Contribution of the cotton irrigation network to farm nitrous oxide emissions
B. C. T. Macdonald A D , A. Nadelko B , Y. Chang B , M. Glover C and S. Warneke AA CSIRO Agriculture Flagship, Black Mountain, Canberra, ACT 2601, Australia.
B CSIRO Agriculture Flagship, Narrabri, NSW 2390, Australia.
C CSIRO Land and Water Flagship, Black Mountain, Canberra, ACT 2601, Australia.
D Corresponding author. Email: ben.macdonald@csiro.au
Soil Research 54(5) 651-658 https://doi.org/10.1071/SR15273
Submitted: 21 September 2015 Accepted: 14 April 2016 Published: 25 July 2016
Journal Compilation © CSIRO Publishing 2016 Open Access CC BY-NC-ND
Abstract
Nitrous oxide (N2O) is a potent greenhouse gas, and agriculture is the dominant source of N2O-N emissions. The Australian cotton industry requires high inputs of N to maintain high lint quality and yields; however, over-fertilisation with N is symptomatic of the industry. Up to 3.5% of N fertiliser applied is lost directly from cotton fields as N2O gas. Excess N may also be lost via erosion, deep-drainage, leaching and runoff, and may subsequently form indirect N2O emissions. The estimate by the Intergovernmental Panel on Climate Change (IPCC) suggests that 0.0025 kg N2O-N is produced indirectly from groundwater and surface drainage for each kg N lost via runoff and leaching, although this estimate carries a large degree of uncertainty. This study is the first to address the lack of indirect N2O emission data from irrigated cotton-farming systems. Indirect emissions were determined from total N concentrations in irrigation runoff by using the IPCC emission factor and from measurements of dissolved N2O during the first four irrigations (October–December 2013). Total indirect N2O emissions from the surface of the irrigation network over 3 months when estimated by the dissolved-N2O method were 0.503 ± 0.339 kg ha–1. By contrast, N2O emissions estimated by the IPCC methodology were 0.843 ± 0.022 kg ha–1 irrigation surface area. Over the same period of measurement, direct land-surface emissions were 1.44 kg N2O-N ha–1 field. Despite relatively high emissions per surface area, the irrigation network is only a minor component of the total farm area, and indirect emissions from the irrigation system contribute ~2.4–4% of the total N2O emissions and <0.02% of the applied N fertiliser.
Additional keywords: furrow irrigation, N2O emissions, nitrogen use efficiency (NUE), runoff.
Introduction
In Australia, irrigated cotton is a high-yielding system that requires nitrogen (N) fertiliser inputs to maintain the quality and quantity of yields. Over-fertilisation with N does occur. Comparisons between the internal N-use efficiency (kg lint kg–1 crop N uptake) from commercial crops and cotton grown under optimum N rates demonstrate that during 2009–12 the industry over-fertilised by ~49 kg N ha–1 in 2011 (Rochester 2011) and 25 kg N ha–1 in 2012 (Rochester 2012). Several growers over-fertilised by 80–90 kg N ha–1 (Rochester et al. 2009; Rochester 2011, 2012). A consequence of the excess N is increased production of the greenhouse gas nitrous oxide (N2O).
Nitrous oxide is a potent greenhouse gas with a 100-year warming potential 298 times that of carbon dioxide (Butterbach-Bahl et al. 2013). The N2O molecule is produced as an intermediate compound from two main processes, nitrification and denitrification (Butterbach-Bahl et al. 2013). Rates of N2O emission are controlled by various environmental factors including soil porosity, temperature, microbial community, pH and availability of mineral N e.g. (Eichner 1990; Bouwman 1994; Butterbach-Bahl et al. 2013). Emissions of N2O resulting from fertiliser use and manure management comprise 26–35% of total emissions (Syakila and Kroeze 2011), and increased applications of N fertiliser are positively related to direct N2O emissions (Bouwman 1996; Hinton et al. 2015; Rochester 2003). For cotton, the relationship between N rate and N2O emissions is exponential, with 1.1–3.5% N applied subsequently emitted as N2O at N rates of 280–320 kg N ha–1 (Grace et al. 2016).
Excess N may also be leached (Benjamin et al. 1998) or lost as runoff into the irrigation system (Mchugh et al. 2008). A study of furrow-irrigated cotton in Emerald, Queensland, Australia, with application of 250 kg N ha–1, showed average N runoff to be 18.8 and 11.3 kg N ha–1 for 2001–02 and 2002–03, respectively (Mchugh et al. 2008). In furrow-irrigated maize production systems in Iran, nitrate (NO3–) runoff ranged from 26 to 70 N ha–1 after application of 60 kg N ha–1 (Ebrahimian et al. 2012). Nitrogen species lost via runoff may subsequently undergo denitrification to form N2O in the water column or drain sediments.
Emissions of N2O that occur as a result of the transformation of N species lost from the field (e.g. via volatilisation, runoff and leaching) or movement of dissolved N2O from the field are termed ‘indirect N2O emissions’ (Reay et al. 2005; IPCC 2006). Indirect emissions are thought to be 29–67% of the magnitude of direct emissions (Reay et al. 2003; Syakila and Kroeze 2011; Outram and Hiscock 2012). Current estimates from the Intergovernmental Panel on Climate Change (IPCC) suggest that for each kg N lost via runoff or leaching, 0.0025 kg N2O-N may be produced (emission factor, EF5g = 0.0025) but the range of uncertainty is large at 0.0005 to 0.025 (IPCC 2006). Based on the NO3-N losses reported by Mchugh et al. (2008), we might estimate that ~0.028–0.047 kg N2O-N ha–1 would be produced from furrow-irrigated cotton via indirect emissions. Harrison and Matson (2003) have shown with direct measurement that average emissions of 0.04 N2O-N kg ha–1 day–1 can occur within furrow-irrigated wheat production in Mexico.
