The Dicranemataceae (Gigartinales, Rhodophyta) revisited: molecular data indicate polyphyly in yet another wholly or primarily Australian endemic family
Gerald T. Kraft A * and Gary W. Saunders B
A * and Gary W. Saunders B
A
B
Abstract
The Dicranemataceae was monographed morphotaxonomically by Kraft in 1977, to which the four genera Dicranema Sond., Peltasta J.Agardh., Reptataxis Kraft and Tylotus J.Agardh were attributed. All, save for a species of Tylotus J.Agardh (from east Asia), were endemic to Australia. Additions (in 2006 and 2014 respectively) were the genus Pinnatiphycus N’Yeurt, Payri & P.W.Gabrielson from New Caledonia and Fiji and a new species of Tylotus from Hawaii. General features emphasised by Kraft were similarities of apical and internal structure, zonate tetrasporangia, monoecious gametophytes and placentate cystocarps. The genera did not show uniformity in regard to thallus habits and especially carposporophytes, however, the major differences of which were not accorded any family-level significance. Two later studies, N’Yeurt et al. in 2006 and Kraft et al. in 2014, presented limited molecular data but did not treat the family as a whole or fully resolve relationships between all of the taxa, leaving Kraft’s assumption that the family was monophyletic unchallenged. We address all of the genera, both anatomically and molecularly, and support proposal of two new families, the Peltastaceae and Tylotaceae, in addition to a monogeneric Dicranemataceae. A new genus and species of Peltastaceae, Peltastanomala virantra G.W.Saunders & Kraft, has unique axial and spermatangial anatomies and an unexpected family association with Peltasta. Two additional new genera and species (Chambersius thyrsus G.W.Saunders & Kraft and Huismanophycus marinus G.W.Saunders & Kraft) are even more dissimilar to Peltasta in habit and structure but weakly allied to the Peltastaceae on molecular evidence. Both are therefore regarded as incertae sedis.
Keywords: Dicranemataceae, Gigartinales, molecular-assisted alpha taxonomy, morpho-taxonomy, Peltastaceae fam. nov., phylogenetics, Rhodophyta, Tylotaceae fam. nov.
Introduction
This is our final ‘revisitation’ of a group of exclusively or primarily Australian endemic families that were morphotaxonomically monographed by Kraft (1977a, 1977b, 1978). With the passage of over 45 years, we have tested the conclusions of the early studies against the indications of the extensive molecular data amassed over three decades to determine whether or not these three obscure but morphologically complex family groups are indeed monophyletic, as assumed in Kraft’s (1977a, 1977b, 1978) publications.
First to be examined was Mychodea Hook.f. & Harvey and the Mychodeaceae as these had been treated by Kraft (1978), the validity reaffirmed by Kraft and Saunders (2017) as the largest wholly endemic genus and family of any Australian plant or protist group at those taxonomic levels. Differences in the species composition mostly involved restoration of some that were synonymised in Kraft’s (1978) paper and the recognition of several new species, almost all of these a result of new collections subjected to molecular analyses and resolved as novel and worthy of further investigation.
The second group to be reappraised was the Acrotylaceae, monographed by Kraft (1977a) and later characterised by Kraft and Saunders (2021) as ‘one of the most enigmatic red-algal families’. With only a single confirmed non-Australian member (Ranavalona Kraft, from the southern tip of Madagascar), the molecular results indicated that of the six genera composing the Acrotylaceae in Kraft and Womersley (1994), half should be placed in the new family Clavicloniaceae.
The third family that we will address is the Dicranemataceae, that is (as we hope to show in Fig. 1–11 in many ways even more puzzling a study of systematics than the Acrotylaceae. As monographed by Kraft (1977b), the group consisted of the generitype (Dicranema Sond. with two endemic species), Peltasta J.Agardh (one endemic species), Reptataxis Kraft (one endemic species) and Tylotus J.Agardh (the Australian-endemic type species plus one east-Asian species from Japan, China and Taiwan, and also with a third species later added by Kraft et al. (2014) from the Hawaiian Islands). The features that had been common to all members of this morphologically diverse assemblage were very general: multiaxial growth, zonate tetrasporangia, monoecious gametophytes with sunken, urceolate spermatangial clusters and protuberant, outwardly directed (inwardly only in Dicranema) and elaborately placentate carposporophytes covered by thick ostiolate pericarps. Carpogonial branches were two- or three-celled, with straight or arching trichogynes directed to thallus surfaces, the relationship between presumably fertilised carpogonia and generative auxiliary cells being procarpic in two members (Peltasta and Tylotus), not clearly so or not at all in the rest. Once zygote nuclei were assumed to have been transferred to auxiliary cells, complex fusion cells formed from which multiple gonimoblast initials and filaments issued. What was illustrated in Kraft’s monograph were the very substantial differences among members of the family that take place between carposporophyte initiation and the production of carposporangia. This was not an issue for Kylin (1932) when establishing the family (as the Dicranemaceae), for this was created solely for Dicranema. In regard to the two other established genera later added by Kraft (1977b), Kylin (1956) had placed Peltasta in ‘unsafe positioning’ (unsicherer Stelling) and Tylotus in the Gracilariaceae. Kraft’s (1977b) study and the addition of still other genera and species (Kraft and Womersley 1994) lacked a taxonomic emphasis on exactly how different these were from the family’s generitype in gonimoblast features.
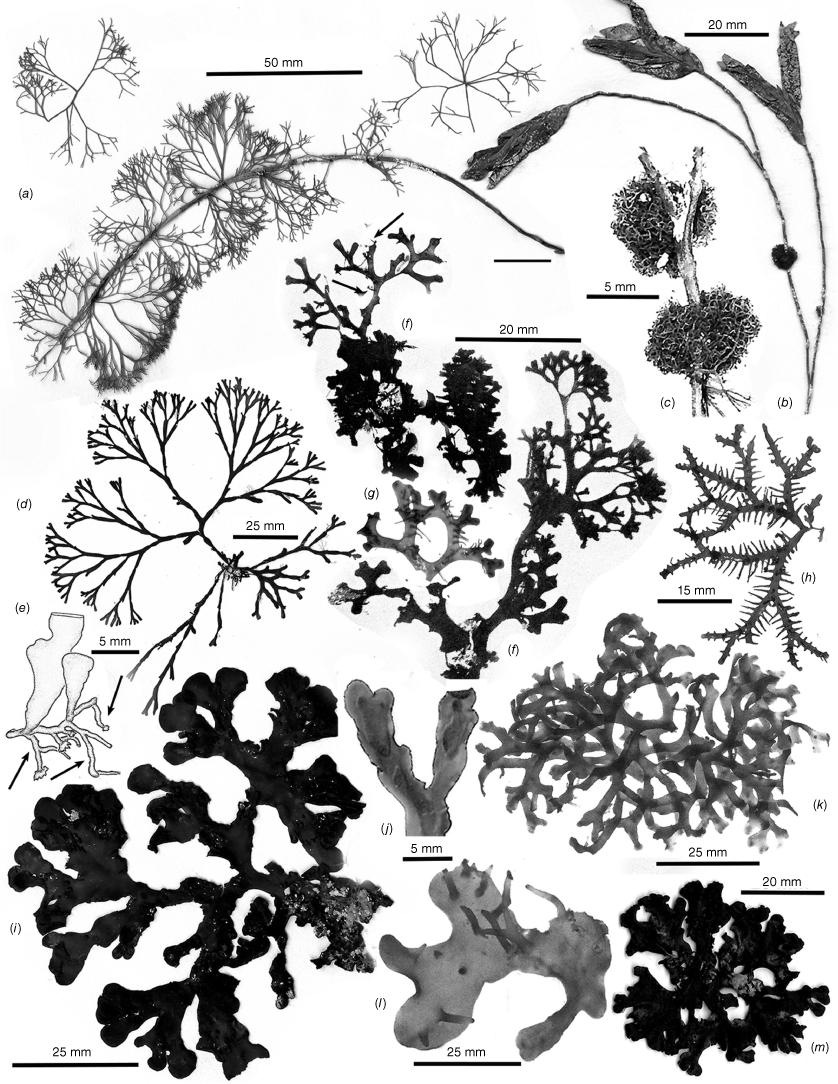
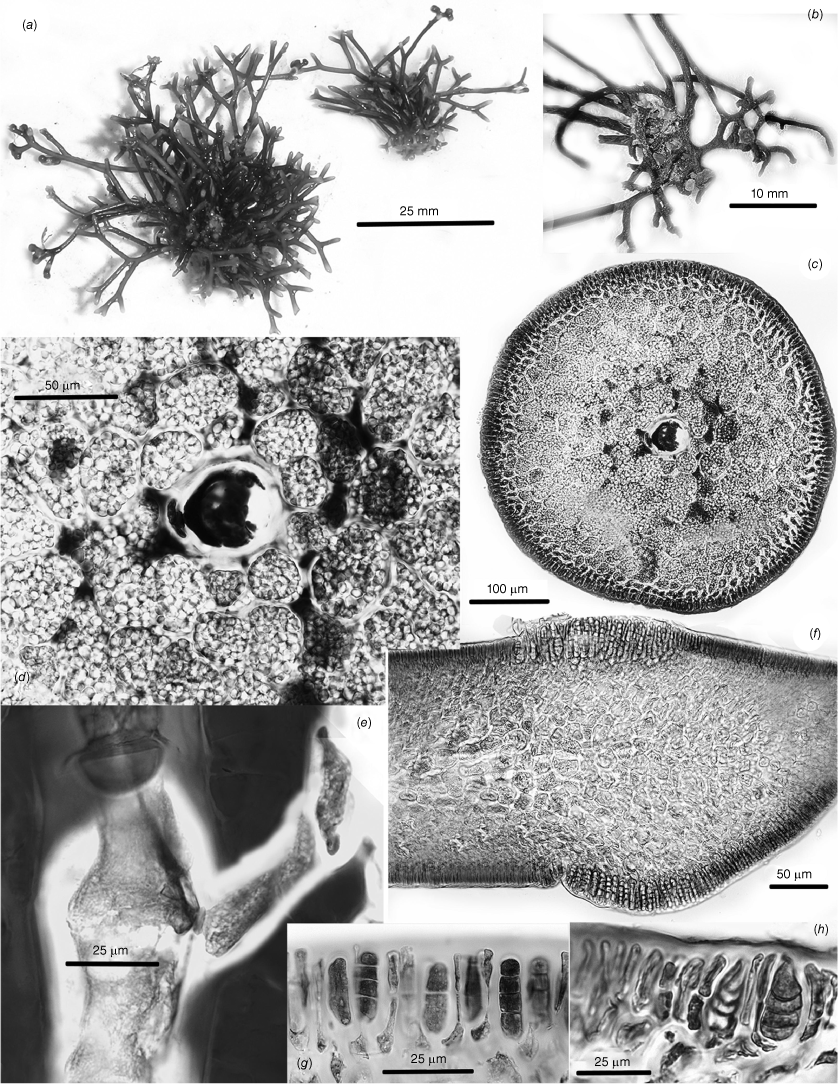
Thallus habits of dicranemataceous species. (a) Dicranema revolutum. A dense aggregation of filiform, dichotomous thalli epiphytic on a stem of Amphibolis antarctica (K-GEN-4168e.1). (b, c) Dicranema cincinnalis. Densely clumped, rounded aggregates of thalli on A. antarctica (b, AD, A44738; c, AD, A44737). (d, e) Peltasta australis. (d) Compressed narrowly linear axes of a complanate, (sub-)dichotomous cystocarpic thallus (K-GEN-4713b). (e) Detail of the anchoring stolons and haptera of a holdfast (K-GEN-6163 (=MELU 23539)). (f, g) Reptataxis rhizophora. (f) Proximally clumped, distally uncongested compressed and (sub-)dichotomous axes. (g) Detail of haptoral anchoring processes (arrow) at the base of a frond (K-LHI- 9914 (=MELU 23390)). (h) Pinnatiphycus menouana. Complanate, compressed and dichotomous primary axes with regularly spaced, distichous filiform laterals on a paratype tetrasporangial specimen (IRD 0038). (i, j) Tylotus obtusatus. (i) A broadly foliose dichotomous thallus rising from a prostrate, haptorally anchored base (AD, A42228 (=K-GEN-4251)). (j) Distal portion of a tetrasporophytic axis with submarginal elongate and submarginal nemathecia (K-GEN-7223). (k, l) Tylotus laqueatus. (k) Uniformly linear and narrow flattened axes of an imbricating recumbent frond. (l) Detail of anchoring haptera at the base of a thallus (HAW-IAA-30653 (K-GEN-11672)). (m) Tylotus lichenoides. A compact thallus with axis widths, dichotomies and dark coloration similar to many specimens of T obtusatus (K-GEN-12207).
