Morphological and molecular evidence refute a broad circumscription for Pultenaea glabra (Fabaceae: Mirbelieae), with implications for taxonomy, biogeography, and conservation
Matthew A. M. Renner A C , Russell L. Barrett
A C , Russell L. Barrett  A * , Steve Clarke B , James A. R. Clugston
A * , Steve Clarke B , James A. R. Clugston  A , Trevor C. Wilson
A , Trevor C. Wilson  A and Peter H. Weston A
A and Peter H. Weston A
A National Herbarium of New South Wales, Royal Botanic Gardens and Domain Trust, Australian Botanic Garden, Locked Bag 6002, Mount Annan, NSW 2567, Australia.
B Blaxland East, Blue Mountains, NSW 2774, Australia.
C Present address: Wildland Consultants, 99 Sala Street, Te Ngae, Rotorua, New Zealand.
Australian Systematic Botany 35(3) 225-277 https://doi.org/10.1071/SB21030
Submitted: 6 August 2021 Accepted: 23 May 2022 Published: 14 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Morphological and single-nucleotide polymorphism data support splitting Pultenaea glabra Benth. into eight species, including one in Victoria, and seven in eastern and northern New South Wales. Six species are newly described, five of which are, like P. glabra, narrow-range endemics within the Greater Blue Mountains World Heritage Area and adjacent sandstone landforms of the Great Dividing Range. The recognition of six new species from what was broadly P. glabra has implications for conservation management, including for P. glabra itself, which has a smaller distribution and more precise habitat requirements than previously thought. One of the new species, P. percussa, is known by a single 1971 gathering only. The occurrence of several narrow-range Blue Mountains endemic Pultenaea species may be explained by the combination of edaphic diversity and topographic complexity, which could act in concert to promote divergences among small, allopatric populations. Hybrids between P. glabra and P. flexilis Sm. are documented for the first time; however, limited evidence for introgression between the two species was observed. The following new taxa are described: Pultenaea aculeata M.A.M.Renner, P.H.Weston & S.Clarke, Pultenaea percussa M.A.M.Renner & P.H.Weston, Pultenaea furcata M.A.M.Renner & R.L.Barrett, Pultenaea mutabilis M.A.M.Renner & P.H.Weston, Pultenaea mutabilis var. angusta M.A.M.Renner, P.H.Weston, & S.Clarke, Pultenaea praecipua M.A.M.Renner & P.H.Weston, Pultenaea praecipua subsp. temperata M.A.M.Renner & R.L.Barrett, and Pultenaea tenebrosa M.A.M.Renner, P.H.Weston & S.Clarke. Lectotypes are designated for Pultenaea villosa var. glabrescens Benth. and Pultenaea weindorferi Reader.
Keywords: Australia, New South Wales, plant taxonomy, rare species, sandstone flora, short-range endemics, speciation, systematics, Victoria.
Introduction
Family Fabaceae is one of the most species-rich plant lineages in Australia, with ~2000 species (Harden 2002), ~1000 of which belong to Acacia in subfamily Caesalpinoideae (Maslin 2001). Most of the remainder belong to the subfamily Faboideae, which is represented in Australia by 140 genera. Within the Australian Faboideae, the tribe Mirbelieae is the largest tribe by species number, with 756 recognised species in 24 accepted genera (Barrett et al. 2021). The most speciose genus of Mirbelieae is the Australian endemic Pultenaea Sm., with 130 species, most of which are shrubs that are conspicuous and charismatic components of mesic-zone sclerophyll communities. Approximatley 90 species are endemic to eastern Australia (Orthia et al. 2005a).
The taxonomy of Pultenaea in eastern Australia was revised in three papers by de Kok and West (2002, 2003, 2004), each paper treating a different informal group circumscribed by whether the ovary was glabrous or hairy, and for the latter whether the leaves were involute, or revolute. Glabrous ovaries included those having hairs around the ovary apex only, this qualification was important because a few species have a completely glabrous ovary, or have ovaries with a tuft of hairs at the apex, including P. alea de Kok, P. altissima F.Muell. ex Benth., P. euchila DC., P. flexilis Sm., P. glabra and P. spinosa (DC.) H.B.Will. (de Kok and West 2002, p. 82). In these polymorphic species, the degrees of ovary hairiness and leaf hairiness were correlated, with continuous variation among individuals in some species (de Kok and West 2002). The inference of continuous morphological variation justified the broad circumscription adopted for these species, including P. glabra, which included two taxa generally regarded as distinct from P. glabra up to that time: P. sp. Olinda (R. Coveny 6616) NSW Herbarium (as Pultenaea sp. E in the Flora of New South Wales; Weston 1991) and Pultenaea weindorferi Reader from Victoria (Corrick 1996). Pultenaea sp. Olinda had previously been recognised ‘on the basis of being a small plant with more incurved leaf’, features of plants that grow ‘in a much drier habitat than is usual for this species’ (de Kok and West 2002, p. 95). Because these, and other, morphological differences were correlated with environment, they were interpreted as ‘insufficient for formal recognition’ (de Kok and West 2002, p. 95). The inclusion of P. sp. Olinda and P. weindorferi increased the distribution of P. glabra to include central eastern Victoria, as well as the Central Tablelands of New South Wales. As a result, de Kok and West (2002, p. 96) concluded that P. glabra ‘is more widespread than previously thought and a new conservation risk code is needed’.
The broad concept adopted by de Kok and West (2002) has already been rejected by the National Herbarium of Victoria, which maintains Pultenaea weindorferi Reader for Victorian populations, and in Queensland the disjunct record of P. glabra (Batianoff & Franks 911011, not specifically cited by de Kok and West 2002, other than as a dot on their map) has been re-identified as the locally vulnerable species P. setulosa Benth. (P. Forster, pers. comm.). The broad circumscription of Pultenaea glabra adopted by de Kok and West (2002) also encompassed several informal, and unpublished, tag-named entities recognised by the National Herbarium of New South Wales, including Pultenaea sp. Shadowgraph Bluff (T. & J. Whaite 3455) NSW Herbarium, P. sp. Lees Pinch (L. A. S. Johnson s.n.: NSW 17642) NSW Herbarium, Pultenaea sp. Newnes (I. R. Telford 5072 & M. D. Crisp) NSW Herbarium, and P. sp. Nullo Mountain (M. A. M. Renner et al. 9032) NSW Herbarium. The status of these entities has not been investigated further since their treatment as P. glabra.
Subsequent to the Pultenaea revision (de Kok and West 2002, 2003, 2004) being published, and especially over the past decade, a considerable resource of herbarium specimens has been established, largely in the course of extensive bushwalking in the Blue Mountains by one of us (S. Clarke). These collections have included new informal entities such as Pultenaea sp. Genowlan Point (NSW 417813), Pultenaea sp. Wolgan Cliffs (S. Clarke 602) and P. sp. Dingo Creek (M. A. M. Renner 9176 & J. M. Cohen) NSW Herbarium that further broaden the amplitude of morphological, ecological, and geographic variation encompassed by P. glabra. Most of these collections were made as plants were encountered rather than as part of any targeted search or formal survey. Realisation that documentation of previously unknown populations would be invaluable regarding general understanding of diversity, conservation, and future taxonomic investigation motivated the collection of a series of specimens from newly discovered populations. Pultenaea sp. Wolgan Cliffs is, a previously unknown, morphologically and ecologically distinct entity discovered on the western side of the Wolgan Valley. Additional populations of P. sp. Wolgan Cliffs were discovered on the eastern and western sides of Wolgan Valley during targeted searches informed by satellite imagery and topographic map interpretation, direct observation from adjacent cliff lines, and knowledge of the country. In 2018, during the early stages of this study, images of a suspected P. glabra from the Dingo Creek catchment on the Newnes Plateau sent to NSW for confirmation of identity proved, on visiting the site at which they occurred, to be a previously unknown morphotype belonging to the P. glabra complex, P. sp. Dingo Creek. Although these informal entities each presents a morphotype different from P. glabra in the strict sense, each is associated with a different microsite, and subjected to different climatic and edaphic conditions, which, through phenotypic plasticity, may explain the observed morphological variation as de Kok and West (2002) proposed.
The aim of this study is to clarify the circumscription, distribution, ecology, and conservation status of P. glabra. We achieve this by establishing the patterns and parameters of morphological and molecular variation among individuals attributed to the P. glabra complex. By quantifying morphology we explicitly address whether morphological variation is continuous among populations, or partitioned into discrete clusters. We then use DArTSeq to capture thousands of genome-wide single-nucleotide polymorphisms (SNPs) to inform fine-scale genetic relationships among individuals. Diversity Arrays Technology generates genome-wide SNP data, which has the capacity to probe population genetic structure, and so identify restrictions in gene flow and reproductive isolation, with a sensitivity beyond any inference possible by using traditional molecular data sources (Melville et al. 2017; Binks et al. 2020). In this study, we test the broad circumscription proposed for Pultenaea glabra, and the status of each tag-named entity using a combination of morphological and molecular datasets, the latter comprising thousands of SNPs. If a broad circumscription of P. glabra were appropriate, we would expect two patterns in our data. First, we would expect a single, broad and variable morphological cluster, perhaps with populations forming distinct, but not discrete, subclusters in response to their exposure to continuous environmental variables underlying the expression of phenotypic plasticity. Second, we would expect to recover limited genetic differentiation among populations and morphotypes, consistent with ongoing gene flow among populations that are all descended from a single ancestral population.
Materials and methods
A combination of field-collected and existing herbarium specimens was used as a source of samples for morphotyping and genotyping; the SNP dataset of 94 samples captured as much of the range of morphological and geographic variation expressed by P. glabra sens. lat. as possible, using existing herbarium material and targeted field collections. Of these, 90 were included in the final SNP dataset (Supplementary Table S1). For field-collected specimens, up to six individuals were sampled from a population, each individual being separated by at least 6 m from other sampled individuals where this was possible. Fresh leaf material was placed onto silica gel for DNA extraction, flowers were placed into 70% ethanol for morphological examination, and shoots were pressed and dried for herbarium specimens. For Pultenaea sp. Genowlan Point and P. sp. Nullo Mountain, a 6-m spacing was not possible because the known populations of both comprise a handful of individuals with a geographic extent of hundreds of square metres only. One population of P. glabra (Woodford Creek) formed a mixed stand with P. flexilis, and we suspected, on the basis of morphology of individuals observed in the field, that some individuals were hybrids. We included one individual of P. flexilis, a species whose status as distinct from P. glabra is universally accepted, from Woodford Creek to facilitate confirmation of the hybrid status of these suspect individuals.
Morphological data collection
Morphological characters were assessed and scored from 97 specimens (see Supplementary Table S1) comprising 90 of the 94 molecular voucher specimens, one specimen of Pultenaea sp. Shadowgraph Bluff, one additional specimen of P. sp. Olinda, and five specimens of P. weindorferi from Victoria (Supplementary Table S1). The final morphological matrix comprised 19 variable characters, including three quantitative and 16 qualitative characters, describing leaf length, width, and hairiness, flower arrangement, branching pattern, leaf-surface texture (Fig. 1), and presence or absence of hairs on various structures. For the quantification of leaf dimensions and hairiness, 10 leaves were selected haphazardly from each specimen, using accidentally dislodged leaves where these were present to mitigate any subconscious ascertainment bias. Leaves were mounted on glass slides with archival glue, and their length and width were measured with mechanical vernier callipers. Leaf hairiness was scored as an estimate of the ventral leaf surface clothed by hairs. Hair coverage was variable but consistent in that the hairy portion of the leaf was always closest to the petiole irrespective of hair density, so hairiness was estimated as the proportion of the total leaf length covered by hairs, from the base to the apex. The mean of the 10 measurements was the character state scored for each specimen. The final dataset comprised three numeric, three ordered, and thirteen factorial characters.
For the collection and analysis of morphological data, and for the descriptions in the taxonomic treatment that follow, we focus on vegetative features because these are present year-round, and, as such, are the primary source of characters on which plants will be identified.
Morphological data analysis
All analyses of the morphological data were completed using the cluster package (ver. 2.1.0, see https://cran.r-project.org/package=cluster) in the package R (ver. 3.5.2, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org). To summarise patterns of morphological variation, we computed a distance matrix using Gower’s metric, which accommodates the mixture of continuous and categorical variables within our morphological dataset, with the ‘daisy’ function, and performed a principal-component analysis (PCA) with the prcomp function.
Molecular data collection
Leaf tissue from living plants and herbarium specimens was placed onto silica gel and freeze-dried. No specimen of Pultenaea weindorferi available to us was suitable for inclusion in the molecular dataset. Freeze-dried leaf material was sent to Diversity Arrays Technology (Canberra, ACT, Australia) for genotyping by sequencing, using a high-density sequencing DArTseq analysis. DArTSeq combines DArT complexity-reduction methods with next-generation sequencing. The complexity-reduction approach, which is based on restriction digestion using a combination of enzymes (PstI/HpaII, PstI/SphI, SbfI/HpaII, SbfI/MseI), had previously been optimised for other species of Pultenaea by Diversity Arrays Technology. For a full description of the DArTSeq methods, see Sansaloni et al. (2010), Killan et al. (2012) and Melville et al. (2017). Read assembly, removal of contaminant sequences, quality control and SNP calling were completed by DArT PL proprietary bioinformatics pipeline (DArTsoft14), which produced 124 208 binary SNPs for 93 individuals, the 94th individual failed to yield useable DNA.
