A new leaf species of Proteaceae and other Gondwanan elements from the early Paleogene Lota–Coronel flora of south–central Chile
Raymond J. Carpenter A * and Stephen McLoughlin B
A * and Stephen McLoughlin B
A
B
Abstract
Leaf fossils collected in 1908 from the Arauco–Concepción Coal Measures of Chile (the Lota–Coronel flora) during a Swedish expedition to southern South America are formally assigned to the important Gondwanan family Proteaceae as Proteaceaefolia araucoensis R.J.Carp. & McLoughlin gen. nov., sp. nov. This is the oldest South American record of macrofossils that can be assigned to Proteaceae with confidence due to the likelihood of the age dating to the latest Paleocene. The fossils lack cuticle but the large, lobed and minutely toothed form is consistent only with extant species of the subfamily Grevilleoideae (notably, Orites excelsus R.Br.) that are confined to eastern Australian rainforests. A new assessment of the Swedish Lota–Coronel collection and review of previous palynological and macrofossil studies, also provide evidence of the strong biogeographic connection that existed between southern South America and Australasia during the early Paleogene, and contradict a traditional view that several Chilean floras of this age consist wholly or largely of Neotropical taxa. Notable austral taxa include Casuarinaceae (as abundant pollen), diverse Podocarpaceae (as both foliage and pollen) and likely Cunoniaceae (leaves). No taxa with clearly Neotropical nearest living relatives have been found to date, but previous conclusions for a warm and very wet early Paleogene climate are supported.
Keywords: Arauco coal, Australasia, climate change, Gondwana, lobed leaf, Neotropics, Orites, Paleogene, plate tectonics, rainforest.
Introduction
Plant fossils housed at the Swedish Museum of Natural History (Naturhistoriska riksmuseet, NRM) include important collections of specimens from the early Paleogene coal mines of Lota and Coronel (hereafter Lota–Coronel), Concepción Province, Biobío Region, Chile, made by P. Dusén during the Nordenskjöld expedition, 1895–1897 (Dusén 1899) and T. G. Halle during the Swedish Magellanic Expedition, 1907–1909 (Skottsberg 1909).
The first taxonomic assessments of Lota–Coronel foliar fossils were undertaken mostly by Engelhardt (1891, 1905), with supplements by Berry (1922). These authors described a few species of ferns and gymnosperms, and over 100 species of angiosperms that included a high diversity of Lauraceae (14 species). Overall, these authors regarded the flora as essentially a Neotropical rainforest assemblage, where:
in every case its elements are represented by modern species of tropical South America east of the Andes, and in the main of forms dwelling in the Amazon basin, especially toward the Peruvian part of the basin, and extending southward into eastern Bolivia [Berry 1922, p. 106]
Physiognomy-based assessments of the flora followed the initial taxonomic determinations and reinforced the notion of a fundamentally Neotropical composition (Hinojosa and Villagrán 1997; Villagrán and Hinojosa 1997; Gayó et al. 2005). However, these authors did not cite Florin (1940), who determined that at least the Lota–Coronel conifers were incorrectly identified and rather belong to the austral family Podocarpaceae. Florin (1940) was also dubious of the angiosperm identifications, none of which has been critically reassessed since Berry’s (1922) publication over 100 years ago. Similarly, no new angiosperm taxa have been described from the flora since that time.
We address this gap in the literature with the description of a new leaf species of Proteaceae from the NRM Lota–Coronel collection. This description is based on a distinctively large, lobed leaf specimen (S165435) obtained by Halle that has an accompanying label identifying the specimen as being proteaceous but that has not been described or illustrated. Furthermore, we discuss the implications of this new species and associated taxa with respect to Gondwanan biogeography and the early Paleogene vegetation history and climate of southern South America, especially with regard to the perceived Neotropical signature of the Lota–Coronel flora.
Materials and methods
Fossil site and age of the sediments
Coal deposits of the mostly offshore Arauco–Concepción Basin (~37–38°S: Fig. 1) of central Chile, west of the Coastal Cordillera, were mined in the Arauco Peninsula, Coronel and Lota regions for ~150 years from the mid-19th century until the late 20th century, and provided the major energy resource for the country. The region is of great geocultural importance, including recognition by the Chilean government of the Lota El Chiflón del Diablo mine as a National Historic Monument. There is also a project underway to establish a UNESCO ‘Litoral del Biobío’ Mining Geopark (Ferraro et al. 2020) and a tentative listing of the Lota Mining Complex as a UNESCO World Heritage cultural site (see https://whc.unesco.org/en/tentativelists/6498/, accessed May 2024). There are numerous old coal mines in the region, including the Buen Retiro mine from which the target fossils of the current study were collected.
Map of Chile and the Arauco region, showing the locations of mine sites at which the NRM Halle and Dusén collections were made (map adapted from Collao et al. 1987; Servicio Nacional de Geología y Minería 2003; Castillo 2021).
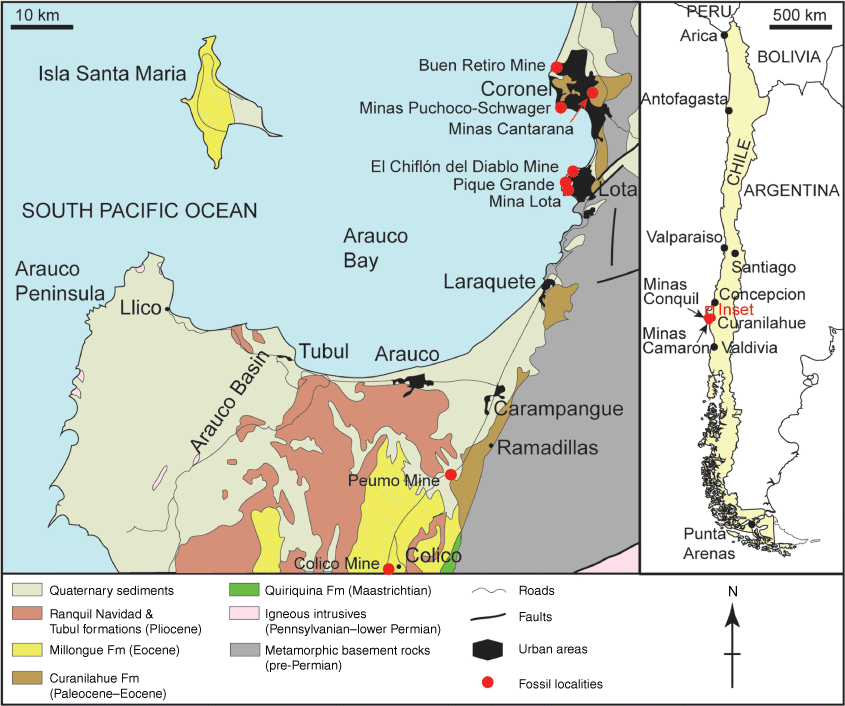
Upper Cretaceous and Cenozoic strata of the Lebu Group (Muñoz-Cristi 1946, 1956) of the Arauco Basin are interpreted to have accumulated as an interdigitating shallow marine to coastal continental succession influenced by a series of transgressions and regressions related to the subsidence and uplift of tectonic blocks associated with the development of the Andean Orogen to the east (Brüggen 1934; Muñoz-Cristi 1968; Wenzel et al. 1975; Pineda 1986; Le Roux and Elgueta 1997). Within the Lebu Group (Fig. 1), marine strata of the uppermost Maastrichtian (Stinnesbeck 1986; Salazar et al. 2010) Quiriquina Formation are unconformably overlain by continental shales and coals intercalated with sandstones of shallow marine origin. These strata have generally been divided into the mixed continental and marine Curanilahue, marine Boca Lebu, continental Trihueco and marine Millongue formations (García 1968; Muñoz-Cristi 1968) from base to top, of which the first two are classified by the Chilean Servicio Nacional de Geología y Minería (2003) as of Paleocene–Eocene age and the latter two of Eocene age. The Curanilahue Formation in turn comprises the terrestrial Lota and Colico members that are separated by fossiliferous glauconitic marine sandstones of the Intercalación Member. The coals, that occur within the basal Lota Member, formed within near-sea-level lagoonal and swamp environments during a regressive interval (Brüggen 1934; Pineda 1986).
Although an early Eocene age has been cited by numerous authors (e.g. Tavera 1942; Zeil 1964; Muñoz-Cristi 1968; Wenzel 1982; Pineda 1986; Collao et al. 1987; Le Roux and Elgueta 1997; Gayó et al. 2005; Malumián and Náñez 2011; Becerra et al. 2013), detailed biostratigraphic reviews by Martínez-Pardo et al. (1997) and Martínez-Pardo and Martínez-Guzmán (1997, 2008) placed the Lota Member succession entirely within the Paleocene, as also interpreted by García (1968) and Suárez et al. (2000) and accepted by many others, including Hinojosa et al. (2006). More recently, in describing an elasmobranch fauna and associated stratigraphic setting in the Concepción area slightly north of Lota–Coronel, Rodriguez et al. (2023) reasoned that the Curanilahue Formation is of latest Thanetian–early Ypresian age (i.e. latest Paleocene–early Eocene). Furthermore, a Paleocene age has recently been inferred from unpublished U–Pb zircon ages (Zambrano et al. 2014; W. Stinnesbeck, pers. comm. 2022) obtained from a sample sourced above the Quiriquina Formation disconformity at the base of the Curanilahue Formation at Hualpen (i.e. resolving a maximum depositional age of 57 Ma for this formation). Four other sedimentary rock samples from the base of the Trihueco Formation to the middle of the Millongue Formation provided U–Pb zircon ages of 50–52 Ma (i.e. within the early Eocene: Ypresian) that corresponds to the maximum depositional age of these samples (W. Stinnesbeck, pers. comm.). Given that the Lota Member coals are overlain by the Intercalación and Colico members of the Curanilahue Formation, and by the Boca Lebu Formation, a minimum age limit for these coals much younger than the Paleocene–Eocene boundary is unlikely and we conclude that the Lota–Coronel leaves were most likely deposited in the latest Paleocene (i.e. latest Thanetian).