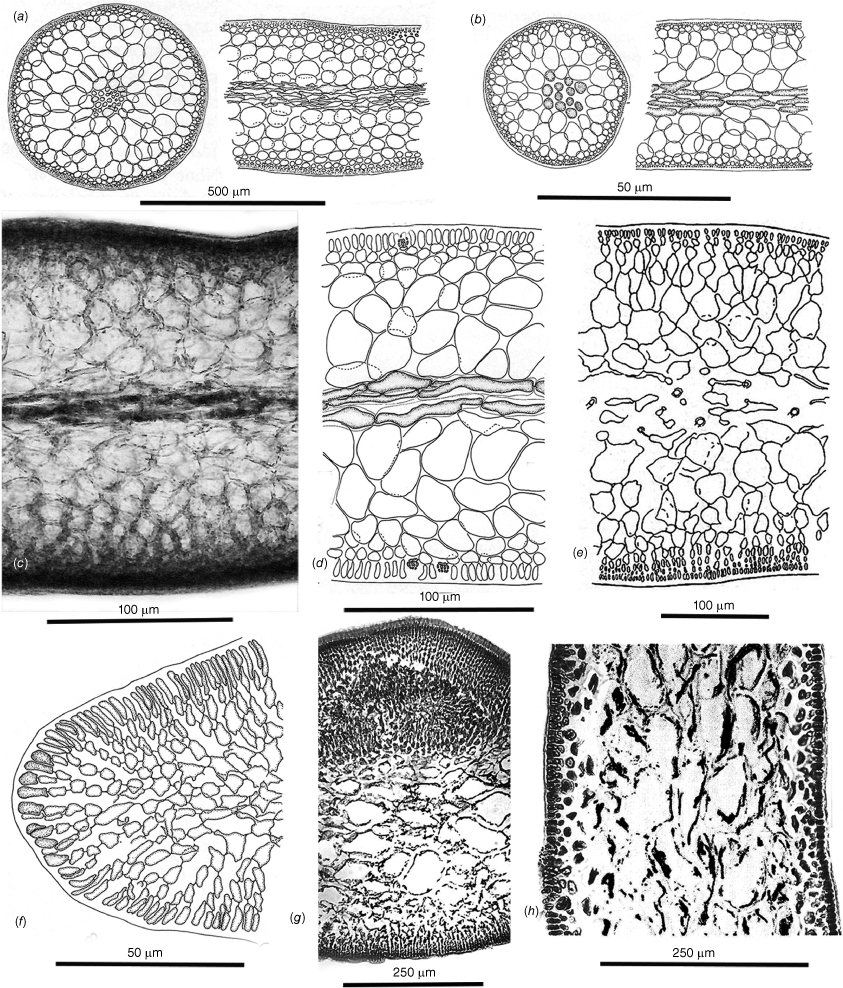
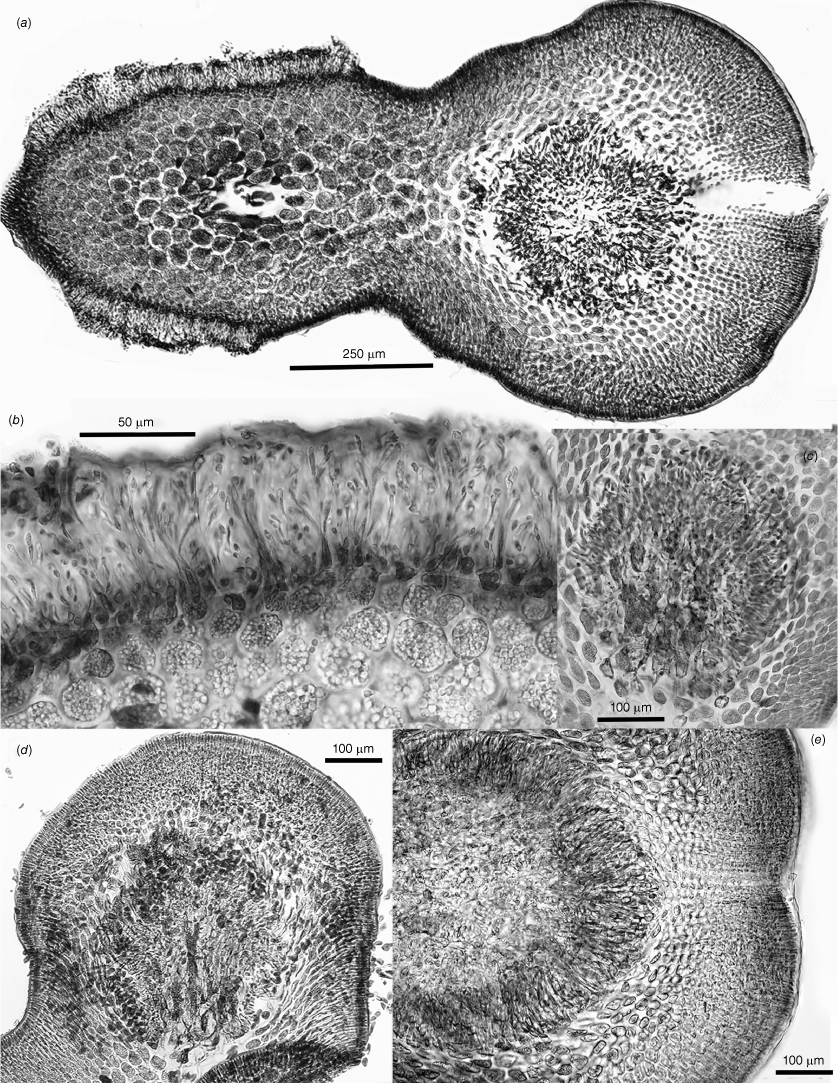
Cross- and longitudinal sections of axes’ cell features. (a) Dicranema revolutum. Cross- and longitudinal sections, the compact narrow cores of longitudinal filaments encircled by broad outer medullas of sub-isodiametric cells and shallow cortexes (AD, A38408). (b) Dicranema cincinnalis. Cross- and longitudinal sections similar to those of the much broader sister species (AD, A44739 (=K-GEN-3546, x.s.; K-GEN-3554, l.s.)). (c, d) Peltasta australis. (c) The darkly pigmented filaments of the narrow central medulla surrounded by hyaline, subisodiametric outer medullary cells (K-GEN-FJS 5268; photo by F.J. Scott). (d) Long-section of spermatangial axis, the transition abrupt between the pigmented cells of the central core and the surrounding hyaline medullary cells that grade to the two- or three-layered cortex (AD, A44740 (=K-GEN-4248)). (e) Reptataxis rhizophora. Cross-section showing the lax aggregation of central medullary filaments and cells, the uncompacted subisodiametric outer medullary cells relative to those in (d), and the deeper and more moniliform layer of cortical cells on the ventral rather than the dorsal surface (MELU 23382 (=K-LHI-9039)). (f, g) Tylotus obtusatus. (f) Multiaxial apical cells (stippled) and the Initial formation of a uniformly ‘cellular’ medulla. (g) Section of a mature frond subtending a cystocarp, the cells of the medulla compact and thick-walled throughout (AD, A42228 (=K-GEN- 4243a)). (h) Tylotus laqueatus. Long-section of a mature frond, the completely ‘cellular’ medulla bounded by evenly shallow cortical layers (K-GEN-11672b).
Tetrasporangial and spermatangial features. (a–c) Dicranema revolutum. (a) Tetrasporangial nemathecia in swollen branch tips (arrows). (b) Progressive stages in the development of deeply inset zonate tetrasporangia. (c) Progressive stages in the development of subsurface catenate spermatangial clusters (a–c, AD, A38408). (d–f) Dicranema cincinnalis. (d) A tetrasporic thallus from the type collection, the nemathecia (arrows) in hooked branch tips (AD, A44737). (e) Initial and deeply inset mature tetrasporangia. (f) Inner cortical clusters of catenate spermatangia (e, f, AD, A44749 (=K-GEN-3546)). (g, h) Peltasta australis. Mature tetrasporangia, distally flush with the thallus surface and paired with a single cortical cell (AD, A8277). (h) Clusters of non-catenate ovoid spermatangia on subsurface mother cells (AD, A44740 (K-GEN-4248)). (i–k) Reptataxis rhizophora. (i) Tetrasporangial nemathecia (arrows) in subapical dorsal patches. (j) Initial and mature tetrasporangia among a palisade of nemathecial filaments (i, j, MELU 23390 (=K- LHI-9914)). (k) Lax spermatangial ampullae borne on inner- cortical mother cells (MELU 23382 (=K-LHI-9039)). (l, m) Tylotus obtusatus. Initials and mature tetrasporangia borne at surface and lower positions on lengthy nemathecial filaments (AD, A41823 (=K-GEN-3980)). (m) Shallow spermatangial ampullae borne on subsurface cortical mother cells (AD, A42228 (=K-GEN-4243a)). (o) Tylotus laqueatus. (n) Oval to ovoid tetrasporangial nemathecia (arrows) on a dorsal frond surface (BISH IAA 1221). (o) A palisade of short vertical nemathecial filaments with terminal tetrasporangia (BISH MSD 8669).
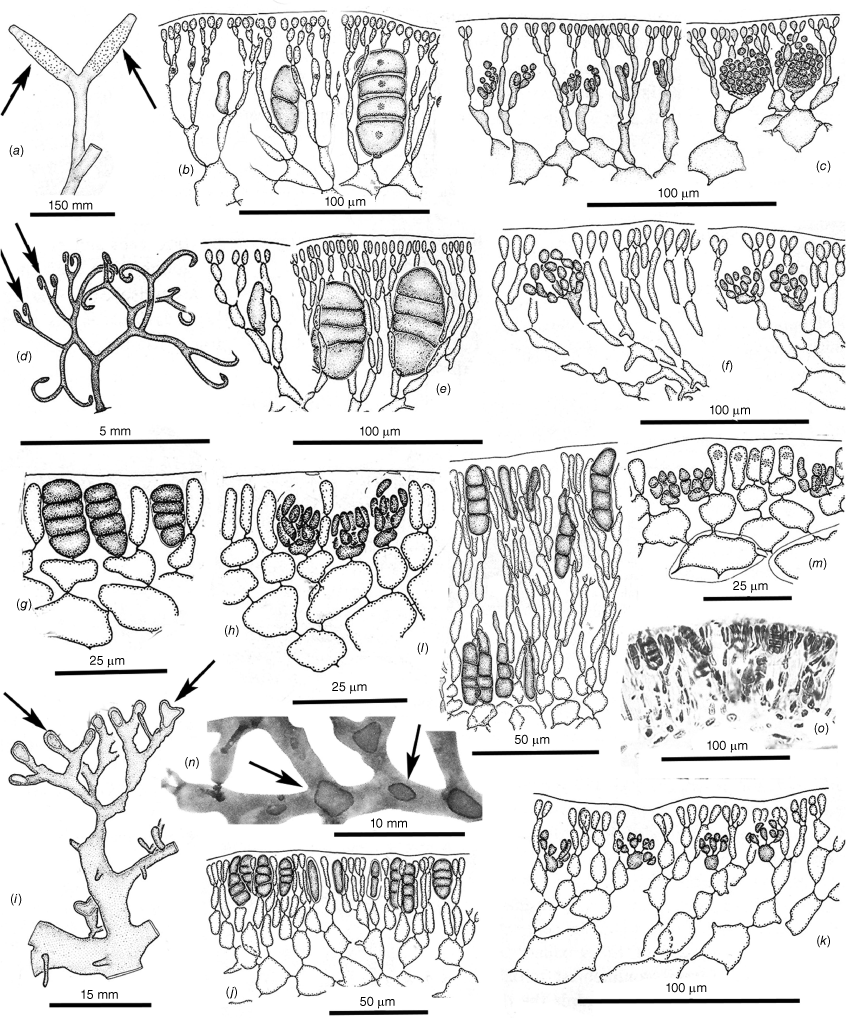
Female reproductive features of Dicranema revolutum (a–l, AD, A38408) and D. cincinnalis (m, AD, A44739 (=K-GEN-3554)). (a) Series of swellings (arrows) made by ostiolate subterminal cystocarps. (b) A single mature cystocarp (arrow) and scars left at sites of sloughed cystocarps on other branch forks. (c) Two-celled carpogonial branches, the straight trichogynes arching toward the surface from supporting cells deep in the cortex. (d–f) Apparent fusions of the bases of trichogynes to seemingly non-procarpic auxiliary cells (arrows) both prior (d) and subsequent (e, f) to early fusion-cell development. (g) Multiple gonimoblast filaments issuing in all directions from a growing fusion cell. (h) Inward development from a fusion cell of a placental mixture of host and gonimoblast filaments. (i) Later stage of placentation of the mixed gonimoblast and vegetative filaments as a cavity over the surface of the carposporophyte develops. (j) Growth of free-standing, unmixed gonimoblast filaments on the leading surface of the placenta, the boundary between the placenta and the female-gametophyte tissue (arrow) indicated by a zone of enlarged and deeply staining gametophyte cells. (k) A mature cystocarp, the ovoid carposporangia borne across the surface of the placenta and directed away from the remnant fusion cell (arrow) toward the opposite side of the bearing axis. (l) Carpospore release from the rupturing pericarp at the site of the ostiole. (m) A shallow carposporophyte placenta spreading inwardly from the fusion cell, with primordia of carposporangia differentiating on the leading surface. (n) Glancing x.s. of a mature cystocarp that nearly fills the parent axis, the ovate carposporangia single and terminal on elongate subtending cells. The shallower and laxer cortex on the left side (arrow) will possibly to be the site of a non-ostiolate opening to the surface that effects spore release.
