More links in the daisy chain: morphology and molecules delimit two new species in Coronidium and two in Leucozoma (Asteraceae; Gnaphalieae)
Timothy L. Collins A C , Alexander N. Schmidt-Lebuhn
A C , Alexander N. Schmidt-Lebuhn  B , Rose L. Andrew
B , Rose L. Andrew  A , Ian R. H. Telford
A , Ian R. H. Telford  A and Jeremy J. Bruhl
A and Jeremy J. Bruhl  A *
A *
A
B
C Present address:
Abstract
Taxonomic uncertainty in Coronidium has existed since its original circumscription. Recent molecular phylogenetic analyses inferred Coronidium to be non-monophyletic and composed of four distinct clades, leading to the erection of Leucozoma and the confirmation that C. scorpioides (Labill.) Paul G.Wilson and related species are more closely related to other Australian Gnaphalieae. The present study focused on the delimitation of those species inferred to be part of Coronidium, Leucozoma and the closely related Helichrysum leucopsideum DC. We gathered DArTseq single-nucleotide polymorphism data and tested species limits by examining genotypic differences, ancestry, and morphological characters observed on herbarium specimens and living collections. Results support the recognition of four new narrowly endemic species, namely, C. batianoffii T.L.Collins & I.Telford, C. bruhlii T.L.Collins, L. alexandri T.L.Collins and L. wollumbin T.L.Collins. Results indicated that the narrow endemic C. fulvidum Paul G.Wilson is a variable hybrid between C. newcastlianum (Domin) Paul G.Wilson and C. rupicola (DC.) Paul G. Wilson, and subspecies of C. oxylepis (F.Muell.) Paul G.Wilson to be a polymorphic aggregate or ochlospecies, the subject of ongoing study. We lectotypify H. elatum A.Cunn ex DC. and Helipterum glutinosum Hook. and provide revised descriptions of all taxa in the genera, their conservation status, a dichotomous key, tables distinguishing closely related taxa and distribution maps.
Keywords: Australia, Compositae, DArT, endemic, Helichrysum, Helichrysum leucopsideum, hybrid, ochlospecies, paper daisy, taxonomy.
Introduction
Background to the study
The daisy genus Coronidium Paul G.Wilson consists of 22 species and 4 subspecies, of which the majority were formerly included in Helichrysum Mill. (Wilson 2008; Walsh 2014). The highest diversity in species of Coronidium is found in the eastern states of Australia, and diversity decreases westwards with two species occurring in South Australia and none in Western Australia and the Northern Territory (Australia’s Virtual Herbarium, see http://avh.chah.org.au, accessed 14 March 2024). Current species delimitation is based on morphological characters found on phyllaries, cypselae, and pappi (Wilson 2008).
The Australian endemic Helichrysum leucopsideum DC. was thought by Wilson (2008) to be a part of Xerochrysum Tzvelev. However, inferred evolutionary relationships from high-copy chloroplast and nrDNA sequences indicated that Coronidium is paraphyletic to Xerochrysum, and H. leucopsideum is more closely related to C. adenophorum (F.Muell.) Paul G.Wilson and C. waddelliae (J.H.Willis) Paul G.Wilson (Schmidt-Lebuhn et al. 2015). In the same study, rhizomatous species of Coronidium with oblanceolate phyllaries termed by Wilson (2008) the ‘C. scorpioides group’ were found to be phylogenetically isolated and possibly part of Chrysocephalum Walp. (Schmidt-Lebuhn et al. 2015) or Leiocarpa Paul G.Wilson (Schmidt-Lebuhn and Bovill 2021). A recent analysis of evolutionary relationships in Coronidium and Xerochrysum applied genome-wide single-nucleotide polymorphism (SNP) data and corroborated the results of Schmidt-Lebuhn et al. (2015), segregating a new genus Leucozoma T.L.Collins containing six species and two subspecies previously included in Coronidium (Collins et al. 2022).
The term ‘ochlospecies’ has been used to describe polymorphic species where hybridisation, apomixis and autogamy are not considered to be the source of the variation and morphology cannot be correlated with geographic distribution (White 1962, 1978; Cronk 1998). The use of the term ochlospecies has been regarded as preferable to the description of large numbers of subspecies in taxonomically difficult groups (Cronk 1998; e.g. Harrington and Gadek 2009; Wilson 2016; Khan et al. 2018). Alternatively, the lack of any clear taxonomic resolution may be interpreted as shortcomings in the power of the data (e.g. Lima et al. 2015; De Salas and Schmidt-Lebuhn 2018). Solutions may lie in under-utilised sources of information (e.g. large-scale ecological data; Hammer et al. 2018). Although there are many species concepts (de Queiroz 2007), taxonomists are increasingly treating species (and subspecies) as independently evolving meta-population lineages (Dayrat 2005; de Queiroz 2005, 2007) and applying multiple data sources to support taxonomic delimitation (e.g. Chang et al. 2018; De Salas and Schmidt-Lebuhn 2018; Hammer et al. 2018; Collins et al. 2019).
Taxonomic questions
Eight phrase names have previously been applied to putative new taxa in Coronidium sens. lat. (Council of Heads of Australasian Herbaria, CHAH, see https://biodiversity.org.au/nsl/services/APC, accessed 14 March 2024). Four synonymised entities are included in the present study (Table 1). The remaining four phrase name entities were not considered here, because they are a part of the C. scorpioides group (Schmidt-Lebuhn et al. 2015).
| Partition | Taxon or phrase name | State: population | |
|---|---|---|---|
| C. oxylepis group (‘Co’) | C. cymosum | Qld: Blackdown Tableland NP | |
| C. aff. cymosum | Qld: Byfield NP | ||
| C. flavum | Qld: Paluma Range | ||
| C. fulvidum | Qld: Atherton Tableland | ||
| C. glutinosum | Qld: Blackdown Tableland NP | ||
| C. gnaphalioides | Qld: Mount King | ||
| C. lanuginosum | Qld: Byfield NP, Canoona, Clairview, Blackbraes NP | ||
| C. newcastlianum | Qld: Atherton Tableland, Newcastle Range | ||
| C. oxylepis subsp. oxylepis | NSW: Yuraygir NP | ||
| C. oxylepis subsp. carnosum | Qld: Great Sandy NP | ||
| C. oxylepis subsp. lanatum | Qld: Coorada, Cracow | ||
| C. rupicola | Qld: Paluma Range | ||
| C. sp. Many Peaks (I.R.Telford 12309) NE Herbarium | Qld: Mount Castle Tower NP | ||
| C. sp. Penrose (C.Burgess 7/Nov/1968) NE Herbarium | ACT: Black Mountain; NSW: Braidwood, Paddys River, Emu Plains | ||
| C. sp. Thulimbah = Helichrysum oxylepis subsp. (Thulimbah R.W.Johnson 2918) | NSW: Roberts Range; Qld: Girraween NP, Stanthorpe | ||
| C. elatum group 2 (‘Ce2’) | C. adenophorum | SA: Kangaroo Island | |
| C. waddelliae | NSW: Kosciusko NP; Vic.: Mount Buffalo | ||
| C. aff. waddelliae | NSW: Moreton NP, Newnes, Paddys River | ||
| H. leucopsideum | Tasmania: St Helens; SA: Kangaroo Island, Tothill Range, Normanville, Blanchetown; WA: Mundaring, Narogin, Stirling Range NP | ||
| Leucozoma | L. boormanii | NSW: Tenterfield, Boonoo Boonoo NP | |
| L. elatum subsp. elatum | NSW: Boonoo Boonoo NP, Gulaga NP, Nethercote Falls, Pokolbin SF, Willi Willi NP; Qld: Maleny, Springbrook | ||
| L. elatum subsp. minus | NSW: New England NP | ||
| L. elatum subsp. vellerosum | NSW: Wollumbin NP | ||
| L. kaputaricum | NSW: Mount Kaputar NP | ||
| L. lindsayanum | Qld: Mount Barney NP | ||
| L. telfordii | Qld: Lamington NP | ||
| L. sp. Tuggolo SF | NSW: Oxley Wild Rivers NP |
Qld, Queensland; NSW, New South Wales; SA, South Australia; Vic., Victoria; WA, Western Australia; NP, National Park; SF, State Forest.
Describing the genus Coronidium with six new species and two new subspecies, Wilson (2008) also raised taxonomic questions regarding a further four taxa.
The broadly distributed and morphologically variable C. oxylepis subsp. lanatum Paul G.Wilson occurs on sandstone hills in central Queensland, with other disjunct populations in south-eastern Queensland, New South Wales and the Australian Capital Territory potentially representing an additional two unrecognised taxa (Wilson 2008).
A population identified as C. rupicola (DC.) Paul G.Wilson with a prostrate habit occurring on cliff edges on Hinchinbrook Island in northern Queensland, subsequently referred to as C. sp. N Qld Headlands, has glabrous to near-glabrous leaves, contrasting with the typical erect habit and woolly abaxial leaf indumentum of C. rupicola (Wilson 2008). Other populations of Coronidium in northern Queensland on Cape Kimberley and Cape Melville have not been confidently determined at the species level in herbaria (AVH, see http://avh.chah.org.au).
A plant broadly similar to Coronidium gnaphalioides (Domin) Jeanes (syn. C. lanosum Paul G.Wilson; Jeanes 2021), but with sparsely woolly leaf indumentum and lacking stipitate glands on the phyllary claw, is restricted to populations in the Many Peaks Range near Gladstone, Queensland, and could not be confidently circumscribed owing to limited collections (Wilson 2008). On the basis of observations of herbarium material at NE, this entity has the phrase-name Coronidium sp. Many Peaks (I.R.Telford 12309) NE Herbarium since 2011, although it is currently treated in the Australian Plant Census as a taxonomic synonym of C. scorpioides (CHAH, see https://biodiversity.org.au/nsl/services/APC). Collins et al. (2022) showed that it is instead part of the C. oxylepis (F.Muell.) Paul G.Wilson group.
The morphologically variable C. lanuginosum (Domin) Paul G.Wilson occurs in various habitats, from coastal headlands to stony, serpentinite inland hills and is thought likely to represent a species complex (Wilson 2008).
In addition to these taxonomic questions, a plant morphologically similar to Leucozoma lindsayanum (Domin) T.L.Collins and occurring ~500 km south in similar cliff-face habitats is thought likely to represent a putative new narrow endemic and has the accepted phrase name C. sp. Tuggolo State Forest (L.M.Copeland 4225) NE Herbarium (CHAH, see https://biodiversity.org.au/nsl/services/APC).
Some species of Coronidium have disjunctions in distribution, altitude and habitat that may constitute barriers to gene flow, leading to genotypic divergence, as has been seen in other taxa (Lipsen et al. 2013; Hammer et al. 2018). The Queensland endemic species C. cymosum Paul G.Wilson has populations restricted to the sandstone plateau at Blackdown Tableland National Park ~170 km inland and at 800-m altitude, and disjunct coastal populations near sea level at Byfield National Park (AVH, see http://avh.chah.org.au). The latter populations are subsequently referred to as C. aff. cymosum in this study. Populations of Coronidium occurring on the foredunes and beaches in the Whitsunday Islands with small leaves, subsequently referred to as C. sp. Whitsunday Islands, have been variously determined as C. oxylepis subsp. lanatum or C. cymosum (AVH, see http://avh.chah.org.au). Variation in leaf shape and petiole presence is seen between populations of C. newcastlianum (Domin) Paul G.Wilson from the Herberton Range (subsequently referred to as C. sp. Stannary Hills) on the Atherton Tablelands and in the Newcastle Range ~300 km to the south-west, the location of the type collection. Coronidium waddelliae occurs in disjunct populations with contrasting habitats from alpine to subalpine habitats in the South Eastern Highlands Bioregion on humic gravelly soils derived from granite, to the Sydney Basin Bioregion (subsequently referred to as C. aff. waddelliae) on skeletal sandy soils (AVH, see http://avh.chah.org.au). Helichrysum leucopsideum is thought to be closely related to C. waddelliae and C. adenophorum (Schmidt-Lebuhn et al. 2015) and has been recorded from disjunct populations in eastern Tasmania, south-eastern New South Wales, coastal Victoria and southern South Australia, the mallee region of western Victoria and eastern South Australia, and the wheat belt of south-western Western Australia (AVH, see http://avh.chah.org.au).
This study aims to test species limits and find evidence of common ancestry or recent gene flow among populations within and among populations of Coronidium, Leucozoma and Helichrysum leucopsideum. Taxa supported here as independently evolving lineages will be described and diagnosed using morphology, recognising species limits congruent with the molecular data where available. Hybrids are inferred by the presence of intermediate morphology or genetic admixture plus geographic co-occurrence of the putative parents. Resolution of the longstanding taxonomic uncertainty in this study group will allow accurate identification and facilitate a sound basis for assessment of conservation status, as well as highlighting taxa with particular horticultural potential.
Materials and methods
Definition and usage of morphological terms, including those describing stylar appendage shape, leaf shape and indumentum, follow the ‘Flora of Australia’ glossary (Orchard and Thompson 1999). Trichome terminology draws on Payne (1978).
Study group
The scope of the present study includes the taxa defined by Wilson (2008) in his Coronidium oxylepis group, as well as the taxa in the recently erected Leucozoma (Collins et al. 2022), the C. elatum Group 2 as defined by Schmidt-Lebuhn et al. (2015) and the closely related Helichrysum leucopsideum. Formally and informally accepted phrase and manuscript names are applied to some populations (Table 1). This approach ensured testing of the current species limits against multiple hypotheses of putative new entities.
Field collection
Field collection was essential to gather high-quality specimens, as well as propagules, and tissue for DNA extraction with sufficient sampling density. Many gatherings of Coronidium and Leucozoma in Australian herbaria, including many type specimens (e.g. C. newcastlianum and C. adenophorum), are fragments and constitute just the inflorescence and upper cauline leaves and do not have the root system, lower leaves or cypselae (T. L. Collins, pers. obs., 2017–2020). Type localities were prioritised to collect topotypes, and other sites with vouchered collections of <20 years old by using details from the Australasian Virtual Herbarium (CHAH, see https://biodiversity.org.au/nsl/services/APC) to increase the likelihood of relocating sites. Fieldwork was conducted between November 2017 and March 2019. Descriptions of habit, habitat and associated vegetation, topography, estimates of population size, and soil were recorded at each collection site. Herbarium specimens, leaf material for DNA extraction, cypselae and cuttings for propagation were collected when found, and when permit conditions allowed. When available, leaf material from at least five plants spaced approximately 5 m apart was placed immediately in nylon bags and then in sealed containers with silica gel desiccant. Herbarium specimens were pressed in the field and later air-dried at ~16°C and 16% relative humidity before freezing for 1 week at −30°C for herbarium biosecurity.
To broaden sampling, additional sources were sought. Collections of cypselae were accessed from the South Australian Seed Conservation Centre, Australian PlantBank, and the Australian National Botanic Gardens. An extended network of colleagues, friends and volunteers collected material when available and permit conditions allowed. A complete list of gatherings used in the present study can be found in Supplementary Tables S1–S3.
Plant propagation and cultivation
Cypselae obtained during field collection and from seedbanks were sown in a commercial seed-raising medium (Searles Seed Raising Mix, JC & AT Searle Pty Ltd, Kilcoy, Qld, Australia) and germinated in a refrigerated incubator (Model: RI250SG, Thermoline Scientific Equipment, Sydney, NSW, Australia), set at 22°C days, 12°C nights and 10-h days and 14-h nights with fluorescent lighting. Germinated seedlings were transplanted to a soil-less potting medium (Searles Professional Potting Mix, JC & AT Searle, Kilcoy, Qld, Australia). Seedlings and plants were grown in an air-conditioned glasshouse, maintaining the maximum temperature below 25°C.
Small numbers of seedling transplants were gathered during field collection when cypselae were unavailable. Seedlings were removed from soil, wrapped in damp newspaper inside a plastic bag and kept cool. On return to the University of New England, seedlings were potted in soil-less potting media and kept under intermittent mist with bottom heat for ~2 weeks before transferring to the glasshouse bench.
Cuttings were collected when cypselae and seedlings were unavailable. Tip cuttings were wrapped in damp newspaper and placed into plastic bags labelled to indicate the identity of the parent plant. On return to the university, cuttings were stripped of older leaves, re-cut, dipped into Clonex Purple Rooting Hormone Gel (Yates Australia, Padstow, NSW, Australia) and potted into Searles Seed Raising Mix before placing under intermittent mist with bottom heat until roots formed, or cuttings perished. Rooted cuttings were transplanted to the soil-less potting medium and transferred to the air-conditioned glasshouse bench.
Morphology
Morphological characters (Table 2), including those used in species descriptions by Wilson (2008), were examined on live plants and on herbarium specimens listed in Tables S1–S3. The choice of characters was developed from preliminary examinations of herbarium specimens, cultivated plants, plants in the field, and from published characters previously applied to species of Coronidium (Wilson 2008).
| Taxon | Habit | Max. leaf length (mm) | Max. leaf width (mm) | Leaf margin indumentum | Abaxial leaf midvein indumentum | Abaxial leaf indumentum | Adaxial leaf indumentum | Outer phyllary claw shape cross-section | Medial phyllary claw shape cross-section | |
|---|---|---|---|---|---|---|---|---|---|---|
| C. cymosum | Erect | 100 | 15 | Glabrous | Tomentose | Felted, glandular | Villous, glandular | Flattened | Flattened | |
| C. sp. Whitsundays | Erect | 40 | 6 | Cobwebby | Tomentose | Felted, glandular | Cobwebby, glandular | Flattened | Semi-terete | |
| C. flavum | Erect | 80 | 15 | Woolly | Tomentose | Woolly, glandular | Tomentose, woolly | Flattened | Flattened | |
| C. fulvidum | Erect | 80 | 15 | Villous | Tomentose | Tomentose, glandular | Villous | Flattened | Flattened | |
| C. newcastlianum | Erect | 80 | 20 | Felted | Felted | Felted | Felted | Sessile | Terete | |
| C. sp. Stannary Hills | Erect | 45 | 15 | Cobwebby | Felted | Woolly, glandular | Felted, glandular | Flattened | Flattened | |
| C. gnaphalioides | Erect | 35 | 10 | Woolly | Felted, tomentose | Felted, tomentose | Felted, tomentose | Semi-terete | Semi-terete | |
| C. sp. Many Peaks | Erect | 70 | 20 | Villous | Tomentose | Tomentose | Villous, floccose | Flattened | Flattened | |
| C. oxylepis subsp. oxylepis | Erect | 150 | 12 | Hirsute, hispid, cobwebby | Hirsute, hispid, cobwebby | Glandular | Hispid, glandular | Semi-terete | Semi-terete | |
| C. oxylepis subsp. carnosum | Prostrate, decumbent | 100 | 20 | Glabrous, glandular | Glabrous, sparse villous | Glabrous, glandular | Glabrous, glandular | Concave | Concave, flattened | |
| C. oxylepis subsp. lanatum | Erect | 60 | 15 | Villous | Villous | Tomentose, glandular | Tomentose, villous | Semi-terete | Semi-terete | |
| C. sp. Penrose | Erect | 100 | 8 | Cobwebby, villous | Hirsute, cobwebby, glandular | Villous, glandular | Hirsute, floccose | Semi-terete | Concave, flattened | |
| C. sp. Thulimbah | Erect | 70 | 15 | Cobwebby | Villous | Villous, glandular | Hirsute | Semi-terete | Semi-terete | |
| C. lanuginosum | Erect | 80 | 20 | Woolly, glandular | Villous, glandular | Villous, glandular | Villous, glandular | Semi-terete | Semi-Terete | |
| C. glutinosum | Erect or shrubby | 40 | 4 | Glandular | Glandular | Glandular | Glandular | Semi-terete | Flattened | |
| C. rupicola | Erect | 120 | 15 | Cobwebby | Tomentose | Felted | Villous, floccose | Flattened | Flattened | |
| C. sp. N Qld Headlands | Shrubby | 40 | 10 | Glabrous | Glabrous | Glandular | Glandular | Semi-terete | Flattened | |
| C. sp. Russell Island | Shrubby | 110 | 25 | Woolly | Felted | Felted | Felted | Concave | Semi-terete |
Max., maximum; NQ, northern Queensland. Definition and usage of morphological terms follow the Flora of Australia glossary (Orchard and Thompson 1999).
DArTseq genotyping and ordination
DNA extraction and DArTseq reduced representation sequencing were conducted by Diversity Arrays Technology, by using the DArTseq approach with medium marker density and proprietary DNA purification (Kilian et al. 2012). This method can be optimised for detecting gene flow in organisms lacking a reference genome (Al-Beyroutiová et al. 2016; Egea et al. 2017; Alam et al. 2018). Details of the DArTseq approach can be found in the Supplementary material under the subheading, ‘DArTseq genotyping’.
The genetic structure within each study-group partition was visualised using principal component analysis (PCA), implemented in the function ‘gl.pcoa’ in the dartR package (ver. 1.0.8, see https://cran.r-project.org/package=dartR; Gruber et al. 2018). The package handles both principal coordinate analysis and PCA. Exploratory filtering of DArTseq loci at different levels of stringency confirmed only minor effects of filtering stringency on the clustering of samples in PCA. Filtering loci and individuals on call rate using the function ‘gl.filter.callrate’ (Gruber et al. 2018) was applied prior to analysis. We respectively excluded loci and individuals with >20 and >30% missing data, ensuring minimal missing data, inclusion of most samples and maximal computational efficiency.
To exclude the potential influence of linkage disequilibrium, sequenced fragments with more than one SNP were thinned, retaining the best allele based on the DArT repeatability measure, then by average polymorphic information content, using the function ‘gl.filter.secondaries’ (Gruber et al. 2018). Finally, locus metrics were recalculated for the filtered data by using the function ‘gl.recalc.metrics’ (Gruber et al. 2018). Filtering based on Hardy–Weinberg equilibrium was not conducted, as the samples represented several distinct species.
Four data partitions were examined, following the results of Schmidt-Lebuhn et al. (2015): (1) all study-group species and putative entities in Coronidium, Leucozoma and Helichrysum leucopsideum, hereafter ‘all samples’; (2) C. oxylepis group, hereafter ‘Co’; (3) Leucozoma; and (4) C. elatum group 2, hereafter ‘Ce2’ (Table 1).
