Conservation genomics uncovers disjunct subspecies and critically low diversity in Zieria obcordata A.Cunn. (Rutaceae)
Eilish S. McMaster A B * , Marco Duretto
A B * , Marco Duretto  C , Jia-Yee S. Yap A and Maurizio Rossetto A
C , Jia-Yee S. Yap A and Maurizio Rossetto A
A
B
C
Abstract
Zieria obcordata A.Cunn. (Rutaceae), an endangered species endemic to central New South Wales, Australia, faces significant conservation challenges due to limited occurrence in two small, isolated populations. Using genome-wide SNPs (DArTseq), we examine genetic relationships and diversity within and between these populations, and make comparisons with other Zieria species. Our results confirm that Z. obcordata is a distinct species, with the Bathurst and Wellington populations showing sufficient genetic divergence to warrant recognition as two subspecies: Z. obcordata subsp. obcordata and Z. obcordata subsp. wuuluman (formally described here). Minor morphological differences further support this classification. Genomic analyses reveal minimal gene flow between the subspecies, along with extremely low heterozygosity and high inbreeding coefficients within each. Compared to other Zieria species, including Z. covenyi, Z. cytisoides, Z. laevigata, Z. odorifera and Z. smithii, both subspecies exhibit exceptionally low genetic diversity, likely due to geographic isolation, genetic drift and inbreeding. We provide conservation assessments for both subspecies and conclude that each qualifies to be listed as Critically Endangered under the New South Wales Biodiversity Conservation Act 2016. We recommend strategies to facilitate gene flow between the subspecies to improve genetic diversity and enhance fitness.
Keywords: conservation genetics, endangered plants, population genetics, Rutaceae, taxonomy.
Introduction
The genus Zieria Sm. (Rutaceae) comprises ~60 species that are endemic to eastern Australia and New Caledonia, many of which have small populations and narrow distributions. Duretto and Forster (Duretto and Forster 2007) hypothesised that the abundance of these species is driven by the preference of the lineages for rocky habitats, often resulting in isolation between populations. Over extended periods, this isolation can contribute to allopatric speciation, with a number of instances of speciation occurring over very short distances (<5 km) (Hogbin and Peakall 1999; Orel et al. 2024). Prior to speciation, these isolated populations can contract and lose genetic diversity through drift and without conservation interventions, can become increasingly inbred and vulnerable to extinction.
We present a genetic study to guide conservation efforts of the Endangered Zieria obcordata A.Cunn., commonly known as granite Zieria. The plants are densely branched shrubs up to 100 cm high (Shelly et al. 2021), with small aromatic, hirsute, cordate leaflets. Only two disjunct populations exist near Wellington and Bathurst (100 km apart) in the Central Western Slopes of New South Wales. Zieria obcordata tends to grow in crevices between granite boulders in eucalypt woodland or shrubland (New South Wales Department of Environment and Conservation 2007), and plants are threatened by wallaby and domestic stock grazing, weed invasion and habitat degradation (Shelly et al. 2021). Such restricted distribution and historically small population sizes make Z. obcordata susceptible to extinction in the near future, hence the Endangered listing under the New South Wales Biodiversity Conservation Act 2016 and the Commonwealth Environment Protection and Biodiversity Conservation Act 1999.
Zieria obcordata is most morphologically similar to Z. odorifera J.A.Armstr. (Duretto and Forster 2008). Phylogenetic analyses of Zieria have been incongruent between chloroplast and nuclear methods; a chloroplast phylogeny placed Z. obcordata in two different clades with the sequenced individual from near Bathurst was closest to Z. citriodora J.A.Armstr. and the individual from near Wellington was closest to Z. cytisoides Sm. (Barrett et al. 2015). However, a more recent phylogeny based on nuclear ribosomal DNA placed Z. obcordata closest to Z. odorifera subsp. williamsii Duretto & P.I.Forst. and Z. adenophora Blakely (Barrett et al. 2018). Phylogenetic analyses of Zieria are complicated by incomplete lineage sorting, hybridisation, ploidy variation and sequence homoplasy (Stace and Armstrong 1992; Barrett et al. 2018). Distinction between the identities of the Z. obcordata populations at Bathurst and Wellington has not been questioned, despite the populations being highly disjunct, restricted and falling into different clades in chloroplast phylogenetic analyses (Barrett et al. 2015).
Observations of Zieria obcordata over >15 years showed that the population sizes at both Wellington and Bathurst vary considerably from year to year. More recently, significant fluctuations in the population size of Z. obcordata have made accurate assessment of population health difficult. In 2016, ~200 mature individuals were observed at Wellington and 800 mature individuals at Bathurst (Shelly 2016). Due to an intense heatwave in December 2019 with several consecutive days with temperatures of 40–45°C, an average of 82% of the plants at various sites at Bathurst and 84% at Wellington perished (Shelly et al. 2021). Following consistent rainfall in 2020, ~12,000 Z. obcordata plants germinated at Wellington and Bathurst, and many of the surviving mature plants showed foliage growth, flowering and fruit production. Site visits in early 2021 confirmed that most seedlings recruited in 2020 had remained alive, representing the largest recruitment event observed for this species (Shelly et al. 2021). This mass germination confirmed the presence of a soil seed bank and co-occurrence with nearby bushfires indicated that smoke cues may play a significant role in stimulating germination in Z. obcordata. Whether or not this mass germination exhausted the soil seed bank is not known.
We present a population genomic study of Zieria obcordata using reduced representation DArTseq designed to support the management of the species by the Saving our Species program of the New South Wales Department of Department of Environment and Heritage (DEH). We aim to provide foundational knowledge to support the long-term conservation of Z. obcordata by testing the species concept within the current phylogenetic framework, determining the extent of genetic differences between the Bathurst and Wellington populations, and assessing genetic health and gene flow within Z. obcordata. Addressing these objectives is essential for developing effective conservation strategies to ensure survival of the species (Rossetto et al. 2021).
Materials and methods
Field sampling
Sampling for this study was conducted by several DEH officers (see Acknowledgements). In total, 20 specimens of Zieria obcordata were collected from a population near Wellington (hereafter referred to as ‘Wellington’) and 62 specimens from a population near Bathurst (hereafter referred to as ‘Bathurst’) that are located ~100 km apart. Each population included distinct sites (Fig. 1): two at Wellington and six at Bathurst. Additionally, six Z. odorifera subsp. copelandii Duretto & P.I.Forst. individuals were sequenced to serve as an outgroup for the Z. obcordata analyses. Vouchers are lodged at the National Herbarium of New South Wales (Table 1).
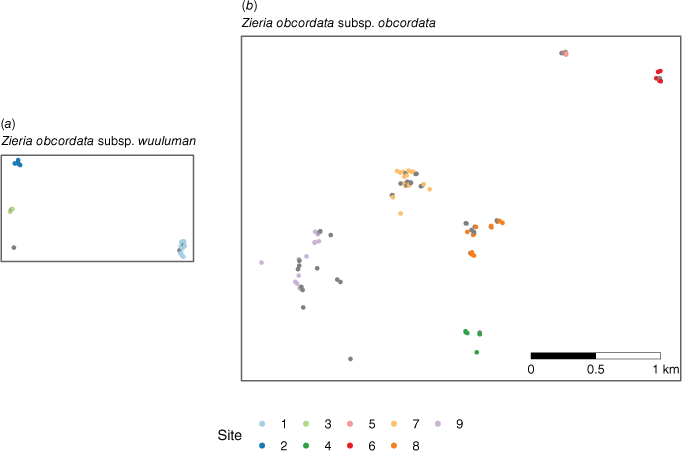
Maps depicting the populations of (a) Zieria obcordata subsp. wuuluman near Wellington and (b) Z. obcordata subsp. obcordata near Bathurst. Each point represents a sample record for the genetic study, colour-coded by site number or survey data (grey points) collected between 2011 and 2022. These data were used to calculate extent of occurrence (EOO) and area of occupancy (AOO) for the conservation listing. Location information has been removed for privacy. Both maps are presented at the same scale.
| Scientific name | Catalogue number | Institution code | Collector | Collection date | Associated media | |
|---|---|---|---|---|---|---|
| Zieria obcordata subsp. wuuluman | NSW1078497 | NSW | Shelly, D. | 10/3/2022 | https://herbariumnsw-pds.s3-ap-southeast-2.amazonaws.com/images/NSW1078497.jpg | |
| Zieria obcordata subsp. obcordata | NSW1078495 | NSW | Shelly, D. | 1/4/2022 | https://herbariumnsw-pds.s3-ap-southeast-2.amazonaws.com/images/NSW1078495.jpg | |
| Zieria obcordata subsp. obcordata | NSW1078496 | NSW | Shelly, D. | 1/4/2022 | https://herbariumnsw-pds.s3-ap-southeast-2.amazonaws.com/images/NSW1078496.jpg | |
| Zieria cytisoides | NSW1069566 | NSW | Taseski, G.M. | 20/5/2020 | https://herbariumnsw-pds.s3-ap-southeast-2.amazonaws.com/images/NSW1069566.jpg | |
| Zieria covenyi | NSW1028827 | NSW | Wilson, T.C. | Jones, M. | Barker, C.H. | 13/6/2019 | https://herbariumnsw-pds.s3-ap-southeast-2.amazonaws.com/images/NSW1028827.jpg |
All are lodged at the National Herbarium of New South Wales (NSW Code).