Lota–Coronel plant fossils
As explained by Wilf (2012, pers. comm., September 2024), the vast bulk of the original Arauco–Concepción fossils described by Engelhardt (1891) appear to be lost. Berry’s (1922) collection at the National Museum of Natural History, Smithsonian Institution, USA (Wilf 2012; Wilf et al. 2017) is not extensive and closure of coal mines, together with degradation of spoil dumps, means that the prospects of obtaining new material are slim. However, the specimen that is the principal subject of the current study is part of the collection of ~200 registered specimens at the NRM obtained by Halle in 1908 from the Buen Retiro mine, ‘near the base of the coal-bearing series, between coal-seams 7 and 8’ (Halle 1940, p. 258). This site is located near the mouth of the Maule River, 5 km north-north-west of central Coronel (Fig. 1). Another major collection from slightly east of Coronel was obtained from Minas Cantarana, and the larger Halle collection also includes sundry material from Minas Puchoco-Schwager and Minas Ramon (Coronel), and the Lota (El Chiflón del Diablo and Pique Grande) and Lebu regions (Minas Conquil and Minas Camaron: Fig. 1). Further fossils from Lota (~9 km south of Coronel) were also collected, presumably on the same expedition, with attribution to ‘Condon 1908’ on the specimen labels. Other Cenozoic material from the Arauco region was collected by Dusén in 1896 (Dusén 1899), mostly from Curanilahue (~170 registered specimens) but also from Lota and Peumo. The Dusén collections also include material from multiple sites in southern Chile that were not investigated in this study.
As described by Florin (1940) and Halle (1940), the Buen Retiro specimens typically occur in a hard, generally light grey shale or claystone matrix. Several such specimens have already been studied in detail and have been robustly identified with the aid of organic preservation: Lygodium skottsbergii Halle (Schizaeaceae) from Buen Retiro (Halle 1940), Dacrycarpus chilensis (Engelh.) Wilf (Podocarpaceae) from Lota and Coronel (Engelhardt 1891, 1905; Wilf 2012), Retrophyllum araucoensis (E.W.Berry) Wilf (Podocarpaceae) from Buen Retiro and Lota (Berry 1922; Florin 1940; Wilf et al. 2017) and Coronelia molinae Florin (?Podocarpaceae) from Buen Retiro (Florin 1940).
The Buen Retiro mine was not listed as a collection locality by Engelhardt (1891) and Berry (1922), although the nearby Puchoco mine in Coronel was, along with Lota. Although there is agreement that the plant material forming the coals and preserved in associated sediments was deposited without significant transport, considering the local vegetation to not have been uniform in composition across the Arauco–Concepción region is reasonable. For example, as discussed by Florin (1940), gradients of edaphic variation related to fluctuating water tables must have been present across the extensive swamps. Nevertheless, floristic and physiognomic differences between the collections from the various localities appear not to be major, and treating all the collected fossils as of more-or-less contemporaneous deposition under broadly the same climatic conditions seems reasonable. However, whether the NRM fossils were collected systematically, e.g. without bias towards more ‘interesting’ or complete specimens, is unknown. Hence, a quantitative evaluation of the collection may not provide a meaningful measure of the richness or dominance of taxa in the palaeoflora. We also note the impossibility of further collecting from precisely the same sites or associated stratigraphic equivalents: the Lota and Coronel sites were active coal mines, and for example, Berry (1922) commented on the loss of fossiliferous shales as waste that was dumped directly into the Pacific Ocean.
None of Halle’s angiosperm fossils housed at the NRM have been described. According to Halle (1940), the fossils were due to be studied by Dr Fredrik Dahlstedt of the Museum but he died in 1939. Berry (1922, pp. 82–86) produced a revised systematic list of the Lota–Coronel flora, based on Engelhardt’s taxonomy, with a few updates from his new collections, and broadly agreed that the flora was composed of numerous families and genera currently well represented in the Neotropics, including Annonaceae (Annona L.), Euphorbiaceae (Mallotus Lour.), Fabaceae (Cassia L.), Sapindaceae (Sapindus L., Thouinia Poit.), Malvaceae (Triumfetta L.), numerous Lauraceae (e.g. Nectandra Rottb.), Myrtaceae (Myrcia DC.) and Rubiaceae (Coussarea Aubl.).
The Lota Member coal seams have been examined for microfossil content by Doubinger (1972), Doubinger and Chotin (1975), Takahashi (1977), Palma-Heldt (1980), Palma-Heldt et al. (2009) and Collao et al. (1987). These studies consistently reported a high abundance of fungal matter, pteridophyte spores and Haloragacidites harrisii (Couper) W.F.Harris pollen that was attributed to Myricaceae but is currently widely accepted to represent Casuarinaceae (see Discussion). The geological evidence for a low-energy coastal depositional setting was supported, especially by the presence of Spinizonocolpites echinatus J.Mull. (Palma-Heldt 1980; Collao et al. 1987; Palma-Heldt et al. 2009), a junior synonym of Spinizonocolpites prominatus (D.J.McIntyre) Stover & P.R.Evans (Pocknall et al. 2022), whose modern affinity is with the mangrove palm Nypa fruticans Wurmb (Arecaceae) (Muller 1968).
Fossil descriptions, photography and preparation
Leaf morphological descriptions follow Ellis et al. (2009). Apart from the new species of Proteaceae, we do not attempt new detailed descriptions or revisions of the other NRM Lota–Coronel fossils. However, given that to our knowledge, none of the angiosperms have been treated in the literature for >100 years, we illustrate and discuss a range of specimens that highlight the nature of the flora and the inherent potential for further research.
Specimens were photographed with Canon EOS 40D and 90D cameras, and images prepared using Photoshop Elements, with adjustment of brightness and contrast where appropriate. Important details of the type specimen were best observed using grayscale (Fig. 2). Cuticles proved difficult to recover owing to degradation and microfracturing; most mesophyll and cuticular material degraded into fine powder upon immersion in acids or household bleach. Attempts were subsequently made to observe epidermal and stomatal details using fluorescence microscopy (with incident-ultraviolet light excitation at ~460–490 nm) using an Olympus BX51 microscope with an Olympus DP71 digital camera.
(a, b) Proteaceaefolia araucoensis specimens from Buen Retiro. (c) A leaf of the very similar extant Australian species Orites excelsus. (a) S165435 (Type specimen). Minute teeth are generally indistinct, however the inset magnification from region x of the apical lobe is interpreted to show a tooth right of t, to which a minor vein below b branches from a secondary vein loop l. (b) S160367. Note the vein loops and branches to minute teeth along the margin on the right. (c) Specimen from Beechmont, Queensland, Australia. Note especially the same form of lobing and marginal teeth as in the fossils. Scale bars: 5 cm for (a) and (c); 1 cm for (b). The scale intervals for (a) inset are 1 mm.

Taxonomy
Proteaceaefolia araucoensis R.J.Carp. & McLoughlin gen. nov., sp. nov.
(Fig. 2.)
Type: S165435 (Fig. 2). Lota Member, Curanilahue Formation; latest Paleocene (?to early Eocene); Buen Retiro coal mine, Concepción Province, Biobío Region, Chile. Swedish Museum of Natural History, Stockholm, Sweden.
Leaves without preserved cuticle, pinnately lobed, serrate, base acute and cuneate. Lobes alternately placed, narrowly oblong to ovate, apically directed, apices acute, sinuses rounded. Teeth small, apically directed. Venation semicraspedodromous where teeth present or inferred, otherwise brochidodromous.
Leaf (Fig. 2) pinnately lobed, evidently sparsely serrate, large (~29 cm long but tip of acute apex missing), ~14.5 cm wide (measured between extremities of lobes), base acute and cuneate; lateral lobes alternately placed, apically directed, apices acute, sinuses rounded, ~11 cm long (measured from primary vein divergence point from midvein to incomplete (L) and indistinct (R) apices) and ~7–8 cm from base of sinus, ~2.5 cm wide, narrowly oblong to ovate (tapering toward apex), asymmetrical (narrower) near sinus; terminal (distal) lobe ~17.5 cm long from distalmost (L) lobe vein junction and ~13 cm from base of sinus, elliptic, ~2.2 cm wide from midvein to margin opposite small sublobe, this ~5 cm long from midvein divergence point. Teeth sparse (~1 per 1.5 cm of margin), minute (~0.5–1 mm long), very indistinct due to poor preservation but directed apically and most evident along margin of terminal lobe. Venation semicraspedodromous where teeth inferred, otherwise brochidodromous; lateral lobe primary veins arising at ~60°, then curved upwards near midvein and running straight to apex at ~45° from midvein, apical sublobe arising at ~45° and running at ~30°; ~10 alternately placed secondary veins per side in major lobes arising at ~80° from primary vein, then running at ~60° before curving up to junction with super-adjacent secondaries; regular intersecondary veins present, distinct admedially but indistinct exmedially; higher order veins not preserved.
The type (S165435) is the only more-or-less complete specimen known and preserved as an impression in pale grey sandy siltstone. S160367 is a leaf fragment only (Fig. 2). This could be part of a non-lobed leaf but is here placed in the new species because of the identical venation and similar width to that of the lobes of the type. The teeth are better preserved than those of the type.
Associated with Halle’s 1908 collection label for the type is a more recent label that names both Halle (in 1908) and P. Dusén (in 1896) as collectors, and another new label provides an identification as Proteaceae and the specimen number (S165435). However, to our knowledge no mention or illustration of this leaf has previously been made in the literature. The reference to Dusén is enigmatic and may be disregarded, since in our understanding he did not collect from Buen Retiro.
The coarseness of the sedimentary matrix precludes clear observation of minute teeth on the margin of the type specimen. However, there is physical evidence of at least one tooth (Fig. 2), and the location of others can be inferred, especially by reference to specimen S160367, where the secondary veins more clearly approach the marginal teeth and deflect apically, i.e. semicraspedodromously) (Fig. 2).
