Micromorphological evidence for mineral weathering pathways in a coastal acid sulfate soil sequence with Mediterranean-type climate, South Australia
R. M. Poch A C , B P. Thomas B C , R. W. Fitzpatrick B C and R. H. Merry B CA Departament de Medi Ambient i Ciències del Sòl, University of Lleida, Catalonia 25198, Spain.
B CSIRO Land & Water/University of Adelaide, Private Bag No. 2, Glen Osmond, SA 5064, Australia.
C Corresponding authors. Emails: brett.thomas@csiro.au; rob.fitzpatrick@csiro.au; richard.merry@csiro.au; rosa.poch@macs.udl.cat
Australian Journal of Soil Research 47(4) 403-422 https://doi.org/10.1071/SR07015
Submitted: 23 January 2008 Accepted: 26 March 2009 Published: 30 June 2009
Abstract
Soil micromorphology, using light microscopy and scanning electron microscopy (SEM), was used to describe detailed soil morphological and compositional changes and determine mineral weathering pathways in acid sulfate soils (ASS) from the following 2 contrasting coastal environments in Barker Inlet, South Australia: (i) a tidal mangrove forest with sulfidic material at St Kilda, and (ii) a former supratidal samphire area at Gillman that was drained in 1954 causing sulfuric material to form from sulfidic material. Pyrite framboids and cubes were identified in sulfidic material from both sites and are associated with sapric and hemic materials. Gypsum crystals, interpreted as a product of sulfide oxidation, were observed to have formed in lenticular voids within organic matter in the tidal mangrove soils at St Kilda. Sulfide oxidation was extensive in the drained soil at Gillman, evidenced by the formation of iron oxyhydroxide pseudomorphs (goethite crystallites and framboids) after pyrite and jarosite, and of gypsum crystals. Gypsum crystals occur where a local source of calcium such as shells or calcareous sand is present. Sporadic oxidation episodes are indicated by the formation of iron oxide and jarosite coatings around coarse biogenic voids. These observations indicate that mineral transformation pathways are strongly influenced by soil physico-chemical characteristics (i.e. oxidation rate, Eh, pH, soil solution chemistry, mineralogy, and spatial distribution of sulfides). This information has been used to illustrate the interrelationships of pyrite, carbonate, gypsum, jarosite, and organic matter and help predict soil evolution under changing hydro-geochemical, redoximorphic, and thermal conditions in soils from coastal environments.
Additional keywords: mineralogy, micromorphology, organic materials, soil classification, pyrite.
Introduction
Acid sulfate soils (ASS) are soils in which sulfuric acid may be produced, is being produced, or has been produced in amounts that have a lasting effect on main soil characteristics (Pons 1973). This general definition includes potential, active (or actual), and post-active ASS, 3 broad genetic kinds that continue to be recognised (e.g. Fanning 2002). ASS form in coastal estuarine and mangrove swamp environments because these waterlogged or highly reducing environments are ideal for the formation of sulfide-containing minerals, predominantly iron pyrite (FeS2). Soil materials that contain sulfides are called sulfidic materials (Isbell 1996) and can be environmentally damaging if exposed to air by disturbance. Exposure results in the oxidation of pyrite, with each mole of pyrite yielding 4 moles of acidity (i.e. 2 moles of sulfuric acid). This process transforms sulfidic material to sulfuric material when, on oxidation, the material develops a pH of ≤4 (Isbell 1996; note that a sulfuric horizon has a pH of ≤3.5 according to Soil Survey Staff 2003).
In Australia, ASS occupy an estimated 215 000 km2, of which 58 000 km2 is coastal and 157 000 km2 is inland. In the coastal zone, 41 000 km2 of ASS is exposed at some point during the tidal cycle, with the remaining 17 000 km2 permanently subaqueous (Fitzpatrick et al. 2008a). These soils commonly underlie coastal estuaries and tidal flats close to where most Australians live. Research into these soils in Australia has largely been limited to tropical and subtropical regions of eastern Australia. In South Australia, with its Mediterranean climate, ASS have different environmental processes operating on them and different properties. They pose different environmental hazards requiring tailored management options. In South Australia, coastal ASS with sulfuric and sulfidic materials occupy ~2410 km2 and have an estimated acid reservoir of 2 Mt (Fitzpatrick et al. 2006, 2008b, 2008c). To investigate the processes and environmental hazards in Mediterranean coastal ASS, we studied the highly degraded wetland at Gillman, adjacent to the city of Port Adelaide in the Barker Inlet, 20 km north of Adelaide and covering an area of ~25 km2 (Fig. 1). From 1954, about 1000 ha of coastal wetland has been progressively drained for urban and industrial development by the construction of levee banks, and a network of drains and tidal floodgates (Fig. 1) (Thomas and Fitzpatrick 2006). The levee bank subdivided the intertidal environment so that initial conditions were similar on both sides of the bank. Subsequently, within the bunded area, drainage and acidification degraded ~460 ha of the land at Gillman (Thomas et al. 2003; Fitzpatrick et al. 2008b, 2008c), while outside the bank the undrained soils are approximately in their original condition and provide a baseline for the assessment of environmental change (Fig. 1).