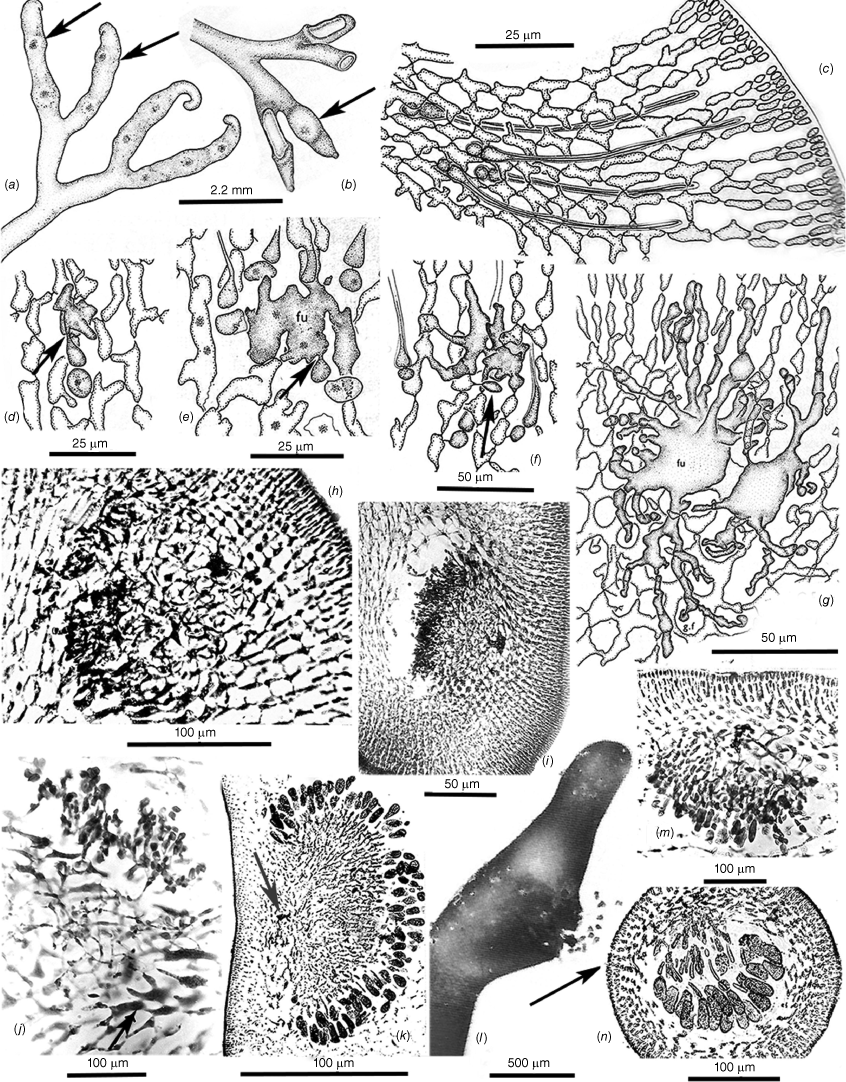
female reproductive features of Peltasta australis (a, c, MELU, 23529 (=K-GEN-6163); b, d–j, AD. A44740 (=K-GEN-4248)). (a) Habit of a cystocarpic gametophyte. (b) Early stages in the subapical development of cystocarps. (c) Fully mature ostiolate cystocarps. (d) Three-celled carpogonial branches on supporting cells in the deep cortex (arrows), the procarpic auxiliary cells slightly swollen and darker than surrounding cortical cells and the centre of the three arrows indicating a carpogonium possibly in the process of fusing to the largest of the putative auxiliary cells. Several vegetative cells in the outer medulla have undergone direct fusion to adjacent cells. (e) An early diploidised auxiliary cell. (f) Multiple fusions between an auxiliary and neighboring cells, the multiple gonimoblasts radiating from the complex. (g) Early carposporophyte development forming a spherical cluster of gonimoblast filaments deep within the vegetative tissue as an ostiole grows downward from the surface to meet the expanding carposporophyte. (h) Detail of the early gonimoblast as this grows through vegetative filaments and connects to gametophytic cells. (i) A mature cystocarp of radiating gonimoblast filaments from an interior placenta of mixed haploid and diploid cells and filaments. (j) Detail of the peripheral gonimoblasts, the aligned filaments of narrow rectilinear cells budding off tiny spherical carposporangia at the surface. Knots of cells of seemingly arrested growth are buried in the filaments.
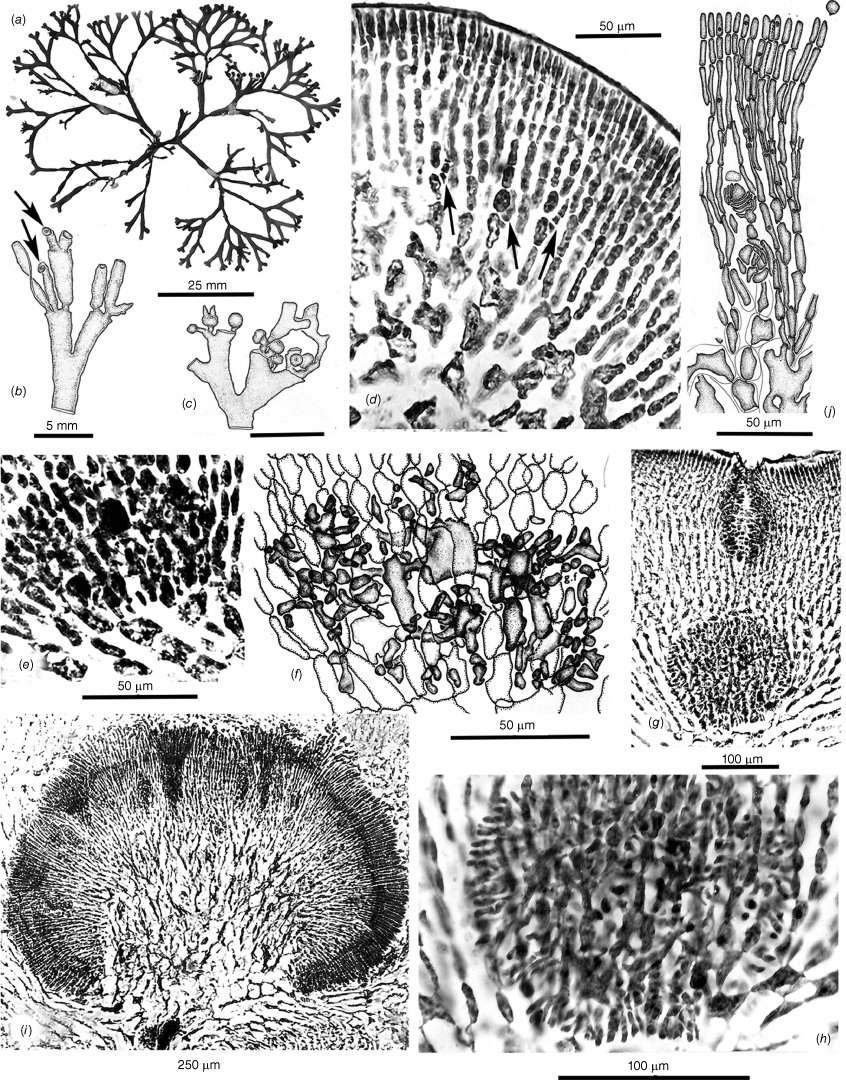
Female reproductive features of Reptatataxis rhizophora (a, MELU, 23390 (=K-LHI-9914); b–h, MELU 23382 (=K-LHI-9039)). (a) Frond habit with mature ostiolate cystocarps. (b) Three-celled carpogonial branches on outer medulla supporting cells, a ‘glandular’ hair (left) and two basal hair cells (stippled) in the inner cortex. (c) Apparent fusion of a diploidised carpogonium with an auxiliary cell and consequent multiple gonimoblast initials (g.i.); trichogyne (tr), supporting cell (su) and hypogynous cell (hy) all indicated. (d) Early stage of fusion-cell formation with possible site of carpogonial fusion (arrow). (e) Mostly lateral growth of gonimoblast filaments from an auxiliary fusion cell (fu), with remnants of the basal and hypogynous cells (hy) of the possible diploidising carpogonial branch (arrow) still intact. (f) A more advanced stage of carposporophyte growth through host gametophytic tissue from a persistent fusion cell. (g) A basally and centrally placentate mature cystocarp, chains of peripheral carposporangia being released through a broadly spreading ostiole. (h) Detail of the catenate carposporangia borne on the central placenta within the pericarp.
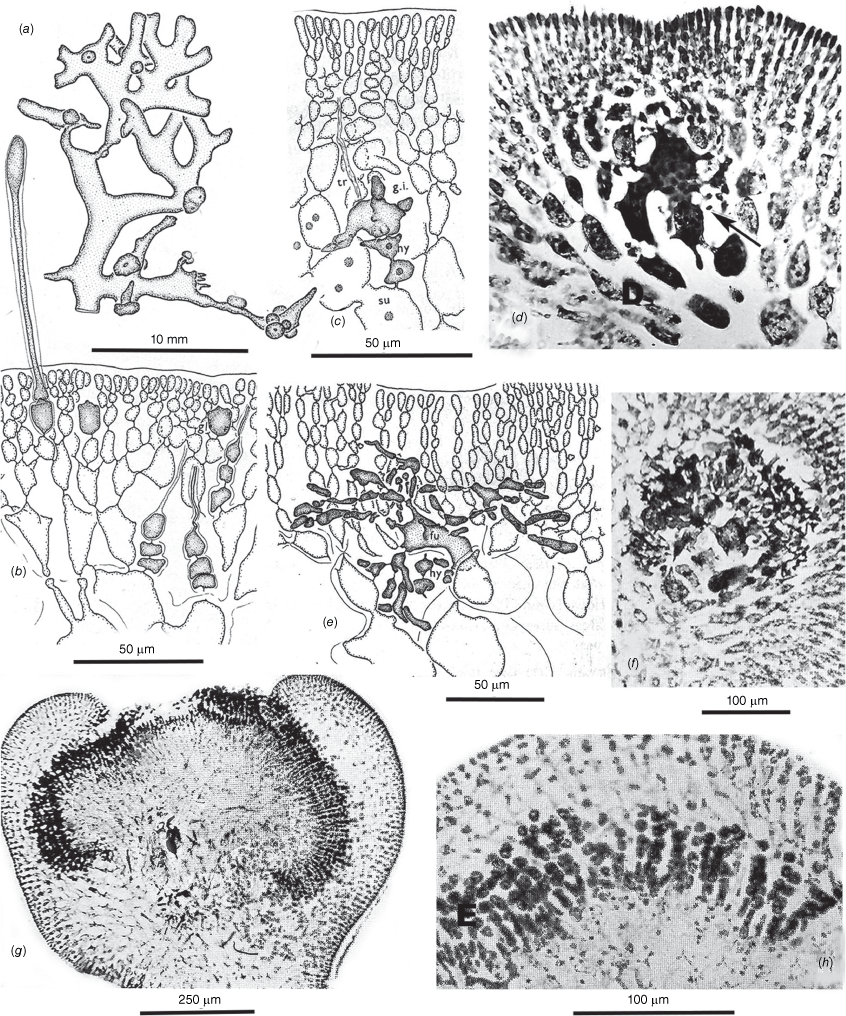
Female reproductive features of Tylotus obtusatus (a–j, l, m, AD, A42228 (=K-GEN-4251); k, AD, A44741 (=K-GEN-1602)). (a) Ventral surface of a young cystocarpic thallus basally anchored by haptera, the undersides of scattered distal cystocarps on the dorsal side visible through the frond. (b, c) Three-celled carpogonial branches attached to the undersides of supporting cells and wrapping around these. (d) Evidence of the incorporation of a presumably diploidised carpogonium resulting in conversion of the supporting to an auxiliary cell (arrow). (e) Likely diploidisation site of an auxiliary cell in the process of forming a fusion cell. (f, g) Stages of early gonimoblast growth, the filaments issuing laterally from the fusion cells into chambers beneath the pericarp. (h) Detail of gonimoblasts growing from one side of the fusion cell of (g). (i) Lateral gonimoblasts growing across a floor of elongate gametophyte cells and forming a consolidated dome of carposporophyte filaments beneath the pericarp. (j, k) Stages in the invagination of the carposporophyte that increase the surfaces for the production of carposporangia, the persistent basal fusion cells still intact. (l) A fully mature cystocarp with invaginated troughs and pockets lined with sporogenous filaments. Breakdown of the pericarp progressing from the ostiole site results in easier carpospore release. (m) Detail of the short, unbranched sporogenous filaments terminating in single spherical carposporangia.
