Deltamethrin reduces survival of non-target small mammals
Amanda R. Goldberg A * , Dean E. Biggins B , Shantini Ramakrishnan C , Jonathan W. Bowser B , Courtney J. Conway D , David A. Eads B and Jeffrey Wimsatt E
A * , Dean E. Biggins B , Shantini Ramakrishnan C , Jonathan W. Bowser B , Courtney J. Conway D , David A. Eads B and Jeffrey Wimsatt E
A Department of Biology, Colorado State University, Fort Collins, CO 80523, USA.
B US Geological Survey, Fort Collins Science Center, Fort Collins, CO 80526, USA.
C Conservation Science Center, New Mexico Forest and Watershed Restoration Institute, New Mexico Highlands University, Las Vegas, NM 87701, USA.
D US Geological Survey, Idaho Cooperative Fish and Wildlife Research Unit, University of Idaho, Moscow, ID 83844, USA.
E Department of Medicine, West Virginia University, Morgantown, WV 26506, USA.
Wildlife Research 49(8) 698-708 https://doi.org/10.1071/WR21153
Submitted: 23 October 2021 Accepted: 28 February 2022 Published: 25 May 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Vector-borne diseases have caused global pandemics and were responsible for more human deaths than all other causes combined in prior centuries. In the past 60 years, prevention and control programs have helped reduce human mortality from vector-borne diseases, but impacts of those control programs on wildlife populations are not well documented. Insecticides are used to reduce vector-borne diseases in several critically endangered animal populations. Although insecticides are often effective at controlling targeted vectors, their effects on non-target species have rarely been examined.
Aims: To evaluate the impact of deltamethrin (an insecticide) on sympatric non-target species in areas affected by sylvatic plague, a lethal flea-borne zoonosis.
Methods: We compared flea control and the effect of deltamethrin application on survival of non-target small mammals (Peromyscus maniculatus, Chaetodipus hispidus, Microtus spp., and Reithrodontomys megalotis) at three study locations in South Dakota, Colorado, and Idaho, USA.
Key results: Deltamethrin treatments were more effective in reducing fleas on P. maniculatus and Microtus spp. than C. hispidus. Following burrow, nest, and bait-station applications of deltamethrin dust, apparent small mammal survival was greater for non-treatment animals than for flea-reduction animals. However, the magnitude of the difference between treated and non-treated animals differed among host species, study location, time interval, and treatment application method.
Conclusions: Our results suggest that considering the impact of deltamethrin on co-occurring non-target species before widespread application in future insecticide applications is warranted.
Implications: Insecticide application methods warrant consideration when designing plague management actions.
Keywords: Chaetodipus, conservation, fleas, insecticide, management, Microtus, non-target, Peromyscus, Reithrodontomys, rodents, survival, Yersinia pestis.
Introduction
Insecticides are applied worldwide as tools to control invasive species, diseases, and pests. One common use of insecticides is to control vector-borne diseases such as malaria, dengue, leishmaniasis, chaga disease, and plague (van den Berg et al. 2012). These control efforts are effective at minimising human and wildlife diseases by reducing the abundance of the disease vectors and, thus, reducing disease transmission (Seery et al. 2003; Eads and Biggins 2019; Biggins et al. 2021a; Goldberg et al. 2021a). The effects of application of these chemicals may have unforeseen consequences on non-target co-existing species via both direct and indirect pathways (Bourguet and Guillemaud 2016); however, such effects have rarely been examined.
Deltamethrin, a synthetic pyrethroid, is an insecticide that is currently distributed within selected prairie dog (Cynomys spp.) colonies in the United States to control plague. Plague is a disease caused by the bacterium Yersinia pestis and fleas are the main vectors (Gage and Kosoy 2005). Plague reduces survival of black-footed ferrets (Mustela nigripes), Utah prairie dogs (C. parvidens), and other species of conservation concern (Biggins et al. 2010; Matchett et al. 2010; Russell et al. 2019). Deltamethrin is an effective conservation tool for reducing the incidence of plague in these species by controlling flea abundance (Seery et al. 2003; Eads and Biggins 2019; Biggins et al. 2021a; Goldberg et al. 2021a), resulting in greater survival of target species (Biggins et al. 2010, 2021b; Matchett et al. 2010; Goldberg et al. 2021a). However, the effects of the insecticide on sympatric non-target small mammals are mostly unknown.
Deltamethrin may have unintended negative effects on small mammals similar to those of other insecticides (Sheffield and Lochmiller 2001; Sánchez-Bayo 2011). For example, acute exposure to organophosphorate insecticides in laboratory white-footed mice (Peromyscus leucopus) affects luteinising hormone secretion, suggesting a possible negative affect on reproduction (Rattner and Michael 1985). Similarly, exposure to 2 g chlorpyrifos bait caused increased mortality of laboratory deer mice (P. maniculatus; Gregory et al. 1993). Adverse effects of deltamethrin and other pyrethroids likely depend on dosage (Rehman et al. 2014; Nieradko-Iwanicka et al. 2015), duration (Brander et al. 2016), and intensity of exposure. Experiments to assess the effects of deltamethrin applications at dosages encountered in field settings on wild populations of small mammals are needed.
We evaluated effects of deltamethrin on small mammals and fleas over 8 years in three states (Colorado, Idaho, and South Dakota). These locations experience different intensities of plague circulation (regular epizootic events followed by periods of enzootic levels versus extremely rare epizootic events and persistent plague at enzootic levels). For instance, both Colorado and South Dakota study areas have documented periodic epizootics in prairie dogs (Griffin et al. 2010; Biggins et al. 2021a), whereas Idaho study sites have no documented epizootics, but serological surveys suggest that plague is present (Goldberg et al. 2021a). Our reference to enzootic and epizootic plague follows a dichotomous definition (Biggins and Eads 2021). Epizootics are characterised by catastrophic population collapses (≥90% mortality over a large area within a short time span), with enzootic plague encompassing the remaining broad spectrum of mortality rates, time scales, and temporal scales (Biggins et al. 2021a). Part of the ongoing discussion over enzootic plague (Colman et al. 2021) might be resolved with more articulate definitions. Because survival of several target mammals has increased when enzootic and epizootic plague is reduced via application of deltamethrin in the western US (Biggins et al. 2010, 2021a, 2021b; Matchett et al. 2010; Tripp et al. 2017; Goldberg et al. 2021a), we hypothesised that similar vector-control measures would increase the survival of non-target small mammals. We posited that varying plague circulation rates would lead to differences in the degree of treatment effect on small mammals at our three study areas.