|
Here we describe the 2 undrained sites at St Kilda and a drained site at Gillman and make comparisons between the oxidised near-surface soil horizons and the taxonomically similar but reduced soil horizons lower in the same soil profile (BG11) at Gillman (Fig. 1). Our study objectives were to examine soil profile changes in sulfidic and sulfuric materials and associated relict horizons in the drained supratidal site and 2 undrained intertidal and tidal sites. We use soil micromorphology and scanning electron microscopy (SEM) to describe and characterise the imprints of biological, physical, chemical, and mineralogical processes. We used these techniques to: (i) define the various sources of organic matter fractions (i.e. sapric and histic materials), (ii) identify mineral associations (e.g. pyrite, gypsum, and jarosite), and (iii) describe micro-scale weathering pathways and mechanisms under changing hydrological and biogeochemical conditions.
Review of previous micromorphological studies
Integration of soil micromorphological research (i.e. using light microscopy and scanning electron microscopy) with pedo-geomorphic studies aids in understanding complex contemporary and relict processes in soils (e.g. Rabenhorst and Lindbo 1998; Stoops 2003). However, literature associated with micromorphological and pedo-geomorhic studies of coastal ASS is not extensive in Australia, especially for areas with Mediterranean climates.
The first micromorphological observations of ASS are those of Van Dam and Pons (1973) on ASS from Thailand, Nigeria, and the Netherlands, focusing on the morphology and occurrence of pyrite. They distinguished between primary pyrite scattered in the matrix or in syn-sedimentary organic matter (such as diatoms or foraminifers) and secondary pyrite associated with organic matter. The morphology of primary pyrite structures has also been used as a proxy to identify a variety of redox-controlled cyclic transitions and depositional environments (e.g. Raiswell and Berner 1985; Boesen and Postma 1988; Wilkin and Barnes 1997). In modern euxenic environments the mean framboid diameters are generally smaller (below 10 µm) and have a narrower size range than for oxic and dysoxic environments (Wilkin et al. 1996). However, the dynamics of these coastal systems, where sediments are subject to daily and seasonally oscillating environmental determinants, mean that caution is needed when using pyrite structure morphologies as a proxy for interpreting the redox conditions of different depositional environments (Roychoudhury et al. 2003). Secondary pyrite often occurs as clusters of pyrite framboids, 20–50 μm in diameter, or scattered framboids when the organic matter decays (Van Dam and Pons 1973). Oxidation proceeds from crack walls (planar voids) into the peds, with the first oxidation products being poorly crystalline iron oxyhydroxides that precipitate along ped walls, impregnating the matrix and producing iron hypocoatings (Van Dam and Pons 1973; Bullock et al. 1985).
Van Dam and Pons (1973) noted that iron oxidation products are usually poorly crystalline iron oxyhydroxides, but can also include crystalline goethite and hematite whenever a dry period permits dehydration of soils. Where calcium is available, gypsum may precipitate in pores as pseudo-rhombohedral crystal infillings or coatings, or as hypo- or quasi-coatings of pores (Van Dam and Pons 1973; Bullock et al. 1985). When calcium is provided by inundation waters, gypsum precipitates above jarosite layers. Cryptocrystalline silica (opal) can precipitate in close proximity to pyrite, and is thought to be derived from weathering of layer aluminosilicate minerals.
Miedema et al. (1974) observed the process of oxidation of pyrite from the outer edge to the inside of the framboids, noting that the first products are poorly crystalline iron oxyhydroxide hypocoatings and pseudomorphs after pyrite framboids, which sometimes have a remnant nucleus of pyrite. In non-calcareous environments, if oxidation proceeds, the pH decreases sufficiently (i.e. pH < 4) that both jarosite and various Fe-oxyhydroxides form. Jarosite may occur immediately adjacent to Fe-oxyhydroxides in similar forms, i.e. as pseudomorphs after framboids but mainly in voids or as hypocoatings and quasicoatings. Fe-hypocoatings may be caused by iron hydrolysis after dissolution of jarosite as H+ is leached. This process also releases Fe3+; therefore, the Fe oxides are immobile and precipitate near jarosite. When the oxidation is faster, the intermediate stage of Fe-oxide formation does not occur and jarosite is formed immediately after oxidation of pyrite to form jarosite pseudomorphs after pyrite framboids.
In calcareous environments, gypsum precipitates as coatings and infillings in pores (Miedema et al. 1974). The distinction between calcareous and non-calcareous environments was shown to be a more rapid precipitation of Fe oxides in the former; therefore, those authors considered the Fe-hypocoatings to be a redoximorphic feature in those soils.
Studies describing the distribution, formation, and chemical and physical properties of ASS in Australia have mainly focused on subtropical to tropical coastal ASS in New South Wales and Queensland (e.g. Willett and Walker 1982; Lin and Melville 1992; White et al. 1993, 1997; White and Melville 1993; Sammut et al. 1994, 1995, 1996; Ahern et al. 1998, 2000). Few published studies have involved coastal ASS from southern Australia, with a Mediterranean climate (e.g. Fitzpatrick et al. 1993; Thomas et al. 2004) characterised by extended hot, dry summers and cool, wet winters. The present study involves a natural and a drained mangrove environment that was until recently under marine-dominated tidal conditions, the only similar study being from East Trinity (Hicks et al. 1999). Most studies are of brackish estuary floodplain and back-swamp environments that were under mangrove vegetation at some time during the Holocene with only minor tidal influences and minimal flushing with sea water (Sammut et al. 1996). Micromorphological studies of ASS in Australia have been conducted by Willett et al. (1992), Fitzpatrick et al. (1993, 1996), Bush and Sullivan (1996, 1998, 1999), and Poch et al. (2004).
Bush and Sullivan (1999) described the morphology, distribution, and composition of pyrite in Holocene estuarine sediments from the north coast of NSW, using SEM; all pyrite (i.e. framboids associated with organic remnants and scattered pyrite in the matrix) was formed in situ, due to microbiological activity, to dislodgement from framboids, or formation from diffusion. Bush and Sullivan (1999) emphasised the importance of soil microscopic features (i.e. coatings of Fe monosulfides, organic matter or aluminosilicates, porosity, and the distribution of pyrite) in the understanding of ASS processes (e.g. estimating oxidation rate and acidification potential of sulfidic materials, which is critical in managing ASS). Sullivan and Bush (2004) further described the micromorphology of iron oxide precipitates in degraded coastal ASS environments and related their mineralogy to water quality.
Fitzpatrick et al. (1993) briefly described the micromorphology of ASS in mangrove swamps at St Kilda, using SEM and TEM analyses, and made comparisons with inland ASS from South Australia and coastal ASS from the Northern Territory. Poch et al. (2004) described a preliminary, thin-section micromorphological study of sulfidic and sulfuric materials in coastal ASS in southern Australia. The present study extends the studies of Poch et al. (2004), Fitzpatrick et al. (1993), and Bush and Sullivan (1999).
Materials and methods
Landscape
The natural elevation of the Barker Inlet region ranges from –1.0 m AHD (Australian Height Datum, 0.0 m AHD approximates mean sea level) in tidal creek channels, to 2.5 m AHD on undulating mounds between tidal creeks. The climate is Mediterranean (cool to mild, wet winters and extended hot, dry summers). Mean annual rainfall is 470 mm, falling mainly between May and September. The high potential evaporation demand (1760 mm/year) exceeds rainfall by almost 4 to 1, and accordingly, local groundwater movement is predominately vertical, with very low lateral seepage rates of 0.3–0.6 m/year (Pavelic and Dillon 1993).
The recent geological evolution of the Barker Inlet region has largely been controlled by global sea level fluctuations (Edmonds 1995). The Holocene St Kilda Formation overlies the Glanville Formation (Pleistocene), which together on-lap the thick alluvial Hindmarsh Clay Formation (Belperio and Rice 1989). Reworking of coastal sediments since sea level stabilisation ~7500 BP resulted in the northerly extension of Le Fevre Peninsula and the Port River outlet (Fig. 1) (Bowman and Harvey 1986). The establishment of extensive seagrass meadows led to the rapid accumulation of marine and estuarine sediments, resulting in coastal progradation throughout the late Holocene (Edmonds 1995). Progradation led to the simultaneous back-barrier development of mangrove swamps, samphire salt marshes, and sabkhas parallel to the shoreline. The embayment is now mostly in-filled except for the Port River estuary. Subsidence rates of 1 mm/year have been documented in the Barker inlet area (Belperio 1993) and are attributed to movement along the Para Fault, ground water extraction, and consolidation of intertidal soils after drying due to bunding (construction of levee banks) (Fig. 1).
The coastal ASS sequences studied are formed in modern intertidal and mangrove swamp deposits. They overlie unconsolidated Holocene coastal marine sediments (St Kilda formation) consisting of saturated, light grey, shelly, and often silty or clayey sands. The morphological, chemical, and mineralogical characteristics are given by Fitzpatrick et al. (1993, 2008b, 2008c), Fitzpatrick and Self (1997), and Thomas et al. (2004). The following 3 ASS were selected for detailed study: (i) profiles 600 and 2610 in a mangrove tidal area at St Kilda, and (ii) profile BG11 in the drained (bunded) supra-tidal samphire area at Gillman (Fig. 1).
St Kilda study area
Profile 600 (34°44′47.00″E, 138°32′15.30″S) is 250 m from the low tide mark at the seaward fringe of the mangrove forest (Fig. 1). This profile supports samphire vegetation and is shallow (up to 0.5 m thick). It consists of a hemic Oa horizon and a sapric C horizon. Profile 2610 (34°44′51.80″E, 138°32′12.10″S) is 2 m thick and 50 m from the low tide mark near the seaward fringe of the mangrove forest (Fig. 1). This profile is close to the coastline and more affected by tides. However, it is affected by more reduced redox conditions than profile 600 (Thomas et al. 2004). It also has hemic materials at the surface (Oa1) and sapric materials at depth (Oa2, Oa3 and C). A detailed schematic soil-landscape cross section displaying localities of both soil profiles in relation to vegetation, geology and soil types is shown in Thomas et al. (2004). Detailed macromorphological descriptions are given in Table 1.
|
Gillman study area
Profile BG11 (34°49′44.82″E, 138°32′40″S) originally developed in a supratidal regime but tidal inundation was totally excluded in 1954 when a bund wall or levee bank was constructed causing oxidation of sulfidic materials. The parent material consists of several layers of lacustrine and tidal sediments deposited under mangrove and samphire vegetation. The quart-rich sands of an old chenier dune system cover a large portion of this area and have a low acid neutralising capacity. Soil pH (field) values <2 were recorded for oxidised soil materials (Thomas et al. 2004). Detailed schematic soil-landscape cross sections at Gillman in the Barker Inlet displaying vegetation, geology, hydrology and soil types are shown in Thomas et al. (2004) and Fitzpatrick et al. (2008b, 2008c).
Sample collection for chemical and mineralogical analysis
Where possible, soil pits were dug to a depth of ~0.5–1.5 m and a gouge auger was used to sample soils down to 3 m. Samples for chemical analysis were either placed in plastic bags with the air removed and double bagged, or placed in screw-top plastic jars filled to capacity. They were then stored in insulated containers and frozen within 8 h of collection. Samples were rapidly oven-dried at 80°C for ASS analysis (Ahern et al. 1998) and dried at 40°C for chemical and mineralogical analyses (Rayment and Higginson 1992).
Sample collection for micromorphology
Horizontal and vertical thin sections were made from undisturbed soil blocks of surface and subsurface soil horizons using the method of Salins and Ringrose-Voase (1994). Soil blocks were collected from two depth intervals in soil profiles 600 (0–0.12 and 0.12–0.32 m) and 2610 (0–0.08 and 0.08–0.30 m). Undisturbed soil blocks were collected using Brewer tins from 5 depth intervals from soil profile BG11 (0.02–0.15, 0.35–0.48, 0.50–0.65, 0.75–0.88, 0.92–1.08, and 1.46–1.61 m). The study and description of the thin sections followed the guidelines and terminology of Stoops (2003). SEM and EDAX studies were made on carbon-coated polished blocks, thin sections, and rough-surfaced soil fragments glued onto stubs. TEM studies were previously carried out on selected clay fractions by Fitzpatrick and Self (1997).
Soil descriptions
A representative profile face in the pit or on a cleaned core face was used to describe each soil profile according to Soil Taxonomy (Soil Survey Staff 2003) and the Australian Soil and Land Survey Field Handbook (McDonald et al. 1990). Macromorphological description and main chemical characteristics of profile BG11 are presented. The following morphological features were described: horizon thickness (m), horizon type (using nomenclature from Soil Survey Staff 2003), and horizon boundary and field texture (McDonald et al. 1990).
Laboratory analyses
Soil pH was measured by direct insertion of a glass spear point combination pH electrode into the (saturated) soil. Samples were incubated for 7 weeks according to the method for determining the presence of sulfidic material described in Soil Taxonomy (Isbell 1996; Soil Survey Staff 2003). Laboratory analyses included electrical conductivity (EC) and pH in a 1 : 5 soil : water extract, hydrogen peroxide-treated pH, titratable acidity, and neutralising capacity (Ahern et al. 1998). Samples were analysed for total carbon and sulfur by high frequency induction furnace with infrared detection (Rayment and Higginson 1992). Total reduced inorganic sulfur was determined by chromium reduction (Sullivan et al. 1999) and is referred to as chromium-reducible sulfur (SCR). A Philips PW1800 microprocessor-controlled X-ray diffractometer using Co-Kα radiation (XRD) was used to confirm mineralogy on finely ground samples.
Results and discussion
Organic matter characteristics and pyrite formation in the intertidal mangrove area, St Kilda
Macromorphology and soil classification
Profiles 600 and 2610 are permanently saturated and occur in intertidal areas with mangroves. They both overlie unconsolidated Holocene coastal marine sediments consisting of saturated light grey, shelly, and often silty or clayey sands (St Kilda formation). They both contain soil materials that qualify as sulfidic material (Tables 1 and 2) because they have a natural pH 7.5 and when incubated as a layer 10 mm thick under moist conditions, while maintaining contact with the air at room temperature, showed a marked drop in pH to ≤4 or less within 8 weeks (Isbell 1996; Soil Survey Staff 2003).