Indirect N2O emissions may be a significant component of the total N2O emissions for Australian cotton systems. However, indirect N2O fluxes from agriculture have not been measured in Australia. Current estimates for indirect emissions rely on the use of IPCC emission factors with high levels of uncertainty. The aims of this study are to quantify indirect N2O losses and to compare indirect with direct N2O emissions, in an Australian furrow-irrigated cotton-farming system.
Materials and methods
Site description and sampling regime
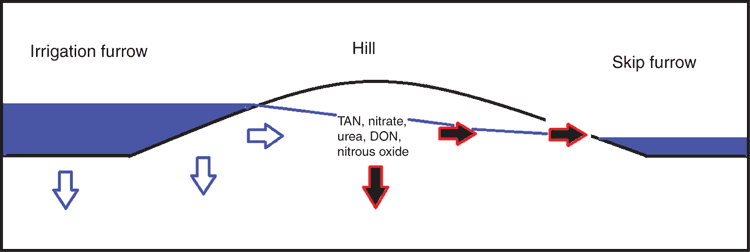
The research was conducted at the Australian Cotton Research Institute (ACRI) at Narrabri, NSW, Australia (30°19ʹS 149°46ʹE). ACRI is located at the geographic centre of cotton production in Australia. The soil at this site is a high shrink–swell medium grey clay overlying brown clay and is classified as a fine, thermic, montmorillonitic Typic Haplustert (Soil Survey Staff 2010). Cotton is grown at ACRI by using furrow irrigation, and on average, the irrigation network contains water 100 days each year. The irrigation network comprises storage ponds, supply channels, head (supply) and tail ditches for each field, furrows through the field, main tail drains and return channels (which return water to the storage ponds) (Fig. 1). Prior to the irrigation season, water is transferred from the river or groundwater source to supply channels, and then to head ditches. Water is then supplied to the irrigation furrows via siphon from the head ditches. Once the water has transited the field, it empties into the tail ditch and runs off into the main tail drains. The return channel takes the water back to a pump that lifts the water either into the storage ponds or back into the head ditch. The cycling of water around the irrigation network occurs within a 12-h period, and the return water is stored until required for a subsequent irrigation.

|
Water sampling and measurements were made at ACRI during the 2013–14 Australian cotton season. Samples were taken over 1-week periods in October, November and December 2013. These are the first 3 months of the season and they coincide with land preparation, sowing and fertilising; active growth phase and complete uptake of the fertiliser N by cotton crop; and the final crop irrigation. The blocks, field drains, channels and storage ponds throughout the irrigation network were sampled in triplicate on an ad hoc basis coinciding with the farm irrigation schedule for the sampling period (Fig. 1).
Dissolved nitrate, organic nitrogen and nitrous oxide in the irrigation network
Electrical conductivity (EC), pH and temperature of the water were measured in situ with a WP-81 field meter (TPS, Brendale, Qld). Samples were filtered (0.45 µm) and analysed for NO3-N, total ammonia N (TAN) and total dissolved N (DTN). Total N (TN) was determined on unfiltered samples; N > 0.45 μm equates to TN – DTN. Nitrate-N and TAN were measured by the cadmium reduction method (Method 4500 Nitrate) and automated phenate method (Method 4500 Ammonia G), respectively (Rice et al. 2012). Samples of TN and DTN were digested by using the persulfate method (Method 4500-N) and the NO3-N concentration in the digest was measured using the cadmium reduction method (Rice et al. 2012). Dissolved organic N (DON) was determined by subtracting mineral N (NO3-N and TAN) from TDN. The calculated DON values, except for six samples (mean –0.2, standard error 0.03) were always >0. After sampling, water samples were stored at 4°C, and analysed within 4–7 days of collection. The detection limit of the NO3-N and TAN analysis was 0.02 mg N L–1.
Dissolved N2O (N2O-Nd) concentrations were determined by using the headspace equilibrium technique (Weiss and Price 1980; Roper et al. 2013). Briefly, during field sample collection, a 6-mL unfiltered water sample was injected into an evacuated 12-mL Exetainer (Labco, Lampeter, UK) and stored at 2−4°C, then returned and analysed in the laboratory within 4–7 days of collection. Harrison and Matson (2003) showed that after 12–48 h there is limited consumption or production of N2O. However, at the longer storage times used in this study, some consumption or production of N2O may have occurred but it is expected to be limited because at temperatures <4°C denitrification is limited (Nowicki 1994). Prior to analysis, samples were allowed to warm to room temperature (~25°C) and 10 mL of helium was injected into each Exetainer. The N2O concentration of the headspace was then measured with a GC-2014 fitted with an electron capture detector (Shimadzu, Kyoto, Japan). The temperature of the laboratory was recorded during sample analysis by an EL-USB-2 data logger (Lascar Electronics, Whiteparish, UK) and used to calculate the N2O-Nd concentration in the analysed water sample, using the approach of Weiss and Price (1980) and Roper et al. (2013).
