adult wallabies at both our sites were significantly larger than this. The weight of female and male dama wallabies on Rotorua farmland averages 4.3 kg and 5.6 kg, respectively, with some males exceeding 10 kg (Williams 1986, in Latham and Warburton 2021). The weight of female and male Bennett’s wallabies in South Canterbury averages 11 kg and 13 kg, respectively, with some males exceeding 20 kg (Wodziki and Flux 1967, in Latham and Warburton 2021). Despite their size advantage, we recorded no instances of either wallaby species exhibiting behavioural dominance over possums. This finding is consistent with a previous unpublished report from New Zealand (Williams 1997), and with a report from Tasmania (Statham et al. 2010), that brushtail possums were aggressive to wallabies. We are not aware of any reason having been proposed for why this happens.
Both wallaby species approached and used bait feeders cautiously, maintaining high levels of vigilance throughout their visits. There was evidence to suggest that Bennett’s wallabies were particularly wary of possums. First, many more encounters were recorded between dama wallabies and possums than between Bennett’s wallabies and possums (229 and 22, respectively), although this is explained in part by possums visiting feeders at a higher rate at Titoki than at Blue Cliffs, and also by the greater daytime activity of Bennett’s wallabies. Second, when possums approached, Bennett’s wallabies always (7 of 7 encounters) moved away from the feeder before the possum exhibited aggression, whereas half of the dama wallabies (51 of 102 encounters) did not leave until the possum exhibited aggression. Third, we recorded 50 encounters where a dama wallaby continued feeding on bait at a feeder site while a possum was present; this outcome was never seen for Bennett’s wallabies.
When a possum was already feeding at a station, both wallaby species often waited at a distance and did not attempt to use the feeder until the possum left of its own accord. Given the limited field of view of the cameras, this ‘waiting’ response will have been underestimated for both species; we speculate that this helps to explain why so few encounters between wallabies and possums were recorded. Statham et al. (2010) and Williams (1997) both suggested that the aggressive behaviour of possums towards wallabies that they observed appeared to be reducing wallabies’ use of bait feeders.
Bennett’s wallabies were also clearly averse to the presence of possum carcases, at least while the carcases were relatively fresh. We did not have an opportunity to conduct an equivalent carcase trial at Titoki, so whether dama wallabies would respond in a similar way requires further study.
Implications for bait feeder control of wallabies
Since possums are common throughout most wallaby habitat on the New Zealand mainland, their pronounced dominance behaviour has the potential to reduce the efficacy of bait feeder control of wallabies. Firstly, when possums are abundant, bait feeders may be emptied of bait before any wallabies have an opportunity to feed at them. This problem was evident at some sites at Titoki, despite possum control there in preparation for our trial. Possums emptying feeders have also been reported by wallaby control field staff at other sites (D. Williams, Bay of Plenty Regional Council, pers. comm.). Possums were less disruptive at Blue Cliffs, but this farm had large-scale possum control efforts in recent years aimed at reducing livestock disease risk and the 2023 feeders were located in high-elevation tussock grasslands where possum activity was very low.
Emptying of feeders by possums (and to a lesser extent by other non-target species such as hedgehogs and rats) has implications for bait feeder design and deployment. All else being equal, larger feeders containing more bait and/or with a more frequent replenishing schedule should reduce the risk of wallabies encountering empty feeders, however both measures would increase the cost of control.
A second problem that we have documented for wallaby management is that the interruption and exclusion of feeding wallabies by arriving possums will increase the risk that the wallaby ingests a sublethal dose of toxin and consequently develops conditioned taste aversion (CTA, or ‘shyness’) to the bait. Bait shyness, which can arise from interrupted feeding, is known to reduce the efficacy of possum bait station control (Morgan et al. 1996). Bait shyness is of greatest concern when acute toxins such as sodium manofluoroacetate (1080) or cyanide-based Feratox® are deployed. The problem can be addressed by pre-feeding with non-toxic bait (Moss et al. 1998) or by using slow-acting anticoagulants such as brodifacoum (Morgan et al. 2002). However, use of anticoagulants on New Zealand’s mainland is usually infeasible due to concerns about non-target deaths and environmental residues (Eason et al. 2010), so poisoning of wallabies relies on being able to successfully deploy relatively acute toxins (e.g. Shapiro et al. 2011).
In this study, both wallaby species often came to the feeders in groups and so intraspecific interactions (such as ‘boxing matches’ between individuals) were relatively common but were not measured. The extent to which possums contribute additional feeding disruption on top of these intraspecific interactions is unclear; however, one qualitative difference is that possums exclude all wallabies, whereas following intraspecific interactions the dominant wallaby presumably gets to complete its feeding bout. If dominant wallabies then die from ingesting toxic bait, subordinate wallabies will thereafter gain undisturbed access to feeders.
We suggest that suppression of the local possum population will help to reduce the risk of emptied bait feeders and disruption of feeding wallabies. One possible approach could be to implement possum control in advance of the feeder operation, as was attempted in our Spring 2022 trial at Titoki. A disadvantage of this approach is that unless a large enough area can be controlled with sufficient intensity, possums will reinvade from surrounding uncontrolled areas before the bait feeder operation is complete. An alternative could be to alternate between non-toxic and toxic bait in the feeders over a number of weeks with the aim of poisoning possums as they arrive, thereby freeing up the bait feeders for later-arriving wallabies. This strategy was attempted during our Winter 2023 trial but was relatively ineffective, presumably because bait station coverage at Titoki was too sparse to achieve a sustained knock-down of the possum population. (This approach was unable to be evaluated at Blue Cliffs as the winter 2023 site had very few possums.)