Molecular data analysis
Data quality control was completed using the R package dartR (ver. 1.1.11, see https://CRAN.R-project.org/package=dartR; Gruber et al. 2018), again in R (ver. 3.5.2). To ensure that downstream analyses were conducted on high-quality SNP data, and to reduce missing-data rates for PCA, structure analysis, and phylogeny reconstruction, we applied a range of fairly stringent filters in the following order. First, we removed loci the call rate of which was less than 0.95, then individuals the call rate was less than 0.75, then kept the best SNPs on the same fragment and discarded other secondary and linked SNPs, then retained loci the repeatability of which was higher than 0.95, and, finally, removed loci the minor allele frequency of which was 0.02 or less, leaving 90 individuals and 2542 SNPs, none of which departed significantly from Hardy–Weinberg equilibrium. We calculated a genomic relatedness matrix for individuals with gl.grm, the dendrogram which is generated by a call to hclust, probably using an unweighted pair group mean algorithm, given it is the mean probability of identity by descent being analysed; and pairwise fixation indices among populations with gl.fst.pop (Supplementary Table S2), as exploratory and summary metrics for the SNP data using functions in dartR, refer to the dartR manual for details.
Genetic structure within the sample was assessed using PCA, which provides a model-free representation of similarities and differences among individuals through a low-dimensional projection of the data that preserves the variance–covariance structure. PCA was conducted with gl.pcoa, which performs a Gower ordination using Euclidean distances, in the adegenet package (ver. 2.1.2, see https://github.com/thibautjombart/adegenet/wiki; Jombart 2008) in R. We investigate the relationships between P. sp. Lees Pinch and P. sp. Newnes in more detail because these two morphotypes formed a single cluster in the PCA including all samples. To achieve this, we reduced the data matrix to include individuals of these two entities only, and performed PCA on this reduced matrix to eliminate the influence of orthogonal variation in other taxa, thus more clearly displaying variation within this subgroup.
We visualised the overall structure, and complexity in the SNP data in an abstract phylogenetic network computed by SplitsTree (ver. 4.16.2, see https://software-ab.informatik.uni-tuebingen.de/download/splitstree/welcome.html; Huson and Bryant 2006) using the Neighbor-net distance transformation (Bryant and Moulton 2004), on uncorrected characters with ambiguous states ignored. Neighbor-net provides a visual summary of incongruence owing to reticulation and other processes, which can be used to guide further investigation of the data. The nexus file that is substrate for analysis was converted from fastA format, output by the dartR package, by PGD Spider (ver. 2.1.1.5, see http://www.cmpg.unibe.ch/software/PGDSpider/PGDSpider_2.1.1.5.zip; Lischer and Excoffier 2012).
The splits network placed three individuals of P. glabra from Hazelbrook on cycles between the single individual of P. flexilis and the other P. glabra individuals (see Results below). One of these three individuals had been tentatively identified as a hybrid at the time of collection, the other two had been collected as P. glabra. To corroborate the hybrid status of these three individuals, we used admixture modelling to assess the genetic structure present in the sample set as a whole, and the ancestry proportions of each individual. Our expectation was that the suspected hybrid individuals would return a signal of mixed ancestral population membership. We used model-based clustering analysis implemented by STRUCTURE (ver. 2.3, see https://bioweb.pasteur.fr/packages/pack@structure@2.3.4; Pritchard et al. 2000), under an admixture model because each of the populations under study had been considered part of a single species, so presumably are in, or were in recent, genetic contact. Structure assigns samples to K distinct genetic groups to minimise deviations from Hardy–Weinberg equilibrium and linkage disequilibrium within each genetic group, using Bayesian inference. For each value of K from 1 to 12, we ran MCMC chains for 100 000 iterations after a burnin of 20 000 iterations, and completed at least three runs for each value of K, to assess and confirm run convergence, and selected the optimal K value by using the delta-K statistic (Evanno et al. 2005). Because unequal sample sizes within populations may lead to suboptimal estimates of K and individual ancestry, we estimated a separate α for each genetic cluster, as recommended by Wang (2017). For the selection of optimal K, we included runs with K of 1–6 only, as higher K values had widely fluctuating likelihoods in runs that failed to converge. We used KFinder (ver. 1.0.0, see https://www.zsl.org/science/software/kfinder) to generate delta K, and perform K selection. Visual summaries of cluster membership in the form of a stacked bar chart were generated using Genesis (ver. 0.2.6, see https://github.com/shaze/genesis; Buchmann and Hazelhurst 2014).
Finally, we estimated a phylogeny from the SNPs by using iQTree (Nguyen et al. 2015), we reduced the dataset to 2160 variable sites, and used ModelFinder (Kalyaanamoorthy et al. 2017), implemented as part of iQTree, to select the optimal substitution model, with an ascertainment bias, for that SNP set, using the Bayesian information criterion (BIC) as the optimality criterion (the best fitting model selected and used in the tree reconstruction was TVMe + ASC + R3). To assess consistency and node support, we used the Shimodaira–Hasegawa-like approximate likelihood-ratio test (Guindon et al. 2010), and 1000 ultrafast bootstrap replicates (Hoang et al. 2018) were used to assess consistency and support for nodes within the phylogeny. We rooted trees on Pultenaea flexilis on the assumption that this species was related to, but not part of, the P. glabra complex. Although this approach to phylogeny reconstruction treats the thousands of independently inherited SNPs as sharing a single gene tree, and so fails to adequately accommodate their independent evolution and inheritance, it may still provide insight into phylogenetic relationships among individuals within the Pultenaea glabra complex, if interpreted cautiously.
Results
Morphological data
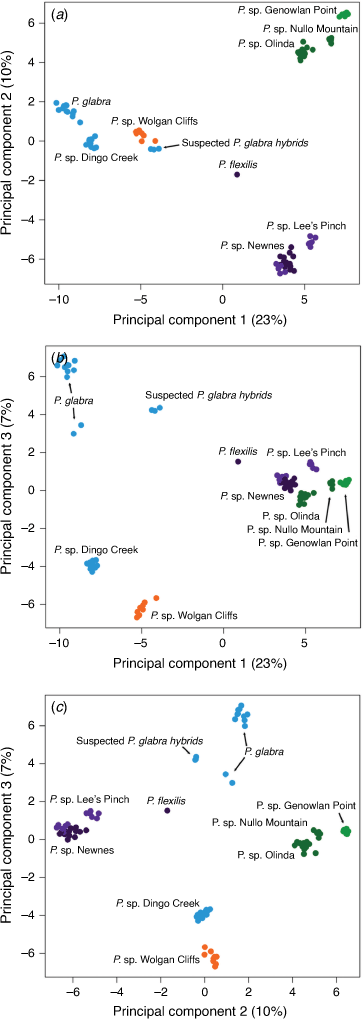
Seven discrete clusters comprising more than three individuals and five singleton or doubleton clusters were separated by the combination of first and second principal components summarising major axes of morphological variation (Fig. 2). Three of the seven multi-individual clusters corresponded to tag-named entities (P. sp. Wolgan Cliffs, P. sp. Olinda, P. sp. Dingo Creek), a fourth corresponded to P. weindorferi, and a fifth corresponded to P. glabra. The other two multi-individual clusters contained two tag-named entities. Pultenaea sp. Genowlan Point and P. sp. Nullo Mountain were resolved in close proximity, but each formed a discrete subcluster. Pultenaea sp. Lees Pinch and P. sp. Newnes were resolved together in a loose cluster, with the P. sp. Lees Pinch population from Mount Irvine overlapping P. sp. Newnes, and the P. sp. Lees Pinch population from Lees Pinch separated as a distinct subcluster. One of the singleton clusters corresponded to P. sp. Shadowgraph Bluff, which is known by a single specimen only, and another singleton corresponded to P. flexilis, which was proximate to but separated from the P. sp. Lees Pinch–P. sp. Newnes cluster. The remaining isolated singleton and doubletons correspond to three individuals of P. glabra, which partially straddled the morphological space between P. glabra and P. flexilis. The closest of these to P. flexilis had been recognised in the field as a putative hybrid, and the separation of the other two individuals on morphological grounds suggested that they may also be hybrids, a possibility we address further below. One individual of P. weindorferi was also separated from the others, this individual is atypical in the long hairs on the leaf surfaces, it may too represent a hybrid. Like P. glabra and P. weindorferi, P. sp. Dingo Creek and P. sp. Olinda formed tight clusters, indicating a degree of morphological consistency across populations, and sampled individuals. By contrast, the three sampled populations of P. sp. Wolgan Cliffs were recovered as discrete subclusters, as were the populations of P. sp. Lees Pinch, suggesting some morphological differentiation among populations of these entities. Regardless, the degree of separation among clusters was always greater than cluster spread, implying that intra-specific variation was less than the degree of inter-specific difference, with the exception of P. flexilis and P. sp. Lees Pinch–P. sp. Newnes, in which case the separation of P. flexilis was less than the spread of individuals within the latter cluster, an interesting result we return to in the discussion. The first principal component captured 65% of morphological variation among individuals, the second component 19%, and the plot of first and second principal components captured 84% of morphological variation within the dataset.
Molecular data
In total, 124 208 binary SNPs with an average call rate of 65.5%, an average scoring reproducibility of 99.3%, and an average polymorphism information content (PIC) of 0.15 were generated by the DArTSeq analysis. Of these, 202 SNPs had a 100% genotype call rate, and 5696 had a call rate of 95%. After filtering, the final SNP matrix contained 90 individuals and 2542 SNPs, with an average call rate of 97.0%, an average scoring reproducibility of 98.9%, an average PIC of 0.18, and a missing data rate of 1.7%.
The similarities and differences among individuals summarised in a genetic relatedness matrix (Fig. 3) suggested three broad genetic groups, and also suggested that suspected hybrids had a mixed ancestry, on the basis of their similarities to both P. glabra and P. flexilis.

|
Principal components analysis
When first and second principal components of the final, filtered SNP matrix were plotted, individuals were resolved into three broad clusters (Fig. 4). One of these clusters comprised P. sp. Olinda with P. sp. Genowlan Point and P. sp. Nullo Mountain, each forming a discrete subcluster in a linear series, with P. sp. Olinda closest to the plot centre, P. sp. Nullo Mountain furthest away and P. sp. Genowlan Point in the middle. The second of the three broad clusters comprised P. sp. Lees Pinch and P. sp. Newnes, with the northern population of P. sp. Lees Pinch, from Goulburn River National Park, separated from the others. The third primary cluster contained P. glabra, P. sp. Dingo Creek, and P. sp. Wolgan Cliffs, each forming a discrete subcluster, with the exception of three P. glabra individuals that were separated from the others. These three individuals, all from the Woodford Creek population, were approximately halfway between the main P. glabra cluster and the single individual of P. flexilis. The first principal component captured 23% of the molecular variation, and the second principal component captured 10% of the variation. The second principal component described differences between P. sp. Newnes and P. sp. Lees Pinch on the one hand, and among P. sp. Olinda, P. sp. Nullo Mountain, and P. sp. Genowlan Point on the other. The second and third principal components together achieved the greatest separation of clusters within a single plot, even though principal component three captured only 7% of molecular variation, it captured differences between P. glabra on the one hand, and P. sp. Dingo Creek and P. sp. Wolgan Cliffs on the other.
|
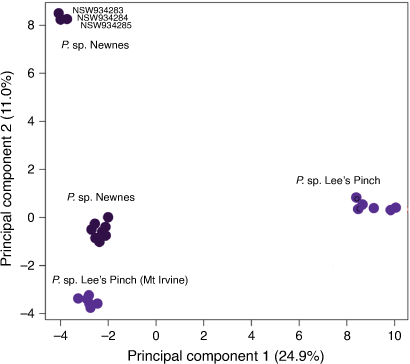
PCA for P. sp. Lees Pinch and P. sp. Newnes only (Fig. 5) separated the northern and southern populations of the former from Lees Pinch and Mount Irvine, as expected, but unexpectedly also subdivided P. sp. Newnes into two groups. One of these groups contained only three individuals, all from the same site amidst other sampled locations for this entity. We confirmed that the call rate across SNPs for these three individuals was >0.95, so the isolation of these three individuals in PCA of SNP data was not an artefact of missing data. Unfortunately, these three individuals were not included in the morphological data matrix, because they were unavailable at the time morphological data were scored. Their morphology is qualitatively the same as other P. sp. Newnes specimens, but given their distinct genetic identity, this warrants further investigation.
|
Splits network
The splits network had a broadly bifurcating structure, with groups of individuals corresponding to tag-named entities and named species resolved around the termini of long narrow-rectangular cycles (Fig. 6). The network contained nine separate clusters of individuals, which correspond to those resolved in the PCA. In general terms, individuals were subdivided into two groups, with P. glabra, P. sp. Dingo Creek and P. sp. Wolgan Cliffs on one side of a long medial branch of rectangular cycles, and the remaining taxa on the other. However, cycles were present throughout the centre network, indicating the presence of incongruent phylogenetic signal. Generally the cycles were also long-rectangular, indicating high weight for signal congruent with a bifurcating, and hence tree-like, structure. However, box-like and complex cycling was associated with three individuals of P. glabra from Woodford Creek, which were mid-way between the main P. glabra cluster and the single individual of P. flexilis. The individual of P. flexilis was itself associated with complex cycling, some linked with the P. sp. Newnes and P. sp. Lees Pinch cluster, which comprised an open web-like network next to P. flexilis. Individuals of P. sp. Lees Pinch from Lees Pinch were connected to the network by a long branch of narrow cycles, linked at its base with the other P. sp. Lees Pinch and P. sp. Newnes on one side, and a broad network of cycles leading to P. sp. Olinda, P. sp. Genowlan Point, and P. sp. Nullo Mountain on the other. Pultenaea sp. Genowlan Point and P. sp. Nullo Mountain were linked to a series of long narrow cycles that connected to the network at the base. In turn, this Pultenaea sp. Genowlan Point and P. sp. Nullo Mountain branch was connected to a shorter branch of rectangular cycles, to which all the P. sp. Olinda individuals were also connected. On the other side of the network, the individuals of P. sp. Wolgan Cliffs were connected to the network by a branch containing long narrow cycles. Pultenaea sp. Dingo Creek was connected to the network next to P. sp. Wolgan Cliffs, on a shorter broader branch that was, in turn, linked to the two individuals of P. glabra from the northern side of the Grose River valley by several rectangular cycles. These two individuals were in turn linked with the other individuals of P. glabra, all from the southern side of the Grose River valley.