Several other Zieria species from previous studies were co-analysed with the new dataset to obtain broader evolutionary context regarding the position of Zieria obcordata and Z. odorifera relative to other Zieria species. This included 2 Z. arborescens Sims subsp. arborescens, 1 Z. arborescens subsp. decurrens J.A.Armstr., 78 Z. covenyi J.A.Armstr., 10 Z. cytisoides, 10 Z. laevigata Bonpl., 1 Z. odorifera subsp. odorifera, 16 Z. pilosa Rudge and 28 Z. smithii Andrews individuals collected from wild populations (Fig. 2). Zeria covenyi is formally listed as an Endangered species under New South Wales and Federal legislation, and Z. odorifera subsp. copelandii as Critically Endangered in New South Wales. We excluded previously sequenced individuals that were identified as hybrids to focus on non-hybrid individuals, as broader insights into speciation and mating patterns in the genus provided by hybridisation were not considered part of the scope of this study.
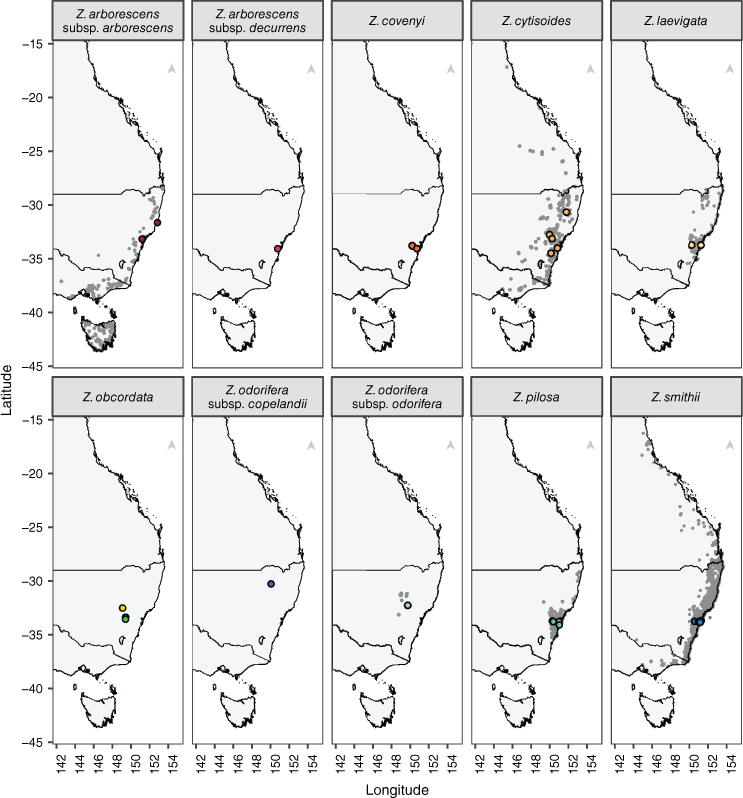
Distribution maps of Zieria species included in this study based on Australian Virtual Herbarium, New South Wales BioNet Atlas and PlantBank records sourced from the Atlas of Living Australia (2025). Each plot represents a species or subspecies distribution. Coloured points show collection sites for the samples used in this study and grey points are Atlas of Living Australia records. In Z. obcordata, the green points indicate Z. obcordata subsp. obcordata collections near Bathurst and the yellow points indicate Z. obcordata subsp. wuuluman collections near Wellington.
All plant collections made for this project were conducted under the Botanic Gardens of Sydney collection permit (SL100569). Vouchers are lodged at the National Herbarium of New South Wales.
Geographic coordinates were recorded for each plant and ~3 g of leaf tissue was collected from each individual plant. Sampling numbers were adjusted based on the known number of individuals and associated distribution patterns to capture genetic diversity across differently sized populations of the threatened species. After collection, samples were stored at −80°C for a minimum of 12 h and freeze-dried before being stored in silica gel to ensure preservation until DNA extraction.
DNA extraction and DArTseq
Genotyping was conducted using medium-density DArTseq, a reduced representation sequencing method implemented by Diversity Arrays Technology Australia Pty Ltd (Canberra, ACT, Australia). The DArTseq method involves a genome restriction digest followed by sequencing of the digested products using an Illumina instrument, with Single Nucleotide Polymorphisms (SNPs) called using proprietary analytical pipelines by DArT Pty Ltd (Jaccoud et al. 2001; Kilian et al. 2012).
Analyses
Genetic relationships among Zieria samples were investigated using two primary datasets: a ‘core’ dataset focusing exclusively on Z. obcordata and Z. odorifera subsp. copelandii, and a multispecies dataset encompassing all known Zieria species. The ‘core’ dataset, designed with fewer closely related species, was particularly suited for analysing species-specific polymorphic sites essential for diversity statistics. By contrast, the multispecies dataset, that includes a broader range of species, provided a comprehensive view of interspecies relationships through principal component analysis (PCA), fixation index (FST) and phylogenetic networks.
All computational analyses were performed in the R statistical environment (ver. 4.3.1, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/) using custom scripts, RRtools, developed in-house, available on GitHub (see https://github.com/jasongbragg/RRtools). Data were imported using new.read.dart.xls.onerow and read.meta.data.full.analyses.df functions, and merged using dart.meta.data.merge. Rigorous filtering criteria were applied to both datasets to ensure robust data quality. Loci with a DArT reproducibility score of 0.96 or lower and monomorphic loci were excluded, and only one SNP was retained per locus using the remove.poor.quality.snps and remove.fixed.snps functions. Loci with more than 30% missing data across genetic groups and samples were removed for the ‘core’ dataset, resulting in a final dataset comprising 2901 loci from 83 samples. Similarly, in the multispecies dataset, loci with more than 80% missing data and samples with more than 80% missing loci were excluded, leaving a dataset of 8528 loci across 180 samples after filtering.
Pairwise kinship between individuals within each dataset was estimated using the PLINK identity by descent (IBD) method (Purcell et al. 2007; Chang et al. 2015), implemented via the snpgdsIBDMoM function in SNPRelate (ver. 1.36.0, see http://rdrr.io/bioc/SNPRelate/; Zheng et al. 2012) using minimum MAF of 5% and maximum locus missingness of 20%. Individuals with a kinship estimate exceeding the theoretical threshold for clonality (Manichaikul et al. 2010) were considered genetically identical and the sample with lower data quality was excluded from subsequent PCA.
PCA was conducted using the adegenet package (ver. 1.7–22, see http://rdrr.io/cran/adegenet/; Jombart 2008) in the multispecies dataset to visualise genetic clustering patterns among different Zieria species. Fixation index (FST) calculations were performed between Z. obcordata sites and other species in the multispecies dataset. The analysis utilised the Weir & Hill relative beta estimator (Weir and Hill 2002) implemented in the snpgdsFst function of SNPRelate (Buckleton et al. 2016) on loci with maximum missingness of 30% and minimum minor allele frequency (MAF) of 5%.
Diversity metrics including observed heterozygosity (HO), unbiased expected heterozygosity (uHE) and unbiased inbreeding coefficient (uFIS) were computed for each species or subspecies in both datasets using the faststats function of fastDiversity (ver. 0.9.0, see https://github.com/eilishmcmaster/fastDiversity; Keenan et al. 2013). Loci with less than 30% missing data and a minimum MAF of 5% were selected for analysis within each genetic group.