Subset partitions of the DArTseq samples (Table 1) were produced from raw data, filtered as above, and from the ‘Ce2’ and Leucozoma partitions were filtered to respectively exclude loci and individuals with >10 and >20% missing data to allow examination of finer-scale genetic differences (Rutherford et al. 2018).
Bayesian genetic clustering
To assess putative hybridisation and account for fine-scale genetic variation, species and putative entities were investigated using the Bayesian Monte Carlo Markov-chain (MCMC) procedure implemented in STRUCTURE (ver. 2.3.4, see https://web.stanford.edu/group/pritchardlab/structure.html; Pritchard et al. 2000; Beaumont et al. 2001; Falush et al. 2003). The program optimises the allele frequencies that characterise K genetic clusters on the basis of a model assuming random mating and linkage equilibrium within ancestral populations. If a model allowing admixture is used, each individual is assigned a proportion of ancestry derived probabilistically from each ancestral population. This approach is appropriate because it does not assume population ancestry a priori. We used the default setting for RECESSIVEALLELES = 0, treating all samples as diploid, because diploids comprised all of the samples (Laport et al. 2016; Collins et al., unpubl. data). STRUCTURE has previously been tested in several diploid–polyploid species complexes (D’hoop et al. 2010; Stöck et al. 2010; Moore et al. 2014), with little effect of the RECESSIVEALLELES flag observed for codominant markers (Moore et al. 2014; Meirmans 2019). Simulations have shown STRUCTURE to be robust in detecting population structure in mixed-ploidy samples (Stift et al. 2019). STRUCTURE analyses were applied to subsets of the study group identified using PCA. Estimation of clusters was performed on 10 replications using K = 1–20 for the ‘Co’ partition, and 10 replications using K = 1–10 for the ‘Ce2’ and Leucozoma partitions. Higher values of K were tested for the ‘Co’ partition because of the greater number of putative entities. Replicate runs were compared and typical (‘major’) clustering modes plotted using CLUMPAK (see https://tau.evolseq.net/clumpak/; Kopelman et al. 2015). Values of K best supported by the data were identified by plotting the natural logarithm of the likelihood and were used to assess the support for each value of K following the reasoning outlined by Pritchard et al. (2000). Caution must be taken when interpreting STRUCTURE barplots, because the algorithm parsimoniously explains variation among individuals and does not provide a parametric model of divergence and admixture (Lawson et al. 2018). Violations of the model assumptions can result from inclusion of samples that are close relatives, population divergence owing to isolation-by-distance, subtle subdivisions nested within diverged groups, and recent genetic bottlenecks, potentially leading to misinterpretation of results (Lawson et al. 2018). We have avoided sampling close relatives, used subsets to probe subtle population structure that may be obscured by hierarchical subdivisions, and avoided basing our conclusions solely on parametric estimates from the model.
Results
Morphology
In all cases, features described here were constant within and across populations seen in the field, with the exception of phyllary colour (Fig. 1). Differences between several species and putative entities were also observed in the leaf and margin indumentum (Fig. 2) and the shape of the medial phyllary claw respectively (Tables 2, 3).
Variation in phyllary morphology between Coronidium newcastlianum and closely related species. (a) C. fulvidum (T.L.Collins 1054 & J.J.Bruhl). (b) C. fulvidum (T.L.Collins 1056). (c) C. newcastlianum × C. rupicola (cultivated ex T.L.Collins 1056 & J.J.Bruhl). (d) C. sp. Stannary Hills (T.L.Collins 1059). (e) C. newcastlianum (T.L.Collins 1064 & J.J.Bruhl). (f) C. newcastlianum (T.L.Collins 1065 & J.J.Bruhl). (g) C. rupicola (T.L.Collins 1060 et al.). (h) C. flavum (T.L.Collins 1071 & J.J.Bruhl). Photographs by J. J. Bruhl (a, b, d–h) and T. L. Collins (c).

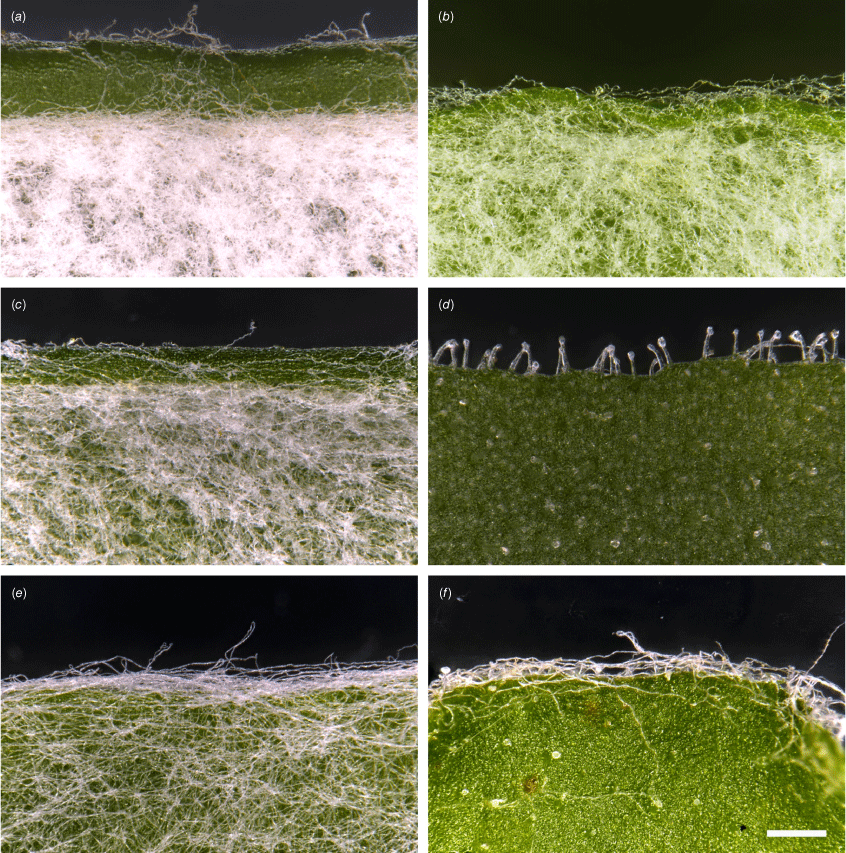
Cauline leaf abaxial and margin indumentum variation among species of Leucozoma and Coronidium. (a) Felted lamina and cobwebby margin (L. lindsayanum, T.L.Collins 1040 & B.Wright). (b) Tomentose lamina and margin (L. elatum subsp. elatum, I.R.Telford 15537). (c) Tomentose lamina and cobwebby to glabrous margin (L. alexandri, T.L.Collins 934 & M.F.Duretto). (d) Hispid and glandular lamina and glandular margin (L. boormanii, T.L.Collins 1086 & I.R.Telford). (e) Tomentose lamina and woolly margin (C. flavum, T.L.Collins 1071 & J.J.Bruhl). (f) Scattered villous and glandular lamina and cobwebby margin (C. lanuginosum, T.L.Collins 1066 & J.J.Bruhl). Photographs by T. L. Collins.
| Taxon | Pop. | Abaxial leaf | Leaf margin | Stalk of capitulum | Outer phyllary claw | Medial phyllary claw | Medial phyllary colour | |
|---|---|---|---|---|---|---|---|---|
| C. sp. N Qld Headlands | Cape Kimberley R.L. Jago 6470 | Glandular | Glabrous | Glandular | Glandular and puberulous abaxially | Glandular abaxially and on margins | Bronze-coloured | |
| C. sp. N Qld Headlands | Hinchinbrook Is. P. Sharpe 1665 | Glandular | Cobwebby | Glandular | Abaxially puberulous | Glandular abaxially, cobwebby and glandular margins | Bronze-coloured | |
| C. sp. N Qld Headlands | Cape Melville P.I. Forster 41461 | Tomentose, glandular | Cobwebby | Tomentose | Glandular and puberulous abaxially | Glandular abaxially and on margins | White | |
| C. rupicola | Paluma Range J.J. Bruhl 2465 | Tomentose, glandular | Cobwebby | Tomentose | Glandular and puberulous abaxially | Glandular and cobwebby abaxially and on margins | Bronze-coloured | |
| C. sp. Russell Island | Russell Island A.R. Field 5206 | Felted and woolly | Felted and woolly | Woolly and cobwebby | Glandular and woolly to puberulous abaxially | Glandular abaxially | Bronze-coloured |
Pop., population and voucher. Definition and usage of morphological terms, including stylar appendage shape, leaf and claw indumentum, follow the Flora of Australia glossary (Orchard and Thompson 1999).
Indumenta and trichome length on leaves and stems observed on cultivated plants were consistent with dried herbarium specimens, although spreading trichomes on live plants became appressed on the dried specimens, an artefact that was likely to be due to the pressing and drying process. Indumenta on cauline leaves varied at a fine scale among species and entities, with septate trichomes, or with sessile or stipitate glands (Fig. 2).
Populations of C. sp. N Qld Headlands could not be relocated at Cape Kimberley, and fieldwork sites on Hinchinbrook Island and Cape Melville could not be accessed, preventing inclusion of this putative entity in the molecular analyses and a more thorough assessment of within- and among-site variation in morphology (Table 3). Comparisons of morphology showed distinct differences between herbarium specimens from Cape Melville and those collected at Cape Kimberley and Hinchinbrook Island (Table 3). Specimens collected at Cape Melville have leaf and claw indumentum similar to those of C. rupicola, but have white medial phyllaries compared with the bronze phyllaries of C. rupicola (Table 3).
Measurements of leaves and phyllary claws differed between C. sp. Whitsundays as distinct from C. cymosum, C. rupicola and C. oxylepis subsp. lanatum. Specimens of C. cymosum have leaves up to 100 mm long and 15 mm wide with glabrous margins and flattened medial phyllary claws, v. leaves 40 mm long and 6 mm wide and medial phyllary claws that are semi-terete in cross-section in C. sp. Whitsundays (Table 2). Coronidium rupicola has leaves up to 120 mm long and C. oxylepis subsp. lanatum has leaves up to 60 mm long. Coronidium rupicola and C. cymosum have flattened medial phyllary claws distinct from the semi-terete medial phyllary claws of C. sp. Whitsundays (Table 2); C. oxylepis subsp. lanatum has medial phyllary claws that are semi-terete, similar to C. sp. Whitsundays, but lacking the dense villous hairs of the latter.
The putative entity C. sp. Many Peaks has villous to woolly outer phyllary claws, distinct from the puberulous and stipitate glands on C. rupicola and the stipitate glands on the outer phyllary claws of C. gnaphalioides. Coronidium sp. Many Peaks and C. gnaphalioides can be further distinguished (Table 2) by the different adaxial leaf indumentum (villous and floccose on C. sp. Many Peaks and felted on C. gnaphalioides). The leaves of C. rupicola (up to 120 mm long and 15 mm wide) and C. sp. Many Peaks (up to 70 mm long and 20 mm wide) are much larger than those of C. gnaphalioides (up to 35 mm long and 10 mm wide). Respective leaf and claw indumenta on plants remained unchanged under cultivation.
Only small differences in leaf indumentum and claw morphology were seen among C. newcastlianum, C. sp. Stannary Hills and C. fulvidum Paul G.Wilson. Small gradations in phyllary colour from white to copper were seen between populations of C. newcastlianum and C. sp. Stannary Hills (Fig. 1), whereas even greater variation in phyllary colour from white to bronze was seen among naturally occurring individuals of C. fulvidum at the type locality, as well as between mother plants and cultivated plants (e.g. Fig. 1c).
Variation in leaf indumentum and claw shape was observed among the different populations of C. oxylepis included in the molecular analyses (Table 2). When confirming these character variations more broadly on herbarium specimens of C. oxylepis, leaf indumentum and claw shape appeared to vary inconsistently. Recognition of any putative taxonomic group could not be made without reference to location data.
Specimens of the putative entity L. sp. Tuggolo State Forest were morphologically distinct from L. lindsayanum, with differences in claw shape (concave on L. sp. Tuggolo State Forest and terete on L. lindsayanum), and also differed from the subspecies of L. elatum (A.Cunn. ex DC.) T.L.Collins. Leucozoma sp. Tuggolo State Forest has glabrous leaf margins and adaxial leaf surfaces, compared with woolly margins and floccose, villous or felted adaxial leaf indumentum on subspecies of L. elatum (Table 4). Leucozoma elatum subsp. vellerosum (Paul G.Wilson) T.L.Collins is also morphologically different to other subspecies of L. elatum, with flattened medial phyllary claws in cross-section, unlike the semi-terete claws on subspecies of L. elatum.
| Taxon | Habit | Max. leaf length (mm) | Max. leaf width (mm) | Leaf margin indumentum | Abaxial leaf midvein indumentum | Abaxial leaf indumentum | Adaxial leaf indumentum | Outer phyllary claw shape cross-section | Medial phyllary claw shape cross sect. | |
|---|---|---|---|---|---|---|---|---|---|---|
| L. boormanii | Erect | 110 | 20 | Hirsute, glandular | Hirsute, glandular | Hirsute, glandular | Hirsute, glandular | Terete | Flattened | |
| L. elatum subsp. elatum | Erect | 180 | 45 | Woolly | Tomentose, glandular | Tomentose, glandular | Floccose, glandular | Semi-terete | Semi-terete | |
| L. elatum subsp. minus | Erect | 140 | 50 | Woolly | Woolly, glandular | Felted, glandular | Villous, floccose, glandular | Semi-terete | Semi-terete | |
| L. elatum subsp. vellerosum | Erect | 150 | 30 | woolly | Tomentose, glandular | Felted, glandular | Felted, floccose, glandular | Semi-terete | Flattened | |
| L. kaputaricum | Decumbent | 70 | 20 | Felted | Felted, glandular | Felted, glandular | Tomentose, glandular | Sessile | Flattened | |
| L. lindsayanum | Decumbent | 70 | 10 | Glabrous | Felted | Felted, glandular | Glabrous, glandular | Sessile | Terete | |
| L. sp. Tuggolo State Forest | Erect | 90 | 10 | Cobwebby or glabrous | Felted, glandular | Felted | Glabrous, glandular | Flattened | Concave | |
| L. telfordii | Erect | 80 | 30 | Felted | Felted | Felted | Cobwebby, floccose | Semi-terete | Concave |
Max., maximum. Definition and usage of morphological terms, pertaining to leaf and claw indumentum, follow the Flora of Australia glossary (Orchard and Thompson 1999).
No distinct differences in morphology were observed between specimens of C. waddelliae and C. aff. waddelliae (Table 5). Only small variations in trichome size and shape were observed among populations of H. leucopsideum, but no distinct differences were seen in leaf size or phyllary claw shape (Table 5).
| Taxon | Habit | Max. leaf length (mm) | Max. leaf width (mm) | Leaf margin indumentum | Abaxial leaf midvein indumentum | Abaxial leaf indumentum | Adaxial leaf indumentum | Outer phyllary claw shape cross sect. | Medial phyllary claw shape cross sect. | |
|---|---|---|---|---|---|---|---|---|---|---|
| C. adenophorum | Erect | 70 | 8 | Hispid, glandular | Hispid, glandular | Hispid, glandular | Hispid, glandular | Semi-terete | Flattened | |
| C. waddelliae | Erect | 40 | 2 | Hispid, glandular | Tomentose | Tomentose, glandular | Hispid, glandular | Semi-terete | Flattened | |
| H. leucopsideum Tas. | erect | 60 | 8 | Cobwebby, hirsute | Silky, tomentose | Silky, glandular | Hispid, glandular | Sessile | Sessile | |
| H. leucopsideum SA | erect | 60 | 5 | Cobwebby, hispid | Cobwebby, tomentose | Tomentose, glandular | Hispid, glandular | Sessile | Sessile | |
| H. leucopsideum WA | erect | 60 | 5 | Cobwebby, hispid | Hirsute, glandular | Cobwebby, glandular | Hispid, glandular | Sessile | Sessile |
Max., maximum; Tas., Tasmania; SA, South Australia; WA, Western Australia. Definition and usage of morphological terms, pertaining to leaf and claw indumentum, follow the Flora of Australia glossary (Orchard and Thompson 1999).
DArTseq genotyping
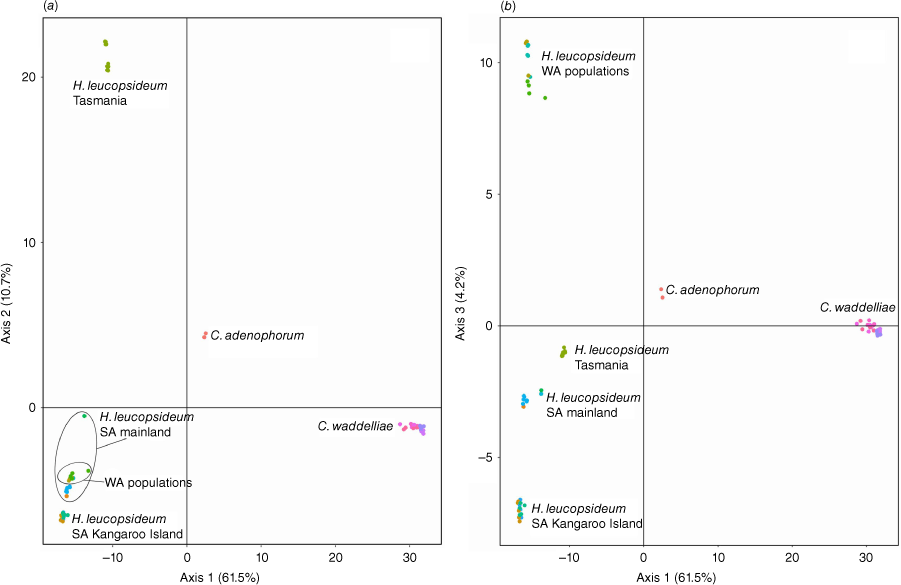
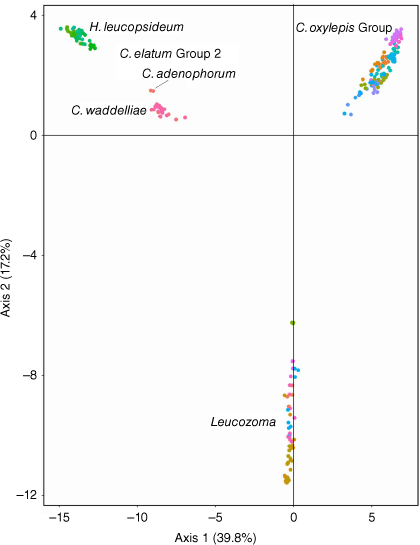
Only 4 of the 287 samples failed to yield high-quality sequence data and were discarded. Filtering to reduce levels of missing data resulted in the removal of only small numbers of samples (Table 6). Observed heterozygosity (HO) was slightly lower than expected heterozygosity (HE) for all ‘Co’ and Leucozoma partition taxa (Tables S4, S5). All taxa had a positive FIS indicative of reduced heterozygosity. Pairwise FST comparisons of C. fulvidum with populations of C. sp. Stannary Hills (0.0579–0.101), and C. newcastlianum (0.255–0.268) were much lower than with C. rupicola (0.399) and C. glutinosum (Hook.) Paul G.Wilson (0.516), with all other ‘Co’ partition taxa indicating even greater divergence (>0.57). Pairwise FST comparisons of L. sp. Tuggolo with L. lindsayanum was high (0.818) and all other Leucozoma partition taxa ranged from the lowest (0.369) with L. elatum subsp. elatum at Boonoo Boonoo NP to the highest (0.973) with L. kaputaricum (Paul G.Wilson) T.L.Collins. Axes 1 and 2 of PCA of the ‘all samples’ study group partition explained 57% of the genetic variation and showed distinct separation of the ‘Co’, ‘Ce2’ and Leucozoma groups (Fig. 3). Samples in the Ce2 partition formed two clusters representing entities in H. leucopsideum and in Coronidium.
Principal coordinates analysis Axes 1 and 2 of 1752 single-nucleotide polymorphism loci representing all species and putative entities in the Coronidium oxylepis group, the C. elatum Group 2, and Leucozoma. Each dot represents a sample coloured by population location.
| Partition | Before filtering | After filtering | |||
|---|---|---|---|---|---|
| Number of samples | Loci | Number of samples (percentage retained) | Loci (percentage retained) | ||
| ‘allsamples’ | 280 | 8595 | 276 (99) | 1752 (20) | |
| ‘Co’ | 153 | 126,902 | 148 (97) | 11,017 (9) | |
| Leucozoma | 55 | 80,503 | 55 (100) | 9994 (12) | |
| ‘Ce2’ | 75 | 103,248 | 73 (97) | 7350 (7) | |
‘Co’, C. oxylepis Group; ‘Ce2’, C. elatum Group 2.
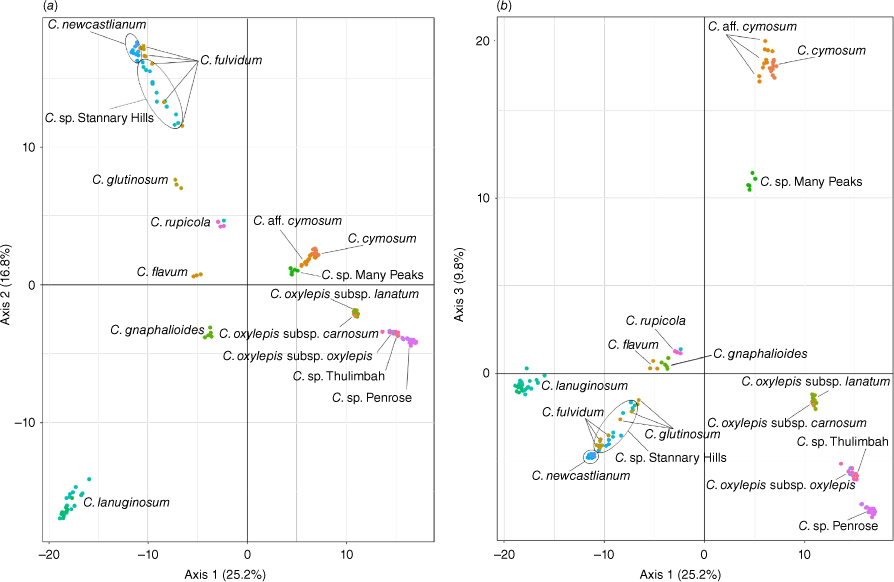
Axes 1 and 2 of PCA of the ‘Co’ partition explained 42% of the genetic variation, and Axes 1 and 3 explained 35%. PCA showed distinct separation of most taxa (Fig. 4a, b). The putative entity C. sp. Many Peaks formed a distinct cluster well separated from C. gnaphalioides. Coronidium cymosum clustered tightly with the putative entity C. aff. cymosum. Populations of C. lanuginosum from coastal headlands and inland hills formed a single cluster. Some taxa and putative entities did not form separated clusters. Coronidium newcastlianum formed a tight cluster adjoining a larger spreading cluster of C. fulvidum and C. sp. Stannary Hills. One sample of C. sp. Stannary Hills can be seen as a blue dot clustered with C. rupicola (Fig. 4a, b), whereas another sample from this gathering, (TLC1055e) clustered with C. glutinosum and C. sp. Stannary Hills (Fig. 4b). Coronidium oxylepis subsp. oxylepis clustered with C. sp. Thulimbah, a putative entity currently included in C. oxylepis subsp. lanatum. Samples of C. oxylepis subsp. lanatum collected near the type locality clustered with samples from the type locality of C. oxylepis subsp. carnosum Paul G.Wilson. Coronidium sp. Penrose formed a distinct cluster in PCA Axes 1 and 3 (Fig. 4b).
