Field-based evaluation of scat DNA methods to estimate population abundance of the spotted-tailed quoll (Dasyurus maculatus), a rare Australian marsupial
Monica Ruibal A E , Rod Peakall A , Andrew Claridge B C and Karen Firestone DA Evolution, Ecology and Genetics, Research School of Biology, The Australian National University, Canberra, ACT 0200, Australia.
B Department of Environment and Climate Change, Parks and Wildlife Group, Planning and Performance Unit, Southern Branch, Queanbeyan, NSW 2620, Australia.
C School of Physical, Environmental and Mathematical Sciences, University of New South Wales, Australian Defence Force Academy, Northcott Drive, Canberra, ACT 2620, Australia.
D School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
E Corresponding author. Email: monica.ruibal@anu.edu.au
Wildlife Research 36(8) 721-736 https://doi.org/10.1071/WR09086
Submitted: 29 June 2009 Accepted: 14 November 2009 Published: 16 December 2009
Abstract
Context. DNA extracted non-invasively from remotely collected scat samples has been used successfully to enumerate populations of a few endangered mammal species. However, scat DNA surveys relying on scent-marking behaviours need to identify if age- or sex-specific variations or seasonal changes in scat scent-marking patterns affect population estimates. Furthermore, owing to the low quantity and quality of scat DNA, a thorough assessment of the technique is needed when it is applied to different species to ensure that individual identification is reliable.
Aims. In the current study, microsatellite genetic profiles derived from 208 remotely collected scats of the spotted-tailed quoll (Dasyurus maculatus), a rare Australian marsupial carnivore, were compared with DNA profiles from tissue of 22 live-trapped individuals from the same study area to critically assess the reliability of the non-invasive method to estimate population abundance.
Methods. Scat samples were collected at scent-marking sites over 4 consecutive months (April–July 2005), 7 weeks of which overlapped with the trapping program to allow direct comparisons of population estimates.
Key results. Combining a multiple-tubes approach with error checking analyses provided reliable genetic tags and resulted in the detection of the majority of the live-trapped population (18 of 22 individuals). Ten additional individuals not known from trapping were also observed from scat DNA. A longer-term sampling regime was required for scats than for trapping to allow direct detection of a large proportion of the population and to provide a comparable population estimate. Critically, the 4-month scat collection period highlighted the importance of performing scat surveys during the mating season when scat scent marking is more frequent, and to avoid sex and age biases in scat marking patterns.
Implications. Non-invasive scat DNA sampling methods that rely on scent-marking behaviours need to consider the duration of the sampling period and temporal differences in behaviours by the sexes and age groups to ensure that meaningful population estimates are achieved.
Acknowledgements
We thank Debbie Claridge, Doug Mills, Fred Ford, Ross Meggs and Pam O’Brien for their assistance in the trapping program, and Alex Martin and Chris Howard and two anonymous reviewers for improving earlier drafts of the manuscript. Funding for this project was provided to M. Ruibal from The Australian National University, Sophie Danforth Conservation Biology Fund, The Australian Academy of Science, M.A Ingram Trust, Estate of the Late Winifred Violet Scott, Wildlife Protection Society of Australia and NSW National Parks and Wildlife Service; K. Firestone and W. Sherwin received an ARC grant linkage grant LP0454947. The primary author was supported by an APA PhD scholarship. All fieldwork and tissue collections were conducted under ethics approvals from the Animal Experimentation Ethics Committee, The Australian National University, F.BTZ.75.04 & F.BTZ.78.04, and under the auspices of a NSW National Parks and Wildlife Service Section 120 Scientific Investigation Licence (S11472).
Banks, S. C. , Piggott, M. P. , Hansen, B. D. , Robinson, N. A. , and Taylor, A. C. (2002). Wombat coprogenetics: enumerating a common wombat population by microsatellite analysis of faecal DNA. Australian Journal of Zoology 50, 193–204.
| Crossref | GoogleScholarGoogle Scholar |
Belcher, C. A. , and Darrant, J. P. (2004). Home range and spatial organisation of the marsupial carnivore, Dasyurus maculatus maculatus (Marsupialia: Dasyuridae) in south-eastern Australia. Journal of Zoology 262, 271–280.
| Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W. , and Mills, D. J. (2007). Aerial baiting for wild dogs has no observable impact on spotted-tailed quolls (Dasyurus maculatus) in a rainshadow woodland. Wildlife Research 34, 116–124.
| Crossref | GoogleScholarGoogle Scholar |
Deuter, R. , Pietsch, S. , Hertel, S. , and Muller, O. (1995). A method for preparation of fecal DNA suitable for PCR. Nucleic Acids Research 23, 3800–3801.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Eggert, L. S. , Eggert, J. A. , and Woodruff, D. S. (2003). Estimating population sizes for elusive animals: the forest elephants of Kakum National Park, Ghana. Molecular Ecology 12, 1389–1402.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Henegariu, O. , Heerema, N. A. , Dlouhy, S. R. , Vance, G. H. , and Vogt, P. H. (1997). Multiplex PCR: Critical parameters and step-by-step protocol. BioTechniques 23, 504–511.
| CAS | PubMed |
Kohn, M. H. , York, E. C. , Kamradt, D. A. , Haught, G. , Sauvajot, R. M. , and Wayne, R. K. (1999). Estimating population size by genotyping faeces. Proceedings of the Royal Society of London. Series B. Biological Sciences 266, 657–663.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Lukacs, P. M. , and Burnham, K. P. (2005). Estimating population size from DNA-based closed capture-recapture data incorporating genotyping error. The Journal of Wildlife Management 69, 396–403.
| Crossref | GoogleScholarGoogle Scholar |
McKelvey, K. S. , and Schwartz, M. K. (2005). Dropout: a program to identify problem loci and samples for noninvasive genetic samples in a capture-mark-recapture framework. Molecular Ecology Notes 5, 716–718.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Navidi, W. , Arnheim, N. , and Waterman, M. S. (1992). A multiple-tubes approach for accurate genotyping of very small DNA samples by using PCR: Statistical considerations. American Journal of Human Genetics 50, 347–359.
| CAS | PubMed |
Smith, D. A. , Ralls, K. , Hurt, A. , Adams, B. , Parker, M. , and Maldonado, J. E. (2006). Assessing reliability of microsatellite genotypes from kit fox faecal samples using genetic and GIS analyses. Molecular Ecology 15, 387–406.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |
Taberlet, P. , and Luikart, G. (1999). Non-invasive genetic sampling and individual identification. Biological Journal of the Linnean Society. Linnean Society of London 68, 41–55.
| Crossref | GoogleScholarGoogle Scholar |
Valiere, N. (2002). GIMLET: a computer program for analysing genetic individual identification data. Molecular Ecology Notes 2, 377–379.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Waits, L. P. , and Leberg, P. (2000). Biases associated with population estimation using molecular tagging. Animal Conservation 3, 191–199.
| Crossref | GoogleScholarGoogle Scholar |
White, G. C. , and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl.), 120–138.
| Crossref | GoogleScholarGoogle Scholar |