A thorough phylogenetic assessment of leaf architecture and venation types across Proteaceae is lacking but we hypothesise that the large, lobed, simple leaf-type of the new species that shows evidence of teeth and semicraspedodromous venation is definitively proteaceous, and is not found in any other taxa, extinct or extant. Circumstantial evidence that this, and other Southern Hemisphere leaf fossils of proteaceous aspect, indeed belong in the family is that Proteaceae pollen is typically diverse and abundant across this region in the Paleogene (Dettmann and Jarzen 1998). Moreover, many such leaves have well-preserved cuticles (or at least with faithfully recorded diagnostic imprints thereof: Carpenter et al. 2014a) showing brachyparacytic stomata and compound trichome bases (i.e. an annular surface scar associated with more than one underlying epidermal cell), that in combination provide strong evidence supporting attribution to the family (Carpenter et al. 2005).
Certain fossil forms of very large lobed leaves from the Northern Hemisphere have historically been assigned to Artocarpus J.R.Forst. & G.Forst. (breadfruits and jackfruits), an extant genus of Moraceae but such leaves are clearly entire-margined and the better-preserved specimens (notably ‘A.’ lessigiana (Lesq.) Knowlt.) are known to have venation and cuticular morphology that indicates affinities with Lauraceae instead (Upchurch and Wolfe 1987; K. R. Johnson, pers. comm. to Manchester 2014).
Proteaceaefolia is necessary because we do not regard any previous genus to be a satisfactory repository for the fossils. These cannot be assigned to Euproteaciphyllum G.J.Jord., R.J.Carp. & R.S.Hill because this genus requires cuticular evidence (Carpenter and Jordan 1997; Jordan et al. 1998). Also, previous genera for impression fossils, many established from the Northern Hemisphere before the late 20th century (e.g. Proteoides Heer), do not define characters that are strong evidence for Proteaceae, and it is extremely doubtful that any of the specimens assigned to these are proteaceous (e.g. Cookson and Duigan 1950; Johnson and Briggs 1963, 1975; Gonzalez et al. 2007; Olde 2017). Even in the Southern Hemisphere, Carpenter et al. (2016) warned against inferring that all species of Banksieaeformis R.S.Hill & Christophel (erected for Banksia-like leaves lacking cuticle: Hill and Christophel 1988) truly represent Banksia or even Proteaceae.
Lobed and small-toothed leaf species of Proteaceae have been described from other Southern Hemisphere Paleogene assemblages, including the cuticle-bearing Megahertzia paleoamplexicaulis R.J.Carp. & Rozefelds (Carpenter and Rozefelds 2023) and Maslinia grevilleoides D. T. Blackburn (Blackburn 1981) from Eocene strata of southern Australia. The known specimens of these two species are approximately half the size of the Arauco–Concepción type specimen. Moreover, Megahertzia A.S.George & B.Hyland can readily be differentiated by the amplexicaul leaf base and although the gross morphological features of the Chilean fossils could satisfy the diagnosis for Maslinia, the lack of cuticle precludes assignment there. The early Eocene Patagonian Lomatia occidentalis (E. W. Berry) Freng. differs in having imparipinnate or pinnatisect leaves (Frenguelli 1943; Gonzalez et al. 2007) with much narrower segments.
Extant Proteaceae is well known for the great variety of foliage forms that change in many cases throughout the development of the plant (or in some cases, of the shoot). According to Johnson and Briggs (1975), Flora treatments (Sleumer 1954, 1955; Virot 1968; Orchard et al. 1995; Prance et al. 2007; Hopkins and Pillon 2020) and personal observations, large pinnately lobed leaves in plants that are older than seedling stage are not found in South America, and are limited to Australasia and Malesia: these occur in subfamilies Persoonioideae (Placospermum C.T.White & W.D.Francis) and especially Grevilleoideae (Alloxylon P.H.Weston & Crisp, Athertonia L.A.S.Johnson & B.G.Briggs, Buckinghamia F.Muell., Darlingia F.Muell., Grevillea R.Br. ex Knight, Heliciopsis Sleumer, Orites R.Br., Megahertzia, Stenocarpus R.Br. and Virotia L.A.S.Johnson & B.G.Briggs) but are absent in the large subfamily Proteoideae. Adult plants having such leaves with small teeth occur exclusively in tree species of eastern Australian rainforests: i.e. Orites excelsus R.Br. and Megahertzia amplexicaulis of the tribe Roupaleae, and Athertonia diversifolia (C.T.White) L.A.S.Johnson & B.G.Briggs (Johnson and Briggs 1975) of tribe Macadamieae subtribe Virotiinae.
The true phylogenetic position of Proteaceaefolia araucoensis, presumably within subfamily Grevilleoideae, cannot be inferred in the absence of cuticular evidence. However, we consider that the fossils are morphologically closest to leaves found within Orites excelsus (Fig. 2) and note with relevant significance that: (1) leaf fossils (O. excelsoides R.J.Carp. & G.J.Jord.) with cuticle distinctively similar to Orites excelsus occur in the early Oligocene of Tasmania (Carpenter and Jordan 1997; Carpenter 2012); (2) follicular fruits assigned to Orites ‘without reservations’ (Gonzalez et al. 2007, p. 254) occur in the early Eocene of Patagonian Argentina. These fruits co-occur with imparipinnately lobed leaves assigned to Lomatia occidentalis but these lack apomorphic support (including cuticular evidence) for referral to that genus and could well belong in other genera (Carpenter 2012), especially within Orites; (3) Pole (1994) reported poorly preserved but small-toothed and probably trilobed leaf specimens from the early to middle Eocene of New Zealand that he assigned to ‘cf. Orites excelsa’; (4) together with O. excelsus, other extant Orites species currently occur in Tasmania, mainland south-eastern Australia and South America, and genetic evidence does not contradict a vicariance explanation for this distribution (Barker et al. 2007). We rejected the option to attribute the fossils to Orites but the future recovery of better-preserved specimens, especially with cuticular details, may allow this.
A full taxonomic review of other specimens from the Buen Retiro site and others of the Arauco–Concepción Coal Measures is beyond the scope of this study. We briefly discuss and illustrate some specimens but for more information on the occurrence, age and current taxonomic attributions of the Chilean Cenozoic plant fossils in the NRM collections, the reader is directed to the searchable database (see https://samlingar.nrm.se/faces/pages/results.xhtml).
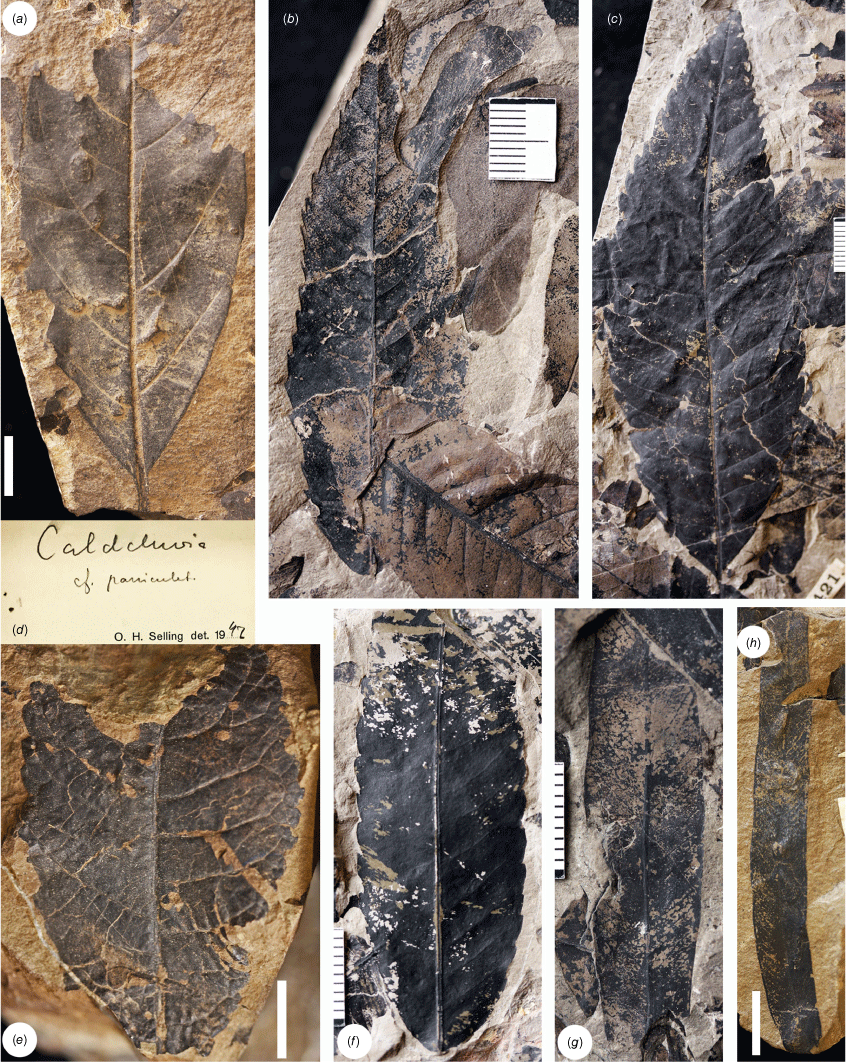
The species previously established from the Chilean assemblages, i.e. Lygodium skottsbergii (Fig. 3), Dacrycarpus chilensis (Fig. 3), Retrophyllum araucoensis (Fig. 3) and Coronelia molinae are all represented in the Buen Retiro collection. Lygodium specimens are abundant, as are numerous sterile foliage specimens labelled as ‘Polypodiopsida’ (Fig. 3) that we confidently refer to Blechnaceae. This foliage is strikingly similar to that of the extant Chilean hard fern, Parablechnum chilense (Kaulf.) Gasper & Salino that intriguingly occurs in the same region of Chile today, but which, on molecular evidence, belongs to a clade that evolved and diversified much more recently than the early Paleogene (Testo et al. 2022). Sterile fronds of this species have a distinctively undulate margin and the same feature is clearly apparent in many of the fossils (Fig. 3). Engelhardt (1891, pp. 642–644 and pl. 2, fig. 1–4) described apparently similar foliage from Lota as Pteris cousiniona Engelh., and other Blechnum L.-like foliage is known from the spatially and temporally similar Quinamávida flora (Troncoso 1992). More recently, Machado et al. (2023) discussed these taxa and other Blechnaceae fossils in light of well-preserved sterile foliage from the Arroyo Chacay flora (of likely early Eocene age) of Argentina described as ‘cf. Parablechnum C.Presl’, also noting strong resemblance to P. chilense and the closely related P. cordatum (Desv.) Gasper & Salino.
