Plantation tree growth responses to P, N, K and minor and trace elements on low fertility savanna soils
Stan J. Rance A B , David M. Cameron B , Carl R. Gosper A C * and Emlyn R. Williams D
A C * and Emlyn R. Williams D
A CSIRO Land and Water, Private Bag 5, Wembley, WA 6913, Australia.
B School of Environment, Science and Engineering, Southern Cross University, Lismore, NSW 2480, Australia.
C Biodiversity and Conservation Science, Department of Biodiversity, Conservation and Attractions, Locked Bag 104, Bentley Delivery Centre, WA 6983, Australia.
D Statistical Consulting Unit, ANU, Canberra, ACT 2600, Australia.
Soil Research 61(3) 255-266 https://doi.org/10.1071/SR21259
Submitted: 18 October 2021 Accepted: 12 September 2022 Published: 19 October 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Widespread soil nutrient limitations in savanna soils typically constrain plantation tree growth, and hence limit economic opportunities in tropical regions. Fertilisation offers an approach to overcome soil nutrient limitations, but requires research on nutrient contents and rates to maximise plant growth while avoiding nutrient imbalances that have stunted plant growth under some fertiliser regimes.
Aims: To test the hypothesis that multiple nutrient limitations exist in savanna soils, with nutrient deficiencies exposed in sequence with fertiliser addition.
Methods: Factorial field experiments tested the growth of the plantation timber species African mahogany (Khaya senegalensis) to applications of phosphorus, potassium, nitrogen and minor and trace elements (referred to as the T treatment) on a kandosol soil near Darwin, Australia.
Key results: Under high stocking rates to induce deficiencies sooner through utilisation of a high proportion of the available nutrient capital, positive responses and interactions to all four main treatments were recorded. There were step-wise responses to phosphorus, potassium, nitrogen and the T treatment. Treatments with greater mean tree growth were more uniform than lesser-growing treatments, even though the largest and smallest individuals were similar across treatments.
Conclusions: Consistent with our hypothesis, correcting one soil nutrient deficiency exposed another in sequence as nutrient reserves were depleted in a drying soil. Variation in tree performance across plots indicates that testing of soil nutrients and fertiliser responses need to be assessed in replicate and dispersed samples.
Implications: Khaya senegalensis demonstrated potential for plantation use in northern Australia with minimal mortality from termites and other causes, if supported with broad-spectrum fertilisation balanced to match plant growth and water availability.
Keywords: fertiliser, Khaya senegalensis, nutrient limitation, phosphorus, plant nutrition, soil limitation, soil variation, tropical soil.
Introduction
Renewed interest in establishing plantation and cropping systems, and making use of available water resources, has prompted expansion of research into the soil, water and economic constraints to agriculture in Northern Australia (Petheram et al. 2014; Office of Northern Australia 2015) and a re-examination of early work. Previous large-scale agricultural developments in Northern Australia have mostly performed poorly, with multiple independent and interacting constraints (Bauer 1985; Ash and Watson 2018).
Similar to many savanna regions globally (Barnard and Fölscher 1980; Pellegrini 2016), the Top End of the Northern Territory of Australia (the area to the north of 15°S) was assessed as generally having poor potential for forestry (Bateman 1955), related to the extended dry season, soils of low nutrient status, termite attack and annual fires (Stocker 1972). To address these limitations and difficulties, research on tree nutrition and termite biology (Cameron 1985; Williams et al. 1985; Story et al. 2010) and soil surveys were initiated to find adequate areas of suitable soils for agricultural crops and plantations. These identified the massive sesquioxidic soils comprising kandosols and podosols as having potential for agricultural crops and plantations (Day 1977; Wells and Harrison 1978; Cameron 1985). Furthermore, contemporary plantation development will need to consider conservation of indigenous cultural heritage, ecological function and biodiversity (Haynes 1978).
Soil from monsoon forest patches, rarely burnt, supported greater plant growth (Stocker 1966) than open forest soil with a long history of regular fires (de Souza et al. 2016; Rance et al. 2020), suggesting both a mechanism for nutrient depletion in savanna soils and the potential for fertilisation to improve plant growth. In omission experiments, application of commercial ‘Complete mixtures’ was associated with improved plant health compared to −N, −P and −K treatments (Stocker 1966), but plantation trees routinely fertilised with a commercial N + P + K mixture often developed unhealthy crowns in their second or third dry season. These observations indicate multiple element soil limitations and nuanced responses to fertilisation that could be examined with factorial design experiments incorporating interactions.
Glasshouse and field experiments with Pinus caribaea var. hondurensis Barr. and Golf. (Belize provenance) showed N and T applications (where T was a combined minor and trace element treatment, including B, Cu, Zn, Mn, Mg, Fe, Mo and S) reduced growth and survival in the absence of P, but the T treatment increased growth on plots treated with P and/or K (Cameron et al. 1981). Further studies indicated the major response was to the T treatment, with further responses to P + T and K + T at some sites, while the healthiest plants were on N + P + T treatments (Cameron et al. 1982). Responses to N, P, K and the T treatment, and interactions, seemed to be common in the region (Rance et al. 2020), and similar to savanna soils on Cape York Peninsula (Isbell et al. 1976), raising questions of whether these responses were peculiar to the sites sampled or more widespread on savanna soils, and were they limited to P. caribaea? The general pattern was a response to P, followed by responses to N, K and the T treatment, not necessarily in that order. Soil pH appeared to play a role in driving these responses (Rance et al. 2020), suggesting that variable response to nutrients may be due to the effects of pH on the availability of exchangeable cations, and/or the microbiome.
African mahogany (Khaya senegalensis (Desr.) A. Juss.) (Meliaceae), one of the more promising plantation exotics tested in the Darwin region (Cameron 1972; Nikles et al. 2008), appeared to be less frequently attacked by termites (Mastotermes darwiniensis Froggat) which had killed many pines on cleared and disturbed sites (Fox and Clarke 1972). It also showed rapid early growth, responded to additional watering and fertilising in Darwin parks and gardens, and produced cabinet-quality timber. The aim of this study is to test the hypothesis, using K. senegalensis, that multiple nutrient limitations exist in savanna soils, with sequential exposure of nutrient deficiencies occurring with fertiliser addition. If this hypothesis is supported, these experiments can inform the contents, rates and balance of broad-spectrum fertilisation to underpin plantation tree growth on savanna soils.
