Priming of carbon decomposition in 27 dairy grazed soils after bovine urine additions
S. M. Lambie A * , N. W. H. Mason A and P. L. Mudge A
A * , N. W. H. Mason A and P. L. Mudge A
A Manaaki Whenua – Landcare Research, Private Bag 3127, Hamilton, New Zealand.
Soil Research 60(2) 124-136 https://doi.org/10.1071/SR20313
Submitted: 6 November 2020 Accepted: 13 August 2021 Published: 4 November 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Soil organic matter (SOM) plays a vital role in carbon (C) storage and agricultural sustainability. Additions of bovine urine to soils can cause positive priming of soil C decomposition and represents a pathway for SOM loss. However, data is limited to a few soils.
Aims: We investigated the priming response to bovine urine of 27 dairy grazed pasture soils from the North Island of New Zealand.
Methods: Soils from Allophanic, Gley, Recent and Brown soil orders were collected. 14C-labelled dairy cow urine was applied (1000 kg N ha−1) to undisturbed soil cores and carbon dioxide (CO2) fluxes measured (25°C) for 21 days. Urine applications were repeated, and CO2 measured for a further 21 days (25°C). Water was the control treatment.
Key results: CO2 fluxes rapidly increased after both urine additions by 86 ± 1% 24 h after the first urine addition, and 68 ± 4% after the second. Positive, negative and no priming were observed, and the mean absolute deviation of priming ranged between 200 and 1000 μg C g−1, and variability was greater after the second urine addition. Urine induced changes in pH and electrical conductivity (EC) had no effect on priming, and soil C contents were correlated to cumulative CO2, but not priming, and varied over time.
Conclusions: Factors affecting soil priming remain elusive and priming was highly variable within and between soil types.
Implications: The impacts of bovine urine on C pools requires further investigation to determine if, or when, urine patches are potential pathways for soil C loss.
Keywords: buffering capacity, carbon, cumulative urine effect on soil electrical conductivity, cumulative urine effect on soil pH, negative priming, pasture, positive priming, soil organic matter.
Introduction
Loss of soil organic matter (SOM) reduces the potential of soil to deliver ecosystem services, such as carbon (C) sequestration and nutrient retention (Lal 2014; Orwin et al. 2015). One of the pathways for loss of SOM is positive priming of soil C decomposition (Fontaine et al. 2004), which occurs when substrate addition increases the mineralisation of SOM (Kuzyakov et al. 2000). Bovine urine can stimulate positive priming and is a potential pathway for reducing SOM in intensively grazed pastures (Uchida et al. 2011; Lambie et al. 2013). Priming is a microbially-mediated process (Janson 1958; Kuzyakov et al. 2000) and in urine-affected soils, this may result from direct introduction of urine C substrates or indirectly from urine facilitated solubilisation of soil C (Shand et al. 2000; Lambie et al. 2012b).
Much of the literature assessing soil C priming responses to urine has used artificial urine (e.g. Kool et al. 2006). However, Lambie et al. (2013) found that artificial urine is a poor substitute for real urine when assessing C cycling and ∼5% of soil C concentration was lost as carbon dioxide (CO2) during a priming event in repacked cores of sandy loam applied with real urine (Allophanic soil). Uchida et al. (2011) found 0.4–0.6% of soil C concentration was lost as CO2 in repacked cores of clay loam (Oxidic soil). This limited data suggests the priming response following urine addition may be positive, but variable in magnitude across soil orders.
Abiotic factors such as soil pH and electrical conductivity (EC) affect microbial function (e.g. Degens et al. 2001; Cao et al. 2016) and may therefore, affect the prevalence of priming events and SOM loss. Bovine urine contains large amounts of urea (Doak 1952), which is hydrolysed by urease, during which hydrogen ions are consumed and soil pH increases (Cabrera et al. 1991; Tabatabai 1994), increasing soil C solubilisation (Lambie et al. 2012b). This increase in soil pH varies considerably and is dependent on the initial soil pH, urea concentration (Cabrera et al. 1991) and whether artificial or real urine is added to the soil (Lambie 2012). Urine’s high salt load can elevate soil EC (Haynes and Williams 1992), contributing to acid neutralising capacity (Lambie et al. 2012a) and dissolution of SOM (Menneer et al. 2001; Arienzo et al. 2009). Soil carbon content is the main driver determining soil pH buffering capacity (Curtin and Trolove 2013) and may affect the magnitude of C solubilisation in response to urine additions and therefore, indirect soil priming.
In intensive pastoral systems, the same area of soil may receive repeated urine deposition in subsequent grazing rotations (Pleasants et al. 2007; Moir et al. 2011). Kelliher et al. (2005) found that two sequential additions of pure urea led to a doubling of CO2 fluxes. Although soil respiration differs between urea solutions and real urine (Lambie 2012), it is possible that a second addition of urine would also lead to an enhanced priming response. The area of a paddock subject to repeat urine addition is small (Pleasants et al. 2007), but should sequential additions of urine enhance priming, these small areas of land would contribute disproportionately to soil C losses.
To inform whether urine additions are a potential pathway for C loss under intensive grazing, we assessed the soil priming response following a first and second bovine urine addition to a range of soils from four soil orders (collected from across the North Island of New Zealand) and assessed if soil pH, EC, and carbon content affected priming.
Materials and methods
Soil sampling
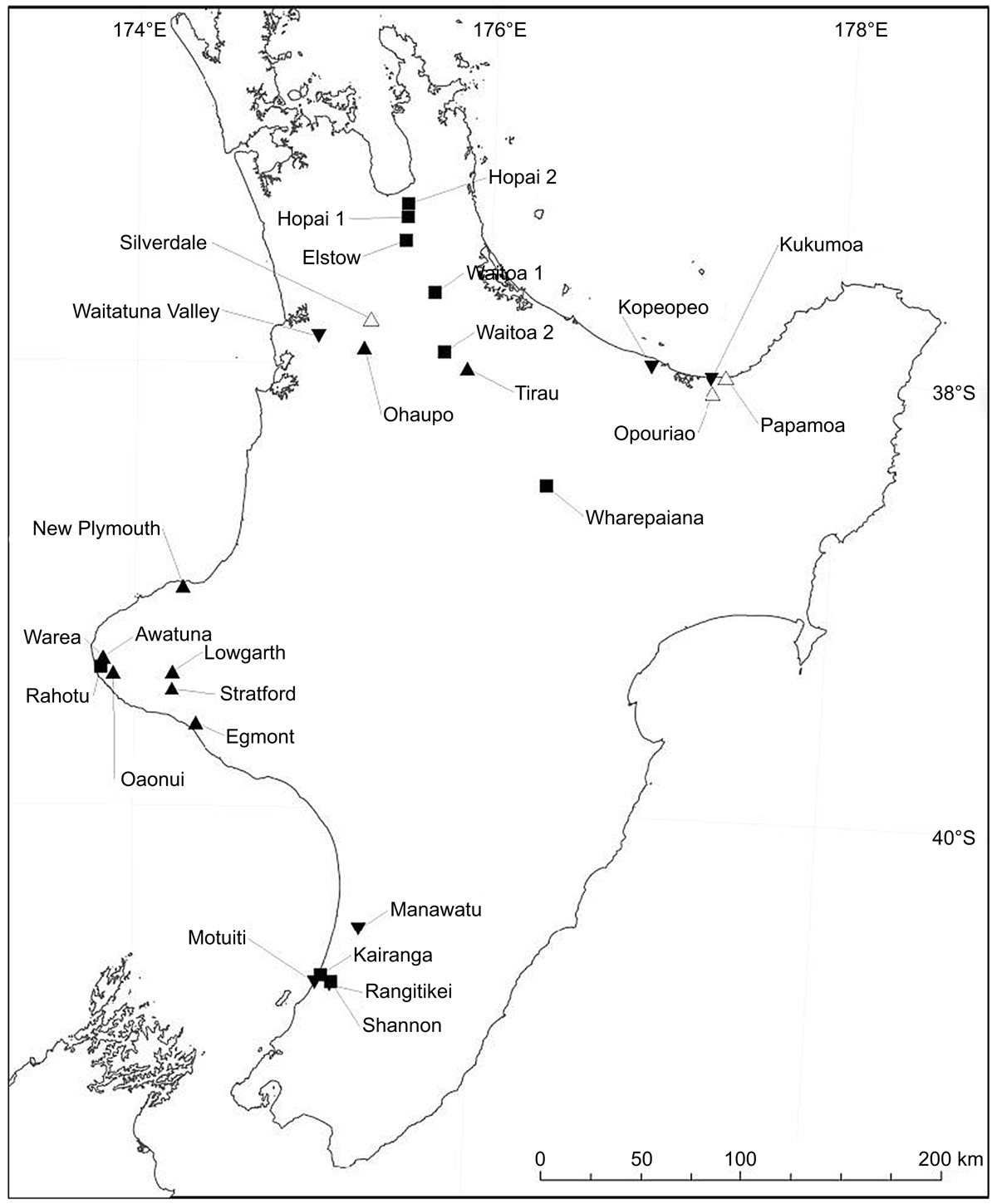
Sampling sites (n = 27) in the North Island of New Zealand (Fig. 1) were selected using the New Zealand National Soils Database; a database containing point soil profile data including physio-chemical and land use data. We selected sites from long-term dairy production within Allophanic (n = 9), Gley (n = 9), Recent (n = 6) and Brown (n = 3) soil orders (Supplementary Table S1). The pasture composition was predominantly ryegrass (Lolium perenne L.) and clover (Trifolium repens L.) at all sites. Soils samples were collected in August and September 2014. Where possible, sites were sampled 2 weeks after grazing to enable visual identification of urine patches and these areas were excluded from sampling.
