Can flexible timing of harvest for translocation reduce the impact on fluctuating source populations?
Simon J. Verdon A B D , William F. Mitchell
A B D , William F. Mitchell  C and Michael F. Clarke A B
C and Michael F. Clarke A B
A Research Centre for Future Landscapes, La Trobe University, Kingsbury Drive, Melbourne, Vic. 3086, Australia.
B Department of Ecology, Environment and Evolution, La Trobe University, Kingsbury Drive, Melbourne, Vic. 3086, Australia.
C School of Biological Sciences, Monash University, Rainforest Walk, Melbourne, Vic. 3800, Australia.
D Corresponding author. Email: S.Verdon@latrobe.edu.au
Wildlife Research 48(5) 458-469 https://doi.org/10.1071/WR20133
Submitted: 6 August 2020 Accepted: 13 February 2021 Published: 16 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Context: Species translocations are used in conservation globally. Although harvest for translocation may have negative impacts on source populations, translocation programs rarely explore ways of minimising those impacts. In fluctuating source populations, harvest timing may affect its impact because population size and trajectory vary among years.
Aims: We explored whether the timing and scale of harvest can be altered to reduce its impact on a fluctuating source population of Mallee Emu-wrens, Stipiturus mallee; an endangered passerine in south-eastern Australia. Mallee Emu-wren populations fluctuate with ~5–10-year drought–rain cycles.
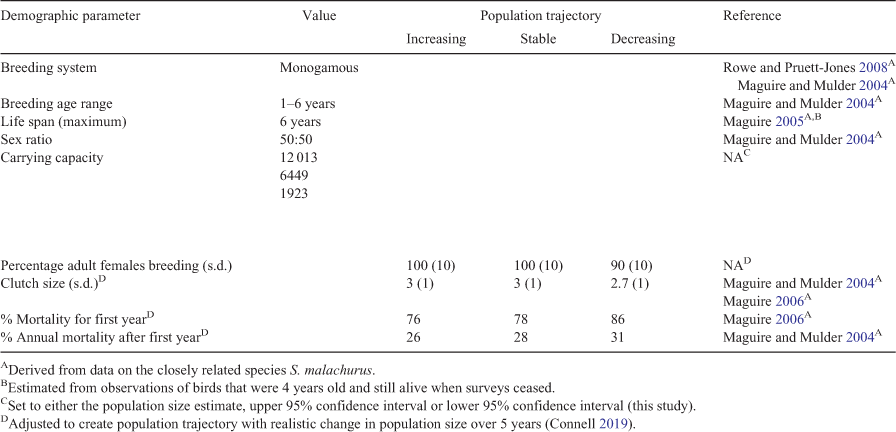
Methods: We used population viability analysis (PVA) to compare the impact of five harvest scales (no harvest, 100, 200, 300 or 500 individuals) under three population trajectories (increasing, stable or decreasing) and two initial population sizes (our model-based estimate of the population size and the lower 95% confidence interval of that estimate). To generate a model-based estimate of the population size, we surveyed 540 sites (9 ha), stratified according to environmental variables known to affect Mallee Emu-wren occurrence. We used an information-theoretic approach with N-mixture models to estimate Mallee Emu-wren density, and extrapolated results over all potential habitat.
Key Results: We estimate that in spring 2019, the source population consisted of 6449 individuals, with a minimum of 1923 individuals (lower 95% confidence interval). Of 48 harvest scenarios, only seven showed no impact of harvest within 5 years (15%). Those seven all had increasing population trajectories and carrying capacity set to equal initial population size. Twenty-six populations showed no impact of harvest within 25 years (54%). These were either increasing populations that had reached carrying capacity or decreasing populations nearing extinction.
Conclusions: Initial population size, carrying capacity, harvest scale and population trajectory were all determinants of harvest impact. Given the importance of carrying capacity, further research is required to determine its role in the Mallee Emu-wren source population.
Implications: Harvesting Mallee Emu-wrens after high-rainfall years will have the least impact because source populations are likely to be large with increasing trajectories. For fluctuating source populations, flexibility in the timing of harvest can reduce its impact and should be considered during translocation planning.
Keywords: abundance, conservation management, conservation planning, endangered species, population modelling, population viability, population management, threatened species.
Introduction
Translocations are increasingly used in the management of threatened species (Seddon et al. 2014; Berger-Tal et al. 2020). Translocations aim to supplement small populations or establish new populations, most often within a species’ current range or former range (Seddon et al. 2007; IUCN 2013). When assessing the net benefit conferred to a species by a translocation program, it is important to consider both the benefits of translocation for the recipient population and the risk of harvest for the source population (Pérez et al. 2012). However, many translocation programs do not have capacity to assess the impact of harvest on the source population (Berger-Tal et al. 2020).
Barriers to assessing impact on source populations include time and budgetary constraints, a lack of basic biological information regarding species’ rates of reproduction and mortality and a lack of information regarding the size of source populations (Clarke et al. 2003; Wolf et al. 2015; Garnett and Geyle 2018; Furlan et al. 2020). An estimate of population size is an important pre-requisite for assessing impact on source populations in a population viability analysis (PVA) framework (Lande et al. 2003). It is difficult and costly to precisely estimate population size, especially for widespread, low-density populations or species that are difficult to detect (Clarke et al. 2003; Wolf et al. 2015; Garnett and Geyle 2018). Likewise, the necessary data on species’ demography, breeding system and vital rates are rarely available for input to PVA models (Wolf et al. 2015). Because these parameters influence dynamics of source populations post-harvest, poor data quality for these parameters leads to uncertainty when deciding among management strategies and may reduce the utility of PVA (Miller et al. 2019).
Despite potential uncertainty, many translocation programs use PVA to inform decisions regarding impacts of harvest and harvest strategy (Armstrong and Reynolds 2012). This is because information regarding species biology and population size may take decades to acquire, and species targeted in translocation programs often face a level of extinction risk that requires immediate intervention (Burgman and Possingham 2000; Martin et al. 2012; Wolf et al. 2015). In many cases, PVA is still best placed to compare the relative impacts of harvest scenarios, even if estimates of population change and extinction risk are prone to high levels of uncertainty (Burgman and Possingham 2000; Reed et al. 2002; Armstrong and Reynolds 2012).
Assessing the impact of harvest is even more complex for populations that frequently undergo large-scale fluctuations in population size (Southgate and Possingham 1995; Bode and Brennan 2011; Céré et al. 2015). For example, many arid-zone species undergo population ‘boom-and-bust’ cycles, i.e. substantial increase in population size during years of above-average rainfall, followed by population decline in the following years (Holmgren et al. 2006; Céré et al. 2015). Fluctuations in population size and population trajectory present challenges to assessing impacts of harvest on source populations because (a) any estimate of population size is relevant for only a short period and (b) the impact of harvest is likely to vary among years depending on subsequent conditions for recruitment.