Principal coordinates analysis of 11,017 single nucleotide polymorphism loci representing all species and putative entities in the Coronidium oxylepis group. (a) Axes 1 and 2. (b) Axes 1 and 3. Each dot represents a sample coloured by population location.
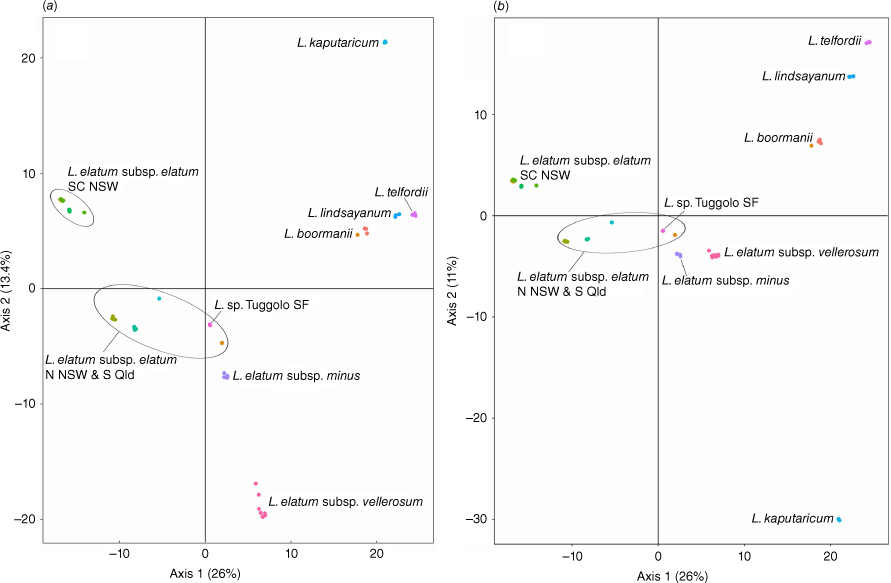
Axes 1 and 2 of the PCA of Leucozoma explained 39.4% of the genetic variation and showed distinct separation of most taxa (Fig. 5). The putative entity L. sp. Tuggolo State Forest clustered close to a single sample of L. elatum subsp. elatum from Boonoo Boonoo National Park, which is part of a very loose grouping of northern New South Wales and south-eastern Queensland populations. Populations of L. elatum subsp. elatum from the South Coast Bioregion in New South Wales formed a tighter, separate cluster. The two other subspecies of L. elatum formed tight, well-separated clusters.
Principal coordinates analysis of 9994 single-nucleotide polymorphism loci representing all species and putative entities in Leucozoma. (a) Axes 1 and 2. (b) Axes 1 and 3. Each dot represents a sample coloured by population location.
Axes 1 and 2 of PCA of the ‘Ce2’ partition explained 72.2% of the genetic variation and showed distinct separation of all taxa (Fig. 6). Tasmanian populations of H. leucopsideum formed a separate cluster to South Australian and Western Australian populations in Axis 2, whereas in Axis 3, Western Australian populations were the most distant. Populations of the putative entity C. aff. waddelliae from the Sydney Basin Bioregion clustered with populations of C. waddelliae from alpine and subalpine habitats.
Population genetic structure
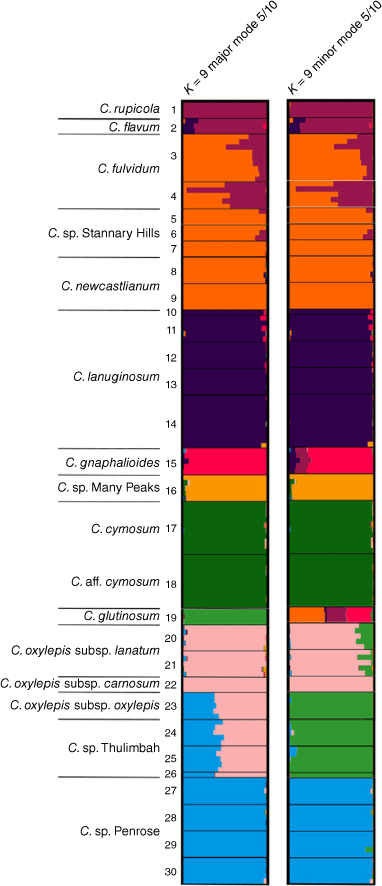
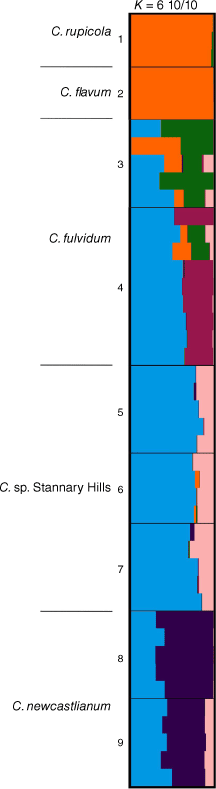
For species and putative entities in ‘Co’, the log-likelihood of the model (Supplementary Fig. S1) did not increase with increasing values of the number of groups K beyond K = 9. STRUCTURE bar plots at K = 9 were divided into two clustering modes, each representing 5 of the 10 runs. Both major and minor modes suggested no common ancestry between C. gnaphalioides and C. sp. Many Peaks (Fig. 7). By contrast, C. newcastlianum and C. sp. Stannary Hills were inferred to have common ancestry, which was also shared among C. fulvidum populations in a mixture with either C. rupicola or C. flavum Paul G.Wilson. In the major mode, common ancestry was indicated among subspecies and putative entities (i.e. C. sp. Thulimbah and C. sp. Penrose) in C. oxylepis, whereas three distinct groups were indicated in the minor mode (Fig. 7).
STRUCTURE bar plots, for species and putative entities in the Coronidium oxylepis group for major and minor clustering modes K = 9. Minor modes represent alternative clustering hypotheses to the major mode. Bar plots show each individual as a horizontal bar divided into segments on the basis of the proportion of ancestry suggested for 1–20 subpopulations across the 10 runs; n = 286. Fine black lines delineate sampling locations, numbered as in Table S4.
For a subset of species of Coronidium comprised of C. rupicola, C. flavum, C. fulvidum, C. sp. Stannary Hills and C. newcastlianum, the log-likelihood of the model did not increase beyond K = 6 (Fig. S2). STRUCTURE bar plots at K = 6 inferred a complex mixture of shared ancestry among C. rupicola, C. flavum, and some populations of C. fulvidum (Fig. 8). Parapatric populations of C. rupicola and C. flavum in the Paluma Range were indicated to share similar ancestry, whereas some difference in genetic composition was suggested among C. fulvidum, C. sp. Stannary Hills and C. newcastlianum.
STRUCTURE bar plots for Coronidium rupicola, C. flavum, C. fulvidum, C. sp. Stannary Hills, and C. newcastlianum, major clustering modes K = 6. Bar plots show each individual as a horizontal bar divided into segments on the basis of the proportion of ancestry suggested for 1–10 subpopulations across all of the 10 runs; n = 44. Fine black lines delineate sampling locations, numbered as in Table S5.
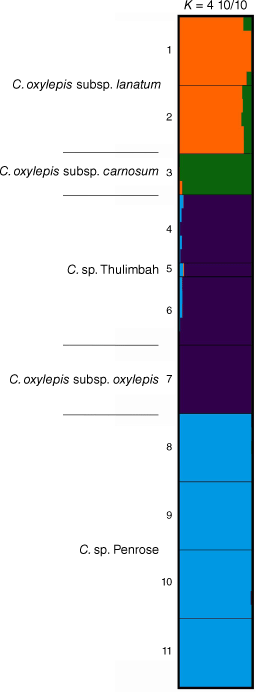
Bayesian clustering of the subspecies and putative entities (i.e. C. sp. Thulimbah and C. sp. Penrose) of C. oxylepis indicated that the log-likelihood of the model (Fig. S3) did not increase with increasing values of the number of groups K, beyond K = 4. STRUCTURE bar plots at K = 4 (Fig. 9) were congruent with the minor mode of clustering in analysis of the ‘Co’ partition (Fig. 7). They suggested three distinct groups, with common ancestry inferred between C. oxylepis subsp. oxylepis and the putative entity C. sp. Thulimbah (Fig. 9). Approximately 4–12% of C. oxylepis subsp. carnosum ancestry appeared to be shared with populations of C. oxylepis subsp. lanatum (Fig. 9).
STRUCTURE bar plots for Coronidium oxylepis subsp. lanatum, C. oxylepis subsp. carnosum, C. sp. Thulimbah, C. oxylepis subsp. oxylepis, and C. sp. Penrose, major clustering modes K = 4. Bar plots show each individual as a horizontal bar divided into segments on the basis of the proportion of ancestry suggested for 1–10 subpopulations across all of the 10 runs; n = 49. Fine black lines delineate sampling locations, numbered as in Table S4.
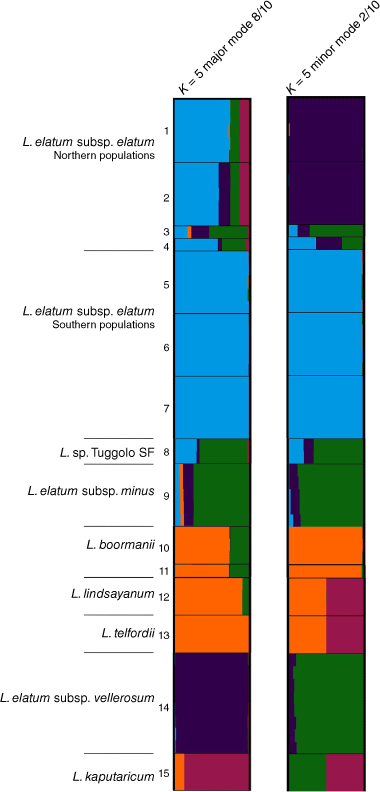
For species and putative entities in Leucozoma, the log-likelihood of the model (Fig. S4) did not increase beyond K = 5. STRUCTURE bar plots at K = 5 suggested that northern populations of L. elatum subsp. elatum shared ancestry with the two other subspecies of L. elatum and comprise a complex mixture of ancestry compared with southern populations (Fig. 10). Shared ancestry was inferred among L. lindsayanum, L. telfordii (Paul G.Wilson) T.L.Collins, and L. boormanii (Maiden & Betche) T.L.Collins. Bayesian analysis of allele frequencies suggested that a majority of the ancestry (~65%) of the putative entity L. sp. Tuggolo State Forest is shared with L. elatum subsp. minus (Paul G.Wilson) T.L.Collins. Varying proportions of L. sp. Tuggolo State Forest ancestry (1–30%) were suggested across the 10 runs from either L. elatum subsp. elatum or L. elatum subsp. vellerosum, and probability intervals from the raw STRUCTURE output supported this inference. Leucozoma elatum subsp. vellerosum was genetically distinct, with <10% of allele frequencies suggesting recent shared ancestry (Fig. 10).
STRUCTURE bar plots for species and putative entities in Leucozoma, major clustering modes K = 5. Bar plots show each individual as a horizontal bar divided into segments on the basis of the proportion of ancestry suggested for 1–10 subpopulations across major and minor modes of the 10 runs; n = 56. Fine black lines delineate sampling locations, numbered as in Table S6.
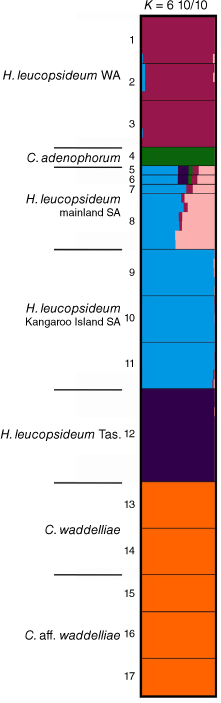
For species and putative entities in ‘Ce2’, the log-likelihood of the model (Fig. S5) did not increase beyond K = 6. STRUCTURE bar plots at K = 6 suggested that there was no common ancestry among C. adenophorum, C. waddelliae and H. leucopsideum. Tasmanian, South Australian and Western Australian populations of H. leucopsideum were indicated to have different ancestries (Fig. 11). Populations of H. leucopsideum from the South Australian mainland were suggested to have a mix of ancestry, shared among Kangaroo Island (~50–60%), Tasmanian (~15%) and Western Australian (~3–9%) populations, as well as a unique cluster (~20–40%), in contrast to nearby populations on Kangaroo Island that comprised a single cluster. Shared ancestry was suggested between populations of C. waddelliae from alpine areas in the South Eastern Highlands Bioregion and the putative entity C. aff. waddelliae from the Sydney Basin Bioregion.
STRUCTURE bar plots for species and putative entities in Coronidium adenophorum, C. waddelliae, C. aff. waddelliae, Helichrysum leucopsideum, major clustering modes K = 6. Bar plots show each individual as a horizontal bar divided into segments on the basis of the proportion of ancestry suggested for 1–10 subpopulations across the 10 runs; n = 73. WA, Western Australia; SA, South Australia; Tas., Tasmania. Fine black lines delineate sampling locations, numbered as in Table S7.
Discussion
This study is the first to apply molecular approaches to delimit species within the established genus Coronidium and the newly recognised genus Leucozoma. Genetic differences between the genera were clearly illustrated by PCA (Fig. 3), corroborating earlier results (Schmidt-Lebuhn et al. 2015; Collins et al. 2022) and indicating the large genetic differences between H. leucopsideum and the remainder of the Coronidium elatum Group 2.
The putative entity C. sp. Many Peaks was included in C. gnaphalioides by Wilson (2008), but C. sp. Many Peaks has villous to woolly outer phyllary claws and much larger leaves (Table 2), distinct from the smaller leaves and stipitate glands on the outer phyllary claws of C. gnaphalioides. Claw indumentum of C. sp. Many Peaks is more like that of C. cymosum, and these species were previously inferred to be sisters with >0.95 posterior probability, with C. gnaphalioides in a separate clade (Collins et al. 2022). The multiple lines of evidence, including distinct morphology, distinct genotype clusters (Fig. 4) and absence of recent gene flow or common ancestry (Fig. 7), support the recognition of C. sp. Many Peaks at the species level as Coronidium bruhlii T.L.Collins.
Morphology clearly separates C. sp. Whitsundays from C. cymosum (including C. aff. cymosum), C. oxylepis subsp. lanatum and C. rupicola, with which it has been confused. Coronidium sp. Whitsundays has much smaller leaves and a hairy, semi-terete claw (Table 2). Label data indicate that C. sp. Whitsundays has a limited distribution, growing on the foredunes and beaches of the Whitsunday Island group. On the basis of the morphological differences, we recognise C. sp. Whitsundays as a distinct entity at species level as Coronidium batianoffii T.L.Collins & I.Telford.
Variation in habit and habitat seen between inland and coastal populations of C. lanuginosum was not supported by examinations of specimen morphology (Table 2) and analyses of the molecular data (Fig. 4, 7) did not support recognition of any populations as distinct entities.
A complex mixture with some common ancestry was suggested between C. fulvidum, C. sp. Stannary Hills and C. newcastlianum (Fig. 8). Molecular data indicated little genetic differentiation between C. fulvidum and C. sp. Stannary Hills, with PCA of SNPs showing the genotypes as a loose cluster of samples (Fig. 3). Wilson (2008) applied morphological differences, including phyllary shape and colour, and leaf and claw indumentum to distinguish C. fulvidum and C. newcastlianum. Variation in phyllary length and colour was seen both between populations of C. fulvidum (Fig. 1a, b) and between seedlings in the successive generation (Fig. 1c) that may be the result of introgression between C. newcastlianum and C. rupicola. Expression of variation in morphological characters by F1 hybrids and later generations commonly occurs as a mosaic of forms (Rieseberg et al. 1993) and has been widely recorded in Gnaphalieae and more broadly in Asteraceae (reviewed in Abbott 2017). Coronidium fulvidum has a very limited distribution in the Great Dividing Range east of Herberton, despite large areas of continuous potential habitat where it has never been recorded (AVH, see http://avh.chah.org.au). Some populations of C. fulvidum have coppery-coloured phyllaries that are intermediate in size between those of C. rupicola and C. newcastlianum, and plants of C. fulvidum grown during this study had coppery phyllaries larger and paler than those in the mother plant, although they were similar in size to those seen on C. sp. Stannary Hills (Fig. 1), whereas Coronidium flavum has larger, yellow phyllaries rather than the narrow coppery phyllaries of C. fulvidum and C. rupicola. The molecular data and morphological variation (Table 2) seen in this study suggested that C. fulvidum is a morphologically variable hybrid between C. newcastlianum and C. rupicola and should not be recognised as a distinct species.
The leaf variation between C. newcastlianum, with winged petioles, and C. sp. Stannary Hills, with sessile leaves, may represent local adaptation, by reduced leaf area and exposure to desiccation, across environmental gradients between the Atherton Tablelands at ~1000-m altitude and the Newcastle Range at ~600–750 m. Populations near Mount Garnet (e.g. D.E.Symon 4897) and Millstream Falls National Park (e.g. P.I.Forster 30893) have leaves with attenuate leaf bases and are intermediate in leaf and petiole shape between populations at Newcastle Range and those on the Atherton Tablelands. The continuum of variation in leaf and petiole morphology and weak genetic differentiation support the current taxonomy with C. sp. Stannary Hills and C. newcastlianum as populations within a single, morphologically variable species.
Subspecies of C. oxylepis could not be easily diagnosed using morphology alone, and molecular data indicated only weak genetic divergence, with suggested common ancestry among some subspecies (Fig. 7, 9). Coronidium oxylepis meets some of the criteria for definition as an ochlospecies, being strongly polymorphic but weakly polytypic, showing partial correlation of morphology with geography and ecology, complexity of variation not being due to hybridisation, and being geographically and ecologically widespread (Cronk 1998). Historical range shifts in response to Pleistocene climatic oscillations have been suggested as a causal factor for the development of ochlospecies (Prance 1979). The uncorrelated morphological and genotypic differences found in this study could also be due to incomplete reproductive isolation between entities at an early stage of evolutionary divergence (de Queiroz 2005, 2007), as has been found in other Gnaphalieae (e.g. De Salas and Schmidt-Lebuhn 2018). Further research is warranted on the breeding system, and molecular analyses of a greater number of populations, with dense within-population sampling, may help in understanding the relationships among different populations of C. oxylepis and to delimit taxonomic entities.
Bayesian analysis provided some support for C. sp. Thulimbah, being a product of hybridisation between C. oxylepis subsp. lanatum and C. sp. Penrose, and C. oxylepis subsp. oxylepis to be a hybrid of C. oxylepis subsp. carnosum and C. sp. Penrose (Fig. 7). A more focused Bayesian analysis indicated that C. sp. Penrose, C. oxylepis subsp. lanatum and C. oxylepis subsp. carnosum comprised largely unique ancestry (Fig. 9), supporting the hypothesis that C. sp. Penrose represents a distinct entity. However, this result may be interpreted alternatively as population divergence owing to isolation-by-distance (Lawson et al. 2018). Surprisingly, C. oxylepis subsp. oxylepis and C. sp. Thulimbah were indicated to share common ancestry or recent gene flow (Fig. 9) instead of the expected association between C. oxylepis subsp. lanatum and C. sp. Thulimbah. Coronidium oxylepis subsp. oxylepis and C. sp. Thulimbah are distinguished by differences in leaf length, width and indumenta, and occur in different ecosystems (coastal dunes and granitic tablelands respectively; Table 2). It is possible that a recent genetic bottleneck violated the model assumptions (Lawson et al. 2018), and this hypothesis is supported by the small and scattered populations recorded during field work.
Most species of Leucozoma were genetically distinct, forming discrete and well separated clusters in the PCA (Fig. 5), but there was evidence of shared ancestry or recent gene flow between some species. The PCA also showed homogenous southern and heterogeneous northern populations of L. elatum subsp. elatum. Bayesian analyses suggest the northern populations have some shared ancestry with L. elatum subsp. vellerosum, L. elatum subsp. minus and L. kaputaricum. Leucozoma elatum subsp. minus also has ancestry in common with L. sp. Tuggolo State Forest, L. lindsayanum, L. telfordii and L. boormanii, but is easily distinguished from these species by leaf size and indumentum (Table 4). The putative entity L. sp. Tuggolo State Forest has been treated as a morphological variant of L. lindsayanum or L. elatum by some Australian herbaria (T. L. Collins, pers. obs., 2018). Leucozoma sp. Tuggolo State Forest is morphologically distinct with concave medial phyllary claws, erect to decumbent habit and leaves up to 90 mm long, compared with terete medial phyllary claws, decumbent habit and leaves up to 70 mm long on L. lindsayanum; L. elatum has semi-terete medial phyllary claws and leaves up to 180 mm long that are covered by a floccose indumentum adaxially, unlike the glabrous adaxial leaf surface on L. sp. Tuggolo State Forest (Table 4). The unique ancestry found here in L. sp. Tuggolo State Forest provides additional evidence supporting recognition as a species, Leucozoma alexandri T.L.Collins.