|
At each sampling site, from three randomly selected points, avoiding obvious dung and urine patches, two undisturbed soil cores (0.1 m diameter × 0.1 m deep) were taken for CO2 measurement, 12 cores taken to measure soil pH and EC over time and one core for bulk density measurement (Fig. 2). Ten of the soil cores for pH and EC measurements were smaller diameter (0.05 m diameter × 0.1 m deep) due to limited space in the constant temperature facility and the large amount (>1000) of cores required. Bulk density cores (0.1 m diameter × 0.1 m deep) were measured for fine earth bulk density; whereby the weight of the soil was divided by the volume of soil, minus the volume of roots and stones (McKenzie et al. 2002).

|
All cores were retained in a stainless-steel liner, wrapped in clear wrap, and refrigerated (4 ± 1°C) until required. Before urine addition, cores were trimmed of grass to eliminate grass decomposition contributing to CO2 fluxes during the incubation. Nylon was attached to the base of each core to retain the soil within the liners, and all the cores were adjusted to 60% of gravimetric water holding capacity as per Harding and Ross (1964) and Lambie et al. (2013). Briefly, 30 g of field moist soil was placed in a funnel with a stoppered outlet, water was added to the funnel to above the soil surface by 10 mm. The soil was left to saturate overnight, the stopper removed from the base of the funnel and the soil left to drain for 2 h. The gravimetric water content determined at saturation is the water holding capacity. Water holding capacity ranged between 54% and 196% with an average of 115% ± 22. The post-adjustment moisture content at 60% of water holding capacity equated to a soil moisture content of 32–118%, with an average of 69% ± 2 moisture content.
Urine collection and application for CO2 measurement
Sixty litres of dairy cow urine were collected from many cows, fed on the ryegrass–clover pasture, during milking in early September 2014 (Stokes Farm, Taupiri, North Island, New Zealand). The cow urine was bulked, subsampled in triplicate, and analysed for total C and total N (LECO TruMac; LECO Corp., St Joseph, MI, USA). The carbon content of the urine was 1.00% ± 0.03 and the nitrogen content 0.40% ± 0.02. One-third of the urine was mixed with 125 mL 14C urea (4.6 MBq) and the labelled and un-labelled urine frozen until needed. Urea in urine does not breakdown as rapidly as pure urea in soils and is not the dominant carbon compound in bovine urine but contributes ∼90% of loss of C from urine over time (Lambie 2012). 14C urea was selected to trace urine-C dynamics due to limited alternatives but enabled comparative measures between soils.
14C urine was applied to three replicate (Fig. 2) cores from each sampling site at a rate of 1000 kg N ha−1 (Haynes and Williams 1993); which equated to 2500 kg C ha−1 and 196 mL of urine. The same volume of deionised water was applied to a further three replicate cores as a control treatment to enable correction for changes in moisture on microbial activity (Orchard and Cook 1983).
Solutions were applied within the unsaturated hydraulic conductivity of the various soils to minimise surface ponding and maximise matrix flow of the added solution. The moisture content of the cores after urine/water addition ranged between 38% and 132% with an average of 80% ± 3. The undisturbed cores were sufficiently sealed against the edge of the liner to prevent edge flow of the solutes between the soil and liner. Following urine/water application, the cores were left to drain until no further liquid was detected, which was usually within 3 h of the end of solution application. This period of drainage was not included in the trapping period for CO2 collection as CO2 emitted from the drained urine would have confounded the collection of soil CO2 data. The leachate from the cores was collected and the volume recorded. The volume of fluid retained during the first urine addition was 55 mL ± 3 (28% ± 2 of added urine), which equated to retention of 1100 ± 68 mg C kg−1 and 440 ± 27 mg N kg−1. 14C content of the leachate was measured to determine the amount of 14C urine-C retained in each core. Leachate (0.2 mL) was added to scintillation cocktail (5 mL), the vials shaken to mix, and left to rest in the dark for least 30 min before analysis to ensure that bubbles in the cocktail solution had dissipated. The 14C content was then determined using scintillation counting (TriCarb 2900TR, PerkinElmer, Waltham, MA, USA).
Twenty one days after the first urine addition, we applied a second urine addition. In New Zealand, during periods of high grass production, a 21-day grazing rotation is commonly used. Therefore, we used 21-day incubations as a representative of a worst-case scenario for the shortest possible length between urine additions. As it is unlikely that a single urine patch would receive a subsequent urine application with identical characteristics (e.g. Doak 1952), 40 L of fresh urine were collected, as above, near the end of September 2014 from the same herd on the same farm. The second urine collection had a lower N content (C = 0.82% ± 0.02, N = 0.33% ± 0.01), but the same C:N ratio, and therefore a slightly greater volume of urine was applied (238 mL) to maintain the 1000 kg N ha−1 and 2500 kg C ha−1 application rate. The moisture content of the cores ranged between 30% and 147% on Day 21, before the second urine addition, with an average of 71% ± 2. After urine/water addition, the moisture content of the cores ranged between 35% and 143% with an average of 76% ± 3. The volume of fluid retained in the soil after the second urine addition was 30 mL ± 1 (12% ± 0.4 of added urine) and equating to retention of 475 ± 18 mg C kg−1 and 191 ± 7 mg N kg−1.