Nitrous oxide emissions: direct (terrestrial cropping area) and indirect (irrigation network) emissions
To enable the comparison of the direct and indirect emissions, the report fluxes are relative to the source. Thus, the direct emissions are the function of area (ha) of land surface and the indirect emissions are in terms of area of irrigation network.
Direct (terrestrial cropping area) N2O emissions
Direct (or terrestrial) N2O emissions were calculated from the equation of Macdonald et al. (2015):

where x is the fertiliser rate, and in this case the average rate was 200 kg N ha–1.
Indirect (irrigation network) N2O emissions
The N2O flux from the irrigation network surface (13 ha) was calculated using (i) dissolved N2O concentrations (Cole and Caraco 2001; Clough et al. 2007) and (ii) IPCC emission factors (IPCC 2006).
(i) Dissolved N2O method. Indirect N2O fluxes (N2O-Ndf) were estimated from N2O-Nd concentrations according to Eqn 2:

where N2O-Nd(water) (μmol m–3) is the measured concentration of N2O in the water, N2O-Nd(eq) (μmol m–3) is the concentration the water would have if it were in equilibrium with the atmosphere N2O concentration, and k is the gas transfer coefficient (m s–1) (Cole and Caraco 2001; Clough et al. 2007).
The gas transfer coefficient, ktotal, was calculated as the sum of the transfer velocities attributed to wind (kwind m s–1) and water (kwater m s–1) speed, and these were calculated using Eqns 3 and 4 (Clough et al. 2007; Wanninkhof 1992):


where u10 (m s–1) is the wind speed at 10 m above the height of the water body; Sc (dimensionless) is the Schmidt number for N2O; D (m2 s–1) is the temperature- and salinity-dependent diffusion coefficient of N2O in water; U (m s–1) is the velocity of water, which was measured using an OTT Flow Meter (OTT, Kempton, Germany); and h (m) is the average depth of the water body. Where water speed was unavailable, kwind was used instead of ktotal.
The wind speed at 10 m height was calculated from measured wind speeds (ACRI weather station) by using the logarithmic wind profile law (Eqn 5):

where Z0 is the ‘effective roughness height’, here assumed to be 0.001 m; and U1 and U2 (m s–1) are the wind speeds at heights Z1 and Z2, respectively (Kubik et al. 2011). Sc and D were calculated in R, using the package ‘marelac’, from measured water salinity and temperature and atmospheric pressure (R Development Core Team 2015; Soetaert et al. 2014).
The average daily N2O-Ndf flux (kg N2O-N m–1 ha–1) was calculated by using Eqn 2. During the irrigation season, the period for which the irrigation network contains tail water is ~15 days.
(ii) IPCC EF5 method. The default IPCC emission factor for leaching and runoff (EF5) of 0.0075 has three components: emission factors for groundwater and surface drainage (EF5g = 0.0025), rivers (EF5r = 0.0025) and estuaries (EF5e = 0.0025) (IPCC 2006). Given that water for cotton irrigation usually remains on site, the EF5g was used to calculate the indirect emissions using Eqn 6:

where  (mg L–1) is the average concentration of TN in the tail and main tail drain water during each of the first four irrigations; v is the volume of water discharging into the field per irrigation (assumed to be 250 000 L ha–1, which is 25% efficiency of a 100-mm application); n is the number of irrigations within the month; and A is the surface area of the irrigation network (here 13 ha) and B the irrigation area (here 188 ha).
(mg L–1) is the average concentration of TN in the tail and main tail drain water during each of the first four irrigations; v is the volume of water discharging into the field per irrigation (assumed to be 250 000 L ha–1, which is 25% efficiency of a 100-mm application); n is the number of irrigations within the month; and A is the surface area of the irrigation network (here 13 ha) and B the irrigation area (here 188 ha).
All indirect losses are reported on the water surface area and the direct emissions on the land surface area.
Data analyses
All analyses were performed in R (R Development Core Team 2015). Analyses of variance were used to examine influenced by location and sampling time on the measured parameters (EC, pH, TN, NO3–, and N2O-Nd) using the model: water chemistry = location + sampling time.
Linear regression was used to determine (i) the relationship between N2O concentration and the other water chemistry parameters, and (ii) the relationship between the two different methods used to calculate indirect N2O emissions. Where data did not meet assumptions of equal variance, generalised least-squares procedures (in the ‘nlme’ package) were used as an alternative (Hay-Jahans 2011; Pinheiro et al. 2015).
Results
Water chemistry: EC, pH and nitrogen species in the irrigation network
There was a significant effect of location and month on the distribution of EC, pH, TN and NO3-N of the water sampled (Tables 1–4) Values of EC, TN, NO3-N, DTN and N > 0.45 μm all increased after the irrigation water transited the field. Conversely, the pH of the water decreased during the transit (Table 1). Throughout the season, the EC and concentrations of the different N species followed a similar pattern, with EC, TN, NO3-N, DTN and DON peaking during December (Table 2). The water chemistry of the tail water shows that the concentration of N in the DON fractions was often as large as the NO3-N fraction (Tables 1 and 2). There was a positive correlation between EC and NO3– concentration of the discharge water (P < 0.001, r2 = 0.51).

|
Dissolved N2O-N concentration
The N2O-Nd concentrations followed a similar pattern to that of the other N species with time of different sampling and locations, but concentrations were highly variable. Differences in N2O-Nd concentration due to location were not significant (Tables 1 and 3) despite N2O-Nd concentrations tending to increase in the tail ditch and the main tail drain. Average concentrations of N2O-Nd ranged from 0.395 ± 0.045 µg L–1 (supply channel) to 2.15 ± 1.34 µg L–1 (tail drain) in the irrigation network for the 3 months of measurement.