Fluctuating source populations also present an opportunity for translocation programs; if the capacity of a source population to recover post-harvest depends on the prevailing population trajectory, then harvest can be timed to promote the recovery of the source population. To the best of our knowledge, the present study is the first to examine whether harvesting of a fluctuating source population can be timed to optimise the rate of post-harvest recovery in the context of threatened species translocations.
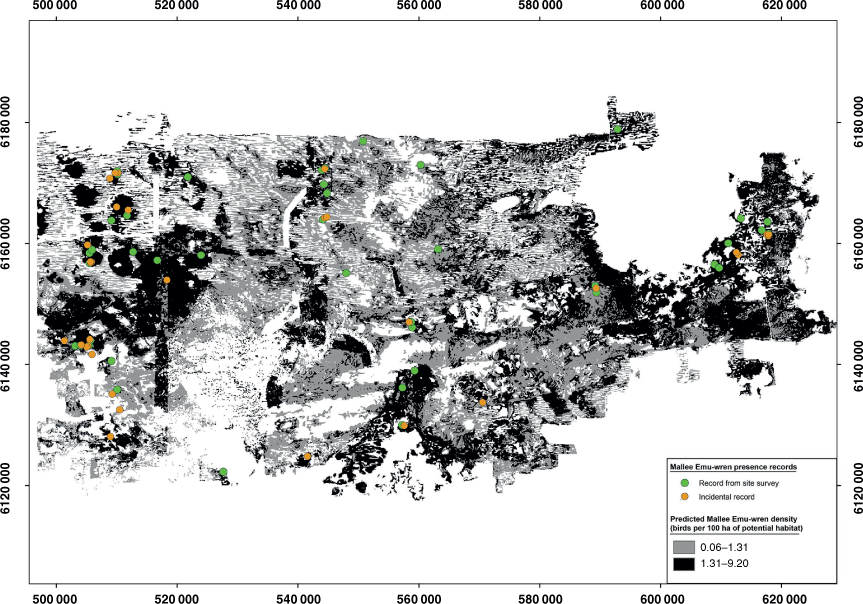
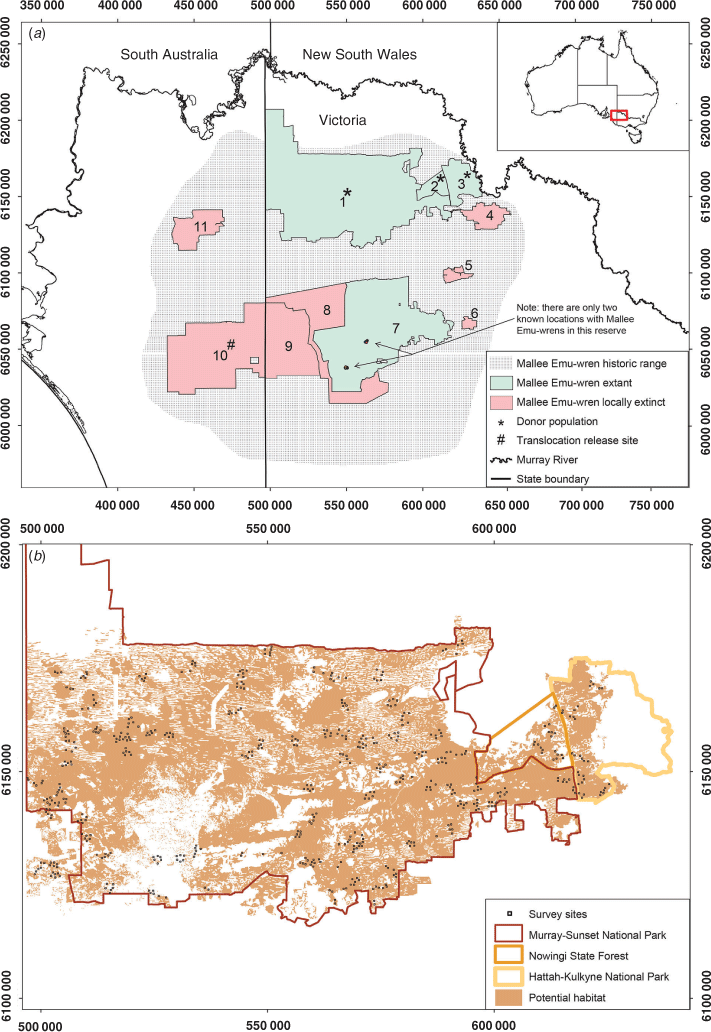
The present study focussed on the Mallee Emu-wren, Stipiturus mallee, an endangered passerine endemic to the Murray–Mallee region of south-eastern Australia (Fig. 1; Higgins et al. 2001). Despite being sedentary and occupying their territories throughout the year, Mallee Emu-wrens are difficult to detect because they are poor flyers and, when disturbed, often shelter in dense hummock-grass rather than flush (Higgins et al. 2001). In addition, Mallee Emu-wrens have a soft and high-pitched call, are very small (5 g) and are present at low densities in a large (~700 000 ha) reserve network (Higgins et al. 2001; Brown et al. 2009; Menkhorst et al. 2017).
|
More than half of the historic range of the Mallee Emu-wren was cleared for agriculture during the past century and almost all remaining habitat is on public reserves (Fig. 1; White 2006; Brown et al. 2009; Garnett et al. 2011). Over the past four decades, drought and large (>10 000-ha) wildfires have contributed to the Mallee Emu-wren’s decline in these public reserves (Paton et al. 2009; Garnett et al. 2011). A wild-to-wild translocation program aims to re-establish a population of Mallee Emu-wrens in Ngarkat Conservation Park in South Australia through re-introduction of birds from a source population in Victoria (Fig. 1; Mitchell et al. 2021). However, the likely impact of harvest on the source population is unknown for two main reasons. First, the most recent estimate of the Mallee Emu-wren population size (16 821 individuals) used data from 1999–2006 (Brown et al. 2009). Because of ongoing declines in this species, obtaining a contemporary estimate of population size is a critical first step in comparing the impact of harvest scenarios on the source population (Brown et al. 2009; Garnett et al. 2011; Verdon et al. 2019). Second, the influence of the prevailing population trajectory on post-harvest recovery is poorly understood for this species (Mitchell et al. 2021).
A recent study found that rates of Mallee Emu-wren occurrence undergo significant changes over short periods as a result of fluctuations in annual rainfall (Connell 2019). Although Connell (2019) did not directly measure changes in abundance, increases in abundance after years of above-average rainfall (‘Big-Wet’ years) have been observed in many species in arid and semiarid environments (Holmgren et al. 2006), and it is likely that the observed increase in the rate of Mallee Emu-wren occurrence was associated with increased abundance. Connell (2019) was limited by a small number of Mallee Emu-wren records. As a result, formal analysis comparing years was not possible. However, when resurveying sites at 4-year intervals, Mallee Emu-wren occurrences increased by 40% between ‘Drought’ (2006–2008) and ‘Big-Wet’ (2011–2012) periods and decreased by 65% from ‘Big-Wet’ to ‘post Big-Wet’ (2015–2016) periods (Connell 2019).