We constructed a phylogenetic network based on Euclidean distances between individuals in the multispecies dataset using RSplitsTree (ver. 0.1.0, see https://github.com/IVS-UZH/RSplitsTree). This network was visualised in R with ggplot2 (ver. 3.4.2, see https://ggplot2.tidyverse.org; Wickham 2016) and tanggle (ver. 1.0.0, see http://rdrr.io/github/KlausVigo/tanggle/). Phylogenetic network analysis allows for the representation of complex evolutionary relationships, such as reticulate evolution and hybridisation events that are not adequately captured by traditional tree-based methods (Huson 1998).
Distribution maps were made by retrieving records of the study species from the Atlas of Living Australia (ALA) (Belbin et al. 2021) using the galah package (ver. 2.0.2, M. Westgate, M. Stevenson, D. Kellie and P. Newman, see https://galah.ala.org.au/R). Records were filtered to only contain Australian Virtual Herbarium, New South Wales BioNet Atlas and PlantBank records, and subsequently plotted using the ozmaps (ver. 0.4.5, see https://github.com/mdsumner/ozmaps) and ggsn packages (ver. 0.5.3, see https://github.com/oswaldosantos/ggsn).
The extent of occurrence (EOO) and area of occupancy (AOO) were calculated for populations near Wellington and Bathurst. This was undertaken using the metadata from samples collected for genomic analysis combined with DEH survey data from 2011 onwards. We used the official IUCN R package, red (ver. 1.6.1, see https://rdrr.io/cran/red/; Cardoso 2017), to calculate the EOO and AOO for each subspecies individually and for the species as a whole.
Plant descriptions
Vouchers and all material for the species (all specimens listed as types or under Additional Specimens Examined for each subspecies in Taxonomy below) and related species, lodged at the National Herbarium of New South Wales were examined and the scored morphological data combined to produce the descriptions and determine morphological variation across the species. Specimens were examined by eye and under a stereo microscope, and measurements were made with a ruler with 0.5-mm divisions. All morphological characters, where organs were present, were scored to document the full range of morphological variation displayed on each specimen. Data from all specimens were subsequently combined to produce the descriptions.
Results
Zieria obcordata demonstrated high genetic distinctiveness from other Zieria species, including those previously identified as potentially phylogenetically close, such as Z. cytisoides (Barrett et al. 2015) and Z. odorifera (Barrett et al. 2018). However, this distinctiveness was not uniform across all comparisons; notably, both Z. odorifera subsp. copelandii and Z. odorifera subsp. odorifera appeared to be almost as closely related to Z. obcordata as the two Z. obcordata subspecies were to each other. These relationships were consistently observed across PCA (Fig. 3a) and phylogenetic network (Fig. 3b), confirming the unique genetic identity of Z. obcordata, while highlighting the varying degrees of relatedness among closely associated species.
Population genomic analyses of the multispecies dataset. (a) Principal component analysis (PCA) of Zieria SNP genotypes. Point shape and colour indicate species or subspecies. (b) Phylogenetic network of Zieria samples based on Euclidean distances between genotypes. Tip shape and colour indicate species or subspecies and labels are adjacent. (c) Heatmap of pairwise geographic distance (km) rounded to the nearest 100 m (lower-left triangle) and pairwise fixation index (FST; upper-right triangle). Groups are either species or subspecies level (for outgroups) or by site (for Z. odorifera subspecies).
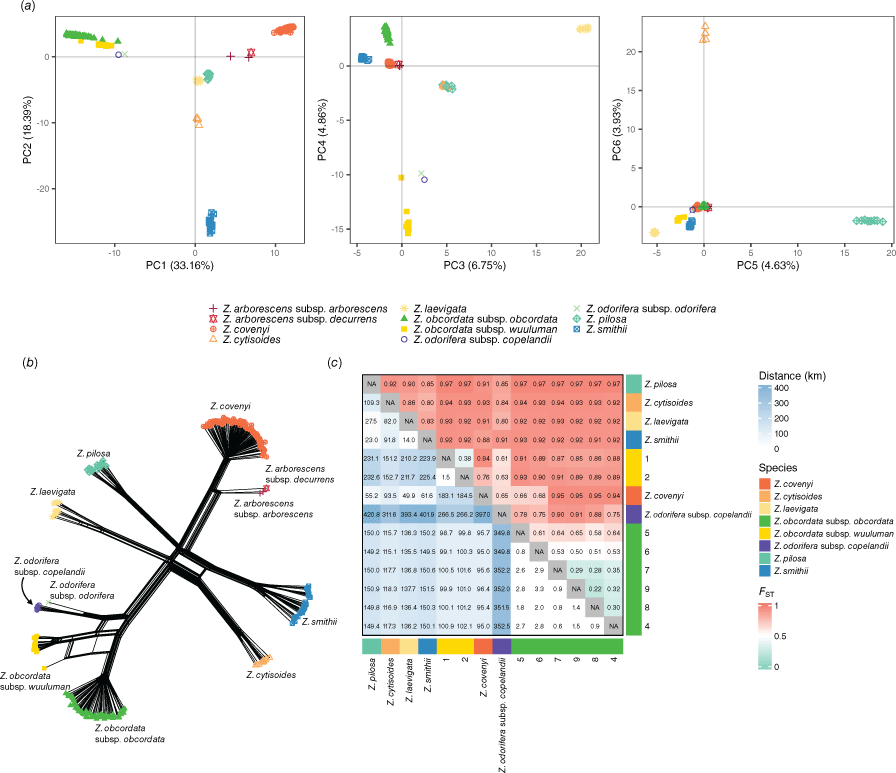
Substantial genetic differentiation was observed between the Wellington and Bathurst populations of Z. obcordata. This differentiation was evident in the PCA, where the two populations were separated along PC4 that explained 4.86% of the variation in the multispecies dataset (Fig. 3a). Additionally, the phylogenetic network analysis showed the two populations as highly distinct, although one individual at Wellington exhibited an intermediate genotype (Fig. 3b). Fixation index (FST) values indicated that the genetic distinction between the Wellington and Bathurst populations (FST 0.85–0.93) was comparable to species-level differentiation (Fig. 3c) (McMaster et al. 2024). Morphological characters that distinguished the populations were the presence or absence of glandular tubercula on the stems and cocci: for the Bathurst population these were not distinct or only partially so and for the Wellington population these were present to a marked degree (see Taxonomy). Consequently, we described these populations as distinct subspecies: Z. obcordata subsp. obcordata from Bathurst, as this matched the type collection and Z. obcordata subsp. wuuluman McMaster & Duretto, a name new to science, from Wellington (see Taxonomy below).
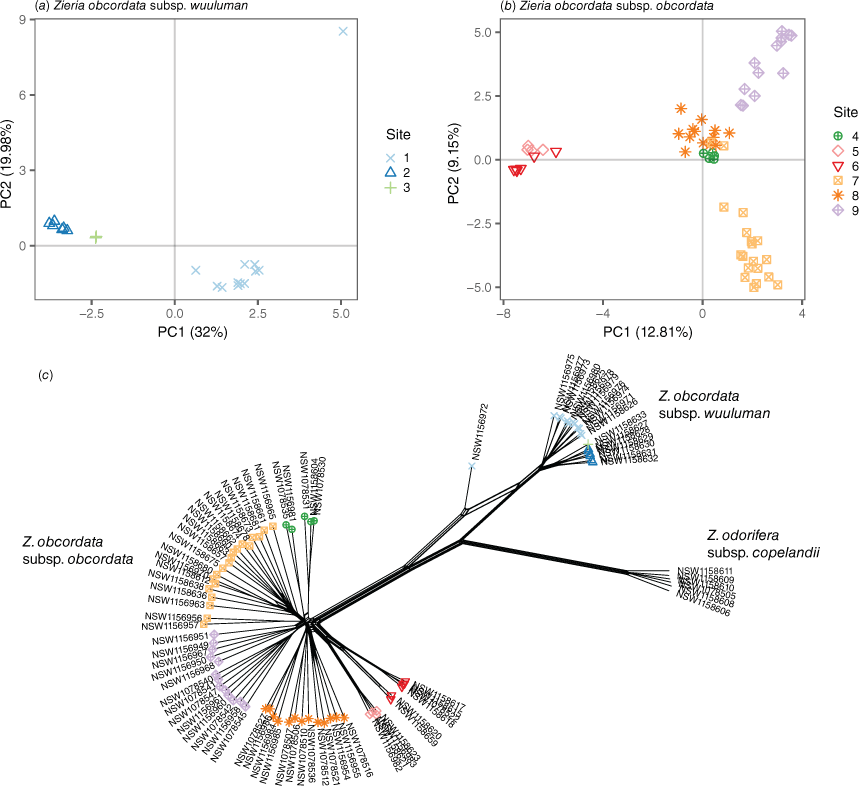
Even within populations, gene flow between sites in the Z. obcordata subspecies was somewhat limited; between the Z. obcordata subsp. wuuluman sites FST was 0.38 and in Z. obcordata subsp. obcordata, FST was as high as 0.65 between sites, indicating limited gene flow across short distances (>3 km; Fig. 3c). This limited gene flow has led to pronounced genetic divergence between sites, reflected in the distinct clustering of samples by site observed in both the PCA and phylogenetic networks (Fig. 4).