Indirect N2O emissions
Dissolved N2O method. The cumulative N2O-N loss from the irrigation water surface during the first four irrigations between October and December 2013 was 0.503 ± 0.338 kg ha–1 (Table 5).

|
IPCC EF5 method. Average total N concentrations for water sourced from tail ditches and main tail drains over the four irrigations was 28.96 ± 6.903 mg L–1. This corresponded to a cumulative N2O-N emission of 0.843 ± 0.022 kg ha–1 from the irrigation water surface, representing a field leaching loss of 23.31 ± 0.61 kg N ha–1 during the first four irrigations between October and December 2013 (Table 5).
There was a strong, positive linear relationship between monthly N2O fluxes calculated by using the IPCC EF5g and dissolved N2O methods (P < 0.05, R2 = 0.99). However, the disparity between the two methods increased with higher N2O emissions, and total N2O emissions estimated using the IPCC method were 46% higher than under the dissolved method.
Land-surface direct N2O-N emissions
During the cotton season, direct emissions of N2O-N from the land surface were, on average, 16 g N2O-N ha–1 day–1 (Macdonald et al. 2015). The cumulative direct N2O-N emission off the entire cotton farm over the season (150 days) was 2.42 kg N2O-N ha–1. During the period of indirect measurements (90 days), the direct N2O-N emissions off the cotton farm were 1.45 kg ha–1.
Discussion
Electrical conductivity, pH, dissolved nitrate and organic nitrogen in the irrigation network
The water chemistry of the irrigation water was modified during its transit through the cotton field (Table 1). Nitrate and DON were the main components of TN present in the irrigation water, and both N species were lost from the cotton field (Table 1). The measured NO3-N concentrations are similar to those from studies within the Australian cotton industry (Mchugh et al. 2008; Weaver et al. 2013) and other irrigated cropping systems (Harrison et al. 2005). Salt and other nutrients accumulate as a result of evaporation from the furrow surface (Noborio et al. 1996) and are remobilised during the first flush at the beginning of an irrigation. Irrigated furrows were less saline than non-irrigated furrows, suggesting that movement of water from irrigated to skip furrows transits through the adjacent hill, removing salts and N, which are then lost via runoff (Fig. 2). The differences in the N concentrations between the sampling events (Table 2) are likely due to the mineralisation or organic N within the hill releasing ammonium and NO3-N, which can be mobilised by the irrigation water.
Further, we observed significant variation in the water N concentration during irrigation and between irrigations. The soil physical and moisture characteristics also vary within each furrow and mound, and as a result, the irrigation water and dissolved N compounds will transit through the soil at different rates. It is evident from the measured concentrations that the flux of the DON pool must be as important as the NO3-N in the measured furrow-irrigated system (Tables 1 and 2). The DON is being sourced from the mound as the water passes through from the irrigated furrow to the skip furrow. DON, like NO3-N, can undergo transformation and conversion into N2O-Nd in the water column and on the sediment surfaces (Nevison 2000; Tiedje et al. 1982). All N species lost into the irrigation system can potentially undergo subsequent transformations to form N2O-Nd within the water column and drain sediments (Nevison 2000; Harrison and Matson 2003).
Dissolved N2O-N
The N2O-Nd in irrigation water may be sourced from N2O produced within the field, or from subsequent denitrification or nitrification reactions in the water column. Irrigated cotton fields provide optimal conditions for denitrification, including microbial available carbon, nitrate and anaerobic environment. The much lower N2O-Nd concentrations in our study than in other studies (Harrison et al. 2005; Outram and Hiscock 2012) are likely due to fields being irrigated when there is a 75-mm water deficit in the soil profile. At such water contents, the formation of N2O in the surface soil would be negligible (Weier et al. 1993; Davidson et al. 2000), and the measured terrestrial atmospheric flux rates in cotton systems at these soil moistures are small relative to those after emissions that occur when irrigation has ceased (Mahmood et al. 2008; Scheer et al. 2013). There is no pool of N2O-N to move from the soil during the irrigation, and typically denitrification and N2O-N emissions occur 1–2 days after the irrigation has ceased. Further, our site is in a semi-arid, irrigated cropping region of Australia, whereas many of the other indirect emissions studies were conducted in areas of higher rainfall (Outram and Hiscock 2012; Risk et al. 2013; Kaushal et al. 2014), which are more conducive to shallow groundwater fluxes of N2O-Nd.
There was no relationship between N2O-Nd and the other N components. This is in contrast to several studies that have demonstrated a relationship between N2O-Nd and NO3-N concentrations (Harrison and Matson 2003; Reay et al. 2005; Beaulieu et al. 2009, 2011; Warneke et al. 2011) or NH4-N concentrations (Xia et al. 2013). Water in the cotton irrigation system is transient, only retained for a short period, due to the cessation of irrigation once mounds are ‘wet up’ and short field lengths (<500 m). There is also no lateral groundwater discharge into the canals.
Runoff from the cotton field at the ACRI experimental farm is negligible within 12 h of the start of the irrigation; however, on commercial farms, field irrigations occur over longer periods, due to the field length exceeding 1000 m. An increase in contact time between water and the soil surface could maximise N2O-Nd production from TN in the irrigation tail water. Further indirect N2O emissions, resulting from N loading in the irrigation water, may continue downstream (e.g. in storage ponds) and as the irrigation networks dry down, neither of which were measured in this study.