Leucozoma elatum subsp. vellerosum is morphologically distinct with leaves up to 150 mm long and 30 mm wide, felted leaf indumentum covering both adaxial and abaxial surfaces, and flattened medial phyllary claws, compared with L. elatum subsp. elatum which has larger leaves up to 180 mm long and 45 mm wide, floccose and glabrescent adaxial leaf surface, and semi-terete medial phyllary claws (Table 4). There is also strong genetic differentiation between L. elatum subsp. elatum and L. elatum subsp. vellerosum, with only very small proportions of shared ancestry in northern populations of L. elatum subsp. elatum. These multiple lines of evidence support recognition at species rank, as L. wollumbin T.L.Collins.
Morphological characters and molecular analyses were informative for delimiting C. adenophorum and C. waddelliae and corroborated the conclusion that C. aff. waddelliae populations in the Sydney Basin Bioregion are not a distinct entity. The morphological characters studied here on H. leucopsideum indicated only minor variation in leaf indumentum, with no other discontinuities seen (Table 5). By contrast, genotypic differences were much greater, illustrated by the distinct separation of H. leucopsideum from C. adenophorum and C. waddelliae (Fig. 3), and confirming the uncertain systematic placement of these three species (Collins et al. 2022). There was no indication of common ancestry among Tasmanian, Kangaroo Island and Western Australian populations, possibly owing to isolation by distance and subsequent genetic drift. Further research is required to resolve the taxonomy and systematics of this enigmatic species.
Conclusions
Multiple lines of evidence, including distinct genotypic differences, lack of recent gene flow and distinct morphology, support the taxonomic recognition at species rank of C. bruhlii, C. battianoffii, L. alexandri and L. wollumbin (Table 7). Molecular and morphological data indicate C. fulvidum to be a variable hybrid between C. rupicola and C. newcastlianum. An expanded description for C. newcastlianum is provided below.
| New name | Phrase name or synonym | |
|---|---|---|
| C. bruhlii | C. sp. Many Peaks | |
| C. batianoffii | C. sp. Whitsundays | |
| L. alexandri | L. sp. Tuggolo State Forest | |
| L. wollumbin | L. elatum subsp. vellerosum |
Recognition and description of these four new species, together with basic information on ecology, distribution and conservation status provide a taxonomic service to conservation and land managers, enabling detailed assessment of conservation status and facilitating conservation action where required.
This study was unable to find genetic and co-varying morphological differences separating C. waddelliae from C. aff. waddelliae, supporting the hypothesis that the disjunct populations represent a single species found in alpine and subalpine habitats (Wilson 2008). Genetic variation in Helichrysum leucopsideum populations did not co-vary with the morphological characters, and we retain the current taxonomic treatment as a single species.
On the basis of the evidence presented here, it is preferable to retain the current taxonomic treatment of C. oxylepis, with C. sp. Penrose and C. sp. Thulimbah being included in C. oxylepis subsp. lanatum, because the three subspecies of Wilson (2008) are distinguishable by using morphological characters. This study has added some understanding of a likely recent diversification in subspecies of C. oxylepis, with suggested common ancestry among morphologically diverged populations of this potential ochlospecies, but further study is required to completely show the taxonomic boundaries.
Taxonomy
Taxa are listed alphabetically within genera. Type specimens for the names treated below have been examined either directly (indicated by ‘!’) or as images from CANB and on JSTOR Global Plants (indicated by an asterisk, ‘*’, see https://plants.jstor.org/). Distribution data are based on specimens seen at BRI, CANB, NE and NSW, and use IBRA regions (Department of the Environment and Energy 2016). Where sufficient data are available, conservation status is given on the basis of International Union for Conservation of Nature Red List Categories and Criteria: Version 3.1. (International Union for Conservation of Nature and Natural Resources 2022).
| 1. | Fruit pericarp opaque, idioblasts elliptic, pappus persistent or breaking unevenly above pappus–pericarp union...Leucozoma Fruit pericarp translucent, idioblasts linear, pappus persistent or breaking evenly above pappus–pericarp union to retain a ‘corona’ of pappus bases...Coronidium |
Leucozoma T.L.Collins, Taxon 71(5): 1056 (2022)
Type: Leucozoma elatum (A.Cunn. ex DC.) T.L.Collins
Perennial herbs. Indumentum woolly, villous, hirsute, tomentose, felted, floccose, cobwebby, glabrescent or with stipitate glands. Leaves alternate; ovate, elliptic, or lanceolate. Capitula predominantly homogamous, discoid, rarely heterogamous, disciform. Receptacle ±flat, epaleate. Phyllaries white or pink, multiseriate, scarious, spreading when mature; claw of intermediate and inner bracts terete, semi-terete, concave or flattened, abaxially with stipitate glands, cartilaginous; prominent central vascular bundle extending into the lamina; stereome undivided. Florets mostly bisexual, some outer florets female; corolla narrowly cylindrical, broadened above, lobes short, ovate; anther tails slender, equal to or exceeding collar, appendage ovate, outwardly concave; style arms slender; style apex clavate, ovate, triangular or deltoid, and papillose. Cypsela cylindrical to oblongoid; pericarp crustaceous, opaque and brown to fawn-coloured, striated, glabrous, with elliptic idioblasts; testa pale brown. Pappus bristles filiform, denticulate, proximally smooth where united in a ring, and persistent or breaking a short distance above it.
| 1. | Stylar appendages clavate...2 Stylar appendages deltoid or ovate...5 |
| 2. | Cauline leaves abaxial indumentum felted or tomentose; with or without stipitate glands...3 Cauline leaves abaxial indumentum not felted or tomentose; with stipitate glands...Leucozoma boormanii |
| 3. | Cauline leaves adaxial indumentum cobwebby, floccose or glabrous...4 Cauline leaves adaxial indumentum felted...Leucozoma wollumbin |
| 4. | Cauline leaves 25–140 mm long, 5–50 mm wide; capitula 40–50 mm in diameter (including phyllaries); phyllary apex acute...Leucozoma elatum subsp. minus Cauline leaves 30–80 mm long, 10–30 mm wide; capitula 30–40 mm in diameter (including phyllaries); phyllary apex apiculate to cuspidate...Leucozoma telfordii |
| 5. | Outer phyllary claw flattened or semi-terete in cross-section...6 Outer phyllaries sessile...7 |
| 6. | Erect, short-lived perennial or annual herb...Leucozoma elatum subsp. elatum Erect, pendulous or decumbent perennial shrub...Leucozoma alexandri |
| 7. | Cauline leaves adaxial indumentum tomentose and with stipitate glands; outer phyllaries white and translucent...Leucozoma kaputaricum Cauline leaves adaxial indumentum with stipitate glands and occasional or scattered long hairs; outer phyllaries pink or white and opaque...Leucozoma lindsayanum |
Leucozoma alexandri T.L.Collins, sp. nov.
Type: AUSTRALIA: New South Wales: Northern Tablelands: Oxley Wild Rivers National Park, Paradise Rocks, ~40 km E of Walcha, 800 m NNE of Paradise Trig, 27 Aug. 2013, L.M.Copeland 4519 & P.Lupica (holo: NSW; iso: BRI, CANB, K, MO, NE 101979!, NSW, US).
Coronidium sp. Tuggolo State Forest (L.M.Copeland 4225) NE Herbarium: CHAH, Austral. Pl. Census (2020) [accessed 20 Feb. 2020].
Distinguished from L. lindsayanum by the stems and branches felted with septate trichomes and with shorter stipitate glands (v. felted or woolly, or glabrescent with corky bark on older stems), stipitate leaf glands restricted to the abaxial midvein (v. restricted to leaf lamina), flattened outer phyllary claw (v. sessile), and concave medial phyllary claw (v. flattened). Distinguished from L. elatum and L. wollumbin by the stem felted with septate trichomes (v. woolly to glabrescent in L. elatum and cobwebby to woolly in L. wollumbin) and the shorter and narrower leaves 25–90 mm long (v. 25–180 mm long in L. elatum and 35–100 in L. wollumbin)
Decumbent to erect, perennial, tap-rooted, subshrub. Stems and branches felted with septate trichomes and with shorter stipitate glands, internode length 2–5 mm. Basal leaf rosette absent at flowering. Cauline leaves ovate to lanceolate, 25–90 mm long and 5–10 mm wide, base amplexicaul and attenuate, margin revolute and cobwebby or glabrous, apex acute and mucronate; abaxial indumentum felted to tomentose, midvein indumentum felted and with stipitate glands; adaxial leaf surface glabrous with stipitate glands on midvein. Foliaceous bracts subtending capitula 10 mm long, margin villous and with stipitate glands. Flowering stems branched, capitula 40–50 mm in diameter (including phyllaries), terminal. Outer phyllary claw flattened and with stipitate glands abaxially; white and opaque, basal margin villous, abaxial surface glabrous, apex acute and occasionally incised. Medial phyllary claw concave and with stipitate glands abaxially and on margins; abaxially white, apex cuspidate to acute. Stylar appendages ovate. Cypsela cylindrical to oblongoid, 2.5 mm long and 0.75 mm in diameter, pericarp grey–brown, idioblasts present. Pappus persistent, ~5.5 mm long. (Fig. 12.)
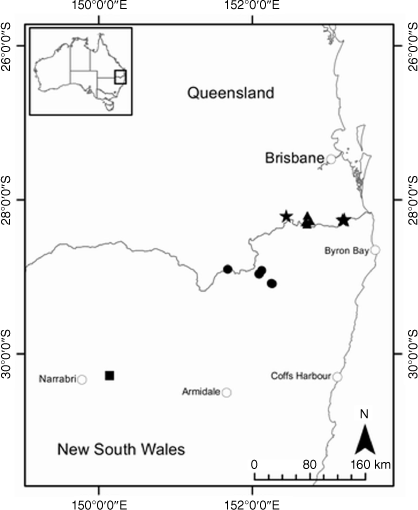
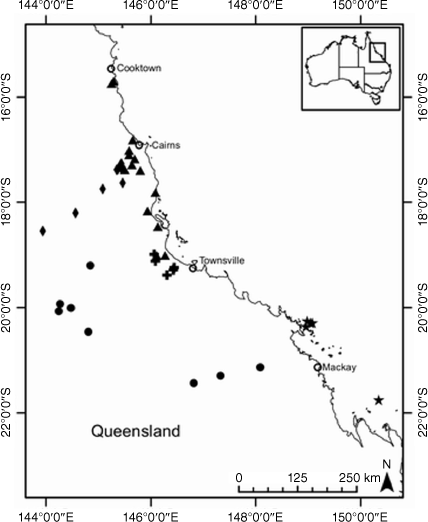
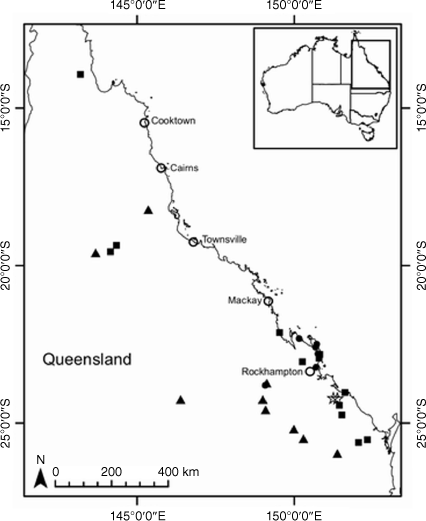
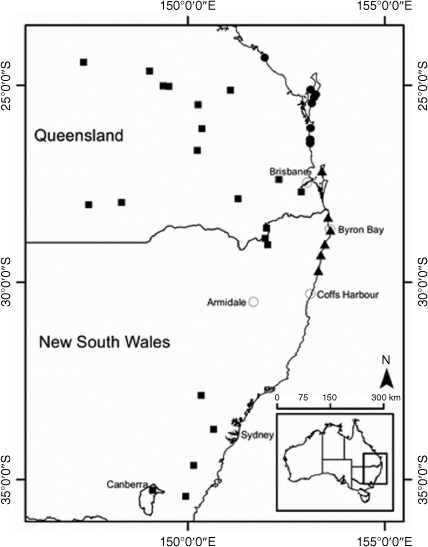
Restricted to the south-eastern gorges of the New England escarpment, NSW North Coast and New England Tableland bioregions (Fig. 13).
Cracks in vertical cliff faces and crevices between boulders in skeletal soils derived from metasediments.
Erect habit in cultivation. Showy white phyllaries and compact habit provide great horticultural potential.
Only known from two populations ~70 km apart. Given the restricted distribution and small population sizes, we suggest ‘Vulnerable’ status is appropriate under the International Union for Conservation of Nature and Natural Resources (2022) because it fulfils the criteria of Categories VU D1 and D2.
The specific epithet is derived from the given name of systematic botanist Alexander Nikolai Schmidt-Lebuhn (1976–), of CSIRO and the Centre for Australian National Biodiversity Research in Canberra, in recognition of his work on the Australian Asteraceae.
NEW SOUTH WALES: Northern Tablelands: Oxley Wild Rivers National Park, Paradise Rocks, 10 Nov. 2015, T.L.Collins 934 & M.F.Duretto (NE!, NSW!); Tuggolo State Forest, ~60 km S of Walcha, 4 Dec. 2008, L.M.Copeland 4332 (BRI, CANB*, K, MO, NE!, NSW, US); ~60 km S of Walcha, 6 Apr. 2007, L.M.Copeland 4225 (CANB, NE!, NSW).
Leucozoma boormanii (Maiden & Betche) T.L.Collins, Taxon 71(5): 1056 (2022)
Helichrysum boormanii Maiden & Betche, Proc. Linn. Soc. New South Wales 30(3): 366 (1905); Helichrysum boormanii var. typicum Domin, Biblioth. Bot. 22(89): 1224 (1930), nom. inval.; Coronidium boormanii (Maiden & Betche) Paul G.Wilson, Nuytsia 18: 316 (2008).
Type: Boonoo Boonoo, New South Wales, Nov. 1904, J.L.Boorman (lecto, fide Paul G.Wilson, Nuytsia 18: 316 (2008): NSW 230757*; isolecto: BRI AQ0370524*; MEL 1585991).
Erect, short-lived perennial, tap-rooted herb. Stems and branches with stipitate glands on septate stipes, internode length 3–10 mm. Basal leaf rosette absent at flowering. Basal leaves spathulate, base amplexicaul and auriculate, margin with stipitate glands, apex acute and mucronate; abaxial leaf surface with stipitate glands; midvein with stipitate glands; adaxial leaf surface with stipitate glands. Cauline leaves elliptic to lanceolate, 50–110 mm long and 5–20 mm wide, base attenuate and auriculate, margin with stipitate glands, apex acute and mucronate; abaxial leaf surface with stipitate glands, midvein indumentum hirsute and with stipitate glands; adaxial leaf surface with stipitate glands. Foliaceous bracts subtending capitula 12–15 mm long, margin cobwebby and with stipitate glands. Flowering stems branched or unbranched, capitula 45–60 mm in diameter (including phyllaries), terminal. Outer phyllary claw slender, cross-section oval, and with stipitate glands abaxially; phyllary lamina translucent, white, basal margin cobwebby or hispid, abaxial surface glabrous, apex cuspidate. Medial phyllary claw flattened, with stipitate glands on abaxial surface and on margins; phyllary lamina abaxially white, apex cuspidate to acute. Stylar appendages clavate. Cypsela cylindrical to oblongoid, ~2 mm long and 0.75 mm in diameter, pericarp brown, idioblasts present. Pappus persistent, ~7.5 mm long.
Restricted to the New England Tableland Bioregion in the southern Granite Belt, Queensland, and in the Tenterfield area on Roberts Range and the Great Dividing Range, along the New South Wales and Queensland border (Fig. 14).
Leucozoma boormanii is common on roadsides where it may benefit from regular disturbance and water runoff from the road. Showy white phyllaries provide horticultural potential.
Despite a restricted distribution, L. boormanii occurs in several National Parks. Road maintenance activities may damage some populations. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Darling Downs: Wallangarra, Nov. 1906, Dalton s.n. (NSW 519165!). NEW SOUTH WALES: Northern Tablelands: Boonoo Boonoo State Forest, road to Basket Swamp, 29 Oct. 2006, J.J.Bruhl 2452 & I.R.Telford (NE!, NSW!); Mount Donaldson Gully, ~30 km W of Tenterfield, 15 Nov. 1992, J.B.Williams s.n. (CANB, NE!); Mount Lindesay Road, ~12.2 km from Tenterfield, 16 Sep. 2018, T.L.Collins 1086 & I.R.Telford (BRI!, CANB!, NE!, NSW!); Great Dividing Range, Thunderbolts Gap, 12 km NE of Tenterfield, 1 Nov. 2007, T.Vollbon 43 (NE!); Timbarra Plateau, ~30 km SE of Tenterfield, 27 Apr. 2010, L.M.Copeland 4406 (CANB, NE!, NSW); Demon Nature Reserve, ~30 km SE of Tenterfield, 15 Mar. 1997, J.T.Hunter 5078 & N.J.Beresford-Smith (NE!).
Leucozoma elatum (A.Cunn. ex DC.) T.L.Collins, Taxon 71(5): 1056 (2022)
Helichrysum elatum A.Cunn. ex DC., Prodr. 6: 193 (1838); Gnaphalium elatum (A.Cunn. ex DC.) Sch.Bip., Bot. Zeitung (Berlin) 3: 171 (1845), nom. illeg. non Lam. (1788); Helichrysum albicans var. commune Domin, Biblioth. Bot. 89: 668 (1929); Coronidium elatum (A.Cunn. ex DC.) Paul G.Wilson, Nuytsia 18: 317 (2008).
Type citation: ‘in Novâ-Hollandiâ orient. et merid. (herb. mus. Par.!) in umbrosis littoreis distr. Illawarra et circà Port Jackson (A. Cunn.!)’. Type: ‘shaded woods on the coast near Port Jackson’, 1817, A.Cunningham 115 (lecto here designated: G-DC G00470686*).
Helichrysum albicans Sieber ex Spreng., Syst. Veg. 17th edn, 3: 482 (1826), nom. illeg. non A.Cunn. (1825). Type: ‘Novae Hollandia’, F.Sieber 346 (syn: HAL0111205*, MPU012643*).
Erect, perennial or annual, tap-rooted herb. Stems and branches cobwebby, woolly or glabrescent, and with stipitate glands; internode length 5–25 mm. Basal leaf rosette absent at flowering. Cauline leaves ovate to elliptic (with winged, stem-clasping petiole), 25–180 mm long and 2–50 mm wide, base amplexicaul and attenuate; margin undulate or sinuate, and cobwebby to woolly; apex acute and mucronate; abaxial indumentum cobwebby, woolly, felted or tomentose, and with stipitate glands, midvein indumentum tomentose or woolly, and with stipitate glands; adaxial indumentum cobwebby, villous, floccose to glabrous, and with stipitate glands. Foliaceous bracts subtending capitula (with papery apices) 5–12 mm long, margin cobwebby to woolly. Flowering stems branched or unbranched, capitula 30–50 mm in diameter (including phyllaries), terminal. Outer phyllary claws cross-section semi-terete, abaxial surface puberulous and with stipitate glands. White, basal margin fimbriate and cobwebby; phyllary lamina abaxial surface glabrous, apex acute (occasionally minutely incised). Medial phyllary claws cross-section semi-terete, with stipitate glands abaxially; phyllary lamina abaxially white, apex acute (and minutely incised). Stylar appendages clavate to ovate. Cypsela cylindrical to oblongoid, ~2–3 mm long and 0.75 mm in diameter, pericarp brown, ovate idioblasts. Pappus persistent, ~7.5 mm long.
We here designate G-DC G00470686 as lectotype of Helichrysum elatum A.Cunn. ex DC. because it is the specimen from Cunningham’s gathering held in Geneva and seen by de Candolle. The citation of ‘iso: G-DC barcode G00470661’ under Helichrysum albicans (Collins et al. 2002, p. 1056) was erroneous and should be disregarded.
Leucozoma elatum (A.Cunn. ex DC.) T.L.Collins subsp. elatum
Erect, short-lived perennial or annual herb. Cauline leaves ovate to elliptic, 30–180 mm long and 2–45 mm wide; abaxial indumentum tomentose and with stipitate glands, midvein indumentum tomentose and with stipitate glands. Capitula 30–45 mm in diameter (including phyllaries). Stylar appendages ovate.
Widespread from south-eastern Queensland to eastern Victoria in the South Eastern Queensland, NSW North Coast, New England Tablelands Sydney Basin, and South East Corner bioregions (Fig. 13).
Open shrubby eucalypt forests on soils derived from sandstone, granite, and volcanics including basalt, from near sea level up to ~1000-m altitude.
Observations in cultivation and in the field indicate that plants of L. elatum subsp. elatum are perennial, but under adverse conditions may flower and fruit in less than 1 year. There are large variations in peduncle length and leaf width between some populations. Showy white phyllaries indicate horticultural potential.
Widespread and common, occurring in numerous conservation reserves. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Moreton: 10 km from Maleny, 18 Nov. 2005, S.Shaw s.n. (BRI AQ0735726); McPherson Range, Springbrook, 7 Dec. 2018, I.R.Telford 15537 (BRI!, CANB!, NE!, NSW!). NEW SOUTH WALES: Northern Tablelands: Boonoo Boonoo State Forest, 29 Oct. 2006, J.J.Bruhl 2451 & I.R.Telford (CANB!, NE!, NY!, US!); Moogem State Forest, 17 Oct. 1998, R.J.Bayer s.n. (CANB*, NSW). North Coast: North Brother Mountain, Camden Haven State Forest, 11 Sep. 1985, G.J.White s.n. (NE!); Dyers Crossing, 20 Sep. 1947, L.A.J.Gilbert 19 (NE!). Central Coast: Malabar Headland (west) National Park, 7 Oct. 2019, T.L.Collins 1184 (CANB!, NE!, NSW!); 800 m W of North Era Beach, 7 Sep. 1953, B.G.Briggs s.n. (NE!). South Coast: Gulaga National Park, Mount Dromedary Trail, 27 Jan. 2018, T.L.Collins 1176 & M.Harris (CANB!, NE!, NSW!); Nullica State Forest, Pipeclay Road, 3 Feb. 2019, T.L.Collins 1180 (CANB!, NE!, NSW!). VICTORIA: East Gippsland: Mount Elizabeth Natural Feature – Scenic Reserve, 17 Sep. 1984, A.C.Beauglehole 77117 (CANB*, MEL); Reedy Creek Gully, 10 km direct NE of Cann River township, 5 May 1976, J.Piggin s.n. (CANB*).