|
These soils contain horizons with different kinds of organic soil materials as defined by the amount of rubbed fibre content (sapric has <17% by volume rubbed fibre, hemic >40% rubbed fibre, and fibric >75% rubbed fibre) (Soil Survey Staff 2003). Hence, horizons that contain predominantly sapric material have a high degree of decomposed plant materials (from which the organic materials are derived).
Profile 600 consists of a hemic Oa horizon overlying a sapric C horizon and classifies as a Terric Sulfihemist (Soil Survey Staff 2003) and Protothionic Histosol (FAO 1998). Profile 2610 consists of a thin layer of hemic material at the near surface (Oe1) with thick underlying layers of sapric material (Oa2, Oa3, and Ca) and classifies as a Terric Sulfisaprist (Soil Survey Staff 2003) and Protothionic Histosol (FAO 1998). Both profiles classify as Histic-Sulfidic Intertidal Hydrosols according to the Australian Soil Classification (Isbell 1996).
Micromorphology
Detailed micromorphological descriptions are presented in Table 3. In the sulfidic material of both profiles, pyrite framboids were identified with different degrees of fragmentation, embedded in the sapric material (Fig. 2d). Sulfur from sea water is the major source of sulfur, and sulfur transformations result in the accumulation of organic sulfur in the sapric material. Availability of Fe is probably the limiting factor for the formation of pyrite in this environment. The fragmentation of the framboids is possibly due to wave and tidal action (Fitzpatrick et al. 1993) and bioturbation (Fig. 3e). Mangroves are dying in localised parts of the intertidal swamp areas near profile 2610 due to the erosion of soil around the base of large trees. Eutrophication has adversely affecting mangrove growth through retardation of pneumatophore growth and retraction from highly reduced areas, and is exacerbated by regional subsidence raising the average tidal water level (Thomas et al. 2004). Eutrophic conditions have developed as the nutrient content of metropolitan seawater has increased, resulting in loss of seagrass (Posidonia sp.) and increased growth of sea lettuce (Ulva spp.), a source of fine organic matter. Increased input of fine (sapric) organic matter washing into the mangrove forest accumulates at profile 2610, where it forms unstable sediments that become extremely reducing as it decomposes (Thomas et al. 2004). This sapric material is trapped from the seawater by the outer mangroves before it reaches profile 600 further inland (Fig. 1).