Examples of Lota–Coronel fossils collected by Halle from Buen Retiro (a, b, e–j) and by Dusén from Peumo (c, d). (a, b) Lygodium skottsbergii; (a) S165197 sterile, (b) S165201 fertile specimens. These specimens were illustrated by Halle (1940, plate 1, fig. 3, 7 respectively). (c) S160125 Dacrycarpus chilensis. This specimen was illustrated by Florin (1940, plate 6, fig. 10), although he attributed the collection to Halle from Minas Conquil. (d) S160127-01 Retrophyllum araucoensis. This specimen was illustrated by Florin (1940, plate 1, fig. 12). (e–h) cf. Parablechnum; (e) S160536 attributed to Polypodiopsida on accompanying label, (f) S160401, (g) 160417-02 attributed to Polypodiopsida on accompanying label, (h) S160441. Note the undulate margin shown in (g) and (h). (i, j) Likely Proteaceae; (i) S160390, (j) S160375. Note in inset that UV fluorescence revealed the presence of brachyparacytic stomata on this specimen. (k) S160513-02 likely Lauraceae with suprabasally actinodromous venation. Scale bars: 1 cm for foliage specimens, 50 µm for cuticle in (j) inset.

Angiosperm leaves and especially leaf fragments are common. The specimens of Proteaceaefolia araucoensis and several other fragments of likely Proteaceae leaves have been observed only from Buen Retiro – S160375 and S160390 appear to be parts of toothed, pinnatisect leaves with organic remains (Fig. 3) that are not assigned to P. araucoensis due to insufficient diagnostic features. Nevertheless, UV fluorescence yielded evidence at least of stomata with brachyparacytic subsidiary cells on S160375, the arrangement that is typical of Proteaceae. Leaves of likely several species of Lauraceae (Fig. 3, 4) occur throughout. These are of uncertain affinities but include at least one taxon with suprabasally actinodromous venation (Fig. 3). Cunoniaceae-like and Elaeocarpaceae-like specimens include those from Buen Retiro (S160421-01) that are accompanied by a label with the determination ‘Caldcluvia cf. paniculata’ by O.H. Selling in 1942 (Fig. 4). We agree with this determination based on overall leaf morphology, including the nature of the craspedodromous venation and the cunonioid teeth. A few leaves with intramarginal veins and the type of high-angled secondaries typical of Myrtaceae were observed from Buen Retiro (Fig. 4). Similar foliage occurs at Peumo (Fig. 4). Some leaves were identified by Selling as comparable with Sterculiaceae and we especially note a lobed, basally actinodromous leaf (Fig. 5) as reasonably belonging to Malvales. Perhaps the most striking fossil described by Engelhardt (1891, p. 645, pl. 1, fig. 1) is Sabal ochseniusi Engelh., a section of palmate, deeply folded palm foliage from Curanilahue in which the presence of a costa may be interpreted. A further Curanilahue specimen, missing any evidence of a costa, was collected by Dusén in 1896 (Fig. 5) and Berry (1922, p. 91) noted another from Concepción. Various other foliage types are present, including evidence of compound leaves and leaf specimens with apical ‘drip-tips’ (Fig. 5).
Examples of Lota–Coronel fossils collected by Halle from Buen Retiro (b–d, f, g) and Minas Cantarana Site 1 (a) and Dusén from Peumo (e, h). (a) S160606 likely Lauraceae. (b, c) cf. Caldcluvia paniculata (Cunoniaceae). These specimens and others occur on the same rock, suggesting that these are leaflets originating from a compound leaf. (b) S160421-01-04, (c) S160421-01-01. (d) Original label accompanying (b, c). (e) S160016 incomplete Elaeocarpaceae-like or Cunoniaceae-like leaf. (f) S160527-0203 Elaeocarpaceae-like or Cunoniaceae-like leaf. (g, h) Likely Myrtaceae; (g) S160523-01, (h) S160014. Scale bars: 1 cm.
Examples of Lota–Coronel fossils collected by Halle from Buen Retiro (c, e, g, l, m) and Minas Cantarana (h, i, k) and Dusén from Curanilahue (a, b, d, f, j). (a) S160756 likely Malvales. (b) S165532 likely coryphoid palm attributed to Sabalites ochseniusii on accompanying label. (c) S160417-02-03 possible monocot. (d–m) Unidentified dicotyledonous angiosperms. (d) S165531-01 attributed to Myrica sp. on accompanying label. (e) S160492. Note drip-tip. (f) S160872-01. Note vein detail. (g) S160542. (h) S160497. These may be leaflets originating from the same compound leaf. (i) S160950 with asymmetrical base suggesting that this represents a leaflet originating from a compound leaf. (j) S160779 attributed to Sterculiaceae on accompanying label but note the small teeth. (k) S160569-01 attributed to Sterculiaceae on accompanying label. (l) S160444-01 possibly Lauraceae. (m) S160545-01. Scale bars: 1 cm.
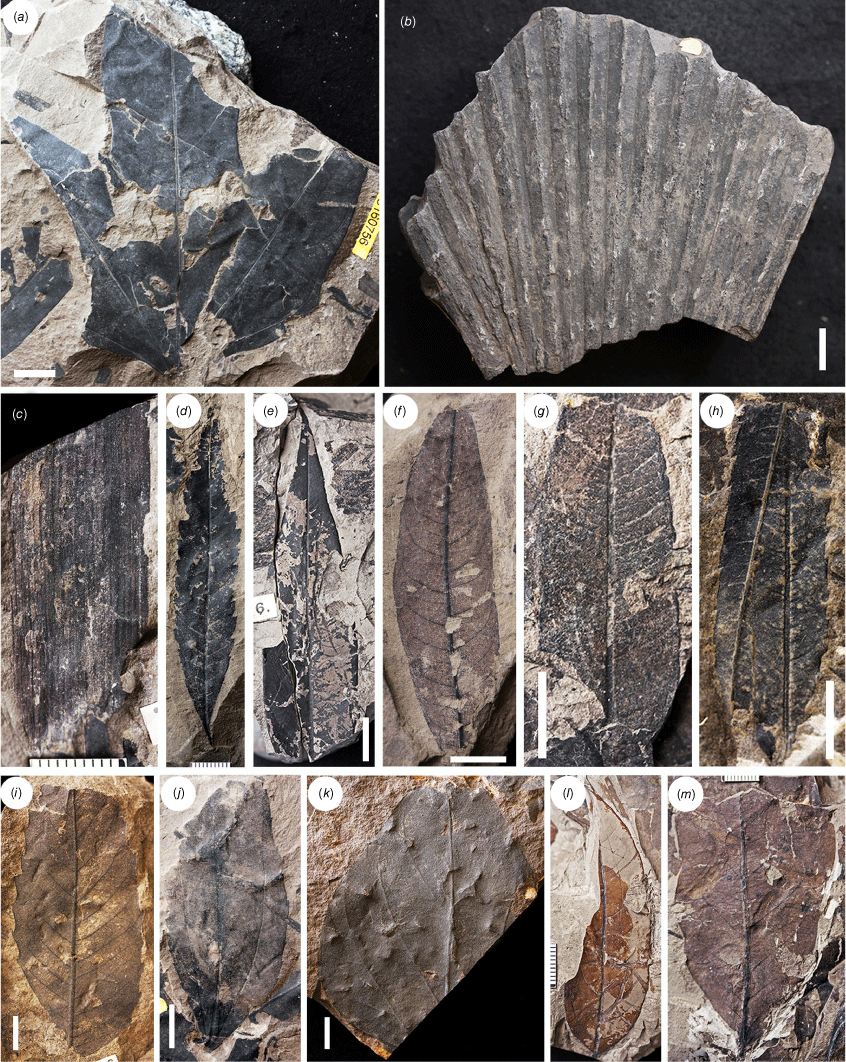
As discussed by Wilf et al. (2014), a specimen assigned to Zamia L. by Engelhardt (1891) from Coronel is evidently lost but could represent a monocot. Several incomplete Buen Retiro specimens also possess parallel venation consistent with monocot affinities (Fig. 5).
Discussion
Proteaceae in the early Paleogene of South America
We establish the presence of a new Proteaceae leaf species in the latest Paleocene of southern South America and highlight that the affinities are with extant Australasian taxa. The species is also the oldest convincing leaf record of the family in the Americas, with the previous oldest being several early Eocene taxa from Laguna del Hunco, Patagonian Argentina (Gonzalez et al. 2007). Other southern South American early Paleogene (late Paleocene or early Eocene) floras, including some that have been collected and researched extensively, lack records of Proteaceae macrofossils. These floras are from strata exposed at Caleta Cocholgüe (Gayó et al. 2005) and Quinamávida (Troncoso 1992) in Chile, the Ligorio Márquez Formation that crops out on the Chile–Argentina border (Troncoso et al. 2002; Carpenter et al. 2018), and, in Patagonian Argentina, the Danian Salamanca and Peñas Coloradas formations (Iglesias et al. 2021) and the lower member (Eocene) of the Rio Turbio Formation (Panti 2018). Further south, in Antarctica, several late Paleocene leaf impressions from the Cross Valley Flora of Seymour Island were referred to Proteaceae by Dusén (1908) and two taxa were accepted by Tosolini et al. (2013) at family level. However, as noted by Doktor et al. (1996) with regard to the Eocene La Meseta Formation and also accepted more recently by Tosolini et al. (2023), these and other fossils from Seymour Island are too poorly preserved for unequivocal assignment to the family. Similarly, fossil woods unequivocally attributable to Proteaceae have not been documented from the Antarctic Peninsula region (Oh et al. 2020; Tilley 2024).