Materials and methods
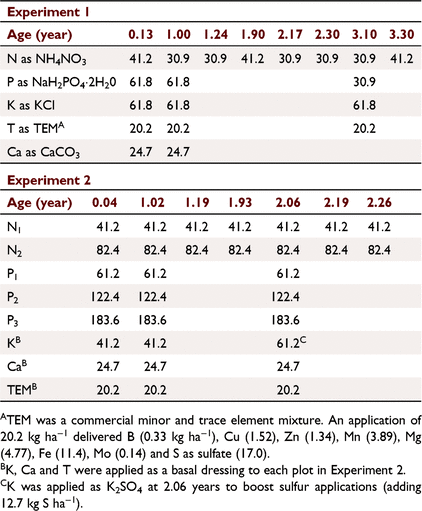
A site survey indicated a low incidence of the termite Mastotermes darwiniensis before clearing. The native eucalypt open forest had been cleared for plantation development, adjacent to the field experiment of Cameron et al. (1981) and where the soil was collected for glasshouse experiments (Rance et al. 1983). Two field experiments were established on a red kandosol soil derived from lower Cretaceous sandstone near Howard Springs (12°27′S, 131°3′E, lateritic red earth, Wells and Harrison 1978), using a species previously untested in timber plantations in northern Australia, K. senegalensis. First, an experiment replicating the earlier P. caribaea field and glasshouse N × P × K × T factorial experiments (Experiment 1) (Cameron et al. 1981; Rance et al. 1983), and the second examined the N × P interaction, with the K and the T treatment of Experiment 1 applied as a basal dressing (Experiment 2).
Most of the nutrient capital was in the top 10 cm of the soil profile (Table 1). Prior to planting, the site was mowed and cultivated, plots surveyed and pegged and each plot of 49 trees (7 × 7) established with the inner 25 trees (referred to as the ‘net plot’) measured and sampled. At planting, 3332 4-month-old K. senegalensis seedlings raised in a nursery were separated into two approximately equal groups, the tallest and shortest for planting in the isolation rows, and the most uniform for the net plots. They were stripped of their polythene tubes and planted into mattock holes at 1.5 m × 1.5 m spacing (4444 stems ha−1) in mid-December 1969 on days following overnight rain. This was a much higher planting density than the plantation standard at that time of 1111 stems ha−1 but was chosen to limit the area of each experiment to minimise soil heterogeneity across the site, reduce the area for weed control and constrain the trees to extract nutrient requirements from limited soil volumes (Richards 1961) in testing early responses to treatments. The close spacing was expected to allow the trees to rapidly occupy the site and compete with weeds, although it was anticipated that competition between trees for water and light could mask treatment effects within a few years.

|
Experimental designs
Experiment 1 was a 24 factorial (Cochran and Cox 1957) testing the main treatments N, P, K and a minor and trace element treatment (referred to as the T treatment), with the 16 treatments replicated twice. The N × P × K × T interaction was confounded into blocks of two plots by four, with the long axes of the blocks parallel with the contours.
Experiment 2 examined a sub-set of Experiment 1 treatments, a 3 × 4 factorial testing three levels of N by four levels of P (Cochran and Cox 1957), replicated three times, with the K and T treatments of Experiment 1 applied as a basal dressing. The N0P0 treatment was an unfertilised control, the N1 and P1 levels near the estimated optimum for early growth, and the higher rates at ‘luxury levels’ with potential to inhibit growth (Table 2).
|
Termites (Coptotermes acinaciformis Froggatt) had been observed removing superphosphate applied in pockets adjacent to trees (unpubl. data), so fertiliser was broadcast by hand evenly across the plots, although it was recognised that this could also encourage competing weed growth, and there could be differences regarding losses to volatilisation, soil wash and leaching. Nitrogen was applied as NH4NO3, P as NaH2PO4·2H2O, K as KCl, Ca as CaCO3 and T was a commercial mixture of minor and trace elements (TEM) (Table 2). The fertiliser applications at 0.13 and 1 year after planting were made after mowing weeds, followed by rotary hoeing to incorporate the fertiliser into the soil. The second cultivation severed surface feeding roots and checked tree growth, and cultivation was not repeated. Weed control, primarily by mowing but initially also including hand removal around seedlings, commenced within a few weeks of planting. Mowing was carried out again in the first wet season before the grass set seed, using a motorised scythe adjusted to cut above the litter layer, the cuttings remaining on site to protect the soil surface and to maintain nutrient capital (Cunningham 1963). Mowing was repeated four times in the second wet season, and twice on some plots in the third. The basal K application of Experiment 2 at age 2.06 years was changed to K2SO4 to boost sulfur applications after S was identified as one of the elements of importance in the T treatment (Rance et al. 1983).
Mean height at planting was 16.5 cm. Heights (H) of Experiment 1 were measured at age 0.68 year by extending height poles along the stem to the stem tip; H and diameters at 15 cm over bark (D15, using Vernier callipers for small trees and girth tapes for larger trees) at age 1.47 years; H, D15 and diameter at breast height (DBH, 1.3 m) at age 2.51 years; and H and DBH at 3.59 years. At the 3.59-years measurement the crowns were too dense to see the stem tips from below the crowns and an observer was stationed above the crowns to direct the height poles. Only DBH was measured at age 8.7 years. Experiment 2 heights were measured at 0.63 year, H and D15 at 1.41 years and H, D15 and DBH at 2.46 years.
By 1.4 years, D15, low in the stem in the relatively evenly tapered section of the stem above the butt swell, allowed an estimate of stem volume to be made. Diameter at ground level (DGL) was estimated by extrapolation:
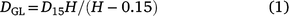
and similarly for DBH for trees taller than 5.5 m, and volumes (Vc) estimated from H and DGL as cones.