Wilson, G. J. , Frantz, A. C. , Pope, L. C. , Roper, T. J. , Burke, T. A. , Cheeseman, C. L. , and Delahay, R. J. (2003). Estimation of badger abundance using faecal DNA typing. Journal of Applied Ecology 40, 658–666.
| Crossref | GoogleScholarGoogle Scholar |

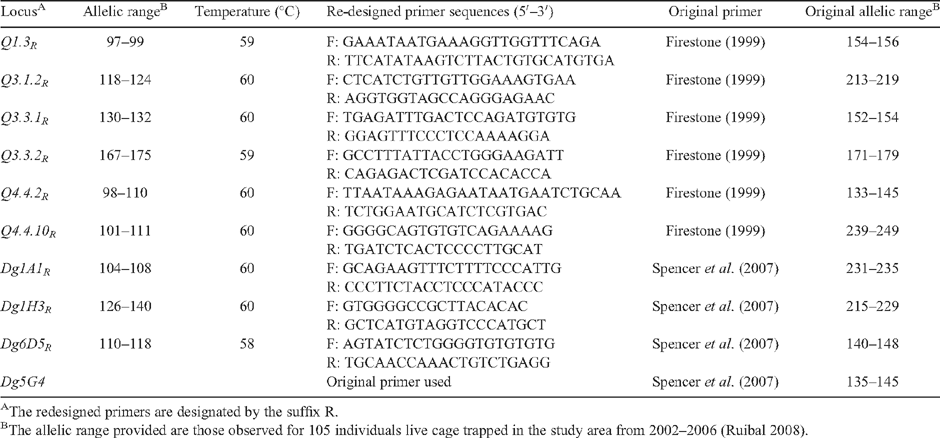
|

|

|

|