Leucozoma elatum subsp. minus (Paul G.Wilson) T.L.Collins, Taxon 71(5): 1056 (2022)
Coronidium elatum subsp. minus Paul G.Wilson, Nuytsia 18: 318 (2008). Type: NEW SOUTH WALES: Northern Tablelands: Point Lookout, New England National Park, 19 Aug. 1969, I.R.Telford 1149 (holo: CBG 29740 [in CANB]!).
Erect, perennial, shrubby herb. Cauline leaves ovate (with minutely-winged, stem-clasping petiole), 25–140 mm long and 5–50 mm wide; abaxial indumentum felted and with stipitate glands and prominent woolly lateral veins, midvein indumentum woolly and with stipitate glands. Capitula 40–50 mm in diameter (including phyllaries). Stylar appendages clavate.
Restricted to the eastern escarpment of the plateau of the New England Tableland Bioregion (Fig. 13).
Shrubby eucalypt woodlands, on steep slopes, cliff-tops, and rock outcrops, on granite and basalt, over 1000-m altitude.
Despite a small distribution, most populations are protected in conservation reserves. The threats posed by climate warming may severely affect this species. We recommend a status of ‘Vulnerable’ under the International Union for Conservation of Nature and Natural Resources (2022), because it fulfils the Criterion VU D1.
NEW SOUTH WALES: Northern Tablelands: ~45 km ENE of Glen Innes along Gwydir Highway, 7 Sep. 2005, L.M.Copeland 3951 (BRI, CANB, NE!, NSW); Gibraltar Range, Glen Elgin State Forest, 10 Apr. 2005, L.M.Copeland 3928 (NE!); Dorrigo, 1 Oct. 1955, D.R.Hayward s.n. (NE 5475!); slopes SE of Cathedral Rock, near Ebor, 25 Sep. 1974, J.M.B.Smith 17 (NE!); Cathedral Rock National Park, Woolpack Rocks track, 11 Sep. 1988, J.B.Williams s.n. (NE!); New England National Park, Weeping Rock, 4 Oct. 2002, J.J.Bruhl 2079, I.R.Telford & J.G.West (NE!); Oxley Wild Rivers National Park, 7 Feb. 2007, L.M.Copeland 4166 (NE!, NSW); Werrikimbe National Park, Spokes Hill, 5 Nov. 1977, J.B.Williams s.n. (NE!).
Leucozoma kaputaricum (Paul G.Wilson) T.L.Collins, Taxon 71(5): 1056 (2022)
Coronidium kaputaricum Paul G.Wilson, Nuytsia 18: 315 (2008). Type: AUSTRALIA: New South Wales: Northern Tablelands: Nandewar Range, Mount Kaputar National Park, The Governor, 13 Nov. 1995, I.R.Telford 12080 (holo: PERTH 06072976!; iso: CANB!, K!, MEL!, NE 68558!, NSW!).
Pendulous or decumbent perennial herb with corky crown. Stems and branches felted or woolly, and with stipitate glands; internode length 1 mm. Basal leaf rosette absent at flowering. Cauline leaves ovate to obovate, or elliptic (with winged stem-clasping petioles); 35–70 mm long and 5–20 mm wide, base amplexicaul and attenuate, margin felted, apex acute and mucronate; abaxial indumentum felted and with stipitate glands, midvein indumentum felted and with stipitate glands; adaxial indumentum tomentose and with stipitate glands. Foliaceous bracts subtending capitula 5–15 mm long, margin felted to villous. Flowering stems branched, capitula 20–35 mm in diameter (including phyllaries), terminal. Outer phyllaries sessile, translucent white, basal margin villous and hispid, abaxial surface glabrous, apex cuspidate to acute (occasionally incised). Medial phyllary claw flattened in cross-section and with stipitate glands on abaxial surface; phyllary lamina abaxially white, apex cuspidate. Stylar appendages ovate. Cypsela cylindrical to oblongoid, 3 mm long and 1 mm in diameter, cross-section oblongoid to cylindrical, pericarp straw–brass-coloured, idioblasts present. Pappus persistent, ~6.5 mm long.
Restricted to the summit plateau of Mount Kaputar on the Nandewar Range, in the New England Tableland Bioregion (Fig. 14).
Conserved in Mount Kaputar National Park, but given the very small distribution, and limited capacity to migrate in response to climate warming, we recommend a status of ‘Vulnerable’ under the International Union for Conservation of Nature and Natural Resources (2022) because it fulfils the criteria of VU D1 and D2.
NEW SOUTH WALES: Nandewar Range: Mount Kaputar National Park, 8 Mar. 1994, A.M.Lyne 1300 & J.Lyne (CANB*); Nandewar Range, Mount Kaputar National Park, 21 Aug. 1996, I.R.Telford 12377 & S.Donaldson (CANB!, NE!); Mount Kaputar National Park, track to The Governor, 16 Nov. 2000, J.J.Bruhl 1985 & I.R. Telford (NE!); Mount Kaputar NP, The Governor, 9 May 2019, R.L.Palsson 259 (NE!).
Leucozoma lindsayanum (Domin) T.L.Collins, Taxon 71(5): 1056 (2022)
Helichrysum lindsayanum Domin, Biblioth. Bot. 22(89): 1224 (1930); Coronidium lindsayanum (Domin) Paul G.Wilson, Nuytsia 18: 323 (2008). Type citation: ‘Süd-Queensland: Mount Lindsay, FRASER 1829; ibidem (‘on the rocks, 5000 ft [∼1524 m],’), W. HILL 25 XII. 1857’. Type: ‘Mount Lindsay’, Queensland, 1829, C.Fraser 61 (lecto, fide Paul G.Wilson, Nuytsia 18: 323 (2008): K 000899133*; residual syn: MEL 2105253* [annotated as ‘lectoparatype’ by Paul G.Wilson]).
Helichrysum elatum var. fraseri Benth., Fl. austral. 3: 621 (1867), p.p. [excluding populations at Port Curtis]. Type citation: ‘Rocks of Mount Lindsay, at an altitude of 5000 ft [∼1524 m], Fraser, W.Hill; Port Curtis, M’Gillivray’.
Pendulous or decumbent perennial, tap-rooted subshrub-like herb. Stems and branches felted or woolly, or glabrescent (with corky bark on older stems); internode length 1–3 mm. Basal leaf rosette absent at flowering. Cauline leaves lanceolate, 30–70 mm long and 5–10 mm wide, base amplexicaul and attenuate, margin revolute and glabrous, apex acute and mucronate; abaxial indumentum felted or tomentose, and with short stipitate glands; abaxial midvein indumentum felted; adaxial leaf surface with stipitate glands and occasional or scattered long hairs. Foliaceous bracts subtending capitula 7–10 mm long, margin villous. Flowering stems branched or unbranched, capitula 40–50 mm in diameter (including phyllaries), terminal. Outer phyllaries sessile and with stipitate glands abaxially, opaque, pink or white; basal margin villous, abaxial surface glabrous, apex acute (occasionally incised). Medial phyllary claw flattened and with stipitate glands abaxially, lamina pink or white, apex cuspidate to apiculate (occasionally incised). Stylar appendages deltoid to ovate. Cypsela cylindrical to oblongoid, 2.25 mm long and 0.75 mm in diameter, cross-section with oblique angles, pericarp grey–brown, idioblasts present. Pappus persistent, ~5 mm long.
Restricted to the McPherson Range on Mount Barney and associated volcanic remnants in the South Eastern Queensland Bioregion (Fig. 14).
Cliff faces with humic sediments in cracks and crevices in granophyre, on Mount Barney; elsewhere on acid volcanics particularly rhyolite, above 600-m altitude.
Decumbent habit retained in cultivation. In early collections cited in the typification, the locality of Allan Cunningham’s collection is misspelled as ‘Mount Lindsay’. Because of an early cartographical error, the Mount Lindesay visited by Cunningham is now known as Mount Barney (Telford 1990).
We recommend a rating of ‘Least Concern’ under the International Union for Conservation of Nature and Natural Resources (2022). Recent extreme wildfires and limited capacity to migrate in response to climate warming could rapidly reduce population sizes and area of extent and we recommend periodic monitoring to assess conservation status.
QUEENSLAND: Moreton: Glennies Pulpit, 2 km SE of Moogerah Dam, 8 Oct. 1992, P.I.Forster 11938 (BRI!); Mount Maroon, summit plateau, 19 Aug. 1996, I.R.Telford 12360 (CANB!, NE!); Mount Barney National park, Mount Maroon, 23 Sep. 2018, H.T.Kennedy 1 (BRI!, NE!); Mount Barney National Park, SE ridge walking track, 21 Mar. 2018, T.L.Collins 1040 & B. Wright (BRI!, CANB!, NE!); Mount Barney, East Peak, 14 Oct. 1998, A.S.Benwell 322 (NE!); Mount Gillies, rhyolite clifflines with scattered vegetation, 19 Oct. 1992, P.I.Forster 12069 (BRI!, MEL); Mount Ernest, McPherson Range, 14 Sep. 1990, P.I.Forster 7384 (BRI!, CANB*, MEL, PERTH).
Leucozoma telfordii (Paul G.Wilson) T.L.Collins, Taxon 71(5): 1056 (2022)
Coronidium telfordii Paul G.Wilson, Nuytsia 18: 323 (2008). Type: Mt Merino, McPherson Range, Qld–NSW border, 30 Sep. 1973, I.R.Telford 3357 (holo: CANB 050131!; iso: A!, BRI!, K!, L!, NE 75142!).
Helichrysum sp. 1: T.D.Stanley in T.D.Stanley & E.M.Ross, Fl. South-eastern Queensland 2: 537 (1986).
Erect, perennial, tap-rooted subshrub-like herb. Stems and branches felted or glabrescent, internode length 5–25 mm. Basal leaf rosette absent at flowering. Cauline leaves ovate to elliptic (with minutely winged, stem-clasping petiole), 30–80 mm long and 10–30 mm wide, base amplexicaul and attenuate, margin entire, felted or glabrous, apex acute and mucronate; abaxial indumentum felted, midvein indumentum felted (with a pair of secondary veins prominent abaxially on lower leaves and adaxially on higher leaves); adaxial indumentum cobwebby or floccose (with sparsely scattered stipitate glands on midvein towards the base). Foliaceous bracts subtending capitula 7–9 mm long, margin felted. Flowering stems branched, capitula 30–40 mm in diameter (including phyllaries), terminal. Outer phyllary claw semi-terete, puberulous and with stipitate glands abaxially; phyllary lamina translucent and white, basal margin fimbriate and cobwebby, abaxial surface glabrous, apex apiculate (occasionally incised). Medial phyllary claw concave, with stipitate glands on abaxial surface and on margins; phyllary lamina abaxially white, apex cuspidate (occasionally incised). Stylar appendages clavate. Cypsela cylindrical to oblongoid, 1.75 mm long and 0.75 mm in diameter, pericarp brown, idioblasts absent. Pappus persistent, ~5 mm long.
Restricted to the South Eastern Queensland Bioregion where the main distribution lies along the McPherson Range from Mount Merino, south to Echo Point, with a single record from the Main Range at The Steamers (Fig. 14).
Cliff edges and steep slopes of basalt escarpment on margins of Nothofagus moorei forest on the McPherson Range; at The Steamers in Eucalyptus campanulata open forest.
The specimen collected at The Steamers lacks the prominent leaf venation of McPherson Range plants.
Given the very small distribution, recent 2020 extreme wildfires and limited capacity to migrate in response to climate warming, we recommend a rating of ‘Vulnerable’ under the International Union for Conservation of Nature and Natural Resources (2022), because it fulfils the criteria of VU D1 and D2.
QUEENSLAND: Moreton: Roberts Plateau, May 1918, J.Shirley s.n. (NSW 518860!); on exposed rocks under stunted Beech Trees, Macpherson Range, Jan. 1919, C.T.White s.n. (BRI 360974!); McPherson Range, Lamington National Park, Robert’s Plateau, Nov. 1921, W.D.Francis s.n. (BRI AQ247971!); McPherson Range, Lamington National Park, 12 Apr. 1996, J.B.Williams s.n. (NE!); McPherson Range, Mount Merino, 12 May 1970, I.R.Telford 1549 (CANB!, NE!); Lamington National Park, Tooloona Lookout, 7 Dec. 2018, T.L.Collins 1144 (BRI!, CANB!, NE!). Darling Downs: The Steamers E of Emu Vale, 3 Oct. 1997, A.R.Bean 12427 (BRI!). NEW SOUTH WALES: North Coast: Limpinwood Nature Reserve, Yargabullang Lookout, 6 Nov. 2005, I.R.Telford 12888 (CHR!, NE!, NSW!); Tyalgum Ridge, Macpherson Range, 3 Dec. 1977, L.Haegi 1527 (NSW!).
Leucozoma wollumbin T.L.Collins, sp. nov.
Type: AUSTRALIA: New South Wales: North Coast: Wollumbin National Park, Mount Warning just below summit, 25 Oct. 2018, T.L.Collins 1095, J.J.Bruhl & I.R.Telford (holo: NSW!; iso: CANB!, NE 108726!).
Coronidium elatum subsp. vellerosum Paul G.Wilson, Nuytsia 18: 318 (2008); Leucozoma elatum subsp. vellerosum (Paul G.Wilson) T.L.Collins, Taxon 71(5): 1057 (2022). Type: Mount Warning, 14 km south-west of Murwillumbah, New South Wales, 19 Aug. 1996, I.R.Telford 12351 & S.Donaldson (holo: CANB!; iso: NSW!, NE!).
Distinguished from L. elatum subsp. elatum by the perennial lifecycle (v. annual or short-lived perennial), shrubby habit (v. erect), clavate stylar appendages (v. ovate), flattened medial phyllary claws (v. semi-terete), and felted leaf indumentum adaxially (v. floccose–glabrescent). Distinguished from L. elatum subsp. minus by the flattened medial phyllary claws (v. semi-terete) and felted leaf indumentum adaxially (v. villous).
Erect, perennial, tap-rooted subshrub-like herb. Stems and branches cobwebby, woolly, and with stipitate glands. Basal leaf rosette absent at flowering. Cauline leaves ovate to lanceolate (with winged, stem-clasping petiole), 35–100 mm long and 7–30 mm wide; base attenuate; margin entire, cobwebby or woolly; apex acute and mucronate; abaxial indumentum felted to tomentose, midvein with stipitate glands; adaxial indumentum felted, and with stipitate glands. Foliaceous bracts subtending capitula ~15 mm long (with papery apices), margin villous to cobwebby, and with stipitate glands. Flowering stems branched or unbranched, capitula 45–50 mm in diameter (including phyllaries), terminal. Outer phyllary claw semi-terete, with stipitate glands abaxially; phyllary lamina white, basal margin fimbriate and cobwebby, abaxial surface glabrous, apex acute. Medial phyllary claw flattened, with stipitate glands on abaxial surface; phyllary lamina abaxially white, apex cuspidate to apiculate and minutely incised. Stylar appendages clavate. Cypsela cylindrical to oblongoid, 2.3 mm long and 0.5 mm in diameter, pericarp brown, idioblasts absent. Pappus persistent, ~7 mm long. (Fig. 15.)
Known only from the summit area of Wollumbin National Park in the NSW North Coast Bioregion (Fig. 13).
Trachyandersite cliff faces and steep slopes, in cracks and crevices with humic sediments, above 1000-m altitude.
Showy white phyllaries and silvery leaves provide good horticultural potential. Leucozoma wollumbin has been grown at the Australian National Botanic Gardens (ANBG) in Canberra after Stuart Donaldson and Ian Telford collected cuttings in 1996, along with the herbarium specimens that were to become the type of Coronidium elatum subsp. vellorosum. The species was released in 2001 by the ANBG as the floral emblem of the International Year of Volunteers and registered with the Australian Cultivar Registration Authority as Helichrysum elatum ‘Helping Hand’ (see https://www.anbg.gov.au/gnp/gnp-extras/coronidium-helping-hand.html), erroneously referred later to Coronidium telfordii.
Given the very small distribution, and limited capacity to migrate in response to climate warming, we recommend a rating of ‘Vulnerable’ under the International Union for Conservation of Nature and Natural Resources (2022) because it fulfils the Criteria VU D1 and D2.
The new species epithet is the traditional name of the type locality and acknowledges the continuing importance of the sacred mountain to the culture of the Nganduwal, Galibal, Githabul and Widjabal peoples (Herb Roberts, pers. comm., 2020). It is a noun in apposition (ICN Art. 23.1, Shenzhen Code; Turland et al. 2018).
NEW SOUTH WALES: North Coast: summit Mount Warning, Nov. 1898, W.Forsyth s.n. (NSW 519032!); Mount Warning, 28 Sep. 1973, I.R.Telford 3260 (CANB!); Wollumbin National Park, Mount Warning mountainside ~100 m below summit, 25 Oct. 2018, T.L.Collins 1096 & J.J.Bruhl (CANB!, NE!, NSW!); Wollumbin National Park, Mount Warning mountainside ~130 m below summit, 25 Oct. 2018, T.L.Collins 1097 & J.J.Bruhl (CANB!, NE!, NSW!).
Coronidium Paul G. Wilson emend. T.L.Collins
Type: Coronidium oxylepis (F.Muell.) Paul G.Wilson
Indumentum woolly, silky, hirsute, hispid, glabrous, glabrescent, puberulous, villous, tomentose, felted, floccose, cobwebby, or with stipitate glands. Leaves alternate; ovate, obovate, lanceolate, oblanceolate, or linear. Capitula predominantly homogamous, discoid, rarely heterogamous, disciform. Receptacle ±flat, epaleate. Phyllaries white, pink, yellow or straw-, bronze-, or coppery-coloured, multiseriate, scarious, spreading when mature; claw of intermediate and inner bracts terete, semi-terete or flattened, abaxially with stipitate glands or villous hairs, cartilaginous; prominent central vascular bundle extending into the lamina; stereome undivided. Florets bisexual, or rarely some outer florets female; corolla narrowly cylindrical, broadened above, lobes short, ovate; anther tails slender, equal to or exceeding collar, appendage ovate, outwardly concave; style arms slender; style apex truncate, rounded, ovate, triangular to narrowly triangular, and strongly papillose. Cypsela cylindrical or oblongoid; pericarp very thin and translucent, smooth, glabrous, with linear cells (idioblasts); testa brown. Pappus bristles filiform, denticulate, and proximally smooth where united in a persistent ring and breaking closely above it.
The circumscription of the genus used here excludes taxa previously included in the C. elatum and C. scorpioides groups of Wilson (2008). The putative hybrid C. newcastlianum × C. rupicola is included in the key, as well as all other taxa in Coronidium.
Stipitate glands are best observed on young leaves. With age, abrasion removes the gland leaving the trichome stipe and giving a hispid appearance to the leaf surface.
| 1. | Phyllaries yellow, straw- or bronze-coloured...2 Phyllaries white or white and pink...13 |
| 2. | Stipitate glands absent from cauline adaxial and abaxial leaf surfaces; other hair types mostly present...3 Stipitate glands present on both cauline leaf surfaces, or only present on abaxial surface; other hair types mostly present also...6 |
| 3. | Outer phyllaries sessile; stylar appendages clavate to ovate ...C. newcastlianum × C. rupicola Outer phyllaries with flattened or semi-terete claws; stylar appendages deltoid to ovate...4 |
| 4. | Foliaceous bracts subtending capitula 5–8 mm long and woolly...C. sp. Russell Island Foliaceous bracts subtending capitula absent...5 |
| 5. | Cauline leaf adaxial surface with floccose to villous indumentum; outer phyllary claw flattened, villous to woolly and with stipitate glands abaxially; distribution restricted to the Many Peaks Range west of Gladstone, Queensland...C. bruhlii Cauline leaf adaxial surface with felted to tomentose indumentum; outer phyllary claw semi-terete, and with stipitate glands abaxially; distributed through a broad region of central Queensland from west of Mackay to the Einasleigh Uplands Bioregion west of Townsville...C. gnaphalioides |
| 6. | Stipitate glands absent on cauline leaf adaxial surface...7 Stipitate glands present on cauline leaf adaxial surface...9 |
| 7. | Phyllaries yellow...C. flavum Phyllaries bronze or straw-coloured...8 |
| 8. | Most cauline leaves lanceolate, 50–120 mm long, margin revolute and cobwebby; capitulum 15–25 mm in diameter (including phyllaries), outer phyllaries opaque and bronze-coloured; stylar appendages ovate...C. rupicola Most cauline leaves oblanceolate to lanceolate, 20–60 mm long, margin flat and villous; capitulum 20–30 mm in diameter (including phyllaries), outer phyllaries translucent or straw-coloured; stylar appendages narrowly triangular...C. oxylepis subsp. lanatum |
| 9. | Stems and branches villous or glabrescent, and with scattered stipitate glands...10 Stems and branches cobwebby to tomentose, with or without stipitate glands...11 |
| 10. | Most cauline leaves spathulate, 30–100 mm long, margin entire, glabrous, or with scattered villous hairs and stipitate glands; stylar appendages narrowly triangular...C. oxylepis subsp. carnosum Most cauline leaves oblanceolate to elliptic, 20–40 mm long, margin revolute and glabrous; stylar appendages deltoid to ovate...C. sp. N Qld Headlands |
| 11. | Cauline leaf abaxial surface hairless and with stipitate glands, midvein indumentum cobwebby, hirsute, hispid, or glabrescent...C. oxylepis subsp. oxylepis Cauline leaf abaxial surface (including midvein) felted to tomentose, and with stipitate glands...12 |
| 12. | Most cauline leaves 30–40 mm long and 3–6 mm wide, apices acute and mucronate; foliaceous bracts subtending capitula to 5 mm long; outer phyllaries straw-coloured ...C. batianoffii Most cauline leaves 50–100 mm long and 10–15 mm wide, apices cuspidate and fimbriate; foliaceous bracts subtending capitula absent; outer phyllaries bronze-coloured...C. cymosum |
| 13. | Cauline leaves linear, 2–4 mm wide...C. glutinosum Cauline leaves ovate, obovate, oblanceolate or elliptic, 6–20 mm wide...14 |
| 14. | Cauline leaf margin revolute or undulate, indumentum of cobwebby, villous to woolly hairs or occasionally hairless, with stipitate glands; stylar appendages narrowly triangular...C. lanuginosum Cauline leaf margin not revolute, indumentum of felted hairs, non-glandular; stylar appendages clavate to ovate...C. newcastlianum |
Coronidium batianoffii T.L.Collins & I.Telford, sp. nov.
