East rarely meets West: a revised delimitation for Pultenaea (Fabaceae: Mirbelieae) with reinstatement of Euchilus and three new genera from south-west Western Australia
Russell L. Barrett A B # * , James A. R. Clugston
A B # * , James A. R. Clugston  A C D # , Lindy A. Orthia E , Lyn G. Cook F , Michael D. Crisp F , Brendan J. Lepschi G , Terry D. Macfarlane
A C D # , Lindy A. Orthia E , Lyn G. Cook F , Michael D. Crisp F , Brendan J. Lepschi G , Terry D. Macfarlane  H , Peter H. Weston A and Carolyn F. Wilkins H I
H , Peter H. Weston A and Carolyn F. Wilkins H I
A
B
C
D
E
F
G
H
I
Handling Editor: Limin Lu
Abstract
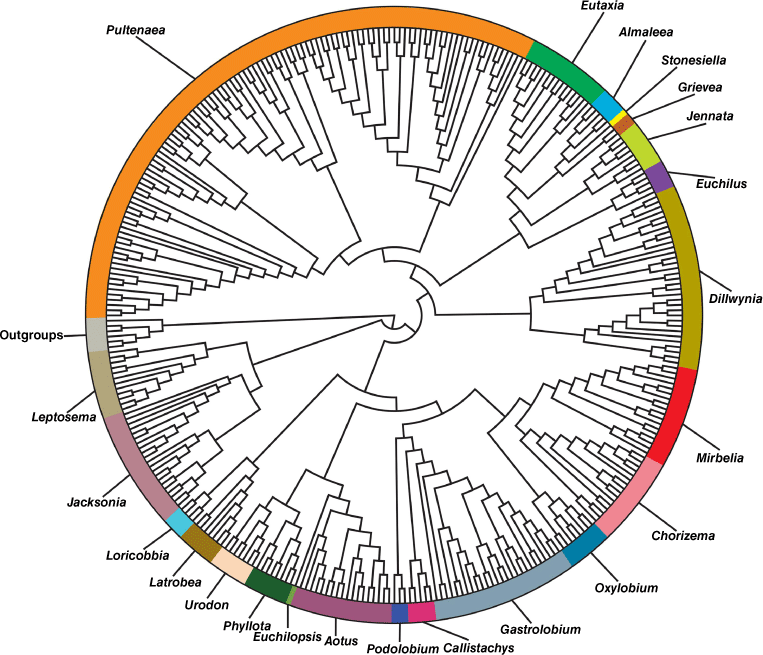
Circumscription of the large genus Pultenaea Sm. has been contentious since shortly after description. We draw on recently generated phylogenomic data to provide a fully resolved phylogeny of Pultenaea and related genera based on near-complete species level sampling for the genus. Phylogenomic data divide Pultenaea sens. lat. into five independent lineages, corresponding to previously identified clades, that we recognise as distinct genera. Pultenaea sens. str. contains most of the currently recognised species and as circumscribed here, all of the species are endemic to eastern Australia except for P. tenuifolia R.Br. & Sims that extends across the Nullarbor into Western Australia. The genus Euchilus R.Br. is reinstated for eight species, all endemic to south-west Western Australia except for E. elachistus (F.Muell.) R.L.Barrett & Orthia that also occurs in South Australia. Three new genera are described, with all of the constituent species endemic to south-west Western Australia: Grievea R.L.Barrett, Clugston & Orthia, with two species, Jennata R.L.Barrett, Clugston & Orthia, with nine species and Loricobbia R.L.Barrett, Clugston & Orthia with six species. Pultenaea adunca Turcz. remains unplaced but we exclude this species from our concept of Pultenaea. Twenty-one new combinations are made: Euchilus aridus (E.Pritz.) R.L.Barrett & Orthia, E. calycinus subsp. proxenus (Orthia & Chappill) Orthia & R.L.Barrett, E. daena (Orthia & Chappill) Orthia & R.L.Barrett, E. elachistus (F.Muell.) R.L.Barrett & Orthia, Grievea brachytropis (Benth. ex Lindl.) R.L.Barrett & Orthia, G. craigiana (C.F.Wilkins, Orthia & Crisp) Orthia & R.L.Barrett, Jennata brachyphylla (Turcz.) R.L.Barrett & Clugston, J. empetrifolia (Meisn.) R.L.Barrett & Clugston, J. ericifolia (Benth.) R.L.Barrett & Clugston, J. indira (Orthia & Crisp) Orthia & R.L.Barrett, J. indira subsp. monstrosita (Orthia) Orthia & R.L.Barrett, J. indira subsp. pudoides (Orthia) Orthia & R.L.Barrett, J. radiata (H.B.Will.) R.L.Barrett & Clugston, J. strobilifera (Meisn.) R.L.Barrett & Clugston, J. verruculosa (Turcz.) R.L.Barrett & Clugston, Loricobbia aspalathoides (Meisn.) R.L.Barrett & T.D.Macfarl., L. ochreata (Meisn.) R.L.Barrett & T.D.Macfarl., L. pauciflora (M.B.Scott) R.L.Barrett & T.D.Macfarl., L. pinifolia (Meisn.) R.L.Barrett & T.D.Macfarl., L. reticulata (Sm.) R.L.Barrett & T.D.Macfarl. and L. skinneri (F.Muell.) R.L.Barrett & T.D.Macfarl.
Keywords: Australia, biodiversity hotspot, bush-peas, new genera, phylogenetic classification, plant taxonomy, south-eastern Australia, systematics, Western Australia.
Introduction
Pultenaea Sm. was originally established based on a single species, P. stipularis Sm. (Smith 1794). James Edward Smith, an English nurseryman of note, had a particular interest in Australian peas and named many of the genera currently recognised in the tribe Mirbelieae (Smith 1794, 1798, 1805, 1808a, 1808b, 1808c). In the first decade after the genus was named, another 15 species were added (Schrader and Wendland 1797; Wendland 1798, 1799; Willdenow 1799; Andrews 1803, 1804; Ventenat 1804; Labillardière 1805; Persoon 1805; Smith 1805) but only nine of these remain in the genus as currently circumscribed. In an early review of genera allied to Pultenaea, Smith’s Decandrous Papilionaceous Plants, Smith (1805, p. 503) remarked that:
It is scarcely necessary to observe, that many plants have been forced into Pultenaea that have little to do with it. I mean not to censure those who have done so, but rather to approve of it, unless they had felt themselves competent to establish new genera on solid grounds.
These words hold true today and both sentences carry a unique weight of history. Many species have been named in Pultenaea that are currently placed in other genera. However, many genera currently recognised in Mirbelieae had also already been named by this time. Subsequently named genera in Mirbelieae are: Oxylobium Andrews (Andrews 1807); Euchilus R.Br., Eutaxia R.Br., Gastrolobium R.Br. and Podolobium R.Br. (Brown 1811); Jacksonia R.Br. ex Sm. (Smith 1811); Isotropis Benth., Leptosema Benth. and Phyllota (DC.) DC. ex Benth. (Bentham 1837); Latrobea Meisn. (Meisner 1848); Urodon Turcz. (Turczaninow 1849); Euchilopsis F.Muell. (Mueller 1882); Almaleea (Crisp and Weston 1991); and Stonesiella (Crisp et al. 1999). Smith’s name Daviesia reticulata Sm., long included in Pultenaea, finally has a home in our new genus, Loricobbia R.L.Barrett, Clugston & Orthia.
This paper originated at the 150 Conference held in Melbourne in September 2003 (Barrett et al. 2005), where phylogenetic data generated by Lindy Orthia and colleagues were presented in a session on generic concepts in the Australian flora. Even at that time, the urgent need to generate additional data in the hope of resolving critical relationships in the tribe Mirbelieae was recognised. Orthia et al. (2005a, 2005b, 2005c) highlighted that the trend for placing species in Pultenaea over the preceding 200 years had clearly rendered the current concept of Pultenaea paraphyletic. However, the phylogeny of the clades recovered could not be resolved for lack of support for the topology recovered. Definition of Pultenaea has remained challenging largely due to the lack of diagnostic characters for the genus, particularly following the broad concept adopted by Bentham (1864), who openly acknowledged that the concept may not have been a natural one. Nevertheless, Orthia et al. (2005c) did identify clear morphological clades within the Western Australian species placed in Pultenaea at that time that correlate well with clades identified in our phylogenetic analyses (Table 1).
| Clade | Current genus | Number of species | |
|---|---|---|---|
| Clades A, B, C | Pultenaea sens. str. | 150+ | |
| Clade D | Euchilus | 8 | |
| Clade E | Jennata | 9 | |
| Clade F | Loricobbia | 6 | |
| Pultenaea brachytropis | Grievea | 2 | |
| Pultenaea adunca | Unplaced (cf. Grievea) | 1 | |
| Pultenaea neurocalyx | Eutaxia | – | |
| Pultenaea barbata | Phyllota | – |
Morphology, phylogenetics and classification
The seminal work of Sands (1975) recognised that variation within Pultenaea, as defined at the time, was equivalent to that of numerous recognised genera within what is currently recognised as the tribe Mirbelieae (Crisp and Weston 1987, 1995). Crisp and Weston (1987) identified a potential synapomorphy for Pultenaea: scarious, keeled stipules that are fused behind the petiole. This conclusion stimulated further research that resulted in the recognition of two new genera, Almaleea and Stonesiella, and the transfer of some species to Eutaxia and Urodon (Crisp and Weston 1987, 1995; Crisp et al. 1999; Wilkins et al. 2010). Crisp and Weston (1987) also noted that the Western Australian species Pultenaea ericifolia did not have fused stipules.
Most of the species formerly included in Pultenaea in Western Australia (16 of 24) do have free stipules (Orthia et al. 2005c). Orthia et al. (2005c) list 17 Western Australian species, however, the record of P. juniperina Labill. appears to be based on a cultivated plant and that of P. vestita R.Br., an erroneous identification, sometimes being confused with Jennata indira subsp. monstrosita (Orthia) Orthia & R.L.Barrett due to the dense indumentum on the leaves that is otherwise unusual in this segregate genus. Pultenaea craigiana C.F.Wilkins, Orthia & Crisp was described as an additional species from southern Western Australia (Wilkins et al. 2009). Pultenaea linearifolia Strid, described among diverse ‘novelties’ by Strid (1987) and not mentioned in subsequent literature, was first synonymised with P. adunca (Green 1987) and later with Dillwynia divaricata (Turcz.) Benth. (Western Australian Herbarium, FloraBase, see http://florabase.dpaw.wa.gov.au/).
We present a new classification of Pultenaea in the sense of Orthia et al. (2005c), recognising five genera (and removing two other anomalous species) based on comprehensive analyses of phylogenomic data using the Angiosperms353 nuclear bait kit (Clugston, Barrett, Murphy, Renner, Weston, Cook, Jobson, Lepschi and Crisp, unpubl. data; Fig. 1).
Lineage evolution and generic concepts
Plant diversity is high in both southwestern and southeastern Australia. This is particularly high in heath communities, and banksia and eucalypt woodlands, the primary habitats for species in the Mirbelia clade. The floras of both regions are highly distinct in many ways, yet also share many connections that are often >25 million years old (Bickford et al. 2004; Crisp et al. 2004; Crisp and Cook 2009; Nge et al. 2020, 2021; Tian et al. 2024) and many of these evolutionary connections predate the aridification of Central Australia and uplift of the Nullarbor Plain. Sister genera (and infrageneric groups) are commonly separated across the Nullarbor divide therefore definition of distinct clades as genera in the east and west is not an uncommon situation.
In Mirbelioid peas, there are several reasons for recognising eastern and western clades as distinct genera in specific cases. Stability of names is promoted by the separation of the western Pultenaea clades, as the genus would otherwise need to be expanded to include Almaleea, Stonesiella and Eutaxia to remain monophyletic. Inclusion of these three genera in Pultenaea would also reduce rather than improve morphological diagnosability of the genus (Crisp and Weston 1991; Crisp et al. 1999; Crisp and Cook 2003a, 2003b; Chappill et al. 2007).
Orthia et al. (2005a, 2005b) provide a detailed discussion of possible relationships within the Pultenaea group relative to the Mirbelia clade of Mirbelieae (as defined by Crisp and Cook 2003b) but the data available unfortunately failed to resolve key relationships between the major clades. The precautionary principle has been applied for the last 20 years and taxonomists have refrained from proposing generic changes within Mirbelieae during that time.
Coincident with a post-doctoral fellowship grant from the Australian Biological Resources Study, the Genomics for Australian Plants (GAP) initiative provided support that enabled species-level sequencing for most genera of the Mirbelia clade using the Angiosperms353 targeted bait kit and downstream data processing on the GADI server (Johnson et al. 2018; McLay et al. 2021; Nauheimer et al. 2021; Baker et al. 2021, 2022; Jackson et al. 2023; Simpson et al. 2024). This enabled unprecedented testing of relationships within Mirbelieae, especially at genus level. In contrast to early Sanger sequence-based analyses, our results resolved almost every node in the reconstructed phylogeny with a high level of confidence, enabling a robust classification to be developed (Clugston, Barrett, Murphy, Renner, Weston, Cook, Jobson, Lepschi and Crisp, unpubl. data).
The GAP initiative partnered with the Plant and Fungal Tree of Life (PAFTOL) project led by the Royal Botanic Gardens, Kew, to create a genus-level phylogenetic framework for Angiosperm diversity (Baker et al. 2021, 2022; Jackson et al. 2023; Simpson et al. 2024). The most significant combined output from PAFTOL and GAP has been a new global phylogeny including over 50% of angiosperm genera and almost all genera of Mirbelieae (Zuntini et al. 2024). The structure of this tree is consistent with the topology presented in Fig. 1 except for the placement of Urodon that may be anomalous in the PAFTOL tree due to low gene recovery. Combined with our present results, these papers are a testament to the broad utility of the Angiosperms353 targeted bait kit for phylogenetic resolution.
While we have had to wait 20 years for the generation of detailed genomic data for the tribe, we are gratified that new data remove the significant uncertainty that surrounded relationships within the tribe based on only three markers. We are now confident of phylogenetic relationships within the broader Pultenaea clade and can move forward.
Our aim has always been to minimise the number of taxonomic changes required, while circumscribing genera that are recognisable by morphological characters. Translating these data into generic concepts, acceptance of Almaleea, Stonesiella and Eutaxia speaks of the utility of these groups as natural, diagnosable units, and offers a logical reason for the recognition of additional Western Australian clades that are also morphologically diagnosable, at generic rank. All the genera in the core Pultenaea clade (Euchilus–Pultenaea in Fig. 1) are essentially geographically isolated in either eastern or western Australia, with only two variable species crossing the Nullarbor, one in each direction (Orthia et al. 2005c). The recognition of four additional genera highlights the uniqueness of the Western Australian biodiversity hotspot and adds three endemic genera to this region. The bush-pea genus Pultenaea sens. str. is essentially restricted to southeastern and eastern Australia and can now be readily morphologically defined.
This neat biogeographical separation is convenient for the communication of new generic concepts as all but two species in Western Australia are here transferred to other genera, therefore the past concept of Pultenaea becomes in consequential in that state. In eastern Australia, the well-known genus Pultenaea is retained entirely, except for a single species in South Australia (Craigie and Lang 2014) that belongs in Euchilus. As only 24 species are moving to one reinstated and three new genera, we hope that the degree of nomenclatural stability enabled by this minimal approach to taxonomic change will increase the likelihood of acceptance of the genera we propose to recognise.