Materials and methods
Study areas
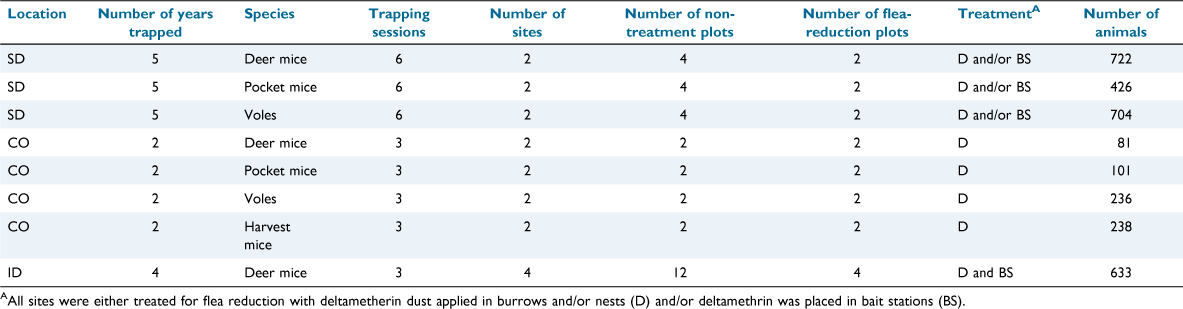
We captured small mammals at two study sites in Badlands National Park, Pennington County, South Dakota, from 2013 to 2017, at two study sites in Larimer County, Colorado, from 2010 to 2011 on private land (see Biggins et al. 2021b for more details), and at four study sites in Adams County, Idaho, from 2014 to 2017 on Forest Service, and private land (see Goldberg et al. 2021a for more details). Each site contained at least one or more flea-reduction treatment plots paired with one or more non-treatment (control) plots (Table 1). South Dakota was the only study location where large-scale flea control was implemented (our study locations were adjacent to plague-managed prairie dog towns). Idaho study locations were located on sites that contained larger ground squirrels that would typically be the target of plague management treatments (part of a study evaluating the presence of plague; see Goldberg et al. 2021a for more details).
|
The two South Dakota study sites were at 785 m elevation on inactive black-tailed prairie dog (C. ludovicianus) colonies in mixed-grass prairie originally dominated by western wheatgrass (Pascopyrum smithii), blue grama (Bouteloua gracilis), and other native species. Plague decimated these colonies in 2011, and rapid growth of numerous invasive plants such as cheatgrass (Bromus tectorum), Canada thistle (Cirsium arvense), giant mullein (Verbascum thapsus), bindweed (Convolvulus sp.), and yellow sweet clover (Melilotus officinalis) became the dominant vegetative cover. Partially collapsed prairie dog burrows (in some cases treated with deltamethrin dust) were often occupied by small mammals (e.g. P. maniculatus).
The two juxtaposed Colorado sites were at 1810 m elevation in the Rocky Mountain foothills, 4 km south of Livermore, Colorado. The shrub–grassland mosaic was dominated by cheatgrass (B. tectorum), western wheatgrass (P. smithii), needle-and-thread (Hesperostipa comata), green needlegrass (Nassella viridula), and blue grama (B. gracilis), with shrub patches of three-leaf sumac (Rhus aromatica) and mountain mahogany (Cercocarpus montanus) on upland sites. Two small, intermittent, stream drainages contained dense riparian vegetation with cottonwoods (Populus deltoides and P. angustifolia) and shrub thickets of American plum (Prunus americana), hawthorn (Crataegus macracantha) and chokecherry (Prunus virginiana).
Idaho sites were within or close to the Payette National Forest and located on a mix of mesic and xeric open meadows (containing a mixture of grasses and forbs) or mixed conifer forests (dominated by Ponderosa pine (Pinus ponderosa) and Douglas fir (Pseudotsuga menziesii) trees). Elevation ranged from 1221 to 1708 m.
Treatment
We delivered 0.05% deltamethrin dust (DeltaDust®; Bayer Environmental Science, Cary, NC, USA) to flea-reduction plots by using combinations of bait stations and dust sprayers. A backpack dust applicator (no longer in production) was used in South Dakota and Colorado. The unit had a hand-operated lever that energised a diaphragm pump, delivering dust under sufficient pressure to infuse the fine powder into nests or burrows. The pressure often allowed dust to be observed as it exited the far sides of woodrat nests or connected burrow openings up to 2 m away from the treated opening. In contrast, we used a small, hand-operated, squeeze bulb dust applicator (B&G bulb duster M1150, Jackson, GA, USA; Goldberg et al. 2021a) in Idaho that delivered dust under much lower pressure. We also used bait stations to bring animals into contact with deltamethrin. Each bait station included a carpet strip treated with 0.06% deltamethrin solution of Suspend® SC (Bayer Environmental Science, Cary, NC, USA), following the procedure of Bronson and Smith (2002). Amounts of deltamethrin applied to the carpet were not determined. We placed the treated carpet strips under 43.2-cm-long pieces of inverted vinyl gutter material to create the bait stations.
In South Dakota, we dusted burrows in 2013 and at one site in 2014 at a rate of 4–6 g per burrow. Prior to dusting, we sprayed dust into plastic bags and weighed the expelled dust to establish approximately how much dust was being deployed in each burrow. We used bait stations at the second site in 2014 and all sites in subsequent years. We deployed 44.4 bait stations per hectare. We dusted burrows in early June. We treated carpet in bait stations with Suspend SC® initially in May or June of each year, then retreated them with DeltaDust in July and September (except in 2017, when dust was reapplied to bait stations in July, August, and September). When first deploying bait stations each year, we placed a small handful of equine sweet feed and peanut butter mixture under the gutter on the ground (not on the treated carpet) to encourage small mammal use.
We treated the Colorado sites twice annually (once in the spring before trapping each year and another in the summer) using the backpack duster. Application rates were about 2–4 g per small mammal burrow, except for woodrat (Neotoma sp.) nests (stick nests, rock crevices, and other crevices with nesting material), which were dusted at higher rates proportional to the size of the nest (104 g dust per nest of average volume of 198.6 L = 0.52 g/L; Biggins et al. 2021b).