CO2 measurement
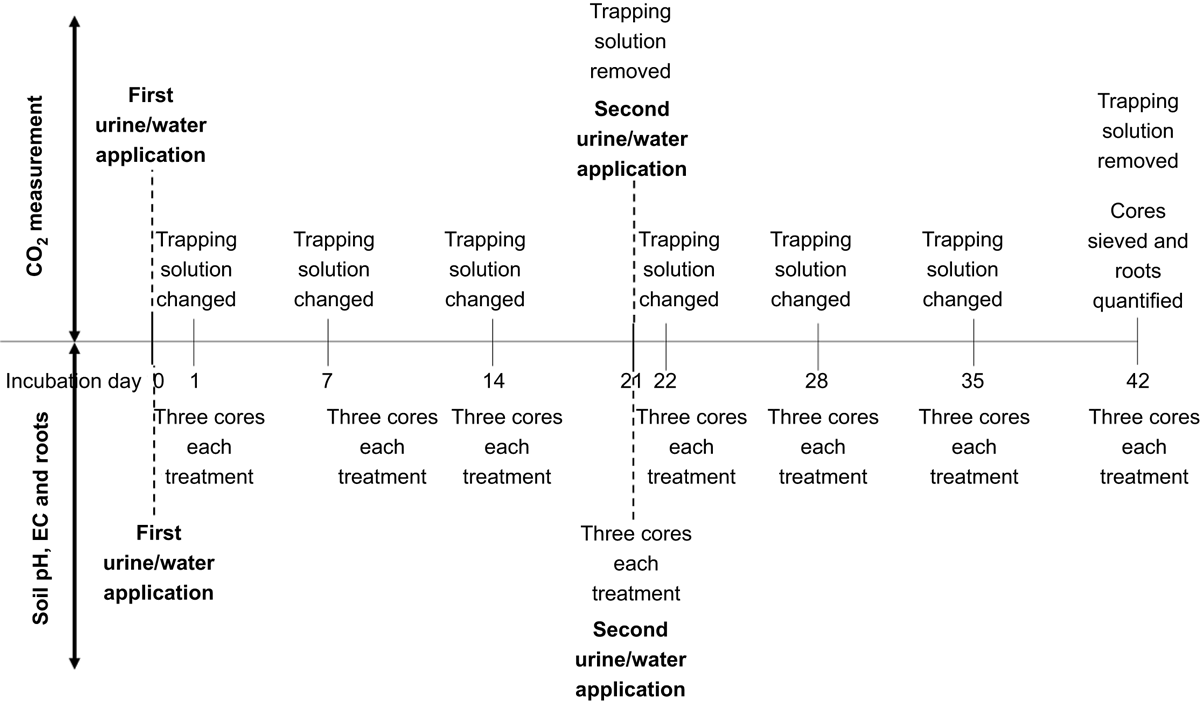
Capture of CO2 was undertaken to assess microbial activity, and therefore priming, response to urine additions (e.g. Lambie et al. 2013; Zhang et al. 2017; Chen et al. 2019; You et al. 2020). Following solution application, each core was placed into 2.2 L plastic containers (Sistema, Auckland, New Zealand), with 40 mL of 2 M sodium hydroxide solution to trap CO2, sealed, and incubated at 25°C, in the dark, for 21 days (Lambie et al. 2013). The trapping solution was changed on Days 1, 7, 14 and 21 of the incubation (Fig. 3). The incubation containers were ventilated in a fume cupboard for 30 min each time the trapping solution was changed. Total CO2 fluxes (combined soil and urine) was determined by back titration of the trapping solutions with 0.1 M hydrochloric acid against a phenolphthalein indicator after precipitation of carbonates with excess barium chloride (Saggar et al.1999). The 14C content of the trapping solution was measured to determine the contribution of urine-derived CO2 to the total CO2 trapped. Trapping solution (0.6 mL), water (0.4 mL) and cocktail solution (5 mL) were shaken to mix and left in the dark for at least 30 min before scintillation measurement (TriCarb 2900TR, PerkinElmer, Waltham, MA, USA).
|
On Day 21, following the second urine addition, the cores were left to drain and incubated as above for a further 21 days. The trapping solutions were changed on Days 1, 7, 14 and 21 of the second incubation (Days 22, 28, 35 and 42 of the total incubation), and CO2 and 14CO2 fluxes were determined as above (Fig. 3). At the termination of the experiment, the moisture content of the soils ranged between 30% and 136% with an average of 74% ± 2.
Soil chemistry
Un-labelled urine or water was applied to fifteen 0.05 m diameter (0.1 m deep) and three 0.1 m diameter (0.1 m deep) cores for each sampling site at the same application rate as for CO2 measurement, which equated to 49 mL of urine/water for the 0.05 m cores and 196 mL to the 0.1 m diameter cores. After solution application, cores were incubated at 25°C in the dark, in loosely covered trays, and maintained at constant moisture content. After 21 days of incubation, the remaining cores received a second urine/water application and were treated as described above (Fig. 3). As for the CO2 measurement cores, more urine was required for the second urine application to maintain the 1000 kg N ha−1 application rate, which equated to 60 mL of urine or water.
Three 0.05 m diameter cores, from each sampling site, were removed from incubation 1, 7, 14, 22, 28 and 42 days after solution addition and three 0.1 m diameter cores were removed from the incubation 21 days after solute addition and tested for root biomass contents, pH and EC measurement (Fig. 3). The cores were sieved to 4 mm and the root biomass collected, washed, dried (60°C), and weighed to estimate the change in root biomass over time. A subsample from each core was air dried, sieved to 2 mm, and tested for pH in a 1:2.5 water:soil slurry and EC in a 1:5 water:soil slurry (Blakemore et al. 1987). At the termination of the experiment (Day 42 incubation), the cores used for CO2 measurement were also sieved and the root biomass quantified.
Priming calculations
Priming was determined using a 14C mass balance according to Lambie et al. (2013). Briefly, the amount of 14C urine retained by the soil was determined by subtracting the amount of 14C in the leachate from that applied in the urine. As the cores with 14C labelled urine applications were required to remain intact after the first incubation period for the continued CO2 measurement during the second incubation period, the 14C content of the soil could not be directly measured following the first incubation period. To be consistent between the first and second incubation the amount of urine-C left in the soil following the first urine addition was calculated as below:
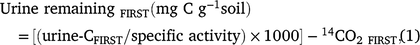
where urine-CFIRST was the urine-C retained (Bq g−1 soil) after the first urine addition, specific activity was specific activity of urine (25 694 Bq g−1 urine-C), and 14CO2 FIRST was the carbon dioxide from mineralisation of the urine-C (14CO2-C g−1) during the first 21-day incubation.
Following the second urine addition, urine-C remaining in the soil would have been a combination of urine-C remaining after the first and second urine additions. Therefore, the total amount of urine remaining (mg g−1) from both urine additions was calculated as below:
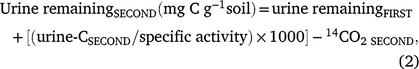
where urine remainingFIRST was the urine-C remaining after the first urine addition as determined in Eqn 1 (mg C g−1 soil), urine-CSECOND was the urine-C retained (Bq g−1 soil) after the second urine addition, specific activity was the specific activity of urine (Bq g−1 urine-C), and 14CO2 SECOND was carbon dioxide produced from mineralisation of the urine-C (CO2-C g−1) during incubation from days 21 to 42.
Priming was then determined as per Eqn 3:
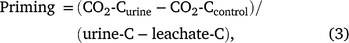
where CO2-Curine is cumulative respiration from the urine treatment (mg CO2-C g−1); CO2-Ccontrol is cumulative respiration from the water treatment (mg CO2-C g−1); urine-C is C added in the urine (mg C g−1); leachate-C is C in the leachate (mg C g−1), which is representative of the amount of urine-C retained as outlined in the equations above.