Biogeography
East–west disjunctions point to vicariance events early in the evolution of the Mirbelia clade (Crisp and Cook 2003b; Barrett et al. 2021), likely preceding speciation within the genera recognised here. Vicariance events are estimated to have occurred between 12 and 20 Ma (Crisp et al. 2004), and may have promoted speciation within individual clades. This may possibly have been associated with the onset of aridification across inland Australia and uplift of the Nullarbor Plain. Further speciation is subsequently likely to have occurred within the current range area of these clades.
Separation of clades recognised here as genera may also have been assisted by chromosomal change, as most of the major clades within Mirbelieae have distinct chromosome numbers (see Sands 1975; Keighery 1984). Chromosomal arrangement may have been a driver of generic separation during a period of rapid radiation as such changes commonly form complete breeding barriers (de Storme and Mason 2014). Such changes may have occurred in response to changing climates, concurrent with the onset of aridification in Australia (Crisp et al. 2004; Crisp and Cook 2009).
Segregate genera
In arriving at what we consider to be the most appropriate generic boundaries, we assessed both phylogenetic relationships and the ease with which these related groups can be identified using morphological characteristics. Comparative morphological data for the Western Australian species previously included in Pultenaea and the sister genera are presented in Table 2. Euchilus R.Br. is reinstated for eight species with distinctively enlarged upper calyx lobes, seven of which are endemic to south-west Western Australia, the eighth extending across the Nullarbor into South Australia. Nine species, a number of which form a poorly defined complex, are recognised as a new genus, Jennata R.L.Barrett, Clugston & Orthia. These species all share a head-like inflorescence and strongly incurved (semi-terete) leaves. Two species whose relationships have been uncertain are here placed in a new genus, Grievea R.L.Barrett, Clugston & Orthia, and the presence of fused stipules in these species may represent a reversal of the character state in that clade.
| Current paper | Pultenaea Sm. sens. str. | Euchilus R.Br. | Jennata R.L.Barrett, Clugston & Orthia | Grievea R.L.Barrett, Clugston & Orthia | Stonesiella Crisp & P.H.Weston | |
|---|---|---|---|---|---|---|
| Cladistic group in Orthia et al. (2005c) | Clades A–C | Clade D (P. quaerita group) | Clade E (P. ericifolia group) | P. brachytropis | ||
| Included species | 150+ species | E. aridus, E. calycinus, E. daena, E. elachistus, E. obcordatus, E. purpureus, E. rotundifolius, E. spinulosus | J. brachyphylla, J. empetrifolia, J. ericifolia, J. indira, J. radiata, J. strobilifera, J. verruculosa, J. sp. Mt Lesueur, J. sp. southern | G. brachytropis, G. craigiana | S. selaginoides | |
| Habit | Prostrate, to 5 m | Prostrate to erect, to 1.8 m | Prostrate to erect, to 1.2 m | Erect, 0.15–0.5(–1) or 1–1.5 m | Erect to 2.5 m | |
| Leaf arrangement | Alternate, spiral or 3-whorled | Decussate, 3-whorled, or spiral | Spiral | Spiral | Spiral | |
| Leaf margins | Flat, recurved, revolute or involute | Flat, incurved, involute or recurved | Involute | Involute or revolute to strongly recurved | Incurved | |
| Stipules | Very conspicuous, fused behind petiole | Distinct, not fused, but stipule material A present behind petiole in some species | Distinct, not fused, no material behind petiole | Distinct, fused behind petiole | Minute, not fused, no material behind petiole | |
| Inflorescence type | Terminal determinate, terminal indeterminate or axillary and solitary indeterminate | Weakly terminal or axillary and solitary indeterminate | Terminal indeterminate | Axillary and solitary indeterminate or terminal determinate | Terminal determinate | |
| Inflorescence bracts | Absent | Absent | Usually scarious | Absent or golden brown with a reduced leaf-like blade | Caducous, scale-like | |
| Flower bract texture | Scarious | Scarious and pale to red–brown, commonly enlarged or herbaceous, leaf-like and green | Scarious, rarely herbaceous and leaf-like | Absent or scarious | Herbaceous, leaf-like | |
| Bracteoles | Present or absent | Present | Present | Present | Present | |
| Calyx arrangement, sepal width | Two-lobed, sepals ±equal to unequal | Two-lobed, sepals unequal | Two-lobed, sepals unequal | Two-lobed, sepals unequal | Two-lobed, sepals ±equal | |
| Upper calyx lobes | Usually triangular, not enlarged to ∼2× as broad | Greatly enlarged, elliptic–obovate or ovate | Narrowly triangular or deltate | Falcate or rounded, fused for length | Broad–triangular, fused for length | |
| Standard petal shape | Reniform to circular | Reniform or circular | Reniform or circular | Broadly ovate or strongly reniform | Ovate | |
| Standard petal apex | Emarginate | Emarginate | Emarginate | Emarginate | Emarginate | |
| Standard claw | Short to long (1.2–5.1 mm) | Short (1–2.2 mm) | Long (2.5–6.2 mm) | Short (2–2.3 mm) | Short (~2 mm) | |
| Wing arrangement | Held over keel, coherent for entire length or sometimes held apart | Held over keel, coherent for length or held apart | Often coherent or overlapping at apex | Held apart, not coherent | Coherent and overlapping at apex | |
| Wing and keel arrangement | Wings conceal or expose keel | Wings conceal or expose keel | Wings conceal keel | Wings conceal keel | Wings conceal keel | |
| Keel size relative to wing | ±Equal | ±Equal | Much smaller or ±equal | Much smaller | Smaller | |
| Ovary shape | Laterally flattened | Laterally flattened | Elongate ovoid | Laterally flattened or ovoid | Ovoid | |
| Style | Hooked or curved to almost straight, glabrous or hairy at base | Straight to curved or hooked, glabrous | Hooked, glabrous | Hooked to curved, glabrous | Filiform, gently incurved, villous | |
| Inside of ovaries and pods | Glabrous | Glabrous | Glabrous | Glabrous | Glabrous | |
| 2n = | 12–32 | 16 or 18 | 14 | Unknown | Unknown | |
| Distribution | E Australia (to SE coast of W Australia) | SW WA | SW WA | WA, SW forest or SE coast | Tasmania |
| Current paper | Almaleea Crisp & P.H.Weston | [no data], cf. Almaleea | Eutaxia R.Br. | Latrobea Meisn. | Loricobbia R.L.Barrett, Clugston & Orthia | |
|---|---|---|---|---|---|---|
| Cladistic group in Orthia et al. (2005c) | P. adunca [cf. Almaleea] | Clade F (P. reticulata group) | ||||
| Included species | A. cambagei, A. capitata, A. incurvata, A. paludosa, A. subumbellata | P. adunca | 24 species | L. brunonis, L. colophona, L. diosmifolia, L. genistioides, L. hirtella, L. pinnaculum, L. recurva, L. tenella, L. sp. South Coast | L.aspalathoides, L. ochreata, L. pauciflora, L. pinifolia, L. reticulata, L. skinneri | |
| Habit | Erect, 0.4–1.2 m | Prostrate to 1 m | Prostrate to erect, 0.15–0.6 m | Prostrate to 1 m | 0.3–3(–5) m | |
| Leaf arrangement | Spiral | Spiral | Decussate, alternate, spiral or in whorls of 3 | Spiral | (Alternate or) spiral | |
| Leaf margins | Flat or concave | Involute | Flat to concave | Flat, recurved or involute | Flat or recurved | |
| Stipules | Not fused, minute | Not fused, material present behind petiole, minute or appearing absent | Absent or inconspicuous, not fused | Absent or minute, caducous | Fused | |
| Inflorescence type | Terminal determinate | Axillary indeterminate | Axillary and solitary indeterminate or 2–7 terminal | Axillary and solitary indeterminate or terminal determinate | Terminal determinate | |
| Inflorescence bracts | Forming an involucre at base of inflorescence | Absent | Absent or herbaceous, leaf-like | Absent [or herbaceous, leaf-like] | Scarious or leaf-like | |
| Flower bract texture | Herbaceous, leaf-like | Herbaceous, leaf-like | Absent or thin-textured | Herbaceous, leaf-like | Scarious | |
| Bracteoles | Present | Present | Present | Present | Present | |
| Calyx arrangement | Two-lobed, sepals unequal | Two-lobed, sepals ±equal | Two-lobed, sepals unequal | Symmetrical, sepals equal | Two-lobed, sepals unequal | |
| Upper calyx lobes | Enlarged, broadly triangular | Sinuously triangular to falcate | Triangular to ovate | Narrowly triangular | Narrowly triangular | |
| Standard petal shape | Reniform or round | Reniform | Broadly elliptic to broadly ovate | Sagittate | Round | |
| Standard petal apex | Emarginate | Emarginate | Emarginate | Hooded and peaked to obtuse | Emarginate, sometimes almost obtuse | |
| Standard claw | Short to long (1.1–3.0 mm) | Long (~5 mm) | Short to long (0.9–4.4 mm) | Short (0.7–1.6 mm) | Short or long (1.5–4.0 mm) | |
| Wing arrangement | Often coherent or overlapping at apex | Coherent and overlapping at apex | Often coherent or overlapping at apex | Variable | Not coherent except sometimes near apex | |
| Wing and keel arrangement | Wings conceal keel | Wings conceal keel | Wings conceal keel | Keel concealed or protrudes through wings | ±Equal but keel protrudes through wings | |
| Keel size relative to wing | Much smaller | Much smaller | ±Equal | ±Equal or larger | Larger | |
| Ovary shape | Ovoid | Elliptic | Elongate ovoid | Laterally flattened | Ovoid to laterally flattened | |
| Style | Compressed, hooked at the apex | Hooked, glabrous | Straight, curved or hooked slightly below the apex | Curved or hooked, glabrous | Straight to curved or hooked, glabrous | |
| Inside of ovaries and pods | Glabrous | Glabrous | Glabrous | Glabrous | Sparsely hairy | |
| 2n= | 14 | Unknown | 14 or 16 | 14 | 8 | |
| Distribution | SE Australia | SW WA coast | SW & SE Aust | SW WA coast & Stirlings | SW WA forests |
A clade of six species recovered as sister to Latrobea has been included in Pultenaea for most of the history (Loricobbia reticulata being the sole exception, having first been described as Daviesia reticulata and also combined as Jacksonia reticulata (Sm.) DC.). None of these species have ever been placed in Latrobea, despite largely sympatric distributions. This provides support for the recognition of these two lineages as separate genera based on historical usage. We describe this distinctive lineage as the new genus Loricobbia R.L.Barrett, Clugston & Orthia and diagnostic characters distinguishing the two genera are provided in Table 3.
| Latrobea | Loricobbia | ||
|---|---|---|---|
| Stipules | Absent or caducous | Fused | |
| Inflorescence and floral bract texture | Absent or herbaceous, leaf-like | Scarious or leaf-like | |
| Calyx arrangement | ±Symmetrical, sepals ±free | Asymmetrical, upper sepals fused | |
| Standard petal shape | Sagittate, apex hooded and peaked to obtuse | ±Round, apex emarginate, sometimes almost obtuse | |
| Ovary shape | Laterally flattened | Ovoid | |
| Pods inside | Glabrous | Hairy | |
| Chromosome number (2n) | 14 | 8 |
The enigmatic species Pultenaea adunca Turcz. unfortunately remains unplaced in this classification, as no material was available for inclusion in the phylogenomic study. We are not confident to place this species within any genus based on morphology and available Sanger sequence data. Pultenaea adunca may be best placed in Almaleea or Grievea where the alliance clearly lies (Table 2) or possibly in a novel genus and additional studies are underway. We accept P. tenuifolia R.Br. as the only species of Pultenaea (as we here define the genus) that is native to Western Australia, though the main distribution is in eastern Australia and application of this name to Western Australian populations is worthy of further study.
Current taxonomy
We recognise that significant revision of species concepts within Pultenaea is still required (Renner et al. 2022; Telford et al. 2022; Barrett et al. 2024). We hope that settling generic boundaries will also encourage the description of novel species in remaining species complexes in Pultenaea and in the segregate genera recognised here.
Materials and methods
Full generic descriptions are provided below for Euchilus, Grievea, Jennata, Loricobbia and Pultenaea. Descriptions of these genera are aggregated based on those provided for species described in de Kok and West (2002, 2003, 2004), Orthia et al. (2005c) and Wilkins et al. (2010) with additional character assessment from specimens held at NSW and field photographs. References to previously published descriptions and illustrations are provided for each taxon and these are found under the respective combinations in Pultenaea unless otherwise indicated. Images of type specimens were located using the websites listed in Table 4.
See Orthia et al. (2005c, p. 165) and Barrett et al. (2021) for issues regarding ‘Pultenaea’ [Phyllota] barbata. This species has been confirmed as belonging in Phyllota but taxonomic resolution of species boundaries and application of names is the subject of ongoing research.
| 1. | Stipules minute, appearing absent or early caducous...2 Stipules present, prominent...4 |
| 2. | Standard petal sagittate...Latrobea Standard petal reniform...3 |
| 3. | No beard on style; leaves involute; bracteoles scarious...‘Pultenaea’ adunca Beard on style; leaves revolute; bracteoles leaf-like...‘Pultenaea’ [Phyllota] barbata |
| 4. | Two upper calyx lobes much larger than three lower lobes, with the depth of sinus between two upper lobes >depth of sinus between upper and lower lobes; upper lobes ±obliquely elliptic and widest near middle; stipules free...Euchilus Two upper calyx lobes ±equal in size to three lower lobes or if upper lobes larger than lower lobes then upper lobes are fused for almost entire length and sinus between these <sinus between upper and lower lobes; upper lobes ±triangular or narrowly triangular and widest at base; stipules free or fused...5 |
| 5. | Ovaries and pods with hairs on the inside; stipules fused...Loricobbia Ovaries and pods glabrous inside; stipules free or fused...6 |
| 6. | Stipules fused to at least 1/3 of the length behind the petiole...7 Stipules not fused behind the petiole...9 |
| 7. | Leaf margins revolute to strongly recurved...Grievea brachytropis Leaf margins incurved or involute...8 |
| 8. | Leaf straight or gently incurved towards an acute apex...Pultenaea tenuifolia sens. lat. Leaf recurved in upper 1/3; apex clavate, obtuse...Grievea craigiana |
| 9. | Flower subtending bracts leaf-like; stipules minute or cryptic...‘Pultenaea’ adunca Flower subtending bracts scarious with reduced bract ‘leaf’; stipules prominent...Jennata |
| Database | URL | Date accessed | |
|---|---|---|---|
| Australasian Virtual Herbarium | https://avh.chah.org.au/ | 14 July 2023 | |
| Herbarium Berolinense | http://ww2.bgbm.org/Herbarium/ | 19 July 2023 | |
| Natural History Museum | https://data.nhm.ac.uk/search# | 19 July 2023 | |
| JSTOR Plants | https://plants.jstor.org/ | 7 July 2023 | |
| JACQ | https://www.jacq.org/#database | 14 July 2023 | |
| Kew Herbarium Catalogue | http://apps.kew.org/herbcat/gotoHomePage.do | 14 July 2023 | |
| The Oxford University Herbaria | https://herbaria.plants.ox.ac.uk/bol/oxford/Herbaria | 19 July 2023 | |
| Naturalis Bioportal | https://bioportal.naturalis.nl/ | 14 July 2023 | |
| Komarov Botanical Institute | https://en.herbariumle.ru/ | 19 July 2023 | |
| NYBG Steere Herbarium | https://sweetgum.nybg.org/science/vh/media-search/ | 19 July 2023 | |
| Muséum National d’Historie Naturelle | https://science.mnhn.fr/institution/mnhn/item/search/form | 19 July 2023 | |
| Sweden’s Virtual Herbarium | http://herbarium.emg.umu.se/standard_search.html | 17 July 2023 | |
| Smithsonian National Museum of Natural History | https://collections.nmnh.si.edu/search/botany/ | 19 July 2023 |
Taxonomy
Euchilus R.Br. in W.T.Aiton, Hortus Kew 2nd edn, 3: 17 (1811)
[Orthographic variants include ‘Euchylus’, Poir. in F.Cuvier (ed.), Dict. sci. nat. 2nd edn, 15: 516–517 (1819); ‘Euchilos’ Spreng., Gen. pl. 1: 366 (1830)]
Pultenaea sect. Euchilus (R.Br.) F.Muell., Fragm. 1(1): 8 (1858). Type species: Euchilus obcordatus R.Br.