We treated rodent burrows at the four Idaho study sites each spring prior to the start of the first day of trapping each year (April and May) with the hand-held bulb dusters at a rate of 2–4 g per burrow. Dusters were calibrated as in South Dakota. Study sites were 1.8–3.3 ha in size and burrow density differed among sites (A. Goldberg, pers. obs., because the burrow density was not recorded). We applied dust within the study area and an additional 30-m buffer surrounding each treatment plot. In addition, we deployed 31 bait stations per hectare at least 1 week prior to the start of each trap session (see Goldberg et al. 2021a for more details). In the first year, we dipped each piece of carpet in a bucket of solution. In subsequent years, we sprayed each carpet segment with solution by using a hand-held chemical sprayer until the carpet was visually saturated. Once dried, we placed the treated carpet underneath the inverted gutter. We placed bait stations throughout the sites. In addition, we smeared the underside of the gutters with 5 mL of peanut butter to encourage small mammal use, while controlling food contact to the treated carpet to reduce ingestion risk. In Idaho, bait stations were placed within trapping plots twice per year, including once prior to the start of the first trap session when dust was applied to treatment plots and once prior to the start of the second trap session (Goldberg et al. 2021a). Bait stations were deployed for 9–34 days and were typically removed at the end of each trapping session (give or take a few days, depending on our schedule and weather).
Study animals
We captured deer mice (P. maniculatus) at all three study areas. We captured voles in Colorado (Microtus ochrogaster) and South Dakota (primarily M. ochrogaster and several M. pennsylvanicus). We captured pocket mice (Chaetodipus hispidus) in both Colorado and South Dakota. We captured harvest mice (Reithrodontomys megalotis) only in Colorado (Table 1). All five species are potential plague hosts; some are hypothesised to contribute substantially to the persistence of plague during enzootic periods and to plague circulation during epizootic outbreaks (Gage and Kosoy 2005).
Trapping
We captured small mammals at each study location for 2–5 years and three to six trap sessions per year (Table 1). We used two types of traps, namely, Sherman traps (HB Sherman Inc., Tallahassee, FL, USA; folding 8 × 9 × 23 cm at all study areas; non-folding 7.5 × 7.5 × 25 cm in Colorado only), and Tomahawk live traps (Tomahawk Live Trap Co., Tomahawk, WI, USA; 13 × 13 × 41 cm traps and 15 × 15 × 50 cm traps in Idaho; and folding 14 × 14 × 31 cm traps in Colorado and Idaho). We set Sherman traps in the evening and checked them in the morning. We also set Tomahawk traps in the evening and checked them the following morning in Colorado (Biggins et al. 2021b), and set in the early morning and checked ~2 h later in Idaho (Goldberg et al. 2021a). At each study location, we captured small mammals on paired treatment and non-treatment plots simultaneously within each site. Trapping intervals (the length of time between trap sessions) averaged 24 days for South Dakota, 33 days for Colorado, and 30 days for Idaho.
Handling procedures
We processed animals either at trap locations or at a processing station located adjacent to the study plot. We anesthetised animals with isoflurane in induction chambers, by using protocols similar to those described in Biggins et al. (2010). The size of the induction chamber and dose of isoflurane varied on the basis of species body size (Goldberg et al. 2021a; Biggins et al. 2021b). Isoflurane anesthetises the host and its fleas, which facilitates combing, counting, and collecting fleas. Animals were removed from the induction chamber when all motor functions visibly stopped and breathing slowed (animals were immediately removed and monitored for signs of distress). Once an animal was removed and inspected for steady breathing, we immediately placed it over a collection bin and combed vigorously with a flea comb for approximately 30 s across the entire body surface (combing with or against the hairs varied by study/individual handler). Combed fleas fell into the bin where they were counted and if collected, placed in vials with a 70% ethyl alcohol solution. We marked all animals with metal ear tags in each ear (National Band and Tag Co., Newport, KY, USA, Model 1005-1), and marked some individuals with a passive integrated transponder (PIT) tag (Biomark Inc., Boise, ID, USA, Model HPT12 or AVID, Norco, CA, USA, Model MUSICC, 12 mm). We released all animals, after they recovered from anaesthesia, at the location where they were captured.
Data analysis
To evaluate the effectiveness of the flea reduction treatments, we used a generalised logistic regression model (GLM) implemented in program R version 3.6.2 (R Core Team 2017). We used flea prevalence as our response variable (whether one or more fleas were detected versus no fleas detected). Although flea abundance is an important metric for plague transmission (Tripp et al. 2009; Biggins et al. 2021a), the count of fleas on host bodies is likely to undercount the true abundance of fleas because it is likely that we missed some fleas while combing (Eads et al. 2013) and counts from hosts do not account for fleas that may parasitise individuals in their nests and burrows, but that are not attached to the host at the time we trap them (Krasnov et al. 2021). Hence, prevalence is likely to be a more reliable measurement than is abundance for assessing ectoparasites (Krasnov et al. 2021) and presence/absence of fleas represents the most extreme effectiveness or not of the treatment. We included predictor variables for Treatment (flea-removal and non-treatment), Session, and Species (except for Idaho, where we sampled a single species). We also did not include harvest mice in any models because of small sample sizes. We included a full suite of potential models, including all two-way interactions. We ran models for each of the three study locations separately because the number of sites and species varied among them. We used a corrected Akaike’s information criterion (AICc; Akaike 1974; Burnham et al. 2011) to compare models.
To evaluate small mammal survival, we used Cormack–Jolly–Seber (CJS) models implemented in RMark (Laake 2013; R Core Team 2017) to estimate survival at each of the three study locations separately in program MARK (White and Burnham 1999). We ran RMark in program R version 3.6.2 (R Core Team 2017). We separated analyses for the three study locations because they differed by efficacy of flea-reduction, number of trapping sessions, and host species. We included the following covariates as potential predictors of apparent survival (Phi): Time (trapping interval), Species (not included for Idaho), and Treatment. We used a 30-day time step and the average trapping intervals were: 0.8 for South Dakota, 1.1 for Colorado, and 1.0 for Idaho. We included the following covariates for recapture probability (p): Time (trap session), Species, Site (within study location), and Year. We used Akaike’s Information Criterion corrected for small sample size (AICc) to compare a suite of candidate models in program MARK and used the model with the lowest AICc to simultaneously estimate survival and recapture probability at each study location (Akaike 1974). We calculated the change in survival due to Treatment (non-treatment and flea-reduction) by dividing survival of non-treatment animals by survival of flea-reduction animals.
Results
Effectiveness of flea reduction
Flea prevalence at non-treated sites was greatest in South Dakota for all three species, namely, 60% on voles in South Dakota compared with 26% in Colorado, 49% on deer mice in South Dakota compared with 32% in Idaho and 6% in Colorado, and 12% on pocket mice in South Dakota compared with 3% in Colorado (Fig. 1).