Population genomic analyses of Zieria obcordata subspecies. Principal component analysis (PCA) of (a) Zieria obcordata subsp. wuuluman and (b) Zieria obcordata subsp. obcordata SNP genotypes. Point shape and colour indicate collection site. (c) Phylogenetic network of Z. obcordata samples based on Euclidean distances between genotypes. Tip shape and colour site, and sample IDs are adjacent. The intermediate genotype sample NSW1156972 is evident in the PCA (a) and phylogenetic network (c).
Both subspecies of Z. obcordata exhibited extreme depletion of genetic diversity. HO was markedly low, with values of 0.036 in Z. obcordata subsp. obcordata and 0.076 in Z. obcordata subsp. wuuluman (Table 2). Correspondingly, uFIS was extremely high, at 0.896 and 0.736 for Z. obcordata subsp. obcordata and Z. obcordata subsp. wuuluman respectively. High levels of inbreeding also contributed to the elevated FST between the two subspecies, as inbreeding rapidly differentiates allele frequencies in isolated populations. The genetic diversity metrics of Z. obcordata were notably lower than in other species of Zieria. For example, Z. covenyi showed an HO of 0.29 and a uFIS of 0.091, Z. smithii exhibited an HO of 0.355 and a uFIS of 0.104, and Z. laevigata had an HO of 0.302 and a uFIS of 0.175. Notably, Z. odorifera subsp. copelandii had very high HO and negative uFIS (Table 2), indicative of ploidy variation or hybridisation that warrants further investigation.
| Species | HO | uHE | uFIS | Loci | n | |
|---|---|---|---|---|---|---|
| Z. obcordata subsp. obcordata* | 0.036 | 0.346 | 0.896 | 416 | 60 | |
| Z. obcordata subsp. wuuluman* | 0.076 | 0.288 | 0.736 | 106 | 19 | |
| Z. odorifera subsp. copelandii* | 0.828 | 0.497 | −0.665 | 471 | 6 | |
| Z. covenyi | 0.29 | 0.318 | 0.091 | 362 | 78 | |
| Z. cytisoides | 0.315 | 0.284 | −0.11 | 304 | 10 | |
| Z. laevigata | 0.302 | 0.366 | 0.175 | 280 | 10 | |
| Z. smithii | 0.355 | 0.396 | 0.104 | 423 | 28 |
Groups marked with an asterisk (*) were calculated from the ‘core’ SNP dataset and those without an asterisk were calculated using the multispecies dataset. Clones were excluded from the analysis, and loci were filtered to maximum missingness of 30% and minimum minor allele frequency of 5% for each species or subspecies. Zieria pilosa did not have 100 or more polymorphic loci after filtering, therefore we excluded the species from this analysis.
| EOO | AOO | ||
|---|---|---|---|
| Z. obcordata subsp. wuuluman | 1 | 8 | |
| Z. obcordata subsp. obcordata | 4 | 16 | |
| Z. obcordata combined | 242 | 24 |
Chromosome numbers reported for the species in this study varied, with Z. covenyi having 2n = 54, Z. cytisoides 2n = 72, Z. odorifera 2n = 36 and Z. smithii 2n = 72 (Stace and Armstrong 1992). Despite these differences, we found no genomic evidence of recent ploidy expansion (e.g. increased heterozygosity or unusual kinship patterns) in the populations examined, except for Z. odorifera subsp. copelandii.
Discussion
We present a focused conservation genomic assessment of Zieria obcordata with taxonomic implications. Our genomic findings of the two geographically disjunct populations support the recognition of Z. obcordata subsp. obcordata from near Bathurst and Z. obcordata subsp. wuuluman from near Wellington. The genetic distinction was comparable to that between other species and subspecies, and in combination with the minor morphological characters (see Taxonomy), provided strong support for recognising these as distinct subspecies.
Our analyses reveal an almost complete absence of gene flow between the Bathurst and Wellington populations, with the exception of a single admixture event identified in an individual from Wellington exhibiting an intermediate genotype. This individual might have resulted from accidental seed or pollen transfer between sites during management or possibly sequencing contamination (e.g. tissue from two individuals sequenced together). Assuming this admixed individual is not erroneous, this, combined with the minor morphological differences observed between the populations, supports treating these populations as subspecies rather than distinct species. Speciation within Zieria can occur over much shorter distances than the substantial 100 km disjunction between Z. obcordata subsp. wuuluman and Z. obcordata subsp. obcordata (Hogbin and Peakall 1999; Hogbin and Crisp 2003; Neal et al. 2019; Orel et al. 2024), consistent with the observed genetic divergence.
Genetic isolation between the Z. obcordata subspecies is likely compounded by inbreeding tolerance. Breeding systems in Zieria are highly variable, with some species exhibiting strong self-incompatibility (Lopresti et al. 2023) and others readily self-fertilising (Armstrong 2002). Although Armstrong (2002) reported Z. obcordata as self-incompatible, our data, showing low heterozygosity and high inbreeding coefficients, show that inbreeding is possible. Prolonged isolation and inbreeding have reduced genetic diversity in Z. obcordata, particularly compared to other analysed Zieria species including Z. cytisoides, Z. laevigata and Z. smithii. The exceptionally low diversity in Z. obcordata is unusual even among other threatened Zieria with limited ranges; for example, the endangered Z. covenyi retains higher genetic diversity (Table 2), consistent with prior studies on other endangered species such as Z. baeuerlenii J.A.Amstr. (Sharma 2001) and critically endangered species Z. buxijugum J.D.Brigs & J.A.Armstr., Z. formosa J.D.Brigs & J.A.Armstr. and Z. parrisiae J.D.Brigs & J.A.Armstr. (Orel et al. 2024). In the absence of intervention, continued inbreeding could lead to further genetic differentiation and diversity loss, reducing the ability of the subspecies to adapt to environmental challenges such as climate change and disease (Booy et al. 2000).
Despite concerns about diversity loss resulting from sustained inbreeding, the observed capacity to recruit suggests that Z. obcordata might not be as susceptible to inbreeding depression as species with self-incompatibility mechanisms (Duthie and Reid 2016; Fujii et al. 2016). Deleterious mutations that can contribute towards inbreeding depression may be purged upon recruitment (Arunkumar et al. 2015), allowing the populations to maintain some level of fitness despite low genetic diversity and small isolated populations (Razanajatovo et al. 2019). This situation may illuminate mechanisms driving exceptional taxonomic diversity within the genus: specific habitat preferences, geographic isolation, small population sizes (Armstrong 2002) and reproductive biology – all contributing to increased genetic drift and differentiation. This situation highlights the complex interplay between inbreeding, genetic drift and recruitment dynamics that may mitigate some inbreeding impacts while still presenting significant conservation challenges through reduced adaptive potential.
Based on the strong genetic divergence between the Bathurst and Wellington populations but only minor morphological differences (the presence or absence of glandular tubercula on the stems and cocci, see Taxonomy), we propose describing these as Zieria obcordata subsp. obcordata and Z. obcordata subsp. wuuluman. Although Darwin’s (1883) observation that distinctions between species and subspecies are inherently arbitrary holds true, genetic evidence suggests that these subspecies are on a trajectory toward speciation. The purpose of this work is not to add a subspecies name for the sake of naming alone; rather, our goal is to identify taxonomic and management units critical for effective conservation. Recognising these populations as subspecies acknowledges the unique conservation challenges and ensures that the high risk of extinction is formally recognised.