Indirect N2O-N emissions: N2O-Ndf and N2O-NEF5g
There was a strong positive relationship between the two methods used to calculated monthly N2O flux. Although both methods gave estimates of N2O emissions within the same order of magnitude, the IPCC method returned an emission rate 65% higher than that calculated by the dissolved N2O method. Differences between the dissolved N2O and IPCC methods may have occurred through the dissolved N2O method underestimating amounts of N2O-N produced from the water surface. Alternatively, there are uncertainties associated with the current IPCC EFs for indirect emissions. Although the current EFs have been reduced from previous estimates owing to large discrepancies between measured and IPCC estimated fluxes (Nevison 2000; Reay et al. 2005; Clough et al. 2007), the range of uncertainty for EF5 is still large, from 0.0005 to 0.025 (IPCC 2006).
European measurements form much of the basis for the IPCC EFs (Reay et al. 2012). Use of local emission factors, or models that account for local climatic conditions, soil characteristics and land management, will then reduce the uncertainty in flux estimates (Reay et al. 2012). A definite need exists to quantify and understand better the processes controlling indirect N2O emissions within the Australian cotton industry. This, in turn, will provide a better platform for policy decisions and discussions of potential mitigation strategies.
Magnitude of indirect emissions
The irrigation network area on a typical irrigated cotton farm may represent only 6.5% of the farm area. Despite having a flux rate per hectare comparable to that of the direct land-surface emissions, at the farm scale, the indirect emissions are a minor component of the N2O inventory. The indirect emissions estimated by both methods, from the whole farm, were ~2.4–4% of the magnitude of direct land-surface emissions and <0.02% of the fertiliser applied (260 kg ha–1) to the farm. These are similar to the values reported by Harrison et al. (2005) for furrow-irrigated wheat production in Mexico.
Sampling and measurement of indirect emissions
A key issue in the determination of indirect emissions on a per-hectare basis is the accurate quantification of the fate of the water within the irrigation network. The tail water in semi-arid, irrigated cotton systems is typically recirculated and re-used on-farm. Tail water is returned to the farm storage, stored briefly (24 h), and mixed with river or groundwater and returned to the fields for the next field irrigation. The duration and location of the tail water storage will change during each day of the irrigation period depending on farm watering requirements. We have assumed that the indirect emission is mainly sourced from the tail water because of the N loading from the field and is equally spread across the irrigation network.
In this study, point measurements of N components and N2O occurred only during the period of an irrigation (<12 h), and they were concentrated at the cotton field. During this time, N that has been leached from the fields may be transformed into N2O, NOx or N2 in the storage ponds and either emitted or assimilated in the water column or in the drain sediments. In addition, although many studies of indirect emissions have focused on the emissions of N2O from the water surface, there may be significant N2O emissions from the sediments once the canals are drained. Sediments can sequester NO3-N from the water column (García-García and Gómez 2009), which can lead to significantly higher indirect emissions if irrigation water is allowed to pond and is not re-used. The complexity of these biogeochemical pathways would explain the large uncertainties associated with estimating N2O emissions by using N2O-Nd concentrations.
Reducing indirect N2O emissions
The key to reducing indirect N2O-N emissions from cotton irrigation networks is to control the N supply to the irrigation water. Improvements in the efficiency of water and N use would reduce the export of total N and, hence, lower the potential for indirect and N2 emissions.
Indirect N2O-N emissions may be reduced by maximising the use of plant-available N already present in the water. The tail water contains large amounts of dissolved N, which could be used to fertilise adjacent fields. Reducing water return-times to the field is likely to increase the amounts of N that can be re-used; however, a better understanding is required of the rates of transformation for optimisation of N recycling in the cotton irrigation network.
Conclusions
Estimates of N2O emissions from the surface waters of a cotton irrigation network are now possible. The concentrations of N2O-Nd and N2O-NEF5g are 0.503 ± 0.338 and 0.843 ± 0.022 kg ha–1 irrigation surface, respectively, over 90 days. Overall, the indirect emissions from the surface of the irrigation network are not a significant component of the N2O inventory for Australian cotton systems, because the irrigation network covers only a small area relative to the entire land surface of the farm. The measurement of indirect emissions from irrigated cotton production is not straightforward, owing to the ad hoc re-use and storage of water. Additional N2O emissions are likely to occur downstream of the field within storages and main tail drains during the irrigation season and as the channels dry down. Nitrogen fertilisation due to the re-use of drainage water and subsequent field N2O-N emission could also contribute to indirect emissions. Overall, the IPCC EF5g and the dissolved N2O indirect flux estimation methods were in agreement, and the EF5g could be used to estimate indirect fluxes provided local calibration was undertaken. The irrigation network is a prime mitigation target for minimising losses of dissolved N components via denitrification. Rapid re-use of N-enriched tail water, reducing N loss via runoff, and improving efficiency of water and N use are potential methods to reduce N losses.
Acknowledgements
This research was funded by the Australian Federal Government Department of Agriculture and Water Resources, Filling the Research Gap Grant ‘Indirect emissions of nitrous oxide from broad-acre irrigated agriculture’ and the Cotton Research and Development Corporation. Many thanks to Seija Tuomi for her help with the water chemistry analyses. We acknowledge the late Ian ‘Rocky’ Rochester for his pioneering cotton nutrition research and advice on this paper. We also thank the CSIRO internal and Soil Research reviewers for their efforts.