|
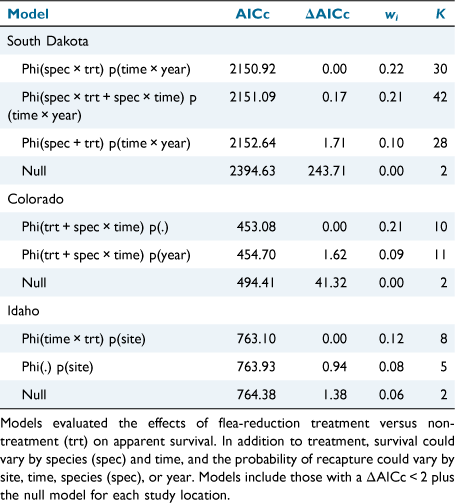
Treatment was included in the top-competing models (ΔAICc < 2.0) for all three study locations (Table 2). Overall, deltamethrin treatment was more effective at reducing fleas on deer mice and voles than on pocket mice (Fig. 1). Estimates from the most-competitive models suggested that flea prevalence was 0.81–6.25 (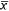 = 2.43) times greater on non-treated deer mice and 0.86–5.16 (
= 2.43) times greater on non-treated deer mice and 0.86–5.16 ( = 1.77) times greater on non-treated voles than on treated animals, and the pattern was consistent among the three study locations. In contrast, flea prevalence did not differ between treatments in pocket mice in either Colorado or South Dakota (Fig. 2).
= 1.77) times greater on non-treated voles than on treated animals, and the pattern was consistent among the three study locations. In contrast, flea prevalence did not differ between treatments in pocket mice in either Colorado or South Dakota (Fig. 2).

|

|
Small mammal survival
All three top competing models for South Dakota included species and time, while the top two models with most of the model weight included the interaction between species and treatment (Table 3). Both the top-competing models for Colorado included an interaction between species and time and the additive effect of treatment (Table 3). Idaho had three highly competitive models including the null model (Table 3). The top model in Idaho included the interaction between treatment and time, and the model weight increased by a factor of 1.60 compared with the second top-competing model (Table 3).
|
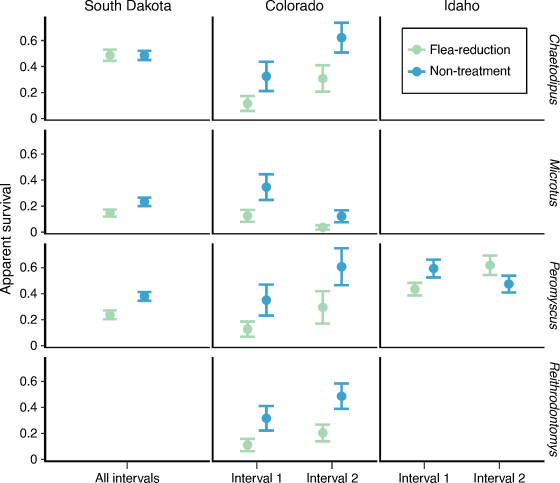
Apparent survival in the first interval following application of DeltaDust® was greater for non-treatment animals than for treated animals (except pocket mice in South Dakota where survival was similar among treatments; Fig. 3). Deer mice in Idaho showed the opposite trend during the second interval after dust had been applied (Fig. 3). Estimates from the most competitive models suggested that survival was: 0.63–1.30 times lower on treated than on non-treated deer mice, 0.30–0.63 times lower on treated than on non-treated voles, and 0.36–1.00 times lower on treated than non-treated pocket mice (Fig. 3).
|
Discussion
In contrast to our hypothesis, most non-target species at all three study locations (South Dakota, Colorado, and Idaho) showed a negative response in apparent survival to deltamethrin. This result is surprising, given the opposite responses that have been reported for larger rodents that were the target of the treatments (prairie dogs, woodrats, and chipmunks) at these same study locations (Eads et al. 2018; Biggins et al. 2021b; Goldberg et al. 2021a) and at other study locations (Biggins et al. 2010). Most animals had a significant reduction in flea loads and flea prevalence at the three study locations (Fig. 1). Exceptions were pocket mice and harvest mice, which had few fleas even on non-treatment plots (Fig. 1). Vector-control treatments were most effective at reducing flea prevalence in South Dakota and Idaho for deer mice and voles (for additional discussion on flea control with small mammals, see Eads et al. 2020; Goldberg et al. 2021a).
In our assessment, individual small mammals functioned as the sampling unit, and we detected a negative effect of deltamethrin treatment on survival for all four species. The underlying modes of exposure and mechanisms of mortality are unknown, but may include a variety of direct and indirect pathways (e.g. inhalation of aerosolised deltamethrin, consumption of tainted bait by being placed near insecticide [although we believe this is a minimal risk in Idaho where the peanut butter was located above the insecticide and not a factor in Colorado], or consumption of the insecticide when oral grooming). If we had used small mammal abundance as the response variable, we may have failed to detect an effect (e.g. Dombro 2016) because high reproductive rates (r-selection) may overshadow a negative treatment effect on survival.