Conservation strategies should prioritise experimental tests of the fitness and reproductive biology of the subspecies to support the long-term viability of these populations. This is crucial for determining whether subspecies exhibit biological differences that are not fully understood but are relevant to management (Dost are et al. 2010). We also recommend investigating the viability of assisted gene flow between the Bathurst and Wellington populations through ex situ approaches to enhance genetic diversity and overall fitness (Wilder et al. 2020; Rodger et al. 2024). This approach is supported by evidence showing that gene flow increases heterozygosity, adaptive potential, fitness and long-term population viability (Frankham 2015, 2016). The risk of outbreeding depression in this case is minimal, as these closely related subspecies share a recent common ancestry (Bell et al. 2019). Additionally, members of Zieria are known to readily outcross and hybridise (Armstrong 2002), increasing the likelihood of successful intervention (Chan et al. 2019).
Although we recognise that this approach will affect the speciation process and potentially erode the distinct characteristics used to differentiate the subspecies (Seehausen 2006), we believe that the primary objective of conservation should be to preserve genetic diversity and species viability. If facilitating gene flow results in the subspecies being synonymised in the future due to reduced differentiation, we consider this an acceptable trade-off, as long as the overall fitness and long-term survival of the species is enhanced. This intervention is particularly warranted in this case, as both subspecies face increasing threats from human-driven factors such as climate change, invasive species, altered fire regimes and habitat fragmentation (New South Wales Department of Environment and Conservation 2007). The subspecies also show greater genetic diversity loss and inbreeding than other small-range Zieria species, placing these at higher extinction risk. Maintaining isolated, inbred populations may preserve subspecific distinctions but risks worsening genetic depletion and limiting adaptive potential. We see controlled ex situ testing as a valuable approach to assess this strategy, balancing the preservation of subspecific traits until the fitness benefits can be confirmed (Wilder et al. 2020).
Currently, Z. obcordata is listed as Endangered under both New South Wales and Federal legislation. To highlight the critical conservation need of the two taxonomically distinct populations, here recognised as subspecies, we propose that each subspecies of Z. obcordata qualifies for listing as Critically Endangered due to the highly reduced population size and extent of occurrence, future threats and not being in formal conservation reserves (see Taxonomy; IUCN Species Survival Commission 2012). If this proposal is accepted and the subspecies are formally listed, the original species listing will need to be delisted. This heightened conservation status is warranted due to the geographic isolation, extremely small populations sizes, current threats, lack of formal protection in reserves and heightened genetic depletion compared to other Zieria species.
Taxonomy
Zieria obcordata A.Cunn. in B.Field (ed.), Geogr. Mem. New South Wales 330 (1825)
Type: NEW SOUTH WALES. Macquarie River, Oct. 1822, A.Cunningham 92 (syn:lectotype, designated here: K [K000717168 image!]; isolecto: BM [BM001015520 image!], BRI [AQ021623 image!], K [K000717167 image!], MEL [MEL62119 image!]).
Spreading to prostrate aromatic shrub to 50 cm high and 100 cm wide. Branchlets terete, leaf bases not decurrent, smooth or slightly to prominently glandular–tuberculate, pubescent with simple hairs; hairs to ~0.5 mm long. Leaves trifoliolate; petiole 1.5–3.5 mm long, pilose, glandular tuberculate; central leaflet cuneate–obovate, 2–9 mm long, 1.5–5 mm wide, margins glandular crenulate and slightly undulate, flat to recurved, apex rounded to obcordate; adaxial surface slightly glandular–verrucose, sometimes only near margins, hirsute; abaxial surface not glandular–verrucose on lamina, hirsute, midrib slightly raised, hirsute, glandular–verrucose, sometimes sparsely so, secondary venation obscure; lateral leaflets similar to terminal leaflets and slightly shorter to slightly longer. Inflorescence shorter than leaves, 1–3-flowered; peduncle 1–2.5(–9) mm long, not glandular tuberculate; pedicle 1–3 mm long, not glandular tuberculate; prophylls leaflike, smaller than leaves, simple or trifoliolate, to 5 mm long. Sepals 0.75–1.5 mm long and wide, deltate, slightly glandular–verrucose, hirsute abaxially. Petals valvate, 1.0–2.5(–4) mm long, very pale pink to creamy white, deciduous with fruit, adaxial surface with few hairs or glabrous, abaxial surface pilose. Disc glabrous, with four antisepalous lobes. Stamens 4, opposite sepals; filaments glabrous or with few hairs adaxially, slightly glandular–verrucose apically; anther glabrous, without an apiculum. Ovary glabrous, becoming pubescent with maturity; style glabrous, short, narrow; stigma slightly wider than style, 4-lobed. Cocci not apiculate, not glandular–verrucose though glandular or glandular–verrucose with large hemispherical glands, pubescent, 2.5–4 mm long, 2–2.5 mm wide. Seed black, striated, 2.0–2.5 mm long, 1.2–2.0 mm wide (Fig. 5).
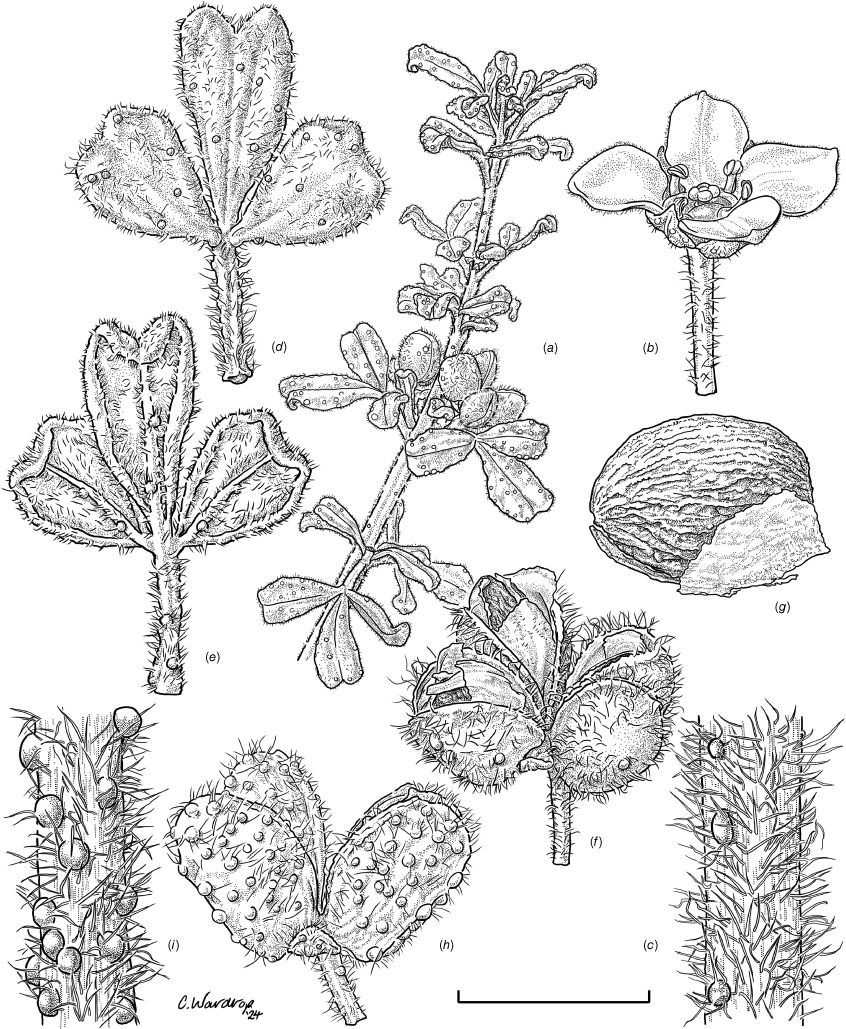
Zieria obcordata subsp. obcordata (NSW847717): (a) fruiting habit; (b) flower; (c) stem detail; (d) leaf, abaxial view; (e) leaf, adaxial view; (f) fruiting cocci; (g) seed, lateral view. Zieria obcordata subsp. wuuluman (NSW368782): (h) fruiting cocci; (i) stem detail. Scale bar: (a) 8 mm; (b, d, e, f, h) 4 mm; (c, i) 1.6 mm; (g) 2 mm. The morphological character that distinguished the populations was the presence or absence of glandular tubercula (bumps) on the stems and cocci: for the Bathurst population (Zieria obcordata subsp. obcordata) these were not distinct or only partially so and for the Wellington population (Zieria obcordata subsp. wuuluman) these were present to a marked degree (see Taxonomy). Illustration by Catherine Wardrop.