Although there is little pre-Eocene macrofossil evidence of Proteaceae in South America, several pollen morphospecies have been recognised in the south of the continent from the Campanian onwards (Askin and Baldoni 1998) and proteaceous pollen is common in Eocene strata of Seymour Island (Askin 1991). The chronology of records and typically much lower diversity than in the Australian region has usually indicated eastwards spread from a southern Australian diversification centre via Antarctica (Askin and Baldoni 1998; Dettmann and Jarzen 1998; Cantrill 2018). Similarly, the general scarcity of proteaceous pollen and absence of grevilleoids in Maastrichtian–Eocene strata of the Salta Basin in the north-western corner of Argentina (Quattrocchio and Volkheimer 1990; Llorens et al. 2022) is consistent with a southern origin, although Lamont et al. (2024) proposed an arrival from northern South America instead. Whatever the true history of the family in the region, we consider that the pre-Eocene paucity of macrofossils is likely an indication that Proteaceae plants were not common in the South American vegetation at that time or had low fossilisation potential due to one or a combination of plants being of small stature, not sclerophyllous or growing in habitats remote from depositional sites.
Regarding the Arauco coals, Proteaceae pollen was evidently neither common nor diverse, and neither of the two Proteacidites Cookson ex R.A.Couper spp. recorded by Palma-Heldt (1980) and Collao et al. (1987) are usefully informative for affiliation to extant taxa.
Does the Lota–Coronel flora have a Neotropical signature?
Romero (1986), following Engelhardt (1891, 1905) and Berry (1922), placed the Lota–Coronel flora in the ‘northern, purely American Province’ (p. 457) of his Neotropical Paleoflora, consisting of genera (‘or their ancestors’) currently occurring exclusively within the Neotropical Phytogeographic Region of South America defined by Cabrera and Willink (1973). Gayó et al. (2005) continued to regard the Lota–Coronel flora as part of the Neotropical Paleoflora but with a revised list of >80% Neotropical and pantropical and <20% Australasian generic composition (citing Villagrán and Hinojosa 1997). The same composition was also used by Hinojosa (2003, 2005; see also Hinojosa et al. 2006; Quattrocchio et al. 2013), a co-author with Villagrán on the Gayó et al. (2005) paper, who reclassified the Lota–Coronel flora as part of his Gondwanic Paleoflora. Regardless of these potentially confusing alternative names, the acceptance of wholly or dominantly Neotropical floristic affinities in these studies is surprising, especially given that >80 years ago Florin (1940, p. 24) not only found that the Lota–Coronel conifers were incorrectly identified but also questioned the reliability of the angiosperm identifications, these being based only on external morphology and with many of the specimens being fragmentary.
We strongly agree with Florin, and emphasise that the only Lota–Coronel foliar fossils that have been rigorously identified to genus, i.e. Lygodium Sw., Dacrycarpus (Endl.) de Laub., Retrophyllum C.N.Page and Coronelia Florin (none of which were referred to in the phytogeographical studies above) do not have exclusively Neotropical affinities. Thus, Lygodium is currently globally widespread, mostly in the tropics, with L. skottsbergii evidently belonging to a complex of species that was distributed across a vegetated Antarctica in the Paleogene (Rozefelds et al. 2017). Dacrycarpus is currently found only in Australasia, Malesia and South-east Asia with D. chilensis closely matching D. dacrydioides (A.Rich.) de Laub. of New Zealand (Florin 1940; Wilf 2012). Retrophyllum represents an interesting case of a Gondwanan genus with disjunct species in Australasia and northern South America, where the latter occurrence most likely resulted from a rare, deep northwards migration of an austral taxon (Wilf et al. 2017). Coronelia is of uncertain affinity but we agree with Florin (1940) that this belonged to Podocarpaceae. The known distribution is purely austral, with well-preserved foliage being found also in the Ligorio Márquez Formation, Argentina (Carpenter et al. 2018), Australian Eocene floras in Tasmania (Townrow 1965) and Queensland (R. J. Carpenter, unpubl. data), and the Miocene of New Zealand (R. J. Carpenter, unpubl. data) to date. Although more taxonomic work is required, the presence of Caldcluvia paniculata (Cav.) D.Don-like leaves at Buen Retiro is further evidence of austral affinities because the extant species is endemic to Patagonian rainforests and the closest relatives are restricted to Australasia. Species of Parablechnum with foliage similar to the Buen Retiro fossils do occur in the Neotropics but these species occur mostly in montane areas and overall, the genus is typical of southern temperate zones (Testo et al. 2022).
Among the Lota–Coronel taxa that have been unjustifiably linked to Neotropical genera, the proposed Lauraceae taxa are exemplary: at least 14 species from 9 genera were originally named (Engelhardt 1891; Berry 1922) but numerous authors have since noted the impossibility of confidently assigning lauraceous leaf fossils to extant genera, even when cuticle is well preserved (e.g. Hill 1986; Bannister et al. 2012; Carpenter et al. 2018). Therefore, the abundant latest Cretaceous to early Paleogene records of the family from Lota–Coronel and other Patagonian and Antarctic Peninsula floras could alternatively represent lineages currently found remotely from the Neotropics, such as in Australasia.
Our brief assessment of the early studies and existing collections from Lota–Coronel sites other than Buen Retiro shows Sabal ochseniusi (Arecaceae) to be of interest as a possible Neotropical element. Although Sabal Adans. is currently found only in this region, many authors (e.g. Horn et al. 2009; Matsunaga and Smith 2021) have concluded that certain inference of closest affinity with Sabal is not possible because similar palmate or costapalmate foliage occurs widely in the large, mostly pantropical Coryphoideae, and palmate foliage also occurs elsewhere in the family. Similarly, the specimen assigned to the Neotropical cycad Zamia L. by Engelhardt (1891) was not verified by any cuticular evidence and may represent another cycad or monocot (Wilf et al. 2014).
Similarly to the macrofossils, the biogeographic significance of the Lota–Coronel pollen flora has been overlooked or evidently misinterpreted in previous studies. Although many of the >40 spore and pollen taxa (e.g. Cyathidites Couper spp., Gleicheniidites M.E.Dettmann, Myrtaceidites Cookson & K.M.Pike, the tricolpates) listed by Doubinger (1972), Doubinger and Chotin (1975), Palma-Heldt (1980) and Collao et al. (1987) have imprecise or widely distributed modern affinities, there is scant evidence of taxa with definite and exclusively Neotropical relatives, whereas there is abundant evidence of those with austral relatives (Doubinger and Chotin 1975). These taxa include ?Araucariacites Cookson sp. (?Araucariaceae), Podocarpidites Cookson spp. (Podocarpaceae) and Proteacidites spp. (Proteaceae) and of particular note, Haloragacidites harrisii. This last pollen species is widely accepted as belonging to the exclusively Australasian family Casuarinaceae (e.g. Macphail 2007), not Myricaceae, as treated by all the abovementioned authors. Importantly, Casuarinaceae macrofossils (Gymnostoma L.A.S.Johnson), including foliage and staminate inflorescences with in situ H. harrisii pollen, also occur in the early Eocene Laguna del Hunco deposits of Argentina, and dispersed pollen grains have been found in numerous early Paleocene to Eocene palynoassemblages from other Patagonian localities (Zamaloa et al. 2006). The long history of Nypa Steck-like pollen was recently reviewed by Pocknall et al. (2022), although these authors did not include the records of Spinizonocolpites from southern South America. Nypa abundance and diversity evidently reached a global peak during the warm Paleocene and Eocene intervals, including across northern South America, but became extinct in the Americas since that time, and was reduced to the single species N. fruticans elsewhere. We consider that the presence of Nypa in the Arauco coals was likely a consequence of southward range expansion, but the genus is clearly no longer a Neotropical genus.
Angiosperm leaf fossils from other Paleogene assemblages in central Chile and the Patagonian region, such as Quinamávida (Troncoso 1992), Ligorio Márquez (Troncoso et al. 2002) and the Río Turbio Formation (Panti 2016, 2018; Vento and Prámparo 2018) have been assigned to taxa described by Engelhardt (1891, 1905) and Berry (1922) from Lota–Coronel, although in many cases, with severe reservations because of poor preservation, including lack of cuticular evidence (e.g. Troncoso et al. 2002). Although of floristic and physiognomic interest, these studies contribute little to the question of whether or not Neotropical elements were present. By contrast, detailed reviews of the wider South American palaeobotanical (especially palynological) evidence by Jaramillo and Cardenas (2013) and Jaramillo (2023) show that early Paleogene global warming events did not result in expansion of Neotropical forests into higher southern latitudes, nor broad migration into the tropics from temperate regions. These authors proposed that although thermal regimes may have been suitable, and some individual lineages could extend or change ranges, marked differences in both the variability and total amounts of solar insolation were likely limiting at the biome level.
Our work on the Lota–Coronel flora adds to other evidence that the strongest early Paleogene southern South American floristic connections were with the Australasian region, not the Neotropics, via an ice-free and vegetated Antarctica (e.g. Zamaloa et al. 2006; Wilf et al. 2009, 2014, 2016, 2017; Gandolfo et al. 1988, 2011; Hermsen et al. 2012; Wilf 2012; Carvalho et al. 2013; Knight and Wilf 2013; Macphail et al. 2013; Carpenter et al. 2014b; Gandolfo and Hermsen 2017; Escapa et al. 2018; Jud et al. 2018; Rossetto-Harris et al. 2020) that was connected until final separation at the end of the Eocene (McLoughlin 2001). Many of the pan-Antarctic lineages persist only in subtropical rainforest associations of Australasia, including several genera within the World Heritage Gondwana Rainforests of eastern Australia and others in Malesia (Kooyman et al. 2014).