|
|
|
Near-surface layers with predominantly hemic material
Surface horizons (Oe) of profiles 600 and 2610 have high porosities (80%) and contain a high proportion of fibres. These layers are classified as hemic material because they contain moderately decomposed organic materials. In thin section, many of the recognisable organic residues, which form from rubbed fibre, are anisotropic due to the preservation of cellulose (Figs 2 and 3).
Surface horizons of both profiles contain primarily a mixture of yellowish, amorphous organic matter (highly decomposed organic matter) and recognisable organic tissues and plant organs (less decomposed fibre). The latter are altered to different degrees due to physical breakdown by fauna and chemical alteration, namely the blackening and formation of phlobaphene, a brown tannin product of oxidation that is very resistant to further decomposition (Bullock et al. 1985). Near the surface, profile 2610 has more reddish, phlobaphene-rich organic residues than profile 600.
Subsurface layers with predominantly sapric material
Sapric materials contain less recognisable organic tissue (i.e. fibres) and more yellow, gelatinous, amorphous organic matter (Figs 2a, b and 3a, b). Lee and Manoch (1974) described a brown amorphous matrix in drained sapric material from Wisconsin, with a strong aggregation in pellets due to faunal activity. In both profiles, the sapric material has a lower porosity consisting of vesicles and vughs. This gives the deeper horizons a lower permeability than the near surface layers where hemic materials dominate and give rise to more conducting porosities due to packing pores between fibres. De Coninck et al. (1974) reported that amorphous organic material has several forms that are mixed with clay and have a granular structure due to faunal activity. The amorphous material has a layered-like structure alternating with horizontal tissue and organ residues in the deeper horizons. In some areas cracks have formed, possibly due to desiccation during sample preparation. The amorphous material has a yellowish colour with opaque, highly reflective spots that occur inside yellowish aggregates distant from the pore walls. The coarse material consists of fine muscovite flakes, diatoms, and quartz grains.
Some blackened fibres appear to have transformed to opaque intercalations as they are often found around tissue residues (Fig. 3a, b). These intercalations consist of amorphous black materials. Less frequently, the black material forms hypocoatings in the yellow organic groundmass. Microprobe analysis of the yellow organic groundmass indicates a higher clay content than in the black organic materials. Implications are that the yellow organic materials are older and more stable. A common characteristic is the high Na, Cl, and S contents, indicating the capacity of the amorphous organic matter to scavenge and retain these elements from seawater.
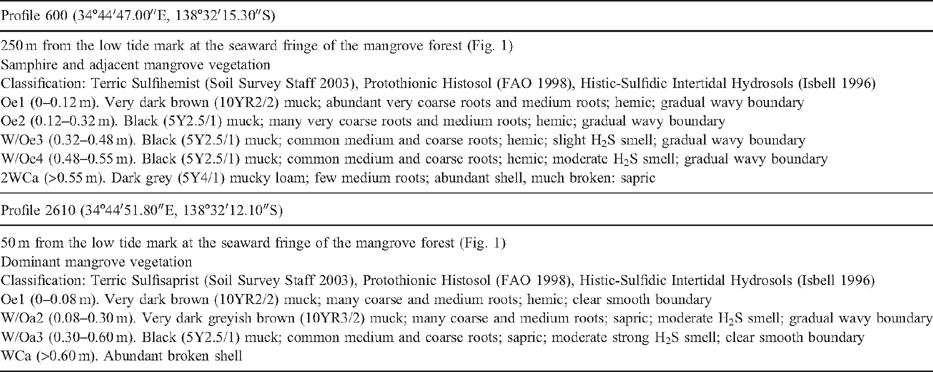
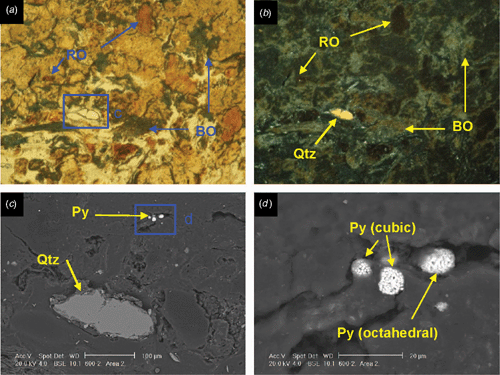
Pyrite crystals and framboids
SEM images of sulfidic material from horizon Oe2 in profile 600 identified pyrite cubes and framboids (maximum diameter 10 μm), embedded and dispersed in organic matter (Fig. 2). Framboids composed of cuboid pyrite crystals exist in close proximity to framboids made up of octahedral pyrite crystals (Fig. 2d). Analysis of the composition of compact and dispersed framboids shows mainly pyrite (Fe2S), with only a few having monosulfide (FeS2-x) composition. This is unexpected given the S/Fe ratio of the organic matter, mostly >2, which should promote the formation of monosulfides. This suggests that Fe may be the limiting factor for the formation of pyrite in these soils and this is also supported by the absence of large framboids (Roychoudhury et al. 2003). A large grain of chalcopyrite was identified in a blackened tissue residue in the sulfidic material. It is likely to be contamination, a primary mineral grain from the crushed rock levee bank ~200 m north of profile 600 sample site. Some inclusions of detrital CaCO3 and halite crystals have been observed in tissue residues.