Aerial photographs of the site were taken at 1.55 years, and foliar samples were collected from each plot at age 1.63 years (August, dry season). Ten leaflets were collected from the most recent fully expanded undamaged pinnate leaves from each of nine trees in each net plot, from the net plot corners and alternate trees within the plot, wiped with a damp cloth to remove dust, dried at 65°C and ground to pass a 0.42-mm mesh sieve. Sample analysis was outsourced (Associated Laboratories of Australia, Pty. Ltd., Perth). The N concentration was determined by an auto analysis procedure, and P, K and S by X-ray fluorescence.
In the second and third dry season, soil moisture contents down the profile were examined as an indicator of likely moisture stress and in relation to changing crown conditions. Neutron moisture meter access tubes were installed along plot boundaries by taking 40-mm diameter cores using a hand auger to a depth of 4.6 m (maximum depth constrained by auger handle length) with measurement intervals of every 10 cm. Samples were completed in 1 day and timed to relate changing crown conditions with soil moisture, mainly the onset of leaf shedding, and were not spatially or temporally replicated in a systematic way to minimise changing water infiltration patterns at the next wet season. Precipitation observations were used for context of the timing of leaf flush.
Litter samples were collected from 1.5 m × 1.5 m quadrats between four trees near the centre of 12 plots from Experiment 2 at age 2.85 years. Dry season winds had scattered leaves on some outer plots. This was in the build-up to the wet season, after the first storm had dislodged dead leaves and restored soil moisture to surface layers of the profile, but before significant litter decay.
Statistical analysis
Factorial analyses of variance were carried out on all data using the statistical package GenStat (VSN International Inc.). For Experiment 1, the external replicates provided an error term with 14 d.f. for testing the effect of treatments (N, P, K and T) and interactions on plant growth (height, diameter and volume) and foliar nutrient concentrations. For Experiment 2, there were 22 d.f. for the error term for testing the effect of treatments (three levels of N and four levels of P) and interactions on plant growth and foliar nutrient concentrations.
Results
Weeds, pests and canopy closure
Weeds, mainly grasses to ~2 m (Sorghum sp.), soon overtopped the trees (0–0.25 year after planting) with weed growth responses a precursor of later tree growth. In an unusual extended wet spell in the second wet season (~1 year), trees with newly expanded growth flushes suffered a fungal attack, tentatively identified as a species of Colletotrichum, which killed the top 2–50 cm of the stem. Grasshoppers attacked stem growing tips of trees in the resting phase between growth flushes during the second dry season (~1.5 years). In both cases, two or more new leaders extended from below the damage, with marked kinks in the stem and a significant downgrade of stem quality. By this time trees in some plots were approaching canopy closure (Fig. 1a).
Four trees had been killed by termites (Mastotermes darwinensis) at age 1.4 years, and 3% of trees in Experiment 1 and 7% in Experiment 2 by 2.46 years, without any apparent preference for treatments. Killed trees were ring-barked by the termites, and bark flaked away, mostly within 15 cm of the ground, with gallery and exploratory holes higher in the stem. Surface layers of larger roots had been heavily grazed, with occasional deeper explorations to 2–4 mm. The colony was eliminated with a bait containing 0.1 g of Mirex™ (Paton and Miller 1980). There were few deaths from other causes.
In the second and third dry season, trees in the fastest growing plots shed leaves and slowed in growth first, allowing trees in slower growing plots to catch up, before they too shed foliage later in the dry season. Limited soil sampling indicated that leaf shedding coincided with drying of the soil profile, to a depth of 4.6 m at age 2.85 years. By the end of the dry seasons, litter had covered the ground in both experiments. Most of the litter layer at the end of the first dry season was grass cuttings, a mixture of grass cuttings and K. senegalensis leaves at the end of the second, and nearly all K. senegalensis leaves at the end of the third. Litter in Experiment 2 at age 2.85 years, near the end of the dry season was 2.4 Mg ha−1 in the N0P0 treatment and a mean of 28.4 Mg ha−1 in N + P plots. During the third wet season, crowns in most treatments formed a dense continuous interlocked canopy, little direct sunlight reached the ground, stem tips grew into zones of higher light intensity, leading to stems with poor form (Fig. 1b). By the end of the third wet season (age 3.2 years) almost all the leaflet litter had decomposed, only the woody leaf petioles remaining, leaving the soil surface almost unprotected.
Experiment 1
By age 0.30 year, there seemed to be more vigorous top growth in response to P applications, but no obvious response to other treatments. By age 0.51 year, plots treated with N + P were taller than those without either. By 0.57 year, a response to K applications seemed likely, but there was a delay before those observations were reflected in measurements. Heights measured at age 0.68 year, late in the dry season, confirmed greater growth with P addition (P < 0.001, Fig. 2a), and trees on N1P0 plots had much darker green leaves than those on N1P1 plots, but looked less healthy than N0P0 trees. Height growth increased with the onset of the second wet season, and in response to K applications (Fig. 3). At later measurements there were significant main effect increases of H, D15 and DBH in response to P and K applications (P < 0.001 and P < 0.01, respectively, Fig. 2a, b). While P and K applications both increased growth, crown conditions indicated a P × K interaction. The Vc summed for each net plot showed substantial increases: a three-fold increase at age 1.47 years (mean K0P0 = 2.40 m3 ha−1, K1P0 = 3.36, K0P1 = 4.82, K1P1 = 7.23; least significant difference [l.s.d.] = 1.69), and similarly at age 2.51 years, (13.8, 17.1, 22.3, 33.1 m3 ha−1; l.s.d. = 6.5), and age 3.59 years (26.7, 30.0, 36.4, 53.4 m3 ha−1 respectively, l.s.d. = 7.0, P < 0.01) (Fig. 2c).