The Lota–Coronel palaeoenvironment
The nearest inferred extant relatives of Proteaceaefolia araucoensis are rainforest trees confined to warm, wet and humid climates in Australasia, consistent with physiognomic evidence for such climates in the early Paleogene of southern South America based on leaf fossils from both the Lota–Coronel flora and floras of similar age and geographic location (Ligorio Márquez and Caleta Cocholgüe) (Romero 1986, 1993; Hinojosa and Villagrán 1997; Villagrán and Hinojosa 1997; Gayó et al. 2005). None of the other nearest living relatives of the Lota–Coronel fossils that has reasonably well-justified identifications contradict this conclusion, and some of the angiosperms in the assemblage have extended apices likely representing drip tips for shedding water in strongly humid settings (Fig. 5). Together with the noted high abundance of fungal bodies and pteridophyte spores in the Arauco coal (Collao et al. 1987), Haloragacidites harrisii (Casuarinaceae; presumably Gymnostoma) pollen is of particular importance as an indicator of both palaeohabitat and palaeoclimate. This species represents up to ~43% of the total pollen composition in the samples examined by Collao et al. (1987), levels that likely indicate the type of dominance attained by extant Gymnostoma species of warm, lowland peat forest habitats of Borneo on poorly drained soils (Brunig 1990). In terms of the other most reliably identified nearest living relatives, the presence of Lygodium, Dacrycarpus, Retrophyllum, Lauraceae and likely Cunoniaceae and coryphoid palms among the macrofossils, and Nypa and Gymnostoma among the microfossils, do not contradict the inference of a warm, mesic Arauco palaeoclimate, as all these taxa have overwhelmingly modern tropical to subtropical distributions in generally high rainfall and humid habitats.
Conclusion
Our study shows the Paleocene presence of a Proteaceae species in southern South America whose closest relatives are Australasian, and should promote greater interest in the palaeobotanical importance of the Arauco–Concepción coal flora and further elucidation of the historical development of Gondwanan floras in general.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study. Details of all NRM specimens are available from the Palaeobiology Department’s database by contacting the second author or from https://samlingar.nrm.se/faces/pages/results.xhtml
Declaration of funding
S. McLoughlin is funded by a Swedish Research Council VR grant (2022-03920). R. J. Carpenter received funding from the Wenner-Gren Foundation and the Swedish Research Council (VR Grant 2018-04527) to visit the Swedish Museum in 2022, when this project was conceived.
Acknowledgements
We are very grateful to Yayuan Chen and Gustav Nesset Mattsson for assistance with photographing the fossils. Thanks are extended to both reviewers and the journal’s editorial staff for prompt and helpful assessments that led to improvements in our manuscript.
References
Askin RA (1991) Eocene terrestrial palynology of Seymour Island. Antarctic Journal of the United States 26, 44-46.
| Crossref | Google Scholar |
Askin RA, Baldoni AM (1998) The Santonian through Paleogene record of Proteaceae in the southern South America–Antarctic Peninsula Region. Australian Systematic Botany 11, 373-390.
| Crossref | Google Scholar |
Bannister JM, Conran JG, Lee DE (2012) Lauraceae from rainforest surrounding an early Miocene maar lake, Otago, southern New Zealand. Review of Palaeobotany and Palynology 178, 13-34.
| Crossref | Google Scholar |
Barker NP, Weston PH, Rutschmann F, Sauquet H (2007) Molecular dating of the “Gondwanan” plant family Proteaceae is only partially congruent with the timing of Gondwanan break-up. Journal of Biogeography 34, 2012-2027.
| Crossref | Google Scholar |
Becerra J, Contreras-Reyes E, Arriagada C (2013) Seismic structure and tectonics of the southern Arauco Basin, south-central Chile (~38°S). Tectonophysics 592, 53-66.
| Crossref | Google Scholar |
Berry EW (1922) The flora of the Concepcion–Arauco coal measures of Chile. Johns Hopkins University Studies in Geology 4, 73-143.
| Google Scholar |
Blackburn DT (1981) Tertiary megafossil flora of Maslin Bay, South Australia: numerical taxonomic study of selected leaves. Alcheringa 5, 9-28.
| Crossref | Google Scholar |
Cantrill DJ (2018) Cretaceous to Paleogene vegetation transition in Antarctica. In ‘Transformative paleobotany: papers to commemorate the life and legacy of Thomas N. Taylor’. (Eds JF White Jr, K Kingsley, C Harper, Q Chen, SK Verma, L Brindisi, X Chang, A Micci, M Bergen) pp. 645–659. (Academic Press: London, UK) 10.1016/B978-0-12-813012-4.00027-9
Carpenter RJ (2012) Proteaceae leaf fossils: phylogeny, diversity, ecology and austral distributions. Botanical Review 78, 261-287.
| Crossref | Google Scholar |
Carpenter RJ, Jordan GJ (1997) Early Tertiary macrofossils of Proteaceae from Tasmania. Australian Systematic Botany 10, 533-563.
| Crossref | Google Scholar |
Carpenter RJ, Rozefelds AR (2023) Leaf fossils show a 40-million-year history for the Australian tropical rainforest genus Megahertzia (Proteaceae). Australian Systematic Botany 36, 312-321.
| Crossref | Google Scholar |
Carpenter RJ, Iglesias A, Wilf P (2018) Early Cenozoic vegetation in Patagonia: new insights from organically preserved plant fossils (Ligorio Márquez Formation, Argentina). International Journal of Plant Sciences 179, 115-135.
| Crossref | Google Scholar |
Carpenter RJ, Hill RS, Jordan GJ (2005) Leaf cuticular morphology links Platanaceae and Proteaceae. International Journal of Plant Sciences 166, 843-855.
| Crossref | Google Scholar |
Carpenter RJ, Jordan GJ, Hill RS (2016) Fossil leaves of Banksia, Banksieae and pretenders: resolving the fossil genus Banksieaephyllum. Australian Systematic Botany 29, 126-141.
| Crossref | Google Scholar |
Carpenter RJ, McLoughlin S, Hill RS, McNamara KJ, Jordan GJ (2014a) Early evidence of xeromorphy in angiosperms: stomatal encryption in a new Eocene species of Banksia (Proteaceae) from Western Australia. American Journal of Botany 109, 1486-1497.
| Crossref | Google Scholar |
Carpenter RJ, Wilf P, Conran JG, Cúneo NR (2014b) A Paleogene trans-Antarctic distribution for Ripogonum (Ripogonaceae: Liliales)? Palaeontologia Electronica 17(3.39A), 9.
| Crossref | Google Scholar |
Carvalho MR, Wilf P, Hermsen EJ, Gandolfo MA, Cúneo NR, Johnson KR (2013) First record of Todea (Osmundaceae) in South America, from the early Eocene paleorainforests of Laguna del Hunco (Patagonia, Argentina). American Journal of Botany 100, 1831-1848.
| Crossref | Google Scholar | PubMed |
Collao S, Oyarzun R, Palma S, Pineda V (1987) Stratigraphy, palynology and geochemistry of the lower Eocene coals of Arauco, Chile. International Journal of Coal Geology 7, 195-208.
| Crossref | Google Scholar |
Cookson IC, Duigan SL (1950) Fossil Banksieae from Yallourn, Victoria, with notes on the morphology and anatomy of living species. Australian Journal of Scientific Research, Series B: Biological Sciences 3, 133-165.
| Crossref | Google Scholar |
Dettmann ME, Jarzen DM (1998) The early history of the Proteaceae in Australia: the pollen record. Australian Systematic Botany 11, 401-438.
| Crossref | Google Scholar |
Doktor M, Gaździcki A, Jerzmańska A, Porębski SJ, Zastawniak E (1996) A plant and fish assemblage from the Eocene La Meseta Formation of Seymour Island (Antarctic Peninsula) and its environmental implications. Palaeontologia Polonica 55, 127-146.
| Google Scholar |
Doubinger J (1972) Evolution de la flore (pollen et spores) au Chili central (Arauco), du Crétacé supérieur au Miocéne. Compte Rendu des séances de la Societé de Biogeographie 49, 17-25 [In French].
| Google Scholar |
Doubinger J, Chotin P (1975) Etude palynologique de lignites Tertiaires du basin d’Arauco Concepcion (Chile). Revista Espanola de Micropaleontologia 7, 549-565 [In French].
| Google Scholar |
Dusén P (1899) Über die tertiäre Flora der Magallans-Länder. In ‘Wissenschaftliche Ergebnisse der Schwedischen Expedition nach den Magallans-Länder 1895–97. Band I. Geologie, Geographie und Anthropologie’. (Ed. O Nordenskjöld) pp. 87–108. (Lithographisches Institut des Generalstabs: Stockholm, Sweden) [In German]
Engelhardt H (1891) Über Tertiärpflanzen von Chile. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 16, 629-692 [In German].
| Google Scholar |
Engelhardt H (1905) Bemerkungen zu chilenischen Tertiarpflanzen. Abhandlungen der Naturwissenschaft lichen Gesellschaft Isis in Dresden 1905, 69-72 [In German].
| Google Scholar |
Escapa IH, Iglesias A, Wilf P, Catalano SA, Caraballo-Ortiz MA, Cúneo NR (2018) Agathis trees of Patagonia’s Cretaceous-Paleogene death landscapes and their evolutionary significance. American Journal of Botany 105, 1345-1368.
| Crossref | Google Scholar | PubMed |
Ferraro FX, Schilling ME, Baeza S , Oms O, Sá AA (2020) Bottom-up strategy for the use of geological heritage by local communities: Approach in the “Litoral del Bíobío” Mining Geopark project (Chile). Proceedings of the Geologists’ Association 131, 500-510.
| Crossref | Google Scholar |
Florin R (1940) The Tertiary fossil conifers of south Chile and their phytogeographical significance. Kungliga Svenska Vetenskapsakademiens Handlingar 19, 1-107.
| Google Scholar |
Frenguelli J (1943) Proteáceas del Cenozoico de Patagonia. Notas del Museo de La Plata 8, 201-213 [In Spanish].
| Google Scholar |
Gandolfo MA, Hermsen EJ (2017) Ceratopetalum (Cunoniaceae) fruits of Australasian affinity from the early Eocene Laguna del Hunco flora, Patagonia, Argentina. Annals of Botany 119, 507-516.