As we predicted, the effect of deltamethrin on small-mammal survival varied among species and locations. Pocket mice in South Dakota did not show any reduction in survival regardless of treatments. Idaho had the smallest difference in deer mice survival between treatments, and an opposite trend during the second interval when the only new treatment applications were Suspend SC bait stations. Several potential explanations may explain why certain species and/or locations were affected differently by the treatments, including the following: (1) methods of insecticide delivery, which varied by location, have differential effects on survival; (2) variation in frequency of insecticide application, have differential effects on survival; (3) variation in habitat use causes variation in effects of insecticide; (4) interspecific differences in physiology cause differential responses to insecticide treatments; (5) environmental conditions affect deltamethrin persistence and toxicity; (6) variation in effects of insecticide on arthropod (prey) abundance; and (7) it is likely that circulation rates of plague varied over space and time. Idaho deer mice had a lower reduction in apparent survival owing to treatment than did deer mice at the other two locations. The difference in survival may reflect differences in method of dust application. In Idaho, the small hand-held bulb dusters may have aerosolised less dust. Furthermore, dust was not applied at subsequent intervals in Idaho; deltamethrin was subsequently applied only by using bait stations treated with Suspend SC. In comparison, we used backpack dusters multiple times per field season at the Colorado and South Dakota study locations. Future studies designed explicitly to assess the effects of insecticide applications on rodent survival and flea control by using varied equipment and application frequencies would help inform optimal application methods to reduce unintended negative effects on non-target species. All target species where DeltaDust® has been used to reduce plague risk have shown a net positive benefit in survival. Multiple studies evaluating the effect of dusting on squirrels (Cynomys spp., Urocitellus spp., and Neotamias spp.) and woodrats (Neotoma spp.) have shown that non-treatment plots had reduced survival or density compared with plots with flea-reduction treatments (Biggins et al. 2010, 2021a, 2021b; Ramakrishnan 2017; Goldberg et al. 2021a). Perhaps larger animals are less susceptible to potential adverse effects of deltamethrin treatment than are small mammals (simply mass to insecticide ratio). However, woodrats and squirrels may also be more susceptible to plague and the net effect of reduction in plague exposure overshadows any negative impact that insecticides may have on these species. Final outcomes of net effect on survival in small mammals thus becomes difficult to predict and would depend on the rates of plague circulation (and perhaps circulation of other vector-borne diseases). Our results seem noteworthy because any positive effects of flea removal were apparently insufficient to overcome the negative effect that likely emanated from direct toxicity of the insecticide on these smaller, non-target species. Many small rodents consume arthropod prey, suggesting that reductions in prey biomass might be important; however, effects on deltamethrin applications on common arthropod prey items appear minimal (e.g. Dombro 2016).
The net effect concept (the idea that flea-reduction treatment leads to increased survival rates that overshadow any negative effect that insecticides may have) raises another important point relative to estimating the impacts of plague on wild rodent populations of small mammals. Biggins et al. (2021b) listed nine factors that might lead these types of treatment-control studies to underestimate the true effect of plague. If pesticides used for vector control tend to depress non-target host-survival rates in the absence of plague circulation, this would be the 10th factor that could cause underestimation of the effect of plague on survival of wild mammal populations. The net positive effect of vector control for larger rodents does not preclude the presence of a pervasive negative effect that was consistently overwhelmed by the benefits of vector control in the studies cited above.
Indirect effects can be potentially pervasive in these studies. Decreasing the circulation of plague in woodrats in Colorado, and in chipmunks and ground squirrels in Idaho, might have benefitted the associated small mammals via herd effects (Biggins et al. 2021b; Goldberg et al. 2021a). However, the attendant increases in survival rates of targeted species might also have resulted in population densities that could be detrimental to associated species via interference or exploitative competition. The increased competition hypothesis is less plausible because populations of target species were not noticeably increasing during these studies. For example, it is likely that the woodrat population declined during the Colorado study (Biggins et al. 2021b). Furthermore, it appears that the treatments were less effective at reducing fleas on small mammals than on squirrels and woodrats. Overall, flea prevalence was reduced in small mammals across all three locations by 48%. Conversely, burrow treatments with deltamethrin in Utah initially reduced fleas by 96% for white-tailed prairie dogs (C. leucurus) and by 98% for Utah prairie dogs (Biggins et al. 2010). Results for Mexican woodrats (Neotoma mexicana) in Colorado were similar, with insecticide treatments at nests producing >90% reduction in flea prevalence (Biggins et al. 2021b). Northern Idaho ground squirrels (U. brunneus) and Columbian ground squirrels (U. columbianus) had over 97% fewer fleas on deltamethrin-treated plots (Goldberg et al. 2021a), whereas deer mice had 71% fewer fleas and yellow-pine chipmunks (Neotamias amoenus) had only 24% fewer fleas on the same treatment plots than on non-treatment plots in Idaho (Goldberg et al. 2021a). Why treatments seem less effective at reducing fleas for smaller animals still needs to be evaluated, but may be due to our inability to locate and treat very small burrows, and/or bait stations are perhaps less utilised by smaller species or less effective at treating them. In addition, flea species composition varies considerably among rodent species; some flea species might be less susceptible to deltamethrin because of direct resistance or other factors. Furthermore, different flea species peak in abundance seasonally and treatment may not be applied at the optimal time for all flea species (Krasnov et al. 2002; Hubbart et al. 2011).
The use of DeltaDust® and other insecticides is a critical plague-management tool (Barnes et al. 1972; Seery et al. 2003; Hoogland et al. 2004; Biggins et al. 2010; Tripp et al. 2016). These insecticides are currently the primary method being employed across the western US for reducing harmful plague impacts on listed species such as the Utah prairie dog (Seery et al. 2003; Eads and Biggins 2019) and the black-footed ferret (Matchett et al. 2010). Insecticides are also potential management tools in other systems where species of conservation concern may be affected by plague such as the Peñasco least chipmunk (Neotamias minimus atristriatus; U.S. Fish and Wildlife Service 2021). Although the injectable F1 and F1-V vaccines have shown promise at protecting some rodent species from plague (Anderson et al. 1998; Heath et al. 1998), subcutaneous vaccinations are too labour intensive to implement on a broad scale; however, an injectable vaccine is the primary tool in protecting black-footed ferrets (Rocke et al. 2004; Matchett et al. 2010). Additionally, an orally delivered plague vaccine is under development, but early versions of the vaccine have provided far less protection than deltamethrin and have not yet proven to be practical for effective plague management for black-footed ferrets (Rocke et al. 2017; Biggins et al. 2021a).
Management agencies are likely to need to weigh the benefits of deltamethrin dusting (e.g. reduced incidence of plague among squirrels and rats) with the potential negative impacts it may have on non-target small mammal populations (e.g. mice and voles herein). These decisions will likely be location- and target-species specific. For instance, areas where a mouse or vole species of conservation concern resides, management agencies may need to use alternative methods to control plague as opposed to dusting burrows. Whereas some locations may have low small-mammal diversity (such as those prairie dog towns sampled by Dombro 2016) where a small potential reduction in deer mice may not be a concern. Further research is needed to fully understand the mechanism causing the negative impacts by deltamethrin in mice and voles. As these mechanisms are better understood, perhaps effective methods can be identified that reduce flea loads, minimise harmful impacts on mice and vole populations, and provide operational plague mitigation at large scales. Additionally, the evolution of vector resistance to any insecticide is an important consideration when designing potential treatment regimens (Eads et al. 2018).
Plague is a disease that directly causes moderate to substantial declines in mammal populations around the world annually (Biggins and Kosoy 2001; Gage and Kosoy 2005). Furthermore, associated species are indirectly affected by loss of food and habitat owing to small-mammal population declines (Eads and Biggins 2015). We need management solutions that reduce the harmful impact of plague on small-mammal populations. Although the use of insecticides may be an imperfect solution, vector control is currently the best option available to address many conservation needs. However, it is imperative that we understand the biological impacts of these insecticides at individual, population, and ecosystem levels.