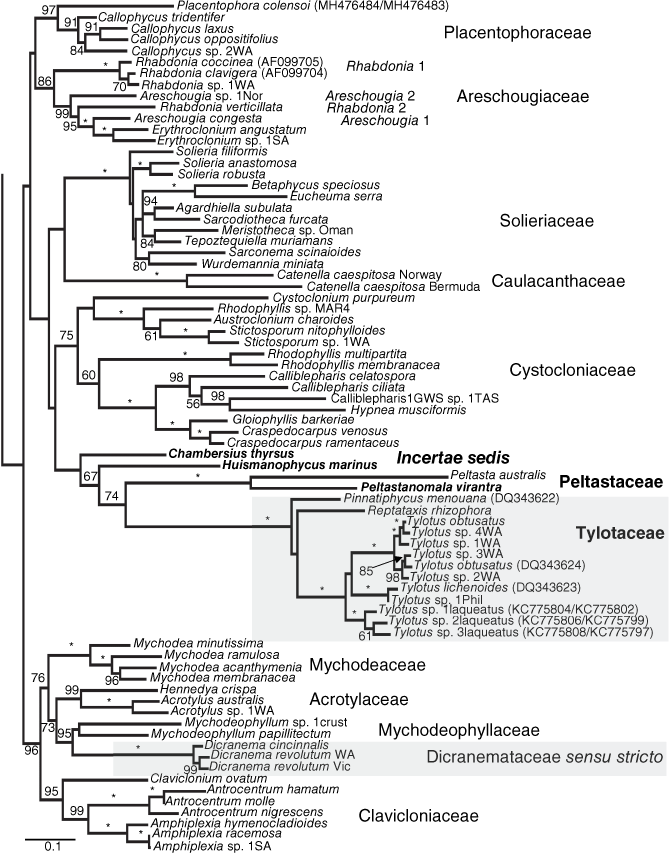
Maximum likelihood tree generated from the concatenated alignment (COI-5P, rbcL, LSU). Values at nodes represent bootstrap support values >50% with full support (100%) indicated by an asterisk. Three major lineages previously assigned to the Dicranemataceae highlighted in grey. New taxa in bold. Data sourced from GenBank indicated after each taxon name in parentheses as rbcL only or COI-5P/rbcL when both genes were available for a genetic group. Scale is substitutions per site.
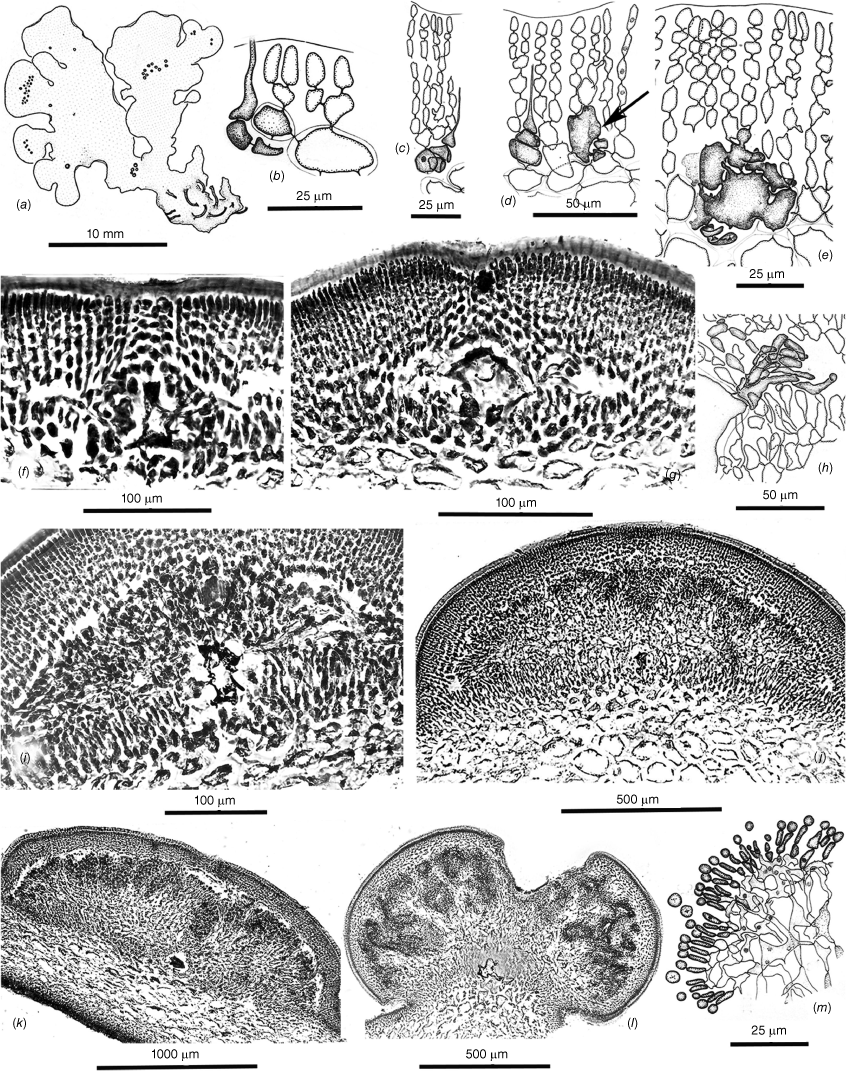
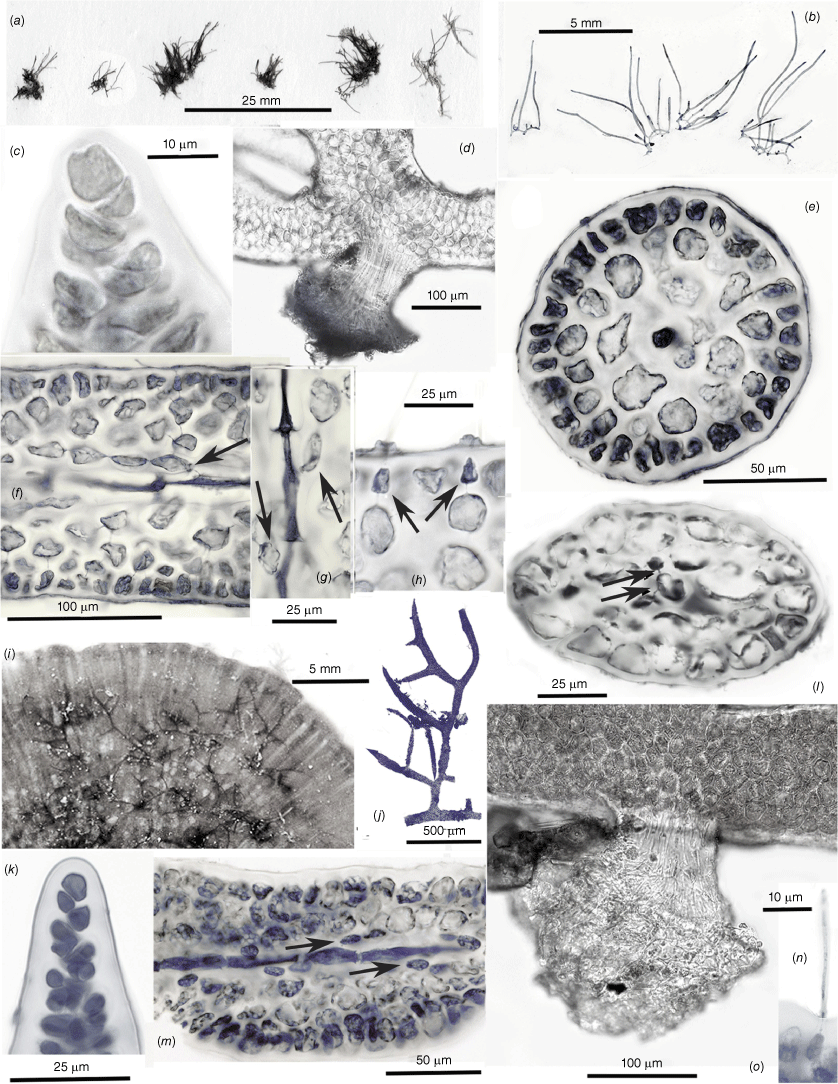
Habits, cross- and long-sections and tetrasporangial features of Peltastanomala virantra (a, c, d, UNB, GWS032778; b, e–h, UNB, GWS032779). (a) Wet habits of the holotype clusters of monoecious gametophytes. (b) Detail of the basal haptera and stolons. (c) Axis cross-section illustrating the prominent central-axial filament ringed by a deep pseudoparenchymatous medulla surrounded by a shallow inner and surface cortex. (d) Detail of the central-axial filament, the basal cell of a single periaxial filament (its basal cell on the left of the cetral cell) and starch-filled cells of the medulla. (e) Long-section of a central-axial cell and the proximal two cells of the single periaxial filament. (f) Long-section of a distal branch axis with opposite tetrasporangial nemathecia. (g) Undivided (left), bisected (centre) and mature basally attached zonate tetrasporangia and accompanying one- or two-celled sterile paraphyses. (h) Detail of one- and two-celled paraphyses paired with atypically rotund tetrasporangia.
Spermatangial and cystocarpic features of Peltastanomala virantra (a–e, UNB, GWS032778). (a) Cross-section of an axis on which a lateral cystocarp is developing opposite surface layers of chambers housing spermatangial filaments. (b) Detail of the surface chambers of fountain-like filaments with terminal spermatangia. (c) An early stage of radial carposporophyte growth, fused cells in the centre and mixed gonimoblast and host gametophyte filaments spreading circumferentially. (d) A basally placentate carposporophyte showing fused cells centrally and intermixed gonimoblast and gametophyte filaments at a stage prior to the development of carposporangial filaments. (e) Apical portion of a mature carposporophyte covered by a dense layer of sporogenous filaments beneath a developing ostiole in the pericarp.