Structure analysis
Each method for K selection chose a different value of K, 4 with delta K, 5 with parsimony, 6 with Pritchard and 2 with Evanno method. Because delta K reached a plateau at K = 4 (not shown), we consider K values of 4, 5 and 6 in our description of Structure results. The broad pattern of genetic ancestry across the different values of K was one of three main ancestral genetic groups (Fig. 7). Pultenaea sp. Olinda, P. sp. Genowlan Point, and P. sp. Nullo Mountain together comprised one of these groups, regardless of the number of inferred ancestral populations. Pultenaea sp. Lees Pinch and P. sp. Newnes comprised another, with the exception that at K = 6, the population of P. sp. Lees Pinch from Lees Pinch was attributed a distinct genetic ancestry. Most of the variation in genetic ancestry among the different numbers of inferred ancestral populations was associated with P. sp. Wolgan Cliffs and P. sp. Dingo Creek. At K = 3, these two entities were attributed a shared genetic ancestry with P. glabra. At K = 4, P. sp. Wolgan Cliffs was attributed a unique genetic ancestry. At K = 5, P. sp. Wolgan Cliffs was attributed a mixed ancestry comprising a unique major component and a minor component shared with P. sp. Dingo Creek. The latter was, in turn, the major component of P. sp. Dingo Creek, whose minor component was shared with P. glabra. At K = 6, P. sp. Wolgan Cliffs was attributed to its own single ancestral group, but this was shared in part with P. sp. Dingo Creek. Three individuals that were suspected of being hybrids between P. glabra and P. flexilis had mixed ancestry with all values of K, and all three shared half their ancestry proportion with the unique cluster accorded to P. flexilis at K = 6, the only K value at which P. flexilis formed its own cluster, a result we attribute to the small sample size for this species (see, for example, Pritchard et al. 2000). At lower K, P. flexilis was attributed a mixed ancestry including three major components, and the suspected hybrids reflected these proportional ancestries attributed to P. flexilis in turn.
IQ tree
Six clades corresponding to species were recovered by IQtree in the unrooted phylogeny (Fig. 8), which suggests that P. glabra, P. sp. Dingo Creek and P. sp. Wolgan Cliffs are closely related, possibly monophyletic, and that P. sp. Olinda and P. sp. Genowlan Point are possibly sister species. The population of P. sp. Lees Pinch from Lees Pinch was resolved sister to the remainder of the P. sp. Lees Pinch and P. sp. Newnes complex. The three individuals of P. sp. Newnes that were isolated from the remainder of P. sp. Newnes in the PCA were resolved in a fully supported lineage of their own, which was sister to the remainder of the P. sp. Newnes and P. sp. Lees Pinch complex.
Putative hybrids between P. glabra and P. flexilis all grouped with P. flexilis with strong support, and well apart from P. glabra, in this phylogeny. However, whether they formed a monophylum or not cannot be resolved without knowledge of, and perhaps inclusion of, the appropriate outgroup.
Discussion
Patterns of morphological and genetic differentiation among populations present compelling evidence against P. glabra being a single variable species. Rather than forming, or even tending towards, a continuum of morphological and genetic variation, the P. glabra complex in the Blue Mountains World Heritage Area and surrounds comprises seven discrete morphological and genetic clusters. Each of the eight genetic clusters has the attributes associated with species, they are genetically distinct, they are morphologically different and diagnosable, and they occur in different microsites and climates. The correlated differences may be explained by each having a unique evolutionary history involving a response to selective pressures associated with each microsite and habitat. Together with the Victorian P. weindorferi, the P. glabra complex comprises eight morphological and genetic clusters that warrant formal recognition as species.
There has been only one previous study of Pultenaea population genetic structure, on the West Australian P. pauciflora M.B.Scott (Millar and Byrne 2013), which comprised two genetic clusters. One cluster contained two widely separated populations within sclerophyll forests dominated by Eucalyptus wandoo Blakely. The close relationship despite geographic separation was presumably because, until recently, those populations belonged to a nearly continuous population system (Millar and Byrne 2013). The other cluster within P. pauciflora occurred in forest dominated by Eucalyptus marginata Donn ex Sm., and the two clusters lacked obvious morphological differences. The landscapes in which P. pauciflora grow are predominantly flat, and geographically uniform. By contrast, the eastern coast of Australia, and the Blue Mountains in particular, contain landscapes of considerable topographic complexity, within which the P. glabra complex exhibits fine geographic structuring of both genetic and morphological diversity. Six different clusters recovered in the SNP data are also morphologically distinct, and two further morphological entities, one from Kanangra Boyd National Park (P. sp. Shadowgraph Bluff) and another from Victoria (P. weindorferii) are almost wholly allopatric, and some are associated with different microsites. For example, Pultenaea glabra sens. str. occurs in riparian vegetation alongside streams in gullies, or on the margins of hanging swamps, whereas Pultenaea sp. Olinda is often associated with exposed sandstone pagodas, where it grows in crevices or cracks on the pagodas themselves, or in shallow soils accumulated on pavements around the pagodas. Correlated differences in distribution, microsite occupancy, morphology, and genetic identity, together with the complete absence of morphological and genetic intermediates and the absence of evidence for ongoing gene flow among genetic clusters, all support the recognition of each of the morphological and genetic entities as distinct evolutionary lineages. There is broad agreement that:
species should represent robust independently evolving lineages that remain largely intact when in contact with close relatives—in accord with the intent of the Evolutionary Species Concept (Wiley 1978) and the Generalised Lineage Species Concept (de Queiroz 1998, 2007) [Coates et al. 2018, p. 3].
On the basis of phenotypic and genetic divergence, there is a case for separating species of P. glabra, because it is currently broadly circumscribed. This case is strengthened when ecological and geographic differences are also considered. As a result, we conclude that, as currently circumscribed, P. glabra comprises eight species, including seven in the Greater Blue Mountains, plus Kanangra Boyd National Park and P. weindorferi in Victoria. All eight of these species differ from one another by degrees equivalent to, or greater than, the morphological and genetic distances observed separating P. glabra from P. flexilis, two species whose status as distinct is beyond doubt. Framed in terms of that comparison, the case for conferring species status to the eight entities is strong.
The segregation of P. glabra into eight species, (one with two subspecies and another with two varieties, see discussion below) has significant conservation management implications, not only for the six newly recognised species, but for P. glabra itself, which has a smaller geographic range, and more precise habitat requirements than previously thought. Pultenaea glabra is confined to eastern catchments of the Great Dividing Range on Narabeen Sandstones, where it is an edaphic specialist in soils with reliable subsurface moisture associated with gully heads, streamsides, and edges of hanging swamps. The eastern-most occurrence of P. glabra is at Woodford Creek, on Narabeen sandstone, which abuts the transition from Narabeen to Hawkesbury sandstone. There are no records of P. glabra further east, on the Hawkesbury Sandstone itself.
Three individuals from the eastern-most and lowest-elevation population of P. glabra (Woodford Creek) had mixed ancestry, and were likely primary hybrids between P. flexilis and P. glabra, given their ancestry proportions. However, even though probable F1s occurred at this site, there was little evidence of introgression in the other P. glabra sampled. The fertility of primary hybrids between P. glabra and P. flexilis would be worth investigating, as would their ability to backcross to both parents. Possible hybrids between Pultenaea flexilis and Pultenaea stipularis were noted by de Kok and West (2002). Pultenaea glabra was placed in a clade with P. flexilis and P. stipularis by chloroplast markers in the phylogenetic study of Orthia et al. (2005b). Hybridisation may explain the phylogenetic grouping of such morphologically disparate species, may contribute to the lack of congruence among nuclear and plastid gene trees, and the general lack of resolution of phylogenetic relationships in molecular phylogenetic studies of Pultenaea (Orthia et al. 2005a, 2005b). Pultenaea flexilis may not, in fact, be a close relative of P. glabra, given the genetic distance separating these two species on the basis of genome-wide SNPs, and the morphological differences among them, but this remains to be established on the basis of more extensive molecular datasets, including multiple low-copy nuclear loci.
Pultenaea glabra itself exhibits significant geographic population genetic structure, the samples from Mount Tomah are genetically distinguished from populations on the southern side of the Grose Valley, differing by a fixation index of 11.7% from the most similar of the two southern populations. Despite being genetically distinct, the individuals in the northern population are morphologically indistinguishable from those in southern P. glabra. Arguments for recognising morphologically cryptic, genetically divergent, species on the basis of DNA-sequence differences exist, and have been utilised (Johnson and Cairns-Heath 2010). However, the genetic separation of P. glabra populations north and south of the Grose River might be expected, given the intervening distance and improbability of direct genetic contact through pollen or seed dispersal. In this instance, we note the existence of evolutionary significant units within P. glabra, but do not think that these warrant recognition as separate species, given their shared phenotype and habitat, an interpretation that follows the precedent set by the identification of cryptic population genetic structure within the Western Australian Pultenaea pauciflora (Millar and Byrne 2013). Interestingly, on the basis of genome-wide SNPs, the northern P. glabra is partly intermediate between southern P. glabra and P. sp. Dingo Creek, with which it shares some incongruent genetic signal. This pattern could have four explanations. It could be geographic, involving molecular drift of an isolated population established following a dispersal event without morphological change in northern P. glabra. Alternatively, it could be due to historical subdivision of a continuous population exhibiting clinal variation, the products of which are P. sp. Dingo Creek, and northern and southern P. glabra. The genetic distinctness of the northern P. glabra could also represent a signal of historical or ongoing introgression between P. sp. Dingo Creek and the northern P. glabra. Finally, the pattern of relationships could represent an historical introgression or reticulation event involving northern P. glabra and P. sp. Wolgan Cliffs, that produced P. sp. Dingo Creek as a hybrid, which has subsequently stabilised and experienced genetic drift, or divergent selection. More sampling of northern and upper mountain populations of P. glabra is required before these alternatives can be adequately addressed.
Pultenaea sp. Dingo Creek is a surprising addition to the diversity of Pultenaea in the GBMWHA and surrounding sandstone landscapes, because it was unknown before this study, and was not represented in herbarium collections held at NSW. This may reflect the species having a small geographic range, it is known only from the Dingo Creek catchment on the northern side of the Newnes Plateau, and although it is patchily distributed, it is locally common where it does occur.
Two threatened phrase-named entities, Pultenaea sp. Olinda and P. sp. Genowlan Point, are morphologically and genetically distinct from P. glabra and deserve species status, as noted above. Pultenaea sp. Olinda, together with P. sp. Genowlan Point and P. sp. Nullo Mountain, form a distinct genetic cluster, and it is perhaps surprising that P. sp. Olinda and P. sp. Genowlan Point are closely related, given the differences in their overall form. Pultenaea sp. Genowlan Point and P. sp. Nullo Mountain are closely related, and morphologically similar, but present strikingly different facies in the field because of their different leaf width (wider in P. sp. Genowlan Point). We did not identify any other distinguishing vegetative or reproductive morphological characters, but did not have access to flowering or fruiting material of P. sp. Nullo Mountain, so the possibility of additional differences cannot be discounted. Given these two entities form morphologically consistent and diagnosable, isolated but closely related, entities, a conservative approach would be to recognise them as subspecies. This we do, which affords both entities formal recognition, reflects the known degree of morphological difference, and leaves open the possibility for subsequent elevation of these subspecies to full species rank when the morphology of the Nullo Mountain plants is more completely known, and if such an elevation proves appropriate.
A more challenging situation is presented by Pultenaea sp. Lees Pinch and Pultenaea sp. Newnes. These entities represent distinct morphotypes, which differ in leaf width with the distribution of leaf widths being discontinuous and non-overlapping. They also differ in details of their floral morphology, the standard and keel of P. sp. Lees Pinch have conspicuous red markings, the standard is 50% broader for its height and is slightly cordate at the base, and the wings have a rounded auricle. The bracteole apex lays over or exceeds the sinus between the upper and lower calyx lobes, and the flowers of P. sp. Newnes emit a strong fragrance of vanilla ice-cream (not just vanilla alone), which was not noted in association with flowering P. sp. Lees Pinch, although this should be confirmed. Both morphotypes belong to the same genetic cluster, although P. sp. Lees Pinch from Goulburn River National Park is genetically divergent, with a fixation index of 32–38% between it and the other P. sp. Lees Pinch from Mount Irvine and P. sp. Newnes, values roughly equal to those between other morphological entities that are also genetically distinct. This may reflect its geographic isolation; however, the isolation of three individuals of P. sp. Newnes from the other individuals of this morphotype is not correlated with geographic distance. Those three individuals were all from the same site, which is mid-way between two other sampling sites. Individuals from both grouped in the main P. sp. Newnes cluster. The genetic relationships within and among P. sp. Lees Pinch and P. sp. Newnes are complex and somewhat unexpected, and further investigation is required, which raises the question of how to formalise these two entities given current knowledge.