| Crossref | Google Scholar | PubMed |
Gandolfo MA, Dibbern MC, Romero EJ (1988) Akania patagonica n. sp. and additional material on Akania americana Romero and Hickey (Akaniaceae), from Paleocene sediments of Patagonia. Bulletin of the Torrey Botanical Club 115, 83-88.
| Crossref | Google Scholar |
Gandolfo MA, Hermsen EJ, Zamaloa MC, Nixon KC, González CC, Wilf P, Cúneo NR, Johnson KR (2011) Oldest known Eucalyptus macrofossils are from South America. PLoS ONE 6, e21084.
| Crossref | Google Scholar | PubMed |
Gayó E, Hinojosa LF, Villagrán C (2005) On the persistence of tropical paleofloras in central Chile during the early Eocene. Review of Palaeobotany and Palynology 137, 41-50.
| Crossref | Google Scholar |
Gonzalez CC, Gandolfo MA, Zamaloa MC, Cúneo NR, Wilf P, Johnson KR (2007) Revision of the Proteaceae macrofossil record from Patagonia, Argentina. Botanical Review 73, 235-266.
| Crossref | Google Scholar |
Halle TG (1940) A fossil fertile Lygodium from the Tertiary of southern Chile. Svensk Botanisk Tidskrift 34, 257-264.
| Google Scholar |
Hermsen EJ, Gandolfo MA, Zamaloa CMC (2012) The fossil record of Eucalyptus in Patagonia. American Journal of Botany 99, 1356-1374.
| Crossref | Google Scholar | PubMed |
Hill RS (1986) Lauraceous leaves from the Eocene of Nerriga, New South Wales. Alcheringa 10, 327-351.
| Crossref | Google Scholar |
Hill RS, Christophel DC (1988) Tertiary leaves of the tribe Banksieae (Proteaceae) from south-eastern Australia. Botanical Journal of the Linnean Society 97, 205-227.
| Crossref | Google Scholar |
Hinojosa LF (2005) Cambios climáticos y vegetacionales inferidos a partir de Paleofloras Cenozoicas del sur de Sudamérica. Revista Geológica de Chile 32, 95-115 [In Spanish].
| Crossref | Google Scholar |
Hinojosa LF, Villagrán C (1997) Historia de los bosques del sur de Sudamérica I: antecedentes paleobotánicos, geológicos y climáticos del Terciario del cono sur de América. Revista Chilena de Historia Natural 70, 225-239 [In Spanish].
| Google Scholar |
Hinojosa LF, Armesto JJ, Villagrán C (2006) Are Chilean coastal forests pre-Pleistocene relicts? Evidence from foliar physiognomy, palaeoclimate, and phytogeography. Journal of Biogeography 33, 331-341.
| Crossref | Google Scholar |
Hopkins HCF, Pillon Y (2020) Virotia azurea (Proteaceae: Macadamieae), a striking new species endemic to New Caledonia and notes on V. francii and V. leptophylla. Candollea 75, 89-98.
| Crossref | Google Scholar |
Horn JW, Fisher JB, Tomlinson PB, Lewis CE, Laubengayer K (2009) Evolution of lamina anatomy in the palm family (Arecaceae). American Journal of Botany 96, 1462-1486.
| Crossref | Google Scholar | PubMed |
Iglesias A, Wilf P, Stiles E, Wilf R (2021) Patagonia’s diverse but homogeneous early Paleocene forests: angiosperm leaves from the Danian Salamanca and Peñas Coloradas formations, San Jorge Basin, Chubut, Argentina. Palaeontologia Electronica 24, a02.
| Crossref | Google Scholar |
Jaramillo C (2023) The evolution of extant South American tropical biomes. New Phytologist 239, 477-493.
| Crossref | Google Scholar | PubMed |
Jaramillo C, Cardenas A (2013) Global warming and Neotropical rainforests: a historical perspective. Annual Review of Earth and Planetary Sciences 41, 741-766.
| Crossref | Google Scholar |
Johnson LAS, Briggs BG (1963) Evolution in the Proteaceae. Australian Journal of Botany 11, 21-61.
| Crossref | Google Scholar |
Johnson LAS, Briggs BG (1975) On the Proteaceae—the evolution and classification of a southern family. Botanical Journal of the Linnean Society 70, 83-182.
| Crossref | Google Scholar |
Jordan GJ, Carpenter RJ, Hill RS (1998) The macrofossil record of Proteaceae: a review with new species. Australian Systematic Botany 11, 465-501.
| Crossref | Google Scholar |
Jud NA, Gandolfo MA, Iglesias A, Wilf P (2018) Fossil flowers from the early Palaeocene of Patagonia, Argentina, with affinity to Schizomerieae (Cunoniaceae). Annals of Botany 121, 431-442.
| Crossref | Google Scholar | PubMed |
Knight CL, Wilf P (2013) Rare leaf fossils of Monimiaceae and Atherospermataceae (Laurales) from Eocene Patagonian rainforests and their biogeographic significance. Palaeontologia Electronica 16(3.26A), 39.
| Crossref | Google Scholar |
Kooyman RM, Wilf P, Barreda VD, Carpenter RJ, Jordan GJ, Sniderman JM, Allen A, Brodribb TJ, Crayn D, Feild TS, Laffan SW, Lusk CH, Rossetto M, Weston PH (2014) Paleo-Antarctic rainforest into the modern Old World Tropics: the rich past and threatened future of the “southern wet forest survivors”. American Journal of Botany 101, 2121-2135.
| Crossref | Google Scholar | PubMed |
Lamont BB, He T, Milne LA, Cowling RM (2024) Out of Africa: Linked continents, overland migration and differential survival explain abundance of Proteaceae in Australia. Perspectives in Plant Ecology, Evolution and Systematics 62, 125778.
| Crossref | Google Scholar |
Le Roux J, Elgueta S (1997) Paralic parasequences associated with Eocene sea-level oscillations in an active margin setting: Trihueco Formation of the Arauco Basin, Chile. Sedimentary Geology 110, 257-276.
| Crossref | Google Scholar |
Llorens M, Pérez Loinaze VS, Narváez PL, Zelaya AM, Pérez Pincheira E, Gorustovich S (2022) A mid-latitude Maastrichtian palynological record from the Yacoraite Formation (Salta Group), northwestern Argentina. Cretaceous Research 140, 105332.
| Crossref | Google Scholar |
Machado MA, Passalia MG, Vera EI, Yañez A (2023) Ferns from the Arroyo Chacay flora (Huitrera Formation, Eocene) Río Negro Province, Argentina. Review of Palaeobotany and Palynology 313, 10489.
| Crossref | Google Scholar |
McLoughlin S (2001) The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Australian Journal of Botany 49, 271-300.
| Crossref | Google Scholar |
Macphail M, Carpenter RJ, Iglesias A, Wilf P (2013) First evidence for Wollemi Pine-type pollen (Dilwynites: Araucariaceae) in South America. PLoS ONE 8, e69281.
| Crossref | Google Scholar | PubMed |
Malumián N, Náñez C (2011) The Late Cretaceous–Cenozoic transgressions in Patagonia and the Fuegian Andes: foraminifera, palaeoecology, and palaeogeography. Biological Journal of the Linnean Society 103, 269-288.
| Crossref | Google Scholar |
Manchester SR (2014) Revisions to Roland Brown’s North American Paleocene flora. Acta Musei Nationalis Pragae – B. Historia Naturalis 70, 153-210.
| Crossref | Google Scholar |
Martínez-Pardo R, Martínez-Guzmán R (1997) La Formación Curanilahue del Paleógeno Carbonifero de la Cuenca de Arauco–Concepción, Chile Central: el Ocaso de la “opinio-estratigrafia” como herramienta habitual en al trabajo geológico nacional. In ‘VIII Congreso Geologico Chileno’, 13–17 October 1997, Antofagasta, Chile. pp. 520–524. (Universidad Catolica del Norte) Available at https://repositorio.sernageomin.cl/handle/0104/19966 [In Spanish].
| Google Scholar |
Martínez-Pardo R, Martínez-Guzmán R (2008) Cronomicropaleontología de la Cuenca de Arauco–Concepción, Chile Central: la tafoflora de Lota, el evento megatérmico global del límite Eoceno-Paleoceno y las extinciones masivas. In ‘I Simposio – Paleontologia en Chile’, 2–3 October 2008, Santiago, Chile. (Eds A. Rubilar, D. Rubilar, C Gutstein) pp. 151–153. (Asociación Chilena de Paleontología) Available at https://www.achp.cl/manejador/resources/i-simposiopaleochile-libro-de-actas-24-abril.pdf [In Spanish].
| Google Scholar |
Martínez-Pardo R, Martínez-Guzmán R, Vilches-Guzman G (1997) El límite Paleoceno-Eoceno en la cuenca carbonífera de Arauco–Concepción, Chile Central. In ‘VIII Congreso Geológico Chileno’, 13–17 October 1997, Antofagasta, Chile. pp. 530–533. (Universidad Católica del Norte) Available at https://repositorio.sernageomin.cl/handle/0104/19968 [In Spanish].
| Google Scholar |
Matsunaga KKS, Smith SY (2021) Fossil palm reading: using fruits to reveal the deep roots of palm diversity. American Journal of Botany 108, 472-494.
| Crossref | Google Scholar | PubMed |
Muller J (1968) Palynology of the Pedawan and Plateau Sandstone Formations (Cretaceous–Eocene) in Sarawak, Malaysia. Micropaleontology 14, 1-37.
| Crossref | Google Scholar |
Muñoz-Cristi J (1946) Estado actual del conocimiento sobre la geología de la provincia de Arauco. Anales Facultad de Ciencias Físicas y Matemáticas, Universidad de Chile 3, 30-63 [In Spanish].