A pattern emerged, repeated in each of the measurements, which is typified by the response in Vc, and Fig. 4 shows the additive effects of N, P and K at 1.47, 2.51 and 3.59 years. There were small responses to P or K, and a response to N with P or P + K (P < 0.1). In short, N, P and K were all required for optimum growth. The relative increase from N0P0K0 to N1P1K1 decreased over time, representing the effects of increased competition. However, Vc increments from age 1.44 to 3.59 years showed significant P × K (P < 0.01) and N × P (P < 0.05) interactions, and there was a small response to the T treatment (T0 30.1 m3 ha−1, T1 34.2 m3 ha−1, P < 0.05, l.s.d. = 3.7), with most of the T response expressed in N + P + K plots.
|
|
At the final measurement at age 8.7 years, competition between trees was intense, masking treatment effects, although few trees had died apart from those ring-barked by termites. Only DBH was measured, increments were decreasing, but the P × K interaction persisted (P0K0 7.82 cm, P1K0 8.38 cm, P0K1 8.15 cm, P1K1 10.19 cm; l.s.d. = 0.70 cm; P < 0.01), and there was a small response to the T treatment (T0 8.38 cm, T1 8.90 cm; l.s.d. 0.46 cm; P < 0.05). The mean N0P0K0T0 was 7.40 cm and mean N1P1K1T1 was 10.43 cm, equivalent to a doubling of the mean tree basal area.
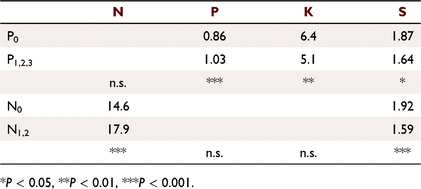
Mean foliar N, P, K and S concentrations at 1.66 years were all increased by their matching treatments (P < 0.001): N (N0 15.3 mg g−1, N1 20.5); P (P0 0.77, P1 1.04); K (K0 3.34, K1 5.59); and S (T0 0.71, T1 0.80). The N and P applications reduced foliar S concentrations (P < 0.001) as occurred in Experiment 2 (see Table 3), but not in tandem with growth responses. The S responses can be summarised with the N × P × T interaction. Mean S concentration in N0P0T0 treatments was 0.87 mg g−1, highest in N0P0T1 treatments (1.06 mg g−1), lower with increased growth in N1P1 treatments (0.54 mg g−1), and the others not significantly different from N0P0T0 (l.s.d. = 0.10 mg g−1). An indication of S content can be made from the product of Vc and foliar S concentration, indicating that the S uptake was diluted in extra growth. A similar mismatch was shown with the N × P × K interaction. The highest S concentrations were in N0P0K0 treatments (1.03 mg g−1), and P and K applications increased growth and reduced foliar S concentrations.
|
Experiment 2
By 0.63 year the height increments showed significant responses to N and P applications, but no significant differences between application rates or the N × P interaction (mean N0 0.13 m, N1,2 0.20 m, P < 0.01; P0 0.09 m, P1,2,3 0.21 m, P < 0.001). There was a hint that the N2 application rate might be more than optimal. By age 1.41 years there were significant responses to P and N at the N1 level, but trees in the N2P0 treatment were similar to the N0P0 treatment and looked unhealthy, with dark green leaves. There was little response to applications higher than the N1P1 treatment (Fig. 5), and N2 treatment trees continued to fall behind the N1 treatment. By 1.55 years, plots with N plus higher P applications were approaching canopy closure (Fig. 1a). These responses were repeated at the 2.46-years measurement, with the N and P responses both P < 0.001. When Vc was summed for each plot at age 2.46 years, N0P0 totalled 17.5 m3 ha−1 and N1P1 37.4 m3 ha−1, with negligible increase with higher application rates, and N1P0 significantly greater than N2P0 (P < 0.05).
|
|
The N applications increased foliar N concentrations at 1.66 years but N1 and N2 treatments did not significantly differ; similarly, P applications increased foliar P concentrations, but higher levels of P application did not significantly differ. The K and S were applied as basal dressings, and N applications reduced foliar S concentrations, and P applications reduced foliar K and S concentrations, similar to Experiment 1 (Table 3).
All treatments had a few large trees as well as some small trees, and the tallest and shortest trees in each treatment were similar. When heights of survivors of the 75 net-plot trees in the three replications of each treatment at age 2.46 years were arranged in ascending order, they separated into two groups. Those in the treatments with lower mean height (N0P0 and N2P0) had a steady increase in the height of ascendingly ordered individuals across the sample (Fig. 6), whereas those in other treatments (N1P0, N0P3, N1P1 and N1P2) had most individuals closer to the plot means and thus were more uniform.
Discussion
Consistent with trends identified by previous studies in savannas locally and globally using a variety of tree species (Cameron et al. 1981, 1982; Pellegrini 2016; Rance et al. 2020), the main response was to P applications, consistent with low soil nutrient concentrations (Table 1). However, little additional growth response occurred, or adverse effects emerged, from recurrent P application rates higher than ~60 kg P ha−1 (Table 2, Fig. 5). The K applications had similar effects to P, although usually marginally lower in magnitude and with a P × K interaction (Fig. 4) as found by Cameron et al. (1982).
In contrast, responses to N on Top End soils have been inconsistent. In the nursery, N applied to the soil as urea or ammonium nitrate without P, at rates commonly used in agriculture, made seedlings unhealthy, while N applied as dilute foliar sprays increased growth (unpubl. data), indicating unexpected soil interactions. In Experiment 1, a response to N was mainly expressed in the presence of P or P + K (Fig. 4). There was evidence for a step-wise response; correcting one deficiency exposing the next in train. This was similar to, but more pronounced than, that of an adjacent experiment of the same design planted with P. caribaea var. hondurensis, where the N, K and the T treatments applied without P appeared to be detrimental, but there were positive responses when applied with P (Cameron et al. 1981). These responses could vary with conditions, and to N:P ratios. Application rates of the N1P1 treatment in Experiment 2 were estimated to be near the optimum, and this confirmed the observation that high N applications were associated with poor growth (Fig. 5). The NH4NO3 applied at rates of N1 with P improved growth, although it is likely that much of the applied N would have been taken up by competing weeds in the first 2 years. These results were similar to those of Richards (1961), working with P. taeda L. in Queensland, who found a widespread response to P, but high levels of available N in the soil depressed germination, survival and early growth, and that nitrate N was more deleterious than ammonium N. Thus, N may depress growth when P is deficient but increase growth when added with P, with an understanding of their effects only achieved by considering them together.