(a–h) Chambersius thyrsus (UNB, GWS016630). (i–o) Huismanophycus marinus (UNB, GWS025656). (a) Pressed specimens of the holotype collection. (b) Habits of wet-preserved specimens from the type collection. (c) Oblique division of the uniaxial apical cell. (d) Multicellular cable of hapteral anchoring filaments. (e) Cross-section of a terete uniaxial erect axis. (f) Long-section of a mature axis, a cell of the central-axial filament bearing a single periaxial basal cell (arrow) and filament from which the cortical tissues are derived. (g) Successive central-axial cells each giving rise to a single periaxial cell (arrows) and filament. (h) Darkly pigmented basal portions of ‘glandular’ hairs (arrows) with evanescent distal portions that erupted through surface collars and craters. (i) Diminutive members of the holotype collection epiphytic on the dorsal surface of Sonderophycus capensis (Montagne) M.J. Wynne. (j) Wet habit of the holotype specimen. (k) Oblique division of the uniaxial apical cell. (l) Cross-section of a compressed uniaxial axis, the central axial cell pit-connected (arrows) to two periaxial cells. (m) Long-section showing apparent single periaxial cells (arrows) and filaments. (n) The embedded base and extended portion of a cortical hair cell. (o) The short, dense cable of filaments of a hapteral holdfast.
Little work was undertaken on the Dicranemataceae between 1977 and the present but three things did affect the family in the hiatus. As a result of intrepid field work, the new genus Pinnatiphycus N’Yeurt, Payri & P.W.Gabrielson (2006) was discovered at great depths in New Caledonia and Fiji, the sole species most appropriately named P. menouiana for the valiant efforts of Jean-Louis Menou to collect and photograph the type and other collections from 30- to 70-m depths. This study also presented an NJ tree showing the new genus nested between Dicranema and two species of Tylotus, the first time that molecular data had featured in a taxonomic treatment focused exclusively on the family. This was followed by a new species of Tylotus, T. laqueatus Kraft, K.Y.Conkl. & A.R.Sherwood (2014) from Hawaii, that was also accompanied by molecular data that placed all three species of Tylotus in a family grouping with Pinnatiphycus and the Lord Howe Island-endemic Reptataxis rhizophora (A.H.S.Lucas) Kraft. Only distantly allied, however, was the generitype of the Dicranemataceae, Dicranema revolutum (C.Agardh) J.Agardh, that was shown to be an outlier to the other genera Kraft placed in the monogeneric family originally established by Kylin in 1932.
A type of digression from serious taxonomic focus took place in pre-molecular-taxonomic times when Norris (1987), in a study of a South African species of Sarcodia J.Agardh, concluded that the Dicranemataceae as portrayed by Kraft (1977b) was so inadequately distinguished from the Sarcodiaceae in terms of essential morphological characteristics that the two should be merged and the former family reduced to synonymy. This suggestion was strongly disputed by Liao et al. (1994) on both anatomical grounds and because of the major differences in the types of carrageenans present, all of which were taken to decisively distinguish and separate the two groups. Silva et al. (1996, p. 287) noted the difference of opinion without making a final judgment, although these authors opted to recognise the Dicranemataceae in the Indian Ocean Catalogue. Although Norris (1987) pointed out some general similarities between the generitypes of the two families, these paled to insignificance when major differences in thallus structure and particularly features of carposporophyte development were compared. When the two genera were later included in a broad molecular survey of the Gigartinales by Saunders et al. (2004), results indicated that not only were the two families distinct but also most likely did not even belong to the same order.
Our revision of the Dicranemataceae begins with observations made from a morphological and anatomical standpoint (Fig. 1–7), particularly in regard to carposporophyte development. We subsequently aim to establish whether we can correlate these genus-level features with the implications of molecular data and from this draw conclusions about the monophyly or polyphyly of the family as conceived by Kraft (1977b), Kraft and Womersley (1994), N’Yeurt et al. (2006) and Kraft et al. (2014). New taxa that emerged during the course of this research are formally proposed and illustrated in Fig. 9–11.
Materials and methods
Anatomical methods
The taxonomic histories, various synonymies and previously published literature are covered in Kraft (1977b) and Kraft and Womersley (1994).
Site, habitat, date and collector(s) data for each of the specimens illustrated in Fig. 1–7 and 9–11 are provided in the ‘Specimen data: sources of morphological illustrations’ section in the Supplementary material. Equipment and procedures employed for anatomical observations are as detailed in Kraft and Saunders (2021). All voucher materials on which illustrations previously published in Kraft (1977b) and Kraft et al. (2014) that are reproduced below are either permanently housed in The State Herbarium of South Australia (AD) or in the case of the Hawaiian-endemic Tylotus laqueatus, the Herbarium Pacificum, Bernice Bishop Museum, Honolulu (BISH). Herbarium accession numbers provided in Kraft (1977b) and Kraft and Womersley (1994) as ADU (for the Womersley herbarium at the University of Adelaide) and MELU (for the Herbarium of The University of Melbourne) are provided unchanged from the earlier publications to avoid confusion but all are currently placed in AD. Many of the collections cited in the ‘Specimen data: sources of morphological illustrations’ section in the Supplementary material are also accompanied by numbers from Kraft’s field notebooks (as K-GEN-xxx or K-LHI-xxx). These specimens have also been transferred to AD where AD designations and labels will ultimately be received.
Specimens cited in the previous monographs of Mychodea and the Mychodeaceae (Kraft and Saunders 2017), and the Acrotylaceae (Kraft and Saunders 2021) have been transferred to AD from the Kraft private herbarium.
Molecular methods
Collections used in our molecular analyses are listed in Table S1 in the Supplementary material. These were variously dried as vouchers (pressed or in vials) with subsamples placed in silica gel for subsequent DNA extraction as outlined in Saunders and McDevit (2012). All specimens were initially screened with COI-5P or rbcL to facilitate an initial assignment to genetic species groups (Saunders and Moore 2013). Data were generated for COI-5P, rbcL and LSU as outlined in Saunders and Moore (2013) or sourced from GenBank in a few cases (indicated in Fig. 8) for each dicranematacean species uncovered and related gigartinalean taxa to round out the phylogenetic analyses. An exception occurred for amplification of the LSU in GWS016630 and GWS025656 for which the new forward primer PeltF1 (TTCGGGSTGTCAGTGYGGGWA) replaced the external forward primer T01N to acquire some data for these interesting specimens. In both cases PeltF1 was used in PCR reactions with T20 or T10N or T08 to acquire PCR products for sequencing (Saunders and Moore 2013). Individual gene alignments were COI-5P (78 taxa, 664 bp), rbcL (90 taxa, 1358 bp) and LSU (70 taxa, 1955 bp). Each alignment was subjected to maximum likelihood (ML) analyses with RAxML (ver. 8.2.11, see https://github.com/stamatak/standard-RAxML; Stamatakis 2014; GTR + I + G model with partitioning by codon for protein coding genes) with branch support calculated using 500 bootstrap replicates. No strong conflicts were detected, and a concatenated COI-5P + rbcL + LSU alignment was constructed (91 sequences, 3977 bp) and analysed as above but with partitioning by gene and codon for protein coding genes and with 1000 bootstrap replicates. This tree was rooted on the Gigartinaceae–Phyllophoraceae clade (Kraft and Saunders 2021) and these families, and taxa of the Chondrymeniaceae and Furcellariaceae (Table S1), were cropped from the final figure to facilitate presentation (Fig. 8).
Morphological results
Critical features of the component genera of the Dicranemataceae as the family currently stands are described and illustrated in the seven figures that follow. Collection data for the illustrated material are supplied in the ‘Specimen data: sources of morphological illustrations’ section in the Supplementary material.
Dicranema revolutum (C. Agardh) J. Agardh (1852, p. 634)
The generitype species is an obligate epiphyte of the seagrass Amphibolis antarctica (Labill.) Sond. & Asch. from subtropical Western Australia, eastward across temperate southern coasts to Victoria and south to Flinders I. in The Bass Strait (Kraft and Womersley 1994). The thalli are erect, filiform and laxly to compactly dichotomous but not complanate (Fig. 1a). Axes are composed of a narrow central core of compact filaments surrounded by a broad band of large hyaline, (sub-)isodiametric cells bounded by a thin compact cortex of small, pigmented cells (Fig. 2a). Tetrasporangia occur subterminally in slightly swollen nemathecia at branch forks (Fig. 3a) and are basally pit-connected to mother cells at the base of vertical cortical filaments (Fig. 3b). At maturity the sporangia are immersed two or three cortical layers below surface cortical cells (Fig. 3b). Spermatangia are catenate and form in scattered ampullar clusters deeply inset from the branch surface (Fig. 3c). Gametophytes are monoecious, the cystocarps protuberant, ostiolate and single or in short series near branch apices (Fig. 4a, b). Large cavities remain when outer pericarps and mature carposporophytes and carposporangia are shed (Fig. 4b). Carpogonial branches are two-celled, the trichogynes smoothly cylindrical and arching toward the branch surface from deeply positioned supporting cells (Fig. 4c). Presumably diploidised carpogonia appear to initially fuse at the bases of the trichogynes to apparently non-differentiated auxiliary cells situated within adjacent cortical filaments (Fig. 4d), the auxiliary cells subsequently commencing to join with cells in neighbouring filaments to form elaborate fusion cells (Fig. 4e, f) that issue numerous gonimoblast filaments in all directions (Fig. 4g). A matrix of intermixed and secondarily pit-connected and fused cells of gonimoblast and vegetative filaments forms from the fusion cell and develops mostly toward the centre of the host axis (Fig. 4h) whereas a cavity above the placenta opens up above the growing carposporophyte (Fig. 4i). The leading surface consolidates with further growth of the mixed placental filaments and gives rise to a layer of free dendroid gonimoblasts (Fig. 4j). Large obovoid to lachrymose terminal carposporangia subsequently cover the surface (Fig. 4k) beneath the roof of the pericarp, where release is made through an ostiole on the side of the cystocarp opposite the diploidised auxiliary cell (Kraft 1977b, fig. 3c) or through the breakdown of the pericarp that begins at the ostiole (Fig. 4l).
Dicranema cincinnalis Kraft (1977b, p. 228)
Thalli are confined to the stems of the seagrass Amphibolis antarctica and recorded from western Yorke Peninsula, South Australia to Westernport, Victoria. The erect axes form tightly curled, ball-like clusters of slender (200–250 μm in diameter) dichotomous filaments (Fig. 1b, c, 3d). Vegetative structure is basically that of D. revolutum but layers encircling the medullary filaments are fewer and somewhat laxer in these much thinner axes (Fig. 2b). Tetrasporangia form in slightly swollen and hooked ends of branch forks (Fig. 3d), the cells basally pit-connected and at maturity borne on inner-cortical mother cells similarly inset from the surface as in D. revolutum (Fig. 3e). Deeply inset clusters of catenate spermatangia (Fig. 3f) are also similar to but smaller than those of the generitype species. Carpogonial branches, fusion-cell formation and gonimoblast initiation are much as in D. revolutum (Kraft 1977b, fig. 4F, G) but placental size and depth (Fig. 4m; Kraft 1977b, fig. 5B) are much reduced owing to the thinness of the axes. Mature cystocarps take virtually the whole interior of the bearing axis and no ostiole has been observed although filaments to one side of the surrounding pericarp (Fig. 4n, arrow) are much laxer and may be where the pericarp splits to release carpospores. On rare occasions, two carposporophytes originating from opposite sides of the bearing branch meet in the middle and completely fill the centre of the axis (Kraft 1977b, fig. 13E).
Peltasta australis J.Agardh (1892, p. 102)
Thalli are recorded from West Island, South Australia, to Western Port (Phillip Island), Victoria and the east coast of Tasmania (Kraft and Womersley 1994, p. 327). Fronds are erect on rocky substrata and narrowly linear, complanate, compressed and dichotomously branched (Fig. 1d, 5a). Anchorage is by a series of short basal stolons and haptera (Fig. 1e). Axes are bluntly rounded and slightly flared at the multiaxial tips, and mature cross- and long-sections (Fig. 2c) consist of a narrow core of deeply pigmented filaments surrounded by a wide expanse of colorless, subisodiametric cells (Fig. 2c) bordered on both sides by a cortex of ovoid surface cells subtended by a single layer of small isodiametric cells (Fig. 2d). Tetrasporangia are basally pit-connected to cells of the subsurface layer and mostly accompanied by a single elongate companion cell (Fig. 3g). Male gametes form in subsurface ampullae and consist of elongate spermatangia that are released through surface pores in the colorless cuticle (Fig. 3h). The monoecious gametophytes (Fig. 5a) initiate cystocarps in subapical swellings (Fig. 5b) and produce carposporophytes within globular, ostiolate pericarps at maturity (Fig. 5c). Carpogonial branches are three-celled (Fig. 5d; Kraft 1977b, fig. 6G) and form deep in the cortex on supporting cells that subtend slightly swollen auxiliary cells (Fig. 5d) with which the presumably fertilised carpogonia directly fuse (Fig. 5d, central arrow). Vegetative cells to the interior of the extended cortex housing the carpogonial branches become frequently linked to adjacent cells by direct fusions (Fig. 5d) even prior to zygote formation. The presumably diploidised auxiliary cell links up and fuses with cells of adjacent vegetative filaments, and issues numerous gonimoblast initials and filaments circumferentially (Fig. 5e, f). As the carposporophytes placentate (Fig. 5g, h), an ostiole progressively differentiates from the surface and extends deeply downwardly to the gonimoblasts (Fig. 5g). The ultimate development of gonimoblasts on the central placental core consists of dense arrays of long, parallel files of elongate cells (Fig. 5i) that bud off spherical carposporangia and contain varying numbers of knotted cells (Fig. 5j) that appear to be islands of arrested growth.