Given that the Mallee Emu-wren source population is known to fluctuate among years, it is important to determine the degree to which the impact of harvest depends on the population trajectory and the population size at the time of harvest. We aimed to (a) estimate the Mallee Emu-wren population size and test whether initial population size influences capacity for population recovery and (b) identify the timing of harvest (i.e. when population is increasing, stable or decreasing) and scale of harvest (number of birds) with the least negative impacts.
Methods
Estimating population size
Study area
We surveyed the Mallee Emu-wren source population in north-western Victoria (Fig. 1). Birds were harvested from this population for ‘Phase 1’ translocations of Mallee Emu-wrens in 2018 and will be used to source birds for any future translocations (Mitchell et al. 2021).
We restricted analysis to ‘Triodia Mallee’ vegetation (henceforth, ‘Potential Habitat’; Fig. 1; Haslem et al. 2010). Within this part of the species’ range, Mallee Emu-wrens are found in this vegetation type only due to a close association with the hummock-grass, Triodia scariosa (often called spinifex), which they use for foraging, nesting and protection from predators (Higgins et al. 2001; Verdon et al. 2020). Wyperfeld National Park was not included in the present study because the population size in this reserve is likely to be less than 200 individuals (Chris Hedger, pers. comm.; Brown et al. 2009).
Study design
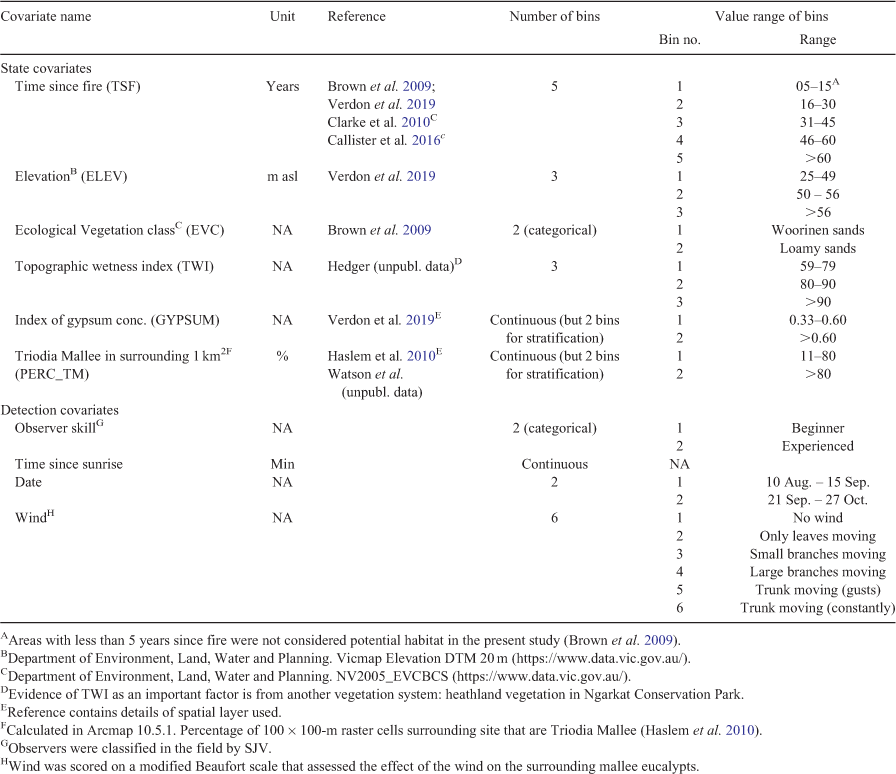
Selection of covariates. This study design is based on random sampling of sites, stratified according to important covariates of Mallee Emu-wren occurrence determined in earlier studies (Table 1). Covariate values were binned wherever previous studies indicated that such bins were appropriate and have an ecological basis (Table 1). In addition to the covariates selected for Mallee Emu-wren state models, covariates were required for Mallee Emu-wren detection models (Table 1). We used four detection covariates in total (Table 1).
|
Survey site selection. We selected 540 survey sites (9 ha each). We distributed sites across the 120 unique covariate combinations according to the area of potential habitat covered by each combination. We increased the proportion of sites allocated to more common combinations of covariates, at the expense of rarer combinations because we judged that the combinations that cover the greatest area would have the greatest effect on the final population estimate. We used a log(x) + 1 transformation to distribute the number of sites per combination. This transformation was necessary to ensure that rarer combinations still received adequate replication and that all combinations were surveyed (Supplementary material S1).
Survey method. Our survey protocol included an average of 2.8 (2–4) repeat surveys at 540 sites. Surveys (n = 1508) consisted of a single person conducting a 45-min 9-ha (300 × 300 m) area search, walking 1500 m per survey. All surveys were conducted in the austral spring of 2019 (10 August – 27 October). Each person surveyed three to six sites per day and surveys started at dawn (±1 h).
Surveys began by playing 1 min of the Mallee Emu-wren contact calls in the centre of the site, followed by 2 min of listening. All call playback was performed using the surveyor’s smartphone at full volume. Playback of the contact call was repeated wherever appropriate habitat was encountered. At the end of the survey, the surveyor returned to the centre of the site and played a 1 min recording of the Mallee Emu-wren territorial song, followed by 2 min of listening. The territorial song was played only at the centre to reduce the likelihood of ‘calling birds in’ from outside of the site. Surveyors used georeferenced pdf maps on their smartphones for navigation (Avenza Maps 3.9.1). Surveyors recorded the location of each Mallee Emu-wren group, the number of birds present and their sexes.
Analysis
Statistical approach. We used N-mixture modelling to estimate Mallee Emu-wren population size. N-mixture models incorporate a binomial model to estimate the detectability of a species (detection model) and a Poisson or negative binomial model to estimate the density of a species (state model; Kéry and Royle 2015). Pre-requisites for effective N-mixture modelling and our capacity to meet them are outlined in Table S1.
Broadly, this statistical approach involved conducting repeat surveys of sites that differed in their environmental conditions. By surveying sites on multiple occasions, we were able to model Mallee Emu-wren detectability and use this estimate of imperfect detection to estimate the density of Mallee Emu-wrens per 9-ha survey site.
For N-mixture modelling we used the ‘unmarked’ package in R version 3.6.1 (Fiske and Chandler 2011; R Core Team 2019). We undertook an information-theoretic approach, using Akaike’s information criterion (AIC) to determine the most parsimonious model (Akaike 1974). We restricted candidate detection models to univariate because of issues with over-fitting. As a result, we had four candidate detection models, each with one covariate (Table 2). We included no state covariates when comparing candidate detection models.