Larger pyrite framboids (up to 50 μm) are well formed in horizon Oa2 of profile 2610 (Fig. 3). They occur as nests of framboids up to 100 μm wide, but also frequently as loosely packed and compact singular framboids in the organic groundmass. Pyrite cubes range from 0.5 to 2 μm. The occurrence of non-framboidal pyrite was rare. Fine gypsum crystals occur in the groundmass as nests of very fine prismatic crystals up to 2 μm in length within tissue residue (Fig. 3d). The morphology of the prismatic gypsum crystals indicates they have formed in situ at very low pH values (Cody and Cody 1988). Tissue channels may provide sufficient aeration during low tides to cause oxidation of the pyrite or sulfide ions retained in the organic matter to lower the pH and promote in situ gypsum precipitation. Carey et al. (2002) observed euhedral, prismatic gypsum crystals up to 1 mm long in the roots of nursery-grown pine seedlings, and attributed their formation to substrate dryness and excessive S supply through fertilisation, causing precipitation of gypsum in the root channel. The acidic conditions would be responsible for the prismatic shape, as in our case. However, the gypsum may also be an artefact of drying during thin section preparation, when buffering from seawater is negligible; halite crystals would also be expected with desiccation, but were absent. The source of calcium is likely to be detrital calcareous material or shell fragments. Pyrite oxidation is more limited in the sapric groundmass because the porosity of the amorphous organic material is low, consisting of non-conducting pores as vughs and vesicles.
Micromorphologically, profile 2610 shows better formation (semi-spheroidal framboids and euhedral crystals) of pyrite, a higher degree of decomposition of organic matter (less blackened residues) and a less conductive porosity (implying lower hydraulic conductivity) than profile 600. The pyrite structures suggest a more stable, biogeochemical environment (Wilkin et al. 1996) in profile 600 with limited availability of reactive Fe. This is supported by the soil textures and the more reduced redox conditions measured in profile 2610 (Thomas et al. 2004). In contrast, Rabenhorst and Lindbo (1998) reported that more black organic matter forms in soils with ‘more wetness’ than in soils undergoing podsolisation, which have more light (brown) organic matter. These variations demonstrate that soils texture, porosity, and organic matter type, as well as physical disturbances such as bioturbation and variations in root density, can all contribute to creation of microcosms within coastal soils, resulting in the co-existence of contrasting pyrite structures on the micro-scale.
Pedogenic processes in drained coastal soil (BG11)
Macromorphology and soil classification
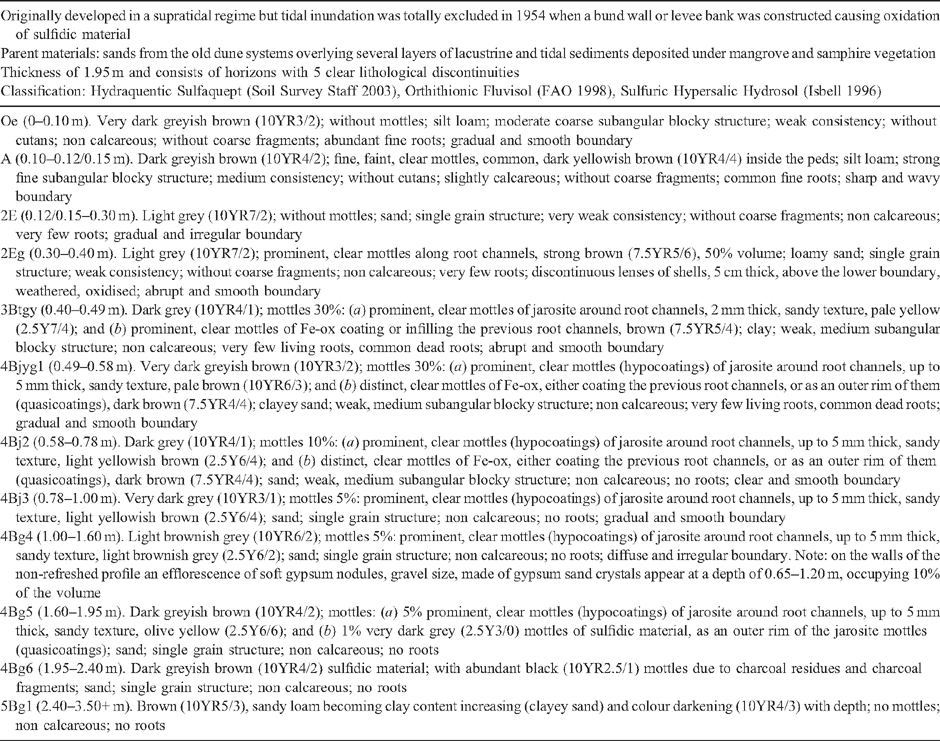
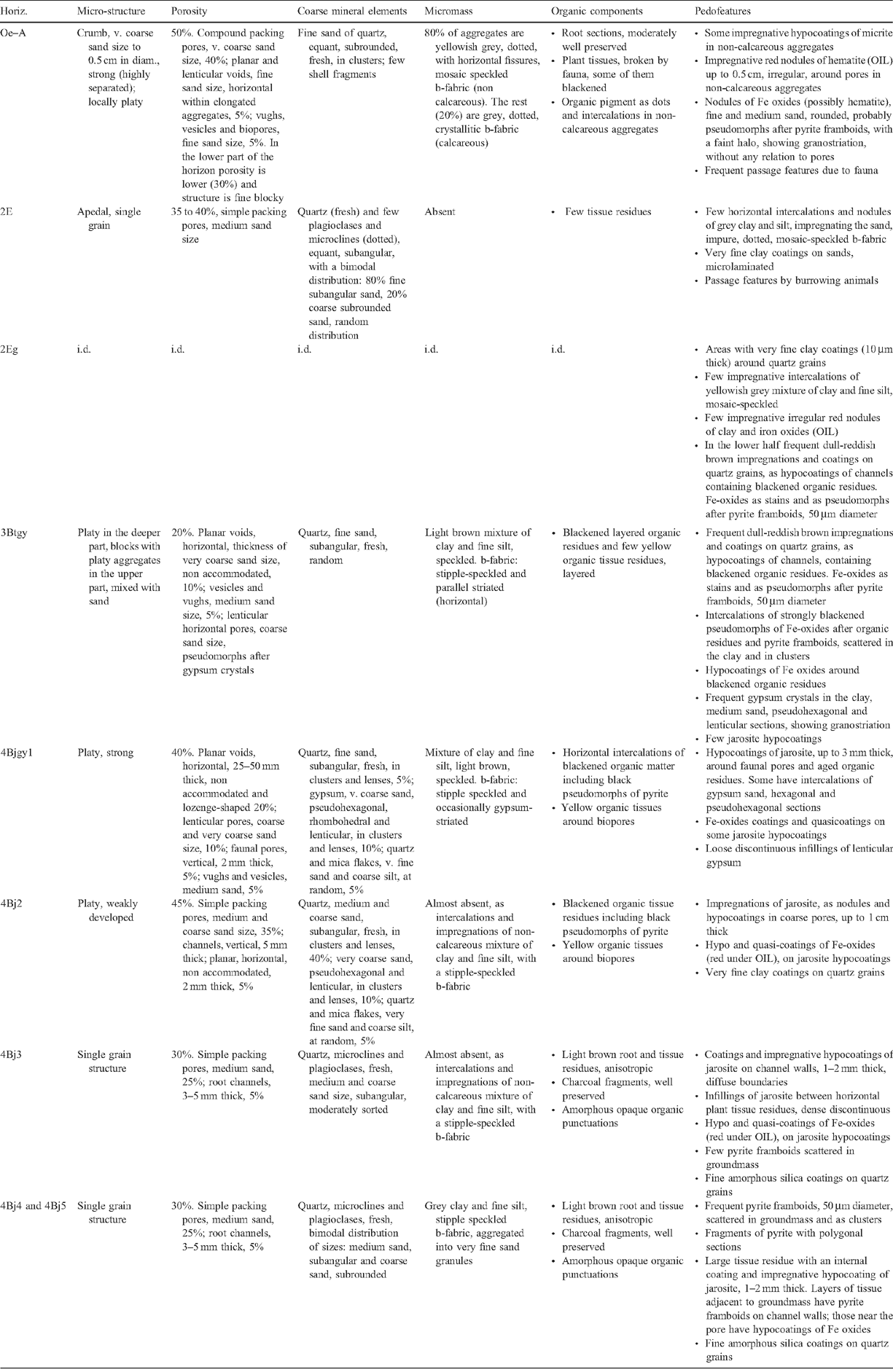
Soil profile BG 11 has a thickness of 1.95 m and consists of horizons with 5 clear lithological discontinuities (Table 4). A ‘sulfuric horizon’ is defined as comprising either mineral or organic soil material (15 cm or more thick) that has both pH < 3.5 and bright yellow jarosite mottles (Tables 4 and 5). The soil has a sulfuric horizon and classifies as a Sulfuric Hypersalic Hydrosol (Isbell 1996), Hydraquentic Sulfaquept (Soil Survey Staff 2003) and Orthithionic Fluvisol (FAO 1998). The properties of profile BG11 reflect the presence of the lithological discontinuities and the processes that have acted on them–waterlogging followed by desiccation of the swamp. The present micro- and macromorphology are summarised in Fig. 4 and Tables 3, 4. The complete micromorphological descriptions are in Table 6.
|