A restricted species known from two small areas near Bathurst and Wellington, New South Wales, Australia. Specific geolocation details have been suppressed to protect the species.
Grows in previously cleared areas in Eucalyptus and Callitris woodland and shrubland on granitic hillsides with a weedy understory of herbs and grasses. Brachychiton has been recorded from some populations.
Flowers have been recorded from September to December, and March and May; fruit have been recorded from September to March.
Cunningham (1825) indicated that the original material of Zieria obcordata was collected from near the Macquarie River. Both populations, here recognised as subspecies, occur near the Macquarie River in the Bathurst and Wellington areas. The two subspecies are distinguished morphologically by the absence or presence of glandular tubercles on the cocci and stems. The isolectotypes at the Queensland Herbarium (BRI) and National Herbarium of Victoria (MEL) have cocci that are smooth, therefore the form from the Bathurst area is the type subspecies and the population near Wellington is newly described below as Z. obcordata subsp. wuuluman.
Armstrong (2002), when revising the genus Zieria, designated one of the syntypes of Z. obcordata lodged at Kew as the holotype without discussion. As outlined by McNiell (2014) this is not considered to be a valid approach and the type collections listed by Armstrong (2002) are syntypes. Zieria obcordata is lectotypified above against the specimen at Kew that Armstrong (2002) designated the holotype as this is still the logical specimen to use, containing the appropriate data (collector, location, date, etc.) and being in good condition.
Zieria obcordata is currently listed as an Endangered species in both New South Wales and Australian legislation. The species has an active conservation plan (New South Wales Department of Environment and Conservation 2007; Saving our Species Program 2020). Both subspecies have been assessed as Critically Endangered (see below under each subspecies) and the species listing requires review. Formally listing each subspecies separately would be appropriate.
Zieria obcordata subsp. obcordata
Branchlets not prominently glandular–verrucose or with scattered glandular tubercles. Cocci smooth, glandular but not prominently glandular–verrucose.
Confined to a number of subpopulations near Crackerjack Rock, west of Bathurst, Central Tablelands, New South Wales.
Zieria obcordata subsp. obcordata is localised and known from several subpopulations that occur over a limited area that is ~3.5 km across. The species has been extensively surveyed in the area through the Saving Our Species program. The area is heavily disturbed and though stock and wildlife have been excluded from some plants, these are an ongoing threat to plants and successful recruitment outside the small, protected areas. The subspecies is found entirely on private property, in heavily disturbed areas and therefore not secure in the long term. Future threats include changes of land use, such as increased stocking or clearing, changes in conservation management and climate change. The overall population likely constitutes slightly more than 250 adult individuals scattered over a wide area. Shelly et al. (2021) indicated that the subspecies had significant reduction in plant numbers (an average loss of 82% of plants at selected sites) due to drought conditions from 2017 to 2019. Significant recruitment (thousands of seedlings) has occurred following recent wet conditions (Shelly et al. 2021) but the success rate of these seedlings is not known. The EOO of this subspecies is 4 km2 and the AOO is 16 km2 (Table 3). The assessment below is based on adult plants.
As far as could be determined, Zieria obcordata subsp. obcordata meets the criteria for listing as a Critically Endangered Species under the New South Wales Biodiversity Conservation Act 2016 (see also IUCN Species Survival Commission 2012; IUCN Standards and Petitions Committee 2024). According to the New South Wales Biodiversity Conservation Regulation 2017, Clause 4.3 (Restricted Geographic Distribution of Species and Other Conditions; equivalent to IUCN Standards and Petitions Committee 2024, Criterion B), the subspecies qualifies for this status due to its extremely limited range (EOO <100 km²) and severely fragmented population, consisting of one main population with several small subpopulations. Additionally, ongoing decline is evident in both the number of subpopulations and habitat quality. The species primarily grows in a rural setting on private land with minimal formal protection, and its survival is further threatened by herbivory and invasive weeds, both of which are well documented.
In addition, Zieria obcordata subsp. obcordata meets the requirements to be listed as an Endangered Species under Clause 4.4 (Low numbers of mature individuals of the species and other conditions; equivalent to IUCN Standards and Petitions Committee 2024, Criterion C) as there are probably between 250 and 500 mature individuals, the number of individuals in each subpopulation is ≤250 and continued decline can be inferred (see discussion under Clause 4.3 above); and as a Vulnerable Species under Clause 4.5 (Low total numbers of mature individuals of species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion D) as the number of mature individuals is ≤1000 and Clause 4.7 (Very highly restricted geographic distribution of species–vulnerable species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion D2) as the AOO is ≤20 km2. This subspecies does not appear to be eligible to be listed under Clauses 4.2 (Reduction in population size of species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion A) or Clause 4.6 (Quantitative analysis of extinction probability; equivalent to IUCN Standards and Petitions Committee 2024, Criterion E).
NEW SOUTH WALES. Central Tablelands: ‘Little Waco’, 1340 Ophir Rd, Rock Forest, 17 Dec. 2015 G.P.Phillips 29 & A.E.Orme (NSW847720); 1391 Ophir Rd, Rock Forest, North Jack site, 17 Dec. 2015, G.P.Phillips 28 & A.E.Orme (CANB n.v., NSW); North Jack site, ~1 km S along track from Ophir Road turn-off ~17 km WNW from Bathurst, 8 Apr. 2008, P.Carmen 377, C.Hook, R.Armstrong & K.Bollard, (NSW930639); ~12 km west of Bathurst, Oct. 2015, T.Bangel s.n. (NSW857221); Crackerjack Rock, W of Bathurst, 27 Oct. 1963, C.K.Ingram s.n., (NSW75929); Bathurst, 32 Pine Ridge Road, 5 May 2011, M.F.Mulvaney 1 (NSW); 1.3 km direct NNE of the summit of Crackerjack Rock, ~17 km W of Bathurst via Ophir Road, 26 Sep. 1988, J.D.Briggs 2433 (CANB n.v., NSW); 1.3 km direct north-north-east of the summit of Crackerjack Rock, ~17 km west of Bathurst via Ophir Road, 26 Sep. 1988, J.D.Briggs 2433 (CANB n.v., NSW); ~1 km north-east of Crackerjack Rock, 11 Mar. 1994, R.L.Johnstone 44 (NSW); Crackerjack Rock, W of Bathurst, 27 Oct. 1963, C.K.Ingram s.n. (NSW); ‘The Rocks’ property W of Bathurst, 23 Nov. 2001, L.R.Cole s.n. (NSW608324); ‘Lunch Site’ on North Jack property, Ophir Rd, Rock Forest, 11 Dec. 2019, G.P.Phillip 982 & D.Shelly s.n. (NSW); North Jack property, Ophir Rd, Rock Forest, 12 Dec. 2019, G.P.Phillips 983 & D.Shelly (NSW); Crackerjack North, near Crackerjack Rock, 15 km West of Bathurst, 1 Apr. 2022, D.Shelly s.n. (NSW1078495); Pine Ridge Road, 15 km west of Bathurst, 01 Apr. 2022, D.Shelly s.n. (NSW1078496); ‘Little Waco’, Ophir Rd, Rock Forest, 11 Dec. 2019, G.P.Phillips 981 & D.Shelly (CANB n.v., NSW1059946).
Zieria obcordata subsp. wuuluman McMaster & Duretto, subsp. nov.
Type: NEW SOUTH WALES. Bulbudgeree Station near Wuuluman, 15 km east-north-east of Wellington, 18 Oct 1978, J.A.Armstrong 1267 (holo: NSW368782!; iso (all n.v.): AD, HO, K, L, MEL).
Differs from the typical subspecies in having glandular–verrucose branchlets (v. with or without scattered glandular tubercules) and cocci that are prominently glandular–verrucose with large hemispherical glands (v. smooth or slightly glandular tuberculate; Fig. 5).
Armstrong (2002, p. 405, fig. 92), as Zieria obcordata.
Known from two subpopulations on ‘Bulbudgeree’ Station near Wuuluman, east-north-east of Wellington.
The subspecific epithet refers to Wuuluman, a local placename. Verbal permission to use the name was granted to Eilish McMaster over the telephone by the Wellington Local Aboriginal Land Council (4 July 2024).