For the state models, we had six covariates and candidate models fell into the following three categories: additive models; two-way interaction models; two-way interaction models with additive covariates. For additive models, candidate models were selected by beginning with the global model and by using stepwise removal of covariates until only one covariate remained. For interaction models, we compared all possible two-way interaction models. For two-way interaction models with additive covariates, we began with the most parsimonious two-way interaction model plus the remaining four covariates included as additive terms. We then used stepwise removal of additive terms until the two-way interaction and only one additive term remained. This model development process led to a total of 24 candidate state models, all of which included the most parsimonious detection covariate in the detection component of the N-mixture model. We selected the candidate N-mixture model with the lowest AIC as the most parsimonious model and used this model to develop the Mallee Emu-wren population estimate.
We used a zero-inflated Poisson distribution for all N-mixture models (Kéry and Royle 2015). We evaluated goodness of fit for the final model using the parametric bootstrapping function ‘parboot’ in the ‘unmarked’ package in R. Goodness-of-fit testing compares the observed data with the quantity that we would expect to see under the model when we use simulated datasets generated with the model’s distribution. We ran 100 bootstrap simulations using the most parsimonious model. We used the Freeman–Tukey test to test for a significant deviation between our dataset and the simulated datasets (Kéry and Royle 2015).
We also undertook an informal assessment of the capacity of the model to predict the occurrence of Mallee Emu-wrens in the study area. We used predicted Mallee Emu-wren density to divide potential habitat in the study area into two categories of equal area, namely, Predicted high-density or Predicted low-density (183093 ha each). We used incidental occurrence records of Mallee Emu-wrens collected during fieldwork as an independent dataset (24 presence records >200 m from the nearest survey site). We compared the number of incidental records in each density category with the number of records that was predicted by the model to be in each density category. We report the expected and observed values for comparison.
We used the ‘raster’ package in R to predict Mallee Emu-wren density, estimate population size and calculate 95% confidence intervals across potential habitat in the study area (Hijmans et al. 2015).
Comparing the impact of harvest scenarios
Harvest scenarios
We compared the impact of harvest under three different prevailing population trajectories (increasing, stable and decreasing) and compared the impact of five different scales of harvest (no harvest, 100, 200, 300 or 500 birds harvested). We made these comparisons with the initial population size set to either the population estimate, or the lower 95% confidence interval, derived from the present study.
We compared the impact of harvest scenarios over two different ‘prediction horizons’. First, we plotted post-harvest change in population size (mean ± standard deviation) over a 5-year period to assess short-term impact. The 5-year prediction horizon is important because (a) it is a realistic timeframe over which a population may maintain an increasing or decreasing population trajectory in this system due to the cyclical nature and duration of drought and non-drought periods (Letnic and Dickman 2006) and (b) it is a relevant timeframe for the planning and funding of translocation programs (IUCN 2013).
Second, we used a 25-year prediction horizon to compare the number of years over which the impact of harvest was observable. The 25-year prediction horizon adds uncertainty because the prevailing population trajectory is likely to change over this period. Despite this, the 25-year prediction horizon provides important information regarding the potential for long-term impacts of harvest on source populations. For this component, we present the number of years required under each harvest scenario for the source population size to intersect with the population size predicted under a ‘no harvest’ scenario.
PVA parameterisation
We used Vortex10 software to parameterise a non-spatial, individual-based PVA model that simulates changes in Mallee Emu-wren population size over time (Lacy and Pollak 2020). Using an annual time-step, models simulated individual birds as they are born, mature, breed and die. Models were structured by age and sex, so that mate limitation at low densities, could be incorporated (Lacy and Pollak 2020). Models were also stochastic, meaning they included variation in the simulated population trajectory due to chance differences in the survival and reproductive rates among individuals in the population (i.e. demographic stochasticity). We ran 1000 iterations of each harvest scenario simulation. We used a non-spatial PVA because studies of Mallee Emu-wren genetics found a lack of genetic structuring, indicating gene flow through dispersing birds moving across potential habitat (Brown et al. 2013).
All simulations except for ‘no harvest’ scenarios included a one-off harvest at Year 1. Given that demographic information for the Mallee Emu-wren is limited, we substituted vital-rate estimates from the Southern Emu-wren, Stipiturus malachurus, a closely related species that has been studied in greater detail (Table 2; IUCN 2013). Models also incorporated uncertainty around these parameters (Table 2). Where demographic data are limited for a species, using the vital rates of a closely related species is recommended by the IUCN (2013) Guidelines for Re-introductions and other Conservation Translocations. However, clearly, such a strategy is flawed because even closely related species may differ in terms of their vital rates. Ultimately, delaying the analysis required for this research until more data on Mallee Emu-wren vital rates is not a viable option because harvesting of this species for translocations is already underway. Nevertheless, future work that elucidates Mallee Emu-wren vital rates would improve population modelling for this species.
To simulate each of the prevailing population trajectories (increasing, stable, decreasing), we altered parameters relating to reproductive output, juvenile mortality and adult mortality to achieve rates of population change consistent with those observed in drought and Big-Wet periods (Table 2; Connell 2019).
Carrying capacity
We set the carrying capacity to the upper 95% confidence interval of the population size estimate (the present study). To test the influence of carrying capacity on our PVA model outputs, we repeated PVA simulations with carrying capacity set to the same value as initial population size. As a result, simulated populations with an otherwise increasing trajectory could not increase over years. However, these populations did have higher reproductive rates and lower mortality rates.
Results
Estimating population size
We detected Mallee Emu-wrens on 75 occasions in 42 of 540 survey sites (7.8%). This comprised 50 independent groups and 25 resightings of groups that were detected in a previous survey round(s). Multiple groups of Mallee Emu-wrens were detected at eight sites, indicating that the 9-ha site size was sufficiently large to incorporate two Mallee Emu-wren territories in some instances.
Model selection
The N-mixture model with the lowest AIC included wind as the sole detection covariate and all six candidate state covariates, with an important interaction between time since fire and elevation i.e. Estimate ± 95% confidence interval did not overlap zero (Tables S2, S3).
Goodness of fit
The bootstrap P-value for the model based on the Freeman–Tukey’s statistic was 0.38, indicating that our model provided an adequate fit to the data. Eighteen incidental Mallee Emu-wren records (75%) were in the category ‘predicted high-density of Mallee Emu-wrens’ with the remaining six records (25%) being in the ‘predicted low-density of Mallee Emu-wrens’ category (Fig. 2). This result is very similar to the model predicted rates of 19 records (78%) occurring in the ‘high-density Mallee Emu-wrens’ category and five records (22%) occurring in the ‘low-density Mallee Emu-wrens’ category.