|

|
|
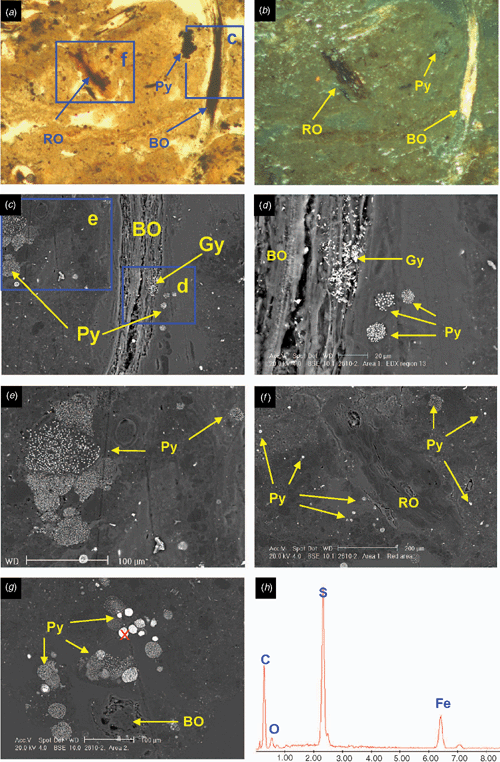
The surface horizons (Oe and A) have a well-developed crumb structure, formed by a mixture of calcareous and non-calcareous aggregates that are partly layered, with horizontal planar and lozenge-shaped (lenticular) voids, showing granostriation (Fig. 4a, b). The lenticular voids are attributed to gypsum lenses formed in water-saturated conditions which dissolved after the soil was drained. The A horizon also shows nodules of Fe-oxides in the aggregates, not related to pores, replacing pyrite framboids formed under past anaerobic conditions. Following drainage, their rapid oxidation in a calcareous environment produced the in situ precipitation of Fe-oxides, a process also observed by Miedema et al. (1974). The soil structure has been altered by biological activity (introduction of pasture grasses) and ped formation. Jarosite is absent because of the presence of CaCO3.
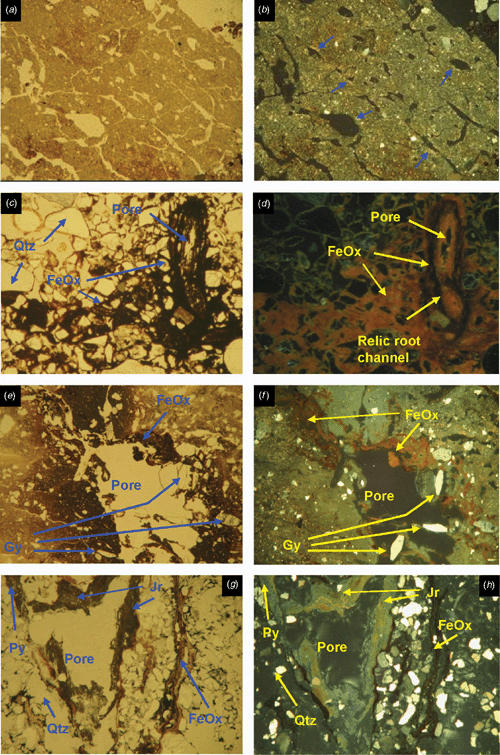
The underlying E horizons consist of sands that lack organic matter except near the lower boundary (horizon 2Eg), where there are frequent iron coatings and hypocoatings near plant remains (Fig. 4). Iron nodules are clay-coated pseudomorphs of pyrite framboids (Fig. 6c, d) which have originated from rapid pyrite oxidation in a calcareous environment, similar to the overlying Oe horizon (Figs 5 and 6).
|