Open to dense, prostrate, procumbent or spreading to erect, multi-stemmed rounded shrubs, sometimes rhizomatous, 0.1–1.8 m high, 0.2–2.0 m wide. Stems and lower branches glabrous or glabrescent, or with persistent hairs. Branchlets apically with sparse to dense, appressed to ascending hairs, glabrescent, without tubercles, not spinescent. Stipules red–brown to red–black or black, persistent or breaking irregularly with age, the bases not fused but some stipule tissue remnant behind petioles, ovate to triangular, glabrous to sparsely hairy, 0.7–5.0 mm long. Leaves appressed to reflexed or divergent to ascending, decussate, sometimes in whorls of 3 (rarely 4) or spiralled; petiole subterete or pulvinate, not decurrent, 0.3–1.0 mm long; blade elliptic to obovate or oblong to linear, sometimes clavate to oblanceolate, straight or recurved, 1.1–12.9(–15.5) mm long, 0.5–4.0 mm wide, concolourous or discolourous, abaxial surface pale to olive, mid-green, bright green or grey–green to reddish-purple, vein and margins sometimes darker, midvein prominent or not, glabrous or with sparse, white, appressed or erect hairs, dense on new growth, smooth (rarely weakly verruculose); adaxial surface not concealed, glabrous or sparsely to densely hairy; margins flat to incurved or involute, or recurved; apex obtuse to rounded, mucronate or aristate, obcordate, slightly mucronate or sometimes recurved, sometimes minutely notched. Inflorescence with solitary axillary flowers, grouped towards apex, indeterminate. Inflorescence bracts absent. Peduncle absent. Flower subtending bracts scarious and pale to red–brown, commonly enlarged or herbaceous, leaf-like and green. Pedicels straight (sometimes twisted or tortuous), (0.1)–0.5–11.5 mm long. Bracteoles usually persistent slightly below the calyx, glabrous or sometimes sparsely hairy, scarious, dark red or red–brown, ovate to lanceolate, 0.8–6.0 mm long, 0.1–1.3 mm wide, apex acuminate. Hypanthium 0.6–1.8 mm long. Calyx glabrous to densely hairy, not ribbed; lobes asymmetrical, adaxial two larger, upper and lower lobes coherent for 25–100% of length; adaxial lobes elliptic to obovate, 2.0–7.8 mm long, apex usually obtuse, sometimes acute; middle and lateral abaxial lobes narrowly triangular to lanceolate, 1.0–4.8(–6.5) mm long; apex of abaxial lobes acute to acuminate. All petals vary in colour from yellow, gold, deep yellow or orange–yellow through pinkish to entirely red, apices often darker. Standard with a claw 1.0–2.2 mm long; lamina with flares of red following veins on upper surface surrounding a basal, ovate pale eye, circular to reniform, non-auriculate, 3.3–9.0 mm long, 3.9–9.7 mm wide, apex slightly emarginate. Wings held over keel, coherent for length or held apart, yellow with red markings towards apex, 3.0–7.2 mm long, 1.2–2.5 mm wide. Keel ±equal to the wings, lamina pale yellow to reddish with pale red apex, 2.8–7.5 mm long, 1.2–3.0 mm wide. Staminal filaments unequal, 2.5–5.5 mm long; anthers 0.2–0.6 mm long. Gynoecium with a stipe 0.2–0.6 mm long; ovary ovate, 1.2–3.5 mm long, laterally flattened, with dense, appressed hairs white, straight, evenly distributed or glabrous on body but hairy on margins; style straight to curved or hooked, 1.0–4.2 mm long, glabrous; stigma scarcely capitate. Pod not seen or elliptic or obovate, laterally flattened, apically dehiscent, 3.0–6.6 mm long, 2.0–3.7 mm wide, the outer surface glabrous or with sparse to dense hairs; the inner surface glossy, glabrous. Seed not seen or one per pod, reniform, round or ovate, smooth, pale brown to black with mottled brown markings or black spots, 1.3–2.6 mm long; aril absent or whitish. 2n = 16 or 18.
Distinguished by the greatly enlarged upper calyx lobes, the lower three often reduced and obscure on first examination, similar in this respect to Euchilopsis linearis (Benth.) F.Muell. but this species has distant, alternate leaves (opposite or sometimes 3-whorled in Euchilus). Euchilus differs from Pultenaea in having free stipules. Euchilus differs from Jennata in opposite or sometimes 3-whorled (v. consistently spiralled) leaves; inflorescence bracts absent (v. present, scarious); upper calyx lobes greatly enlarged, elliptic to obovate (v. only slightly enlarged, triangular); standard claw short (v. long); ovary laterally compressed (v. ovoid); 2n = 16 or 18 (v. 14). Euchilus differs from Grievea in the free (v. fused) stipules; floral bracts herbaceous, leaf-like (v. absent or scarious); upper sepals fused for ~1/3 length (v. full length); keel ±equal to the wings (v. much smaller).
The epithet is from the Greek eu- (well-) and cheilos (lipped), presumably in reference to the enlarged upper calyx lobes.
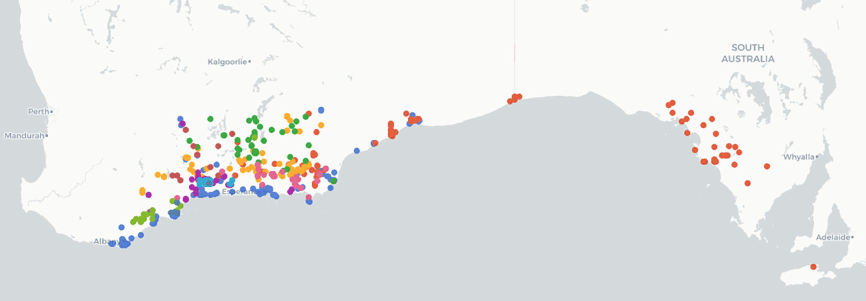
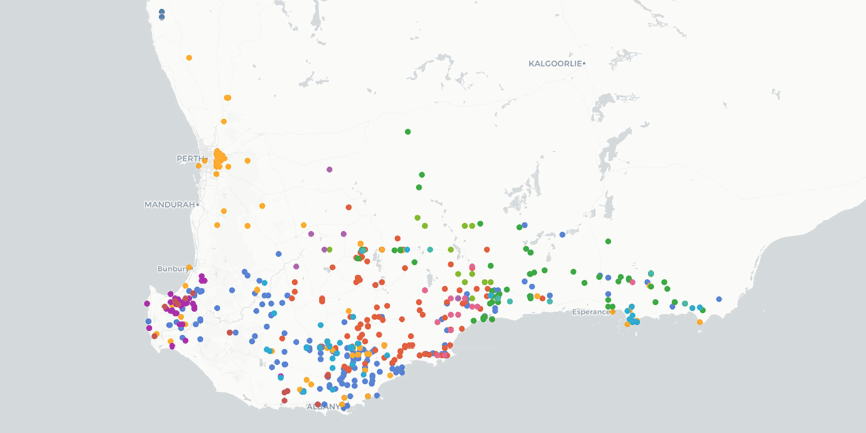
Roe, Coolgardie and Eucla botanical districts in Western Australia. Euchilus elachistus (F.Muell.) R.L.Barrett & Orthia is the only species in the genus that extends across the Nullarbor Plain into South Australia, occurring on the Nullarbor Plain, Eyre Peninsula and Kangaroo Island (Fig. 2).
Distribution of Euchilus species based on data from the Australasian Virtual Herbarium (see https://avh.ala.org.au/, accessed 2 November 2023). Colours represent individual species.
With the exception of Euchilus purpureus Turcz., Grieve (1998) grouped these species together on the basis of having ‘leaves opposite or in whorls of 3’.
| 1. | Leaf margins strongly recurved (particularly clear in young leaves)...2 Leaf margins flat or incurved...4 |
| 2. | Average leaf length:width ratio >4:1; leaves spiralled...E. purpureus Average leaf length:width ratio <4:1; leaves decussate or whorled...3 |
| 3. | Pedicels longer than leaves; branchlets with straight appressed hairs...E. rotundifolius Pedicels shorter than leaves; branchlets with curly hairs...E. elachistus |
| 4. | Calyx lobes and leaves aristate...E. spinulosus Calyx lobes and leaves acute or mucronate but not aristate...5 |
| 5. | In bud upper calyx lobes are coherent with lower lobes for ≥75% of the length; upper calyx lobe apex acute...6 In bud upper calyx lobes are coherent with lower lobes for ≤67% of the length; upper calyx lobe apex obtuse...7 |
| 6. | Prostrate shrub; leaves densely hairy, leaf hairs erect...E. daena Erect shrub; leaves sparsely hairy; leaf hairs usually appressed...E. aridus |
| 7. | Leaves usually whorled; leaf length:width ratio ≥4:1...E. calycinus (8) Leaves decussate; leaf length:width ratio ≤2:1...E. obcordatus |
| 8. | Leaves oblong or slightly obovate and broadly U-shaped in cross section...E. calycinus subsp. calycinus Leaves clavate and strongly obovate and involute...E. calycinus subsp. proxenus |
Euchilus aridus (E.Pritz.) R.L.Barrett & Orthia, comb. nov.
Pultenaea arida E.Pritz. in F.L.E.Diels & E.Pritzel, Bot. Jahrb. Syst. 35: 258 (1904).
Type citation: ‘in distr. Coolgardie meridionalis pr. Gilmores in eucalyptetis lutosis flor. m. Nov. (D. 5275)’. Type: Western Australia: Coolgardie, Nov. [1901], F.L.E.Diels 5275 (holo: B [probably destroyed]; iso: MEL 2057349 [fragment ex B]!).
Williamson (1922, pl. VII); Grieve (1998, p. 423, fig.); Orthia et al. (2005c, pp. 159–162).
Euchilus calycinus Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 276 (1853)
Pultenaea calycina (Turcz.) Benth., Fl. austral. 2: 121 (1864). Type citation: ‘Drum. V. n. 75.’ Type: Western Australia: Swan River, 1849 [?1850], J.Drummond V 75 (holo: KW 001001203!; iso: BM 000544712!, CGE, E 00288364!, G 00370626!, G 00370629!, K 000119026! K 000119027! K 000119073!, NSW 37050!, OXF [Image 0054628P]!, P 02297472!, PERTH 01027441!, PERTH 01027859!, W).
New names published by Turczaninow are accepted as having holotypes (even though duplicates commonly exist) as only the set of specimens now held at KW was examined by Turczaninow (Marchant 1990; Mosyakin and de Lange 2019; Mosyakin et al. 2019; Kellermann et al. 2022).
Williamson (1920, pl. XIV); Woolcock and Woolcock (1989b, p. 178, fig., p. 184, pl.); Grieve (1998, p. 423, fig.); Orthia et al. (2005c, p. 169).
Euchilus calycinus Turcz. subsp. proxenus (Orthia & Chappill) Orthia & R.L.Barrett, comb. nov.
Pultenaea calycina subsp. proxena Orthia & Chappill, Austral. Syst. Bot. 18(2): 171, fig. 9c (2005). Type: Western Australia: [locality withheld for conservation reasons] E of Ravensthorpe along road to Esperance, 5 Nov. l992, T.D.Macfarlane TDM 2122 & H.R.White (holo: CANB 508683!; iso: CANB 508665, PERTH 04947886!).
Orthia et al. (2005c, p. 171, fig. 9c); Wilkins et al. (2011, p. 139, pl.).
Euchilus daena (Orthia & Chappill) Orthia & R.L.Barrett, comb. nov.
Pultenaea daena Orthia & Chappill, Austral. Syst. Bot. 18(2): 171–172, fig. 9d (2005). Type: Western Australia: [locality withheld for conservation reasons] W of Ravensthorpe, 27 Oct. 1997, B.J.Lepschi & B.A.Fuhrer BJL 3743 (holo: PERTH 05013267!; iso: CANB 504753!).
Pultenaea sp. Fitzgerald (B.J.Lepschi & B.A.Fuhrer BJL 3743) WA Herbarium (FloraBase 1998; accessed November 2023).
Orthia et al. (2005c, p. 171, fig. 9d).
We have chosen not to alter the termination of the species epithet daena with the transfer to Euchilus as the name originates from the Avestan language and Orthia et al. (2005c) took the primary meaning to be ‘that which was revealed’, rather than the Zoroastrian goddess of the same name. We therefore treat the epithet as an indeclinable noun.
Photographs of representative Euchilus species. (a, b) Euchilus daena. (c, d) E. elachistus. (e, f) E. obcordatus. (g, h) E. rotundifolius. Photograph credits: (a) M.D. Crisp; (b, e, f) R.L. Barrett; (c, d) T. Hammer; (g, h) L. Fedec. (a from APII, © M.D. Crisp, 2000; c, d, f, g from iNaturalist).

Euchilus elachistus (F.Muell.) R.L.Barrett & Orthia, comb. nov.
Gastrolobium elachistum F.Muell., Fragm. 9(75): 67 (1875); Pultenaea elachista (F.Muell.) Crisp, J. Adelaide Bot. Gard. 6(1): 66 (1982). Type citation: ‘In eremo interiore trans sinum Fowler’s Bay; E. Giles.’ Type: Western Australia, Eucla, [1882], J.Oliver s.n. (neo, designated by R.P.J. de Kok & J.G. West, Austral. Syst. Bot. 17(3): 288 (2004): AD 98046057*; isoneo: K).
Pultenaea cymbifolia J.M.Black, Trans. & Proc. Roy. Soc. South Australia 39: 96, pl. 10, fig. 1–4 (1915). Type: South Australia: Kangaroo Island, between Kingscote and Cassini, May 1914, H.W.Andrew 2 (syn: AD 98046065, AD 98046066, K 000119044*); loc. id., H.W.Andrew 4 (syn: NSW 39213).
Pultenaea sp. Clyde Hill (K.R.Newbey 8236) WA Herbarium (FloraBase 1998, accessed November 2023).
Williamson (1920, pl. XIV), as P. cymbifolia; Crisp (1982, p. 66); Weber (1986, fig. 363b); Woolcock and Woolcock (1989b, p. 179, fig.); Grieve (1998, p. 423, fig.); de Kok and West (2004, p. 288); Orthia et al. (2005c, p. 172); Craigie and Lang (2014, p. 50, fig. 21l–p, pl. 15l).
de Kok and West (2004, p. 288) list material at AD as the holotype of Pultenaea cymbifolia but overlooked the multiple gatherings by Andrew from this location and date, therefore all are correctly treated as syntypes.
Euchilus obcordatus R.Br. in W.T.Aiton, Hortus kew. 2nd edn, 3: 17 (1811)
Pultenaea heterochila F.Muell., Fragm. 4(24): 21 (1863) [nom. nov., non P. obcordatus Andrews (1809)]; Pultenaea obcordata (R.Br.) Benth., Fl. austral. 2: 120 (1864), nom. illeg.; Pultenaea quaerita Orthia, Austral. Syst. Bot. 18(2): 193 (2005), nom. illeg., nom. superfl. Type citation: ‘Nat. of the South-west coast of New Holland. Robert Brown, Esq.’ Type: Western Australia: King George Sound, December 1801, R.Brown [Iter Austral. 4089] (lecto, designated by L.A.Orthia et al., Austral. Syst. Bot. 18(2): 194 (2005): BM 000833607!; isolecto: CANB 278380!, DBN 0001093, E 00288352!, E 00288353!, K 000118886!, K 000118889!, K 000118890!, MEL, n.v.