Indications of unhealthy growth appeared on several plantation species across a variety of sites in the Top End, usually on new growth near the stem tip late in the second, third or fourth dry season (Fig. 7). Applications of N + P in the field increased growth of P. caribaea var. hondurensis during the first wet season (Cameron et al. 1982), and increased the incidence of ramicorns and multiple leaders in the second or third wet season, most pronounced on plots with the fastest growing trees. These were on trees recovering from dead stem tips, some with fasciation, also observed in adjacent native vegetation. Interactions of P × T and K × T were recorded at two Top End sites (Cameron et al. 1982), and an N × P × T interaction was recorded on two similar Howard Springs kandosols in the glasshouse (Rance et al. 1983) and on other sites (Rance et al. 2020), with all three required for increased growth. A response to the T treatment seemed to be developing in Experiment 1 at age 3.49 years (mid dry season, just short of P < 0.05), mostly expressed in the N + P treatments, indicating low soil reserves of at least one element in the T treatment. Sulfur was identified as an element in the T treatment in short supply (Rance et al. 1983, 2020), and N and P applications reduced foliar S concentrations (Table 3). Accordingly, K was added to the basal dressing to Experiment 2 not as KCl but as K2SO4 at 2.06 years to boost the S application by an extra 12.7 kg S ha−1 (Table 2).
Fasciation was consistent with Zn deficiency, hinting that Zn might be deficient in a drying soil profile. Symptoms ranging from short needles in pines through to fasciation were corrected by foliar applications of Zn chelate (Rance et al. 1982), although there was little response to soil applications. Uptake and distribution of Zn in P. caribaea was slow when Zn supply was low (Mcgrath and Robson 1984), and the rate of Zn mobility to younger tissues was particularly depressed in Zn-deficient plants (Loneragan et al. 1979). The first two applications of the T treatment added 2.7 kg Zn ha−1 to the soil surface (Table 2), a significant proportion probably taken up by weeds, perhaps not enough to induce a growth response in the first few years.
Multiple element responses were recorded on similar soils on Cape York Peninsula (Isbell et al. 1976; Winter and Jones 1977), indicating similar responses across the savanna zone in northern Australia. Soil pH ~5.5 at Howard Springs (Table 1) was within the range where some elements become less available to plants, and some, e.g. Al, more so, possibly contributing to inconsistent responses and nutritional disorders (Rance et al. 2020). Ammonium nitrate addition lowered soil pH to <5.5 in the glasshouse (Rance et al. 2020), to levels associated with reduced mycorrhiza development and seedling growth (Richards and Wilson 1963), while P had the opposite effect. In acid soils some of the P can be compounded with Fe or Al and become unavailable to plants (Haynes and Mokolobate 2001).
Concentrations of total N and P declined with soil depth (Isbell and Smith 1976; Day 1977; Cameron et al. 1981; Jones et al. 1985; Table 1). As the soil profile dries with progression of the dry season, uptake of N and P becomes increasingly restricted to moister soils deeper in the profile. Thus, growth rates in better performing treatments likely became limited by soil water availability, although to demonstrate this conclusively increased spatial and temporal replication of soil water sampling would be required. Under drying conditions during the dry season, it is likely that N and P, and probably other elements, were translocated from lower crown and inner crown leaves before abscission to new growth at the crown tops (Lamb 1976; Saur et al. 2000), more accumulating in the upper growing shoots translocated from the lower crown than was being taken up from the drying soil (Pate and Arthur 2000). The N + P + K applications increased Vc three-fold over controls in Experiment 1 at age 1.47 years (Fig. 4), but the relative difference decreased between 1.47 and 3.59 years, representing the effects of increased competition related to a drying soil profile.
A 10-fold increase in litterfall from N0P0 to N + P treatments indicated different crown dynamics and nutrient cycling. For instance, leaf fall at that time coincided with soil dried to wilting point to ~4.6 m. Assuming differences in N and P concentrations in foliar samples in relation to treatments at 1.66 years (Table 3) were reflected in mower cuttings and litter, this indicated even greater differences of nutrient cycling between N0P0 plots and N + P plots. An experimental approach to include water status on the effects of nutrients and carbon assimilation, and hence the growth of trees, was outlined by McMurtrie and Wolf (1983).
Potential explanations for the presence of a few vigorous trees in poorly-performing plots, most noticeable in N0P0 and N2P0 of Experiment 2 (Fig. 6), include the expression of genetic variability in a challenging environment, or small-scale variation of site nutrient capital. If better-performing trees represent fitter genotypes, selection for such genotypes and provenances could be a means of adapting to temperature and soil moisture variation components of climate change. If better-performing (and worse-performing) trees are a result of small-scale variation in soil conditions, it could be a reflection of past events. Past events that could substantially affect soil nutrients include ash beds from trees felled by occasional events like cyclones, nesting sites of ants or termites where foraging workers had concentrated nutrients, and interactions between soil conditions and mycorrhizal associations. The potential for fine-scale variation in soil nutrients needs to be considered when collecting foliar samples and soil samples for profile description or nutrient content.
The exposure of multiple soil nutrient limitations in this study, expressed in a step-wise manner, is consistent with a progressive run down of nutrient capital through recurrent fires and site clearing. This site, like much of the Top End, had been burned annually or near-annually in the years before establishing the experiment, and probably for much longer (Russell-Smith et al. 2003). Essential elements N, P, S, K and Zn are volatilised at temperatures less than 1000°C, which are experienced with burning plant material (Raison et al. 1985, 1993; Wanthongchai et al. 2008). Late dry season high-intensity fires would volatilise much of the nutrient capital in the litter layer, and more in a crown fire, leading to a progressive run down of nutrient capital, leaving refractive elements like Fe, Mn, Ca and Al in the ash bed (Raison et al. 1985; Cook 1994; McIntosh et al. 2005; Rance et al. 2020). In the tropics, a significant proportion of the nutrient capital is in the living biomass, and removing this biomass exports this limited capital off site (Raison et al. 1982; Corbeels et al. 2005; Mendham et al. 2014). Establishing new plantings in the savanna zone, likely to include exotic species not well adapted to low fertility soils, will require fertiliser applications to compensate for the loss of nutrients associated with site preparation, but done in a way to minimise run-off, and protection from fire. This will require a nuanced approach to plantation development, considering landscape context and ecological and cultural values including carbon stocks (Haynes 1978).