Data availability
The data that support this study are available at https://doi.org/10.5066/P9N8SWMV (Goldberg et al. 2021b).
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
Idaho research funding was provided by the US Forest Service (GCK-159) and the US Fish and Wildlife Service (GCK-163). Funding for the South Dakota study was provided by the US Geological Survey (USGS) and National Park Service joint NRPP program, the US Fish and Wildlife Service and USGS Science Support Partnership Program, and supplemented by direct support from Badlands National Park and the Fort Collins Science Center (FORT) of the USGS. Funding for the Colorado study was provided by West Virginia University and US Geological Survey (FORT). Colorado data collection was performed under the auspices of West Virginia University IACUC protocol #10-0404.
Acknowledgements
We are deeply indebted to the numerous crew leaders and field assistants who helped collect data for all three studies. The Hixon family and their ranch employees, D. Evans Mack, J. Galloway, A. Egnew, J. Almack, and R. Richards provided logistical support in Idaho. Idaho data collection was performed under the auspices of University of Idaho IACUC protocol #2015-53. We thank J. Hughes, the Associate Editor, and two anonymous reviewers for constructive comments on a previous version of the article. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
References
Akaike, H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control 19, 716–723.| A new look at the statistical model identification.Crossref | GoogleScholarGoogle Scholar |
Anderson Jr, GW, Heath, DG, Bolt, CR, Welkos, SL, and Friedlander, AM (1998). Short-and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice. The American Journal of Tropical Medicine and Hygiene 58, 793–799.
| Short-and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice.Crossref | GoogleScholarGoogle Scholar |
Barnes, AM, Ogden, LJ, and Campos, EG (1972). Control of the plague vector, Opisocrostis hirsutus, by treatment of prairie dog (Cynomys ludovicianus) burrows with 2% carbaryl dust. Journal of Medical Entomology 9, 330–333.
| Control of the plague vector, Opisocrostis hirsutus, by treatment of prairie dog (Cynomys ludovicianus) burrows with 2% carbaryl dust.Crossref | GoogleScholarGoogle Scholar | 4626587PubMed |
Biggins, DE, and Eads, DA (2021). Utah prairie dog population dynamics on the Awapa Plateau: precipitation, elevation, and plague. Journal of Mammalogy 102, 1289–1297.
| Utah prairie dog population dynamics on the Awapa Plateau: precipitation, elevation, and plague.Crossref | GoogleScholarGoogle Scholar |
Biggins, DE, and Kosoy, MY (2001). Influences of introduced plague on North American mammals: implications from ecology of plague in Asia. Journal of Mammalogy 82, 906–916.
| Influences of introduced plague on North American mammals: implications from ecology of plague in Asia.Crossref | GoogleScholarGoogle Scholar |
Biggins, DE, Godbey, JL, Gage, KL, Carter, LG, and Montenieri, JA (2010). Vector control improves survival of three species of prairie dogs (Cynomys) in areas considered enzootic for plague. Vector-Borne and Zoonotic Diseases 10, 17–26.
| Vector control improves survival of three species of prairie dogs (Cynomys) in areas considered enzootic for plague.Crossref | GoogleScholarGoogle Scholar | 20158328PubMed |
Biggins, DE, Godbey, JL, and Eads, DA (2021a). Epizootic Plague in Prairie Dogs: Correlates and Control with Deltamethrin. Vector-Borne and Zoonotic Diseases 21, 172–178.
| Epizootic Plague in Prairie Dogs: Correlates and Control with Deltamethrin.Crossref | GoogleScholarGoogle Scholar | 33481692PubMed |
Biggins, DE, Ramakrishnan, S, Rocke, TE, Williamson, JL, and Wimsatt, J (2021b). Enzootic plague reduces survival of Mexican woodrats (Neotoma mexicana) in Colorado. Ecosphere 12, e03371.
| Enzootic plague reduces survival of Mexican woodrats (Neotoma mexicana) in Colorado.Crossref | GoogleScholarGoogle Scholar |
Bourguet D, Guillemaud T (2016) The hidden and external costs of pesticide use. In ‘Sustainable Agriculture Reviews, vol 19’. (Ed. E Lichtfouse) pp. 35–120. (Springer: Cham, Switzerland)
| Crossref |
Brander, SM, Gabler, MK, Fowler, NL, Connon, RE, and Schlenk, D (2016). Pyrethroid pesticides as endocrine disruptors: Molecular mechanisms in vertebrates with a focus on fishes. Environmental Science and Technology 50, 8977–8992.
| Pyrethroid pesticides as endocrine disruptors: Molecular mechanisms in vertebrates with a focus on fishes.Crossref | GoogleScholarGoogle Scholar | 27464030PubMed |
Bronson, LR, and Smith, CR (2002). Use of liquid deltamethrin in modified, host-targeted bait tubes for control of fleas on sciurid rodents in northern California. Journal of Vector Ecology 27, 55–62.
| 12125873PubMed |
Burnham, KP, Anderson, DR, and Huyvaert, KP (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65, 23–35.
| AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons.Crossref | GoogleScholarGoogle Scholar |
Colman, RE, Brinkerhoff, RJ, Busch, JD, Ray, C, Doyle, A, Sahl, JW, Keim, P, Collinge, SK, and Wagner, DM (2021). No evidence for enzootic plague within black-tailed prairie dog (Cynomys ludovicianus) populations. Integrative Zoology 16, 834–851.
| No evidence for enzootic plague within black-tailed prairie dog (Cynomys ludovicianus) populations.Crossref | GoogleScholarGoogle Scholar | 33882192PubMed |
Dombro LM (2016) Ecological effects of deltamethrin insecticide in prairie dog colonies of western South Dakota. Auburn University Auburn, AL, USA.