Reptataxis rhizophora (A.H.S.Lucas) Kraft (1977b, p. 241)
This genus is knonw to be endemic to Lord Howe Island that lies at the southern limit of consolidated coral-reef formation in the Tasman Sea approximately one-third of the way between the east coast of Australia and New Zealand. Thalli consist of narrow, cartilaginous, flattened and mostly dichotomous axes that are erect or sprawled on rocky substrata, the fronds broader and more densely branched proximally (Fig. 1f) and attached by narrow haptera both basally and along the lengths and at the ends of prostrate branches (Fig. 1f arrows, g). Axes are laxly filamentous centrally, the medulla surrounded by broad layers of enlarged subisodiametric cells bounded by surface layers that are deeper on one side than the other (Fig. 2e). Tetrasporangia are formed in well-demarcated subapical nemathecia (Fig. 3i) in which these are flush with the surface and tightly jacketed by two-celled cortical paraphyses (Fig. 3j). Spermatangial ampullae are borne on subcortical mother cells and produce two-celled chains of rounded spermatia (Fig. 3k). Mature cystocarps are protuberant, the thick pericarps ostiolate and present both marginally and on flat surfaces (Fig. 6a). Frequent in the cortex are two-celled ‘glandular’ hairs that leave deeply staining basal portions when the part extended beyond the frond surface is shed (Fig. 6b). Carpogonial branches are three-celled and borne on subcortical supporting cells (Fig. 6b). There is uncertainty as to whether the auxiliary cell and diploidised carpogonia branch are procarpic but the gonimoblast filaments (Fig. 6e) issuing from the fusion cell initially grow mostly inwardly and laterally but later form a mostly outwardly oriented placenta of mixed vegetative and gonimoblast filaments (Fig. 6f). The mature cystocarp has a broad hemispherical placenta (Fig. 6g) on which a surface palisade of catenate carposporangia is borne (Fig. 6h), the sporangia shed through a broad ostiole (Fig. 6g).
Pinnatiphycus menouana N’Yeurt, Payri & P.W.Gabrielson (2006, p. 423)
This monotypic genus is recorded from New Caledonia and Fiji. Thalli are shown to be complanate, compressed to flattened and sprawling, arising from a basal disk or short stolon (N’Yeurt et al. 2006, fig. 4, 5) that anchors a short terete stalk that broadens through an apophysis (N’Yeurt et al. 2006, fig. 4, 5) to blades above that are attached to the substratum at intervals by slender haptera (N’Yeurt et al. 2006, fig. 5). Unique among the genera is the abundance of regularly spaced distichous, terete and simple or occasionally bifid horizontal marginal laterals (Fig. 1h; N’Yeurt et al. 2006, fig. 3–6). Axes consist of a broad core of compact filaments of elongate cells tightly encircled by a broad zone of radially aligned (in cross section) filaments of equal width and a three- or four-celled outer cortex (N’Yeurt et al. 2006, fig. 7–9). Spermatangia occur in crowded, deep ampullar pits (N’Yeurt et al. 2006, fig. 16) and tetrasporangia are flush with the surface and accompanied by elongate paraphyses (N’Yeurt et al. 2006, fig. 17, 18) within swollen tips of laterals (N’Yeurt et al. 2006, fig. 6, 19). Carpogonial branches are three-celled (N’Yeurt et al. 2006, fig. 11, 12) but auxiliary-cell position and features of diploidisation are not documented. A fusion cell persists in the centre of an extensive placenta (N’Yeurt et al. 2006, fig. 13), the terminal carposporangia borne on short spreading filaments three or four cells in length (N’Yeurt et al. 2006, fig. 14) and discharged through an apical ostiole (N’Yeurt et al. 2006, fig. 13).
Tylotus obtusatus (Sond.) J.Agardh (1876, p. 428)
Thalli of this variably contoured species are recorded from Champion Bay (Geraldton), Western Australia, to Inverloch, Victoria (Kraft and Womersley 1994, p. 330). The thalli are typically darkly pigmented, almost black and coarsely foliose, smooth margined, basically dichotomous and erect or repent from prostrate basal portions (Fig. 1i) anchored by scattered stout haptera (Fig. 7a). Some thalli are shades of mahogany brown, especially when tetrasporangial (Fig. 1j). Thalli are ‘cellular’ (pseudoparenchymatous) throughout, derived from a broad cluster of marginal initials (Fig. 2f), the interior cells of mature axes becoming compact and thick-walled (Fig. 2g). Tetrasporangia form in elongate nemathecia (Fig. 1j) and grow on and among lengthy palisade filaments, the sporangia mostly flush with the surface but with scattered others, possibly non-functional, occurring at the bases or subsurface along the lengths of the filaments (Fig. 3l). Male ampullae are borne on subsurface mother cells and form tight clusters of two-celled spermatangial filaments (Fig. 3m). Cystocarps form on dorsal surfaces of fronds (Fig. 7a) and produce three-celled carpogonial branches at various depths in the cortex, the carpogonia lying in proximity to the supporting cell (Fig. 7b), and borne on basal and hypogynous cells that partially wrap around what will become the auxiliary cell (Fig. 7c, d). There is direct evidence of procarpy in that the presumably diploidised carpogonium clearly fuses with the supporting cell (Fig. 7d, arrow) and that this fusion precedes the formation of a compact fusion cell. Subsequent gonimoblast growth is lateral from the fusion cell (Fig. 7f) and spreads across a basal, presumably nutritive, layer of gametophytic cells as the ostiole differentiates and a cavity opens beneath the thick pericarp (Fig. 7g, h). The fusion cell persists throughout extensive carposporophyte growth in which gonimoblast and host gametophyte interconnections appear to occur mostly or only along the carposporophyte base (Fig. 7i, j) rather than in a placental mixture. The leading surface of the carposporophyte begins to invaginate with maturity (Fig. 7j, k), producing increased surfaces on which gullies and pockets (Fig. 7l) are lined with short filaments with terminal spherical carposporangia (Fig. 7m).
Tylotus laqueatus Kraft, K.Y.Conkl. & A.R.Sherwood (2014, p. 21)
This species is endemic to the Hawaiian Islands, based on very infrequent records made from Oahu and Maui. Fronds are regularly dichotomous to subdichotomous, of uniformly narrow widths and often hummocked and imbricating (Fig. 1k), becoming broader and more irregularly contoured proximally (Kraft et al. 2014, fig. 7, 9). Anchorage is by numerous scattered haptera at the bases (Fig. 1l) and along the lengths (Fig. 3n, Kraft et al. 2014, fig. 11–17) of distal fronds. Cross sections are composed of compact pseudoparenchyma throughout the interior, bounded by a shallow, even cortex on both sides (Fig. 2h). Occurring in the outer cortex are numbers of short, ‘glandular’ hairs (Kraft et al. 2014, fig. 19–21). Tetrasporangia occur in irregularly rounded sori scattered along the dorsal lengths of fronds (Fig. 3n), the tetrasporangia flush with the surface on relatively short parallel nemathecial filaments (Fig. 3o), with relatively few buried at deeper levels (Kraft et al. 2014, fig. 24). Spermatangia occur in shallow subcortical ampullae (Kraft et al. 2014, fig. 25–27). Carpogonial branches are three-celled and infrequently encountered, although the carpogonium appears to be in proximity to the supporting cell (Kraft et al. 2014, fig. 28). Early gonimoblast development and basal placentation (Kraft et al. 2014, fig. 30), and deep invaginations of the leading carposporophyte edge (Kraft et al. 2014, fig. 29) and short carposporangial filaments (Kraft et al. 2014, fig. 34) appear to be very similar to those of T. obtusatus but a major difference is the implantation of some gonimoblasts of the former into the inner surface of the domed pericarp, where these reverse direction and also produce spore-bearing filaments into the cystocarp chamber (Kraft et al. 2014, fig. 29, 31, 32).
Tylotus lichenoides Okamura (1921, p. 98, pl. 173, fig. 8–15)
This long-obscure species is recorded from Japan, China and Taiwan (M. D. Guiry and G. M. Guiry, AlgaeBase, see https://www.algaebase.org). The thallus illustrated (Fig. 1m) appears very similar to common forms of T. obtusatus in the dark coloring and generally dichotomous flabellate axes but a wide variety of frond morphological characteristics and branching patterns has been illustrated by other authors (Arasaki 1964, p. 97, number 349; Segawa 1967, pl. 66, number 425; Tanaka and Nakamura 2004, p. 169; Tseng 1983, pl. 61, fig. 1). The best illustrations of internal structures are those in the protologue of Okamura (1921, pl. 173).
Fig. 9–11 illustrate the salient features of three previously undescribed genera and species that were discovered during the course of collections (see ‘Specimen data: sources of morphological illustrations’ section and Table S1 in the Supplementary material) for molecularly assisted taxonomic studies. Formal descriptions of these new taxa are provided below.
Molecular results
COI-5P data were generated for a single specimen of Dicranema cincinnalis, this being distinct from D. revolutum (n = 1) by 17–18 substitutions (~2.6%). However, D. revolutum from WA (n = 1; type locality) differed from Victorian individuals (n = 3, all with identical COI-5P sequences) by nearly the same level of divergence (14 substitutions, 2.1%), raising the possibility of cryptic species in this genus. These three genetic groups were tightly clustered and a distant sister to the family Mychodeophyllaceae (Fig. 8). Individuals collected as Peltasta australis from Tasmania and Victoria (n = 3) were identical in COI-5P and sister to, although strongly divergent from, two novel New South Wales collections (74–75 substitutions; ~11%) recognised here as a new genus and species (Peltastanomala virantra G.W.Saunders & Kraft, Fig. 8). Significantly, the peltastacean species formed a lineage independent of the Dicranemataceae sensu stricto in our phylogenetic analyses, rendering that family polyphyletic, thereby necessitating recognition of the new family Peltastaceae (Fig. 8). The COI-5P varied by 0–1 substitutions, this being consistent with a single species, for the seven specimens of Repatataxis rhizophora from Lord Howe Island. This species (and Pinnatiphycus menouiana) joined the various Tylotus spp. in a lineage sister to the previous Peltasta assemblage and likewise well removed from Dicranemataceae sensu stricto, supporting recognition of the new family Tylotaceae (Fig. 8). COI-5P data uncovered a wealth of diversity in the genus Tylotus. Genetic groups with more than one sequence (e.g. T. obtusatus, n = 3, and sp. 1WA, n = 5) had low within genetic group variation (0–6 substitutions, 0.9%) whereas between genetic group variation ranged from 16 to 63 substitutions (2.4–9.5%). Additional genetic groups were uncovered with rbcL (Tylotus sp. 2WA and sp. 3WA) and adding in data from GenBank brought the total number of genetic groups within this genus to 11 (Fig. 8). Two additional novel lineages that were not identified in the field as belonging to this complex were sister to the Peltastaceae and Tylotaceae assemblage although highly divergent in sequences; these are described below as Chambersius thyrsus G.W.Saunders & Kraft and Huismanophycus marinus G.W.Saunders & Kraft (Fig. 8) and not formally placed in a family, pending knowledge of the reproductive anatomy and possibly other close relatives still to be discovered.
The formal proposals of new taxa follow.