While the contrived close spacing in these experiments established a monoculture and rapidly identified nutrient responses, and grass understoreys often reduce tree growth (Tomlinson et al. 2019), a wider spacing with minimal weed control would protect the soil surface and maintain a biodiverse soil microbiome (Squire 1977; Simard et al. 1997; Corbeels et al. 2005; Mendham et al. 2014; Tomlinson et al. 2019), and nutrient capital in litter would be largely returned to the system in the following wet season (Neary et al. 1999; McIntosh et al. 2005; Liddicoat et al. 2019). Adding fertiliser to low fertility soils can induce deficiencies and imbalances, making conditions favourable for opportunistic ‘copiotrophic’ bacteria, more susceptible to adverse effects (Liddicoat et al. 2019), and make conditions favourable for weed species (Prober and Wiehl 2011) and possibly for termites or other pests. Hence, a plantation system of wider spacing with an understorey should be more stable, more closely mimicking the structure of the pre-clearing eucalypt savanna. Optimal stocking, perhaps as high as 1000 stems ha−1 for straight stems for saw logs, or lower to encourage branching for fruit trees, and methods and rates of fertiliser application will require further investigation.
Data availability
The authors will provide raw data upon reasonable request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by CSIRO and the Australian Government.
Acknowledgements
We acknowledge the Larrakia traditional owners of country where this work was conducted and their continuing connection to land, sea and community. We pay our respects to them and their cultures and to their elders both past, present and emerging. We thank B.N. Richards and H.D. Waring for input on planting design, Doug Johnston for refining the treatments, George Petru for templates for statistical analysis, S. Aldrick for fungal identifications, W. Hunziker and G. Raseta for establishment of the experiments, H. Deane and D. Hearne for foliar sampling and preparation and field staff of the Forest Research Institute, in particular, G. Milne, J. Cusack and P. Lukitsch for weed control, measuring and fertilising. We thank the three reviewers for constructive feedback that has improved the manuscript.
References
Ash A, Watson I (2018) Developing the north: learning from the past to guide future plans and policies. The Rangeland Journal 40, 301–314.| Developing the north: learning from the past to guide future plans and policies.Crossref | GoogleScholarGoogle Scholar |
Barnard RO, Fölscher WJ (1980) Nutrient interactions in acid tropical soil. Tropical Agriculture 57, 333–341.
Bateman W (1955) ‘Forestry in the Northern Territory.’ Leaflet No. 72. (Forestry and Timber Bureau: Canberra, Australia)
Bauer FH (1985) A brief history of agriculture in north-west Australia. In ‘Agro-research for the semi-arid tropics: north-west Australia’. (Ed. RC Muchow) pp. 12–31. (University of Queensland Press: St Lucia)
Black CA (1965) ‘Methods of soil analysis.’ (American Society of Agronomy: Madison)
Cameron DM (1972) Nutrition of Khaya senegalensis in the Northern Territory. In ‘The Australian forest-tree nutrition conference’. (Ed. R Boardman) pp. 223–230. (Forestry and Timber Bureau: Canberra)
Cameron DM (1985) Forest crops. In ‘Agro-research for the semi-arid tropics: north-west Australia’. (Ed. RC Muchow) pp. 165–178. (University of Queensland Press: St Lucia)
Cameron DM, Rance SJ, Williams ER (1981) Fertilizer responses of Pinus caribaea var. hondurensis on a tropical red earth. Australian Forest Research 11, 35–45.
Cameron DM, Rance SJ, Williams ER (1982) Effects of fertilizers on growth, form and concentration of nutrient in the needles of Pinus caribaea var. hondurensis in the Northern Territory. Australian Forest Research 12, 105–119.
Chapman HD, Pratt PF (1961) ‘Methods of analysis for soils, plants and waters.’ (University of California: Berkeley)
Cochran WG, Cox GM (1957) ‘Experimental designs.’ (John Wiley & Sons: New York)
Cook GD (1994) The fate of nutrients during fires in a tropical savanna. Australian Journal of Ecology 19, 359–365.
| The fate of nutrients during fires in a tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Corbeels M, McMurtrie RE, Pepper DA, Mendham DS, Grove TS, O’Connell AM (2005) Long-term changes in productivity of eucalypt plantations under different harvest residue and nitrogen management practices: a modelling analysis. Forest Ecology and Management 217, 1–18.
| Long-term changes in productivity of eucalypt plantations under different harvest residue and nitrogen management practices: a modelling analysis.Crossref | GoogleScholarGoogle Scholar |
Cunningham RK (1963) The effect of clearing a tropical forest soil. Journal of Soil Science 14, 334–345.
| The effect of clearing a tropical forest soil.Crossref | GoogleScholarGoogle Scholar |
Day KJ (1977) ‘Fertility studies on three red earth soils of the Daly Basin, Northern Territory.’ (Land Conservation Section, Animal Industry and Agriculture Branch, Department of the Northern Territory: Darwin, N.T.)
de Souza MC, Rossatto DR, Cook GD, Fujinuma R, Menzies NW, Morellato LPC, Habermann G (2016) Mineral nutrition and specific leaf area of plants under contrasting long-term fire frequencies: a case study in a mesic savanna in Australia. Trees 30, 329–335.
| Mineral nutrition and specific leaf area of plants under contrasting long-term fire frequencies: a case study in a mesic savanna in Australia.Crossref | GoogleScholarGoogle Scholar |
Fox RE, Clarke NB (1972) The incidence of termites in eucalypts in the Darwin area. Australian Forest Research 5, 29–36.
Haynes CD (1978) Land, trees and man (Gunret, Gundulk, Dja Bining). Commonwealth Forestry Review 57, 99–106.