Type: Queensland: Port Curtis District: South Percy Island, 50 km NE of Arthur Point, Shoalwater Bay, 26 Oct. 1989, G.N.Batianoff 11468, I.Champion, P.Thompson & H.A.Dillewaard (holo: BRI AQ460844!).
[Coronidium cymosum auct. non Paul G.Wilson: Paul G.Wilson, Nuytsia 18: 306 (2008), p.p. (populations on sandy beaches and forest margins with cauline leaves 30–40 mm long and 3–6 mm wide, capitula 15–20 mm in diameter, and with cobwebby outer phyllary claws)].
Distinguished from Coronidium cymosum, with which it has been confused, by outer phyllary claws puberulous and with stipitate glands (v. outer phyllary claws with stipitate glands only), smaller leaves (30–40 mm long and 3–6 mm wide, v. 50–100 mm long and 10–15 mm wide), and capitula 15–20 mm in diameter (v. 20–25 mm in diameter), and the presence of foliaceous bracts subtending the capitula to 5 mm long (v. bracts absent).
Erect, annual or short-lived perennial herb. Stems and branches cobwebby, tomentose, and with stipitate glands; internode length 5–10 mm. Basal leaf rosette absent at flowering. Seedling and basal leaves not seen. Cauline leaves oblanceolate, 30–40 mm long and 3–6 mm wide, base attenuate, margin revolute and cobwebby, apex acute and mucronate; abaxial indumentum felted, tomentose, and with stipitate glands; midvein indumentum cobwebby and tomentose; adaxial indumentum cobwebby and with stipitate glands. Foliaceous bracts subtending capitula up to 5 mm long, margin cobwebby and with stipitate glands. Flowering stems branched. Capitula 15–20 mm in diameter (including phyllaries), terminal. Outer phyllary claw flattened, puberulous and with stipitate glands abaxially; phyllary lamina straw-coloured, basal margin villous and fimbriate, abaxial surface villous, apex acute and fimbriate. Medial phyllary claw semi-terete, villous and with stipitate glands abaxially; phyllary lamina abaxially straw- or bronze-coloured, apex subulate. Stylar appendages narrowly triangular. Mature cypsela not seen, idioblasts present. Pappus persistent, ~6 mm long. (Fig. 16)
Only known from the Whitsunday and Percy Island groups in the Central Mackay Coast Bioregion, Queensland (Fig. 17).
Restricted to the Whitsunday Islands National Park; however, C. batianoffii has not been collected for 35 years and must be regarded as ‘Data Deficient’ under the International Union for Conservation of Nature and Natural Resources (2022). Population sizes and the threats from climate change and tourism development are poorly known, and we recommend urgent survey and monitoring to determine conservation status.
The specific epithet is derived from the surname of botanist George Nicholaivich Batianoff (1945–2009) and honours his work on the Queensland flora, including collection of the type material for this species.
QUEENSLAND: North Kennedy District: Whitsunday Island. Dunal area. Common herb growing on foredunes, 9 Nov. 1985, G.N.Batianoff 3645 (BRI!); Whitsunday Island. Common in sandy forest land near the sea, 10 June 1934, C.T.White 10145 (BRI!); Whitehaven Beach, Whitsunday Island, 26 Feb. 1985, C.Warrian 208 (BRI!); Hamilton Island E of Proserpine, 27 Dec. 2010, L.Benson WIH1 (BRI!).
Coronidium bruhlii T.L.Collins, sp. nov.
Type: CULTIVATED: University of New England Botany glasshouse, T.L.Collins 1186 (holo: BRI!; iso: CANB!, NE 110438!) [propagated from cuttings ex AUSTRALIA: Queensland: Port Curtis District: Castle Tower National Park, Mount Stanley, R.L.Palsson 203, R.L.Andrew & J.J.Bruhl, NE 106224].
Coronidium sp. Many Peaks (I.R.Telford 12309) NE Herbarium: CHAH, Austral. Pl. Census (2020) [accessed 20 Feb. 2020].
[Coronidium gnaphalioides auct. non (Domin) Jeanes: J.A.Jeanes, Muelleria 39: 109 (2021), p.p. (populations with leaves 20–70 mm long and 7–20 mm wide, and with flattened outer and medial phyllary claws)].
Distinguished from C. gnaphalioides, with which it has been confused, by the larger leaves 20–70 mm long and 7–20 mm wide (v. leaves 20–35 mm long and 7–10 mm wide), and the flattened outer and medial, villous to woolly and glandular phyllary claws (v. outer and medial phyllary claws semi-terete in cross-section, glandular and without villous to woolly hairs).
Erect perennial, tap-rooted, herb. Stems and branches woolly, internode length 5–20 mm. Basal leaf rosette absent at flowering. Seedling and basal leaves not seen. Cauline leaves spathulate or obovate, 20–70 mm long and 7–20 mm wide, base amplexicaul and attenuate, apex obtuse to acute, and mucronate; margin villous; abaxial indumentum tomentose, midvein indumentum tomentose; adaxial indumentum floccose to villous. Foliaceous bracts subtending capitula absent. Flowering stems branched, capitula 15–20 mm in diameter (including phyllaries), terminal, in panicles, or solitary. Outer phyllary claw flattened, villous to woolly and with stipitate glands abaxially; phyllary lamina straw-coloured, basal margin fimbriate, abaxial surface glabrous, apex acute and fimbriate. Medial phyllary claw flattened, villous and with stipitate glands abaxially; phyllary lamina abaxially straw- to bronze- coloured, apex subulate. Stylar appendages narrowly triangular. Cypsela cylindrical to oblongoid, 1.25 mm long and 0.5 mm in diameter, pericarp translucent to cream-coloured, idioblasts present. Pappus persistent, ~3.5 mm long. (Fig. 18)
Restricted to the Many Peaks Range in the Brigalow Belt North Bioregion, Queensland (Fig. 19).
Coronidium sp. Many Peaks (I.R.Telford 12309) NE Herbarium has previously been mistakenly listed as a synonym of C. scorpioides (Labill.) Paul G.Wilson (CHAH, see https://biodiversity.org.au/nsl/services/APC). Collins et al. (2022) showed that it is instead part of the C. oxylepis group.
Collection notes indicate small populations scattered mid-slope across Mount Castle Tower and Mount Stanley in Castle Tower National Park. Given the restricted distribution and poorly known population sizes, ‘Vulnerable’ status is appropriate under the International Union for Conservation of Nature and Natural Resources (2022) because it fulfils the criteria of Categories VU D1 and D2.
Coronidium cymosum Paul G.Wilson, Nuytsia 18: 306 (2008)
Type: Queensland: Leichhardt: Blackdown Tableland, ~35 km SE of Blackwater (campsite at old stockyard on Mimosa Creek), 3 Sep. 1971, R.J.Henderson, L.Durrington & P.Sharpe 948 (holo: BRI AQ 00014196*; iso: CANB 303554, MEL 2111963).
Genus (Aq247974) sp. (Blackdown Tableland R.J.Henderson + H948): A.E.Holland in P.D.Bostock & A.E.Holland (eds), Census Queensland Fl. 2007: 27 (2007).
Erect, perennial, tap-rooted herb. Stems and branches cobwebby to tomentose, and with stipitate glands. Basal leaf rosette absent at flowering. Seedling leaves oblanceolate, 70–100 mm long and 15–25 mm wide, base amplexicaul and attenuate, margin cobwebby, apex cuspidate; abaxial indumentum tomentose, and with stipitate glands, midvein indumentum tomentose; adaxial indumentum floccose to villous. Cauline leaves elliptic to lanceolate, 50–100 mm long and 10–15 mm wide, base amplexicaul and attenuate, margin revolute and glabrous, apex cuspidate; abaxial indumentum felted to tomentose, and with stipitate glands; midvein indumentum tomentose; adaxial indumentum villous on midvein towards leaf base and with stipitate glands. Foliaceous bracts subtending capitula absent. Flowering stems branched, capitula 20–25 mm in diameter (including phyllaries), terminal. Outer phyllary claw flattened, puberulous and with stipitate glands abaxially; phyllary lamina bronze-coloured, basal margin fimbriate and cobwebby, abaxial surface villous, apex cuspidate and fimbriate. Medial phyllary claw flattened to semi-terete, villous and with stipitate glands abaxially; phyllary lamina abaxially bronze-coloured, apex subulate. Stylar appendages narrowly triangular. Cypsela cylindrical to oblongoid, 1.5 mm long and 0.5 mm in diameter, pericarp straw–brass-coloured, idioblasts present. Pappus persistent, ~5 mm long.
Restricted to the Brigalow Belt North and Central Mackay Coast bioregions, Queensland. Recorded from ~800-m altitude in the Blackdown Tableland National Park and near sea-level on the coast north of Yeppoon (Fig. 19).
Considerable variation in habitat and leaf shape occurs between populations found on coastal sands and those on inland sandstone ranges. Molecular data indicated that coastal and range populations were genetically congruent.
The species is common with populations of mature individuals estimated in the hundreds to thousands in the Blackdown Tableland and Byfield National Parks in 2018, and thus is considered to be of ‘Least Concern’ under the criteria of the International Union for Conservation of Nature and Natural Resources (2022).
QUEENSLAND: Port Curtis: Mount Wheeler, summit of southern peak, 7 Aug. 1983, P.I.Forster 13804 (BRI!); Clinton Lowland, Shoalwater Bay area, 10 Feb. 1992, E.J.Thompson 75 (BRI!, DNA); 2 km SE of Mount Gibraltar, Shoalwater Bay Training Area, 21 Feb. 2012, A.R.Bean 31555 (BRI!); Shoalwater Bay Army Training Area, 26 July 2010, R.Date s.n. (BRI AQ851472!); Byfield National Park, Stockyard Point Track, 16 Nov. 2018, T.L.Collins 1133 (BRI!, CANB!, NE!). Leichhardt: Blackdown Tableland National Park, entrance to Munall Creek camping area, 18 Nov. 2018, T.L.Collins 1137 (BRI!, CANB!, NE!); Blackdown Tableland National Park, Mimosa Creek Camping Area, 21 Nov. 2000, L.M.Copeland 2652, J.J.Bruhl & I.R.Telford (BRI!, CANB!, NE!, NSW!).
Coronidium flavum Paul G.Wilson, Nuytsia 18: 311 (2008)
Type: Queensland: North Kennedy: 18.5 km W of Paluma, 22 Oct. 1989, P.C.Jobson 967 (holo: MEL 223774*; iso: BRI AQ481153, CBG 9103497 [in CANB]).
Helichrysum newcastlianum var. (Bluewater Creek A.R. Bean 3784): A.E.Holland in R.J.F.Henderson (ed.), Queensland Pl. Names & Dist. 29 (1997).
Perennial, tap-rooted, erect herb. Stems and branches cobwebby to woolly, internode length 5–15 mm. Basal leaf rosette absent at flowering. Seedling and basal leaves not seen. Cauline leaves ovate to obovate or lanceolate, 30–80 mm long and 7–15 mm wide, base amplexicaul and auriculate, apex subulate and mucronate, margin woolly; abaxial indumentum tomentose to woolly, and with stipitate glands, midvein indumentum tomentose to villous; adaxial indumentum woolly to tomentose. Foliaceous bracts subtending capitula absent. Flowering stems branched, capitula 25–30 mm in diameter (including phyllaries), terminal, on long peduncles. Outer phyllary claw flattened, puberulous and with stipitate glands abaxially; phyllary lamina yellow, basal margin villous and fimbriate, abaxial surface villous, apex acute and fimbriate. Medial phyllary claw flattened and villous abaxially; phyllary lamina yellow abaxially, apex cuspidate. Stylar appendages deltoid to ovate. Cypsela cylindrical to oblongoid, 1.5 mm long and 0.5 mm in diameter, pericarp translucent or cream (with maroon seed coat visible), idioblasts present. Pappus persistent, ~4 mm long.
Recorded from the Wet Tropics Bioregion in the hills bordering the Paluma Range National Park north-west of Townsville, Queensland (Fig. 17).
Very attractive inflorescence with pale yellow phyllaries. May hold future horticultural potential if compact forms are found or developed.
Most recorded populations are estimated to have fewer than 1000 mature individuals, are outside Paluma Range National Park and are on private pastoral land and road reserves. Although C. flavum occurs in low-productivity habitats on rocky slopes with skeletal soils and is, therefore, unlikely to be affected by cropping, the restricted distribution and small populations suggest that this species may be seriously threatened in the future by stochastic events such as intense wildfires, land clearing and careless land management, and therefore must be regarded as ‘Vulnerable’ under the International Union for Conservation of Nature and Natural Resources (2022) Category VU D1.
QUEENSLAND: North Kennedy: 19.5 km from Paluma along road to Hidden Valley, 1 Aug. 1996, I.R.Telford 12148 & S. Donaldson (CANB!); Ewan–Paluma road, ~18.8 km W of Paluma, 4 July 2018, T.L.Collins 1071 & J.J.Bruhl (BRI!, CANB!, NE!); Hellhole Creek, 1 km from ‘Taravale’ Homestead along road to Paluma, 11 Nov. 2006, I.R.Telford 13000 & J.J.Bruhl (NE!); Bluewater Creek Road, NW of Townsville, 10 Nov. 1991, A.R.Bean 3784 (BRI!); S of Boghole Creek and N of Greenvale railway, 21 Aug. 2008, H.Wood s.n. (BRI AQ860984!); S side of Pattersons Gorge, 35 km W of Townsville, 27 Apr. 2002, R.J.Cumming 20561 (BRI!); North Kennedy district Queensland, winter 1950, J.Hennelly 113 (CANB*).
Coronidium glutinosum (Hook.) Paul G.Wilson, Nuytsia 18: 311 (2008)
Helipterum glutinosum Hook. in T.L.Mitchell, J. Exped. Trop. Australia 361 (1848); Helichrysum glutinosum (Hook.) Benth., Fl. Austral. 3: 621 (1867), nom. illeg. non A.Braun. (1841); Helichrysum elatum var. glutinosum (Hook.) C.Moore, Handb. Fl. N. S. W. 281 (1893). Type: [Queensland], 5 Sep. 1846, T.L.Mitchell s.n. (lecto here designated: K 000899132*; residual syn: MEL 604817*; MEL 604816*; NSW 519138*; GH 8849*).
Erect or shrubby, perennial, tap-rooted herb. Stems and branches villous and with stipitate glands, internode length 1 mm. Basal leaf rosette absent at flowering. Seedling leaves spathulate, shorter and broader than cauline leaves, 10–25 mm long and 7–10 mm wide, base amplexicaul and attenuate, margin villous, apex obtuse to apiculate; abaxial indumentum silky and with stipitate glands, midvein indumentum villous; adaxial indumentum villous (trichomes with substantially thickened bases). Cauline leaves linear, 30–40 mm long and 2–4 mm wide, base amplexicaul, margin revolute and with stipitate glands, apex acute and mucronate; abaxial indumentum occasionally villous and with stipitate glands, midvein with stipitate glands; adaxial surface with stipitate glands. Foliaceous bracts subtending capitula 5–7 mm long, margin fimbriate and with stipitate glands. Flowering stems branched, capitula 25–30 mm in diameter (including phyllaries), terminal. Outer phyllary claw semi-terete and with stipitate glands abaxially; phyllary lamina pink or white, basal margin fimbriate, abaxial surface glabrous, apex subulate to cuspidate. Medial phyllary claw flattened, and with stipitate glands abaxially; phyllary lamina white or occasionally pink, apex subulate (irregularly incised). Stylar appendages ovate. Cypsela cylindrical to oblongoid, 1.75 mm long and 0.5 mm in diameter, cross-section cylindrical, pericarp translucent or cream, idioblasts present. Pappus persistent, ~5.5 mm long.
Widespread in the Brigalow Belt South and Brigalow Belt North bioregions, Queensland (Fig. 19).
Occurring in several conservation reserves and National Parks, and common in suitable habitats across the distribution; we recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Leichhardt: 19.7 km E of Jericho, 20 July 1975, A.D.Chapman 1277 (BRI, CANB*, K, L); Marston Station, NE of Blackall and S of Jericho, 5 Nov. 2014, C.D.Kilgour 2210 (BRI!); Blackdown Tableland National Park, 19 Nov. 2018, T.L.Collins 1138 (BRI!, CANB!, NE!); Dawson Range, 13 June 1977, M.D.Crisp 3005 (BM, BRI, CANB*); Expedition Range 23 km W of Bauhinia Downs, 8 Feb. 1961, R.W.Johnson 2048 (BRI!); Lonesome National Park [Expedition National Park, Lonesome section], 25 Aug. 1990, D.L.Jones 6301 & B.E.Jones (CANB*); on Nathan Gorge Road 22.5 K [22.5 km] from Cracow, 15 July 1990, P.I.Forster PIF7058 (BRI!, CANB, MEL, PERTH); Isla Gorge, near lookout, 27 Sep. 2000, I.R.Telford 12423, J.J.Bruhl & L.M.Copeland (NE!); Messmate Mountain, Langtree paddock, ‘Toondahra’, 17 Mar. 1984, P.I.Forster 1744 (BRI!). Maranoa: Chesterton Range, 9 Sep. 1993, R.W.Purdie 4412 (CANB*).
Coronidium gnaphalioides (Domin) Jeanes, Muelleria 39: 109 (2021)
Podolepis gnaphalioides Domin, Biblioth. Bot. 22(89): 1230 (1930). Type citation: QUEENSLAND. ‘Mt Remarkable sowie in den Savannenwäldern bei Pentland (DOMIN III. 1910)’. Type: Mt Remarkable, iii.1910, K.Domin 9049 (lecto, fide J.A. Jeanes Muelleria 39: 109 (2021): PR 531701*); in xerodrymic apud opp Pentland, iii.1910, K.Domin 9050 (residual syn: PR 531702*).
Coronidium lanosum Paul G.Wilson, Nuytsia 18: 309 (2008). Type: Mt King, ~96 km NNE of Hughenden, Queensland, 1 Apr. 1998, E.J.Thompson HUG692 (holo: BRI AQ0573739*).
Helichrysum sp. (Belyando River V.J.Neldner + 3459): A.E.Holland in R.J.F.Henderson (ed.), Queensland Vasc. Pl.: Names & Dist. 39 (1994).
Erect, perennial, tap-rooted, herb. Stems and branches tomentose to woolly, internode length 10–20 mm. Basal leaf rosette absent at flowering. Seedling leaves oblanceolate, obovate, or spathulate, 20–40 mm long and 7–10 mm wide, base amplexicaul and attenuate, margin felted to tomentose, apex acute and mucronate; abaxial indumentum felted, midvein indumentum felted; adaxial indumentum tomentose. Cauline leaves oblanceolate to obovate, 20–35 mm long and 7–10 mm wide, base amplexicaul and attenuate, apex acute and mucronate, margin woolly; abaxial indumentum felted to tomentose, midvein indumentum felted to tomentose; adaxial indumentum felted to tomentose. Foliaceous bracts subtending capitula absent. Flowering stems branched, capitula 12–18 mm in diameter (including phyllaries), terminal. Outer phyllary claw semi-terete, and with stipitate glands abaxially; phyllary lamina translucent and straw-coloured, basal margin fimbriate, abaxial surface glabrous, apex subulate. Medial phyllary claw semi-terete and with stipitate glands on abaxial surface; phyllary lamina abaxially yellow to straw-coloured, apex subulate (minutely incised). Stylar appendages narrowly triangular. Cypsela cylindrical to oblongoid, 1.5 mm long and 0.5 mm in diameter Pericarp translucent or cream (with maroon seed coat visible), idioblasts present. Pappus persistent, ~3 mm long.
Distributed through a broad region of central Queensland from west of Mackay to the Einasleigh Uplands Bioregion west of Townsville, Queensland (Fig. 17).
Most known populations are outside conservation areas and are on private pastoral land and road reserves. While C. gnaphalioides occurs in low-productivity rangeland habitats, the restricted distribution suggests that this species may be seriously threatened in the future by land clearing and careless land management, and therefore must be regarded as ‘Vulnerable’ under the International Union for Conservation of Nature and Natural Resources (2022), Category VU D1.
QUEENSLAND: North Kennedy: 30 km SW of Greenvale, 31 May 2000, R.Cumming 19607 (BRI*); Pretty Plains Station, Mount King, 3 July 2018, T.L.Collins 1069 & J.J.Bruhl (BRI!, CANB!, NE!); near source of Poison Creek, 11 Apr. 1935, S.T.Blake 8548 (BRI*); Mount Sturgeon Station, 13 Nov. 1931, C.E.Hubbard 7574 & C.Winders (BRI*); White Mountains National Park, 23 June 1992, A.R.Bean 4638 (BRI*); White Mountains National Park, 11 Apr. 2000, M.B.Thomas 1581 & E.J.Thompson (BRI*). South Kennedy: 14.8 km N of Belyando River crossing, 15 May 1991, V.J.Neldner 3459 & E.J.Thompson (BRI*); ‘Redcliffe Vale’ W of Mackay, 12 Apr. 2012, A.R.Bean 31809 & I.Champion (BRI*); 20 km E of Mount Coolon tip, 26 Nov. 2010, S.Hardy HMC1 (BRI*).
Coronidium lanuginosum (A.Cunn. ex DC.) Paul G.Wilson, Nuytsia 18: 313 (2008)
Helichrysum lanuginosum A.Cunn. ex DC., Prodr. 6: 193 (1838); Helichrysum albicans var. lanuginosum (A.Cunn. ex DC.) Domin, Biblioth. Bot. 22(89): 1222 (1930). Type: Rodds Bay, Queensland, May 1819, A.Cunningham 110 (holo: G-DC G00470649*; iso: CANB 00436693* [as A. Cunningham 266]).
Helichrysum boormanii var. tryonii Domin, Biblioth. Bot. 22(89): 1224 (1930). Type: South Percy Island, Queensland, 5 Mar. 1906, H.Tryon (first-step lecto, fide Paul G.Wilson Nuytsia 18: 313 (2008); no herbarium indicated: ? n.v.; isolecto: BRI 364942); Port Denison, Queensland, E.Fitzalan (residual isosyn: MEL 262825).
Helichrysum boormanii var. gillivrayi Domin, Biblioth. Bot. 22(89): 1224 (1930). Type: Queensland, Port Curtis, November 1847, John MacGillivray ‘Voyage of Rattlesnake, Botany No. 52.’ (holo?: n.v.).