Population estimate
We estimate that in the austral spring of 2019, the Mallee Emu-wren source population consisted of 6449 individuals (lower95% CI: 1923, upper95% CI: 12013).
Comparing the impact of harvest scenarios
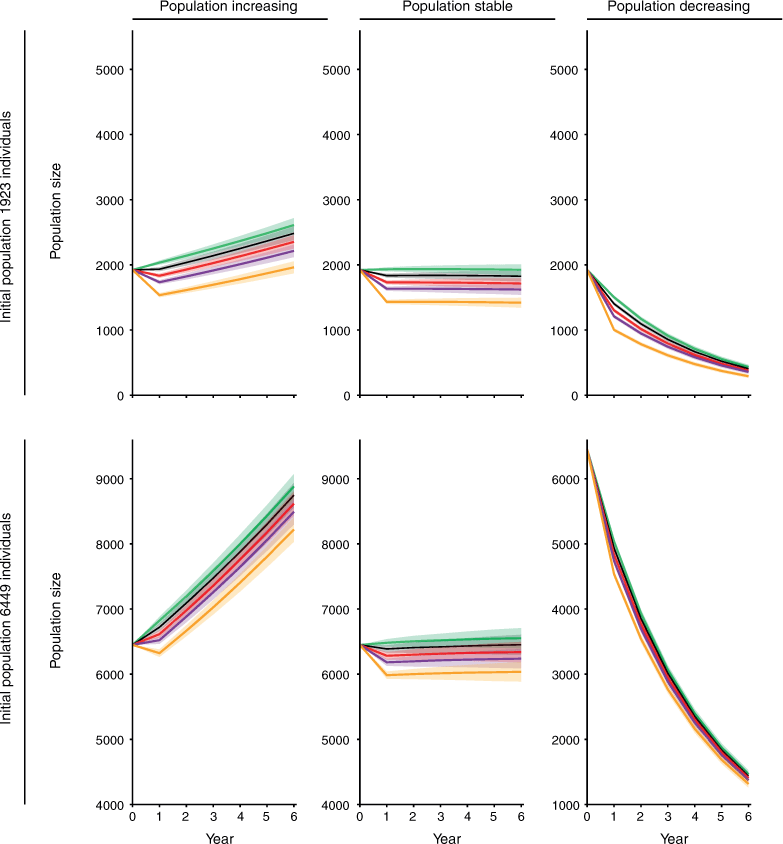
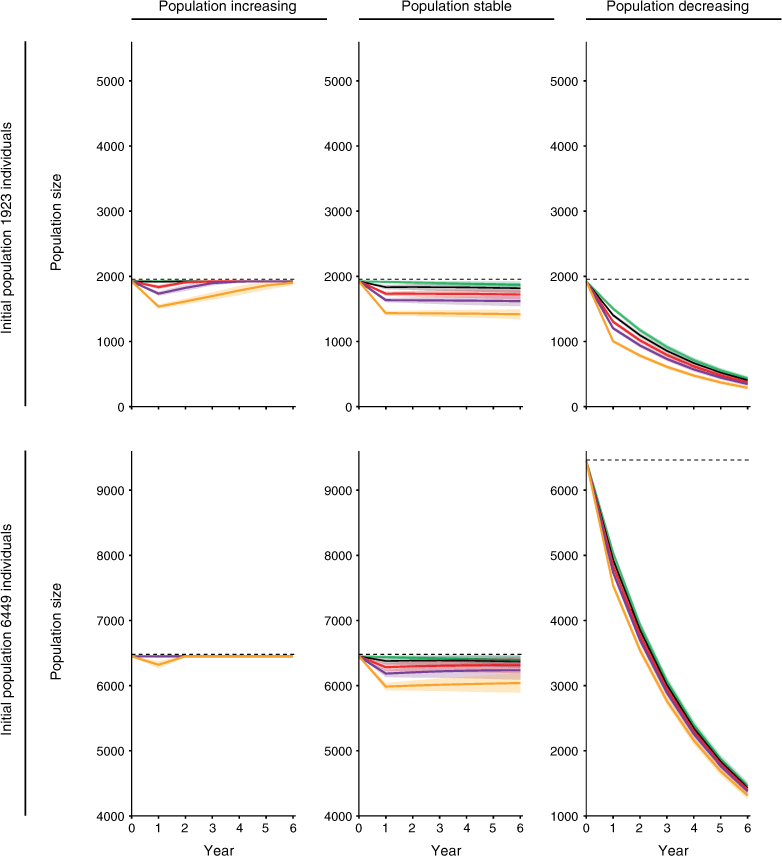
In all, 7 of the 48 harvest scenarios showed no impact of harvest within 5 years (Table 3, Figs 3, 4). These scenarios all had increasing population trajectories and carrying capacity set to equal the initial population size (Table 3, Fig. 4). For simulations with an initial population size of 6449, the impact of harvest lasted for only 1 year at all the scales of harvest we tested (Table 3, Fig. 4). Simulations with an initial population size 1923 showed an impact of harvest for a slightly longer period, but still showed no impact of harvest within 5 years for three of the four scales of harvest tested (Table 3, Fig. 4).
A further 19 harvest scenarios showed no impact of harvest within the 25-year prediction horizon used in the present study (Table 3). Four simulations that had an increasing population trajectory, initial population size of 6449 and carrying capacity set to the upper 95% confidence interval showed no impact of harvest after 15–20 years (Table 3). This timeframe was associated with the time required for populations to reach the greater level set for carrying capacity. Fourteen simulations that had a decreasing population trajectory showed no impact of harvest after 19–23 years. This timeframe was associated with those populations nearing extinction (<50 individuals remaining).
Despite variation in its impact between simulations, harvest did not change the prevailing population trajectory of any simulated population (Figs 3, 4).
Discussion
Initial population size, carrying capacity, scale of harvest and population trajectory all influenced the impact of harvest on simulated Mallee Emu-wren source populations. Although translocation planners have little capacity to alter initial population size or carrying capacity, they can decide the number of birds to take (scale of harvest) and they can time harvest to coincide with a favourable population trajectory. Our study indicated that these measures can reduce the impact of harvest on fluctuating source populations.
Comparing the impact of harvest scenarios
Of the scenarios tested, the impact of harvest on the source population was least when the population was increasing, near carrying capacity and large (6449 rather than 1923 individuals). The influence of harvest scale on the impact of harvest depended on other simulation parameters. Restricting harvest to 300 individuals reduced the impact of harvest for two of the four scenarios with an increasing population trajectory. For the remaining two scenarios, one showed little impact of harvest at all scales of harvest tested and the other did not recover under any of the scales of harvest tested.