One possibility for improved Bennett’s wallaby control could be to exploit their daylight feeding activity by developing and deploying timed-release feeders. If quantities of bait were released each morning, wallabies would have 8 h or more during which they could feed without interruption by possums. Although this is not the peak foraging time for Bennett’s wallabies, it is a period during which possums will not interfere with wallabies’ feeding and so baiting success might be higher than at night. However, robust automated feeders of this type would be much more expensive than passive feeder designs, so the affordability of deploying a large grid of timed feeders is uncertain. The approach is unlikely to be helpful for dama wallaby, given the near-complete overlap of the dama wallabies’ activity period with that of possums.
Given the pronounced behavioural dominance of possums over wallabies reported here, we conclude that possum population suppression is likely to increase the efficacy of wallaby bait feeder control efforts. Intensive suppression will be needed over large areas; however, if continual reinfestation of feeders by possums is to be prevented, this will significantly increase overall operational costs. Broadcast baiting (i.e. using aerial or hand-sown baits widely distributed across the landscape) is an alternative approach to preventing possums dominating access to bait – but can be constrained by risk to livestock, other non-target species, and other stakeholder concerns. Further research, or research-by-management, will be needed to determine the cost-effectiveness of incorporating possum population suppression into large-area wallaby baiting operations.
Data availability
Study data are archived at Manaaki Whenua – Landcare Research and are available on reasonable request to the corresponding author.
Declaration of funding
This research was funded by the Tipu Mātoro National Wallaby Eradication Programme, under Contract MPI-C0033367.
Acknowledgements
We appreciate guidance in project design provided by Travis Ashcroft (MPI), by representatives of the Tipu Mātoro National Wallaby Eradication Programme, and by Bruce Warburton (MWLR). We thank the land managers for providing access to the field sites. Grant Morriss, Peter Sweetapple and Becky Goodsall (MWLR) and Jessica Day (‘Day In The Bush’) assisted with fieldwork and image analysis.
References
Eason C, Henderson R, Hix S, MacMorran D, Miller A, Murphy E, Ross J, Ogilvie S (2010) Alternatives to brodifacoum and 1080 for possum and rodent control—how and why? New Zealand Journal of Zoology 37, 175-183.
| Crossref | Google Scholar |
Havlin P, Caravaggi A, Montgomery WI (2018) The distribution and trophic ecology of an introduced, insular population of red-necked wallabies (Notamacropus rufogriseus). Canadian Journal of Zoology 96, 357-365.
| Crossref | Google Scholar |
Latham ADM, Latham MC, Warburton B (2019) Current and predicted future distributions of wallabies in mainland New Zealand. New Zealand Journal of Zoology 46, 31-47.
| Crossref | Google Scholar |
Latham ADM, Latham MC, Warburton B (2023) Spatial ecology of invasive Bennett’s wallaby in South Island, New Zealand. Wildlife Research 50, 1109-1122.
| Crossref | Google Scholar |
Lentle RG, Hume ID, Stafford KJ, Kennedy M, Springett BP, Haslett S (2005) The temporal organisation of the feeding behaviour of four species of wallaby: examining chronobiological and homeostatic influences. Australian Journal of Zoology 53, 117-29.
| Crossref | Google Scholar |
Microsoft AI for Earth (2020) MegaDetector overview. Available at https://github.com/microsoft/CameraTraps/blob/main/archive/megadetector.md#megadetector-overview [Accessed 12 May 2024]
Morgan DR, Morriss G, Hickling GJ (1996) Induced 1080 bait-shyness in captive brushtail possums and implications for management. Wildlife Research 23, 207-211.
| Crossref | Google Scholar |
Moss ZN, O’Connor CE, Hickling GJ (1998) Implications of prefeeding for the development of bait aversions in brushtail possums (Trichosurus vulpecula). Wildlife Research 25, 133-138.
| Crossref | Google Scholar |
MPI (2022) Aotearoa New Zealand Wallaby Strategy. Ministry of Primary Industries, Wellington. Available at https://www.mpi.govt.nz/dmsdocument/54358-Aotearoa-New-Zealand-Wallaby-Strategy-Full-version [Accessed 15 May 2024]
R Core Team (2023) ‘R: a language and environment for statistical computing (version 4.2.3).’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.r-project.org/ [Accessed 20 April 2023]
Shapiro L, Ross J, Adams P, Keyzer R, Hix S, MacMorran D, Cunningham C, Eason C (2011) Effectiveness of cyanide pellets for control of dama wallabies (Macropus eugenii). New Zealand Journal of Ecology 35, 287-290.
| Google Scholar |
Warburton B (1986) Wallabies in New Zealand: history, current status, research, and management needs. Forest Research Institute Bulletin 114, 1-29.
| Google Scholar |
Warburton B (1990) Control of Bennett’s and tammar wallabies in New Zealand using Compound 1080 gel on foliage baits. Wildlife Research 17, 541-546.
| Crossref | Google Scholar |