Pultenaea sp. Lees Pinch and P. sp. Newnes differ most conspicuously in leaf width. The geographic occurrence of these two morphotypes exhibits broad overlap, but they are not known to co-occur. In the eastern-fall catchments of the Blue Mountains, P. sp. Lees Pinch appears to be associated with occurrences of shale, or at least of a shale influence in soils. By contrast, the occurrences of P. sp. Newnes are not intimately associated with shale formations. The morphological and geographical discontinuity between these two morphotypes correlates with weak molecular differentiation between P. sp. Newnes and southern populations of P. sp. Lees Pinch, which range from 11 to 17%, but these are approximately half that separating the two populations of P. sp. Lees Pinch, at 36% (Supplementary Table S2). The genetic distinctness of the northern P. sp. Lees Pinch population may be associated with its geographic isolation. However, there are differences in leaf texture and shape, and flower colour between the Mount Irvine and Lees Pinch populations of P. sp. Lees Pinch, with plants from Mount Irvine population having thinner leaves that are narrower, on average, and narrower for their length. Other collections of P. sp. Lees Pinch from the western side of the Great Dividing Range all represent the coriaceous morphotype. The apparently consistent expression of this coriaceous morphotype, its occurrence in western-fall catchments, and the significant genetic divergence between it and the thin-leaved plants from the eastern-fall catchments of the Blue Mountains suggest that P. sp. Lees Pinch may constitute a complex that requires further investigation. We do not pursue that investigation here, primarily because P. sp. Lees Pinch is probably more closely related to P. flexilis than P. glabra. Indeed the Mount Irvine form of P. sp. Lees Pinch is one of two elements, the other being P. flexilis, on which Dillwynia teucrioides DC. was based (this apparently confusing situation is discussed later, under the revised synonymy associated with Pultenaea flexilis) and that invalid name has long been placed in synonymy of P. flexilis. The admixture of elements within specimens and the synonymy are both understandable, given the overall similarity of these two species. In herbarium collections, P. sp. Lees Pinch has often been misidentified as P. flexilis. A broader geographic sampling that includes the various expressions of both P. sp. Lees Pinch and P. flexilis, along with more intensive sampling within P. sp. Newnes, will be required to facilitate a full resolution of species circumscription within this complex. Our results suggest that sampling across as broad a range of populations as possible is important, because a lot of variation is partitioned among, rather than within, populations. For the moment, we simply flag the need for further study.
Pultenaea weindorferi is morphologically distinct from P. glabra, and its recognition as a species is appropriate. Pultenaea weindorferi is geographically isolated from the remainder of the P. glabra complex, and is endemic to Victoria. One of the individuals included in our study had long-pilose leaves and was resolved isolated from the rest of the P. weindorferi cluster in PCA of morphological data, which may indicate that it was a hybrid, possibly with P. humilis.
Pultenaea sp. Shadowgraph Bluff is also morphologically isolated from P. glabra, and distinct from all other members of the P. glabra complex. It is also incompatible with the circumscription of all other known Pultenaea. The only known specimen of P. sp. Shadowgraph Bluff differs from P. glabra in both vegetative and reproductive characters, including the short, inrolled-clavate leaves with mamillose lower surface, and the production of flowers in the axils of vegetative leaves on shoot sectors not associated with internode contraction, and which continue normal vegetative growth. Pultenaea sp. Shadowgraph Bluff was collected in the Holland River catchment, on ‘shale slopes’ ~0.25 miles (~400 m) south of Shadowgraph Bluff. That puts the collecting locality in the vicinity of the firetrail crossing of Hollanders River. A search for this species alongside Hollanders River in late 2017 was unsuccessful, suggesting that the specimen may have been gathered from a plant growing further upslope, and additional survey work to relocate this species should be undertaken.
The formal recognition of eight species of what was Pultenaea glabra sens. lat. provides some further insights into biogeographic patterns, particularly in the Greater Blue Mountains World Heritage Area (GBWHA) and adjacent sandstone landscapes (Goldbery 1972). Currently, no formally described Faboideae are endemic to the GBMWHA and adjacent sandstone landscapes, although Phyllota humifusa Benth. is endemic to hill country to the immediate south of the GBMWHA. This is somewhat unusual given that the GBMWHA and adjacent sandstone landscapes support a highly diverse vascular flora, including more than 100 species of Eucalyptus L’Hér. (Hager and Benson 2010), several of which are endemic, including Eucalypus bensonii L.A.S.Johnson & K.D.Hill, E. copulans L.A.S.Johnson & K.D.Hill, E. cunninghamii Sweet, E. laophila L.A.S. Johnson & Blaxell, and E. cannonii R.T.Baker. In another diverse lineage of Fabaceae whose radiation is centred on Australia, there are species known to be endemic to the GBMWHA and adjacent sandstone landscapes, including Acacia asparagoides A.Cunn., A. clunies-rossiae Maiden, and A. flocktoniae Maiden. Other species and genera endemic, or nearly endemic to the Blue Mountains include Alania Endl. (Boryaceae) Atkinsonia ligustrina (A.Cunn. ex F.Muell.) F.Muell., Carex klaphakei K.L.Wilson, Euphrasia bowdeniae W.R.Barker, Grevillea evansiana McKee, Leionema sympetalum (Paul G.Wilson) Paul G.Wilson, Lepidosperma evansianum K.L.Wilson, Leucochrysum graminifolium (Paul G.Wilson) Paul G.Wilson, Machaerina johnsonii (K.L.Wilson) K.L.Wilson, Olearia quercifolia Sieber ex DC., Persoonia hindii P.H.Weston & L.A.S.Johnson, Persoonia recedens Gandoger, Phebalium bifidum P.H.Weston & M.J.Turton, Philotheca obovalis (A.Cunn.) Paul G.Wilson, Prostanthera hindii B.J.Conn, and Prostanthera stricta R.T.Baker (Department of Environment and Conservation 2006). Six of the species we describe here are endemic to the GBMWHA and adjacent sandstone landforms, and highlight two themes of broad relevance. The first is the existence of previously unrecognised diversity in this region of considerable topographic complexity, some elements of which are related to the difficulty accessing sites where species grow. The second is the degree of edaphic and possibly also climatic specialisation that these species exhibit. All the new species appear to be edaphic specialists to some degree, and all but one could be considered narrow-range endemics. These features, interesting in their own right, become intriguing with the possibility that the P. glabra complex is monophyletic, a circumstance that combines divergence along topographic, climatic and edaphic axes with morphological adaptation and change, in a relatively small geographic area, at least by Australian standards. There is precedent for geographic isolation among plants in the GMBWHA, facilitating local adaptation and divergence from studies of population genetic structure in Blue Mountains mallee ashes, which resolved complex patterns of relatedness, including divergences associated with geographic isolation, and previously unrecognised narrow-range endemic taxa (Rutherford et al. 2018).
The topographic and edaphic diversity associated with landforms derived from the Narrabeen sediments (Goldbery and Holland 1973; Herbert 1997) contrasts with much of the remainder of the Australian continent, which is characterised by its low elevation and relief. In addition to being relatively high-relief, the underlying geology and landform combine to produce a landscape containing a range of edaphic environments in relatively close proximity. This topographic complexity is evidently relatively young compared to other regions of Australia. The Tertiary basalts that overly what remains of the plateau were deposited 14–19 million years ago, when the landscape was not so dissected and had lower local relief (Mayne et al. 1974; Martyn 2018), which may impose a maximum upper bound on the age of species currently specialising on microsites particular to the present-day eroded landform.
The resolution of a several narrow-range, allopatric, edaphic and topographic specialists within P. glabra may provide further circumstantial evidence in support of the geographic speciation model proposed to explain Eucalyptus green mallee ash diversity in the Blue Mountains World Heritage Area (Rutherford et al. 2018). Possibly, topographic complexity combines with edaphic variability and climatic gradients, including rainfall and temperature, to reproductively isolate allopatric populations by vertical and horizontal distance, and by topographic barriers. Once isolated, allopatric populations are able to drift or be driven apart in response to local selective pressures. The microsites occupied by some of the species uncovered in this study, including P. sp. Wolgan Cliffs and P. sp. Dingo Creek, are of naturally limited area, and it is likely that population sizes are therefore naturally small, which may favour divergence in response to local selection, or drift. If the P. glabra complex is monophyletic, then it may comprise an adaptive geographic radiation with considerable morphological divergence among descendant species.
The degree of morphological and genetic discontinuity resolved within the P. glabra complex stands in contrast to the broad circumscription proposed by the most recent revision of Pultenaea (de Kok and West 2002), which attributed many character differences to various sources of intra-specific variation (de Kok and West 2002, 2003, 2004), without empirical confirmation. Unfortunately for herbarium-based studies, every observation of morphological difference among specimens is confounded by genotype because each gathering (or set of duplicate specimens) represents a different individual, or set of individuals. Therefore, disentangling the relative contributions of genotype and environment to phenotype is fraught with unavoidable difficulty, and must be approached with care during herbarium-based studies, particularly when observations of any given phenotype are limited. The past two decades have seen extensive collections documenting both the distribution and morphology of entities within the P. glabra complex compiled, and the resulting specimen resource, which was not available to de Kok and West (2002), demonstrates morphological consistency within, and lack of continuity among, the species subsumed within P. glabra, which our study has demonstrated are also genetically distinct. Nevertheless, the inappropriate inference of broad amplitudes of morphological variation within P. glabra suggests that other broadly circumscribed and polymorphic Pultenaea species may also represent complexes requiring further investigation.
Taxonomic treatment
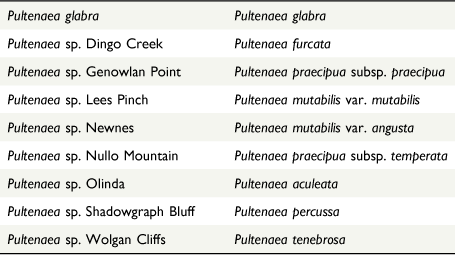
For the remainder of this paper, we refer to the taxa recognised by their scientific names, all of which are formalised below. To facilitate reference to the hypothesis-testing component of this study above, and the formal hypotheses of relationship, we propose as a result below, a translation table linking the phrase-named morphospecies and the formal taxa corresponding to them is presented in Table 1.
Key to species of the P. glabra complex, all of which have glabrous, or near-glabrous, ovaries|
1. Inflorescence with nodes not contracted, and leafy shoots continue vegetative growth after flower production. Each flower subtended by a normal leaf. Branching architecture irregular |
|
2. Leaf apex acute to acuminate with short apiculus. Leaf cells on abaxial leaf surface smooth or with low mamillae. Orange-pigmented cells in lower subepidermis only. Style glabrous or with hairs on ventral surface only, immediately above ovary |
|
3. Leaves linear, margins inrolled, upper surface with narrow channel. Leaf cells on abaxial surface with low mamillae. Calyx outer surface villous. Standard tapering at base, not embracing wings |
|
4. Calyx glabrous or with sparse short antrorse hairs. Leaves linear or oblanceolate (or obovate), not spreading at right angles to stem, or if spreading at right angles, then not closely spaced, stipule apices short, not divergent or recurved |
|
5. Leaves 3.6–14.0 (average 6.7) mm long, with a long pungent apiculus. Stipules on mature shoot sectors with lobes divergent and strongly reflexed. Flowers shortly pedicellate. Bracteole papery. Keel vibrant deep-red |
|
6. Leaves longer than 6.0 mm, linear to narrow oblanceolate, apex apiculate; stipules attached close to the middle of the calyx tube |
|
7. Abaxial leaf surface cells bulging, and with indistinct fine granular ornamentation. Cells on adaxial surface with sparse, low columnar ornamentation, adaxial surface pale green |
|
8. Leaves on average 0.8 mm wide (range 0.6–1.2 mm) |
|
9. Leaves on average 0.8 mm wide (range 0.4–1.0 mm) |
|
1. Pultenaea glabra Benth., Fl. Austral. 2: 125 (1864)
Erect, usually single-stemmed, woody shrub up to 2.2 m tall, openly branched in upper third into a spreading crown; branching pattern furcate or whorled; branchlets pale yellow–green, glabrous. Stipules red–brown or red–purple at base, black–brown towards the tips, aging to black–brown throughout, furcate, lobes triangular, straight, not arched or recurved, sometimes acuminate, keeled and coriaceous medially, membranous marginally; red–brown, lamina and lobe margins denticulate; divided to ~0.6 times length when associated with sterile leaves. Leaves linear to linear-oblanceolate, but only faintly oblanceolate, 9.4–20.9 (average 14.5) mm long, 0.8–2.1 (average 1.4) mm wide, flat, margins plane; adaxial surface with glaucous bloom owing to sparse, low pill-box shaped papillae, glabrous; apex apiculate, red–brown. Leaf anatomy with subepidermis of faintly pigmented orange–brown cells one tier deep immediately above the ventral epidermis either side of the median vascular trace and extending two-thirds of the way to the leaf edge; three vascular traces present, the medial largest, lacking enlarged cells below the medial trace. Inflorescence terminal on shoot that ceases growth; leafy throughout although leaf length decreases from the base to the apex of the inflorescence; internodes contracted; stipules associated with flowers broader than those associated with leaves, with shorter lobes and longer disc. Flowers shortly pedicellate, pedicels glabrous, but with long red–brown trichomes present in axils. Calyx heavily suffused with red on margins, otherwise pale green; outer surface glabrous, inner surface with a floccose covering of short, dense hairs towards and on lobe margins; lobes apiculate; margins hyaline, bordered wine-red, medially green. Bracteoles narrow triangular, acuminate, with broad membranous light brown or fawn ‘wings’ either side of a coriaceous medial band, extending beyond adjacent calyx sinus; glabrous or with a few short marginal hairs. Corolla mid-yellow, other pigments largely absent; standard with very faint red markings at base; keel paler than standard, pale yellow with a green wash that intensifies towards the apple-green base; standard broadly ovate with cordate base, folded along the medial line and so embracing the wings and keel, margins weakly inrolled; wings narrow obovate above a narrow stipe, basal auricle narrow, its apex broadly rounded but asymmetric; keel asymmetrically obovate–elliptic above a narrow stipe, with straight upper margin and evenly rounded apex, its basal auricle triangular, with a narrowly rounded apex. Ovary and style glabrous. Pods ~5 mm long (Fig. 9, 10).