To minimise the impact of harvest on source populations, we recommend that translocation planners use population modelling to define limits on harvests and to identify the timing of harvests that has the least negative impact on source populations. In the case of the Mallee Emu-wren, we recommend limiting harvests to less than 300 birds and aiming to undertake future harvests when the source population has an increasing trajectory. However, we acknowledge that translocation planning decisions must weigh potential for negative impacts on the source population against potential benefits of supplementing a population or establishing a new population (Pérez et al. 2012; IUCN 2013). Given that none of the harvest scenarios tested changed the trajectory of the Mallee Emu-wren source population, translocation planners may determine that the potential benefits of translocation outweigh the costs of harvest for this species (Pérez et al. 2012; Colomer et al. 2020). Such decisions are complex and must be made on a case-by-case basis after considering the level of threat faced by the source population, the likelihood of successful translocation, the benefits of successful translocation and the impact of harvest on the source population.
Feasibility of flexible timing of harvest
In the case of the Mallee Emu-wren, timing harvest to coincide with an increasing population trajectory is logistically feasible because evidence suggests that Mallee Emu-wren populations fluctuate in a predictable way in response to years of above average rainfall or ‘Big-Wet’ years (Connell 2019). This provides translocation planners with a method by which they can easily monitor a proxy for the Mallee Emu-wren population trajectory, through national weather and climate monitoring infrastructure (http://www.bom.gov.au/climate/data/). By delaying harvest until during or directly after Big-Wet years, translocation programs may also benefit from improved condition at the release site, thereby increasing the likelihood of establishment success while also minimising the potential for negative impacts on the source population (Berger-Tal et al. 2020). However, access to funders that allow delayed implementation of translocation is still likely to be a limiting factor for many translocation programs (Berger-Tal et al. 2020).
Whereas predicting the prevailing trajectory of the Mallee Emu-wren source population is relatively straightforward, significant time and effort are required to maintain an up-to-date estimate of the population size (Garnett and Geyle 2018; the present study). In addition, because Mallee Emu-wrens are distributed over a large area and are difficult to detect, estimates of their population size are prone to substantial uncertainty around the true number of individuals present (Knape and de Valpine 2012; Kéry and Royle 2015; this study). Any decisions regarding future translocations will have to be made in the context of uncertainty around the impact of harvest on the source population because of the uncertainty around the true population size (Dimond and Armstrong 2007).
The influence of carrying capacity
When carrying capacity was set to equal the initial population size, more simulated populations recovered to ‘no harvest’ levels. In addition, populations that recovered at both levels of carrying capacity required fewer years to do so when carrying capacity was set to equal initial population size. However, the scenario with the least impact of harvest remained the same regardless of whether carrying capacity was set at initial population size or at the upper 95% confidence level. Therefore, we conclude that the level used for carrying capacity strongly influenced results but PVA models nevertheless appear robust to changes in the level of carrying capacity used (Lande et al. 2003).
The increased rate of recovery observed when carrying capacity was set to initial population size is not surprising (Lande et al. 2003; Knape and de Valpine 2012). Carrying capacity limits population growth through density-dependent effects (Lande et al. 2003). After harvest, survival and reproductive capacity of the remaining individuals increases as density-dependent effects are temporarily reduced (Lande et al. 2003; Miller et al. 2019). Given the lack of information regarding Mallee Emu-wren carrying capacity at the source site, we recommend invoking the precautionary principle for this parameter, i.e. setting a high carrying capacity at the source site to ensure that potential for recovery in the Mallee Emu-wren source population is underestimated, rather than overestimated (Miller et al. 2019). Further field-studies on this topic may shed light on the effect of Mallee Emu-wren carrying capacity on population dynamics. For example, Mallee Emu-wren source sites from ‘Phase 1’ translocations (harvested in 2018) are currently being monitored to determine whether density dependence influences the rate of recovery post-harvest at a fine scale (25 ha; Brook and Bradshaw 2006; Mitchell et al. 2021).
Conclusions
We recommend that flexible timing of harvest be explored for any species with fluctuating source populations for which translocations are planned. However, incorporating flexible timing of harvest in translocation planning is likely to be more feasible for species whose populations fluctuate in a somewhat predictable manner, for example, arid-zone species that increase after Big-Wet years. For the Mallee Emu-wren, timing harvest to coincide with an increasing source population (after Big-Wet years) is likely to reduce the impact of harvest on that population. The extent to which carrying capacity is limiting population growth in the Mallee Emu-wren source population remains unresolved (Brook and Bradshaw 2006). Research on the influence of carrying capacity on source populations generally, and the Mallee Emu-wren source population specifically, will help develop appropriate harvest rates for future translocations.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
We gratefully acknowledge the following funding sources: Birdlife Australia; the Department of Environment, Land, Water and Planning; Zoos Victoria; Parks Victoria and the Holsworth Wildlife Research Endowment.
Acknowledgements
This research was undertaken in accordance with the Wildlife Act 1975 and National Parks Act 1975 under the scientific research permit 10 008 824 and La Trobe University animal ethics approval 19 018. This research was undertaken in consultation with the Threatened Mallee Birds Conservation Action Plan Implementation Team and supported by 27 field workers, most of whom donated their time. Mallee Emu-wren contact calls and territorial song were provided by Luke Ireland and Tom Hunt.
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control 19, 716–723.| A new look at the statistical model identification.Crossref | GoogleScholarGoogle Scholar |
Armstrong, D. P., and Reynolds, M. H. (2012). Modelling reintroduced populations: the state of the art and future directions. In ‘Reintroduction biology: integrating science and management’. (Eds Ewen, J. G., Armstrong, D. P., Parker, K. A. and P. J. Seddon.) pp. 165. (John Wiley & Sons.)
Berger-Tal, O., Blumstein, D. T., and Swaisgood, R. R. (2020). Conservation translocations: a review of common difficulties and promising directions. Animal Conservation 23, 121–131.
| Conservation translocations: a review of common difficulties and promising directions.Crossref | GoogleScholarGoogle Scholar |
Bode, M., and Brennan, K. E. C. (2011). Using population viability analysis to guide research and conservation actions for Australia’s threatened malleefowl Leipoa ocellata. Oryx 45, 513–521.
| Using population viability analysis to guide research and conservation actions for Australia’s threatened malleefowl Leipoa ocellata.Crossref | GoogleScholarGoogle Scholar |
Brook, B. W., and Bradshaw, C. J. (2006). Strength of evidence for density dependence in abundance time series of 1198 species. Ecology 87, 1445–1451.
| Strength of evidence for density dependence in abundance time series of 1198 species.Crossref | GoogleScholarGoogle Scholar | 16869419PubMed |
Brown, S., Clarke, M. F., and Clarke, R. H. (2009). Fire is a key element in the landscape-scale habitat requirements and global population status of a threatened bird: the Mallee Emu-wren (Stipiturus mallee). Biological Conservation 142, 432–445.
| Fire is a key element in the landscape-scale habitat requirements and global population status of a threatened bird: the Mallee Emu-wren (Stipiturus mallee).Crossref | GoogleScholarGoogle Scholar |
Brown, S., Harrisson, K. A., Clarke, R. H., Bennett, A. F., and Sunnucks, P. (2013). Limited population structure, genetic drift and bottlenecks characterise an endangered bird species in a dynamic, fire-prone ecosystem. PLoS One 8, e59732.
| Limited population structure, genetic drift and bottlenecks characterise an endangered bird species in a dynamic, fire-prone ecosystem.Crossref | GoogleScholarGoogle Scholar | 24376814PubMed |
Burgman, M., and Possingham, H. (2000). Population viability analysis for conservation: the good, the bad and the undescribed. In ‘Population Viability Analysis for Conservation’. (Eds A. G. Young, G. M. Clarke, and D. Clarke.) pp. 97–112. (Cambridge University Press.)