Two residual syntypes are known: Cultivated at Kew Gardens 1807–8 from seed of King George’s Sound’ (BM 000544710); Cultivated at Kew Gardens (DBN 0001092) (see Nelson 2004, p. 68; Mabberley and Moore 2022, pp. 378–379).
Williamson (1920, pl. XIV); Woolcock and Woolcock (1989b, p. 180, fig.; p. 181, pl.); Grieve (1998, p. 422, fig.); Orthia et al. (2005c, p. 193), as P. quaerita.
Euchilus purpureus Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 276 (1853)
Pultenaea purpurea (Turcz.) Crisp & Orthia, Austral. Syst. Bot. 18(2): 191 (2005). Type citation: ‘Drum. V. n. 70.’ Type: Western Australia: ‘Nova Hollandia’, 1849, Drummond V 70 (holo: KW 001001204!; iso: BM 000544697!, G 00370627! G 00370637!, K 000118891!, K 000118892!, K 000858741!, ?M 0219166!, MEL 35097!, NSW 37049!, P 02297474! PERTH 01025813!, PERTH 01025821, W).
Pultenaea conferta Benth., Fl. austral. 2: 118–119 (1864). Type citation: ‘W. Australia. Drummond, 5th coll. N. 70.’ Type: Western Australia: ‘Nova Hollandia’, 1849, Drummond V 70 (lecto, here designated: K 000118891!; isolecto: BM 000544697!, G 00370627! G 00370637!, K 000118892!, K 000858741!, KW 001001204!, ?M 0219166!, MEL 35097!, NSW 37049!, P 02297474! PERTH 01025813!, PERTH 01025821, W).
Williamson (1920, pl. XIV); Grieve (1998, p. 418, fig.), both as Pultenaea conferta; Orthia et al. (2005c, p. 191).
Euchilus rotundifolius Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 277 (1853)
Pultenaea rotundifolia (Turcz.) Benth., Fl. austral. 2: 121 (1864). Type citation: ‘Drum. V. n. 78.’ Type: Western Australia: Swan River Colony, 1849, Drummond V 78 (holo: KW [photo seen]; iso: BM 000544711!, CGE, G 00370636!, G 00370649!, K 000118994!, K 000118995!, K 000118996!, K 000118997!, NSW 37052!, W).
Euchilus crinipodus F.Muell., Fragm. 1(6): 145 (1859). Type citation: ‘In montibus East Mount Barren, Fitzgerald et Phillips Ranges’. Type: Western Australia: East Mount Barren, s. dat., F.Mueller s.n. (syn: K 000119040!); Fitzgerald Ranges, 1861 [date of receipt at BM rather than collection date?], G.Maxwell 280 (syn: BM 000839151!); Doubtful Island Bay [Fitzgerald River area], G.Maxwell 242 (syn: BM 000915976!).
Bossiaea strigillosa Benth., Fl. austral 2: 157 (1864). Type: Western Australia: S.W. Australia, s. dat., J.Drummond 5th Coll. ? 81 (lecto, designated by J.H.Ross, Muelleria 15: 32 (2001): K 000278224!).
Williamson (1920, pl. XIV); Woolcock and Woolcock (1989b, p. 182, fig.); Grieve (1998, p. 422, fig.); Orthia et al. (2005c, p. 198).
Euchilus spinulosus Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 275 (1853)
Pultenaea spinulosa (Turcz.) Benth., Fl. austral. 2: 121 (1864). Type citation: ‘Drum. V. n. 71.’ Type: Western Australia: Nova Hollandia, Swan River Colony, 1849, Drummond V, 71 (holo: KW 001001206!; iso: BM 000544713!, CGE, E 00288343!, G 00370633!, G 00370652!, K 000119055!, K 000119056!, K 000119057!, NSW 37051!, P 02928545! PERTH 01027891!, W).
Williamson (1920, pl. XIV); Woolcock and Woolcock (1989b, p. 183, fig.); Grieve (1998, p. 423, fig.); Orthia et al. (2005c, p. 200).
Pultenaea wudjariensis Orthia is not recombined in Euchilus as the species is considered to be a sterile hybrid between Euchilus calycinus subsp. proxenus and E. rotundifolius (Wilkins et al. 2011); Western Australian Herbarium, FloraBase, see http://florabase.dpaw.wa.gov.au/).
Grievea R.L.Barrett, Clugston & Orthia, gen. nov.
Type species: Grievea craigiana (C.F.Wilkins, Orthia & Crisp) Orthia & R.L.Barrett [≡ Pultenaea craigiana C.F.Wilkins, Orthia & Crisp].
Open to dense, bushy erect, multi-stemmed rounded shrubs, 0.15–1.5(–2.5) m high, 0.3–1(–2.5) m wide. Stems and lower branches glabrescent. Branchlets apically with sparse to dense, appressed straight hairs, glabrescent, without tubercles, not spinescent, ascending. Stipules pale gold to transparent or red–brown becoming black, persistent or caducous with age, the bases fused to each other across the stem, narrow–triangular or almost linear, scarious, glabrous, 0.6–4.0(–6.0) mm long. Leaves appressed, ascending, inclined or divergent, spirally arranged, petiole subterete or pulvinate, not decurrent, ~1 mm long; blade narrowly obovate to obovate or oblong to linear, 1.3–19 mm long, 0.5–3.0 mm wide, abaxial surface yellow–green or bright to dark green, with scattered, white appressed hairs on new growth, soon glabrescent or glabrous, sometimes verruculose; adaxial surface concealed or sparsely hairy; margins incurved to involute or revolute to strongly recurved; apex recurved, appearing obtuse but actually acute and mucronate. Inflorescence with solitary axillary flowers, grouped towards apex or flowers in terminal, determinate clusters. Inflorescence bracts absent or leaf-like. Peduncle absent. Flower subtending bracts golden brown, ~4.8 mm long, 2.4 mm wide, leaf strongly reduced or absent, stipules enlarged, margins ciliate, body glabrous and shiny, scarious, filmy. Pedicels straight, 0.2–0.5 mm long. Bracteoles persistent slightly below the calyx, red– or golden brown, elliptic to ovate, glabrous, scarious, 0.9–1.3 mm long, 0.4–0.6 mm wide or ~4.0 mm long, 1.5 mm wide, apex acute. Hypanthium 0.4–0.6 or ~3 mm long. Calyx sparsely to densely hairy, not prominently ribbed; lobes asymmetrical, adaxial two larger, valvate, upper and lower lobes fused for 50–90% of length; adaxial lobes falcate or rounded, 0.5–0.8 mm long; middle and lateral abaxial lobes 1.5–2.4 mm long; apex of all lobes acute or upper lobes rounded. Standard with a claw 2.0–3.3 mm long; lamina yellow with flares of red following veins on upper surface surrounding a basal, ovate, pale lemon or yellow eye, broadly ovate or reniform, non-auriculate, 3.5–4.2 mm long, 5.3–8.2 mm wide or ~10.5 mm long, ~10.5 mm wide, apex emarginate. Wings held apart, not coherent, lamina centre with red markings or yellow, towards apex yellow or red, 4–4.3 mm long, 1.4–1.8 mm wide or ~8.2 mm long, ~1.5 mm wide. Keel much shorter than the wings; lamina dark red fading to the base and tip with narrow yellow margin or white to cream; straight, scarcely obovate, 3.3–5.5 mm long, 1.3–2.1 mm wide. Staminal filaments unequal, 1.7–4.6 mm long; anthers cream, adaxial anthers 0.2–0.4 mm long. Gynoecium without a stipe; ovary 1.1–1.5 mm long, laterally flattened or elliptic, with dense, appressed, straight white hairs ~0.4 mm long, evenly distributed; style hooked, 1.5–2.1 mm long, with scattered hairs throughout or ~3.1 mm long and glabrous; stigma capitate. Pod ellipsoid or obovate, inflated, apically dehiscent, 3.6–4.3 mm long, 2.2–2.6 mm wide or ~5 mm long, ~4 mm wide, the outer surface with sparse or dense, white appressed hairs; the inner surface glossy, glabrous. Seed not seen or one per pod, ovoid, smooth, pale greenish-brown with irregular black markings; aril yellow–white, translucent and surrounding the hilum. 2n = unknown.
Differs from both Euchilus and Jennata in the fused (v. free, minute) stipules. Differs from both Almaleea and Stonesiella in the fused (v. free, minute) stipules; and floral bracts being absent or golden brown with a reduced leaf-like blade (v. herbaceous, leaf-like).
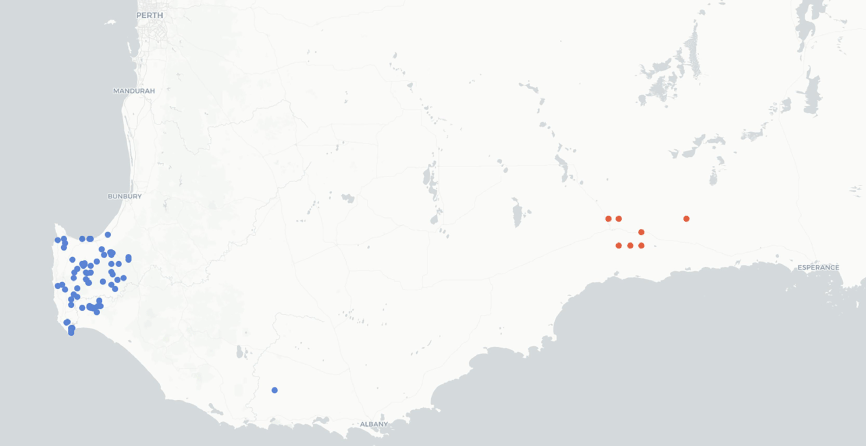
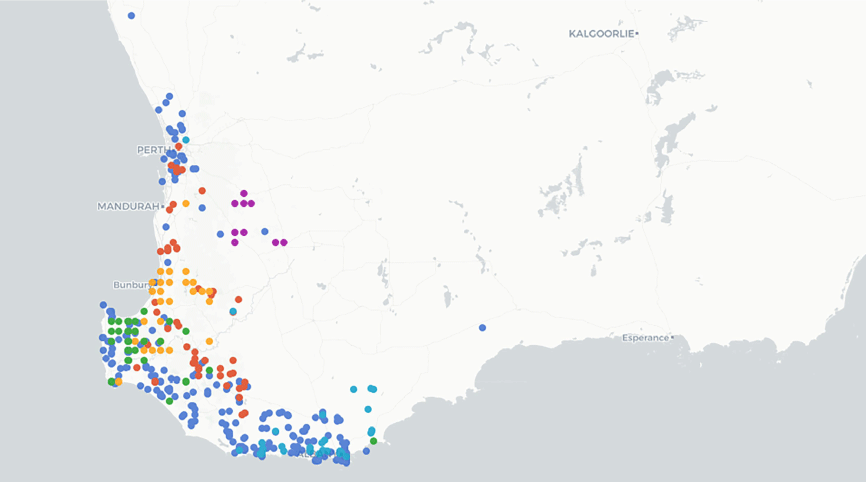
A genus of two species, both endemic to the south coast of Western Australia (Fig. 4).
Distribution of Grievea species based on data from the Australasian Virtual Herbarium (see https://avh.ala.org.au/, accessed 2 November 2023). Colours represent individual species.
The new genus honours the late Brian John Grieve (1907–1997) who became the foundation Head of Botany at The University of Western Australia in 1947 and Professor of Botany in 1957 (Grieve 1975). He was initially co-author and later sole author of a popular series of field guides, How to Know Western Australian Wildflowers (Blackall and Grieve 1965, 1974, 1980, 1981, 1988; Grieve and Blackall 1975, 1982; Grieve 1982, 1998) that is yet to be superseded. (See also a biography in Orchard 1999.)
As postulated by Wilkins et al. (2009), Grievea craigiana is most closely related to G. brachytropis, the two species sharing fused stipules and floral and inflorescence characters, including the blunt, red-tipped calyx. The two species are readily distinguished by the involute leaf margins in G. craigiana, v. strongly recurved to revolute in G. brachytropis.
| 1. | Leaves strongly recurved to revolute...G. brachytropis Leaves involute...G. craigiana |
Grievea brachytropis (Benth. ex Lindl.) R.L.Barrett & Orthia, comb. nov.
Pultenaea brachytropis Benth. exLindl., Edwards’s Bot. Reg. 27: 40 [Misc. Notices no. 76] (1841). Type citation: ‘from Port Augusta, on the South-west coast of New Holland, whence seeds were sent to Capt. James Mangles, R.N. by Mrs Molloy,…’. Type: Cultivated in United Kingdom [ex Augusta, Western Australia], 1841, Hort. Lindley s.n. (holo: K 000119003!).
Pultenaea drummondii Meisn. in J.G.C.Lehmann (ed.), Pl. Preiss. 2: 219–220 (1848). Type citation: ‘Swan River, Drummond coll. II. No. 127.’ Type: Western Australia: Swan River Colony, 1844, Drummond II, 127 (lecto, here designated: NY 00026870!; isolecto: BM 000544686!, K 000119053!, K 000119054!, K 000119076!, LD 1803458!, OXF [Image 0054629Q]!).
Williamson (1920, pl. XIII); Woolcock and Woolcock (1985, p. 79); Grieve (1998, p. 420, fig.); Scott and Negus (2002, p. 108, fig. 1); Wheeler (2002, p. 774, fig.); Scott (2019, p. 83, pl.), all as Pultenaea drummondii; Orthia et al. (2005c, pp. 168–169).
We here designate the sheet at NY as the lectotype of Pultenaea drummondii as this is where Meisner’s herbarium is currently located and whether other duplicates were examined when the description was prepared is unclear.
Photographs of Grievea species. (a, b) Grievea brachytropis cultivated at the Australian National Botanic Gardens, Canberra. (c, d) G. craigiana near Ravensthorpe. Photograph credits: (a, b) M.D. Crisp. (© Australian National Botanic Gardens 1985); (c, d) G.F. Craig (Voucher: G.F.Craig 10805; PERTH).
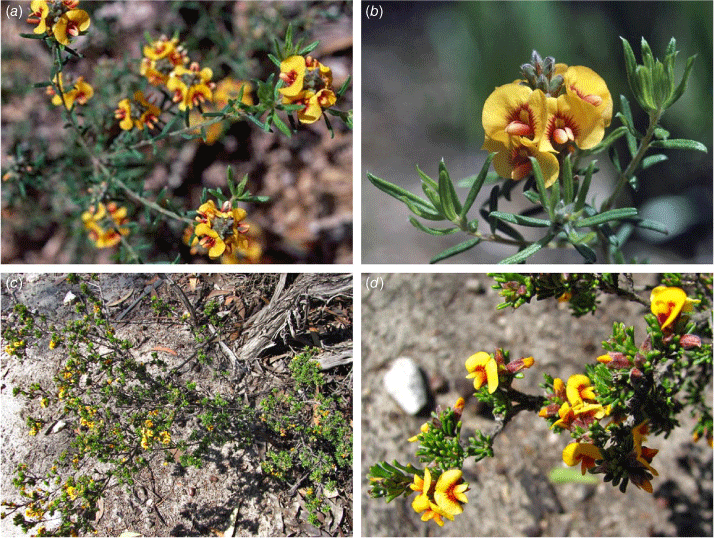
Grievea craigiana (C.F.Wilkins, Orthia & Crisp) Orthia & R.L.Barrett, comb. nov.