Dicranemataceae Kylin, Acta Univ. Lund. 2 28(8), p. 65 (1932), emend Kraft & G.W.Saunders
The Dicranemataceae consists of Dicranema and the two Australian-endemic species D. revolutum and D. cincinnalis. This single genus member differs from the family as characterised by Kraft (1977b) and Kraft and Womersley (1994) in that the genus Dicranema is the sole member. Critical anatomical and morphological features of the emended taxon are provided in the morphological results as above (Fig. 1a–c, 2a, b, 3a–f and 4a–n). Molecular results situate the Dicranemataceae sensu stricto in an isolated position among the Acrotylaceae, Mychodeaceae and the Mychodeophyllaceae, with which this shares very few anatomical particulars. Dicranema is far removed from the positions occupied by all members of the other two families that the group is being divided into.
Peltastaceae Kraft & G.W.Saunders, fam. nov.
Type: Peltasta J.Agardh.
Thalli either multiaxial or uniaxial, terete to compressed, the medulla of narrow, darkly pigmented filaments or consisting of a single stout central-axial filament, each cell of which produces a single periaxial cell (rarely two) and cortical filament. Anchorage by basal haptera or stolons. Tetrasporangia zonate, basally pit-connected, scattered or in nemathecia. Thalli monecious, the spermatangia in ampullae sunken in the cortex or in cavities borne in extensive outgrowths from the cortex surface. Carpogonial branches three-celled, the auxiliary cell procarpic in the generitype (Peltasta australis), the gonimoblast initials and filaments radiating in all directions from a complex of the auxiliary cell and fused adjacent vegetative cells, deeply embedded beneath a down-growing ostiole and ultimately basally and interiorly placentate. Carposporangia borne singly on parallel filaments across the surface of the placenta.
This group of two genera sits as a well-delimited group next to members of the former Dicranemataceae assigned below to the new family Tylotaceae (Fig. 8). Sister to the generitype of the family (Peltasta australis) is the new genus and species Peltastanomala virantra. The separate but deeply rooted, non-reproductive Chambersius thyrsus and Huismanophycus marinus have virtually no habit or anatomical features in common with the more distantly clustered Peltastaceae and Tylotaceae and are hence treated as incertae sedis until such time as cystocarpic features and molecular studies of additional, related taxa may better clarify the family affinities.
Peltastanomala G.W.Saunders & Kraft, gen. nov.
Type: Peltastanomala virantra G.W.Saunders & Kraft.
A monotypic genus, the description as for the single species, P. virantra.
Named for the relationship to Peltasta, as is strongly indicated by the molecular data but differing in many anatomical aspects (such as the prominent uniaxial, rather than multiaxial structure and especially the unique configurations of the spermatangial cavities), making this highly anomalous and counter-intuitively included in the Peltastaceae.
Peltastanomala virantra G.W.Saunders & Kraft, sp. nov.
Type: NEW SOUTH WALES: Coffs Harbour (30°18′17″S, 153°08′53″E), thalli on rock at 4 m off Muttonbird Island, 12 Dec. 2012, G.W. Saunders & K.R. Dixon s.n., (holo: Connell Memorium Herbarium, University of New Brunswick. UNB-GWS032778 (fig. 9A) (cystocarpic); iso: UNB-GWS032779 (tetrasporangial). Type DNA barcode: PP866174 (COI-5P), PP866280 (rbcL).
Thalli cartilaginous, growing in dense bushy tufts composed of erect, terete to slightly compressed axes 2–6 cm in height (Fig. 9a) and arising from a basal tangle of stout haptera and solons (Fig. 9b). Thalli uniaxial, the axes 400–1000 µm in diameter, consisting of a prominent central-axial filament surrounded by a broad, dense layer of pseudoparenchymatous cells packed with floridean starch grains, this bounded by a one or two-layered inner cortex and a single-layered surface (Fig. 9c). Central-axial cells 45–55 µm in diameter, each with a single periaxial filament in a rotating sequence (Fig. 9c–e). Tetrasporangia 18–26 by 5.5–7.7 µm, produced in encircling subapical nemathecia (Fig. 9f), the tetrasporangia zonate, basally pit-connected to bearing cells and each accompanied by a one or two-celled paraphysis. Tetrasporangia normally smoothly rectilinear (Fig. 9f, g), occasionally more rotund, irregularly contoured and possibly non-functional in older axes (Fig. 9h). Gametophytes monoecious (Fig. 10a), the spermatangia borne singly at the ends of narrow dendroid filaments within thinly bounded cavities 65–80 µm deep that arise from surface cortical cells (Fig. 10a, b). Procarpy and diploidisation not observed, early gonimoblasts growing radially from a complex of fused auxiliary-cell and adjacent vegetative cells (Fig. 10c), with maturity the carposporophyte basally placentate within a thick pericarp (Fig. 10a, d), at maturity forming a palisade layer 100–150 µm deep of filaments bearing single carposporangia beneath an initially narrow ostiole (Fig. 10e).
Chambersius G.W.Saunders & Kraft, gen. nov.
Type: Chambersius thyrsus G.W.Saunders & Kraft.
A monotypic genus, the description as for the single species Chambersius thyrsus.
Named in honour of Prof. T. Carrick Chambers, who at The University of Melbourne became, at the age of 37, one of the youngest-ever professorial departmental heads. Prof. Chambers was responsible for the first author’s appointment to the Botany School in 1974, and his leadership and support were greatly admired and deeply appreciated by those who served with or under him. Prof. Chambers left The University of Melbourne in 1986 to become the director of the Royal Botanic Garden of Sydney, continuing his productive administrative and research activities both there and after his retirement. In 1996, he was honoured as a Member of the Order of Australia (AM) for his excellence as a ‘teacher and researcher on conservation issues and botanical concepts’ (see https://www.anbg.gov.au/biography/chambers-thomas-carrick.html).
Chambersius thyrsus G.W.Saunders & Kraft, sp. nov.
Type: VICTORIA: Point Lonsdale (38°16′35″S, 144°37′14″E), thalli on sandstone cobbles at 4.27 m on the reef seaward of the end of Lawrence Road, 02 Feb. 2010, G.W. Saunders, L.G.K. Kraft & K.R. Dixon s.n. (holo: UNB-GWS016630 (fig. 11A), non-reproductive. Type DNA barcode: HM918282 (COI-5P), PP866286 (rbcL).
Thalli filiform (Fig. 11a, b), 3–8 mm in length, the erect axes growing from a single, obliquely dividing apical cell (Fig. 11c) and arising from a creeping base anchored by tight clusters of parallel filaments forming short haptera (Fig. 11d). Erect axes 120–170 µm in diameter, the prominent central-axial cells surrounded by a 2–3 layered cortex of sub-isodiametric cells and a single-celled surface-cortical layer (Fig. 11e). Central-axial cells elongate, with trumpeted ends and producing a single periaxial cell (rarely two), and subsequent filament giving rise to the cortical layers (Fig. 11f, g). Cortical hairs extending from ovoid to triangular bases in the surface cortex and passing through the cuticle through a papillate surface pore (Fig. 11h). Reproductive structures are not known.
A ‘thyrsus’ is a staff associated with Dionysus, who carried this as he went about his daily godly duties in ancient Greece. This play on words pays tribute to the figurative ‘staff of authority’ that Prof. Chambers bore as Professorial Chairman of the School of Botany and as Director of the Royal Botanic Gardens, Sydney, earning throughout his academic and administrative services the deep respect and appreciation of his institutional colleagues.
Huismanophycus G.W.Saunders & Kraft, gen. nov.
Type: Huismanophycus marinus G.W.Saunders & Kraft
A monotypic genus, the description as for the single species Huismanophycus marinus.
Named for Dr John M. Huisman, whose major studies have contributed greatly to the taxonomy of numerous red-algal species, genera, families and orders. Dr Huisman’s monographs of Western Australia’s green, brown and red algae, especially from the previously rarely studied warm-temperate and tropical north-west of that vast state, are encyclopaedic.
Huismanophycus marinus G.W.Saunders & Kraft, sp. nov.
Type: WESTERN AUSTRALIA: ‘The Basin’, Rottnest Island (31°59′21″S, 115°32′09″E), thalli on Sonderophycus capensis at −3.0 m, 18 Nov. 2010, K. & R. Dixon & G. Bolton (holo: UNB-GWS025656 (fig. 11I), non-reproductive. Type DNA barcode: PP866183 (COI-5P), PP866282 (rbcL).
The single collection on which this taxon is based consists of recumbent to prostrate epiphytes on the fan-shaped dorsal surfaces of Sonderophycus capensis (Mont.) M.J.Wynne (Fig. 11i). Thalli fragmenting easily, one of the largest portions reaching 1.55 cm in length with axes 30–70 µm in width (Fig. 11j). Irregularly produced laterals tapering to needle-like points imparting a Hypnea-like appearance to the fronds (Fig. 11j). Growth from a single obliquely dividing apical cell (Fig. 11k), the initially terete axes becoming compressed and 90–110 µm wide by 60–70 µm thick (Fig. 11j, l). The central-axial filament persisting in lower cross-sections and surrounded by one or two layers of rounded hyaline cortical cells and a surface layer of similarly sized rounded to angular cells (Fig. 11l, m). Axial cells occasionally pit-connected to two periaxial cells (Fig. 11l, arrows) but usually only one is present and rotated on successive axial cells (Fig. 11m, arrows). Surface cells occasionally extending into deciduous hairs (Fig. 11n). Prostrate axes anchored basally and along the lengths by compact short haptera that issue from the under sides (Fig. 11o). Reproductive structures not known.
Casting the genus named to honour Dr Huisman in the second declension enables the species name that follows suit with a masculine ending to be the exact nominative-case spelling of his middle name, thus allowing a double-dip of tribute in connection to the marine studies for which he is renown (as emphasised by Kraft 2019, p. 227).
Tylotaceae Kraft & G.W.Saunders, fam. nov.
Type: Tylotus J.Agardh
Family consisting of the genera Tylotus, Pinnatiphycus and Reptataxis.
Thalli multiaxial, attached basally or also along the fronds by stout terete haptera. Fronds flattened to compressed, centrally either completely pseudoparenchymatous (Tylotus) or with a narrow filamentous medulla surrounded by a pseudoparenchymatous cortex. Tetrasporangia zonate, in nemathecia. Gametophytes monoecious, spermatangia in ampullar pits containing a single mother cell ringed distally by one- or two-celled spermatangia. Carpogonial branches three-celled, auxiliary cells clearly procarpic in Tylotus, not yet determined in the other genera. Gonimoblasts multiple and radial in Reptataxis and Pinnatiphycus, becoming basally placentate; gonimoblasts in Tylotus initially horizontal from a large fusion cell, extending across and fusing with cells of a basal layer of gametophyte cells. Cystocarps ostiolate, carposporophytes domed and with much-invaginated surfaces, the carposporangia terminal on elongate subtending cells; carposporophytes of Pinnatiphycus and Reptataxis centrally composed of mixed or fused gonimoblast and vegetative cells or filaments, the carposporangia forming fanned chains of 2–3 in Pinnatiphycus and catenate parallel chains of 4–6 in Reptataxis.
Discussion
We illustrate disjuncts that can be revealed when looking at the alpha-taxonomy of red-algal families from both morphological and molecular standpoints. In regard to the current Dicranemataceae as configured by both wholly anatomical studies (Kraft 1977b; Kraft and Womersley 1994) and two later publications with inclusions of molecular data drawn from only a partial examination of the constituent genera (N’Yeurt et al. 2006; Kraft et al. 2014), the lesson is that the entirety of the family’s genera needs to be analysed to accurately assess the monophyly of a group. What emerges from a study such as ours is that when a family that was defined morphologically prior to the molecular era is shown to be polyphyletic by these new techniques, indications as to which vegetative and reproductive characteristics could have been important markers of family-level differences in earlier times can follow.
These perspectives indicate that the restriction of the Dicranemataceae to the single genus Dicranema should have been clear from the beginning (apologies to Harald Kylin). It differs from all former members of the group in habit and habitat features (such as the filiform, obligately epiphytic thalli; Fig. 1a–c), and reproductively in the catenate spermatangia (Fig. 3c, f), and by two- rather than three-celled carpogonial branches (Fig. 4c). Additionally, the exclusively inward development of the carposporophyte (Fig. 4h, i) and, in the type species, development of the ostiole on the side of the bearing axis above the inwardly growing carposporophyte surface (rather than above the auxiliary cell; Fig. 4k, l; Kraft 1977b, fig. 14A, C) is a unique set of features that correlates convincingly with the distance the clade falls from all the other genera formerly placed in the Dicranemataceae (as is shown by the molecular data of Fig. 8). We show that molecular indications from two closely related populations of D. revolutum from Western Australia and Victoria, and those of the only other member of the genus, D. cincinnalis, constitute a well-supported family. Dicranema cincinnalis is particularly unique in having the thinnest axes of any red alga to house such a large suite of morphologically complex carposporophyte stages, Kraft (1981, p. 12, fig. 1.3) having characterised these as leading to ‘The most morphologically complex cystocarp type in the Rhodophyta’.