References
Beaulieu JJ, Arango CP, Tank JL (2009) The effects of season and agriculture on nitrous oxide production in headwater streams. Journal of Environmental Quality 38, 637–646.| The effects of season and agriculture on nitrous oxide production in headwater streams.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjt12iurc%3D&md5=a0958fd7e6e347abd19141fa6c4034b7CAS | 19244484PubMed |
Beaulieu JJ, Tank JL, Hamilton SK, Wollheim WM, Hall RO, Mulholland PJ, Peterson BJ, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Grimm NB, Johnson SL, McDowell WH, Poole GC, Valett HM, Arango CP, Bernot MJ, Burgin AJ, Crenshaw CL, Helton AM, Johnson LT, O’Brien JM, Pottern JD, Sheibley RW, Sobota DJ, Thomas SM (2011) Nitrous oxide emission from denitrification in stream and river networks. Proceedings of the National Academy of Sciences of the United States of America 108, 214–219.
| Nitrous oxide emission from denitrification in stream and river networks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXms1Kisw%3D%3D&md5=edce586a6fdae573c33c90389d9da1d2CAS | 21173258PubMed |
Benjamin JG, Porter LK, Duke HR, Ahuja LR, Butters G (1998) Nitrogen movement with furrow irrigation method and fertilizer band placement. Soil Science Society of America Journal 62, 1103–1108.
| Nitrogen movement with furrow irrigation method and fertilizer band placement.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXls1Gjtrw%3D&md5=e7869d98258e0084bdd7c61921b7cee8CAS |
Bouwman AF (1994) ‘Method to estimate direct nitrous oxide emissions from agricultural soils.’ (National Institute of Public Health and Environmental Protection: Bilthoven, The Netherlands)
Bouwman AF (1996) Direct emission of nitrous oxide from agricultural soils. Nutrient Cycling in Agroecosystems 46, 53–70.
| Direct emission of nitrous oxide from agricultural soils.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXotlCiuw%3D%3D&md5=5f648d45683c40a80fb498f46f7cde7fCAS |
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philosphical Transactions of the Royal Society B 368, 1–13.
Clough TJ, Buckthought LE, Kelliher FM, Sherlock RR (2007) Diurnal fluctuations of dissolved nitrous oxide (N2O) concentrations and estimates of N2O emissions from a spring-fed river: implications for IPCC methodology. Global Change Biology 13, 1016–1027.
| Diurnal fluctuations of dissolved nitrous oxide (N2O) concentrations and estimates of N2O emissions from a spring-fed river: implications for IPCC methodology.Crossref | GoogleScholarGoogle Scholar |
Cole JJ, Caraco NF (2001) Emissions of nitrous oxide (N2O) from a tidal, freshwater river, the Hudson River, New York. Environmental Science & Technology 35, 991–996.
| Emissions of nitrous oxide (N2O) from a tidal, freshwater river, the Hudson River, New York.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXosVGitA%3D%3D&md5=733d03cc6ee2fe1aab70eb5da113e3a8CAS |
Davidson EA, Keller M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils. Bioscience 50, 667–680.
| Testing a conceptual model of soil emissions of nitrous and nitric oxides using two functions based on soil nitrogen availability and soil water content, the hole-in-the-pipe model characterizes a large fraction of the observed variation of nitric oxide and nitrous oxide emissions from soils.Crossref | GoogleScholarGoogle Scholar |
Ebrahimian H, Liaghat A, Parsinejad M, Playán E (2012) Distribution and loss of water and nitrate under alternate and conventional furrow fertigation. Spanish Journal of Agricultural Research 10, 849–863.
| Distribution and loss of water and nitrate under alternate and conventional furrow fertigation.Crossref | GoogleScholarGoogle Scholar |
Eichner MJ (1990) Nitrous oxide emissions from fertilized soils: summary of available data. Journal of Environmental Quality 19, 272–280.
| Nitrous oxide emissions from fertilized soils: summary of available data.Crossref | GoogleScholarGoogle Scholar |
García-García V, Gómez R (2009) Nitrogen retention in natural Mediterranean wetland-streams affected by agricultural runoff. Hydrology and Earth System Sciences 13, 2359–2371.
| Nitrogen retention in natural Mediterranean wetland-streams affected by agricultural runoff.Crossref | GoogleScholarGoogle Scholar |
Grace P, Shcherbak I, Macdonald B, Scheer C, Rowlings D (2016) Emission factors for estimating fertiliser-induced nitrous oxide emissions from clay soils in Australia’s irrigated cotton industry. Soil Research 54, 598–603.
| Emission factors for estimating fertiliser-induced nitrous oxide emissions from clay soils in Australia’s irrigated cotton industry.Crossref | GoogleScholarGoogle Scholar |
Harrison J, Matson P (2003) Patterns and controls of nitrous oxide emissions from waters draining a subtropical agricultural valley. Global Biogeochemical Cycles 1, 1080–1093.