Distribution and ecology
The known distribution of Pultenaea glabra extends from the Kings Tableland in the south to the vicinity of Mount Tomah in the north. The most easterly known population occurs at Hazelwood Dam at Lawson, and the most westerly population on the south side of the Grose River is at Katoomba, with historical records further west from Mount Victoria, whose source populations may or may not be extant. Collections of Pultenaea glabra from the upper Grose River catchment between Blackheath and Mount Tomah are in the vicinity, but to the east, of Mount Victoria and may represent more recent gatherings from the same ‘Mount Victoria’ population. The only population currently known on the northern side of the Grose River occurs ~3 km west of Mount Tomah on the southern side of the Bells Line of Road. Outside of this area, all records of Pultenaea glabra are based on other species, including P. euchila, P. mutabilis, P. aculeata, and P. weindorferi, among others. A previous report from Parramatta is based on the type of P. villosa var. glabrescens, which is in fact a synonym of P. flexilis, and a previous report from Blackheath is based on P. euchila. Records of P. glabra from Lithgow, Hartley and Sydney, could not be confirmed during our study and are likely to be based on misidentifications, or on cultivated material. We have not seen specimens on which the historical record from Dubbo is based, but suspect this too is a misidentification. Reports from Victoria are all referable to P. weindorferi, those from Queensland to other, unrelated species. Pultenaea glabra in the stricter sense resolved here is endemic to a smaller range than previously appreciated, and couples this narrower geographic distribution with fairly precise microsite preferences. Pultenaea glabra is associated with sites in close proximity to free-flowing water, or on sites with reliable subsurface soil moisture. Riparian vegetation alongside streams including on streambanks and alluvial terraces, and short or tall vegetation in gully heads including on the margins of hanging swamps may all contain P. glabra as a component of the shrub layer. All known sites occur over Narrabeen group sandstone bedrock, in soils that are either peaty (as at Bullaburra) or with a high clay content (as at Empire Pass, Lawson), depending on the microsite. Pultenaea glabra may occur as scattered individuals within a mixed shrub layer beneath Eucalyptus-dominated forest with more or less continuous canopy, as at Mount Tomah, or as a dominant component of streamside riparian vegetation, with many contiguous plants in dense shrubbery beneath a discontinuous forest canopy, as at Hazelwood Dam, and Frederica Falls.
Recognition
Pultenaea glabra possesses a combination of characters encompassing many aspects of form, from the branching architecture through to leaf micromorphology and anatomy, that facilitate ready identification of plants, in both herbarium and field. The branching architecture of P. glabra is distinctive, being furcate or whorled, because shoots are terminated by inflorescences, and vegetative growth is continued by branches that issue from below the inflorescences. The form of the inflorescence is also characteristic, because within the inflorescence, internodes separating sequential flowers are much shorter than those separating adjacent leaves in vegetative shoot sectors. Further, within the inflorescence, the leaves and their pedicels subtending each flower are progressively reduced in size from the base to the apex of the inflorescence, to the point where they are not expressed. The stipules subtending and within the inflorescence are large and imbricate. The calyx is glabrous, and the bracteoles are coriaceous and also glabrous. The branchlets are glabrous, as are the oblanceolate leaves, which are discolourous, being glaucous above due to the presence of low columnar papillae. Characters that discriminate P. glabra from other species of the P. glabra complex, and other relatives, are presented in Table 2.

|
Pultenaea glabra may hybridise with P. flexilis to produce hybrid individuals that grow to maturity alongside individuals of P. glabra. The hybrids are more similar to P. glabra than to P. flexilis in leaf morphology, because they have narrow oblanceolate leaves bearing a glaucous bloom on the adaxial surface, rather than the oblong leaves lacking a glaucous bloom as in P. flexilis. However, the leaves of hybrid individuals are consistently wider than the leaves of pure P. glabra individuals, with a measured range of 1.5–2.8 mm and an average width of 1.9 mm, compared with a range of 0.8–2.1 mm and an average width of 1.4 mm in P. glabra. Hybrids are readily distinguished from pure P. glabra when they are in flower, because their flowers are not contracted in a terminal inflorescence, and although they may group towards the end of shoots, they are separated by internodes of more or less normal length, and are subtended by normal leaves (Fig. 11) as in P. flexilis (Fig. 12).
Pultenaea glabra has been confused with P. mutabilis, but is readily distinguished by the patterns of flower production, the leaf shape, as detailed in Table 2, but perhaps most readily by the branchlets, which are hairy with appressed antrorse hairs in P. mutabilis, and glabrous in P. glabra.
Variation
There is some variation among individuals in flower size; individuals in the population on the northern side of the Grose River may have larger flowers than those on the southern side, but individuals with large and small flowers are both present in this population.
Notes
Pultenaea villosa var. glabrescens Benth. was listed as a synonym of P. glabra by de Kok and West (2002). However, the type specimen (K 000118985) is a better match with P. flexilis, the synonymy of which we transfer this name, below.
Conservation status
The geographic distribution of P. glabra is smaller than previously understood, and its microsite preferences are narrower than previously recognised. Most of the known populations of P. glabra occur within or immediately adjacent to the suburban corridor flanking the Great Western Highway, on council or private land. Some populations are under threat from invasive weeds including Ligustrum sinense Lour. and Lonicera japonica Thunb. ex Murray, possibly facilitated by elevated nutrient levels in runoff from upstream peri-urban areas. Populations on the northern side of the Grose River were burnt during the 2019–2020 fire season, with the likely result that the all adult plants were killed, although this has not been confirmed. Like other members of the P. glabra complex, P. glabra probably experiences extreme fluctuations in the numbers of adult individuals as a result of fire, and recovering populations are vulnerable to depletion by short fire-return times. As circumscribed here, Pultenaea glabra may qualify for listing as Endangered under the IUCN criteria (IUCN 2012), given the extent of occurrence is less than 5000 km2, the quality of habitats is likely to continue to decline as weed invasions progress, and severe fire years result in extreme fluctuations in the number of mature individuals.
Specimens examined
NEW SOUTH WALES: Central Tablelands: Mt Tomah, ~100 m downslope from Bells Line of Road and ~3.5 km east of Mt Tomah, 10 Oct. 2013, C.D. Kilgour 3011 (NSW 908496); Mount Victoria, Oct. 1910, W.F. Blakely s.n., (NSW 38077); Wentworth Falls, Sep. 1893, R.T. Baker s.n., (NSW 467168); Wentworth Falls, 20 Oct. 1959, D.F. Blaxell s.n., (NSW 100242); Wentworth Falls, Jan. 1898, R.T. Baker s.n., (NSW 467169); Wentworth Falls (Bodington Road), Hanging Swamp, Blue Mountains, near Bodington Hospital, 23 June 1999, J. Anderson s.n., (NSW 431580); Within grounds of Bodington Aged Care, Tableland Road, Wentworth Falls, 1 Feb. 2006, S. Rose s.n., (NSW 738435); 5 km S of Wentworth Falls, 300 m along the road N of the entrance of the car park of Queen Victoria hospital. On left side of road if travelling north, 5 Dec. 2000, R.P.J. de Kok 910, L. Orthia, C. Servase, (NSW 506489); Kings Tableland, Wentworth Falls, 6 1/2 miles [~10.4 km] by road from the Western Highway, Apr. 1962, C.E.B.H. Burgess s.n., (NSW 61038); Wentworth Falls, 24 Dec. 1950, H. St. John 24897, (NSW 38082); Andersons Firetrail turnoff, Kings Tablelands Rd, near Wentworth Falls, 2 Oct. 1985, A. Auld 36, (NSW 218436); Leura, Jan. 1908, J.T. Wilson s.n., (NSW 38071); Katoomba Falls, 29 Nov. 1932, W.F. Blakely s.n., (NSW 38080); 7.6 km SE of Queen Victoria Hospital, Wentworth Falls on the McMahons Lookout Rd, close to Anderson Fire Trail, 17 Sep. 1985, R.G. Coveny 12151, W. Bishop, R.O. Makinson, (NSW 218666); Leura, ex Herbarium F.A. Rodway no. 1742, Apr. 1916 H.M.R. Rupp s.n., (NSW 38074); Leura, Jan. 1915, A.A. Hamilton s.n., (NSW 38078); Leura Cascades, 1 Nov. 1985, A. Auld 32, (NSW 218441); Leura Falls, Dec. 1907, A.A. Hamilton s.n., (NSW 38073); Leura Falls, Nov. 1913, A.A. Hamilton s.n., (NSW 467166); Hazelbrook, Sep. 1914, A.A. Hamilton s.n., (NSW 38075); Adelina Falls, Lawson, 7 Oct. 1986, R.G. Coveny 12363, K.U. Kramer, M.D. Tindale, (NSW 507730); Lawson, track to Frederica Falls, 26 Oct. 1982, R.G. Coveny 11311, P.D. Hind, T. James, (NSW 467167); Bullaburra, below Boronia Road and Boronia Reserve on south side of Great Western Highway, 6 Oct. 2018, M.A.M. Renner 9044 & L.J. Gray, (NSW 1052694); ibid, 6 Oct. 2018, M.A.M. Renner 9045 & L.J. Gray, (NSW 1052712); 6 Oct. 2018, M.A.M. Renner 9046 & L.J. Gray, (NSW 1052713); 6 Oct. 2018, M.A.M. Renner 9047 & L.J. Gray, (NSW 1052714); 6 Oct. 2018, M.A.M. Renner 9048 & L.J. Gray, (NSW 1052715); 6 Oct. 2018, M.A.M. Renner 9049 & L.J. Gray, NSW1052716; Central Coast: Hazelbrook, road to dam, Woodford Creek, 26 Sep. 2018, M.A.M. Renner 9037 & R.L. Barrett, (NSW 1052815); 26 Sep. 2018, M.A.M. Renner 9038 & R.L. Barrett, (NSW 1052816); 26 Sep. 2018, M.A.M. Renner 9040 & R.L. Barrett, (NSW 1052818); 26 Sep. 2018, M.A.M. Renner 9041 & R.L. Barrett, (NSW 1052819); 3.5 km W of Mt Tomah, 1 Oct. 2019, M.A.M. Renner 9160 & L.J. Gray, (NSW 1058858); 1 Oct. 2019, M.A.M. Renner 9161 & L.J. Gray, (NSW 1058861).
2. Pultenaea furcata M.A.M.Renner & R.L.Barrett, sp. nov.
Diagnosis
Distinct in the glabrous, linear leaves, with abaxial surface cells each bearing a low triangular mamilla, the glabrous branchlets, the inflorescence terminating leafy shoots, and villous calyx.
Spreading multi-stemmed shrub up to 2.5 m tall, openly branched, bark red–brown to grey–brown, slightly fissured at base. Branchlets yellow–green, glabrous. Stipules red–brown aging to dark brown, divided to just over halfway, lobes diverging, keeled to close to the apex, keels in each lobe eccentric, prominent and convergent at the top of lamina, then running parallel down either side of the leaf insertion; disc flaring slightly towards base but not by much; overall, the disc is quite rectangular; margins irregularly and sparsely toothed. Leaves glaucous above, dull mid-green below; linear to narrow oblanceolate, 7.3–18.7 (average 12.0) mm long by 0.4–1.0 (average 0.7) mm wide, margins upturned to inrolled, when dry obscuring the adaxial surface, glabrous, but a couple of hairs are present on either side of the pulvinus; apex mucronate, orange–brown; abaxial surface with low triangular mamillae formed by single epidermal cells; adaxial surface glaucous, bearing numerous pill-box cuticular papillae; three vascular traces present, medial largest; adaxial epidermis hyaline or slightly tan-pigmented, no orange-pigmented cells are present. Inflorescence terminal on shoot that ceases growth; leafy throughout, although leaf length decreases from the base to the apex of the inflorescence; internodes contracted; within the inflorescence leaves reduced to a red–brown, hair-bearing spine fused with the stipule, between the two stipule keels, and not extending as far as the apex of the stipule lamina. Flowers in clusters of 7–12, shortly pedicellate to nearly sessile, pedicel villous; long red–brown trichomes in the flower axil adjacent the pedicel. Calyx green with wine-red margins; copiously ciliate in bud, hairs evenly distributed and retained through to fruit; densely villous throughout including tube and lobes, hairs tending to be longer at the apex of the tube; lobes long, narrow, acuminate and pungent-tipped; lobe margins floccose or ciliolate with dense short curled hairs; lobe apices obtuse with an acuminus; inner surface of lobes with a hyaline margin subtended by a dark brown to black band, medial part green; dorsal calyx lobes conspicuously reflexed. Bracteoles bronze–brown, villous hairy to near their apex, apex mucronate; papillae present on their uppermost margins. Corolla orange–yellow, with faint red semicircular marking at base of standard, otherwise lacking red markings, keel paler than rest of the flower; greenish-cream to pale yellow; standard rotund above a basal stipe, folded not flat at flower maturity, apex emarginate; wings laterally splayed, ligulate to slightly rectangular–obovate above a long basal stipe, upper and lower margins straight, slightly divergent towards rounded apex, basal auricle a broad rounded triangle. Keel asymmetrically elliptic, upper margin linear, lower margin evenly curved, broadest at mid-point, apex broadly rounded, basal auricle triangular with the auricle apex rounded. Anthers and pollen cream. Ovary glabrous, style with a few sparse sharp, stiff, hairs on the lower surface. Pods not seen (Fig. 13, 14).