Zieria obcordata subsp. wuuluman is highly localised and known with certainty from a single population with four subpopulations occurring in a limited area ~1 km across. The species has been extensively surveyed in the area through the Saving our Species program. The area is heavily disturbed, and though stock and wildlife have been excluded from some plants, these remain ongoing threats to plants and successful recruitment outside these small, protected areas. The subspecies occurs entirely on private property, in heavily disturbed areas and is therefore not secure in the long term. Shelly et al. (2021) indicated that the subspecies had significant reduction in plant numbers (an average loss of 84% of plants at selected sites) due to drought conditions between 2017 and 2019. Significant recruitment (thousands of seedlings) occurred following recent wet conditions (Shelly et al. 2021) but the success rate of these seedlings is not known. Future threats include changes in land use, such as increased stocking or clearing and climate change. The overall population consists of ≤250 adult individuals. The EOO of this subspecies is 1 km2 and the AOO is 8 km2. The assessment below is based on adult plants.
As far as could be determined, Z. obcordata subsp. wuuluman meets the requirements to be listed as a Critically Endangered Species under the New South Wales Biodiversity Conservation Act 2016 (see also IUCN Species Survival Commission 2012; IUCN Standards and Petitions Committee 2024). Following the criteria as set out in the New South Wales Biodiversity Conservation Regulation 2017, under Clause 4.3 (Restricted geographic distribution of species and other conditions; equivalent to IUCN Standards and Petitions Committee 2024, Criterion B) the subspecies is very highly restricted (EOO is <100 km2), the population is severely fragmented (one population with several subpopulations), continued decline can be inferred for both the number of subpopulations (many small) and the quality of the habitat as the species is growing in a rural setting on private land with limited formal protection, and herbivory is well documented.
In addition, Z. obcordata subsp. wuuluman meets the requirements to be listed as an Endangered Species under Clause 4.4 (Low numbers of mature individuals of species and other conditions; equivalent to IUCN Standards and Petitions Committee 2024, Criterion C) and Clause 4.5 (Low total numbers of mature individuals of species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion D) as there are ≤250 adult individuals, though some subpopulations have between 50 and 250 individuals; and as a Vulnerable Species under Clause 4.5 (Low total numbers of mature individuals of species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion D) and Clause 4.7 (Very highly restricted geographic distribution of species–vulnerable species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion D2) as the species is known with certainty from one population with an AOO of <20 km2. The subspecies does not appear to be eligible to be listed under Clauses 4.2 (Reduction in population size of species; equivalent to IUCN Standards and Petitions Committee 2024, Criterion A) or Clause 4.6 (Quantitative analysis of extinction probability; equivalent to IUCN Standards and Petitions Committee 2024, Criterion E).
NEW SOUTH WALES. Central Western Slopes: Bulbudgeree Stn, ~16 km ENE of Wellington, 17 Sep. 1988, J.D.Briggs 2376 (CANB n.v., MEL n.v., NSW); Poggy Cottage, ~16 km ENE of Wellington on ‘Poggy Cottage’ (2.6 km SE along the Uungula Road from Gulgong Road), 22 Oct. 2009, P.Carmen 425, D.Taylor & C.Hook (NSW, MEL n.v.); ibid, 22 Oct. 2009, P.Carmen 429, D.Taylor & C.Hook (NSW); Smith’s property, Bulbudgeree, near Wellington, 7.Mar. 1978, C.K.Ingram, P.Althofer, R.G.Coveny s.n. (NSW); Wuuluman, 1947, P.M.Althofer s.n. (NSW4713); Bulbudgerie near Wuuluman [15 km east-north-east of Wellington], 7 Mar. 1978, R.G.Coveny 10060 & C.K.Ingram (BRI n.v., CANB n.v., MEL n.v., MO n.v., NBG n.v., NSW, P n.v.); Property of Poggy Cottage east of Wellington, 10 Mar. 2022, D.Shelly s.n. (NSW1078497).
Data availability
The data that support this study are available in The University of Sydney eScholarship Repository at https://hdl.handle.net/2123/33657
Declaration of funding
This study received support from the Saving our Species program of the New South Wales Department of Climate Change, Energy, the Environment and Water (DCCEEW, formerly the Department for Planning and Environment).
Acknowledgements
We are grateful to the Traditional custodians of the land on which our collections took place. We honour the enduring connection that Indigenous peoples have with their ancestral territories and recognise their historical custodianship of these landscapes. We thank the Wellington Local Aboriginal Land Council for granting us permission to use the local name ‘wuuluman’ for the newly described subspecies. We thank Catherine Wardrop for illustrations of the Zieria obcordata subspecies. We are grateful to Paul and Cathy Carmen, Darren Shelly, Joanna Haddock, Claudia Pilon-Summons, Michaela Jones, Clive Barker, Lachlan Copeland, James Faris, Guy Taseski and Trevor Wilson for data collection efforts. We are grateful to the senior threatened species officer Joanna Haddock for her continued management of the Z. obcordata populations. We thank the surveyors Gaylene Bennison, Darren Shelly, Kate Hammill, Tanya Bangel, Garry Germon, Andrew E. Orme and Gavin Phillips for conducting the surveys that were used to calculate IUCN area metrics. We acknowledge the ongoing support of the Saving our Species program of the New South Wales Department of Environment and Heritage, and the federal Department of Climate Change, Energy, the Environment and Water in conserving these species, and for providing location data for EOO and AOO analyses. We also thank the editors of Australian Systematic Botany and the reviewers for improving the document significantly. All plant collections made for this project were conducted under the Botanic Gardens of Sydney collection permit and we are thankful for their support in facilitating these efforts.
References
Armstrong JA (2002) The genus Zieria (Rutaceae): a systematic and evolutionary study. Australian Systematic Botany 15, 277.
| Crossref | Google Scholar |
Arunkumar R, Ness RW, Wright SI, Barrett SCH (2015) The evolution of selfing is accompanied by reduced efficacy of selection and purging of deleterious mutations. Genetics 199, 817-829.
| Crossref | Google Scholar | PubMed |
Atlas of Living Australia (2025) Occurrence records download on 2025-02-21. [Data file] 10.26197/ala.4c75dff0-c10e-4322-8f23-88601d12418a
Barrett RA, Bayly MJ, Duretto MF, Forster PI, Ladiges PY, Cantrill DJ, Barrett RA, Bayly MJ, Duretto MF, Forster PI, Ladiges PY, Cantrill DJ (2015) A chloroplast phylogeny of Zieria (Rutaceae) in Australia and New Caledonia shows widespread incongruence with species-level taxonomy. Australian Systematic Botany 27, 427-449.
| Crossref | Google Scholar |
Barrett RA, Bayly MJ, Duretto MF, Forster PI, Ladiges PY, Cantrill DJ (2018) Phylogenetic analysis of Zieria (Rutaceae) in Australia and New Caledonia based on nuclear ribosomal DNA shows species polyphyly, divergent paralogues and incongruence with chloroplast DNA. Australian Systematic Botany 31, 16-47.
| Crossref | Google Scholar |
Belbin L, Wallis E, Hobern D, Zerger A (2021) The Atlas of Living Australia: history, current state and future directions. Biodiversity Data Journal 9, e65023.
| Crossref | Google Scholar | PubMed |
Bell DA, Robinson ZL, Funk WC, Fitzpatrick SW, Allendorf FW, Tallmon DA, Whiteley AR (2019) The exciting potential and remaining uncertainties of genetic rescue. Trends in Ecology & Evolution 34, 1070-1079.
| Crossref | Google Scholar | PubMed |
Booy G, Hendriks RJJ, Smulders MJM, Groenendael JMV, Vosman B (2000) Genetic diversity and the survival of populations. Plant Biology 2, 379-395.
| Crossref | Google Scholar |
Buckleton J, Curran J, Goudet J, Taylor D, Thiery A, Weir BS (2016) Population-specific FST values for forensic STR markers: a worldwide survey. Forensic Science International Genetics 23, 91-100.
| Crossref | Google Scholar | PubMed |
Cardoso P (2017) red – an R package to facilitate species red list assessments according to the IUCN criteria. Biodiversity Data Journal 5, e20530.
| Crossref | Google Scholar | PubMed |
Chan WY, Hoffmann AA, van Oppen MJH (2019) Hybridization as a conservation management tool. Conservation Letters 12, e12652.
| Crossref | Google Scholar |
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, s13742-015-0047–8.