Urine derived 14CO2-C was determined using the 14C-urea label. Unpublished data indicated that 94.8% ± 0.3 of urea in bovine urine was degraded within 14 days and contributed ∼90% of urine-C losses over that time (data not shown) and the other carbon compounds in urine would be minimal contributors to 14CO2-C but may have contributed some dilution of the radio-label (Petersen et al. 2004). The fraction of 14C activity in the CO2 was determined by dividing the total amount of activity (Bq g−1) emitted over the course of each 21-day incubation (i.e. 0–21 days, and 22–42 days) by the amount of 14C (Bq g−1) retained by the soil. The total CO2 produced was then multiplied by the fraction of 14C activity to determine the amount of urine-derived CO2. The remaining CO2 was soil derived and is assumed to include microbial biomass turnover and soil C mineralisation. Priming was the sum of priming measured after both urine additions. Priming can be both positive and negative (Fig. 4). Positive priming occurs when the addition of a substrate increases CO2 fluxes above that of the amount of C added in a substrate compared to soil with no substrate added, indicating degradation of soil C pools. Negative priming occurs when CO2 fluxes are less than CO2 produced by degradation of the added substrate and soil with no substrate added indicating retardation of degradation of soil C pools (Blagodatskaya and Kuzyakov 2011).

|
Urine addition can lead to root biomass death and degradation (Richards and Wolton 1975), adding to potential CO2 fluxes from soil. If urine addition led to root death (as shown by a decrease in root biomass over time), then the correction for baseline soil respiration using the control soils would not be sufficient and priming would be overestimated. There was no significant change in root biomass over time in either the water or urine treatments (data not shown), indicating that root death because of urine application was unlikely to have contributed to an increase in CO2 flux from either of the treatments. However, at the end of the incubation when the CO2 cores were assessed for root biomass, there was greater (P < 0.05) biomass in the urine-treated cores than the water-treated cores. The higher root biomass in the urine treatment meant the water control correction may not have fully accounted for root respiration. To account for this, we fitted a linear regression of priming against root biomass and took the relative deviance of the observed value from the fitted value as a root biomass-corrected measure of priming. Urine addition and defoliation can also increase root exudations (Dawson et al. 2000; Paterson et al. 2006; Zhu et al. 2016), any microbial stimulation facilitated by root exudations, post-urine addition, would be accounted for within the non-14C labelled soil fluxes.
The variability of the priming response within each sampling site was assessed using the mean absolute deviation (Geary 1935). Mean absolute deviation was calculated as the mean of the distance from each data point and the mean as calculated below:

where m(X) is the average value of the data set, n is the number of data values and xi is data values in the set.
Cumulative effect of urine on soil pH and EC
Priming is a cumulative measurement, so we determined the cumulative effect of urine on soil pH and EC over time. The cumulative urine effect on soil pH (CUEpH) and EC (CUEec) were calculated as the integrated difference between the water and urine treatments after the first and second urine additions.
Statistical analyses
Differences in soil pH, and EC and soil order effects between the treatments were assessed using ANOVA with Student–Newman–Keuls post hoc analysis (P < 0.05). The normality of each analysis was evaluated by visual assessment of residual plots and data was assessed for difference to zero using one sample, two-tailed, t-tests. Generalised linear models (GLM) were used to test the influence of soil order, CUEpH and CUEec and all interactions between them on priming. General linear regressions were used to assess the relationship between soil C contents and CO2 fluxes on each of the sampling dates. The statistics were undertaken using Genstat 12 (VSN International, Hemel Hempstead, UK), except for the GLM modelling (R Development Core Team 2009, R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/) and regression modelling (Sigma Plot; Systat Software Inc.).
Results
Carbon dioxide fluxes
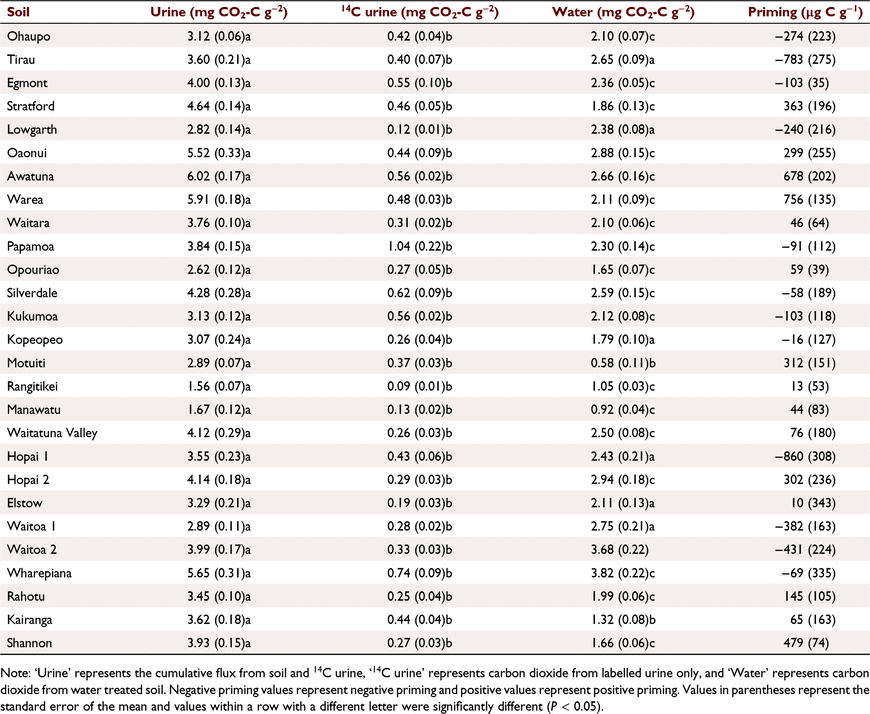
Urine addition significantly increased microbial activity as seen by increases in total CO2 fluxes in 26 of the 27 soils after each urine application (Supplementary Figs S1–S3). On Day 1 of the incubation, urine derived CO2 (determined from 14C) was greater than CO2 flux from the water control (water < 14C urine < total; P < 0.001) (Figs S1–S3). However, after Day 1, CO2 flux from urine was the smallest flux (14C urine < water < total; treatment effect P < 0.001, time effect P < 0.001). The Recent soils emitted the lowest CO2 fluxes in response to urine addition, but the remainder of the soil orders did not differ from one another (Table 1; P < 0.05). Cumulative CO2 fluxes from 14C urine alone was significantly less than total CO2 fluxes (labelled urine plus soil; urine treatment) for all 27 soils (Table 1), indicating the bulk of CO2 produced in the urine treated soils was derived from soil C pools rather than urine-C. In 19 out of 27 soils, total cumulative CO2 was significantly less in the water controls than urine treated soil. Cumulative CO2 in the remaining eight soils exhibited no significant difference between urine treatment and water control. Cumulative CO2 did not differ between 14C urine treatment and water controls in the Motuiti and Kairanga soils.
|
CO2 fluxes (and therefore microbial activity) were greater (P < 0.001) after the first urine addition than the second urine addition when assessed for all soils. CO2 fluxes in the urine-treated soils ranged between 0.13 mg and 0.72 mg CO2-C g−1 day−1 after the first urine addition and between 0.08 mg and 0.52 mg CO2-C g−1 day−1 after the second (Figs S1–S3). On average, CO2 fluxes in the urine-treated soils were 86% ± 1 greater than the water controls 1 day after the first urine addition, and 68% ± 4 greater 1 day after the second urine addition.