Callister, K. E., Griffioen, P. A., Avitabile, S. C., Haslem, A., Kelly, L. T., Kenny, S. A., Nimmo, D. G., Farnsworth, L. M., Taylor, R. S., and Watson, S. J. (2016). Historical maps from modern images: using remote sensing to model and map century-long vegetation change in a fire-prone region. PLoS One 11, e0150808.
| Historical maps from modern images: using remote sensing to model and map century-long vegetation change in a fire-prone region.Crossref | GoogleScholarGoogle Scholar | 27029046PubMed |
Céré, J., Vickery, W. L., and Dickman, C. R. (2015). Refugia and dispersal promote population persistence under variable arid conditions: a spatio-temporal simulation model. Ecosphere 6, 225.
| Refugia and dispersal promote population persistence under variable arid conditions: a spatio-temporal simulation model.Crossref | GoogleScholarGoogle Scholar |
Clarke, R. H., Oliver, D. L., Boulton, R. L., Cassey, P., and Clarke, M. F. (2003). Assessing programs for monitoring threatened species; a tale of three honeyeaters (Meliphagidae). Wildlife Research 30, 427–435.
| Assessing programs for monitoring threatened species; a tale of three honeyeaters (Meliphagidae).Crossref | GoogleScholarGoogle Scholar |
Clarke, M. F., Avitabile, S. C., Brown, L., Callister, K. E., Haslem, A., Holland, G. J., Kelly, L. T., Kenny, S. A., Nimmo, D. G., and Spence-Bailey, L. M. (2010). Ageing mallee eucalypt vegetation after fire: insights for successional trajectories in semi-arid mallee ecosystems. Australian Journal of Botany 58, 363–372.
| Ageing mallee eucalypt vegetation after fire: insights for successional trajectories in semi-arid mallee ecosystems.Crossref | GoogleScholarGoogle Scholar |
Colomer, M. À., Oliva-Vidal, P., Jiménez, J., Martínez, J. M., and Margalida, A. (2020). Prioritizing among removal scenarios for the reintroduction of endangered species: insights from bearded vulture simulation modeling. Animal Conservation 23, 396–406.
| Prioritizing among removal scenarios for the reintroduction of endangered species: insights from bearded vulture simulation modeling.Crossref | GoogleScholarGoogle Scholar |
Connell, J. (2019). Fire and Rain: Investigating how major ecological drivers shape a semi-arid bird community over space and time. Ph.D. Thesis, La Trobe University, Melbourne, Vic., Australia.
Dimond, W. J., and Armstrong, D. P. (2007). Adaptive harvesting of source populations for translocation: a case study with New Zealand robins. Conservation Biology 21, 114–124.
| Adaptive harvesting of source populations for translocation: a case study with New Zealand robins.Crossref | GoogleScholarGoogle Scholar | 17298517PubMed |
Fiske, I., and Chandler, R. (2011). Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software 43, 1–23.
| Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance.Crossref | GoogleScholarGoogle Scholar |
Furlan, E. M., Gruber, B., Attard, C. R. M., Wager, R. N. E., Kerezsy, A., Faulks, L. K., Beheregaray, L. B., and Unmack, P. J. (2020). Assessing the benefits and risks of translocations in depauperate species: a theoretical framework with an empirical validation. Journal of Applied Ecology 57, 831–841.
| Assessing the benefits and risks of translocations in depauperate species: a theoretical framework with an empirical validation.Crossref | GoogleScholarGoogle Scholar |
Garnett, S. T., and Geyle, H. M. (2018). The extent and adequacy of monitoring for Australian threatened bird species. In ‘Monitoring threatened species and Ecological Communities’. (Eds S. Legge, N. Robinson, D. Lindenmayer, B. Scheele, D. Southwell, and B. Wintle.) pp. 43–55. (CSIRO Publishing: Melbourne, Vic., Australia.)
Garnett, S. T., Szabo, J., and Dutson, G. (2011). ‘The action plan for Australian birds 2010.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Haslem, A., Callister, K. E., Avitabile, S. C., Griffioen, P. A., Kelly, L. T., Nimmo, D. G., Spence-Bailey, L. M., Taylor, R. S., Watson, S. J., and Brown, L. (2010). A framework for mapping vegetation over broad spatial extents: a technique to aid land management across jurisdictional boundaries. Landscape and Urban Planning 97, 296–305.
| A framework for mapping vegetation over broad spatial extents: a technique to aid land management across jurisdictional boundaries.Crossref | GoogleScholarGoogle Scholar |
Higgins, P. J., Peter, J. M., and Steele, W. K. (Eds) (2001). ‘Handbook of Australian, New Zealand and Antarctic Birds. Vol. 5: Tyrant flycatchers to chats.’ (Oxford University Press: Melbourne, Vic., Australia.)
Hijmans, R. J., Van Etten, J., Cheng, J., Mattiuzzi, M., Sumner, M., Greenberg, J. A., Lamigueiro, O. P., Bevan, A., Racine, E. B., and Shortridge, A. (2015). ‘R Package ‘raster’’. Available at https://CRAN.R-project.org/package=raster [verified 22 March 2021].
Holmgren, M., Stapp, P., Dickman, C. R., Gracia, C., Graham, S., Gutiérrez, J. R., Hice, C., Jaksic, F., Kelt, D. A., Letnic, M., Lima, M., López, B. C., Meserve, P. L., Milstead, W. B., Polis, G. A., Previtali, M. A., Richter, M., Sabaté, S., and Squeo, F. A. (2006). Extreme climatic events shape arid and semiarid ecosystems. Frontiers in Ecology and the Environment 4, 87–95.
| Extreme climatic events shape arid and semiarid ecosystems.Crossref | GoogleScholarGoogle Scholar |
IUCN (2013). ‘Guidelines for reintroductions and other conservation translocations.’ (IUCN Species Survival Commission: Gland, Switzerland.)