Haynes RJ, Mokolobate MS (2001) Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: a critical review of the phenomenon and the mechanisms involved. Nutrient Cycling in Agroecosystems 59, 47–63.
| Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: a critical review of the phenomenon and the mechanisms involved.Crossref | GoogleScholarGoogle Scholar |
Isbell RF, Smith GM (1976) Some properties of red, yellow and grey massive earths of North Queensland. Technical Paper No. 30. CSIRO Division of Soils, Melbourne. https://doi.org/10.25919/z2sy-c727
Isbell RF, Jones RK, Gillman GP (1976) Plant nutrition studies on some yellow and red earth soils in northern Cape York Peninsula. 1. Soils and their nutrient status. Australian Journal of Experimental Agriculture and Animal Husbandry 16, 532–541.
| Plant nutrition studies on some yellow and red earth soils in northern Cape York Peninsula. 1. Soils and their nutrient status.Crossref | GoogleScholarGoogle Scholar |
Jones RK, Myers RJK, Wright GC, Day KJ, Mayers BA (1985) Fertilisers. In ‘Agro-research for the semi-arid tropics: north-west Australia’. (Ed. RC Muchow) pp. 371–393. (University of Queensland Press: St Lucia)
Lamb D (1976) Variations in the foliar concentrations of macro and micro elements in a fast-growing tropical eucalypt. Plant and Soil 45, 477–492.
| Variations in the foliar concentrations of macro and micro elements in a fast-growing tropical eucalypt.Crossref | GoogleScholarGoogle Scholar |
Liddicoat C, Weinstein P, Bissett A, Gellie NJC, Mills JG, Waycott M, Breed MF (2019) Can bacterial indicators of a grassy woodland restoration inform ecosystem assessment and microbiota-mediated human health? Environment International 129, 105–117.
| Can bacterial indicators of a grassy woodland restoration inform ecosystem assessment and microbiota-mediated human health?Crossref | GoogleScholarGoogle Scholar |
Loneragan JF, Grove TS, Robson AD, Snowball K (1979) Phosphorus toxicity as a factor in zinc-phosphorus interactions in plants. Soil Science Society of America Journal 43, 966–972.
| Phosphorus toxicity as a factor in zinc-phosphorus interactions in plants.Crossref | GoogleScholarGoogle Scholar |
Mcgrath JF, Robson AD (1984) The influence of zinc supply to seedlings of Pinus radiata D. Don on the internal transport of recently absorbed zinc. Functional Plant Biology 11, 165–178.
| The influence of zinc supply to seedlings of Pinus radiata D. Don on the internal transport of recently absorbed zinc.Crossref | GoogleScholarGoogle Scholar |
McIntosh PD, Laffan MD, Hewitt AE (2005) The role of fire and nutrient loss in the genesis of the forest soils of Tasmania and southern New Zealand. Forest Ecology and Management 220, 185–215.
| The role of fire and nutrient loss in the genesis of the forest soils of Tasmania and southern New Zealand.Crossref | GoogleScholarGoogle Scholar |
McMurtrie R, Wolf L (1983) A model of competition between trees and grass for radiation, water and nutrients. Annals of Botany 52, 449–458.
| A model of competition between trees and grass for radiation, water and nutrients.Crossref | GoogleScholarGoogle Scholar |
Mendham DS, Ogden GN, Short T, O’Connell TM, Grove TS, Rance SJ (2014) Repeated harvest residue removal reduces E. globulus productivity in the 3rd rotation in south-western Australia. Forest Ecology and Management 329, 279–286.
| Repeated harvest residue removal reduces E. globulus productivity in the 3rd rotation in south-western Australia.Crossref | GoogleScholarGoogle Scholar |
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36.
| A modified single solution method for the determination of phosphate in natural waters.Crossref | GoogleScholarGoogle Scholar |
Neary DG, Klopatek CC, DeBano LF, Ffolliott PF (1999) Fire effects on belowground sustainability: a review and synthesis. Forest Ecology and Management 122, 51–71.
| Fire effects on belowground sustainability: a review and synthesis.Crossref | GoogleScholarGoogle Scholar |
Nikles DG, Bevege DI, Dickinson GR, Griffiths MW, Reilly DF, Lee DJ (2008) Developing African mahogany (Khaya senegalensis) germplasm and its management for a sustainable forest plantation industry in northern Australia: progress and needs. Australian Forestry 71, 33–47.
| Developing African mahogany (Khaya senegalensis) germplasm and its management for a sustainable forest plantation industry in northern Australia: progress and needs.Crossref | GoogleScholarGoogle Scholar |
Office of Northern Australia (2015) ‘Our north, our future: white paper on developing Northern Australia.’ (Australian Government, Department of Industry, Innovation and Science: Canberra)
Olsen SR, Cole CF, Watanabe FS, Dean LA (1954) ‘Estimation of available phosphorus in soils by extraction with sodium bicarbonate.’ Circular No. 939. (U.S. Department of Agriculture: Washington, D.C.)
Pate JS, Arthur DJ (2000) Uptake, partitioning and utilization of carbon and nitrogen in the phloem bleeding tree, Tasmanian blue gum (Eucalyptus globulus). Australian Journal of Plant Physiology 27, 869–884.
| Uptake, partitioning and utilization of carbon and nitrogen in the phloem bleeding tree, Tasmanian blue gum (Eucalyptus globulus).Crossref | GoogleScholarGoogle Scholar |
Paton R, Miller LR (1980) Control of Mastotermes darwiniensis Froggatt (Isoptera: Mastotermitidae) with Mirex baits. Australian Forest Research 10, 249–258.
Pellegrini AFA (2016) Nutrient limitation in tropical savannas across multiple scales and mechanisms. Ecology 97, 313–324.
| Nutrient limitation in tropical savannas across multiple scales and mechanisms.Crossref | GoogleScholarGoogle Scholar |
Petheram C, Gallant J, Wilson P, Stone P, Eades G, Roger L, Read A, Tickell S, Commander P, Moon A, McFarlane D, Marvanek S (2014) Northern rivers and dams: a preliminary assessment of surface water storage potential for northern Australia. CSIRO Land and Water Flagship Technical Report, Australia.
Prober SM, Wiehl G (2011) Resource heterogeneity and persistence of exotic annuals in long-ungrazed Mediterranean-climate woodlands. Biological Invasions 13, 2009–2022.
| Resource heterogeneity and persistence of exotic annuals in long-ungrazed Mediterranean-climate woodlands.Crossref | GoogleScholarGoogle Scholar |
Raison RJ, Khanna PK, Crane WJB (1982) Effects of intensified harvesting on rates of nitrogen and phosphorus removal from Pinus radiata and Eucalyptus forests in Australia and New Zealand. New Zealand Journal of Forest Science 12, 394–403.