|
The 3Btgy horizon has a similar structure to the lower Oe horizon: layered clays alternating with organic intercalations, lenticular voids, and porostriation. The boundary with the overlying sands is abrupt and precludes any illuvial origin of the clays. Between these horizons are discontinuous lenses of decalcifying shells. The most notable pedo-features are those related to iron accumulations, frequent gypsum lenses in the clay matrix, and jarosite coatings (Figs 5 and 6). The horizontal structure of the clays and the abundance of well-preserved organic matter is due to sedimentation under moist conditions, which favoured pyrite formation. When the water table lowered, Fe-oxide pseudomorphs formed as pyrite was oxidised. Due to the lower permeability of the clays, a perched watertable may have developed on top of that layer. As pH became acidic due to sulfide oxidation, shells began to dissolve and Ca was released allowing gypsum formation. Gypsum crystal growth occurs on shell surfaces. Little jarosite is present in this horizon and is limited to places with less calcium availability, as the shelly layers are discontinuous and irregular. Capillary rise has supplied calcium-enriched groundwater to the clay layer during dry periods, allowing gypsum to precipitate within the clays. This is evident by the poro- and granostriated b-fabrics observed, indicating displacive crystallisation. Gypsum infillings were not observed, but would usually be expected to form by capillary rise of gypsum-saturated water. Pseudohexagonal and lenticular gypsum are found together, which indicates different formation conditions. Organic matter and pH are 2 factors controlling gypsum crystal morphology; lenses would form in neutral to basic pH in presence of organic matter (Cody and Cody 1988). Due to the relatively high solubility of gypsum, several cycles of dissolution–precipitation have occurred since primary gypsum formed. Changes in the formation conditions, mostly Ca concentration and pH of the soil solution, have probably taken place, resulting in different crystal shapes of gypsum. Many of the original gypsum crystals have been dissolved, shown by the abundance of lenticular pores with porostriation. Within the upper part of profile BG11 the lenticular habit of the primary gypsum crystals indicates that they formed at near-neutral pH, due to a calcareous environment.
Horizon 4Bjyg1 shows the same features as the previous horizon, but in a much sandier matrix. Gypsum is generally lenticular, but also rhombohedral and pseudohexagonal. It occurs as intercalations and infillings (Fig. 5e, f). Jarosite has formed around pores as coatings or hypocoatings. Fe-impregnating features occur as quasicoatings or discontinuous coatings on the jarosite coatings. Jarosite coexists with gypsum, since the gypsum may have been formed at the beginning of the oxidation, when availability of calcium was higher. Jarosite is more abundant because as water percolates and gypsum precipitates, the soil solution is depleted of calcium and bicarbonate is neutralised, losing its buffering effect and allowing pH to fall sufficiently for jarosite to form. The appearance of the jarosite-impregnated groundmass with gypsum inclusions does not differ from the jarosite-free zones, suggesting that the jarosite hypo-coatings were formed after gypsum precipitated. According to Van Dam and Pons (1973), if oxidation is slow, sulfate and potassium migrate to pore walls and jarosite is formed as infillings, coatings, or hypocoatings of very fine crystals in pores. Fe coatings (other than quasicoatings) are explained by further hydrolysis of jarosite under the markedly different seasonal climatic conditions. In this case the morphology of Fe oxide coatings indicates they originated directly from pyrite oxidation in organic residues, which are adjacent to pores (Figs 5e, f and 6f). Oxidation of pyrite was rapid, as jarosite has precipitated at the site of pyrite occurrence (see also Van Dam and Pons 1973).
The underlying 4Bj2, 4Bj3, and 4Bj4 horizons have undergone similar processes, but on a still sandier matrix than horizon 4Bj1. Gypsum was difficult to identify in this horizon due to its similar birefringence to quartz, but there is less gypsum because of the depletion of Ca in the soil solution as it percolates down the profile. Jarosite becomes the main product of pyrite weathering along coarse pores, which are the first to aerate during oxidation, but it is also scattered in the groundmass as fine pseudo-cubes 0.5 μm in size (Fig. 6f, g). This cubic morphology of primary jarosite does not necessarily mean a pseudomorph transformation from pyrite, since they have the same crystal habit. EDAX analyses show that these jarosite cubes contain more potassium than the matrix, and also that there is excess sulfur, which suggests that some pyrite is still present (Fig. 6h). Iron oxides are not so related to the organic residues, pointing to a slower oxidation that allows Fe2+ to move further away from the original pyrite crystal site.
Horizon 4Bg5 contains abundant sulfidic materials; pyrite is present as scattered framboids in the groundmass or as aggregate nodules or clusters. There is oxidation only along large pores (relic from mangrove pneumatophores), where jarosite is formed (Fig. 5g, h). The pH is not buffered, as happens in the upper horizons, due to lack of calcite and depletion of bicarbonate. Amorphous silica could also be present (Van Dam and Pons 1973), derived from the weathering of clays, which become unstable at low pH. In our case very thin silica coatings could be present, but there is not much availability of weatherable clay in these 2 horizons to provide silica. NaCl salts have precipitated on quartz grains as needle-like crystals and are likely to be an artefact of drying the soil during thin section preparation.
Conclusions
The micromorphology of the soils provides an insight and confirmation of the processes that have contributed to their formation and development. In the undrained mangrove soils at St Kilda, the micromorphology of organic material indicates that sapric material was more decomposed, less porous, more reduced, and allowed the formation of larger pyrite framboids than hemic material. In these Hydrosols (Histosols), partial oxidation of pyrite along coarse pores forms gypsum because of the presence of calcium (from shells) in the environment. The morphology of pyrite and gypsum crystals indicates conditions of their formation.
The profile at Gillman (profile BG11) is more complex and has changed a great deal following the loss of tidal inundation in 1954. This soil has dried causing rapid oxidation of sulfide minerals in organic residues and acidification of both the soil layers and the groundwater. The sands have limited acid-neutralising capacity and the pH of soil solution is generally <2.5. Oxidation processes, with their products, are clearly evident micromorphologically. In the upper horizons, the rapid oxidation of pyrite in organic residues caused precipitation of iron oxides and lenticular gypsum, which is now being leached out of the profile (Fig. 4). This former back barrier sand ridge contains sulfuric materials and has developed a 2-m-thick sulfuric horizon because pyrite framboids within and surrounding decomposed mangrove pneumatophores have oxidised to form yellow jarosite mottles (4Bj horizons) and acidity where calcium availability is limited. Coatings of jarosite and iron oxides form rapidly along large root channels during periods of aeration. Some small pyrite framboids occur in the underlying sandy sulfuric horizons (horizons 4Bj3, 4Bj4).
Long-term, in situ redox monitoring (B. P. Thomas, unpubl. data) indicates that the large seasonal variation in watertable height (>1 m) may contribute to the re-formation of pyrite and consumption of acidity near the base of the profile during the wetter months, where soil organic matter content is still adequate for reducing conditions to return. Most of the sulfuric acid that has been produced at Gillman is still contained within the soil profile, as acidic groundwater due to the low hydraulic gradients and retained acidity as sulfo-salt efflorescences.
Micromorphological features identified in all soil layers, together with chemical, mineralogical, and physical properties, help explain contemporary and relict processes in the development of these complex coastal acid sulfate soils. Microscopic features also verify soil characteristics that are used to classify these soils (FAO 1998; Soil Survey Staff 2003).
Acknowledgements
The authors thank: staff of the Port Adelaide Enfield Council for their assistance with background information and access to the study sites; Mark Raven (CSIRO Land and Water) for conducting powder X-ray diffraction analyses; Peter Self (formerly CSIRO Division of Soils) and Stuart McClure (CSIRO Land and Water) for conducting SEM and EDAX analyses; and Wayne Hudnall, Texas Tech University, USA, for early interaction, especially with Soil Taxonomy and in thin section preparation of some of the St Kilda profiles.
Belperio AP
(1993) Land subsidence and sea level rise in the Port Adelaide Estuary: Implications for monitoring the greenhouse effect. Australian Journal of Earth Sciences 40, 359–368.
| Crossref | GoogleScholarGoogle Scholar |
and http://www.clw.csiro.au/acidsulfatesoils/index.html
Fitzpatrick RW, Self PG
(1997) Iron oxyhydroxides, sulfides and oxyhydroxysulfates as indicators of acid sulfate weathering environments. Advances in GeoEcology 30, 227–240.
|
CAS |