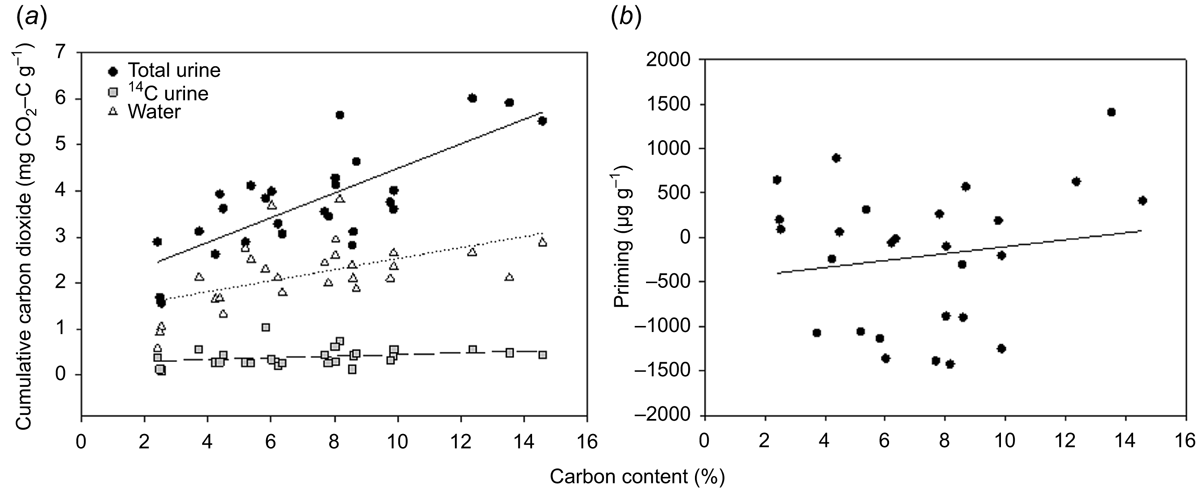
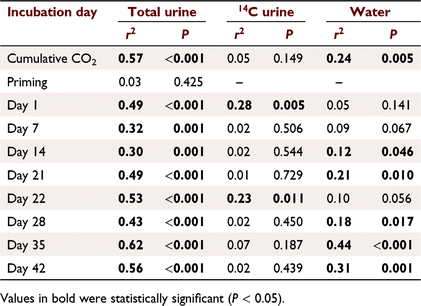
Cumulative CO2 fluxes were positively correlated to soil C contents and explained 54% and 24% of the variation in cumulative CO2 in the total and water treatments (Fig. 5; Table 2). There were no significant relationships between cumulative CO2 fluxes in the 14C urine treatments or with priming (Fig. 5; Table 2).
|
|
The soil C content significantly affected the rate of microbial activity as seen as CO2 fluxes in response to urine addition; however, the strength of this positive relationship varied over time (Fig. S4; Table 2). Total C explained between 30 and 62% of the variability in CO2 fluxes in response to urine addition (Table 2). 14CO2 fluxes were only affected by soil C contents immediately after urine addition where C content explained 23–28% of the variation in CO2 fluxes (Fig. S4). CO2 fluxes in the water controls showed a highly variable relationship with C content (Fig. S4; Table 2). There was a significant positive relationship between these factors on Days 14, 21, 28, 35 and 42, which explained between 12% and 44% of the variation in CO2 fluxes.
Priming
The priming calculation subtracts the amounts of 14CO2 derived from labelled urine and CO2 produced from the water controls after determining the amount of urine-C retained and is indicative of the change in microbial activity in response to urine additions. Negative (retardation of carbon mineralisation), positive (acceleration of carbon mineralisation) and no priming was found in our soils (Table 1). On average, all soils exhibited negative or no significant priming after the urine addition (Fig. 6a). There was also no significant priming (either positive or negative) after the second urine addition and no differences between the soil orders with respect to average priming (Table 1; Fig. 6b).
The mean absolute deviation represents the variation of the priming response. The mean absolute deviation of priming was 200–400 μg C g−1 except for Gley soils after the second urine addition, in which the variation was over 900 μg C g−1 (Fig. 7). Variation in priming response was greater after the second urine addition in Allophanic and Brown soils, lower in Recent soils but twice as great in Gley soils (Fig. 7).
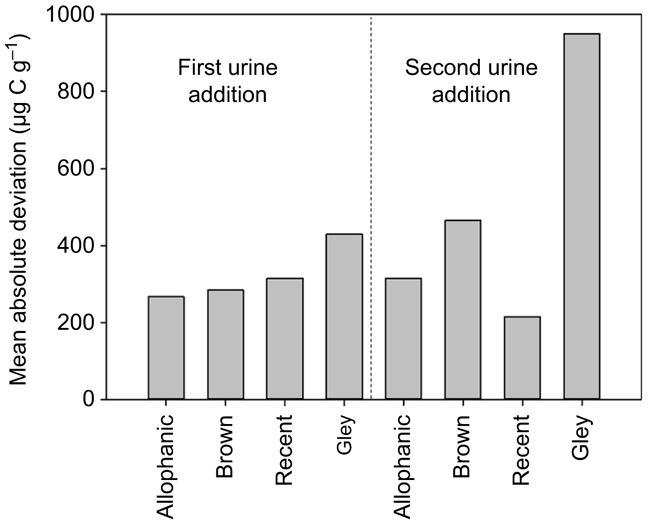
|
Soil pH and electrical conductivity
Soil pH changed little in response to urine addition (Fig. 8), except for Recent soils, where there was a significant increase after both urine additions. The Allophanic and Gley soils also showed a significant (P < 0.05) increase in pH after the second urine addition (Fig. 8). Soil EC significantly increased in all urine-treated soils at all sampling times over the incubation (Fig. 9).
Cumulative urine effects on soil pH (CUEpH) and EC (CUEec) were analysed in relation to priming. There was a significant relationship between CUEec and priming after the second urine addition, which explained about 17% of the variation in priming (Fig. S5), but not after the first urine addition. There was no relationship between CUEpH and priming after either the first or second urine addition. There were also no significant relationships between priming and the maximum soil pH and EC over the 42-day incubation (data not shown).
Discussion
We examined the microbial priming response to two sequential urine additions and investigated the potential for urine facilitated changes in pH and EC changes to affect priming. There was large within soil and within soil order variability indicating soil-specific priming responses.
CO2 fluxes generally exhibited the typical pattern of priming events, with a rapid increase in CO2 fluxes in the urine-treated soils (e.g. Lambie et al. 2013; Boon et al. 2014). With surprisingly little variation, the increase in CO2 fluxes after the first urine addition was 86% ± 1 above the water controls within 24 h of the first urine addition. This is similar to results of Lambie et al. (2013), who found that CO2 fluxes from repacked cores of an Allophanic soil increased by 95%, and Kool et al. (2006), who reported increases of 79% and 84% from undisturbed Podzol soil in the first 24 h after urine addition. However, Uchida et al. (2011) reported considerably greater increases of CO2 around 400% in the first day following urine addition to repacked Oxidic soil.
Allophanic soils exhibited an increase in CO2 fluxes after the second urine addition above the water controls (Fig. 5), possibly due to higher amounts of organic matter and microbial biomass in these soils (Table S1). Certainly, there was some evidence that CO2 fluxes following urine addition were correlated to C contents, although the strength of the relationship varied over the incubation, and C content was likely not the only factor affecting CO2 fluxes over time.