Raison RJ, Khanna PK, Woods PV (1985) Mechanisms of element transfer to the atmosphere during vegetation fires. Canadian Journal of Forest Research 15, 132–140.
| Mechanisms of element transfer to the atmosphere during vegetation fires.Crossref | GoogleScholarGoogle Scholar |
Raison RJ, O’Connell AM, Khanna PK, Keith H (1993) Effect of repeated fires on nitrogen and phosphorus budgets and cycling processes in forest ecosystems. In ‘Fire in Mediterranean ecosystems’. (Eds L Trabaud, R Prodon) pp. 347–363. (CEC: Brussels)
Rance SJ, Cameron DM, Williams ER (1982) Correction of crown disorders of Pinus caribaea var. hondurensis by application of zinc. Plant and Soil 65, 293–296.
| Correction of crown disorders of Pinus caribaea var. hondurensis by application of zinc.Crossref | GoogleScholarGoogle Scholar |
Rance SJ, Cameron DM, Williams ER (1983) Nutritional requirements and interactions of Khaya senegalensis on tropical red and yellow earths. Communications in Soil Science and Plant Analysis 14, 167–183.
| Nutritional requirements and interactions of Khaya senegalensis on tropical red and yellow earths.Crossref | GoogleScholarGoogle Scholar |
Rance SJ, Cameron DM, Gosper CR, Williams ER (2020) Multiple soil element and pH interactions constrain plant performance on tropical soils with a long history of fire. Soil Research 58, 335–345.
| Multiple soil element and pH interactions constrain plant performance on tropical soils with a long history of fire.Crossref | GoogleScholarGoogle Scholar |
Richards BN (1961) ‘Fertilizer requirements of Pinus taeda L. in the coastal lowlands of subtropical Queensland.’ Bulletin No. 16. (Queensland Department of Forestry)
Richards BN, Wilson GL (1963) Nutrient supply and mycorrhiza development in Caribbean Pine. Forest Science 9, 405–412.
| Nutrient supply and mycorrhiza development in Caribbean Pine.Crossref | GoogleScholarGoogle Scholar |
Russell-Smith J, Whitehead PJ, Cook GD, Hoare JL (2003) Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996. Ecological Monographs 73, 349–375.
| Response of Eucalyptus-dominated savanna to frequent fires: lessons from Munmarlary, 1973–1996.Crossref | GoogleScholarGoogle Scholar |
Saur E, Nambiar EKS, Fife DN (2000) Foliar nutrient retranslocation in Eucalyptus globulus. Tree Physiology 20, 1105–1112.
| Foliar nutrient retranslocation in Eucalyptus globulus.Crossref | GoogleScholarGoogle Scholar |
Simard SW, Perry DA, Jones MD, Myrold DD, Durall DM, Molina R (1997) Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388, 579–582.
| Net transfer of carbon between ectomycorrhizal tree species in the field.Crossref | GoogleScholarGoogle Scholar |
Squire RO (1977) Interacting effects of grass competition, fertilizing and cultivation on the early growth of Pinus radiata D. Don. Australian Forest Research 7, 247–252.
Stocker GC (1966) Effects of fires on vegetation in the Northern Territory. Australian Forestry 30, 223–230.
| Effects of fires on vegetation in the Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Stocker GC (1972) ‘The environmental background to forestry in the Northern Territory of Australia.’ Leaflet No. 113. (Forestry and Timber Bureau of Australia)
Story R, Williams MAJ, McAlpine JR, O’Ferrall RE, Hooper ADL (2010) No. 25 Lands of the Adelaide–Alligator area, Northern Territory. CSIRO Land Research Surveys 2010, 1–162.
| No. 25 Lands of the Adelaide–Alligator area, Northern Territory.Crossref | GoogleScholarGoogle Scholar |
Tomlinson KW, Sterck FJ, Barbosa ERM, de Bie S, Prins HHT, van Langeveldt F (2019) Seedling growth of savanna tree species from three continents under grass competition and nutrient limitation in a greenhouse experiment. Journal of Ecology 107, 1051–1066.
| Seedling growth of savanna tree species from three continents under grass competition and nutrient limitation in a greenhouse experiment.Crossref | GoogleScholarGoogle Scholar |
Tucker BM (1974) Laboratory procedures for cation exchange measurements on soils. Technical Paper No. 23. CSIRO Division of Soils: Melbourne.
Verma BC, Swaminathan K, Sud KC (1977) An improved turbidimetric procedure for the determination of sulphate in plants and soils. Talanta 24, 49–50.
| An improved turbidimetric procedure for the determination of sulphate in plants and soils.Crossref | GoogleScholarGoogle Scholar |
Walkley A, Black IA (1934) An examination of the Degtjareft method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science 37, 29–38.
| An examination of the Degtjareft method for determining soil organic matter, and a proposed modification of the chromic acid titration method.Crossref | GoogleScholarGoogle Scholar |
Wanthongchai K, Bauhus J, Goldammer JG (2008) Nutrient losses through prescribed burning of aboveground litter and understorey in dry dipterocarp forests of different fire history. Catena 74, 321–332.
| Nutrient losses through prescribed burning of aboveground litter and understorey in dry dipterocarp forests of different fire history.Crossref | GoogleScholarGoogle Scholar |
Wells MR, Harrison CJ (1978) Land units of the Howard Springs-Humpty Doo area. Internal Report LC 78/8. Land Conservation Unit, Territory Parks and Wildlife Commission, Darwin NT.
Williams J, Day KJ, Isbell RF, Reddy BR (1985) Soils and climate. In ‘Agro-research for the semi-arid tropics: north-west Australia’. (Ed. RC Muchow) pp. 31–92. (University of Queensland Press: St Lucia)
Winter WH, Jones RK (1977) Nutrient responses on a yellow earth soil in northern Cape York Peninsula. Tropical Grasslands 11, 247–255.