Eads, DA, and Biggins, DE (2015). Plague bacterium as a transformer species in prairie dogs and the grasslands of western North America. Conservation Biology 29, 1086–1093.
| Plague bacterium as a transformer species in prairie dogs and the grasslands of western North America.Crossref | GoogleScholarGoogle Scholar | 25817984PubMed |
Eads, DA, and Biggins, DE (2019). Plague management of prairie dog colonies: degree and duration of deltamethrin flea control. Journal of Vector Ecology 44, 40–47.
| Plague management of prairie dog colonies: degree and duration of deltamethrin flea control.Crossref | GoogleScholarGoogle Scholar | 31124240PubMed |
Eads, DA, Biggins, DE, Doherty Jr, PF, Gage, KL, Huyvaert, KP, Long, DH, and Antolin, MF (2013). Using occupancy models to investigate the prevalence of ectoparasitic vectors on hosts: an example with fleas on prairie dogs. International Journal for Parasitology: Parasites and Wildlife 2, 246–256.
| Using occupancy models to investigate the prevalence of ectoparasitic vectors on hosts: an example with fleas on prairie dogs.Crossref | GoogleScholarGoogle Scholar | 24533343PubMed |
Eads, DA, Biggins, DE, Bowser, J, McAllister, JC, Griebel, RL, Childers, E, Livieri, TM, Painter, C, Krank, LS, and Bly, K (2018). Resistance to deltamethrin in prairie dog (Cynomys ludovicianus) fleas in the field and in the laboratory. Journal of Wildlife Diseases 54, 745–754.
| Resistance to deltamethrin in prairie dog (Cynomys ludovicianus) fleas in the field and in the laboratory.Crossref | GoogleScholarGoogle Scholar | 29723100PubMed |
Eads, DA, Biggins, DE, and Gage, KL (2020). Ecology and management of plague in diverse communities of rodents and fleas. Vector-Borne and Zoonotic Diseases 20, 888–896.
| Ecology and management of plague in diverse communities of rodents and fleas.Crossref | GoogleScholarGoogle Scholar | 33074791PubMed |
Gage, KL, and Kosoy, MY (2005). Natural history of plague: perspectives from more than a century of research. Annual Review of Entomology 50, 505–528.
| Natural history of plague: perspectives from more than a century of research.Crossref | GoogleScholarGoogle Scholar | 15471529PubMed |
Goldberg, AR, Conway, CJ, and Biggins, DE (2020). Flea sharing among sympatric rodent hosts: implications for potential plague effects on a threatened sciurid. Ecosphere 11, e93933.
| Flea sharing among sympatric rodent hosts: implications for potential plague effects on a threatened sciurid.Crossref | GoogleScholarGoogle Scholar |
Goldberg, AR, Conway, CJ, and Biggins, DE (2021a). Effects of experimental flea removal and plague vaccine treatments on survival of northern Idaho ground squirrels and two coexisting sciurids. Global Ecology and Conservation 26, e01489.
| Effects of experimental flea removal and plague vaccine treatments on survival of northern Idaho ground squirrels and two coexisting sciurids.Crossref | GoogleScholarGoogle Scholar |
Goldberg AR, Biggins DE, Ramakrishnan S, Bowser JW, Conway CJ, Eads DA, Wimsatt J (2021b) Effects of deltamethrin applications on non-target small mammal populations in South Dakota, Colorado, and Idaho, 2010–2017. U.S. Geological Survey data release.
Gregory, DA, Johnson, DL, and Thompson, BH (1993). The impact of bran baits treated with the insecticides carbaryl, chlorpyrifos and dimethoate on the survivorship and reproductive success of non-target mouse populations. Agriculture, Ecosystems & Environment 45, 95–103.
| The impact of bran baits treated with the insecticides carbaryl, chlorpyrifos and dimethoate on the survivorship and reproductive success of non-target mouse populations.Crossref | GoogleScholarGoogle Scholar |
Griffin, KA, Martin, DJ, Rosen, LE, Sirochman, MA, Walsh, DP, Wolfe, LL, and Miller, MW (2010). Detection of Yersinia pestis DNA in prairie dog–associated fleas by polymerase chain reaction assay of purified DNA. Journal of Wildlife Diseases 46, 636–643.
| Detection of Yersinia pestis DNA in prairie dog–associated fleas by polymerase chain reaction assay of purified DNA.Crossref | GoogleScholarGoogle Scholar | 20688665PubMed |
Heath, DG, Anderson Jr, GW, Mauro, JM, Welkos, SL, Andrews, GP, Adamovicz, J, and Friedlander, AM (1998). Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16, 1131–1137.
| Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine.Crossref | GoogleScholarGoogle Scholar | 9682370PubMed |
Hoogland, JL, Davis, S, Benson-Amram, S, Labruna, D, Goossens, B, and Hoogland, MA (2004). Pyraperm kills fleas and halts plague among Utah prairie dogs. The Southwestern Naturalist 49, 376–383.
| Pyraperm kills fleas and halts plague among Utah prairie dogs.Crossref | GoogleScholarGoogle Scholar |
Hubbart, JA, Jachowski, DS, and Eads, DA (2011). Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi). Journal of Vector Ecology 36, 117–123.
| Seasonal and among-site variation in the occurrence and abundance of fleas on California ground squirrels (Otospermophilus beecheyi).Crossref | GoogleScholarGoogle Scholar | 21635649PubMed |
Krasnov, BR, Burdelova, NV, Shenbrot, GI, and Khokhlova, IS (2002). Annual cycles of four flea species in the central Negev desert. Medical and Veterinary Entomology 16, 266–276.
| Annual cycles of four flea species in the central Negev desert.Crossref | GoogleScholarGoogle Scholar | 12243227PubMed |
Krasnov, BR, Spickett, A, Junker, K, Bugmyrin, SV, Ieshko, EP, Bespyatova, LA, Stanko, M, Khokhlova, IS, and Matthee, S (2021). Parasite counts or parasite incidences? Testing differences with four analyses of infracommunity modelling for seven parasite–host associations. Parasitology Research 120, 2569–2584.
| Parasite counts or parasite incidences? Testing differences with four analyses of infracommunity modelling for seven parasite–host associations.Crossref | GoogleScholarGoogle Scholar | 34137949PubMed |
Laake JL (2013) RMark: an R interface for analysis of capture-recapture data with MARK. Alaska Fisheries Science Centre processed report 2013-01. Alaska Fisheries Science Centre, Seattle, WA, USA.