Kéry, M., and Royle, J. A. (2015). ‘Applied Hierarchical Modeling in Ecology: Analysis of distribution, abundance and species richness in R and BUGS: Vol. 1: Prelude and Static Models.’ (Academic Press.)
Knape, J., and de Valpine, P. (2012). Are patterns of density dependence in the Global Population Dynamics Database driven by uncertainty about population abundance? Ecology Letters 15, 17–23.
| Are patterns of density dependence in the Global Population Dynamics Database driven by uncertainty about population abundance?Crossref | GoogleScholarGoogle Scholar | 22017744PubMed |
Lacy, R. C., and Pollak, J. P. (2020). ‘Vortex: a stochastic simulation of the extinction process. Version 10.3.8.’ (Chicago Zoological Society: Brookfield, IL, USA.)
Lande, R., Engen, S., and Saether, B.-E. (2003). ‘Stochastic population dynamics in ecology and conservation.’ (Oxford University Press.)
Letnic, M., and Dickman, C. R. (2006). Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia. Biodiversity and Conservation 15, 3847–3880.
| Boom means bust: interactions between the El Niño/Southern Oscillation (ENSO), rainfall and the processes threatening mammal species in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Maguire, G. S. (2005). Behavioural ecology of the Southern Emu-wren (Stipiturus malachurus). Ph.D. Thesis, University of Melbourne, Melbourne, Vic., Australia.
Maguire, G. S. (2006). Territory quality, survival and reproductive success in southern emu‐wrens Stipiturus malachurus. Journal of Avian Biology 37, 579–593.
Maguire, G. S., and Mulder, R. A. (2004). Breeding biology and demography of the southern emu-wren (Stipiturus malachurus). Australian Journal of Zoology 52, 583–604.
| Breeding biology and demography of the southern emu-wren (Stipiturus malachurus).Crossref | GoogleScholarGoogle Scholar |
Martin, T. G., Nally, S., Burbidge, A. A., Arnall, S., Garnett, S. T., Hayward, M. W., Lumsden, L. F., Menkhorst, P., McDonald‐Madden, E., and Possingham, H. P. (2012). Acting fast helps avoid extinction. Conservation Letters 5, 274–280.
| Acting fast helps avoid extinction.Crossref | GoogleScholarGoogle Scholar |
Menkhorst, P., Rogers, D., Clarke, R. H., Davies, D., Marsack, P., and Franklin, K. (2017). ‘The Australian Bird Guide.’ (CSIRO Publishing: Melbourne, Vic., Australia.)
Miller, J. A. O., Furness, R. W., Trinder, M., and Matthiopoulos, J. (2019). The sensitivity of seabird populations to density-dependence, environmental stochasticity and anthropogenic mortality. Journal of Applied Ecology 56, 2118–2130.
| The sensitivity of seabird populations to density-dependence, environmental stochasticity and anthropogenic mortality.Crossref | GoogleScholarGoogle Scholar |
Mitchell, W. F., Boulton, R. L., Ireland, L., Hunt, T. J., Verdon, S. J., Olds, L. G. M., Hedger, C., and Clarke, R. H. (2021). Using experimental trials to improve translocation protocols for a cryptic, endangered passerine. Pacific Conservation Biology , .
| Using experimental trials to improve translocation protocols for a cryptic, endangered passerine.Crossref | GoogleScholarGoogle Scholar |
Paton, D. C., Rogers, D. J., Cale, P., Willoughby, N., and Gates, J. A. (2009). Chapter 14: Birds. In ‘Natural history of the Riverland and Murraylands’. (Ed. J. T. Jennings.) (Royal Society of South Australia: Adelaide, SA, Australia.)
Pérez, I., Anadón, J. D., Díaz, M., Nicola, G. G., Tella, J. L., and Giménez, A. (2012). What is wrong with current translocations? A review and a decision‐making proposal. Frontiers in Ecology and the Environment 10, 494–501.
| What is wrong with current translocations? A review and a decision‐making proposal.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2019). ‘R: A language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria.)
Reed, J. M., Mills, L. S., Dunning, J. B., Menges, E. S., McKelvey, K. S., Frye, R., Beissinger, S. R., Anstett, M.-C., and Miller, P. (2002). Emerging issues in Population Viability Analysis. Conservation Biology 16, 7–19.
| Emerging issues in Population Viability Analysis.Crossref | GoogleScholarGoogle Scholar |
Rowe, M., and Pruett-Jones, S. (2008). Reproductive anatomy of male southern emu-wrens (Stipiturus malachurus) and striated grasswrens (Amytornis striatus). Emu-Austral Ornithology 108, 68–73.
| Reproductive anatomy of male southern emu-wrens (Stipiturus malachurus) and striated grasswrens (Amytornis striatus).Crossref | GoogleScholarGoogle Scholar |
Seddon, P. J., Armstrong, D. P., and Maloney, R. F. (2007). Developing the science of Reintroduction Biology. Conservation Biology 21, 303–312.
| Developing the science of Reintroduction Biology.Crossref | GoogleScholarGoogle Scholar | 17391180PubMed |
Seddon, P. J., Griffiths, C. J., Soorae, P. S., and Armstrong, D. P. (2014). Reversing defaunation: restoring species in a changing world. Science 345, 406–412.
| Reversing defaunation: restoring species in a changing world.Crossref | GoogleScholarGoogle Scholar | 25061203PubMed |
Southgate, R., and Possingham, H. (1995). Modelling the reintroduction of the greater bilby Macrotis lagotis using the metapopulation model Analysis of the Likelihood of Extinction (ALEX). Biological Conservation 73, 151–160.
| Modelling the reintroduction of the greater bilby Macrotis lagotis using the metapopulation model Analysis of the Likelihood of Extinction (ALEX).Crossref | GoogleScholarGoogle Scholar |
Verdon, S. J., Watson, S. J., and Clarke, M. F. (2019). Modeling variability in the fire response of an endangered bird to improve fire‐management. Ecological Applications 29, e01980.
| Modeling variability in the fire response of an endangered bird to improve fire‐management.Crossref | GoogleScholarGoogle Scholar |
Verdon, S. J., Watson, S. J., Nimmo, D. G., and Clarke, M. F. (2020). Are all fauna associated with the same structural features of the hummock-grass Triodia scariosa? Austral Ecology 45, 773–787.
White, M. D. (2006). The mallee vegetation of north western Victoria. Proceedings of the Royal Society of Victoria 118, 229–243.
Wolf, S., Hartl, B., Carroll, C., Neel, M. C., and Greenwald, D. N. (2015). Beyond PVA: why recovery under the Endangered Species Act is more than population viability. Bioscience 65, 200–207.
| Beyond PVA: why recovery under the Endangered Species Act is more than population viability.Crossref | GoogleScholarGoogle Scholar |