Pultenaea craigiana C.F.Wilkins, Orthia & Crisp, Nuytsia 19: 192, fig. 1, 2 (2009). Type: Western Australia: southern limit of Ravensthorpe Range, [precise locality withheld for conservation reasons], 5 Nov. 2004, G.F.Craig 6148 (holo: PERTH 07854765!; iso: CANB 694889!; MEL, n.v.).
Pultenaea sp. Kundip (G.F.Craig 6008), Western Australian Herbarium, in FloraBase, http://florabase.dec.wa.gov.au [accessed 16 March 2008].
Wilkins et al. (2009, p. 192, fig. 1, 2); Wilkins et al. (2011, p. 140, pl.).
Jennata R.L.Barrett, Clugston & Orthia, gen. nov.
Type species: Jennata strobilifera (Meisn.) R.L.Barrett & Clugston [≡ Pultenaea strobilifera Meisn.]
Pultenaea sect. Coelophyllum Informal Group D, G.Bentham, Fl. austral. 2: 109 (1864), p.p.
Open to dense, prostrate or spreading to erect, single or multi-stemmed shrubs, 0.1–1.2 m high, 0.2–1.0 m wide. Stems and lower branches glabrous or glabrescent or with persistent hairs. Branchlets apically with sparse to dense, appressed to ascending, velutinous woolly hairs, without tubercles, not spinescent. Stipules red–brown, dark brown or grey, persistent or fraying and turning grey with age, the bases not fused but some stipule tissue remnant behind petioles, lanceolate, deltate or narrowly triangular, glabrous to densely hairy, 0.7–5.3 mm long. Leaves appressed to inclined or divergent to ascending, spiralled; petiole subterete or pulvinate, not decurrent, 0.1–0.5 mm long; blade linear, narrowly oblong to oblanceolate, to obovate, often appearing subterete, straight to strongly recurved along the length, 1.6–14.5 mm long, 0.5–1.1 mm wide, abaxial surface pale to olive to mid-green or bright green, midvein not prominent, glabrous to sparsely white, tangled or erect hairy, smooth or weakly to strongly verruculose; adaxial surface usually not concealed, glabrous or sparsely hairy; margins tightly involute; apex obtuse or acute, mucronate or aristate. Inflorescence with solitary axillary flowers, grouped towards apex, indeterminate. Inflorescence bracts leaf-like or red–brown and scarious (rarely herbaceous and leaf-like), 2.5–9.5 mm long, with free bracteoles to 4.5 mm long, woolly. Peduncle absent. Pedicels absent. Bracteoles usually persistent slightly below the calyx, free or fused, glabrous to densely hairy, scarious, pale brown to red–brown, lanceolate, elliptic to falcate or ovate, 2.3–6.5 mm long, 0.3–1.4 mm wide, apex acute to acuminate. Flower-subtending bracts red–brown, scarious, stipules 3.7–9.5 mm long, woolly. Hypanthium 2.5–4.0 mm long. Calyx glabrous to densely hairy, not ribbed; lobes unequal in size, upper lobes somewhat to distinctly broader, partially fused; adaxial lobes deltate or narrowly triangular, 1.3–4.5 mm long, apex acute; middle and lateral abaxial lobe 1.8–4.5 mm long, narrowly triangular, apex of abaxial lobes acuminate. Standard gold–yellow, yellow, apricot, orange, cream–pink, salmon–pink or vermillion, with a claw 2.5–6.2 mm long; lamina with a red line (sometimes radiating) surrounding a basal, ovate, yellow to white eye, circular to reniform, non-auriculate, 6.2–12.3 mm long, 5.0–11.0 mm wide, apex slightly emarginate. Wings often coherent or overlapping at apex, gold–yellow or pink to dark red, magenta or purple, with red at base, 5.6–10.2 mm long, 1.4–2.1 mm wide. Keel usually ±equal to the wings (but smaller in J. verruculosa, J. sp. Mt Lesueur), lamina red, dark red or black–red or sometimes pink at apex, grading to dark to bright red or pink at base, 5.8–9.5 mm long, 1.0–2.3 mm wide. Staminal filaments unequal, 4.0–7.8 mm long; anthers 0.3–0.6 mm long. Gynoecium without a distinct stipe or sometimes to 0.2 mm long; ovary 0.8–2.1 mm long, lateral section elliptic, velutinous, with hairs evenly distributed; style curved or hooked, 2.8–6.5 mm long, glabrous; stigma scarcely capitate or sometimes bulbous. Pod ovate, apically dehiscent, 3.5–6.0 mm long, 1.5–3.0 mm wide, the outer surface glabrous to densely hairy; the inner surface glabrous. Seed not seen or one per pod, round, ovate or obovate, smooth, light, dark or speckled brown with black spots, 2.0–2.4 mm long; aril whitish. 2n = 14.
Jennata differs from Euchilus in having consistently spiralled (v. opposite or spiral, sometimes 3-whorled) leaves; leaves tightly involute, often appearing subterete with an adaxial groove; inflorescence bracts present, scarious or leaf-like (v. absent); upper calyx lobes only slightly to distinctly enlarged (but not enlarged to the extent found in Euchilus), narrow–triangular or deltate (v. greatly enlarged, elliptic to obovate); standard claw 2.5–6.2 mm long (v. 1–2.2 mm long); ovary ovoid (v. laterally compressed); 2n = 14 (v. 16 or 18). Jennata differs from Grievea in the free (v. fused) stipules; scarious (v. absent) inflorescence bracts; upper calyx lobes being narrowly triangular or deltate (v. falcate or rounded); and standard claw long (v. short; 2–2.3 mm long). Distinguished from Pultenaea by the prominent stipules that are not fused behind the petiole.
A genus of approximately nine species (two not formally named), all endemic to the south coast of Western Australia (Fig. 6).
Distribution of Jennata species based on data from the Australasian Virtual Herbarium (see https://avh.ala.org.au/, accessed 2 November 2023). Colours represent individual species.
The genus name honours the late Jennifer (Jenny) Anne Chappill (1959–2006), who undertook extensive studies on the systematics of the legumes, publishing many new species (Chappill 1995; Doyle et al. 2000; Butcher and Chappill 2001, 2004; Crisp et al. 2005; Chappill et al. 2007, 2008; Wilkins and Chappill 2007a, 2007b, 2007c; Wilkins et al. 2010; https://www.anbg.gov.au/biography/chappill-jennifer-anne.html). The epithet is formed by a contraction of Jennifer (Jen) with the addition of the Latin nata (daughter), meaning that her work provided a foundation for the recognition of this genus. Jenny's legacy lives on through her research publications and the many individuals she trained in plant systematics at The University of Western Australia, including teaching R. L. Barrett as an undergraduate.
Hollister and Thiele (2019) describe some Pultenaea species currently placed in Jennata (e.g. P. ericifolia) as having recurved rather than involute leaves, however this appears to be a character scoring error, presumably confusing the orientation of the leaves.
| 1. | All leaves <4 mm measured in a straight line from base to apex (i.e. if recurved, do not ‘straighten’ before measuring) [leaves usually curved, mucro recurved]...J. empetrifolia Most leaves >4 mm in length...2 |
| 2. | Stipules glabrous...3 Stipule margins ciliate, body of stipule hairy or glabrous...8 |
| 3. | Bracteoles broad, surrounding calyx, apex rounded...J. radiata Bracteoles narrow, not surrounding calyx, apex acuminate...4 |
| 4. | Inflorescence bracts form a light, multi-layered involucre that is not obscured by leaves...5 Inflorescence bracts form a lax involucre or simple whorl that is partly obscured by leaves...7 |
| 5. | Inflorescence bracts fused for 1/2 to 3/4 of length...J. strobilifera Inflorescence bracts fused for ≤2/3 of length...6 |
| 6. | Growing north of 32°30´S...J. ericifolia Growing south of 33°30´S...J. sp. Southern (L.A.Orthia 39) |
| 7. | Growing south of 33°S parallel, style >4 mm long and tapering into stigma ±same diameter as style...J. verruculosa Growing north of 32°S parallel, style <4 mm long bending sharply into bulbous stigma much larger in diameter than style...J. sp. Mt Lesueur (J.S.Beard 7827) |
| 8. | Leaves mucronate; body of bracts and stipules densely hairy to velutinous, hairs on bracts, stipules and stem white and tightly curled...J. brachyphylla Leaves aristate [mucro straight]; body of bracts and stipules glabrous, or if hairy then hairs are ±straight...J. indira (9) |
| 9. | Leaves smooth and glabrous...J. indira subsp. indira Leaves verruculose and hairy...10 |
| 10. | Leaves densely hairy, some hairs short and fuzzy...J. indira subsp. monstrosita Leaves sparsely hairy, hairs long and erect...J. indira subsp. pudoides |
Jennata brachyphylla (Turcz.) R.L.Barrett & Clugston, comb. nov.
Pultenaea brachyphylla Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 279 (1853); Pultenaea verruculosa var. brachyphylla (Turcz.) Benth., Fl. austral. 2: 129 (1864). Type citation: ‘Drum. Coll. V. n. 68.’ Type: Western Australia: Swan River, 1849, J.Drummond V, 68 (holo: KW 001001211!; iso: BM 000544757!, G 00370625!, G 00370669!, K 000119042!, K 000978028!, K 000978029!, MEL 579972!, OXF [Image 0054630I], PERTH 01027883!, W 1889-0005161!).
Williamson (1921, pl. VI), as P. verruculosa var. brachyphylla; Orthia et al. (2005c, p. 165).
Jennata empetrifolia (Meisn.) R.L.Barrett & Clugston, comb. nov.
Pultenaea empetrifolia Meisn. in J.G.C.Lehmann (ed.), Pl. Preiss. 1(1): 76 (1844). Type citation: ‘In Australia occidentali Herb. Preiss. No. 865.’. Type: Western Australia: In the interior, Nov. 1840, L.Preiss [Plantae Preissiana No. 865] (lecto, here designated: NY 00026881!; isolecto: BM 000833631!, LD 1240404!, P 02297487!, S -G-9107!).
Pultenaea verticillata Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 279 (1853). Type citation: ‘Drum. V. n. 64.’ Type: Western Australia: Swan River Colony, 1848, J.Drummond V, 64 (holo: KW 001001216!; iso: BM 000833609!, G 00370635!, G 00370653!, G 00370667!, K 000119041!, NSW 37047!, OXF [Image 0054627O], PERTH 01027867!).
Williamson (1921, pl. VI); Woolcock and Woolcock (1989a, p. 130, fig.); Grieve (1998, p. 421, fig.); Orthia et al. (2005c, p. 174).
We here designated the sheet at NY as the lectotype of Pultenaea empetrifolia, as this is where Meisner’s herbarium is currently located and whether other duplicates were examined when the description was prepared is unclear.
Photographs of representative Jennata species. (a, b) Jennata brachyphylla. (c, d) J. empetrifolia. (e, f) J. ericifolia. (g, h) J. indira subsp. indira. Photograph credits: (a, b) M. Fagg; (c, d, g) L. Fedec; (e, f) R. Cumming; (h) T. Hammer. (a, b from APII, © M.Fagg 2005; c–h from iNaturalist).

Jennata ericifolia (Benth.) R.L.Barrett & Clugston, comb. nov.
Pultenaea ericifolia Benth., Sketch veg. Swan R. xiii (1839), as ‘ericaefolia’.
Type citation: no specimen cited [Western Australia: Swan River Colony]. Type: Western Australia: ‘Swan River’, 1839, J.Drummond I, 248 (lecto, designated by L.A.Orthia et al., Austral. Syst. Bot. 18(2): 178 (2005): K 000978039!; isolecto: BM 000833633!, E 00288363!, G 00370762!, G 00370764!, G 00370772!, G 00370773!, G 00370787!, K 000978040!, ?L 2034474!, P 02297489!, TCD 0014546!, U 1321696!); Western Australia: King George Sound, December 1801, R.Brown [Iter Austral. 5046] (syn: BM!).
Williamson (1921, pl. VI); Wheeler (1987,p. 293); Woolcock and Woolcock (1989a, p. 130, fig.); Grieve (1998, p. 420, fig.); Wheeler (2002, p. 775, fig.); Corrick and Fuhrer (2009, p. 69, pl. 182); Orthia et al. (2005c, p. 178).
Jennata indira (Orthia & Crisp) Orthia & R.L.Barrett, comb. nov.
Pultenaea indira Orthia & Crisp, Austral. Syst. Bot. 18(2): 183 (2005). Type: Western Australia: Cape Arid National Park, along Tagon Road, 3 km south of its junction with Merivale Rd, 25 Sept. 1985, M.G.Corrick 9538 (holo: PERTH 623512!; iso: CBG 8700882! [now at CANB], MEL 0677505!).
Jennata indira subsp. monstrosita (Orthia) Orthia & R.L.Barrett, comb. nov.
Pultenaea indira subsp. monstrosita Orthia, Austral. Syst. Bot. 18(2): 185 (2005). Type: Western Australia: [precise locality withheld for conservation reasons] W of Lake King on hwy, measured from turnoff to Ravensthorpe, 2 Nov. 2002, L.A.Orthia 78 & R.P.J. de Kok (holo: PERTH 07292600!; iso: CANB 643232!, MEL 2278697!, K 000264402!).
Orthia et al. (2005c, p. 185).
Jennata indira subsp. pudoides (Orthia) Orthia & R.L.Barrett, comb. nov.
Pultenaea indira subsp. pudoides Orthia, Austral. Syst. Bot. 18(2): 186 (2005). Type: Western Australia: [precise locality withheld for conservation reasons] NE of Arthur River townsite WA, 15 Sept. 2001, F.Obbens & H.Jenson FO 26/01 (holo: PERTH 06115071!).
Orthia et al. (2005c, p. 186).
Jennata radiata (H.B.Will.) R.L.Barrett & Clugston, comb. nov.
Pultenaea radiata H.B.Will., Proc. Roy. Soc. Victoria 33: 137, pl. VI (1921). Type citation: ‘In National Herbarium, Vic., from Busselton, W.A., 1870 A. and E. Pries, among specimens of P. verruculosa, var. pilosa.’ Type: Western Australia: Busselton, 1870, A. & E. Pries s.n. (lecto, designated by L.A.Orthia et al., Austral. Syst. Bot. 18(2): 195 (2005): MEL 35273!; isolecto: BM 000544778!, K 000119000!, K 000119001!, K H 278/98 88!, MEL 2055276!, NSW 37001!).
Williamson (1921, pl. VI); Woolcock and Woolcock (1989a, p. 131, fig., 136, pl.); Grieve (1998, p. 421, fig.); Wheeler (2002, p. 776, fig.); Scott (2019, p. 83, pl.); Orthia et al. (2005c, p. 195).
Jennata strobilifera (Meisn.) R.L.Barrett & Clugston, comb. nov.
Pultenaea strobilifera Meisn. in J.G.C. Lehmann (ed.), Pl. Preiss. 1(1): 75 (1844). Type citation: ‘In regionibus interioribus Australiae meridionali-occidentalis, m. Oct. Herb. Preiss. No. 1185. et in districtu Minto, m. Nov. 1840. No. 1190. (Drummond n. 247.).’ Type: Western Australia: in the interior regions, Oct. 1840, L.Preiss [Plantae Preissiana No. 1185] (lecto, designated by L.A. Orthia et al., Austral. Syst. Bot. 18(2): 180 (2005): LD 1240524!); Western Australia: Minto district, Nov. 1840, L.Preiss [Plantae Preissiana No. 1190] (syn: G 00370630!, G 00370644!, LD 1240584!, NY 00026902!); Western Australia: ‘Swan River Colony’, 1843, J.Drummond 247 (syn: E 00288342!, G 00370785!, G 00370793!).