Matchett, MR, Biggins, DE, Carlson, V, Powell, B, and Rocke, T (2010). Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana. Vector-Borne and Zoonotic Diseases 10, 27–35.
| Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in Montana.Crossref | GoogleScholarGoogle Scholar | 20158329PubMed |
Matchett, MR, Stanley, TR, McCollister, MF, Eads, DA, Boulerice, JT, and Biggins, DE (2021). Oral Sylvatic Plague Vaccine Does Not Adequately Protect Prairie Dogs (Cynomys spp.) for Endangered Black-Footed Ferret (Mustela nigripes) Conservation. Vector-Borne and Zoonotic Diseases 21, 921–940.
| Oral Sylvatic Plague Vaccine Does Not Adequately Protect Prairie Dogs (Cynomys spp.) for Endangered Black-Footed Ferret (Mustela nigripes) Conservation.Crossref | GoogleScholarGoogle Scholar | 34757815PubMed |
Nieradko-Iwanicka, B, and Borzęcki, A (2015). Subacute poisoning of mice with deltamethrin produces memory impairment, reduced locomotor activity, liver damage and changes in blood morphology in the mechanism of oxidative stress. Pharmacological Reports 67, 535–541.
| Subacute poisoning of mice with deltamethrin produces memory impairment, reduced locomotor activity, liver damage and changes in blood morphology in the mechanism of oxidative stress.Crossref | GoogleScholarGoogle Scholar | 25933966PubMed |
Ramakrishnan S (2017) Impact of enzootic plague on Neotoma mexicana in northern New Mexico. New Mexico Highlands University Las Vegas, NM, USA.
Rattner, BA, and Michael, SD (1985). Organophosphorus insecticide induced decrease in plasma luteinizing hormone concentration in white-footed mice. Toxicology Letters 24, 65–69.
| Organophosphorus insecticide induced decrease in plasma luteinizing hormone concentration in white-footed mice.Crossref | GoogleScholarGoogle Scholar | 3975930PubMed |
R Core Team (2017) ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org
Rehman, H, Aziz, AT, Saggu, S, Abbas, ZK, Mohan, A, and Ansari, AA (2014). Systematic review on pyrethroid toxicity with special reference to deltamethrin. Journal of Entomology and Zoology Studies 2, 60–70.
Rocke, TE, Mencher, J, Smith, SR, Friedlander, AM, Andrews, GP, and Baeten, LA (2004). Recombinant F1-V fusion protein protects black-footed ferrets (Mustela nigripes) against virulent Yersinia pestis infection. Journal of Zoo and Wildlife Medicine 35, 142–146.
| Recombinant F1-V fusion protein protects black-footed ferrets (Mustela nigripes) against virulent Yersinia pestis infection.Crossref | GoogleScholarGoogle Scholar | 15305507PubMed |
Rocke, TE, Tripp, DW, Russell, RE, Abbott, RC, Richgels, KLD, Matchett, MR, Biggins, DE, Griebel, R, Schroeder, G, Grassel, SM, Pipkin, DR, Cordova, J, Kavalunas, A, Maxfield, B, Boulerice, J, and Miller, MW (2017). Sylvatic plague vaccine partially protects prairie dogs (Cynomys spp.) in field trials. Ecohealth 14, 438–450.
| Sylvatic plague vaccine partially protects prairie dogs (Cynomys spp.) in field trials.Crossref | GoogleScholarGoogle Scholar | 28643091PubMed |
Russell, RE, Tripp, DW, and Rocke, TE (2019). Differential plague susceptibility in species and populations of prairie dogs. Ecology and Evolution 9, 11962–11971.
| Differential plague susceptibility in species and populations of prairie dogs.Crossref | GoogleScholarGoogle Scholar | 31695901PubMed |
Sánchez-Bayo F (2011) Impacts of agricultural pesticides on terrestrial ecosystems. In ‘Ecological Impacts of Toxic Chemicals’. (Eds Sánchez-Bayo, PJ van den Brink, RM Mann) pp. 63–87. (Bentham Books: Sharjah, UAE)
| Crossref |
Seery, DB, Biggins, DE, Montenieri, JA, Enscore, RE, Tanda, DT, and Gage, KL (2003). Treatment of black-tailed prairie dog burrows with deltamethrin to control fleas (Insecta: Siphonaptera) and plague. Journal of Medical Entomology 40, 718–722.
| Treatment of black-tailed prairie dog burrows with deltamethrin to control fleas (Insecta: Siphonaptera) and plague.Crossref | GoogleScholarGoogle Scholar | 14596288PubMed |
Sheffield, SR, and Lochmiller, RL (2001). Effects of field exposure to diazinon on small mammals inhabiting a semienclosed prairie grassland ecosystem. I. Ecological and reproductive effects. Environmental Toxicology and Chemistry: An International Journal 20, 284–296.
| Effects of field exposure to diazinon on small mammals inhabiting a semienclosed prairie grassland ecosystem. I. Ecological and reproductive effects.Crossref | GoogleScholarGoogle Scholar |
Tripp, DW, Gage, KL, Montenieri, JA, and Antolin, MF (2009). Flea abundance on black-tailed prairie dogs (Cynomys ludovicianus) increases during plague epizootics. Vector-Borne and Zoonotic Diseases 9, 313–321.
| Flea abundance on black-tailed prairie dogs (Cynomys ludovicianus) increases during plague epizootics.Crossref | GoogleScholarGoogle Scholar | 19492944PubMed |
Tripp, DW, Streich, SP, Sack, DA, Martin, DJ, Griffin, KA, and Miller, MW (2016). Season of deltamethrin application affects flea and plague control in white-tailed prairie dog (Cynomys leucurus) colonies, Colorado, USA. Journal of Wildlife Diseases 52, 553–561.
| Season of deltamethrin application affects flea and plague control in white-tailed prairie dog (Cynomys leucurus) colonies, Colorado, USA.Crossref | GoogleScholarGoogle Scholar | 27195680PubMed |
Tripp, DW, Rocke, TE, Runge, JP, Abbott, RC, and Miller, MW (2017). Burrow dusting or oral vaccination prevents plague-associated prairie dog colony collapse. EcoHealth 14, 451–462.
| Burrow dusting or oral vaccination prevents plague-associated prairie dog colony collapse.Crossref | GoogleScholarGoogle Scholar | 28643090PubMed |
(2021). Endangered and threatened wildlife and plants; endangered species status for the Peñasco least chipmunk and designation of critical habitat. Federal Register 86, 54584–53609.
van den Berg, H, Zaim, M, Yadav, RS, Soares, A, Ameneshewa, B, Mnzava, A, Hii, J, Dash, AP, and Ejov, M (2012). Global trends in the use of insecticides to control vector-borne diseases. Environmental Health Perspectives 120, 577–582.
| Global trends in the use of insecticides to control vector-borne diseases.Crossref | GoogleScholarGoogle Scholar | 22251458PubMed |
White, GC, and Burnham, KP (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139.
| Program MARK: survival estimation from populations of marked animals.Crossref | GoogleScholarGoogle Scholar |