Helichrysum elatum var. fraseri Benth., Fl. Austral. 3: 621 (1867), p.p. [as to the J.MacGillivray collection cited, not as to lectotype, fide Paul G.Wilson Nuytsia 18: 313 (2008)]. Type: Port Curtis, M’Gillivray (residual syn: K n.v.).
Helichrysum boormanii Maiden & Betche, Proc. Linn. Soc. New South Wales 30: 366 (1905), p.p. [as to the syntype, not as to lectotype, fide Paul G.Wilson Nuytsia 18: 313 (2008)]. Type: Atherton, Queensland, Aug. 1901, E.Betche (residual syn: BRI 364944, NSW 519160).
Erect, perennial, tap-rooted, shrub or subshrub. Stems and branches cobwebby, villous, or woolly, and with stipitate glands; internode length 1–20 mm. Basal leaf rosette absent at flowering. Seedling leaves oblanceolate, 30–60 mm long and 6–10 mm wide, base amplexicaul and attenuate, apex acute to apiculate, and mucronate; margin cobwebby to hirsute, and with stipitate glands; abaxial indumentum scattered villous trichomes, and with stipitate glands, midvein indumentum cobwebby and with stipitate glands; adaxial indumentum villous, hispid, and with stipitate glands. Cauline leaves ovate, obovate, or elliptic; 25–80 mm long and 6–20 mm wide, base amplexicaul and attenuate, apex cuspidate to acuminate, and mucronate; margin wavy, revolute or entire, cobwebby, villous to woolly, or hairless and with stipitate glands; abaxial indumentum villous and with stipitate glands, midvein indumentum villous and with stipitate glands; adaxial indumentum villous and with stipitate glands. Foliaceous bracts subtending capitula absent (scarious bracts proceeding down peduncle). Flowering stems branched, capitula 25–40 mm in diameter (including phyllaries), terminal. Outer phyllary claw semi-terete and with stipitate glands abaxially; phyllary lamina white, basal margin fimbriate and hispid, abaxial surface villous to glabrous (coastal populations almost glabrous), apex subulate and fimbriate. Medial phyllary claw semi-terete, villous and with stipitate glands on abaxial surface; phyllary lamina abaxially white, apex subulate (minutely incised). Stylar appendages narrowly triangular. Cypsela cylindrical to oblongoid, 1.5 mm long and 0.5 mm in diameter, cross-section cylindrical, pericarp translucent or cream (with pale yellow seed coat visible), idioblasts present. Pappus persistent, ~6 mm long.
Widespread through eastern Queensland through the Cape York Peninsula, Wet Tropics, South Eastern Queensland, Einasleigh Uplands, Brigalow Belt North, and Central Mackay Coast bioregions, Queensland (Fig. 19).
Rocky headlands in grassy herbfields on the coast, and on rocky and stony hills in shrubby and grassy eucalypt woodlands further inland.
Leaf indumentum with continuous variation in septate hairs from glabrous to villous seen on some specimens on the same plant. Attractive white phyllaries and a variety of growth forms, including compact forms on coastal headlands, suggest excellent horticultural potential.
Although Wilson (2008: 313) selected the H.Tryon gathering from South Percy Island as lectotype of Helichrysum boormanii var. tryonii, he did not indicate in which herbarium the lectotype was held, citing only ‘isolecto: BRI 364942’. This is here considered to be an effective first-step lectotypification by Wilson (ICN Art. 9.17, Shenzhen Code; Turland et al. 2018).
Broadly distributed and found in many conservation reserves and National Parks along the coast. Inland populations are recorded in conservation reserves, and on road reserves and pastoral properties. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Cook: N of Spencer Creek crossing on road to Windsor Tableland, 27 May 1989, D.L.Jones 4425 & M.A.Clements (CANB*, PERTH). North Kennedy: Blackbraes National Park, S side of unnamed crater, 2 July 2018, T.L.Collins 1068 & J.J.Bruhl (BRI!, CANB!, NE!); Kennedy Development Road, ~19.1 km S of Einasleigh River, 2 July 2018, T.L.Collins 1066 & J.J.Bruhl (BRI!, CANB!, NE!). Port Curtis: Bruce Highway, Clairview, 17 Nov. 2018, T.L.Collins 1135 (BRI!, CANB!, NE!); Byfield National Park, Stockyard Point, 15 Nov. 2018, T.L.Collins 1130 (BRI!, CANB!, NE!); Byfield National Park, Waterpark Point, 16 Nov. 2018, T.L.Collins 1132 (BRI!, CANB!, NE!); Bruce Highway, ~1.5 km S of Canoona Obelisk, 18 Nov. 2018, T.L.Collins 1136 (BRI!, CANB!, NE!); Turkey Creek on Rosedale Road N of Bundaberg, 4 Apr. 1975, L.A.Craven 3137 (BRI, CANB*, DNA, L, PERTH); Many Peaks Range, Blackman Gap, 14 Aug. 1996, I.R.Telford 12289 & S.Donaldson (BRI, NE!, NSW). Wide Bay: Kalpowar – Gin Gin Road, Kolan River catchment, 21 June 2011, P.I.Forster 38235 (BRI!); 1 km SE of Fairlies Knob, Seaview Range, 29 May 2000, S.P.Phillips 403 (BRI!); Bluff Mountain Range, W side of Mount Walsh National Park, 15 May 2019, P.Young 2471 (BRI!).
Coronidium newcastlianum (Domin) Paul G.Wilson, Nuytsia 18: 314 (2008)
Helichrysum newcastlianum Domin, Biblioth. Bot. 22(89): 1224 (1930). Type: Newcastle Range, [Queensland], s. dat. [1856], F.Mueller s.n. (holo: n.v.; iso: MEL 2116945*).
Erect, perennial, tap-rooted, herb. Stems and branches felted. Basal leaf rosette absent at flowering. Seedling leaves oblanceolate to obovate (with winged petioles), 15–70 mm long and 8–20 mm wide, base amplexicaul and attenuate, apex obtuse and mucronate, margin felted; abaxial indumentum felted, midvein indumentum felted; adaxial indumentum tomentose. Cauline leaves oblanceolate to obovate, 30–80 mm long and 10–20 mm wide, base amplexicaul and attenuate, apex apiculate and mucronate, margin felted; abaxial indumentum felted, midvein indumentum felted; adaxial indumentum felted to tomentose. Foliaceous bracts subtending capitula ~5 mm long or absent, margin felted. Flowering stems branched, capitula 40–50 mm in diameter (including phyllaries), terminal. Outer phyllary claw sessile; phyllary lamina white or occasionally pink in bud, basal margin fimbriate and hispid, abaxial surface glabrous, apex subulate (occasionally incised). Medial phyllary terete; phyllary lamina abaxially white, apex subulate to cuspidate. Stylar appendages clavate to ovate. Cypsela cylindrical to oblongoid, 1.5 mm long and 0.75 mm in diameter, cross-section cylindrical, pericarp translucent or cream, idioblasts present. Pappus persistent, ~5 mm long.
Plants from populations in the south west of the distribution at Undara Volcanic National Park and the Newcastle Range have a winged petiole. Plants from populations on the Atherton Tablelands have sessile leaves. Intergrades have been collected in the Mount Garnet area and at Millstream Falls National Park. Beautiful white phyllaries, silvery leaves and compact growth habit seen in some populations provide great horticultural potential.
Populations of plants with coppery bronze phyllaries varying in length and occurring near Herberton on the Atherton Tableland, are evidently a hybrid between C. newcastlianum and C. rupicola. These have previously been treated as C. fulvidum Paul G.Wilson.
Occurs along the Great Dividing Range in the Einasleigh Uplands Bioregion of Queensland (Fig. 17).
The species is widespread and common, recorded in conservation reserves with populations of hundreds to thousands of mature individuals, and thus is considered to be of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Cook: Mount Mulligan, 12 Apr. 1984, J.R.Clarkson 5289 (BRI!, DNA, K, MEL, MO, NSW, PERTH, QRS); Herberton Range Ridge Road, 27 June 2018, T.L.Collins 1050 & J.J.Bruhl (BRI!, CANB!, NE!); Atherton–Herberton road, 27 June 2018, T.L.Collins 1053 & J.J.Bruhl (BRI!, CANB!, NE); Atherton–Herberton road, 27 June 2018, T.L.Collins 1054 & J.J.Bruhl (BRI!, CANB!, NE!); Atherton–Herberton road, 27 June 2018, T.L.Collins 1055 & J.J.Bruhl (BRI!, CANB!, NE!); Herberton, track to Anniversary Falls, 27 June 2018, T.L.Collins 1056 & J.J.Bruhl (BRI!, CANB!, NE!); Herberton, track to Anniversary Falls, 27 June 2018, T.L.Collins 1057 & J.J.Bruhl (BRI!, CANB!, NE!); Millstream National Park, S of Ravenshoe, 9 May 2005, P.I.Forster 30893 & K.R.McDonald (BRI!); 30.6K W of Mt Garnet, 26 May 1967, D.E.Symon 4897 (ADW, BRI!, CANB, K, MSC); Undara Volcanic National Park, 1 July 2018, T.L.Collins 1064 & J.J.Bruhl (BRI!, CANB!, NE!); Newcastle Range, 19.7 km W of Einasleigh, 2 July 2018, T.L.Collins 1065 & J.J.Bruhl (BRI!, CANB!, NE!).
Coronidium oxylepis (F.Muell.) Paul G.Wilson, Nuytsia 18: 302 (2008)
Helichrysum oxylepis F.Muell., Fragm. 1: 35 (1858); Helichrysum collinum var. oxylepis (F.Muell.) Maiden & Betche, Census New South Wales Pl. 202 (1916). Type: Moreton Island [Queensland], Aug. 1855, F.Mueller (holo: MEL 1586000*; iso: K 000899129*, NSW 230764*).
Erect or decumbent, perennial, tap-rooted, herb. Stems and branches cobwebby to tomentose, villous or glabrescent, internode length 2–20 mm. Basal leaf rosette present or absent at flowering. Seedling leaves spathulate, 40–70 mm long and 7–15 mm wide; base truncate, amplexicaul or auriculate; margin glabrous, cobwebby, hirsute or hispid; apex acute or apiculate, and mucronate; abaxial leaf surface glabrous or hispid; midvein indumentum glabrous, cobwebby, hirsute, or hispid; adaxial leaf surface glabrous or with scattered hispid trichomes. Cauline leaves spathulate, or lanceolate to oblanceolate, or linear to lanceolate, 20–150 mm long and 3–20 mm wide; base truncate, or amplexicaul and attenuate; margin entire, cobwebby, villous, hirsute, hispid, or glabrous; apex acute, obtuse, apiculate or subulate, and mucronate; abaxial indumentum tomentose and with stipitate glands; midvein indumentum villous, cobwebby, hirsute, hispid, or glabrescent; adaxial indumentum cobwebby, hispid, and with stipitate glands. Foliaceous bracts subtending capitula ~5 mm long or absent. Flowering stems branched or unbranched, capitula 20–35 mm in diameter (including phyllaries), terminal. Outer phyllary claw cross-section concave, or semi-terete, and with stipitate glands abaxially; phyllary lamina straw-coloured to translucent, basal margin fimbriate or hispid, abaxial surface glabrous or with scattered stipitate glands; apex acute, cuspidate or subulate, and fimbriate. Medial phyllary claw cross-section concave to flattened, or semi-terete, and with stipitate glands abaxially; phyllary lamina abaxially straw- to bronze-coloured, apex subulate. Stylar appendages narrowly triangular. Cypsela oblongoid, 1.75–2 mm long and 0.5 mm in diameter, pericarp translucent or cream, idioblasts present. Pappus ~4–5.5 mm long, persistent or breaking evenly ~0.25 mm above pappus–pericarp union.
Coronidium oxylepis (F.Muell.) Paul G.Wilson subsp. oxylepis
Erect herb. Stems and branches cobwebby to tomentose, internode length 5–10 mm. Basal leaf rosette present or absent at flowering. Seedling leaves spathulate, base amplexicaul; margin cobwebby, hirsute, or hispid; apex apiculate and mucronate; abaxial leaf surface glabrous or hispid, midvein indumentum cobwebby, hirsute, or hispid; adaxial leaf surface almost glabrous but for scattered hispid trichomes. Cauline leaves linear to lanceolate, 40–150 mm long and 5–12 mm wide, base truncate; margin entire, cobwebby, hirsute, or hispid; apex acute and mucronate; abaxial surface with stipitate glands, midvein indumentum cobwebby, hirsute, hispid, or glabrescent; adaxial indumentum cobwebby, hispid, and with stipitate glands. Foliaceous bracts subtending capitula absent. Flowering stems branched or unbranched, capitula 30–35 mm in diameter (including phyllaries), terminal. Outer phyllary claw cross-section concave to semi-terete, and with stipitate glands abaxially; phyllary lamina straw-coloured, basal margin fimbriate, abaxial surface glabrous, apex acute and fimbriate. Medial phyllary claw cross-section semi-terete, and with stipitate glands on abaxial surface; phyllary lamina abaxially bronze-coloured, apex subulate. Stylar appendages narrowly triangular. Cypsela oblongoid, 2 mm long and 0.5 mm in diameter. Pappus ~5 mm long.
Restricted to the coastal strip from the Sunshine Coast in southern Queensland in the South Eastern Queensland Bioregion to northern New South Wales near Grafton (Fig. 20).
Recorded flowering April–October, but may continue flowering into early summer in wetter seasons.
Field observations suggest occasional disturbance may be required for germination and recruitment.
Only seven populations have been recorded, and six of these have not been seen for 50–100 years. Populations at Wardell and Jerusalem Creek were not visited, whereas those on Moreton Island and near Byron Bay could not be relocated, possibly owing to past sand mining and recent coastal development. Given the population size of fewer than 100 plants at the most recently recorded population near Minnie Waters, and the possibility that other populations have been lost, this subspecies must be regarded as ‘Endangered’ under the International Union for Conservation of Nature and Natural Resources (2022), Categories EN A1, A2 and A3, or may further be categorised as ‘Critically Endangered’ under CR B1. We strongly urge that further survey work be conducted in Queensland and New South Wales to confirm conservation status and allow development of recovery plans.
NEW SOUTH WALES: North Coast: 1 mile [~1.6 km] N of Pottsville, 1 Sep. 1972, R.Coveny 4402 (BRI!, NSW!); ~1 mile [~1.6 km] S of Byron Bay, 11 Oct. 1969, R.D.Hoogland 11669 (NSW!); Tallow Beach, Byron Bay, 26 Aug. 1973, N.S.Lander 305 (NSW!); Wardell, Oct. 1893, leg. ign. (NSW 518891!); Jerusalem Creek, between Evans Head and Iluka, 27 Sep. 1967, D.J.McGillivray 2702 (NSW!); 4 miles [~6.4 km] S of Yamba, 30 June 1966, D.J.McGillivray 2137 (NSW!); Yuraygir National Park, 13 Apr. 2017, T.L.Collins 960, J.J.Bruhl & I.R.Telford (BRI!, CANB!, NE!, NSW!); Yuraygir National Park, 1 Oct. 2017, T.L.Collins 972 (BRI!, CANB!, NE!, NSW!).
Coronidium oxylepis subsp. carnosum Paul G.Wilson, Nuytsia 18: 304 (2008)
Type: Queensland: Wide Bay: Fraser Island, Eli Creek ~4 miles [~6.4 km] N of Happy Valley on eastern side of island; sandy dune on northern side of creek, 3 May 1967, P.Baxter 813 (holo: BRI AQ248463*).
Helichrysum oxylepis subsp. (Fraser Island P. Baxter 813): A.E. Holland in P.D.Bostock & A.E.Holland (eds), Census Queensland Fl. 43 (2007).
Decumbent or prostrate, perennial, tap-rooted, herb. Stems and branches villous or glabrescent, and with scattered stipitate glands; internode length 2–5 mm. Basal leaf rosette absent at flowering. Seedling leaves spathulate, 40–70 mm long and 7–15 mm wide, base amplexicaul or auriculate, margin glabrous (scattered stipitate glands towards base), apex apiculate and mucronate; abaxial leaf surface glabrous, midvein glabrous (scattered stipitate glands towards base); adaxial leaf surface glabrous. Cauline leaves spathulate, 30–100 mm long and 8–20 mm wide, base amplexicaul and attenuate; margin entire, glabrous, or with scattered villous hairs and stipitate glands; apex obtuse to apiculate; abaxial leaf surface with scattered stipitate glands, midvein glabrous or with occasional villous hairs; adaxial leaf surface with scattered stipitate glands. Foliaceous bracts subtending capitula absent. Flowering stems branched, capitula 20–30 mm in diameter (including phyllaries), terminal. Outer phyllary claw cross-section concave, and with stipitate glands abaxially; phyllary lamina translucent or straw-coloured, basal margin fimbriate and hispid, abaxial surface glabrous, apex subulate and fimbriate. Medial phyllary claw cross-section concave to flattened, and with stipitate glands on abaxial surface; phyllary lamina abaxially straw- to bronze-coloured, apex subulate. Stylar appendages narrowly triangular. Cypsela oblongoid, 2 mm long and 0.5 mm in diameter, pericarp translucent or cream, idioblasts present. Pappus ~4 mm long, persistent or breaking evenly ~0.25 mm above pappus–pericarp union.
South Eastern Queensland Bioregion, from Wide Bay to near Coolum (Fig. 20).
Coastal dunes and sand blows in grassy shrublands, or on foredunes above the high-water mark.
Label data on S.L.Everist 7755 (BRI) states ‘involucral bracts white’ but examination of the specimen shows straw- or bronze-colour, suggesting an artefact of drying and storage, or an error on the label. Specimens with longer, narrower leaves and cobwebby to tomentose stem and leaf indumentum (e.g. T.L.Collins 1121 [BRI, CANB, NE] and B.L.Turner 5625 [BRI]) have morphology intermediate with C. oxylepis subsp. oxylepis and may represent morphological variation or hybrids.
Although restricted to the narrow coastal strip of the South Eastern Queensland Bioregion, many populations of hundreds of mature individuals were recorded in conservation reserves in 2018. Some populations near Peregian, Queensland, may be at risk from coastal development, but this was not confirmed. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Wide Bay: Deepwater National Park, Wreck Rocks, 14 Nov. 2018, T.L.Collins 1127 (BRI!, CANB!, NE!); Fraser Island, N side of Woralie Road on 75 Mile Beach, 13 Nov. 2018, T.L.Collins 1124 & I.R.Telford (BRI!, CANB!, NE!); Fraser Island, Woralie Creek area, 13 Nov. 2018, T.L.Collins 1122 & I.R.Telford (BRI!, CANB!, NE!); Fraser Island, ~2 km N of Happy Valley, 12 Nov. 2018, T.L.Collins 1121 & I.R.Telford (BRI!, CANB!, NE!); Fraser Island, ~6 km N of Eurong along 75 Mile Beach, 12 Nov. 2018, T.L.Collins 1119 & I.R.Telford (BRI!, CANB!, NE!); Kings Bore, ~8 miles [~12.8 km] SSW of Double Island Point, 21 Oct. 1964, S.L.Everist 7755 (BRI!); Alexandria Bay, Noosa National Park, 16 July 1985, P.R.Sharpe 3794 (BRI!); Peregian, in dune sand, 6 Oct. 1965, B.L.Turner 5625 (BRI!).
Coronidium oxylepis subsp. lanatum Paul G.Wilson, Nuytsia 18: 304 (2008)
Type: 12 miles [~19 km] N of Glenhaughton HS [Homestead], Leichhardt District, Q’ld. [Queensland], 14 Oct. 1963, N.H.Speck 1849 (holo: CANB 137894!).
Helichrysum oxylepis subsp. (Thulimbah R.W.Johnson 2918): A.E.Holland in A.E.Holland & P.D.Bostock (eds), Census Queensland Fl. 2007 43 (2007).
Coronidium sp. Penrose (C.Burgess 7/Nov/1968) NE Herbarium: CHAH, Austral. Pl. Census (2020) [accessed 20 Feb. 2020].
Coronidium sp. Lanatum (N.H.Speck 1849) NE Herbarium: CHAH, Austral. Pl. Census (2020) [accessed 20 Feb. 2020].
[Helichrysum collinum auct. non DC.: G.Bentham, Fl. austral. 3: 623 (1866), p.p. (only as to the New South Wales specimens cited)].
Erect, perennial, tap-rooted, herb. Stems and branches cobwebby to tomentose, internode length 10–20 mm. Basal leaf rosette usually absent at flowering, occasionally present. Seedling leaves spathulate, base amplexicaul; margin cobwebby or hirsute; apex apiculate and mucronate. Cauline leaves oblanceolate to lanceolate, 20–60 mm long and 3–15 mm wide, base amplexicaul and attenuate, margin entire and villous, apex subulate and mucronate; abaxial indumentum tomentose and with stipitate glands, midvein indumentum villous; adaxial indumentum tomentose to villous with septate hairs. Foliaceous bracts subtending capitula ~5 mm long with margin hispid and stipitate glands, or absent. Flowering stems branched, capitula 20–30 mm in diameter (including phyllaries), terminal. Outer phyllary claw cross-section semi-terete, and with stipitate glands abaxially; phyllary lamina translucent or straw-coloured, basal margin hispid, abaxial surface glabrous, apex cuspidate to acute, and fimbriate. Medial phyllary claw cross-section semi-terete and with stipitate glands abaxially; phyllary lamina abaxially bronze-coloured, apex subulate. Stylar appendages narrowly triangular. Cypsela cylindrical to oblongoid, 1.75 mm long and 0.5 mm in diameter, pericarp translucent or cream (with yellow seed coat visible), idioblasts present. Pappus ~4.5–5.5 mm long, persistent or breaking evenly ~0.25 mm above pappus–pericarp union.
Widespread across the Brigalow Belt South, South Eastern Queensland, in Queensland, and New England Tableland, Nandewar, Sydney Basin, and the South Eastern Highlands bioregions in New South Wales (Fig. 20).
Variation in leaf size and indumentum, and in capitulum size was not congruent with molecular data, suggesting a recently evolved group or recent rapid expansion. Here, tentatively maintained within the circumscription of Coronidium oxylepis subsp. lanatum following Wilson (2008), the phrase-named groups of populations, Helichrysum sp. Thulimbah (R.W. Johnson 2018) and Coronidium sp. Penrose (C.Burgess 7/Nov/1968) NE Herbarium, need further investigation by denser sampling across the subspecies. See ‘Putative entities of Coronidium requiring further study’ below. We intend to focus further analyses to test the listed putative entities.