Lin C, Melville MD
(1992) Mangrove soil: a potential contamination source to estuarine ecosystems of Australia. Wetlands (Australia) 11, 68–75.
|
CAS |

Raiswell R, Berner RA
(1985) Pyrite formation in euxenic and semi-euxenic sediments. American Journal of Science 285, 712–724.

Roychoudhury AN,
Kostka JE, Van Cappellen P
(2003) Pyritization: a palaeoenvironmental and redox proxy reevaluated. Estuarine, Coastal and Shelf Science 57, 1183–1193.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Sammut J,
White I, Melville MD
(1994) Stratification in acidified coastal floodplains drains. Wetlands (Australia) 13, 49–64.

Sammut J,
White I, Melville MD
(1996) Acidification of an estuarine tributary in eastern Australia due to drainage of acid sulfate soils. Marine and Freshwater Research 47, 669–684.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Sammut J,
Melville MD,
Callinan RB, Fraser G
(1995) Estuarine acidification: the impacts on aquatic biota of draining acid sulfate soils in coastal floodplains. Australian Geographical Studies 33, 89–100.
| Crossref | GoogleScholarGoogle Scholar |

Sullivan LA, Bush RT
(2004) Iron precipitations accumulations associated with waterways in coastal acid sulfate landscapes of eastern Australia. Marine and Freshwater Research 55, 727–736.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Sullivan LA,
Bush RT,
McConchie D,
Lancaster G,
Haskins PG, Clark M
(1999) Comparisons of peroxide-oxidisable sulfur and chromium-reducible sulfur methods for determination of reduced inorganic sulfur in soil. Australian Journal of Soil Research 37, 255–265.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

White I,
Melville MD,
Wilson BP, Sammut J
(1997) Reducing acidic discharges from wetlands in eastern Australia. Wetlands Ecology and Management 5, 55–72.
| Crossref | GoogleScholarGoogle Scholar |

Wilkin RT, Barnes HL
(1997) Pyrite formation in an anoxic estuarine basin. American Journal of Science 297, 620–650.
|
CAS |

Wilkin RT,
Barnes HL, Brantley SL
(1996) The size distribution of framboidal pyrite in modern sediments: an indicator of redox conditions. Geochimica et Cosmochimica Acta 60, 3897–3912.
| Crossref | GoogleScholarGoogle Scholar |
CAS |

Willett IR,
Crockford RH, Milnes AR
(1992) Transformations of iron, manganese and aluminium during oxidation of a sulfidic material from an acid sulfate soil. Catena 21, 297–302.

Willett IR, Walker PH
(1982) Soil morphology and distribution of iron and sulphur fractions in a coastal flood plain toposequence. Australian Journal of Soil Research 20, 283–294.
| Crossref | GoogleScholarGoogle Scholar |
CAS |