CO2 fluxes immediately following the second urine addition were lower in all soils than after the first urine addition. In contrast, Kelliher et al. (2005) found that sequential applications of pure urea to a Gley soil doubled CO2 emissions after the second addition. As bovine urine has a large urea content, we also expected to see increased CO2 following the second urine addition, despite urea applications resulting in a lower CO2 efflux than real urine (Kool et al. 2006; Lambie et al. 2013). The decreased CO2 response to the second urine addition may be due to diminished ability of soil microbes to utilise new substrates 21 days after urine addition. Lambie et al. (2019) showed in a sister experiment that functional capacity (the ability of microbes to utilise added substrates) was inhibited 21-day after bovine urine application compared to pre-incubation and water applied controls. Bertram et al. (2012)) found microbes exhibited signs of stress from 8 days until completion of a 28-day incubation after urine addition. They also found microbes experienced stress in wetter soils compared to drier (70% and 35% water filled porosity) and therefore, our microbes may have experienced substantial stress in response to water adjustment of the soils and the additions of further water or urine over our experiment.
Research into priming response using real urine is limited, but data published to date (e.g. Uchida et al. 2011; Lambie et al. 2013) showed significant positive priming after urine addition but suggested a range in the priming response in different soils. We indeed found considerable variability in both magnitude and direction of priming in our soils, as also reported by Paterson and Sim (2013). The mechanisms driving variability in priming response remain elusive. We found no strong evidence that soil pH and EC changes after urine addition affected priming in the 27 soils we tested. Basal respiration and post-substrate addition moisture contents (Luo et al. 2016) and microbial biomass C (Kuzyakov et al. 2000) have been linked to priming response; however, we found no significant relationships between these factors and priming (Table S2).
The spatial variability of CO2 fluxes has been linked to differences in water contents (e.g. Herbst et al. 2009; Warner et al. 2016; Arias-Navarro et al. 2017), but our soils were adjusted to very similar water contents and there was no indication of a correlation between priming and water content in our soils. Identification of soil chemistry that influences variability of CO2 fluxes is surprisingly rare in the literature; however, Warner et al. (2016) suggested the amount of water-soluble C may be factor. Soil water-soluble C can be directly affected by urine, which contains substantial amounts of C (Lambie 2012) and urine can also increase solubilisation of soil C (Lambie et al. 2012a, 2012b). However, Lambie et al. (2013) found no correlation between water-soluble C and urine priming response. Paterson and Sim (2013) reported that the substrate concentration trigger point for priming could be soil specific and further work assessing urine additions with a range of C concentrations could be valuable in determining if this is the case.
Previous experimentation assessing priming in response to real urine used repacked cores, which would have a more uniform drainage pattern compared to undisturbed cores (Monaghan et al. 1989) due to disruption of pore distributions during repacking. The physical distribution of added substrates within soils affects C mineralisation. For example, Killham et al. (1993) found greater CO2 mineralisation of added 14C-labelled glucose in larger pores, which was further enhanced at lower soil water matric potential. Further, Bouckaert et al. (2013) suggested that the distribution of water among the pores of different sizes, rather than the actual water content influences microbial mineralisation. Nunan et al. (2017) also reported that the microhabitat of microorganisms is affected by pore distribution and ultimately affects microbial function by strongly influencing oxygen and substrate availability. Priming response may also be mediated by only a portion of the soil microbial community (Paterson and Sim 2013) and therefore, their ability to access added substrate may contribute to the variability in priming response.
Sorption processes may also contribute to microbial availability of dissolved organic C (Jardine et al. 1989; Kaiser and Zech 1997) and has not been fully described for urine in soils. While, Lambie et al. (2012b) showed sorption of urine-C does occur in soils, as well as substantial dissolution of soil C compounds, they did not identify the differences in composition between urine added and leachate. Therefore, it is possible that sorption of urine-C compounds may differ in preference for carbon compounds in urine as well as capacity between soils impacting availability of compounds for microbial degradation and therefore variability in priming response.
Much of the literature on urine decomposition uses additions of urea or artificial urine to soils (e.g. Shand et al. 2000; Kelliher et al. 2005) and urea in real urine degrades more in soil than urea in artificial urine (Lovell and Jarvis 1996; Kool et al. 2006; Lambie 2012). For example, Lambie et al. (2013) found that following artificial urine application to an Allophanic soil, urea hydrolysis only contributed to 54% ± 1 of CO2 fluxes. Other C compounds in bovine urine (e.g. hippuric acid, carbodiimide and phenaceturic acid) contribute more C than urea to the total C content of urine (Lambie 2012), and the relative influence of degradation of urine’s different C-containing compounds is unclear. Lambie (2012) found that bovine urine-C has a limited degradability with only 5–15% of urine-C mineralised over 28 days (25°C), and residual urine-C remained in soil 84 days after urine addition with immobilisation in microbial biomass accounting for between 8 and 14% of urine-C (Lambie et al. 2013). Further work exploring the variability in urine-C composition would also inform the priming response to bovine urine.
While we elucidated the priming response to urine across a range of soils, we may have generated more questions than answers around the impact of urine additions on C cycling in agricultural soils. There is a dearth of information on urine-C, be it composition or other attributes, which hinders understanding on how urine-C contributes to the sustainability of intensively grazed systems. The prevalence of the use of artificial urine confuses the issue as there is sufficient data that real bovine urine behaves differently to urea, artificial urine, and other carbon compounds commonly used to assess priming response (e.g. glucose). Further, while priming is a biological process, compositional or functional change in microbial communities under urine patches has predominantly been assessed with respect to N cycling and rarely for C cycling (e.g. Lambie et al. 2019). It is possible that priming is determined by specific organisms being able to physically access added substrate rather than any chemical responses of the soil to urine additions and requires further investigation.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was supported by Capability and Strategic Science Investment Funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment’s Science and Innovation Group.
Acknowledgements
The authors thank Robert Price, Phillippa Rhodes, Scott Bartlam, Alexandra McGill, David Hunter, and the Environmental Chemistry Laboratory for contributing to the technical work for this project. Thanks also to Scott Fraser and Bryan Stevenson for supplying site information, and to Grant Stokes for allowing access to his property for urine collection. Further, the authors recognise the contribution of Leah Kearns and the anonymous reviewers to the manuscript.
References
Arias-Navarro C, Díaz-Pinés E, Klatt S, Brandt P, Rufino MC, Butterbach-Bahl K, Verchot LV (2017) Spatial variability of soil N2O and CO2 fluxes in different topographic positions in a tropical Montane forest in Kenya. Journal of Geophysical Research: Biogeosciences 122, 514–527.| Spatial variability of soil N2O and CO2 fluxes in different topographic positions in a tropical Montane forest in Kenya.Crossref | GoogleScholarGoogle Scholar |
Arienzo M, Christen EW, Quayle W, Kumar A (2009) A review of the fate of potassium in the soil–plant system after land application of wastewaters. Journal of Hazardous Materials 164, 415–422.
| A review of the fate of potassium in the soil–plant system after land application of wastewaters.Crossref | GoogleScholarGoogle Scholar | 18842339PubMed |
Bertram JE, Orwin KH, Clough TJ, Condron LM, Sherlock RR, O’Callaghan M (2012) Effect of soil moisture and bovine urine on microbial stress. Pedobiologia 55, 211–218.
| Effect of soil moisture and bovine urine on microbial stress.Crossref | GoogleScholarGoogle Scholar |
Blagodatskaya E, Kuzyakov Y (2011) Priming effects in relation to soil conditions – mechanisms. In ‘Encyclopedia of agrophysics’. 2011 edn. (Eds J Gliński, J Horabik, J Lipiec) pp. 657–667. (Springer: Dordrecht).