Harrison JA, Matson PA, Fendorf SE (2005) Effects of a diel oxygen cycle on nitrogen transformations and greenhouse gas emissions in a eutrophied subtropical stream. Aquatic Sciences 67, 308–315.
| Effects of a diel oxygen cycle on nitrogen transformations and greenhouse gas emissions in a eutrophied subtropical stream.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtF2msb3L&md5=b212edf5c50a0a971476ba8afaeab8ddCAS |
Hay-Jahans C (2011) ‘An R companion to linear statistical models.’ (CRC Press: Boca Raton, FL, USA)
Hinton NJ, Cloy JM, Bell MJ, Chadwick DR, Topp CFE, Rees RM (2015) Managing fertiliser nitrogen to reduce nitrous oxide emissions and emission intensities from a cultivated Cambisol in Scotland. Geoderma Regional 4, 55–65.
| Managing fertiliser nitrogen to reduce nitrous oxide emissions and emission intensities from a cultivated Cambisol in Scotland.Crossref | GoogleScholarGoogle Scholar |
IPCC (2006) N2O emissions from managed soils, and CO2 emissions from lime and urea application. In ‘IPCC Guidelines for National Greenhouse Gas Inventories’. Vol. 4. Ch. 11. (Institute for Global Environmental Strategies: Hayama, Japan)
Kaushal SS, Mayer PM, Vidon PG, Smith RM, Pennino MJ, Newcomer TA, Duan S, Welty C, Belt KT (2014) Land use and climate variability amplify carbon, nutrient, and contaminant pulses: a review with management implications. JAWRA Journal of the American Water Resources Association 50, 585–614.
| Land use and climate variability amplify carbon, nutrient, and contaminant pulses: a review with management implications.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXovFais7Y%3D&md5=e95272748b43f5eb7c891792010eb3a0CAS |
Kubik ML, Coker PJ, Hunt C (2011) Using meteorological wind data to estimate turbine generation output: a sensitivity analysis. In ‘World Renewable Energy Congress 2011’, Linköping, Sweden. pp. 4074–4081. (Linköping University Press: Linköping, Sweden)
Macdonald BCT, Rochester IJ, Nadelko A (2015) High yielding cotton produced without excessive nitrous oxide emissions. Agronomy Journal 107, 1–9.
| High yielding cotton produced without excessive nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar |
Mahmood T, Ali R, Iqbal J, Robab U (2008) Nitrous oxide emission from an irrigated cotton field under semiarid subtropical conditions. Biology and Fertility of Soils 44, 773–781.
| Nitrous oxide emission from an irrigated cotton field under semiarid subtropical conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlsFCmtLo%3D&md5=ad549b40503292f72c2b360e7542eca3CAS |
Mchugh AD, Bhattarai S, Lotz G, Midmore DJ (2008) Effects of subsurface drip irrigation rates and furrow irrigation for cotton grown on a vertisol on off-site movement of sediments, nutrients and pesticides. Agronomy for Sustainable Development 28, 507–519.
| Effects of subsurface drip irrigation rates and furrow irrigation for cotton grown on a vertisol on off-site movement of sediments, nutrients and pesticides.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhsFekurbI&md5=0340492a9ceb540658d7133dc5b49dedCAS |
Nevison C (2000) Review of the IPCC methodology for estimating nitrous oxide emissions associated with agricultural leaching and runoff. Chemosphere – Global Change Science 2, 493–500.
| Review of the IPCC methodology for estimating nitrous oxide emissions associated with agricultural leaching and runoff.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXos1egsLc%3D&md5=be20b6eaa1520f7aee7192251200e8e6CAS |
Noborio K, McInnes KJ, Heilman JL (1996) Two-dimensional model for water, heat, and solute transport in furrow-irrigated soil: II. Field evaluation. Soil Science Society of America Journal 60, 1010–1021.
| Two-dimensional model for water, heat, and solute transport in furrow-irrigated soil: II. Field evaluation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28Xktl2qs7w%3D&md5=e1a5c2ff27fd05ff86f98449fb112628CAS |
Nowicki BL (1994) The effect of temperature, oxygen, salinity, and nutrient enrichment on estuarine denitrification rates measured with a modified nitrogen gas flux technique. Estuarine, Coastal and Shelf Science 38, 137–156.
| The effect of temperature, oxygen, salinity, and nutrient enrichment on estuarine denitrification rates measured with a modified nitrogen gas flux technique.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXksVaitLc%3D&md5=cb1b8c350c7d68aa962134c0beddc3b0CAS |
Outram FN, Hiscock KM (2012) Indirect nitrous oxide emissions from surface water bodies in a lowland arable catchment: a significant contribution to agricultural greenhouse gas budgets? Environmental Science & Technology 46, 8156–8163.
| Indirect nitrous oxide emissions from surface water bodies in a lowland arable catchment: a significant contribution to agricultural greenhouse gas budgets?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVSqtrnN&md5=f834560096802eb344128cc94464737aCAS |
Pinheiro J, Debroy S, Bates D (2015) nlme: Linear and nonlinear mixed effects models. R package version 3.1–120. R Foundation for Statistical Computing, Vienna. Available at http://CRAN.R-project.org/package=nlme [verified 3 June 2016]
R Development Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf [verified 14 June 2016]
Reay DS, Smith KA, Edwards AC (2003) Nitrous oxide emission from agricultural drainage waters. Global Change Biology 9, 195–203.
| Nitrous oxide emission from agricultural drainage waters.Crossref | GoogleScholarGoogle Scholar |
Reay DS, Smith KA, Edwards AC, Hiscock KM, Dong LF, Nedwell DB (2005) Indirect nitrous oxide emissions: Revised emission factors. Environmental Sciences 2, 153–158.
| Indirect nitrous oxide emissions: Revised emission factors.Crossref | GoogleScholarGoogle Scholar |
Reay DS, Davidson EA, Smith KA, Smith P, Melillo JM, Dentener F, Crutzen PJ (2012) Global agriculture and nitrous oxide emissions. Nature Climate Change 2, 410–416.