| Crossref | Google Scholar |
Cunningham A (1825) A specimen of the indigenous botany of the mountainous country, between the colony sound Port Jackson and the settlement of Bathurst; being a portion of the result of observations made in the months of October, November and December, 1822. In ‘Geographical Memoirs on New South Wales’. (Ed. B Field) pp. 323–336. (John Murray: London, UK) Available at https://gutenberg.net.au/ebooks13/1304421h.html#:∼:text=%7B-,Page%20323,-%7D
Darwin C (1883) ‘On the Origin of Species: By Means of Natural Selection, Or, the Preservation of Favored Races in the Struggle for Life.’ (Appleton) Available at https://books.google.com.au/books?id=M9cPAAAAYAAJ
Dostálek T, Münzbergová Z, Plačková I (2010) Genetic diversity and its effect on fitness in an endangered plant species, Dracocephalum austriacum L. Conservation Genetics 11, 773-783.
| Crossref | Google Scholar |
Duretto M, Forster P (2007) A taxonomic revision of the genus Zieria Sm. (Rutaceae) in Queensland. Austrobaileya 7, 473-544.
| Crossref | Google Scholar |
Duretto M, Forster P (2008) New subspecies for Zieria odorifera J.A.Armstr. (Rutaceae) from northern New South Wales. Austrobaileya 7, 681-690.
| Crossref | Google Scholar |
Duthie AB, Reid JM (2016) Evolution of inbreeding avoidance and inbreeding preference through mate choice among interacting relatives. The American Naturalist 188, 651-667.
| Crossref | Google Scholar | PubMed |
Frankham R (2015) Genetic rescue of small inbred populations: meta-analysis reveals large and consistent benefits of gene flow. Molecular Ecology 24, 2610-2618.
| Crossref | Google Scholar | PubMed |
Frankham R (2016) Genetic rescue benefits persist to at least the F3 generation, based on a meta-analysis. Biological Conservation 195, 33-36.
| Crossref | Google Scholar |
Fujii S, Kubo K, Takayama S (2016) Non-self- and self-recognition models in plant self-incompatibility. Nature Plants 2, 16130.
| Crossref | Google Scholar | PubMed |
Hogbin PM, Peakall R (1999) Evaluation of the contribution of genetic research to the management of the endangered plant Zieria prostrata. Conservation Biology 13, 514-522.
| Crossref | Google Scholar |
Hogbin PM, Crisp MD (2003) Evolution of the coastal neospecies Zieria prostrata (Rutaceae) and its relationship to the Zieria smithii species complex. Australian Systematic Botany 16, 515-525.
| Crossref | Google Scholar |
Huson DH (1998) SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14, 68-73.
| Crossref | Google Scholar | PubMed |
IUCN Species Survival Commission (2012) IUCN Red List categories and criteria, version 3.1. (International Union for Conservation of Nature and Natural Resources: Gland, Switzerland) Available at https://portals.iucn.org/library/node/10315
IUCN Standards and Petitions Committee (2024) Guidelines for Using the IUCN Red List Categories and Criteria. Version 16. (International Union for Conservation of Nature and Natural Resources) Available at https://www.iucnredlist.org/documents/RedListGuidelines.pdf
Jaccoud D, Peng K, Feinstein D, Kilian A (2001) Diversity arrays: a solid state technology for sequence information independent genotyping. Nucleic Acids Research 29, E25.
| Crossref | Google Scholar | PubMed |
Jombart T (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403-1405.
| Crossref | Google Scholar | PubMed |
Keenan K, McGinnity P, Cross TF, Crozier WW, Prodöhl PA (2013) diveRsity: an R package for the estimation and exploration of population genetics parameters and their associated errors. ethods in Ecology and Evolution 4(8), 782-788.
| Crossref | Google Scholar |
Kilian A, Wenzl P, Huttner E, Carling J, Xia L, Blois H, Caig V, Heller-Uszynska K, Jaccoud D, Hopper C, Aschenbrenner-Kilian M, Evers M, Peng K, Cayla C, Hok P, Uszynski G (2012) Diversity arrays technology: a generic genome profiling technology on open platforms. In ‘Data Production and Analysis in Population Genomics: Methods and Protocols’. (Eds F Pompanon, A Bonin) Methods in Molecular Biology, vol. 888, pp. 67–89. (Humana Press: Totowa, NJ, USA) 10.1007/978-1-61779-870-2_5
Lopresti LC, Sommerville KD, Gilpin A-M, Minchinton TE (2023) Floral biology, pollination vectors and breeding system of Zieria granulata (Rutaceae), an endangered shrub endemic to eastern Australia. Australian Journal of Botany 71, 252-268.
| Crossref | Google Scholar |
Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M (2010) Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867-2873.
| Crossref | Google Scholar | PubMed |
McMaster ES, Yap J-YS, McDougall KL, James EA, Walsh N, Jario N, Peterie J, Rossetto M (2024) Embracing biodiversity: multispecies population genomics of leafless Bossiaea species shows novel taxa, population dynamics and conservation strategies. Australian Systematic Botany 37, SB23031.
| Crossref | Google Scholar |
McNiell J (2014) Holotype specimens and type citations: general issues. Taxon 63(5), 1112-1113.
| Crossref | Google Scholar |
Neal WC, James EA, Bayly MJ (2019) Phylogeography, classification and conservation of pink zieria (Zieria veronicea; Rutaceae): influence of changes in climate, geology and sea level in south-eastern Australia. Plant Systematics and Evolution 305, 503-520.
| Crossref | Google Scholar |
New South Wales Department of Environment and Conservation (2007) Zieria obcordata Recovery Plan. (NSW DEC: Sydney, NSW, Australia) Available at https://www.dcceew.gov.au/sites/default/files/documents/zieria-obcordata.pdf
Orel HK, McLay TGB, Guja LK, Duretto MF, Bayly MJ (2024) Genomic data inform taxonomy and conservation of Critically Endangered shrubs: a case study of Zieria (Rutaceae) species from eastern Australia. Botanical Journal of the Linnean Society 205, 292-312.
| Crossref | Google Scholar |
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics 81, 559-575.
| Crossref | Google Scholar | PubMed |
Razanajatovo M, van Kleunen M, Kreft H, Dawson W, Essl F, Pergl J, Pyšek P, Winter M, Weigelt P (2019) Autofertility and self-compatibility moderately benefit island colonization of plants. Global Ecology and Biogeography 28, 341-352.
| Crossref | Google Scholar |
Rodger YS, Dillon R, Monro K, Pavlova A, Coates DJ, Byrne M, Sunnucks P (2024) Benefits of outcrossing and their implications for genetic management of an endangered species with mixed-mating system. Restoration Ecology 32, e14057.
| Crossref | Google Scholar |
Rossetto M, Yap J-YS, Lemmon J, Bain D, Bragg J, Hogbin P, Gallagher R, Rutherford S, Summerell B, Wilson TC (2021) A conservation genomics workflow to guide practical management actions. Global Ecology and Conservation 26, e01492.
| Crossref | Google Scholar |
Saving our Species Program (2020) ‘Granite Zieria’ Zieria obcordata fact sheet. (New South Wales Government) Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Animals-and-plants/Threatened-species/granite-zieria-zieria-obcordata-fact-sheet-200459.pdf
Seehausen O (2006) Conservation: losing biodiversity by reverse speciation. Current Biology 16, R334-R337.
| Crossref | Google Scholar | PubMed |
Sharma IK (2001) Understanding clonal diversity patterns through allozyme polymorphism in an endangered and geographically restricted Australian shrub, Zieria baeuerlenii and its implications for conservation. Biochemical Systematics and Ecology 29, 681-695.
| Crossref | Google Scholar | PubMed |
Shelly D, Irvin M, Faris J (2021) In the nick of time – post-drought recovery of two threatened Zieria species in central-western New South Wales. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 30, 13-16.
| Google Scholar |
Stace HM, Armstrong JA (1992) New chromosome numbers for rutaceae. Australian Systematic Botany 5, 501-505.
| Crossref | Google Scholar |
Weir BS, Hill WG (2002) Estimating F-statistics. Annual Review of Genetics 36, 721-750.
| Crossref | Google Scholar | PubMed |
Wilder AP, Navarro AY, King SND, Miller WB, Thomas SM, Steiner CC, Ryder OA, Shier DM (2020) Fitness costs associated with ancestry to isolated populations of an endangered species. Conservation Genetics 21, 589-601.
| Crossref | Google Scholar |
Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS (2012) A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 28, 3326-3328.
| Crossref | Google Scholar | PubMed |