Distribution and ecology
Pultenaea furcata is known from the upper Dingo Creek catchment, on the eastern side of the Newnes Plateau. Three populations are known, each occurring in slightly different ecological settings. In the upper catchment, Pultenaea furcata grows in deep humic soils within small, steep, south-western facing gullies between sandstone pagodas and outcrops of the Narrabeen Group. Here, it grows within a mixed shrubland with Dillwynia sp., Epacris browniae Colby, Prostanthera sp. and other shrubs. Alongside a tributary of Dingo Creek, P. furcata grows as a dominant component of riparian shrub layer within a shallow gully with a northerly aspect, and beneath a discontinuous Eucalyptus canopy, with Leptospermum sp. At the third known population P. furcata grows on an alluvial terrace beneath a mixed canopy of Leptospermum sp. and Eucalyptus sp. in a sharp gully punctuated by sandstone pagodas.
Recognition
Pultenaea furcata is similar to P. glabra in branching architecture, wherein inflorescences terminate leafy shoots, and vegetative growth is continued by branching immediately below the inflorescence, with the result that branching pattern is furcate to whorled. Pultenaea furcata differs from P. glabra in the linear leaves up to 1.0 mm wide (oblanceolate and up to 2.1 mm wide in P. glabra), the abaxial surface cells each bearing a low triangular mamilla (only bulging and with fine granular ornamentation in P. glabra), and the calyx is villous (glabrous in P. glabra).
Conservation status
Pultenaea furcata may qualify for listing as Critically Endangered, given the small known area of occupancy and extent of occurrence, currently estimated to be less than 5 km2, and extreme fluctuations in the numbers of adult individuals. At the type locality, all but two of the adult plants observed in September 2019 were killed by the fires of October 2019–January 2020. Search and survey work should be undertaken ahead of any listing proposal to establish the likely limits of the distribution of P. furcata, which may include more remote sites along the eastern edge of Newnes Plateau. This species was unknown before this study commenced, suggesting its distribution may well be restricted to the Dingo Creek catchment and relatively inaccessible and remote country to the north thereof.
Etymology
From the Latin furcata, forked, in reference to the bifurcating to whorled branching architecture, which is a function of the terminal determinate inflorescence, and is a distinctive feature shared with only P. glabra within the complex.
Specimens examined
NEW SOUTH WALES: Central Tablelands: Newnes State Forest: Tributary of Dingo Creek at crossing with firetrail, NE side of Newnes Plateau, 27 Sep. 2019, M.A.M. Renner 9170 & J.M. Cohen, (NSW 1058826); ibid, 27 Sep. 2019, M.A.M. Renner 9171 & J.M. Cohen, (NSW 1058827); 27 Sep. 2019, M.A.M. Renner 9172 & J.M. Cohen, NSW1058828; M.A.M. Renner 9173 & J.M. Cohen, 27 Sep. 2019, (NSW 1058829); 27 Sep. 2019, M.A.M. Renner 9174 & J.M. Cohen, (NSW 1058830); 27 Sep. 2019, M.A.M. Renner 9175 & J.M. Cohen, (NSW 1058832); track to Dingo Creek south of Deep Pass Trail, 27 Sep. 2019, M.A.M. Renner 9176 & J.M. Cohen, (NSW 1058819); ibid, 27 Sep. 2019, M.A.M. Renner 9177 & J.M. Cohen, (NSW 1058821); 27 Sep. 2019, M.A.M. Renner 9178 & J.M. Cohen, (NSW 1058822); 27 Sep. 2019, M.A.M. Renner 9179 & J.M. Cohen, (NSW 1058823); 27 Sep. 2019, M.A.M. Renner 9180 & J.M. Cohen, (NSW 1058824); 27 Sep. 2019, M.A.M. Renner 9181 & J.M. Cohen, (NSW 1058825).
3. Pultenaea tenebrosa M.A.M.Renner, P.H.Weston & S.Clarke, sp. nov.
Diagnosis
Distinct in its linear leaves that spread at right angles to the stem, the abaxial leaf surface with bulging cells that bear fine granular ornamentation, the red–brown to black stipules whose lobes are divergent and recurved and completely obscure the branchlets, the calyx with long spreading hairs and coriaceous, pungent, bracteoles that extend as far as, or further than, the adjacent calyx lobe apices.
Spreading multi-stemmed shrub upto 2.0 m tall, peripheral branches nearly horizontal, initiating new branches at base. Branching furcate to fasciculate, irregular. Larger branches and trunk grey–brown with ruminant red–brown traces that become more prominent with branch size. Branchlets yellow–green, almost completely obscured by red–brown recurved stipules. Stipules red–brown aging to dark brown or black, imbricate, furcate, divided for half their length, lobes divergent, sometimes recurved, through as much as 180°; lobe apex acuminate; lamina margins irregularly toothed along their length, the teeth poorly formed but distinct, rounded or acute; stipule lobes eccentrically keeled, the wing on the outside of the keel wider than the inner. Leaves linear to nearly terete, 5.7–16.6 (average 11.1) mm long by 0.4–0.8 (average 0.5) mm wide, spreading at right angles from the stem, dull to mid-green with a hint of yellow below, narrow, inrolled but not completely enclosing the adaxial surface, glabrous; adaxial surface green with a glaucous wash, low pill-box papillae present; abaxial surface discolourous, green laterally (on the surface presented dorsally), red–brown medially (the surface presented ventrally), with a lighter tan–brown central stripe where the midvein is visible; apex tapered to a short point, red–brown; leaf anatomy with ventral epidermal cells hyaline, larger on abaxial surface, subepidermis of orange-pigmented cells present on ventral leaf, three vascular traces present, median trace largest and abutting the orange-pigmented subepidermis; abaxial leaf epidermal cells bulging but not mamillose, bearing granular ornamentation. Inflorescence with flowers closely spaced, but internodes not contracted, with leaves among the flowers progressively decreasing in size at sequential nodes, the shoot continues vegetative growth after flowering; stipules subtending floral leaves similar to sterile stipules, but a little shorter and broader. Flowers sessile. Calyx outer surface with long spreading hairs, the inner surface of each lobe discolourous, margins brown, green below; ventral lobes narrower than the two dorsal lobes, floccose along their median line; the two dorsal lobes floccose along submarginal lines; lobes sometimes bearing scattered hairs on outer surfaces; calyx tube with an indistinct ring of hairs encircling the top of the tube, beneath the sinuses. Bracteoles conspicuous, coriaceous, pungent, weakly keeled, with an indistinct hyaline margin, extending to, or slightly beyond, the level of the calyx lobe apices. Corolla orange–yellow with a faint red flare at the base of standard, keel darker orange–yellow than standard; standard broadly obovate, concave at flower maturity, wings loosely overlaying keel, ligulate above a narrow stipe, almost narrow rectangular with upper and lower margins nearly parallel, apex rounded, base with broad angular auricle; keel asymmetrically elliptic–obovate, upper margin curved, lower margin evenly curved, deepest just beyond mid point towards apex, apex broadly rounded, basal auricle broadly triangular with a rounded apex. Anthers cream, filaments pale orange in dried state. Pollen cream. Ovary and style glabrous (Fig. 15, 16).
Distribution and ecology
Pultenaea tenebrosa is known from the Wolgan Valley, on the cliff complexes on the western side of the valley, and the pagoda-gully complexes on the Sunnyside Plateau on the south-eastern side. The habitat occupied by P. tenebrosa is challenging to access, and the species may be more widely distributed in suitable situations associated with the Wolgan Valley than current records indicate. The initial site on the eastern side of the Wolgan Valley was a chance discovery, and all subsequent sites were located during targeted searches of pagoda habitats in the area. On the western cliff lines of the Wolgan Valley, Pultenaea tenebrosa occurs almost exclusively on ledges immediately below Mount York claystone. Claystone ledges on the eastern side were also searched with no results, so it seems it occurs on such ledges on the western side of the Wolgan Valley only. The ledges below Mount York claystone on the western cliff-lines bear a sparse canopy of Eucalyptus sp. (piperita?), and usually a dense heathy understorey. including Acacia obtusifolia, Allocasuarina sp., Banksia penicillata, Banksia spinulosa, Dracophyllum secundum, Gahnia filifolia, Leptospermum polygalifolium, Styphelia mutica, Styphelia sieberi, Persoonia myrtilloides, Philotheca obovalis, Epacris reclinata and Xanthorrhoea sp. The soils at these sites are sandy loams or sandy clays.
In three of the populations on the western side of the Wolgan Valley, all plants present pre-fire appear to have been killed outright by the Gospers Mountain fire over the summer of 2019–2020. No basal or epicormic resprouting was seen on any of the charred skeletons of pre-fire adults that could still be identified. However, by June 2020, there had been a large flush of post-fire seedling emergence across the sites, with the largest some 15–20 cm tall at 5 June 2021 (Fig. 17). Two sites had at least 100–200 seedlings each, the third site had a modest 16. So, although the fire has resulted in a many-fold increase in the known number of individuals, it has reduced a significant adult proportion of the species, or perhaps the entire global population, given the entire known extent of the species was burnt, to the current crop of post-fire seedlings. This leaves the species particularly vulnerable in the event of another fire before the current cohort matures and reproduces to replenish the soil seed bank.

|
Recognition
Pultenaea tenebrosa can be recognised by its combination of linear leaves that spread at right angles to the stem, and the red–brown to black stipules the lobes of which are divergent and recurved and completely obscure the branchlets. Two other distinctive features of P. tenebrosa are the long spreading hairs on the calyx and the coriaceous, pungent, bracteoles that extend as far as, or further than the adjacent calyx lobes. Pultenaea tenebrosa is similar to P. furcata, but the two can be distinguished by the leaf abaxial surface, which in P. tenebrosa bears bulging cells with fine granular ornamentation, whereas in P. furcata the cells each bear a low triangular mamilla. This character may be useful with sterile specimens whose stipules are not, or are suboptimally, recurved.
Conservation status
Pultenaea tenebrosa may qualify for Critically Endangered under the IUCN criteria, given the extent of occurrence is ~64 km2, the species occurs as small, highly fragmented populations, and the populations experience extreme fluctuations in the numbers of mature individuals. During the fires of 2019–2020, fires burnt over the entire range of this species, and killed all mature individuals at three populations on the western side of Wolgan Valley resurveyed in June 2021. Altered fire regimes and browsing by feral herbivores including goats and probably deer constitute the greatest threats to this species.
Etymology
From the Latin tenebrosa, dark, referencing the habit of this species to grow on sheer cliffs of southerly to south-easterly aspect, and the stems densely covered by red–brown to black stipules.