| Global agriculture and nitrous oxide emissions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XnsFGht7c%3D&md5=9d734bd888ceeea8b0bb3daa91d30b1eCAS |
Rice EW, Caird RB, Eaton AD, Clesceri LS (2012) ‘Standard methods for the examination of water and wastewater.’ 22nd edn. (American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC)
Risk N, Snider D, Wagner-Riddle C (2013) Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Canadian Journal of Soil Science 93, 401–414.
| Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhslels7fP&md5=5c87a21542a4a5d4e0efca0f7757eb4dCAS |
Rochester IJ (2003) Estimating nitrous oxide emissions from flood-irrigated alkaline grey clays. Australian Journal of Soil Research 41, 197–206.
| Estimating nitrous oxide emissions from flood-irrigated alkaline grey clays.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXktFKisrw%3D&md5=3a475561eea619e333bdd85ec4b7f899CAS |
Rochester IJ (2011) Assessing internal crop nitrogen use efficiency in high-yielding irrigated cotton. Nutrient Cycling in Agroecosystems 90, 147–156.
| Assessing internal crop nitrogen use efficiency in high-yielding irrigated cotton.Crossref | GoogleScholarGoogle Scholar |
Rochester IJ (2012) Using seed nitrogen concentration to estimate crop N use-efficiency in high-yielding irrigated cotton. Field Crops Research 127, 140–145.
| Using seed nitrogen concentration to estimate crop N use-efficiency in high-yielding irrigated cotton.Crossref | GoogleScholarGoogle Scholar |
Rochester I, Ceeney S, Maas S, Gordon R, Hanna L, Hill J (2009) Monitoring nitrogen use efficiency in cotton crops. The Australian Cottongrower 30, 42–43.
Roper JD, Burton DL, Madani A, Stratton GW (2013) A simple method for quantifying dissolved nitrous oxide in tile drainage water. Canadian Journal of Soil Science 93, 59–64.
| A simple method for quantifying dissolved nitrous oxide in tile drainage water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXjvVWqsr4%3D&md5=1d06429f1c77d862b481e23388ac6639CAS |
Scheer C, Grace PR, Rowlings DW, Payero J (2013) Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management. Nutrient Cycling in Agroecosystems 95, 43–56.
| Soil N2O and CO2 emissions from cotton in Australia under varying irrigation management.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitVOjsr8%3D&md5=428d497322a79f52965f9aeb6e3e7a96CAS |
Soetaert K, Petzoldt T, Meysman F (2014) marelac: Tools for Aquatic Sciences. R package version 2.1.4. R Foundation for Statistical Computing, Vienna. Available at https://cran.r-project.org/web/packages/marelac/vignettes/marelac.pdf [verified 3 June 2016]
Soil Survey Staff (2010) ‘Keys to Soil Taxonomy.’ 11th edn. (Natural Resources Conservation Service of the United States Department of Agriculture: Washington, DC)
Syakila A, Kroeze C (2011) The global nitrous oxide budget revisited. Greenhouse Gas Measurement and Management 1, 17–26.
| The global nitrous oxide budget revisited.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsValsr%2FL&md5=3e1418c4c151854072e535a43f7a40baCAS |
Tiedje JM, Sexstone AJ, Myrold DD, Robinson JA (1982) Denitrification: ecological niches, competition and survival. Antonie van Leeuwenhoek 48, 569–583.
| Denitrification: ecological niches, competition and survival.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL3s7nslShsw%3D%3D&md5=ebaf7ca7f2407229a689447ddf040cf7CAS | 6762848PubMed |
Wanninkhof R (1992) Relationship between wind speed and gas exchange over the ocean. Journal of Geophysical Research 97, 7373–7382.
| Relationship between wind speed and gas exchange over the ocean.Crossref | GoogleScholarGoogle Scholar |
Warneke S, Schipper LA, Bruesewitz DA, Baisden WT (2011) Rates, controls and potential adverse effects of nitrate removal in a denitrification bed. Ecological Engineering 37, 511–522.
| Rates, controls and potential adverse effects of nitrate removal in a denitrification bed.Crossref | GoogleScholarGoogle Scholar |
Weaver TB, Hulugalle NR, Ghadiri H, Harden S (2013) Quality of drainage water under irrigated cotton in vertisols of the lower Namoi Valley, New South Wales, Australia. Irrigation and Drainage 62, 107–114.
| Quality of drainage water under irrigated cotton in vertisols of the lower Namoi Valley, New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Weier KL, Doran JW, Power JF, Walters DT (1993) Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Science Society of America Journal 57, 66–72.
| Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXisFeqtLk%3D&md5=fd56bf11bee9b78d91071f533d1e8f08CAS |
Weiss RF, Price BA (1980) Nitrous oxide solubility in water. Marine Chemistry 8, 347–359.
| Nitrous oxide solubility in water.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXktFaku7g%3D&md5=17d31b723705da8aef4da69d81c78272CAS |
Xia Y, Li Y, Li X, Guo M, She D, Yan X (2013) Diurnal pattern in nitrous oxide emissions from a sewage-enriched river. Chemosphere 92, 421–428.
| Diurnal pattern in nitrous oxide emissions from a sewage-enriched river.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXitlajurg%3D&md5=4493dce475662f19cc0b3f2032922baeCAS | 23402918PubMed |