| Crossref |
Blakemore LC, Searle PL, Daly BK (1987) ‘Methods for chemical analysis of soils’. (NZ Soil Bureau, Department of Scientific and Industrial Research: Lower Hutt, New Zealand)
| Crossref |
Boon A, Robinson JS, Chadwick DR, Cardenas LM (2014) Effect of cattle urine addition on the surface emissions and subsurface concentrations of greenhouse gases in a UK peat grassland. Agriculture, Ecosystems & Environment 186, 23–32.
| Effect of cattle urine addition on the surface emissions and subsurface concentrations of greenhouse gases in a UK peat grassland.Crossref | GoogleScholarGoogle Scholar |
Bouckaert L, Sleutel S, Van Loo D, Brabant L, Cnudde V, Van Hoorebeke L, De Neve S (2013) Carbon mineralisation and pore size classes in undisturbed soil cores. Soil Research 51, 14–22.
| Carbon mineralisation and pore size classes in undisturbed soil cores.Crossref | GoogleScholarGoogle Scholar |
Cabrera ML, Kissel DE, Bock BR (1991) Urea hydrolysis in soil: effects of urea concentration and soil pH. Soil Biology and Biochemistry 23, 1121–1124.
| Urea hydrolysis in soil: effects of urea concentration and soil pH.Crossref | GoogleScholarGoogle Scholar |
Cao H, Chen R, Wang L, Jiang L, Yang F, Zheng S, Wang G, Lin X (2016) Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Scientific Reports 6, 25815
| Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale.Crossref | GoogleScholarGoogle Scholar | 27170469PubMed |
Chen L, Liu L, Qin S, Yang G, Fang K, Zhu B, Kuzyakov Y, Chen P, Xu Y, Yang Y (2019) Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nature Communications 10, 5112
| Regulation of priming effect by soil organic matter stability over a broad geographic scale.Crossref | GoogleScholarGoogle Scholar | 31704929PubMed |
Curtin D, Trolove S (2013) Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Research 51, 494–502.
| Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics.Crossref | GoogleScholarGoogle Scholar |
Dawson LA, Grayston SJ, Paterson E (2000) Effects of grazing on the roots and rhizosphere of grasses. In ‘Grassland ecophysiology and grazing ecology’. (Eds G Lemaire, J Hodgson, A de Moraes, C Nabinger, PC de F Carvalho) pp. 61–84. (CABI Publishing: New York)
| Crossref |
Degens BP, Schipper LA, Sparling GP, Duncan LC (2001) Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance? Soil Biology and Biochemistry 33, 1143–1153.
| Is the microbial community in a soil with reduced catabolic diversity less resistant to stress or disturbance?Crossref | GoogleScholarGoogle Scholar |
Doak BW (1952) Some chemical changes in the nitrogenous constituents of urine when voided on pasture. The Journal of Agricultural Science 42, 162–171.
| Some chemical changes in the nitrogenous constituents of urine when voided on pasture.Crossref | GoogleScholarGoogle Scholar |
Fontaine S, Bardoux G, Abbadie L, Mariotti A (2004) Carbon input to soil may decrease soil carbon content. Ecology Letters 7, 314–320.
| Carbon input to soil may decrease soil carbon content.Crossref | GoogleScholarGoogle Scholar |
Geary RC (1935) The ratio of the mean deviation to the standard deviation as a test of normality. Biometrika 27, 310–332.
| The ratio of the mean deviation to the standard deviation as a test of normality.Crossref | GoogleScholarGoogle Scholar |
Harding DE, Ross DJ (1964) Some factors in low-temperature storage influencing the mineralisable-nitrogen of soils. Journal of the Science of Food and Agriculture 15, 829–834.
| Some factors in low-temperature storage influencing the mineralisable-nitrogen of soils.Crossref | GoogleScholarGoogle Scholar |
Haynes RJ, Williams PH (1992) Changes in soil solution composition and pH in urine-affected areas of pasture. Journal of Soil Science 43, 323–334.
| Changes in soil solution composition and pH in urine-affected areas of pasture.Crossref | GoogleScholarGoogle Scholar |
Haynes RJ, Williams PH (1993) Nutrient cycling and soil fertility in the grazed pasture ecosystem. Advances in Agronomy 49, 119–199.
| Nutrient cycling and soil fertility in the grazed pasture ecosystem.Crossref | GoogleScholarGoogle Scholar |
Herbst M, Prolingheuer N, Graf A, Huisman JA, Weihermüller L, Vanderborght J (2009) Characterization and understanding of bare soil respiration spatial variability at plot scale. Vadose Zone Journal 8, 762–771.
| Characterization and understanding of bare soil respiration spatial variability at plot scale.Crossref | GoogleScholarGoogle Scholar |
Janson SL (1958) Tracer studies on nitrogen transformations in soil with special attention to mineralisation immobilisation relationship. Annals of the Royal Agricultural College of Sweden 24, 101–361.
Jardine PM, McCarthy JF, Weber NL (1989) Mechanisms of dissolved organic carbon adsorption on soil. Soil Science Society of America Journal 53, 1378–1385.
| Mechanisms of dissolved organic carbon adsorption on soil.Crossref | GoogleScholarGoogle Scholar |
Kaiser K, Zech W (1997) About the sorption of dissolved organic matter to forest soils. Zeitschrift für Pflanzenernährung und Bodenkunde 160, 295–301.
| About the sorption of dissolved organic matter to forest soils.Crossref | GoogleScholarGoogle Scholar |
Kelliher FM, Sedcole JR, Minchin RF, Wan Y, Condron LM, Clough TJ, Bol R (2005) Soil microbial respiration responses to repeated urea applications in three grasslands. Australian Journal of Soil Research 43, 905–913.
| Soil microbial respiration responses to repeated urea applications in three grasslands.Crossref | GoogleScholarGoogle Scholar |
Killham K, Amato M, Ladd JN (1993) Effect of substrate location in soil and soil pore-water regime on carbon turnover. Soil Biology and Biochemistry 25, 57–62.
| Effect of substrate location in soil and soil pore-water regime on carbon turnover.Crossref | GoogleScholarGoogle Scholar |
Kool DM, Hoffland E, Abrahamse SPA, van Groenigen JW (2006) What artificial urine composition is adequate for simulating soil N2O fluxes and mineral N dynamics? Soil Biology and Biochemistry 38, 1757–1763.
| What artificial urine composition is adequate for simulating soil N2O fluxes and mineral N dynamics?Crossref | GoogleScholarGoogle Scholar |
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biology and Biochemistry 32, 1485–1498.
| Review of mechanisms and quantification of priming effects.Crossref | GoogleScholarGoogle Scholar |
Lal R (2014) Societal value of soil carbon. Journal of Soil and Water Conservation 69, 186A–192A.
| Societal value of soil carbon.Crossref | GoogleScholarGoogle Scholar |
Lambie SM (2012) ‘Soil carbon loss under pasture and pine: responses to urine addition’. (University of Waikato: New Zealand)
Lambie SM, Mason NWH, Mudge PL (2019) Bovine urine inhibits microbial function and increases urea turnover in dairy grazed soils. Soil Research 57, 489–499.
| Bovine urine inhibits microbial function and increases urea turnover in dairy grazed soils.Crossref | GoogleScholarGoogle Scholar |
Lambie SM, Schipper LA, Balks MR, Baisden WT (2012a) Carbon leaching from undisturbed soil cores treated with dairy cow urine. Soil Research 50, 320–327.
| Carbon leaching from undisturbed soil cores treated with dairy cow urine.Crossref | GoogleScholarGoogle Scholar |
Lambie SM, Schipper LA, Balks MR, Baisden WT (2012b) Solubilisation of soil carbon following treatment with cow urine under laboratory conditions. Soil Research 50, 50–57.