Specimens examined
NEW SOUTH WALES: Central Tablelands: Gardens of Stone National Park: ~21 km N of Lithgow, ~14 km E of Cullen Bullen, eastern flanks of Sunnyside Ridge in steep gully running into un-named tributary of Carne Creek, 21 Oct. 2015, S. Clarke 703, (NSW 933334); 21 Oct. 2015, S. Clarke 704, (NSW 933333); 21 Oct. 2015, S. Clarke 705, (NSW 933332); 21 Oct. 2015, S. Clarke 706, (NSW 933331); ~23 km N of Lithgow, ~18 km E of Cullen Bullen, eastern side of ridge above an un-named tributary of Carne Creek, 7 Mar. 2015, S. Clarke 612, (NSW 933307); 17 Mar. 2015, S. Clarke 613, (NSW 933308); ~23.5 km N of Lithgow, ~18.5 km E of Cullen Bullen, eastern side of ridge above an un-named tributary of Carne Creek, 17 Mar. 2015, S. Clarke 614, (NSW 933305); 17 Mar. 2015, S. Clarke 615, (NSW 933306); ~23.5 km N of Lithgow, ~18.5 km E of Cullen Bullen, eastern side of ridge in gully running into an un-named tributary of Carne Creek, 21 Oct. 2015, S. Clarke 707, (NSW 933330); 21 Oct. 2015, S. Clarke 708, (NSW 933329); ~24 km N of Lithgow, ~18 km E of Cullen Bullen, eastern side of ridge above an un-named tributary of Carne Creek, 26 May 2015, S. Clarke 623, (NSW 933296); 26 May 2015, S. Clarke 624, (NSW 933297); ~29 km N of Lithgow, ~12.5 km NE of Cullen Bullen, southern cliffline of narrow divide between Wolgan and Capertee valleys, 3 June 2015, S. Clarke 625, (NSW 933293); 3 June 2015, S. Clarke 626, (NSW 933294); 3 June 2015, S. Clarke 627, (NSW 933295); Wolgan State Forest: ~22 km NNW of Lithgow, 7.5 km NE of Cullen Bullen cliffs on western rim of Wolgan Valley, 9 Nov. 2014, S. Clarke 596, (NSW 883686); 9 Nov. 2014, S. Clarke 597, (NSW 883687); 9 Nov. 2014, S. Clarke 598, (NSW 883688); 9 Nov. 2014, S. Clarke 599, (NSW 883689); 9 Nov. 2014, S. Clarke 600, (NSW 883690); 9 Nov. 2014, S. Clarke 601, (NSW 883691); 9 Nov. 2014, S. Clarke 602, (NSW 883692); ~22 km NNW of Lithgow, 7.5 km NE of Cullen Bullen cliffs on western rim of Wolgan Valley, 7 Dec. 2014, S. Clarke 605, (NSW 883679); 7 Dec. 2014, S. Clarke 606, (NSW 883683); 7 Dec. 2014, S. Clarke 607, (NSW 883684); 7 Dec. 2014, S. Clarke 608, (NSW 883685); ~24 km N of Lithgow, 10 km NE of Cullen Bullen, cliffs on western rim of Wolgan Valley, 12 Dec. 2014, S. Clarke 609, (NSW 883674); 12 Dec. 2014, S. Clarke 610, (NSW 883675;) ~23 km N of Lithgow, ~8.5 km NE of Cullen Bullen, cliff lines flanking western side of Wolgan Valley, 27 Sep. 2015, S. Clarke 654, (NSW 933317); 27 Sep. 2015, S. Clarke 655, (NSW 933318); 7 Sep. 2015, S. Clarke 656, (NSW 933319); ~23 km NNW of Lithgow, 8.5 km NE of Cullen Bullen cliffs on western rim of Wolgan Valley, 9 Nov. 2014, S. Clarke 603, (NSW 883693); 9 Nov. 2014, S. Clarke 604, (NSW 883694); ~25 km N of Lithgow, ~8 km NE of Cullen Bullen, ~2 km SE of Baalbone Gap, 27 Sep. 2015, S. Clarke 657, (NSW 933309); ~24 km N of Lithgow, ~10 km NE of Cullen Bullen, cliffs flanking western side of Wolgan Valley, 27 Sep. 2015, S. Clarke 665, (NSW 934289); ~24 km N of Lithgow, ~10 km NE of Cullen Bullen, cliffs flanking western side of Wolgan Valley, 27 Sep. 2015, S. Clarke 666, (NSW 934290); 27 Sep. 2015, S. Clarke 667, (NSW 934291); ~25 km N of Lithgow, ~8 km NE of Cullen Bullen, ~2 km SE of Baalbone Gap, 27 Sep. 2015, S. Clarke 658, (NSW 933310); 27 Sep. 2015, S. Clarke 659, (NSW 933311;) 27 Sep. 2015, S. Clarke 660, (NSW 933312); 27 Sep. 2015, S. Clarke 661, (NSW 933313); 27 Sep. 2015, S. Clarke 662, (NSW 933314); 27 Sep. 2015, S. Clarke 663, (NSW 933315); 27 Sep. 2015, S. Clarke 664, (NSW 933316); ~27.5 km N of Lithgow, ~10.5 km NE of Cullen Bullen, ~0.5 km S of Mount Jamison, southern cliff line of narrow divide between Wolgan and Capertree valleys, 9 May 2015, S. Clarke 620, (NSW 933298); 9 May 2015, S. Clarke 621, (NSW 933299); 9 May 2015, S. Clarke 622, (NSW 933300); Woolpack Gap, ~28.5 km N of Lithgow, ~11 km E of Ben Bullen, 19 Aug. 2015, S. Clarke 649, (NSW 933321); 19 Aug. 2015, S. Clarke 650, (NSW 933320); Woolpack Gap, ~28.5 km N of Lithgow, ~11 km E of Ben Bullen, 19 Aug. 2015, S. Clarke 651, (NSW 933322); 19 Aug. 2015, S. Clarke 652, (NSW 933323); 19 Aug. 2015, S. Clarke 653, (NSW 933324); ~13 km N of Lidsdale, ~8 km NE of Cullen Bullen, W side of Wolgan Valley, 14 Apr. 2014, S. Clarke s.n., (NSW 887775); Newnes State Forest: ~18 km N of Lithgow, ~14 km E of Cullen Bullen, pagoda ridge above an un-named tributary of Carne Creek, 30 Apr. 2015, S. Clarke 616, (NSW 933301); 30 Apr. 2015, S. Clarke 617, (NSW 933302); 30 Apr. 2015, S. Clarke 618, (NSW 933303); 30 Apr. 2015, S. Clarke 619, (NSW 933304); ~18 km N of Lithgow, ~14 km E of Cullen Bullen, pagoda ridge above an un-named tributary of Carne Creek, 21 Oct. 2015, S. Clarke 710, (NSW 933327); 21 Oct. 2015, S. Clarke 711, (NSW 933326); 21 Oct. 2015, S. Clarke 712, (NSW 933325); ~22 km N of Lithgow, ~18 km E of Cullen Bullen, eastern side of ridge running into an un-named tributary of Carne Creek, 25 Jun. 2015, S. Clarke 641, (NSW 933292); 25 Jun. 2015, S. Clarke 642, (NSW 933291); ~22 km N of Lithgow, ~18 km E of Cullen Bullen, running into an un-named tributary of Carne Creek, 25 June 2015, S. Clarke 643, (NSW 933427;) 25 June 2015, S. Clarke 644, (NSW 933428); 25 June 2015, S. Clarke 645, (NSW 933429); ~22 km N of Lithgow, ~18 km E of Cullen Bullen. Near head of gully between pagodas running into an un-named tributary of Carne Creek, 21 Oct. 2015, S. Clarke 698, (NSW 933339); 21 Oct. 2015, S. Clarke 699, (NSW 933338); 21 Oct. 2015, S. Clarke 700, (NSW 933337); 21 Oct. 2015, S. Clarke 701, (NSW 933336); 21 Oct. 2015, S. Clarke 702, (NSW 933335). Ben Bullen State Forest: Cliff-line on western side of Wolgan Valley, E of Bicentennial National Trail, 31 Oct. 2019, M.A.M. Renner 9182 & S. Clarke, (NSW 1058839); 31 Oct. 2019, M.A.M. Renner 9183 & S. Clarke, (NSW 1058840); 31 Oct. 2019, M.A.M. Renner 9184 & S. Clarke, (NSW 1058841); 31 Oct. 2019, M.A.M. Renner 9185 & S. Clarke, (NSW 1058842); 31 Oct. 2019, M.A.M. Renner 9186 & S. Clarke, (NSW 1058844); 31 Oct. 2019, M.A.M. Renner 9187 & S. Clarke, (NSW 1058845).
4. Pultenaea mutabilis M.A.M.Renner & P.H.Weston, sp. nov.
Diagnosis
Pultenaea mutabilis is distinguished from other members of the P. glabra complex by the combination of irregular branching architecture, leaves with little or no ornamentation on the adaxial surface, and indistinct low, granular ornamentation on the abaxial surface, the narrow, linear stipules and papery bracteoles; the inflorescences produced on shoots that continue vegetative growth; flowers produced in the axils of leaves that are identical to those on vegetative shoot sectors; and the internodes separating sequential flowers being the same length as those separating sterile leaves.
Etymology
From the Latin mutabilis, inconsistent, referencing the various morphological expressions of this species that are closely related genetically but that have remarkably disparate field-presentation.
4a. Pultenaea mutabilis M.A.M.Renner & P.H.Weston var. mutabilis
Diagnosis
Pultenaea mutabilis var. mutabilis differs from P. mutabilis var. angusta in its oblanceolate leaves that are 1.3–3.1 (average 2.0) mm wide.
Woody shrub up to 4 m tall, erect with spreading branches, branching weakly whorled, with clusters of branches separated by long unbranched lengths of stem; branchlets densely leafy, green, with antrorse, loosely appressed hairs, then yellow–green. Stipules red–brown changing to brown or black with age; divided to near the base, lobes triangular, diverging, tapering but not long–acuminate; arched; keeled medially, keel faint, eccentric; margins irregular, bearing occasional hairs. Leaves oblanceolate, 9.8–21.7 (average 15.2) mm long, 1.3–3.1 (average 2.0) mm wide, not inrolled, nearly flat; adaxial surface with faint glaucous bloom; sparsely hairy on both ad- and abaxial surfaces; hairs short, antrorse, appressed; petiole hairy, the hairs short and antrorse; leaf cell surfaces smooth on both ad- and abaxial surfaces, adaxial surface hydrophobic; apex with a short apiculus. Leaf anatomy with three vascular traces present, median largest, no enlarged cells below the median vascular trace, ventral epidermis hyaline, no coloured cells except on the margin towards the apex. Inflorescence on leafy shoots that continue vegetative growth, no internode contraction; shoot continuing vegetative growth after flower production; stipules associated with subfloral leaves shorter and wider than those subtending vegetative leaves. Flowers pedicellate, pedicel hairy, hairs short and curled; a cluster of orange–red brown glandular trichomes present in the leaf axil at the base and either side of the pedicel; stipules associated with flowers larger than those on vegetative leaves, broader at base, not so deeply divided, disc quadrate, lobes triangular, not tapering to a narrow filament. Calyx tube yellow–green, lobes apple-green with red–brown margins, apices with or without a differentiated apiculus, often the three ventral lobes have an apiculus, whereas the upper two do not, but this is variable within and among specimens; lobes and tube sparsely hairy with short antrorse hairs; lobe margins densely hairy, hairs short and sometimes curved; lobes with vinous border, medially green. Bracteoles red–brown, narrow linear–lanceolate, exceeding the adjacent sinus or not, hairy on margin and lamina, medial hairs longer and straighter than marginal hairs that are short and curled; lamina base orange and membranous, otherwise green and coriaceous, often suffused with rose-red. Corolla yellow–orange, with a faint red semicircle at base of standard; keel yellow–orange, paler than standard; standard broadly transverse elliptic above a basal stipe; wings laterally splayed; asymmetrically narrow obovate above a narrow basal stipe, upper margin linear, apex broadly rounded, auricle broad and rounded or truncate; keel asymmetrically elliptic, upper margin linear, lower margin continuously curved, deepest at mid-point, apex broadly rounded, basal auricle triangular, acute. Ovary glabrous, style with short antrorse hairs on ventral surface immediately above ovary. Pods around 5 mm long in Blue Mountains plants, glabrous (Fig. 18–20).
Distribution and ecology
Pultenaea mutabilis var. mutabilis is known from higher elevations of the Blue Mountains from Bilpin on the northern side of the Grose River in the south, at Gospers Mountain, at Lees Pinch in Goulburn River National Park, and around Denman, on the southern side of the Hunter Valley in the north. An outlying northern population occurs around Macintyre Falls in the Kwiambal National Park on the north-western slopes. Between Bilpin and Mount Tomah, and including Mount Irvine and Mount Wilson, P. mutabilis var. mutabilis occurs over shale bedrock, on faces and slopes, and broad ridges in wet sclerophyll forest dominated by a range of Eucalyptus species including E. piperita, E. cypellocarpa, and E. radiata. Pultenaea mutabilis var. mutabilis is a common component of the tall shrub layer within wet sclerophyll forests on and around the basalt caps of Mount Tomah, and Mount Irvine, and on the shaley soils surrounding Bilpin. Pultenaea mutabilis var. mutabilis generally grows at higher elevation than P. flexilis, which it replaces as elevation increases from east to west up the Blue Mountains, with overlap around Bilpin where the two species co-occur. At Lees Pinch in the Goulburn River National Park, P. mutabilis var. mutabilis grows over sandstone, within sandstone exposures and on talus slopes surrounding a sandstone outcrop. At Macintyre Falls, P. mutabilis var. mutabilis occurs over granite bedrock, in short dry-sclerophyll forest dominated by Eucalyptus caleyi and Callitris endlicheri with a dense shrub layer. The widely scattered known occurrences, and the variety of bedrock types over which the species may occur, suggest that P. mutabilis var. mutabilis may be widely distributed between its current known northern and southern limits.
All P. mutabilis var. mutabilis plants at Mount Irvine were burnt and killed by the Gospers Mountain Fire over the summer of 2019–2020, with no resprouting evident. However, during the summer and autumn of 2021, numerous seedlings were present, some up to 60 cm tall, suggesting mass recruitment from a pre-existing soil seed bank. At this time, the entire population comprised unflowered juveniles, and whatever ungerminated seed remained in the soil seed bank.
Recognition
Pultenaea mutabilis var. mutabilis can be recognised by the combination of branching architecture, leaf shape and ornamentation, and characters of the stipules and calyx. The branching architecture is irregular, wherein inflorescences are produced on shoots that continue vegetative growth and so do not affect architecture. The flowers are produced in the axils of leaves that are identical to those on vegetative shoot sectors, and the internodes separating sequential flowers are the same length as those separating sterile leaves. The stipules in both flowering and sterile shoot sectors are quite short and narrow, with narrow, straight lobes. The branchlets bear short antrorse hairs. The leaves are quite broad and oblanceolate (1.3–3.0 mm wide) and bear antrorse hairs on the abaxial surface, the cells of which are bulging, not mamillose, and bear fine granular ornamentation. The bracteoles are papery, and usually brown or reddish-brown. This combination of characters should prevent confusion with other members of the P. glabra complex, and most other Pultenaea species. Pultenaea mutabilis var. mutabilis has been confused with P. flexilis, but differs by its oblanceolate, rather than broadly oblong leaves, whose midvein on the abaxial surface is not prominent and covered by two or three rows of enlarged hyaline cells. Further, the leaf apex of P. mutabilis var. mutabilis is acute, whereas the apex of P. flexilis is broader, and tends to be mucronate. Pultenaea mutabilis var. mutabilis can be immediately distinguished from P. flexilis because the midrib on the underside of the leaf is narrow, and not covered by multiple rows of inflated epidermal cells. In Pultenaea flexilis, the midrib occupies approximately one-eighth of the leaf width, is prominent, raised, and pale, often a straw colour. By contrast, the midrib in P. mutabilis var. mutabilis occupies less than one 16th of the leaf width, is not, or hardly, raised, and is weakly differentiated in colour from the leaf. Pultenaea mutabilis var. mutabilis and P. flexilis also differ in leaf shape; the leaves of P. mutabilis var. mutabilis are narrow obovate, and taper evenly from the base to the broadest point, whereas the leaves of P. flexilis are nearly oblong in shape and widen abruptly from the base, such that there is usually an angle in the leaf outline immediately above the petiole, where the margin turns from this widening base into the long margin that extends towards the apex.
Pultenaea mutabilis var. mutabilis is similar to P. mutabilis var. angusta in almost all characters but differs by its consistently wider, oblanceolate leaves. The difference in leaf width is sufficient to impart completely different field aspects to these two taxa.