Pultenaea pteronioides Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 280 (1853). Type citation: ‘Drum. V. n. 67.’ Type: Western Australia: 1849, J.Drummond V, 67 (holo: KW!; iso: BM 000544734!, CGE!, G 00370632!, K 000119028!, NSW 37048!, OXF!, PERTH 01027875!, W!).
Williamson (1921, pl. VI); Grieve (1998, p. 420, fig.); Orthia et al. (2005c, p. 180).
Orthia et al. (2005c) listed an isolectotype of Pultenaea strobilifera at NY, however this specimen is a syntype, being a duplicate of Preiss 1190.
Photographs of representative Jennata species. (a, b) Jennata radiata. (c, d) J. strobilifera. (e, f) J. verruculosa. Photograph credits: (a, b) J. Santore; (c, d) M. Fagg; (e, f) M.D. Crisp. (a, b from iNaturalist; c–f from APII, c, d © M.Fagg 1988; e, f, © Australian National Botanic Gardens 1993).

Jennata verruculosa (Turcz.) R.L.Barrett & Clugston, comb. nov.
Pultenaea verruculosa Turcz., Bull. Soc. Imp. Naturalistes Moscou 26(1): 278 (1853). Type citation: ‘Drum. V. n. 65.’ Type: Western Australia: ‘Swan River Colony’, Cape Riche, 1848–1850, J.Drummond V, 65 (holo: KW!; iso: BM 000544739!, CGE!, E 00288340!, G 00370668!, G 00370670!, K 000978030!, K 000978031!, K 000978032!, K 000119002!, MEL 579971!, NSW 37046!, OXF [Image 0054620H]!, P 02928293! PERTH 01025856!, W 1889-0005164!).
Pultenaea verruculosa var. pilosa Benth., Fl. austral. 2: 129 (1864). Type citation: ‘Cheynes Beach, Oldfield.’ Type: Western Australia: Cheynes Beach, A.Oldfield s.n. (holo: K 000978026!).
Williamson (1921, pl. VI); Woolcock and Woolcock (1989a, p. 132, fig.); Grieve (1998, p. 421, fig.); Wheeler (2002 776, fig.); Orthia et al., p.(2005c, p. 181).
Jennata sp. Mt Lesueur (J.S.Beard 7827) WA Herbarium
Pultenaea sp. Mt Lesueur (J.S.Beard 7827) WA Herbarium (1998)
Pultenaea sp. Mt Lesueur (Beard 7827) [Orthia et al. (2005c), p. 182]
Orthia et al. (2005c, p. 182), as P. sp. Mt Lesueur (Beard 7827).
Jennata sp. southern (L.A.Orthia 39) WA Herbarium
Pultenaea ericifolia ‘southern’ (Orthia 39)
Pultenaea sp. southern (L.A.Orthia 39) WA Herbarium (1998)
Orthia et al. (2005c, p. 180).
Pultenaea verruculosa var. recurva Benth., Fl. austral. 2: 129 (1864).
Type citation: ‘King George’s Sound, Collie.’
Application of this name remains uncertain as authentic type material is yet to be traced. As relatively comprehensive searches for authentic material have been undertaken, either the citation or specimen label is possibly in error and refers to a collection attributed to ‘Bagster’ [Baxter], K 000978033! that is a specimen of Jennata empetrifolia from King George Sound, mounted with a specimen of J. verruculosa. Both collectors were active around King George Sound in the late 1820’s and confusion of attribution is entirely possible.
Loricobbia R.L.Barrett, Clugston & Orthia, gen. nov.
Type species: Loricobbia reticulata (Sm.) R.L.Barrett & T.Macfarlane [≡Daviesia reticulata Sm.].
Pultenaea sect. Aciphyllum Benth., Fl. Austral. 2: 109, 119 (1864). Type species: Pultenaea aciphylla Benth. [=Loricobbia reticulata]
Open to dense, spreading to erect, few- or much-branched or multi-stemmed, rounded or sometimes slender shrubs, 0.3–3(–5) m high, 0.3–1.5(–3) m wide. Stems and lower branches glabrous or glabrescent. Branchlets apically glabrous to densely velutinous, without tubercles, not spinescent. Stipules pale to mid brown, red–brown or mostly transparent with a brown keel, persistent or breaking irregularly and sometimes blackened with age, the bases fused behind the petiole, ovate to deltate, narrowly triangular or almost linear, glabrous to sparsely hairy, 0.7–4.5(–5.5) mm long. Leaves frequently clustered at branchlet apices, appressed to inclined or ascending, usually spiralled (alternate in L. pinifolia); petiole subterete or pulvinate, decurrent (weakly in L. pinifolia), 0.1–2.0 mm long; blade linear to narrowly elliptic or ovate, straight to strongly incurved, 3.0–40 mm long, 0.6–4.0(–7.1) mm wide (1.2–10.4 mm wide in L. reticulata), abaxial surface pale green, yellow–green, grey–green or dark green, prominent pinnately netted venation (sometimes yellow when dry), midvein prominent on older leaves, glabrous or with sparse to dense, white, appressed or erect hairs on new growth, glabrescent, smooth when fresh, ridged and rigid when dried; adaxial surface paler, not concealed, margins flat to incurved (rarely undulate) or revolute; apex acute to acuminate or obtuse, sometimes slightly recurved, mucronate to aristate. Inflorescence terminal, in axillary stems, determinate. Inflorescence bracts scarious or leaf-like, often reduced. Peduncle absent. Pedicels absent or sometimes to 1.0 mm long (or 3.5–8.0 mm long in L. pinifolia and L. skinneri), straight. Bracteoles usually persistent slightly below the calyx (2.0–2.5 mm below the calyx in L. pinifolia and L. skinneri), glabrous to densely hairy, scarious, pale brown, yellow–brown or red–brown, elliptic to ovate, 1.2–9.5 mm long, 0.5–1.5 mm wide, apex acute or obtuse. Flower subtending bracts brown or red–brown, 2.5–7.5 mm long, leaf-like but much reduced, glabrous to densely hairy, scarious. Hypanthium 1.9–5.0 mm long. Calyx sparsely to densely hairy, not ribbed; all lobes ±equal in size, deltate to triangular, but upper lobes fused, hence calyx asymmetrical; adaxial lobes 1.8–3.7 mm long (1.9–7.0 mm long in L. reticulata); abaxial lobes 1.7–3.5 mm long (2.9–6.7 mm long in L. reticulata); apex of all lobes acute to acuminate. Standard with a claw 1.5–4.0 mm long; lamina yellow or gold with red or red–brown markings surrounding a basal, ovate to circular yellow eye, circular, non-auriculate, 7.0–15.0 mm long, 8.3–16.0 mm wide, apex slightly emarginate. Wings not coherent except sometimes near apex, yellow or gold with red base, 7.0–12.0 mm long, 1.2–4.7 mm wide. Keel ±equal to the wings but protruding at apex, lamina pale green or yellow with pink tinge, orange–brown or dark red–brown, 7.5–11.2 mm long, 2.3–4.8 mm wide. Staminal filaments ±equal, 5.0–9.0 mm long (~14 mm long in L. skinneri); anthers 0.5–1.2 mm long. Gynoecium sessile or with a stipe to 0.2 mm long; ovary 1.4–2.3 mm long, elliptic in cross section, velutinous, erect to spreading white hairs, evenly distributed; style straight to curved or hooked, 5.0–10.5 mm long, glabrous; stigma scarcely capitate. Pod ovate (sometimes almost orbicular), apically dehiscent, 4.5–11.0 mm long, 3.5–7.5 mm wide, the outer surface (sometimes glabrous) sparsely to densely hairy; the inner surface sparsely hairy. Seeds 2 per pod, obovate to reniform, smooth, dark brown to black or shiny black, 3.3–5.0 mm long; aril whitish. 2n = 8.
Distinguished from Pultenaea by the presence of scarious inflorescence bracts (absent in Pultenaea) and ovoid (v. laterally flattened) ovaries that are hairy (v. glabrous) inside; and from Latrobea by the fused (v. free) stipules, scarious or leaf-like (v. leaf-like) floral bracts, two-lobed, asymmetrical (v. symmetrical) calyx lobes, emarginate (v. sagittate) standard petal, ovoid (v. laterally flattened) ovary, hairy (v. glabrous) inner surface of pods and 2n = 8 (v. 14); Table 3.
A genus of six species, all of which are endemic to the south coast of Western Australia (Fig. 9).
Distribution of Loricobbia species based on data from the Australasian Virtual Herbarium (see https://avh.ala.org.au/, accessed 2 November 2023). Colours represent individual species.
The new genus honours the late Lorraine (Lori) Cobb (1951–2008), who illustrated the legumes in Grieve (1998) and Wheeler (2002), and taught R. L. Barrett in plant systematics laboratory classes (see also Shepherd and Lyons 2009; https://asbs.org.au/wp-content/uploads/2023/08/08-june-135.pdf#page=10).
Grieve (1998) grouped many of these species together on the basis of ‘leaves rigid, concave, keeled or flat with conspicuous nerves.’ Pultenaea pinifolia and P. skinneri were keyed nearby based on the shared character of ‘Leaves recurved or revolute margins or channelled below.’
| 1. | Leaves without prominent secondary venation...2 Leaves with prominent reticulate or pinnate venation...4 |
| 2. | Leaf margins flat; keel pale green...L. pauciflora Leaf margins recurved; keel red to brown...3 |
| 3. | Leaves >10 mm long; held ascending to inclined...L. pinifolia Leaves <10 mm long; held divergent to reflexed...L. skinneri |
| 4. | Leaf venation pinnate; keel pale green or yellow...L. aspalathoides Leaf venation reticulate; keel red to brown...5 |
| 5. | Stipules partially enclose stem; leaf apex blunt...L. ochreata Stipules do not enclose stem; leaf apex acute to aristate...L. reticulata |
Loricobbia aspalathoides (Meisn.) R.L.Barrett & T.D.Macfarl., comb. nov.
Pultenaea aspalathoides Meisn. in J.G.C.Lehmann (ed.), Pl. Preiss. 1(1): 73 (1844). Type citation: ‘In solo turfoso-subarenoso sylvae et planitiei prope oppidum Albany (Plantagenet) m. Oct. et Nov. 1840, Herb. Preiss. No. 838 et 1195.’ Type: Western Australia: near Albany (Plantagenet), Nov. 1840, L.Preiss [Plantae Preissianae No. 838] (lecto, designated by L.A.Orthia et al., Austral. Syst. Bot. 18(2): 163 (2005): LD 1814385!; isolecto: BR 0000013349769!, G 00370628!, G 00370631!, GOET 005258!, HBG 519213!, K 000119074!, K 000118998!, L 2034567!, L 2034568!, M 0219169!, MO 277386!, NY 00026871!, P 00689376!, P 02297470!, S -G-9112!, W!, W!); Western Australia: near Albany (Plantagenet), 8 Oct. 1840, L.Preiss [Plantae Preissianae No. 1195] (syn: LD 1809713!, NY 00026872!).
Williamson (1920, pl. XIV); Woolcock and Woolcock (1985, p. 75); Grieve (1998, p. 419, fig.); Wheeler (2002, p. 774, fig.); Orthia et al. (2005c, p. 163).
Loricobbia ochreata (Meisn.) R.L.Barrett & T.D.Macfarl., comb. nov.
Pultenaea ochreata Meisn. in J.G.C.Lehmann (ed.), Pl. Preiss. 1(1): 75 (1844). Type citation: ‘In districtu Wellington, d. 5. Dec. 1839. Herb. Preiss. No. 1038.’ Type: Western Australia: Wellington District, 5 Dec. 1839, L.Preiss [Plantae Preissianae No. 1038] (lecto, here designated: NY 00026891!; isolecto: LD 1240464!).
Williamson (1920, pl. XIV); Wheeler (1987, p. 294); Grieve (1998, p. 420, fig.); Wheeler (2002, p. 773, fig.); Orthia et al. (2005c, p. 186).
Loricobbia pauciflora (M.B.Scott.) R.L.Barrett & T.D.Macfarl., comb. nov.
Pultenaea pauciflora M.B.Scott, Bull. Misc. Inform. Kew 1914(10): 378 (1914). Type: Western Australia: Narrogin Experiment Farm, 31 Mar. 1914, F.Stoward 64 (lecto, here designated: K 000119050!; isolecto: MEL 2055266!).
Williamson (1920, pl. XIV); Woolcock and Woolcock (1985, p. 83); Brown et al. (1998, p. 124, pl.); Grieve (1998, p. 419, fig.); Orthia et al. (2005c, p. 187).
There are two syntypes that may well have been a single collection whenScott examined the material, with Williamson subsequently obtaining material from K that is currently held at MEL but we here designate the sheet at K as the lectotype as this history cannot be verified.
Millar and Byrne (2013) found that this taxon has two distinct genetic clusters that likely warrant taxonomic distinction despite no clear morphological separation between the populations.
Loricobbia pinifolia (Meisn.) R.L.Barrett & T.D.Macfarl., comb. nov.
Pultenaea pinifolia Meisn. in J.G.C.Lehmann (ed.), Pl. Preiss. 2(2–3): 220 (1848). Type citation: ‘Swan River, Drummond coll. II. No. 109.’ Type: Western Australia: 1842–3, J.Drummond II, 109 (lecto, here designated: LD 1803394!; isolecto: BM 000833629!, G 00370640!, G 00370642!, K 000119051!, K 000119052!, K 000119071!, K 000119072!, OXF [Image 0054619P]!, P 03062133!, W!; ?isolecto: K H/278/98 280!, K H/278/98 278!).
Williamson (1920, pl. XIV); Grieve (1998, p. 418, fig.); Wheeler (2002, p. 774, fig.); Scott (2019, p. 103, pl.); Orthia et al. (2005c, p. 189).
We here designate the sheet at LD as the lectotype of Pultenaea pinifolia, as Preiss’ primary set of specimens is mostly held there and no material was located at NY where Meisner’s herbarium is currently held.
Photographs of rpresentative species of Loricobbia. (a, b) Loricobbia pinifolia. (c–f) L. reticulata. (g–i) L. skinneri. Photograph credits: (a, b) C.E. Woolcock; (c, e) R.L. Barrett; (d) iNaturalist user pimelea; (f) K. Morris; (g) J. Santore; (h, i) C. Jonkers. (a, b from APII, © Australian National Botanic Gardens 1985; d, f–i from iNaturalist).

Loricobbia reticulata (Sm.) R.L.Barrett & T.D.Macfarl., comb. nov.
(Fig. 11c–f.)
Daviesia reticulata Sm. in A.Rees (ed.), Cycl. 11(21): [Daviesia sp. no. 4] (1808); Jacksonia reticulata (Sm.) DC. in A.P.Candolle (ed.), Prodr. 2: 107 (1825); Pultenaea reticulata (Sm.) Benth., Fl. austral. 2: 119 (1864). Type citation: ‘Mr Menzies, near King George’s Sound, New Holland.’ Type: Western Australia: King George Sound, 1792, A.Menzies s.n. (lecto, here designated: BM 000544700!; isolecto: LINN -HS730-11!, G 00488181!).
Pultenaea aciphylla Benth. in S.F.L.Endlicher et al., Enum. pl. 35 (1837). Type citation: ‘King Georges Sound. (Hügel.)’. Type: Western Australia: King George Sound, C.A.A. von Hügel 105 (holo: W 0046865!).
Pultenaea aciphylla var. latifolia Meisn. in J.G.C.Lehmann (ed.), Pl. Preiss. 1(1): 74 (1844). Type citation: ‘In solo sublimoso distr. Sussex, m. Dec. 1839. Herb. Preiss. No. 1044.’ Type: Western Australia: Sussex District, December 1839, L.Preiss [Plantae Preissiana No. 1044] (holo: LD 1809426!).