Populations in the hundreds and thousands were recorded in conservation reserves or on road reserves in 2018. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Leichhardt: towards Springsure on Tambo–Springsure road, 27 Sep. 2005, M.B.Thomas 3045 (BRI!); Expedition Range, 22 km from Bauhinia Downs roadhouse, 9 Nov. 1993, I.R.Telford 11891 (BRI!); Coorada Station ~200 m SW of Fitzroy Developmental Road, 20 Nov. 2018, T.L.Collins 1139 (BRI!, CANB!, NE!); 5.3 km W of Abercorn, S of Monto, 11 Sep. 1999, A.R.Bean 15317 (BRI!); Cracow–Taroom Road, 21 Nov. 2018, T.L.Collins 1140 (BRI!, CANB!, NE!); State Forest 302 Barakula, 23 Apr. 1994, P.I.Forster 15166 (BRI!). Maranoa: 12 km ENE of Miles, 10 July 2011, B.McLennan 100711–1 (BRI!). Darling Downs: Mount Norman Road, 5 Nov. 2018, T.L.Collins 1099 & I.R. Telford (BRI!, CANB!, NE!); Pegum Road near Stanthorpe, 5 Nov. 2018, T.L.Collins 1100 & I.R. Telford (BRI!, CANB!, NE!); Bald Rock Creek valley, Girraween National Park, 25 Nov. 2018, I.R.Telford 13536 (BRI!, CANB!, NE!). NEW SOUTH WALES: Northern Tablelands: Torrington State Recreation Area, Tin Pot Gully, 25 Jan. 2002, L.M.Copeland 3334, D.Clark & P.J.Clarke (NE!); Roberts Range, ~27 km WNW of Tenterfield, 20 Dec. 2006, I.R.Telford 13134, M.Badham, J.J.Bruhl & D.Caldwell (NE!); Torrington State Recreation Area, 9 Jan. 1997, C.E.Nano 24 & L.M.Copeland (NE!). Southern Tablelands: Penrose, Wingecarribee, 7 Nov. 1968, C.Burgess s.n. (CBG 25693* [in CANB]); Hume Highway dual carriageway, ~1.6 km N of Paddys River bridge, 15 Dec. 2017, T.L.Collins 1004 (CANB!, NE!, NSW!). Central Tablelands: 13 km E of Braidwood, 26 Jan. 2019, T.L.Collins 1172 (CANB!, NE!, NSW!). AUSTRALIAN CAPITAL TERRITORY: Black Mountain, Frith Road on road verge, 6 Dec. 2017, T.L.Collins 990 (CANB!, NE!, NSW!).
Coronidium rupicola (DC.) Paul G.Wilson, Nuytsia 18: 306 (2008)
Helichrysum rupicola DC., Prodr. 6: 190 (1838); Gnaphalium rupicola (DC.) Sch.Bip., Bot. Zeitung (Berlin) 3: 171 (1845). Type: Cape Cleveland, E Australia [Queensland], June 1819, A.Cunningham 89 (holo: G-DC G00470591*; possible iso: BRI AQ0594209, CANB 436696.1, MEL 1585998*).
Helichrysum collinum DC., Prodr. 6: 190 (1838); Gnaphalium endeavourense Sch.Bip. Bot. Zeitung (Berlin) 3: 171 (1845) [non G. collinum Labill. (1806)]. Type citation: ‘in collibus ad ripas flum. Endeavour (lat. 15°) et ad Port Bowen (lat. 23½ °) in Nova Hollandia tropica legit cl. A. Cunningham’, (holo: G-DC G00470637).
Helichrysum rupicola var. danesii Domin, Biblioth. Bot. 22(89): 1225 (1930). Type citation: ‘Walsh Pyramid, DR. J. DANEŠ I. 1910.’ (holo: PR 531623 fide P.G. Wilson Nuytsia 18: 306 (2008)).
Erect, perennial, tap-rooted herb. Stems and branches cobwebby to tomentose, internode length 5–10 mm. Basal leaf rosette absent at flowering. Seedling leaves spathulate, 30–60 mm long and 6–10 mm wide, base amplexicaul and auriculate, margin cobwebby, apex acute and mucronate; abaxial indumentum tomentose, midvein indumentum tomentose; adaxial indumentum villous and floccose. Cauline leaves lanceolate, 50–120 mm long and 7–15 mm wide, base amplexicaul and auriculate, apex acute to apiculate, and mucronate; margin revolute and cobwebby; abaxial indumentum felted and with stipitate glands, midvein indumentum tomentose; adaxial indumentum villous and floccose. Foliaceous bracts subtending capitula 4–6 mm long, margin villous to woolly. Flowering stems branched or unbranched, capitula 15–25 mm in diameter (including phyllaries), terminal. Outer phyllary claw cross-section flattened, slender, puberulous and with stipitate glands abaxially; phyllary lamina bronze-coloured and opaque, basal margin villous to cobwebby, abaxial surface glabrous, apex subulate and fimbriate. Medial phyllary claw flattened, with stipitate glands on abaxial surface and on margin; phyllary lamina abaxially bronze-coloured, apex subulate. Stylar appendages ovate. Cypsela cylindrical to oblongoid, 1.5 mm long and 0.5 mm in diameter, pericarp translucent or cream, idioblasts present. Pappus ~3.5 mm long, persistent or breaking evenly ~0.25 mm above pappus–pericarp union.
Widespread in eastern Queensland in the Einasleigh Uplands, Wet Tropics and Cape York Peninsula bioregions (Fig. 17).
Populations with coppery- or bronze-coloured phyllaries varying in length and occurring near Herberton on the Atherton Tableland, are evidently a hybrid between C. newcastlianum and C. rupicola. These have previously been treated as C. fulvidum Paul G.Wilson.
The possible isotypes of Helichrysum rupicola in CANB (CANB 436696.1) and MEL (MEL 1585998) are cited by Wilson (2008: 306) as isotypes of ‘A. Cunningham 267’. The BRI (BRI AQ0594209) specimen is another element of this gathering. Wilson cited the holotype in G-DC with the same number and indicates that he has seen a microfiche photo of it. The high-resolution image of the G-DC sheet (G-DC G00470591) does not have the number 267, but rather has ‘89’ on the field tag in Cunningham’s handwriting. Although we cannot explain the differences in numbering, Allan Cunningham visited Cape Cleveland only during June 1819 on the voyage of the Mermaid (Curry et al. 2002). Despite the discrepancies in numbering, it seems likely that Cunningham made only one gathering of the species at Cape Cleveland and that all elements mentioned above are parts of that gathering. Nevertheless, the typification of the species should be investigated further.
Populations in the hundreds were recorded in conservation reserves or on road reserves in 2018. We recommend a status of ‘Least Concern’ (International Union for Conservation of Nature and Natural Resources 2022).
QUEENSLAND: Cook: Shiptons Flat, 4 Sep. 1948, L.J.Brass 20008 (A, CANB*); Saddle Hill, Macalister Range, 27 May 1972, J.Wrigley NQ 62 & I.R.Telford (CANB*); Davies Creek National Park, 1 July 2018, T.L.Collins 1062 & J.J.Bruhl (BRI!, CANB!, NE!); Gillies Highway between Lake Barrine and Little Musgrave, 11 Dec. 2010, R.W.Purdie 7986 (BRI, CANB*, CNS); Lakes Drive, Lake Eacham, 27 June 2018, T.L.Collins 1060, J.J.Bruhl & J.R.Clarkson, (BRI!, CANB!, NE!); State Forest Reserve 99, W of Atherton, 14 Oct. 1959, D.I.Bevege 233 (CANB*, NE!); Herberton Range Ridge Road, 27 June 2018, T.L.Collins 1052 & J.J.Bruhl (BRI!, CANB!, NE!). North Kennedy: Cardwell Range, 30 June 1983, I.R.Telford 9261 & G.Butler (AD, CANB*, MEL); Ewan–Paluma road, 4 July 2018, T.L.Collins 1072 & J.J.Bruhl (BRI!, CANB!, NE!); Paluma Range National Park, Little Crystal Creek, 11 Nov. 2006, J.J.Bruhl 2465 & I.R.Telford (NE!).
Coronidium sp. Thulimbah (R.W.Johnson 2918) NE Herbarium
Helichrysum oxylepis subsp. (Thulimbah R.W.Johnson 2918): A.E.Holland in A.E.Holland & P.D.Bostock (eds), Census Queensland Fl. 2007 27 (2007).
Coronidium sp. Penrose (C.Burgess 7/Nov/1968) NE Herbarium: CHAH, Austral. Pl. Census (2020) [accessed 20 Feb. 2020]
Coronidium sp. Russell Island (A.R.Field 5206) NE Herbarium
While this paper was in review, it came to our attention that additional gatherings of this entity had been made (A. R. Field, pers. comm.). Further collections are needed to determine possible morphological variation and taxonomic status. This entity appears to have close affinity to C. rupicola.
Data availability
Morphological data are available in the article and in the accompanying Supplementary material. SNP matrices are available from the first author upon request.
Conflicts of interest
Jeremy Bruhl is an associate editor for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
T. L. Collins gratefully acknowledges a Keith and Dorothy McKay Travelling Scholarship that enabled him to work at CANB; an Australian Biological Resources Study (ABRS) National Taxonomy Research Grant Program (NTRGP) Student Travel Grant to attend the 5th National Postgraduate Training Workshop in Systematics in Adelaide, Australia, and the joint conference of the Australasian Systematic Botany Society and the New Zealand Plant Conservation Network in Wellington, New Zealand; and a University of New England Strategic Scholarship and funding from the School of Environmental and Rural Science.
Acknowledgements
Access to the collection and facilities of the N.C.W. Beadle Herbarium (NE) have been crucial to this study. We thank the curators and staff of the following herbaria for loans of their specimens and access to their collections: AD, BRI, CANB, CNS, HO, MEL, NSW. We also thank the South Australian Seed Conservation Centre, the Australian PlantBank, Royal Tasmanian Botanical Gardens, the Australian National Botanic Gardens, and David Bateman at Randwick City Council for access to seedbanks and living collections. We thank the following Aboriginal Corporations for assistance in gaining permissions to enter Aboriginal land: Quandamooka Yoolooburrabee Aboriginal Corporation, Jabalbina Yalanji Aboriginal Corporation, and Darumbal Enterprises Pty Ltd. Thanks go to Robert Vidler and Herb Roberts for cultural advice and consultation with traditional owners of Wollumbin. Thanks go to Ilse Breitwieser, Mercè Galbany-Cassals, and Nicola Bergh, for constructive comments on the thesis-chapter version of the manuscript, and to Jennifer Tate and Anna Monroe for comments that improved the final version. Thanks go to Karen Wilson and Brendan Lepschi for helpful discussions on nomenclature. Field assistance was generously provided by Alex Buchanan, Mark Harris, Matthew Bashford, Michelle Haby, Bev Overton, John Hosking, and Tanya Howard. Very helpful assistance with field-collection planning was provided by Darren Crayn, Paul Forster, and John Clarkson. Collections were made under the following permits and licences: Queensland Government Permit No. WITK18639817; New South Wales permit number SL100305; South Australia permit number G25787-3; Tasmania permit number TFL17334. Thanks go to Norman Gaywood for help accessing UNE mathematics servers, Malcolm Lambert for help with SEM, Ian Simpson and Rhyan Gorman for help with glasshouse management, and Audrey Larsen for digital enhancement of type images. Use of the NCI high-performance computing cluster was supported by Intersect Merit Allocation ‘gh6’ to R. L. Andrew. The project ‘Integrative taxonomic revision of the everlasting daisy genera Xerochrysum and Coronidium (Asteraceae, Gnaphalieae)’ was supported through funding from the Australian Government’s Australian Biological Resources Study National Taxonomy Research Grant Program. T. L. Collins was supported by a University of New England Strategic Scholarship, and funding from the School of Environmental and Rural Science. Selected specimens of Leucozoma elatum subsp. elatum and Helichrysum leucopsideum were collected by the Ecosystem Surveillance subfacility and all rights pertaining to these specimens are claimed by the University of Adelaide. Specimens were made available for analysis by the Terrestrial Ecosystem Research Network (TERN), supported by the Australian Government through the National Collaborative Research Infrastructure Strategy and the Super Science Initiative. Data were provided under the TERN Attribution-Share Alike Data Licence v.1.0 which allows sharing of the data provided a user shares their new data and products in return. Specimens were provided under equivalent terms.
References
Abbott RJ (2017) Plant speciation across environmental gradients and the occurrence and nature of hybrid zones. Journal of Systematics and Evolution 55, 238-258.
| Crossref | Google Scholar |
Alam M, Neal J, O’Connor K, Kilian A, Topp B (2018) Ultra-high-throughput DArTseq-based silicoDArT and SNP markers for genomic studies in Macadamia. PLoS ONE 13, e0203465.
| Crossref | Google Scholar | PubMed |
Al-Beyroutiová M, Sabo M, Sleziak P, Dušinský R, Birčák E, Hauptvogel P, Kilian A, Švec M (2016) Evolutionary relationships in the genus Secale revealed by DArTseq DNA polymorphism. Plant Systematics and Evolution 302, 1083-1091.
| Crossref | Google Scholar |
Beaumont M, Barratt EM, Gottelli D, Kitchener AC, Daniels MJ, Pritchard JK, Bruford MW (2001) Genetic diversity and introgression in the Scottish wildcat. Molecular Ecology 10, 319-336.
| Crossref | Google Scholar | PubMed |
Chang Y, Ebihara A, Lu S, Liu H, Schneider H (2018) Integrated taxonomy of the Asplenium normale complex (Aspleniaceae) in China and adjacent areas. Journal of Plant Research 131, 573-587.
| Crossref | Google Scholar | PubMed |
Collins TL, Andrew RL, Bruhl JJ (2019) Morphological, phytochemical and molecular analyses define species limits in Eucalyptus magnificata (Myrtaceae) and lead to the discovery of a new rare species. Australian Systematic Botany 32, 12-28.
| Crossref | Google Scholar |
Collins TL, Bruhl JJ, Andrew RL, Telford IRH, Schmidt-Lebuhn AN (2022) Phylogenetic relationships of Xerochrysum, Coronidium and Helichrysum leucopsideum reveal a new genus, Leucozoma (Asteraceae: Gnaphalieae). Taxon 71, 1044-1064.
| Crossref | Google Scholar |
Dayrat B (2005) Towards integrative taxonomy. Biological Journal of the Linnean Society 85, 407-415.
| Crossref | Google Scholar |
de Queiroz K (2005) A unified concept of species and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences 4, 196-215.
| Google Scholar |
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879-886.
| Crossref | Google Scholar | PubMed |
De Salas MF, Schmidt-Lebuhn AN (2018) Integrative approach resolves the taxonomy of the Ozothamnus ledifolius (Asteraceae: Gnaphaliae) species complex in Tasmania, Australia. Phytotaxa 358, 117-138.
| Crossref | Google Scholar |
D’hoop BB, Paulo JM, Kowitwanich K, Sengers M, Visser RGF, van Eck HJ, van Eeuwijk FA (2010) Population structure and linkage disequilibrium unravelled in tetraploid potato. Theoretical and Applied Genetics 121, 1151-1170.
| Crossref | Google Scholar | PubMed |
Egea LA, Mérida-García R, Kilian A, Hernandez P, Dorado G (2017) Assessment of genetic diversity and structure of large garlic (Allium sativum) germplasm bank, by Diversity Arrays Technology ‘genotyping-by-sequencing’ platform (DArTseq). Frontiers in Genetics 8, 98.
| Crossref | Google Scholar |
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164, 1567-1587.
| Crossref | Google Scholar | PubMed |
Gruber B, Unmack PJ, Berry OF, Georges A (2018) dartr: An R package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Molecular Ecology Resources 18, 691-699.
| Crossref | Google Scholar | PubMed |
Hammer TA, Macintyre PD, Nge FJ, Davis RW, Mucina L, Thiele KR (2018) The noble and the exalted: a multidisciplinary approach to resolving a taxonomic controversy within Ptilotus (Amaranthaceae). Australian Systematic Botany 31, 262-280.
| Crossref | Google Scholar |
Harrington MG, Gadek PA (2009) A species well travelled – the Dodonaea viscosa (Sapindaceae) complex based on phylogenetic analyses of nuclear ribosomal ITS and ETSf sequences. Journal of Biogeography 36, 2313-2323.
| Crossref | Google Scholar |
Jeanes AJ (2021) Studies in Podolepis and some related genera (Asteraceae: Gnaphalieae). Muelleria 39, 79-12.
| Crossref | Google Scholar |
Khan G, Godoy MO, Franco FF, Perez MF, Taylor NP, Zappi DC, Machado MC, Moraes EM (2018) Extreme population subdivision or cryptic speciation in the cactus Pilosocereus jauruensis? A taxonomic challenge posed by a naturally fragmented system. Systematics and Biodiversity 16, 188-199.
| Crossref | Google Scholar |
Kilian A, Wenzl P, Huttner E, Carling J, Xia L, Blois H, Caig V, Heller-Uszynska K, Jaccoud D, Hopper C (2012) Diversity arrays technology: a generic genome profiling technology on open platforms. In ‘Data Production and Analysis in Population Genomics’. (Eds F Pompanon, A Bonin) pp. 67–89. (Humana Press: Totowa, NJ, USA)
Kopelman NM, Mayzel J, Jakobsson M, Rosenberg NA, Mayrose I (2015) CLUMPAK: a program for identifying clustering modes and packaging population structure inferences across K. Molecular Ecology Resources 15(5), 1179-1191.
| Crossref | Google Scholar |
Laport RG, Minckley RL, Ramsey J (2016) Ecological distributions, phenological isolation, and genetic structure in sympatric and parapatric populations of the Larrea tridentata polyploid complex. American Journal of Botany 103, 1358-1374.
| Crossref | Google Scholar | PubMed |
Lawson DJ, van Dorp L, Falush D (2018) A tutorial on how not to over-interpret STRUCTURE and ADMIXTURE bar plots. Nature Communications 9, 3258.
| Crossref | Google Scholar | PubMed |
Lima DF, Mauad AVS, da Silva-Pereira V, de Camargo Smidt E, Goldenberg R (2015) Species boundaries inferred from ISSR markers in the Myrcia laruotteana complex (Myrtaceae). Plant Systematics and Evolution 301, 353-363.
| Crossref | Google Scholar |
Lipsen LPJ, Lee C, Whitton J (2013) Genetic and ecological differences between two Utah endemics: US federally threatened Townsendia aprica and its close congener, T. jonesii var. lutea (Asteraceae). Botany 91, 242-250.
| Crossref | Google Scholar |
Meirmans PG (2019) Subsampling reveals that unbalanced sampling affects Structure results in a multi-species dataset. Heredity 122, 276-287.
| Crossref | Google Scholar | PubMed |
Moore AJ, Moore WL, Baldwin BG (2014) Genetic and ecotypic differentiation in a Californian plant polyploid complex (Grindelia, Asteraceae). PLoS One 9, e95656.
| Crossref | Google Scholar |
Payne WW (1978) A glossary of plant hair terminology. Brittonia 30, 239-255.
| Crossref | Google Scholar |
Prance G (1979) Forest refuges: evidence from woody angiosperms. In ‘Biological diversification in the tropics: Proceedings of the Fifth International Symposium of the Association for Tropical Biology’, 8–13 February 1979, Caracas, Venezuela. pp. 137–158. (Colombia University Press: New York, NY, USA)
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155, 945-959.
| Crossref | Google Scholar | PubMed |
Rieseberg LH, Ellstrand NC, Arnold M (1993) What can molecular and morphological markers tell us about plant hybridization? Critical Reviews in Plant Sciences 12, 213-241.
| Crossref | Google Scholar |
Rutherford S, Rossetto M, Bragg JG, McPherson H, Benson D, Bonser SP, Wilson PG (2018) Speciation in the presence of gene flow: population genomics of closely related and diverging Eucalyptus species. Heredity 121, 126-141.
| Crossref | Google Scholar | PubMed |
Schmidt-Lebuhn AN, Bovill J (2021) Phylogenetic data reveal four major clades of Australian Gnaphalieae (Asteraceae). Taxon 70, 1020-1034.
| Crossref | Google Scholar |
Schmidt-Lebuhn AN, Bruhl JJ, Telford IR, Wilson PG (2015) Phylogenetic relationships of Coronidium, Xerochrysum and several neglected Australian species of ‘Helichrysum’ (Asteraceae: Gnaphalieae). Taxon 64, 96-109.
| Crossref | Google Scholar |
Stift M, Kolář F, Meirmans PG (2019) STRUCTURE is more robust than other clustering methods in simulated mixed-ploidy populations. Heredity 123, 429-441.
| Crossref | Google Scholar | PubMed |
Stöck M, Ustinova J, Lamatsch DK, Schartl M, Perrin N, Moritz C (2010) A vertebrate reproductive system involving three ploidy levels: Hybrid origin of triploids in a contact zone of diploid and tetraploid palearctic green toads (Bufo viridis subgroup). Evolution 64, 944-959.
| Crossref | Google Scholar | PubMed |
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (Eds) (2018) ‘International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code)’, adopted by the Nineteenth International Botanical Congress, July 2017, Shenzhen, PR China. Regnum Vegetabile, vol. 159. (Koeltz Botanical Books: Glashütten, Germany) 10.12705/Code.2018
Walsh N (2014) A revision of the Coronidium scorpioides (Asteraceae: Gnaphalieae) complex. Muelleria 32, 16-33.
| Crossref | Google Scholar |
White F (1962) Geographic variation and speciation in Africa with particular reference to Diospyros. Taxonomy and Geography 4, 71-103.
| Google Scholar |
White F (1978) The taxonomy, ecology and chorology of African Ebenaceae I. The Guineo-Congolian species. Bulletin du Jardin Botanique National de Belgique/Bulletin van de National Plantentuin van België 48, 245-358.
| Crossref | Google Scholar |
Wilson PG (2008) Coronidium, a new Australian genus in the Gnaphalieae (Asteraceae). Nuytsia 18, 295-329.
| Crossref | Google Scholar |
Wilson PG (2016) A taxonomic treatment of Chrysocephalum apiculatum and C. semipapposum (Asteraceae: Gnaphalieae). Nuytsia 27, 33-73.
| Crossref | Google Scholar |