| Solubilisation of soil carbon following treatment with cow urine under laboratory conditions.Crossref | GoogleScholarGoogle Scholar |
Lambie SM, Schipper LA, Balks MR, Baisden WT (2013) Priming of soil decomposition leads to losses of carbon in soil treated with cow urine. Soil Research 51, 513–520.
| Priming of soil decomposition leads to losses of carbon in soil treated with cow urine.Crossref | GoogleScholarGoogle Scholar |
Lovell RD, Jarvis SC (1996) Effects of urine on soil microbial biomass, methanogenesis, nitrification and denitrification in grassland soils. Plant and Soil 186, 265–273.
| Effects of urine on soil microbial biomass, methanogenesis, nitrification and denitrification in grassland soils.Crossref | GoogleScholarGoogle Scholar |
Luo Z, Wang E, Sun OJ (2016) A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems. Soil Biology and Biochemistry 101, 96–103.
| A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems.Crossref | GoogleScholarGoogle Scholar |
McKenzie N, Coughlan K, Cresswell H (2002) ‘Soil physical measurement and interpretation for land evaluation’. (CSIRO Publishing: Melbourne)
| Crossref |
Menneer JC, McLay CDA, Lee R (2001) Effects of sodium-contaminated wastewater on soil permeability of two New Zealand soils. Australian Journal of Soil Research 39, 877–891.
| Effects of sodium-contaminated wastewater on soil permeability of two New Zealand soils.Crossref | GoogleScholarGoogle Scholar |
Moir JL, Cameron KC, Di HJ, Fertsak U (2011) The spatial coverage of dairy cattle urine patches in an intensively grazed pasture system. The Journal of Agricultural Science 149, 473–485.
| The spatial coverage of dairy cattle urine patches in an intensively grazed pasture system.Crossref | GoogleScholarGoogle Scholar |
Monaghan RM, Cameron KC, McLay CDA (1989) Leaching losses of nitrogen from sheep urine patches. New Zealand Journal of Agricultural Research 32, 237–244.
| Leaching losses of nitrogen from sheep urine patches.Crossref | GoogleScholarGoogle Scholar |
Nunan N, Leloup J, Ruamps LS, Pouteau V, Chenu C (2017) Effects of habitat constraints on soil microbial community function. Scientific Reports 7, 4280
| Effects of habitat constraints on soil microbial community function.Crossref | GoogleScholarGoogle Scholar | 28655916PubMed |
Orchard VA, Cook FJ (1983) Relationship between soil respiration and soil moisture. Soil Biology and Biochemistry 15, 447–453.
| Relationship between soil respiration and soil moisture.Crossref | GoogleScholarGoogle Scholar |
Orwin KH, Stevenson BA, Smaill SJ, Kirschbaum MUF, Dickie IA, Clothier BE, Garrett LG, van der Weerden TJ, Beare MH, Curtin D, de Klein CAM, Dodd MB, Gentile R, Hedley C, Mullan B, Shepherd M, Wakelin SA, Bell N, Bowatte S, Davis MR, Dominati E, O’Callaghan M, Parfitt RL, Thomas SM (2015) Effects of climate change on the delivery of soil-mediated ecosystem services within the primary sector in temperate ecosystems: a review and New Zealand case study. Global Change Biology 21, 2844–2860.
| Effects of climate change on the delivery of soil-mediated ecosystem services within the primary sector in temperate ecosystems: a review and New Zealand case study.Crossref | GoogleScholarGoogle Scholar | 25891785PubMed |
Paterson E, Sim A (2013) Soil-specific response functions of organic matter mineralization to the availability of labile carbon. Global Change Biology 19, 1562–1571.
| Soil-specific response functions of organic matter mineralization to the availability of labile carbon.Crossref | GoogleScholarGoogle Scholar | 23505211PubMed |
Paterson E, Sim A, Standing D, Dorward M, McDonald AJS (2006) Root exudation from Hordeum vulgare in response to localized nitrate supply. Journal of Experimental Botany 57, 2413–2420.
| Root exudation from Hordeum vulgare in response to localized nitrate supply.Crossref | GoogleScholarGoogle Scholar | 16766600PubMed |
Petersen SO, Roslov P, Bol R (2004) Dynamics of a pasture soil microbial community after deposition of cattle urine amended with [13C]urea. Applied and Environmental Microbiology 70, 6363–6369.
| Dynamics of a pasture soil microbial community after deposition of cattle urine amended with [13C]urea.Crossref | GoogleScholarGoogle Scholar | 15528493PubMed |
Pleasants AB, Shorten PR, Wake GC (2007) The distribution of urine deposited on a pasture from grazing animals. The Journal of Agricultural Science 145, 81–86.
| The distribution of urine deposited on a pasture from grazing animals.Crossref | GoogleScholarGoogle Scholar |
Richards IR, Wolton KM (1975) A note on urine scorch caused by grazing animals. Grass and Forage Science 30, 187–188.
| A note on urine scorch caused by grazing animals.Crossref | GoogleScholarGoogle Scholar |
Saggar S, Parshotam A, Hedley C, Salt G (1999) 14C-labelled glucose turnover in New Zealand soils. Soil Biology and Biochemistry 31, 2025–2037.
Shand CA, Williams BL, Smith S, Young ME (2000) Temporal changes in C, P and N concentrations in soil solution following application of synthetic sheep urine to a soil under grass. Plant and Soil 222, 1–13.
| Temporal changes in C, P and N concentrations in soil solution following application of synthetic sheep urine to a soil under grass.Crossref | GoogleScholarGoogle Scholar |
Tabatabai MA (1994) Soil enzymes. In ‘Methods of soil analysis: Part 2. Microbiological and biochemical properties, 5.2’. (Eds RW Weaver, S Angle, P Bottomley, D Bezdicket, S Smith, A Tabatabai, A Wollum) pp. 775–833. (Soil Science Society of America: Wisconsin, USA)
| Crossref |
Uchida Y, Clough TJ, Kelliher FM, Hunt JE, Sherlock RR (2011) Effects of bovine urine, plants and temperature on N2O and CO2 emissions from a sub-tropical soil. Plant and Soil 345, 171–186.
| Effects of bovine urine, plants and temperature on N2O and CO2 emissions from a sub-tropical soil.Crossref | GoogleScholarGoogle Scholar |
Warner DL, Inamdar SP, Vargas R (2016) ‘Chemical, physical and biological controls on the spatial heterogeneity of annual soil CO2 and CH4 fluxes’. (American Geophysical Union, Fall General Assembly)
You M, Li L-J, Tian Q, He P, He G, Hao X-X, Horwath WR (2020) Residue decomposition and priming of soil organic carbon following different NPK fertilizer histories. Soil Science Society of America Journal 84, 1898–1909.
| Residue decomposition and priming of soil organic carbon following different NPK fertilizer histories.Crossref | GoogleScholarGoogle Scholar |
Zhang X, Han X, Yu W, Wang P, Cheng W (2017) Priming effects on labile and stable soil organic carbon decomposition: pulse dynamics over two years. PLoS ONE 12, e0184978
| Priming effects on labile and stable soil organic carbon decomposition: pulse dynamics over two years.Crossref | GoogleScholarGoogle Scholar | 28934287PubMed |
Zhu S, Vivanco JM, Manter DK (2016) Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize. Applied Soil Ecology 107, 324–333.
| Nitrogen fertilizer rate affects root exudation, the rhizosphere microbiome and nitrogen-use-efficiency of maize.Crossref | GoogleScholarGoogle Scholar |