Williamson (1920, pl. XIV), as P. aciphylla; Woolcock and Woolcock (1987, p. 134, fig.); Wheeler (1987, p. 294); Grieve (1998, p. 419, fig.); Scott and Negus (2002, p. 108, fig. 2); Wheeler (2002, p. 775, fig.); Scott (2019, p. 82, pl.); Orthia et al. (2005c, p. 196).
While Smith may have primarily used the material currently at LINN to write the description of Daviesia reticulata, he is also assumed to have seen the material currently held at BM, and we here designate the sheet at BM as lectotype as this has the more ample material and an original label that bears the species epithet ‘reticulata’.
Loricobbia skinneri (F.Muell.) R.L.Barrett & T.D.Macfarl., comb. nov.
(Fig. 11g–i.)
Pultenaea skinneri F.Muell., Fragm. 8(66): 166 (1874). Type citation: ‘Ad flumen Blackwood-River; McHard.’ Type: Western Australia: Blackwood River, 1873, McHard s.n. (lecto, designated by L.A. Orthia et al., Austral. Syst. Bot. 18(2): 199 (2005): MEL 625090!; isolecto: CAN 143454, n.v., CANB 139645!, MEL 625091!, MEL 2057199!, NY 00026900!, PERTH 00635138!, US 00003019!, US 01107569!).
Williamson (1920, pl. XIII); Woolcock and Woolcock (1987, p. 135, fig.); Wheeler (1987, p. 294); Grieve (1998, p. 418, fig.); Wheeler (2002, p. 775, fig.); Orthia et al. (2005c, p. 199).
Pultenaea Sm., Spec. bot. New Holland 1(3): 35 (1794)
Type species: Pultenaea stipularis Sm.
Pultenaea sect. Eupultenaea Benth., Fl. austral. 2: 108 (1864), nom. inval.
Pultenaea sect. Coelophyllum Benth., Fl. austral. 2: 109 (1864), nom. inval., p.p., excl. Informal Group D.
Open to dense, prostrate or spreading to erect, single or multi-stemmed shrubs or occasionally small trees, sometimes rhizomatous and clonal, 0.05–4.0(–5.0) m high, 0.2–5.0 m wide. Stems and lower branches glabrous, glabrescent or with persistent hairs. Branchlets apically with sparse to dense, appressed to ascending, curved, crisped or erect, ciliate, pilose, sinuous or villose hairs, with or without tubercles, not spinescent, sometimes glaucous. Stipules conspicuous, pale brown, red–brown, dark brown or grey, persistent or fraying and turning grey with age, margins scarious fused (for 1/10–9/10 of length), lanceolate, deltate or narrowly triangular, glabrous to densely hairy, 0.5–11.0 mm long. Leaves divergent to ascending, rarely descending, alternate, spiralled or whorled, rarely (and never exclusively) decussate, rarely fasciculate; petiole subterete or pulvinate, not or shortly decurrent, 0.1–1.5(–3) mm long; blade linear, lanceolate, narrowly oblong, oblanceolate, ovate, obovate, rarely orbicular, or clavate, straight to strongly recurved, 1.0–42.0 mm long, 0.2–20.0 mm wide, concolourous to strongly discolourous, abaxial surface pale, olive, mid-green, bright green or glaucous, sometimes grey–green and new growth sometimes bronze, midvein prominent or not, glabrous to densely white, appressed, matted, spreading or erect hairy, smooth or weakly to strongly verruculose; adaxial surface concealed or not, glabrous to densely hairy; margins flat, involute or revolute; apex obtuse, acute or sometimes acuminate, usually mucronate or aristate, sometimes obtuse. Inflorescence with flowers terminal determinate, terminal indeterminate or axillary and solitary indeterminate. Inflorescence bracts absent. Peduncle present or absent. Flower-subtending bracts usually scarious and pale to red–brown, commonly enlarged or occasionally leaf-like and green, sometimes completely fused to bracteoles, 0.8–7.7 mm long, shortly hairy on margins or glabrous. Pedicels present, 0.5–7.0 mm long. Bracteoles persistent slightly below or variously on the tube of the calyx, free, glabrous to densely hairy, scarious, pale brown to red–brown, lanceolate, elliptic to falcate or ovate, 0.1–7.5 mm long, 0.1–1.4 mm wide, apex acute to acuminate, glabrous to hispid on margins or bracteoles absent in some species. Hypanthium 0.6–3.6 mm long. Calyx glabrous to densely hairy, not or weakly ribbed; lobes ±equal to unequal in size, 0.9–7.8 mm long, upper lobes variously fused, of similar width to lower lobes or up to 2× as broad; adaxial lobes deltate or narrowly triangular, apex acute to acuminate, rarely subobtuse; middle and lateral abaxial lobe narrowly triangular, apex of abaxial lobes acuminate. Standard with a claw 1.2–5.1 mm long; lamina yellow, gold–yellow, apricot, orange, burnt orange or red, rarely pink, 6.0–15.0 mm long, 5.8–15.6 mm wide, with a red line, arch or series of flecks (sometimes radiating) surrounding a basal, ±ovate, yellow to pale yellow eye, circular to reniform, non-auriculate, apex slightly emarginate. Wings held over keel, coherent for length or sometimes held apart, yellow, gold–yellow, orange to deep red or rarely pink, commonly with red at base, 5.0–14.3 mm long, 0.9–2.6 mm wide. Keel ± equal to or slightly longer or shorter than the wings, yellow, gold–yellow, orange to deep red or rarely pink, commonly with red at base, 5.0–14.0 mm long, 1.2–3.7 mm wide. Staminal filaments subequal to unequal, 3.9–12.3 mm long; anthers 0.4–0.6 mm long. Gynoecium without a distinct stipe or sometimes to 0.2 mm long; ovary 0.8–3.9 mm long, lateral section compressed to subelliptic, glabrous to velutinous, with hairs when present white, appressed to erect, evenly distributed or only towards the apex; style hooked or curved, 3.6–9.7 mm long, glabrous or hairy near the base; stigma scarcely capitate. Pod ovate, apically dehiscent, 3.0–10.0 mm long, 2.2–3.9 mm wide, the outer surface glabrous to densely hairy; the inner surface glabrous. Seeds two per pod (often only 1 developing), ovate or obovate, smooth to very finely reticulate, light to dark brown, olive–brown or speckled brown with black spots, 1.2–4.3 mm long; aril whitish, often variously divided. 2n = 12–32.
Pultenaea can be defined by the prominent stipules that are fused behind the petiole. Fused stipules are independently found in Grievea that differs in having the upper calyx lobes falcate or rounded and fused for the full length (v. only partly fused), and a consistently short claw 2.0–2.3 mm long on the standard (v. 1.2–5.1 mm long in Pultenaea); and Loricobbia that has ovoid (v. laterally flattened) ovaries that are hairy (v. glabrous) on the inside.
A genus of over 150 species, almost all of which are endemic to eastern Australia, with a single species with a disjunct distribution across the Nullarbor into Western Australia (Fig. 12).
Distribution of Pultenaea species based on data from the Australasian Virtual Herbarium (see https://avh.ala.org.au/, accessed 2 November 2023). Colours represent individual species.
Two of Bentham’s Pultenaea sections appear to be incongruently essentially based on the same type. However, the International Code of Nomenclature requires that infrageneric names with the prefix Eu- be considered invalid names for the typical (autonym) section and sections including the type species of a genus must also be considered invalid (Turland et al. 2018, Art. 21.3, 22.1, 22.2). In this case, Bentham (1864) erred in not including P. stipularis in sect. Eupultenaea.
Data availability
The data that support this study are available in the article or cited publications.
Conflicts of interest
Dr Russell Barrett and Brendan Lepschi are editors for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practise when handling manuscripts submitted to this journal by an editor. Editors of Australian Systematic Botany are encouraged to publish in the journal and kept completely separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This study was funded by a Postdoctoral Fellowship Grant from the Australian Biological Resources Study (ABRS) National Taxonomy Research Grant Program (NTRGP 4-EHP5TK3) to James Clugston and collaborators at the Royal Botanic Gardens and Domain Trust, Sydney. We acknowledge the contribution of the Genomics for Australian Plants Framework Initiative consortium (https://www.genomicsforaustralianplants.com/consortium/) in the generation of data supporting this publication. The Initiative is supported by funding from Bioplatforms Australia (enabled by NCRIS), the Ian Potter Foundation, Royal Botanic Gardens Foundation (Victoria), Royal Botanic Gardens Victoria, the Royal Botanic Gardens and Domain Trust, the Council of Heads of Australasian Herbaria, CSIRO, Centre for Australian National Biodiversity Research and the Department of Biodiversity, Conservation and Attractions, Western Australia.
Acknowledgements
We thank Gillian Craig for supplying DNA samples and photographs. Curators and staff at CANB, MEL, NSW and PERTH are thanked for assistance while studying specimens in these collections. We thank Russell Cumming, Rob Davis, David Edmonds, Murray Fagg, Loxley Fedec, Tim Hammer, Chris Jonkers, Keith Morris, user pimelea, Joey Santore and the late Collin Woolcock for uploading photographs to iNaturalist or providing these to the Australian Plant Image Index (APII). Anna Monro facilitated permission to use images in the APII and provided extensive notes that improved the formatting and typifications in the paper. Todd McLay and Ian Telford provided constructive feedback on an earlier version of the manuscript.
Author contributions
All authors conceived the study, conducted fieldwork, interpreted results, prepared taxon treatments and wrote and revised the paper.
References
Andrews HC (1803) Pultenaea ilicifolia. The Botanist’s Repository for New, and Rare Plants 5, t. 320.
| Google Scholar |
Andrews HC (1804) Pultenaea rubiifolia. The Botanist’s Repository for New, and Rare Plants 5, t. 351.
| Google Scholar |
Andrews HC (1807) Oxylobium cordifolium. The Botanist’s Repository for New, and Rare Plants 7(101), t. 492.
| Google Scholar |
Baker WJ, Dodsworth S, Forest F, Graham SW, Johnson MG, McDonnell A, Pokorny L, Tate JA, Wicke S, Wickett NJ (2021) Exploring Angiosperms353: an open, community toolkit for collaborative phylogenomic research on flowering plants. American Journal of Botany 108, 1059-1065.
| Crossref | Google Scholar | PubMed |
Baker WJ, Bailey P, Barber V, Barker A, Bellot S, Bishop D, Botigué LR, Brewer G, Carruthers T, Clarkson JJ, Cook J, Cowan RS, Dodsworth S, Epitawalage N, Françoso E, Gallego B, Johnson MG, Kim JT, Leempoel K, Maurin O, Mcginnie C, Pokorny L, Roy S, Stone M, Toledo E, Wickett NJ, Zuntini AR, Eiserhardt WL, Kersey PJ, Leitch IJ, Forest F (2022) A comprehensive phylogenomic platform for exploring the Angiosperm Tree of Life. Systematic Biology 71, 301-319.
| Crossref | Google Scholar | PubMed |
Barrett RL, Hopper SD, Farrer SL (2005) Preface to ‘Generic concepts and modern taxonomy’. Australian Systematic Botany 18(1), i-ii.
| Crossref | Google Scholar |
Barrett RL, Clugston JAR, Cook LG, Crisp MD, Jobson PC, Lepschi BJ, Renner MAM, Weston PH (2021) Understanding diversity and systematics in Australian Fabaceae tribe Mirbelieae. Diversity 13(8), 391.
| Crossref | Google Scholar |
Barrett RL, Clugston JAR, Albrecht DE, Elkan L, Hosking JR, McCune SF, Jobson PC, Orme AE, Palsson RL, Renner MAM, Wardrop C, Weston PH (2024) Revision of the Pultenaea setulosa species complex (Fabaceae: Mirbelieae) including fourteen new species. Australian Systematic Botany 37, SB23014.
| Crossref | Google Scholar |
Bickford SA, Laffan SW, de Kok RPJ, Orthia LA (2004) Spatial analysis of taxonomic and genetic patterns and their potential for understanding evolutionary histories. Journal of Biogeography 31, 1715-1733.
| Crossref | Google Scholar |
Butcher R, Chappill JA (2001) Sphaerolobium macranthum (Leguminosae: Mirbelieae) complex revised. Australian Systematic Botany 14, 155-173.
| Google Scholar |
Butcher R, Chappill JA (2004) A revision of the Sphaerolobium fornicatum complex (Leguminosae: Mirbelieae) from south-west Western Australia. Australian Systematic Botany 17, 423-439.
| Crossref | Google Scholar |
Chappill JA, Wilkins CF, Crisp MD (2007) Taxonomic revision of Jacksonia (Leguminosae: Mirbelieae). Australian Systematic Botany 20, 473-623.
| Crossref | Google Scholar |
Chappill JA, Wilkins CF, Crisp MD (2008) Taxonomic revision of Gompholobium (Leguminosae: Mirbelieae). Australian Systematic Botany 21, 67-151.
| Crossref | Google Scholar |
Crisp MD (1982) Notes on Daviesia and Pultenaea (Fabaceae) in South Australia. Journal of the Adelaide Botanic Gardens 6, 55-66.
| Google Scholar |
Crisp MD, Cook LG (2003a) Molecular evidence for definition of genera in the Oxylobium group (Fabaceae: Mirbelieae). Systematic Botany 28, 705-713.
| Google Scholar |
Crisp MD, Cook LG (2009) Explosive radiation or cryptic mass extinction? Interpreting signatures in molecular phylogenies. Evolution 63, 2257-2265.
| Crossref | Google Scholar | PubMed |
Crisp MD, Weston PH (1991) Almaleea, a new genus of Fabaceae from south-eastern Australia. Telopea 4, 307-311.
| Crossref | Google Scholar |
Crisp MD, Gilmore SR, Weston PH (1999) Phylogenetic relationships of two anomalous species of Pultenaea (Fabaceae: Mirbelieae), and description of a new genus. Taxon 48, 701-714.
| Crossref | Google Scholar |
Crisp MD, Cook LG, Steane DA (2004) Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities? Philosophical Transactions of the Royal Society of London – B. Biologial Sciences 359, 1551-1571.
| Crossref | Google Scholar | PubMed |
de Kok RPJ, West JG (2002) A revision of Pultenaea (Fabaceae) 1. Species with ovaries glabrous and/or with tufted hairs. Australian Systematic Botany 15, 81-113.
| Crossref | Google Scholar |
de Kok RPJ, West JG (2003) A revision of the genus Pultenaea (Fabaceae). 2. Eastern Australian species with velutinous ovaries and incurved leaves. Australian Systematic Botany 16, 229-273.
| Crossref | Google Scholar |
de Kok RPJ, West JG (2004) A revision of the genus Pultenaea (Fabaceae). 3. The eastern species with recurved leaves. Australian Systematic Botany 17, 273-326.
| Crossref | Google Scholar |
de Storme N, Mason A (2014) Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Current Plant Biology 1, 10-33.
| Crossref | Google Scholar |
Grieve BJ (1975) Botany in Western Australia: a survey of progress: 1900–1971. Presidential Address. Journal of the Royal Society of Western Australia 58, 33-53.
| Google Scholar |
Hollister C, Thiele KR (2019) Pea flowers of Western Australia. In ‘Florabase – the Western Australian Flora: interactive keys’. (Department of Biodiversity, Conservation and Attractions) Available at https://florabase.dbca.wa.gov.au/keys/ [Verified 13 May 2024]
Jackson C, McLay TGB, Schmidt‐Lebuhn AN (2023) hybpiper‐nf and paragone‐nf: containerization and additional options for target capture assembly and paralog resolution. Applications in Plant Sciences 11, e11532.