| Google Scholar |
Muñoz-Cristi J (1968) Contribución al conocimiento geológico de la región situada al sur de Arauco y participación de material volcánico en los sedimentos eocenos. In ‘El Terciario de Chile, Zona Central’. (Ed. G Cecioni) pp. 63–93. (Sociedad Geológica de Chile and Andrés Bello: Santiago, Chile) [In Spanish]
Oh C, Philippe M, McLoughlin S, Woo J, Leppe M, Torres T, Park T-YS, Choi H-G (2020) New fossil woods from the early Cenozoic volcano-sedimentary rocks in the Fildes Peninsula, King George Island, and implications for the trans-Antarctic Peninsula Eocene climatic gradient. Papers in Palaeontology 6, 1-29.
| Crossref | Google Scholar |
Olde PM (2017) A preliminary checklist of fossil names in extant genera of the Proteaceae. Telopea 20, 289-324.
| Crossref | Google Scholar |
Palma-Heldt S (1980) Nuevos antecedentes en el estudio palinológico de los mantos carboníferos del terciario de Arauco–Concepción, Chile. Boletim Instituto de Geociencias, Universidad Sao Paulo 11, 161-168 [In Spanish with abstract in Spanish and English].
| Crossref | Google Scholar |
Palma-Heldt S, Quinzio LA, Bonilla R, Cisterna K (2009) Implicancias estratigráficas del primer registro de Notofagadites en el Paleógeno de la Cuenca de Arauco, Región del Bío-Bío, Chile. In ‘XII Congreso Geológico Chileno’, 22–26 November 2009, Santiago, Chile. (Eds M Reich, JP Le Roux) pp. 1–4. (El Departamento de Geología de la Universidad de Chile: Santiago, Chile) [In Spanish]
Panti C (2016) Myrtaceae fossil leaves from the Río Turbio Formation (Middle Eocene), Santa Cruz Province, Argentina. Historical Biology 28, 459-469.
| Crossref | Google Scholar |
Panti C (2018) Fossil leaves of subtropical lineages in the Eocene–?Oligocene of southern Patagonia. Historical Biology 35, 252-266.
| Crossref | Google Scholar |
Pocknall DT, Clowes CD, Jarzen DM (2022) Spinizonocolpites prominatus (McIntyre) Stover & Evans: fossil Nypa pollen, taxonomy, morphology, global distribution, and paleoenvironmental significance. New Zealand Journal of Geology and Geophysics 66, 558-570.
| Crossref | Google Scholar |
Pole M (1994) An Eocene macroflora from the Taratu Formation at Livingstone, North Otago, New Zealand. Australian Journal of Botany 42, 341-367.
| Crossref | Google Scholar |
Quattrocchio ME, Volkheimer W (1990) Paleogene palaeoenvironment trends as reflected by palynological assemblage types, Salta Basin. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 181, 377-396.
| Crossref | Google Scholar |
Quattrocchio M, Martínez M, Hinojosa LF, Jaramillo C (2013) Quantitative analysis of Cenozoic palynofloras from Patagonia (southern South America). Palynology 37, 246-258.
| Crossref | Google Scholar |
Rodriguez D, Ward DJ, Quezada J (2023) Paleontology and stratigraphic implications of a late Paleocene elasmobranch assemblage in Talcahuano, southcentral Chile. Andean Geology 50, 217-247.
| Crossref | Google Scholar |
Romero EJ (1986) Paleogene phytogeography and climatology of South America. Annals of the Missouri Botanical Garden 73, 449-461.
| Crossref | Google Scholar |
Rossetto-Harris G, Wilf P, Escapa IH, Andruchow-Colombo A (2020) Eocene Araucaria Sect. Eutacta from Patagonia and floristic turnover during the initial isolation of South America. American Journal of Botany 107, 806-832.
| Crossref | Google Scholar | PubMed |
Rozefelds AC, Dettmann ME, Clifford HT, Carpenter RJ (2017) Lygodium (Schizaeaceae) in southern high latitudes during the Cenozoic—a new species and new insights into character evolution in the genus. Review of Palaeobotany and Palynology 247, 40-52.
| Crossref | Google Scholar |
Salazar C, Stinnesbeck W, Quinzio-Sinn LA (2010) Ammonites from the Maastrichtian (Upper Cretaceous) Quiriquina Formation in central Chile. Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 257, 181-236.
| Crossref | Google Scholar |
Skottsberg C (1909) Swedish Magellanic Expedition, 1907–1909. The Geographical Journal 33, 289-294.
| Crossref | Google Scholar |
Sleumer H (1954) Proteaceae americanae. Botanischer Jahrbücher 76, 139-211 [In German].
| Google Scholar |
Sleumer H (1955) Proteaceae. Flora Malesiana ser. I 5, 147-206.
| Google Scholar |
Stinnesbeck W (1986) Zu den faunistischen und palökologischen Verhältnissen in der Quriquina Formation (Maastrichtium) Zentral-Chiles. Palaeontographica 194, 99-237 [In German].
| Google Scholar |
Suárez M, de la Cruz R, Troncoso A (2000) Tropical/subtropical upper Paleocene–lower Eocene fluvial deposits in eastern central Patagonia, Chile (46°45′S). Journal of South American Earth Sciences 13, 527-536.
| Crossref | Google Scholar |
Takahashi K (1977) Palynology of the Lower Tertiary Concepción Formation, central Chile. Transactions and Proceedings of the Palaeontological Society of Japan 106, 71-88.
| Crossref | Google Scholar |
Testo WL, de Gasper AL, Molino S, Gabriel y Galán JM, Salino A, de Oliveira Dittrich VA, Sessa EB (2022) Deep vicariance and frequent transoceanic dispersal shape the evolutionary history of a globally distributed fern family. American Journal of Botany 109, 1579-1595.
| Crossref | Google Scholar | PubMed |
Tilley LJ (2024) Composition of Paleocene forests from Antarctica based on fossil wood. Review of Palaeobotany and Palynology 330, 105174.
| Crossref | Google Scholar |
Tosolini A-MP, Cantrill DJ, Francis JE (2013) Paleocene flora from Seymour Island, Antarctica: revision of Dusén’s (1908) angiosperm taxa. Alcheringa 37, 366-391.
| Crossref | Google Scholar |
Tosolini A-MP, Cantrill DJ, Korasidis VA, Francis JE (2023) Palaeocene high-latitude leaf flora of Antarctica Part 2: tooth-margined angiosperms. Review of Palaeobotany and Palynology 314, 104895.
| Crossref | Google Scholar |
Troncoso A (1992) La tafoflora terciaria de Quinamávida (VII región). Boletín del Museo Nacional de Historia Natural, Chile 43, 155-178 [In Spanish].
| Crossref | Google Scholar |
Troncoso A, Suárez M, de la Cruz R, Palma-Heldt S (2002) Paleoflora de la Formación Ligorio Márquez (XI Región, Chile) en su localidad tipo: sistemática, edad e implicancias paleoclimáticas. Revista Geologica de Chile 29, 113-135.
| Crossref | Google Scholar |
Townrow JA (1965) Notes on Tasmanian pines I. Some Lower Tertiary podocarps. Papers and Proceedings of the Royal Society of Tasmania 99, 87-107.
| Crossref | Google Scholar |
Upchurch GR, Wolfe JA (1987) Extinction patterns in Laurales at the Cretaceous–Tertiary boundary. American Journal of Botany 74, 692-693.
| Crossref | Google Scholar |
Vento B, Prámparo MB (2018) Angiosperm association from the Río Turbio Formation (Eocene–?Oligocene) Santa Cruz, Argentina: revision of Hünicken’s (1955) fossil leaves collection. Alcheringa 42, 125-153.
| Crossref | Google Scholar |
Villagrán C, Hinojosa LF (1997) Historia de los bosques del sur de Sudamérica II: análisis fitogeográfico. Revista Chilena de Historia Natural 70, 241-267.
| Google Scholar |
Wenzel O, Wathelet J, Chávez L, Bonilla R (1975) La sedimentación cíclica Meso Cenozoica en la región Carbonífera de Arauco–Concepción, Chile. In ‘II Congreso Ibero-Americano de Geología Económica’, 15–19 December 1975, Buenos Aires, Argentina. pp. 215–237. (Asociación Geológica Argentina: Buenos Aires, Argentina) [In Spanish]
Wilf P (2012) Rainforest conifers of Eocene Patagonia: attached cones and foliage of the extant Southeast Asian and Australasian genus Dacrycarpus (Podocarpaceae). American Journal of Botany 99, 562-584.
| Crossref | Google Scholar | PubMed |
Wilf P, Little SA, Iglesias A, Zamaloa MC, Gandolfo MA, Cúneo NR, Johnson KR (2009) Papuacedrus (Cupressaceae) in Eocene Patagonia: a new fossil link to Australasian rainforests. American Journal of Botany 96, 2031-2047.
| Crossref | Google Scholar | PubMed |
Wilf P, Escapa IH, Cúneo NR, Kooyman RM, Johnson KR, Iglesias A (2014) First South American Agathis (Araucariaceae), Eocene of Patagonia. American Journal of Botany 101, 156-179.
| Crossref | Google Scholar | PubMed |
Wilf P, Stevenson DW, Cúneo NR (2016) The last Patagonian cycad, Austrozamia stockeyi gen. et sp. nov., early Eocene of Laguna del Hunco, Chubut, Argentina. Botany 94, 817-829.
| Crossref | Google Scholar |
Wilf P, Donovan MP, Cúneo NR, Gandolfo MA (2017) The fossil flip-leaves (Retrophyllum, Podocarpaceae) of southern South America. American Journal of Botany 104, 1344-1369.
| Crossref | Google Scholar | PubMed |
Zamaloa MC, Gandolfo MA, Gonzalez CC, Romero EJ, Cúneo NR, Wilf P (2006) Casuarinaceae from the Eocene of Patagonia, Argentina. International Journal of Plant Sciences 167, 1279-1289.
| Crossref | Google Scholar |
Zambrano PA, Encinas A, Buatois LA, Arenillas I, Stinnesbeck W, Nielsen SN (2014) Sedimentology, age and provenance of Paleogene delta systems from Central Chile. In ‘Reunión Argentina de Sedimentología, No. 14, Resúmenes 1, Puerto Madryn’. pp. 299–300. (Asociación Argentina de Sedimentología: La Plata, Argentina) [In Spanish]