The type locality of D. revolutum (Kraft 1977b, p, 223; AlgaeBase, see https://www.algaebase.org) is an almost tropical locality in Western Australia (at 26°26′S, in Shark Bay), close to the northernmost record of the obligate host (at 24°52′S, at Carnarvon), the seagrass genus Amphibolis (Womersley 1984, p. 104; Huisman 2019, p. 398). The range of molecular diversity in the sequenced populations appears to not give an indication as to which populations of this species, common along the whole southern coast of Australia, truly match topotype material that has yet to be collected and sequenced.
The new family that we have designated the Peltastaceae consists of the type species, Peltasta australis and the totally unexpected new monospecific genus Peltastanomala. The generitype is distinctive in being clearly (as opposed to only possibly) procarpic (Fig. 5d), the conversion of completely radial gonimoblasts (Fig. 5g) to a basal and central placentation, and deeply embedded carposporophytes of unique structure in the mature cystocarp (Fig. 5i, j), all of which are highly distinctive and far removed from essential features of the genus Dicranema. A predominantly multiaxial family having a uniaxial member, such as happens in the Acrotylaceae (Kraft 1977a), is not altogether unusual but for so prominent a central-axial filament to be present as in Peltastanomala (Fig. 9c, d), in contrast to a multiaxial generitype species, does seem to be unique. In addition, the male structures (Fig. 10a, b) are so unusual as to have no similarity to those of any other alga.
The two clades represented by the new monotypic genera Chambersius and Huismanophycus (Fig. 8, 11) are surprisingly similar in the diminutive sizes and vegetative structures of the respective type species, particularly in apical and central-axis morphological characteristics, in spite of the single collections being separated by a distance almost the width of the entire continent. Despite this, however, the parallel molecular lineages are deeply rooted and clearly separated, possibly at the family level (Fig. 8). This likely indicates that although detailed studies of southern Australian marine macrophytic algae were made by Womersley over more than 60 years (Kraft 2011), the diminutive ‘fleshy’ forms are less well known and need considerably more focus to establish true alliances and diversity. As is true of the two new genera that we regard as family or families incertae sedis, cystocarpic and more detailed morphological features, in addition to molecular data, will be of great interest when discoveries are finally made.
The largest of the monophyletic groups within the former Dicranemataceae is the Tylotaceae that is defined by sequenced material primarily within two of the three species of Tylotus, and the two monospecific genera Reptataxis and Pinnatiphycus (Fig. 8). This is a family that seems to have radiated from cool-temperate to warm-temperate Western Australian waters, where Tylotus obtusatus and numerous unnamed but closely aligned clades occur (Fig. 8). In genera of warmer subtropical (Reptataxis) and tropical (Pinnatiphycus) Southern Hemisphere islands, and species of Tylotus from tropical–temperate Northern Hemisphere extensions (the Hawaiian-endemic Tylotus laqueatus and the east-Asian T. lichenoides (Guiry & Guiry, 2024)), the impression is of a possible northern migration pattern. One morphological feature uniquely common to the thalli of all the Tylotaceae populations appears to be the type of anchorage that constitutes simple or branched haptera from distal or basal recumbent parts of fronds (Fig. 1l, 7a). The most anatomically distinctive genus of the entire former dicranemataceous members is Tylotus that differs from all others in having no filaments of narrowly elongate cells anywhere in the vegetative axes, in being uniquely procarpic owing to the transfer of the presumed zygote nucleus from the carpogonium to the associated supporting cell (Fig. 7d, e) and anchorage of the carposporophyte by a horizontal basal layer of gametophytic cells (Fig. 7g, i) rather than from a basal and central admixture of fused or secondarily pit-connected gametophyte and gonimoblast cells and filaments (Fig. 6e, f; N’Yeurt et al. 2006, fig. 13). Six collections of Tylotus obtusatus from Western Australia are tightly grouped but separated molecularly and to know which of these correspond to the actual type specimen on which Sonder (1845) based Chondrus obtusatus Sond. is not possible, as this appears to be missing. In the absence of the specimen, a lectotype based on a possible isotype from Geographe Bay on the Indian Ocean coast 220 km south of Perth was chosen by Kraft (1977b; AlgaeBase, see https://www.algaebase.org) but material from only one site (Cozy Corner, three collections belonging to three different genetic groups, Table S1) in that large area has been sequenced. Determination of which of the several Western Australian records may match the collections Sonder had in hand is not possible and therefore none will be provided with formal names at this time (Fig. 8).
We have been fortunate, in our series of publications (Kraft and Saunders 2017, 2021; the present work), to have had the monographs of the three former Australian red-algal families based on completely anatomical and morphological studies made in the 1970s available for comparison. We have also been able to compare the conclusions and speculations arrived at within the monographs with those we currently base on inputs from alpha-molecular taxonomic studies. Observing how the intuitions of the early works stand up to the indications that molecular data can provide, and to find that even precise details of complex carposporophyte development can be misleading or have ambiguous import for phylogenetic assessments at genus and family levels, are interesting. The answer, as expected in our case, is that some guesses about systematic importance made over 45 years ago were fairly predictive of family-level importance, whereas others were inclined to have been relatively far off the mark. A good example of this in the case of persuasive family-level taxonomic conclusions that could have been based on anatomy alone is the genus Tylotus. With the same hindsight that we say Kylin’s Dicranemataceae should never have come to include all the other genera attributed to the family by Kraft (1977a, 1977b), anatomy alone could equally have indicated that Tylotus fully deserved a unique family, being the only genus in the complex to display the suite of unique features outlined above. Molecular evidence does indeed support family status for Tylotus but an anatomist would have been unlikely to have foreseen that Pinnatiphycus and Reptataxis also belong to the family save for the molecular data. The goal of improved systematic understanding in regard to the contents and relationships of some of Australia’s more problematic red-algal families is therefore best served by a combination of detailed anatomical and molecular approaches. Given the complexity of rhodophyte reproductive processes and the frequent challenges to even observing these, our pursuit of monophyletic families in the Mychodeaceae, Acrotylaceae and Dicranemataceae complex of families, materially assisted by molecular inputs, has been a particularly engaging one.
Data availability
The data that support this study are available in the article. All sequence data generated for this study will be available in GenBank and the Dataset DS-DICRANE1 Dicranemataceae on the Barcode of Life Data System (BOLD).
Declaration of funding
This study was funded by the Australian Research Council, the Australian Biological Research Survey and the Schools/Departments of Botany at the Universities of Adelaide, Melbourne and Hawaii. Research at the University of New Bunswick was supported by funding to G. W. Saunders from the Natural Sciences and Engineering Research Council of Canada (RGPIN-2018-03869), the Canada Foundation for Innovation and the New Brunswick Innovation Foundation.
Acknowledgements
The authors thank the many collectors and divers who have assisted with this project over the years (see ‘Specimen data: sources of morphological illustrations’ section and Table S1 in the Supplementary material) and also Prof. Craig Schneider, an anonymous second reviewer, and Mr Brendan Lepschi (a curator at the Australian National Herbarium) for much-appreciated inputs. G. T. Kraft especially acknowledges the late Prof. H. B. S. Womersley, whose critical support and supervision of the thesis work led to the publication of Kraft’s (1977a, 1977b) morphotaxonomic monograph of the Dicranemataceae. G. W. Saunders thanks the many individuals who have contributed to the generation of sequence data in the lab, notably Tanya Moore.
Author contributions
Both authors conceived the study, conducted field work, interpreted results, prepared taxon treatments, and wrote and revised the paper.
References
Agardh JG (1892) Analecta Algologica. Lunds Universitets ÅrsSkrift, Andra Afdelningen, Kongl. Fysiografiska Sällskapets i Lund Handlingar 28(6), 1-182 [In Latin].
| Google Scholar |
Kraft GT (1977a) Studies of marine algae in the lesser-known families of the Gigartinales (Rhodophyta). I. The Acrotylaceae. Australian Journal of Botany 25, 97-140.
| Crossref | Google Scholar |
Kraft GT (1977b) Studies of marine algae in the lesser-known families of the Gigartinales (Rhodophyta). II. The Dicranemataceae. Australian Journal of Botany 25, 219-267.
| Crossref | Google Scholar |
Kraft GT (1978) Studies of marine algae in the lesser-known families of the Gigartinales (Rhodophyta). III. The Mychodeaceae and Mychodeophyllaceae. Australian Journal of Botany 26, 515-610.
| Crossref | Google Scholar |
Kraft GT (2011) In Memoriam: Tribute to Professor Hugh Brian Spencer Womersley (1 November 1922–16 January 2011). Phycologia 50(4), 430-441.
| Google Scholar |
Kraft GT (2019) Book Review: Algae of Australia: Marine Benthic Algae of north-western Australia. 2. Red Algae. John M. Huisman. Phycologia 58(2), 225-227.
| Crossref | Google Scholar |
Kraft GT, Saunders GW (2017) Mychodea and the Mychodeaceae (Gigartinales, Rhodophyta) revisited: molecular analyses shed light on interspecies relationships in Australia’s largest endemic algal genus and family. Australian Systematic Botany 30, 230-258.
| Crossref | Google Scholar |
Kraft GT, Saunders GW (2021) The Acrotylaceae (Gigartinales) revisited: molecular data indicate family-level differences in one of the most enigmatic red algal families. Australian Systematic Botany 34, 305-326.
| Crossref | Google Scholar |
Kraft GT, Conklin KY, Sherwood AR (2014) Tylotus laqueatus, a new species of Dicranemataceae (Gigartinales, Rhodophyta) from the Hawaiian Islands. Phycological Research 62, 16-28.
| Crossref | Google Scholar |
Kylin H (1932) Die Florideenordnung Gigartinales. Lunds Universitets Årssksrift, Ny Följd. Andra Afdelningren 28(8), 1-88 [In German].
| Google Scholar |
Liao M-L, Kraft GT, Munro SL, Craik DJ, Bacic AB (1994) Beta/kappa-carrageenans as evidence for continued separation of the families Dicranemataceae and Sarcodiaceae (Gigartinales, Rhodophyta). Journal of Phycology 29, 833-844.
| Crossref | Google Scholar |
Norris RE (1987) Reproduction in Sarcodia dentata (Suhr) comb. nov. (Gigartinales, Rhodophyceae), with comments on the Sarcodiaceae. British Phycological Journal 22, 147-155.
| Crossref | Google Scholar |
N’Yeurt ADR, Payri CE, Gabrielson PW, Fredericq S (2006) Pinnatiphycus menouana gen. et sp. nov. (Rhodophyta: Dicranemataceae) from New Caledonia and Fiji (South Pacific): vegetative and reproductive morphology and molecular phylogeny. Phycologia 45, 422-431.
| Crossref | Google Scholar |
Okamura K (1921) ‘Icones of Japanese Algae. Vol. IV.’ (Published by the Author: Tokyo, Japan) 10.5962/bhl.title.1618
Saunders GW, McDevit DC (2012) Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. Methods in Molecular Biology 858, 207-222.
| Crossref | Google Scholar | PubMed |
Saunders GW, Moore TE (2013) Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: a summary of current primers, profiles and strategies. Algae 28, 31-43.
| Crossref | Google Scholar |
Saunders GW, Chiovitti A, Kraft GT (2004) Small-subunit rDNA sequences from representatives of selected families of Gigartinales and Rhodymeniales (Rhodophyta) 3. Delineating the Gigartinales sensu stricto. Canadian Journal of Botany 82, 43-74.
| Crossref | Google Scholar |
Sonder OW (1845) Nova Algarum genera et species, quas in itinere ad oras occidentales Novae Hollandiae collegit L. Preiss, Ph.Dr. Botanische Zeitung 3, 49-57 [In Latin].
| Google Scholar |
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9), 1312-1313.
| Crossref | Google Scholar |