| Crossref | Google Scholar | PubMed |
Johnson MG, Pokorny L, Dodsworth S, Botigué LR, Cowan RS, Devault A, Eiserhardt WL, Epitawalage N, Forest F, Kim JT, Leebens-Mack JH, Leitch IJ, Maurin O, Soltis DE, Soltis PS, Wong GK, Baker WJ, Wicket NJ (2018) A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k-medoids clustering. Systematic Biology 68, 594-606.
| Crossref | Google Scholar | PubMed |
Keighery GJ (1984) Chromosome numbers in Western Australian plants. II. Journal of the Royal Society of Western Australia 67, 26-27.
| Google Scholar |
Kellermann J, Mosyakin SL, Clowes C, Udovicic F (2022) Australian species of Rhamnaceae published by Turczaninow, their types, current names and synonyms. Swainsona 36, 135-151.
| Google Scholar |
McLay TGB, Birch JL, Gunn BF, Ning W, Tate JA, Nauheimer L, Joyce EM, Simpson L, Schmidt‐Lebuhn AN, Baker WJ, Forest F, Jackson CJ (2021) New targets acquired: improving locus recovery from the Angiosperms353 probe set. Applications in Plant Sciences 9, e11420.
| Crossref | Google Scholar | PubMed |
Millar MA, Byrne M (2013) Cryptic divergent lineages of Pultenaea pauciflora M.B. Scott (Fabaceae: Mirbelieae) exhibit different evolutionary history. Biological Journal of the Linnean Society 108, 871-881.
| Crossref | Google Scholar |
Mosyakin SL, de Lange PJ (2019) Notes on typification and nomenclature of four taxa of Geraniaceae described by Turczaninow from New Zealand and Australia. Phytotaxa 419, 169-181.
| Crossref | Google Scholar |
Mosyakin SL, McNeill J, Boiko GV (2019) Comments on proper type designation for names of taxa validated by Turczaninow in his Animadversiones, with case studies. Ukrainian Botanical Journal 76, 379-389.
| Crossref | Google Scholar |
Mueller FJH (1882) Notes on some Leguminous plants. The Chemist and Druggist with Australasian Supplement 5(13), 13.
| Google Scholar |
Nauheimer L, Weigner N, Joyce EM, Crayn D, Clarke C, Nargar K (2021) HybPhaser: a workflow for the detection and phasing of hybrids in target capture data sets. Applications in Plant Sciences 9, e11441.
| Crossref | Google Scholar | PubMed |
Nelson EC (2004) William McNab’s herbarium in the National Botanic Gardens, Glasnevin, Dublin (DBN) with catalogues of specimens. Parts 1–4. Scottish Naturalist 116, 43-181.
| Google Scholar |
Nge FJ, Biffin E, Thiele KR, Waycott M (2020) Extinction pulse at Eocene–Oligocene boundary drives diversification dynamics of two Australian temperate floras. Proceedings of the Royal Society of London – B. Biological Sciences 287, 20192546.
| Crossref | Google Scholar | PubMed |
Nge FJ, Biffin E, Waycott M, Thiele KR (2021) Phylogenomics and continental biogeographic disjunctions – insight from the Australian starflowers (Calytrix). American Journal of Botany 109, 291-308.
| Crossref | Google Scholar | PubMed |
Orthia LA, Cook LG, Crisp MD (2005a) Generic delimitation and phylogenetic uncertainty: an example from a group that has undergone an explosive radiation. Australian Systematic Botany 18, 41-47.
| Crossref | Google Scholar |
Orthia LA, Crisp MD, Cook LG, de Kok RPJ (2005b) Bush peas: a rapid radiation with no support for monophyly of Pultenaea (Fabaceae: Mirbelieae). Australian Systematic Botany 18, 133-147.
| Crossref | Google Scholar |
Orthia LA, de Kok RPJ, Crisp MD (2005c) A revision of Pultenaea (Fabaceae: Mirbelieae). 4. Species occurring in Western Australia. Australian Systematic Botany 18, 149-206.
| Crossref | Google Scholar |
Renner MAM, Barrett RL, Clarke S, Clugston JAR, Wilson TC, Weston PH (2022) Morphological and molecular evidence refute a broad circumscription for Pultenaea glabra (Fabaceae: Mirbelieae), with implications for taxonomy, biogeography, and conservation. Australian Systematic Botany 35, 225-277.
| Crossref | Google Scholar |
Sands VE (1975) The cytoevolution of the Australian Papilionaceae. Proceedings of the Royal Society of New South Wales 100, 118-155.
| Google Scholar |
Shepherd KA, Lyons MN (2009) Three new species of Tecticornia (Chenopodiaceae, subfamily Salicornioideae) identified through Salinity Action Plan surveys of the wheatbelt region, Western Australia. Nuytsia 19, 167-180.
| Crossref | Google Scholar |
Simpson L, Cantrill D, Byrne M, Allnutt T, King G, Lum M, Al Bkhetan Z, Andrew R, Baker W, Barrett MD, Batley J, Berry O, Binks R, Bragg JG, Broadhurst L, Brown G, Bruhl JJ, Edwards R, Ferguson S, et al. (2024) The Genomics for Australian Plants (GAP) framework initiative – developing genomic resources for understanding the evolution and conservation of the Australian flora. EcoEvoRxiv [Preprint, published 8 July 2024].
| Crossref | Google Scholar |
Smith JE (1794) ‘A specimen of the botany of New Holland. Volume 1.’ (Davis: London, UK) 10.5962/bhl.title.124861
Smith JE (1798) XVIII. The characters of twenty new genera of plants. Transactions of the Linnean Society of London 4, 213-223.
| Crossref | Google Scholar |
Smith JE (1805) Remarks on the generic characters of the decandrous Papilionaceous plants of New Holland. In ‘Annals of Botany, Vol. 1’. (Eds C Konig, J Sims) pp. 501–512. (R. Taylor and Co., London, UK) Available at https://bibdigital.rjb.csic.es/viewer/14565/?offset=#page=510&viewer=picture&o=bookmark&n=0&q=
Smith JE (1808a) Daviesia. In ‘The Cyclopaedia; or, Universal dictionary of arts, sciences and literature Vol. XI (D–DIS)’. (Ed. A Rees). (Longman, Hurst, Rees, Orme & Brown: London, UK) 10.5962/bhl.title.59683
Smith JE (1808b) XVIII. Specific characters of the decandrous Papilionaceous plants of New Holland. Transactions of the Linnean Society of London 9, 244-267.
| Crossref | Google Scholar |
Smith JE (1808c) Characters of Platylobium, Bossiæa, and of a new genus named Poiretia. Transactions of the Linnean Society of London 9, 301-306.
| Crossref | Google Scholar |
Smith JE (1811) Jacksonia. In ‘The Cyclopaedia; or, Universal dictionary of arts, sciences and literature Vol. XVIII (HIB–INC)’. (Ed. A Rees). (Longman, Hurst, Rees, Orme & Brown: London, UK) 10.5962/bhl.title.59683
Strid A (1987) New species of Beaufortia and Chamaelaucium (Myrtaceae), Drosera (Droseraceae) and Pultenaea (Fabaceae) from SW. Australia. Plant Systematics and Evolution 155, 339-347.
| Crossref | Google Scholar |
Telford IRH, Clugston JAR, Barrett RL (2022) Pultenaea williamsii (Fabaceae: Mirbelieae), a new species endemic to the New England Tableland Bioregion of New South Wales. Telopea 25, 165-171.
| Crossref | Google Scholar |
Tian Q, Stull GW, Kellermann J, Medan D, Nge FJ, Liu SY, Kates HR, Soltis DE, Soltis PS, Guralnick RP, Folk RA, Onstein RE, Yi TS (2024) Rapid in situ diversification rates in Rhamnaceae explain the parallel evolution of high diversity in temperate biomes from global to local scales. New Phytologist 241, 1851-1865.
| Crossref | Google Scholar | PubMed |
Turczaninow PKNS (1849) Decas sexta generum plantarum hucusque non descriptorum adjectis descriptionibus specierum nonnullarum. Bulletin de la Societe Imperiale des Naturalistes de Moscou 22(3–4), 1-38 [In Latin].
| Google Scholar |
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber W-H, Li D-Z, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (Eds) (2018) ‘International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code)’, adopted by the Nineteenth International Botanical Congress, July 2017, Shenzhen, PR China. Regnum Vegetabile 159. (Koeltz Botanical Books: Glashütten, Germany) doi:10.12705/Code.2018
Wilkins CF, Chappill JA (2007a) Three new species of Aotus (Leguminosae: Mirbelieae) from South-Western Australia. Nuytsia 17, 459-468.
| Crossref | Google Scholar |
Wilkins CF, Chappill JA (2007b) Five new species of Eutaxia (Leguminosae: Mirbelieae) from South-Western Australia. Nuytsia 17, 469-482.
| Crossref | Google Scholar |
Wilkins CF, Chappill JA (2007c) Three new species of Latrobea (Leguminosae: Mirbelieae) from South-Western Australia. Nuytsia 17, 483-492.
| Crossref | Google Scholar |
Wilkins CF, Orthia LA, Crisp MD (2009) A new species of Pultenaea (Mirbelieae: Fabaceae) from Kundip, Western Australia. Nuytsia 19, 191-196.
| Crossref | Google Scholar |
Wilkins CF, Chappill JA, Henderson GR (2010) An account of Eutaxia (Leguminosae: Mirbelieae) with a focus on the Western Australian Species. Nuytsia 20, 109-167.
| Crossref | Google Scholar |
Williamson HB (1920) A revision of the genus Pultenaea, Part 1. Proceedings of the Royal Society of Victoria 32, 210-224.
| Google Scholar |
Williamson HB (1921) A revision of the genus Pultenaea, Part II. Proceedings of the Royal Society of Victoria 33, 133-148.
| Google Scholar |
Williamson HB (1922) A revision of the genus Pultenaea, Part III. Proceedings of the Royal Society of Victoria 35, 97-107.
| Google Scholar |
Woolcock CE, Woolcock DT (1985) Bush Peas. The genus Pultenaea – Part 3. Australian Plants 13(102 March), 71-84.
| Google Scholar |
Woolcock CE, Woolcock DT (1987) Bush Peas. The genus Pultenaea – Part 6. Australian Plants 14(111 June), 129-137.
| Google Scholar |
Woolcock CE, Woolcock DT (1989a) Bush Peas - The genus Pultenaea Part 10. Australian Plants 15(119 June), 126-136.
| Google Scholar |
Woolcock CE, Woolcock DT (1989b) Bush Peas. The genus Pultenaea – Part 11. Australian Plants 15(120 September), 177-185.
| Google Scholar |
Zuntini AR, Carruthers T, Maurin O, Bailey PC, Leempoel K, Brewer GE, Epitawalage E, Françoso E, Gallego-Paramo B, McGinnie C, Negrão R, Roy SR, Simpson L, Toledo Romero E, Barber VMA, Botigué L, Clarkson JJ, Cowan RS, Dodsworth S, Johnson MG, Kim JT, Pokorny L, Wickett NJ, Antar GM, DeBolt L, Gutierrez K, Hendriks KP, Hoewener A, Hu A-Q, Joyce EM, Kikuchi IABS, Larridon I, Larson DA, de Lírio EJ, Liu J-X, Malakasi P, Przelomska NAS, Shah T, Viruel J, Allnutt TR, Ameka GK, Andrew RL, Appelhans MS, Arista M, Ariza MJ, Arroyo J, Arthan W, Bachelier JB, Bailey CD, Barnes HF, Barrett MD, Barrett RL, Bayer RJ, Bayly MJ, Biffin E, Biggs N, Birch JL, Bogarín D, Borosova R, Bowles AMC, Boyce PC, Bramley GLC, Briggs M, Broadhurst L, Brown GK, Bruhl JJ, Bruneau A, Buerki S, Burns E, Byrne M, Cable S, Calladine A, Callmander MW, Cano Á, Cantrill DJ, Cardinal-McTeague WM, Carlsen MM, Carruthers AJA, de Castro Mateo A, Chase MW, Chatrou LW, Cheek M, Chen S, Christenhusz MJM, Christin P-A, Clements MA, Coffey SC, Conran JG, Cornejo X, Couvreur TLP, Cowie ID, Csiba L, Darbyshire I, Davidse G, Davies NMJ, Davis AP, van Dijk K-J, Downie SR, Duretto MF, Duvall MR, Edwards SL, Eggli U, Erkens RHJ, Escudero M, de la Estrella M, Fabriani F, Fay MF, Ferreira PL, Ficinski SZ, Fowler RM, Frisby S, Fu L, Fulcher T, Galbany-Casals M, Gardner EM, German DA, Giaretta A, Gibernau M, Gillespie LJ, González CC, Goyder DJ, Graham SW, Grall A, Green L, Gunn BF, Gutiérrez DG, Hackel J, Haevermans T, Haigh A, Hall JC, Hall T, Harrison MJ, Hatt SA, Hidalgo O, Hodkinson TR, Holmes GD, Hopkins H, Jackson CJ, James SA, Jobson RW, Kadereit G, Kahandawala IM, Kainulainen K, Kato M, Kellogg EA, King GJ, Klejevskaja B, Klitgaard BB, Klopper RR, Knapp S, Koch MA, Leebens-Mack JH, Lens F, Leon CJ, Léveillé-Bourret É, Lewis GP, Li DZ, Li L, Liede-Schumann S, Livshultz T, Lorence D, Lu M, Lu-Irving P, Luber J, Lucas EJ, Luján M, Lum M, Macfarlane TD, Magdalena C, Mansano VF, Masters LE, Mayo SJ, Mccoll K, Mcdonnell AJ, Mcdougall AE, Mclay T, Mcpherson H, Meneses RI, Merckx V, Michelangeli FA, Mitchell JD, Monro AK, Moore MJ, Mueller TL, Mummenhoff K, Munzinger J, Muriel P, Murphy DJ, Nargar K, Nauheimer L, Nge FJ, Nyffeler R, Orejuela A, Ortiz EM, Palazzesi L, Peixoto AL, Pell SK, Pellicer J, Penneys DS, Perez-Escobar OA, Persson C, Pignal M, Pillon Y, Pirani JR, Plunkett GM, Powell RF, Prance GT, Puglisi C, Qin M, Rabeler RK, Rees P, Renner M, Roalson EH, Rodda M, Rogers ZS, Rokni S, Rutishauser R, de Salas MF, Schaefer H, Schley RJ, Schmidt-Lebuhn A, Shapcott A, Al-Shehbaz I, Shepherd KA, Simmons MP, Simões AO, Simões A, Siros M, Smidt EC, Smith JF, Snow N, Soltis DE, Soltis PS, Soreng RJ, Sothers CA, Starr JR, Stevens PF, Straub S, Struwe L, Taylor JM, Telford I, Thornhill AH, Tooth I, Trias-Blasi A, Udovicic F, Utteridge T, Del Valle JC, Verboom GA, Vonow HP, Vorontsova MS, de Vos JM, Al-Wattar N, Waycott M, Welker C, White AJ, Wieringa JJ, Williamson LT, Wilson TC, Wong SY, Woods LA, Woods R, Worboys S, Xanthos M, Yang Y, Zhang YX, Zhou MY, Zmarzty S, Zuloaga FO, Antonelli A, Bellot S, Crayn DM, Grace OM, Kersey PJ, Leitch IJ, Sauquet H, Smith SA, Eiserhardt WL, Forest F, Baker WJ (2024) Phylogenomics and the rise of the angiosperms. Nature 629(8013), 843-850.
| Crossref | Google Scholar | PubMed |