Pultenaea mutabilis var. mutabilis could be confused with P. forsythiana Blakely, but P. forsythiana has a long pungent acuminus at the leaf apex, a leaf that widens abruptly above the petiole, as in P. flexilis, and the stems are clothed in numerous, densely packed, orange–red erect and curled hairs. By contrast, P. mutabilis var. mutabilis has a short acuminus, a leaf that tapers gradually from the base, and stems clothed with sparse, hyaline, antrorse and appressed hairs.
Variation
Pultenaea mutabilis var. mutabilis expresses variation in several characters, including the relative lengths of calyx lobes, the length of the bracteoles relative to the calyx, and the hairiness of leaves. Plants from the eastern-fall catchments of the Blue Mountains have thinner leaves that are longer for their length than in plants from western fall catchments on the western side of the Great Dividing Range. Coriaceous-leaved plants also occur at Gospers Mountain, and similar plants occur on the north-western slopes around Macintyre Falls near Wallangra. The Macintyre Falls plants have oblong pods 9–10 mm long (NSW 225730), which are twice as long as those observed on the thin-leaved forms from the Blue Mountains. Although the Macintyre Falls plants are most similar to P. mutabilis, and are certainly not P. flexilis, we note that their status, and the relationships between the thin-leaved and coriaceous-leaved forms from the Greater Blue Mountains Region require further investigation. It is entirely possible that, as circumscribed here, P. mutabilis var. mutabilis comprises a complex of three entities, each warranting some form of formal recognition.
Conservation status
Pultenaea mutabilis var. mutabilis is widely distributed, and locally common at some sites. The distribution of this taxon needs to be fully established before an informed assessment of threat can be made.+
Specimens examined
NEW SOUTH WALES: North Western Slopes, Macintyre Falls Fauna and Flora Reserve, ~22 km NNW of Ashford, 5 Oct. 1990, R.G. Coveny 14432 & R.O. Makinson, (NSW 244919); Macintyre Falls, 3 km S of junction of Macintyre and Severn Rivers, near Wallangra, A.N. Rodd 4094, (NSW 225730). Central Coast: Goulburn River National Park, Lees Pinch, off Wollara Road; track to Lookout then S around base of outcrop, 16 Oct. 2018, M.A.M. Renner 9050 (NSW 1052693); 16 Oct. 2018, M.A.M. Renner 9051 (NSW 1052692); 16 Oct. 2018, M.A.M. Renner 9052 (NSW 1052691); 16 Oct. 2018, M.A.M. Renner 9053 (NSW 1052689); 16 Oct. 2018, M.A.M. Renner 9054 (NSW 1052690); 16 Oct. 2018, M.A.M. Renner 9055 (NSW 1052688). Central Tablelands, Cyrils Rock, 11 miles [~17.7 km] N of Gospers Mountain Army Airstrip, 9 miles [~14.4 km] NE of Glen Davis, on military road, 26 Apr. 1965, D.J. McGillivray 1150 & A.N. Rodd (NSW 77998); Mt Tomah, 17 Feb. 1974, A.N. Rodd 2604 (NSW 474237); Mt Tomah, Nov. 1898, J.H. Maiden (NSW 37524); Mt Wilson, Oct. 1899, J.H. Maiden (NSW 37523); Bowen Creek, SE of Mount Wilson, 9 Oct. 1966, R.G. Coveny s.n. (NSW 474246); Bowen’s Creek, Bilpin–Mt Irvine road, 6 Oct. 1950, E.F. Constable, (NSW 16348); Mount Irvine, 8 Oct. 1953, M. Bowyer (NSW 37519); Mount Irvine, Oct. 1924, M.B. Welch (NSW 474243); Mt Irvine, cemetery on Danes Way, 1 Oct. 2019, M.A.M. Renner 9154 & L.J. Gray (NSW 1058848); 1 Oct. 2019, M.A.M. Renner 9155 & L.J. Gray 9155 (NSW 1058852); Mt Irvine, 1 Oct. 2019, M.A.M. Renner 9156 & L.J. Gray (NSW 1058853); 1 Oct. 2019, M.A.M. Renner 9157 & L.J. Gray (NSW 1058855); 1 Oct. 2019, M.A.M. Renner 9158 & L.J. Gray (NSW 1058856); 1 Oct. 2019, M.A.M. Renner 9159 & L.J. Gray (NSW 1058857). Central Western Slopes: Denman, Oct. 1908, W. Heron (NSW 37525); Wingen Maid Nature Reserve, 10 Nov. 1996, J.R. Hosking 1342, (NSW 413425).
4b. Pultenaea mutabilis var. angusta M.A.M.Renner, P.H.Weston, & S.Clarke, var. nov.
Diagnosis
Pultenaea mutabilis var. angusta differs from P. mutabilis var. mutabilis in its linear leaves that are 0.4–1.0 (average 0.7) mm wide.
Erect single- or multi-stemmed woody shrub up to 4 m tall, stems up to 4 cm in diameter at chest height; bark grey–brown, smooth to weakly fissured at trunk base, irregularly branched, not whorled, sometimes main branches bifurcate with numerous shorter secondary branches. Branching is initially alternate, which is best expressed within leafy shoot sectors; branches fawn–grey; branchlets yellow-green, dull; ridged by a trace running basally from the leaf insertion, yellow–green, sparsely hairy. New growth often distinctly bronzed. Stipules wine-purple, ageing to tan, then black; furcate, lobes long and narrow, straight, not recurved away from the stem, rather they are appressed and straight, or nearly so, most of the time; broadly inserted on the stem, insertion extending at an angle across and down the stem surface, recurved sometimes from the triangular leaf insertion scar; stipule lobes thickened medially like the leaf apex, basal lamina small, keeled medially where the lobe thickening extends through it; lamina margin irregular, with poorly formed projections. Leaves linear, 6.1–18.3 (average 11.1) mm long, 0.4–1.0 (average 0.7) mm wide, inrolled and channelled but dorsal surface visible, discolourous, dull green with only a hint of glaucous bloom above, brighter green below; apex tapered to a short point, red–brown; abaxial surface sparsely and continuously hairy from base to apex, hairs antrorse and usually appressed but sometimes tending to spread, variable in length; adaxial surface green with glaucous bloom, and small cutaneous papillae; abaxial cell surfaces with low, even, granular ornamentation. Leaf anatomy with ventral epidermis hyaline, pigmented hyalodermis absent, but orange-pigmented cells are scattered throughout the spongy mesophyll; three vascular traces are present, medial largest, not separated from epidermis by inflated cells. Inflorescence without evidence of internode compression, flowers in axils of normal vegetative leaves, which occur throughout flower-bearing portion of stem, which continues vegetative growth after flower production; stipules associated with flowers are shorter and wider than sterile leaf stipules. Flowers pedicellate, pedicel bearing sparse, short hairs; a cluster of orange–red brown glandular trichomes present in the leaf axil at the base and either side of the pedicel. Calyx cream–green suffused with red or red–purple, lobes green with dark bronze margins; at maturity may be glabrous or bear a few short hairs on the tube, but always bears long hairs along the midline of each lobe, usually extending below the sinus; the calyx-pedicel junction bears short hairs; when in bud calyx continuously clothed in hairs. Bracteoles wine-purple, triangular, apex not attenuate, shorter or longer than adjacent calyx sinus vertex. Corolla orange–yellow with faint blood-red semi-circular and radiating nectar guides at base of standard; keel orange–yellow; standard transversely elliptic to slightly obcordate, folded, not flat, at flower maturity; wings laterally splayed, asymmetrically obovate, upper margin straight, apex curved, sharper through upper half, auricle triangular, with a rounded to acute apex; keel asymmetrically elliptic, upper margin linear, lower margin continuously curved, deepest at mid point, apex broadly rounded, basal auricle triangular, acute. Anthers cream to pale lemon, filaments translucent. Ovary glabrous, style translucent, with short antrorse hairs on ventral surface immediately above ovary. Pods ~5 mm long (Fig. 21, 22).
Distribution and ecology
The distribution of Pultenaea mutabilis var. angusta follows the western edge of the Higher Blue Mountains sandstone plateau, from the headwaters of Rocky Creek on the northern side of Newnes Plateau in the south to the south-eastern side of Mount Coricudgy in the north. Throughout this distribution, P. mutabilis var. angusta is associated with the western cliff lines of the Narrabeen Group sandstone exposures, where it may grow in steep gully heads, on steep rocky hillsides, gullies between pagodas, or on ledges. Pultenaea mutabilis var. angusta grows in both forest, mallee, and shrubland communities, including dry sclerophyll woodland with Angophora bakeri, Eucalyptus piperita and Eucalyptus macrorhyncha; tall Eucalypt forest containing Eucalyptus oreades and Eucalyptus piperita, with scattered rainforest species including Elaeocarpus reticulatus; on the edge of rainforest; and in mixed shrubbery including Acacia echinula, A. obtusifolia, Boronia pinnata, B. rubiginosa, Conospermum sp., Dillwynia sp., Isopogon dawsonii, Grevillea mucronata, Lepidosperma sp., Leptospermum polygalifolium, L. trinervium, L. macrocarpum, Leucopogon affinis, Styphelia mutica, Persoonia levis, P. linearis, Xanthorrhoea sp. and Zieria cytisoides. The sites on which P. mutabilis var. angusta occurs vary in aspect from south-east through west to north, suggesting the species is tolerant of a wide range of exposures. Soils are generally sandy loams, and may be derived in part from claystone. Flowers are scented with a fragrance remarkably like vanilla ice-cream.
Recognition
Pultenaea mutabilis var. angusta can be recognised by the irregular branching architecture, wherein inflorescences are produced on shoots that continue vegetative growth and so do not affect architecture. The flowers are produced in the axils of leaves that are identical to those on vegetative shoot sectors, and the internodes separating sequential flowers are the same length as those separating sterile leaves. The stipules in both flowering and sterile shoot sectors are short and narrow, with narrow, straight lobes. The branchlets bear short antrorse hairs. The leaves are narrow linear (0.4–1.0 mm wide) and bear antrorse hairs on the abaxial surface, the cells of which are bulging, not mamillose, and bear fine granular ornamentation. This combination of characters should prevent confusion with other members of the P. glabra complex, and most other Pultenaea species. When sterile, and in the herbarium, P. mutabilis var. angusta could be confused with P. furcata, but the latter has prominent triangular mamillae on the abaxial leaf surface that are conspicuous when leaves are examined under magnification even in dried material.
Variation
Pultenaea mutabilis var. angusta expresses variation in several characters, including the relative lengths of calyx lobes, sometimes the medial ventral lobe is longer than the other two ventral lobes, the length of the bracteoles relative to the calyx, which may be shorter than or equal to the adjacent sinus in length, and the hairiness of leaves, which vary from nearly hairless to sparsely hairy through their whole length. Variation in leaf hairiness is expressed by every individual we have examined.
Conservation status
Pultenaea mutabilis var. angusta may qualify for listing as Endangered under the IUCN criteria. The distribution from Newnes State Forest and the Wolgan Valley in the south to at least Mount Coricudgy in the north suggests an extent of occurrence less than 5000 km2. The taxon occurs in highly fragmented and local sites, and usually, but not always, occurs in association with Mount York claystone, on ledges below this formation. The taxon may experience extreme fluctuations in response to fire events, if its susceptibility to fire is similar to other members of the P. glabra complex, including P. mutabilis var. mutabilis. However, the resprouting capacity of P. mutabilis var. mutabilis is unknown, as is the distribution of this taxon between the northern and southern known occurrences, within the largely unmodified area encompassed by Wollemi National Park in between, and as a result its likely area of occupancy and extent of occurrence are difficult to estimate without further survey work.
Etymology
From the Latin angusta, narrow, in reference to the linear leaves, which present the sharpest point of distinction from the typical variety.
Specimens examined
NEW SOUTH WALES. Central Tablelands: Wollemi National Park: 2.7 km SE of Mt Coricudgy, 27 Oct. 1976, I.R.H. Telford 5072 & M.D. Crisp, (NSW 459587); ~40 km NNE of Lithgow, 2.5 km SSW of Glen Davis, Green Gully, 25 Oct. 1976, I.R.H. Telford 5024 & M.D. Crisp, (NSW 459588); ~1.5 km N of Newnes, ~23 km E of Capertee, divide between Little Capertee and Canobla Creeks, 14 Oct. 2015, S. Clarke 687, (NSW 933340); ~30 km N of Lithgow, ~4.5 km SW of Newnes, gully draining narrow divide between Wolgan and Capertee valleys, 30 Sep. 2015, S. Clarke 674, (NSW 934288); ~30 km N of Lithgow, ~5 km S of Newnes, cliffs flanking western side of Wolgan Valley, 30 Sep. 2015, S. Clarke 669, (NSW 934285); Running Stream Creek, ~3 miles [~5 km] ENE of Glen Davis (~30 miles [~48 km] SE of Rylstone, 25 Sep. 1964, E.F. Constable 5098, (NSW 82528); Glow Worm tunnel area, Wolgan Valley, 3 Oct. 1993, H. Brian s.n., (NSW 398520); Halfway between car park and Glow Worm Tunnel (upper end), Wolgan Valley, Oct. 1995, H. Brian s.n., (NSW 398519); ~3 km N of Newnes, ~22 km E of Capertee cliffs, overlooking an un-named tributary of Canobla Creek, 14 Oct. 2015, S. Clarke 680, (NSW 933341); 14 Oct. 2015, S. Clarke 681, (NSW 933342); 14 Oct. 2015, S. Clarke 682, (NSW 933343); 14 Oct. 2015, S. Clarke 683, (NSW 933344); 14 Oct. 2015, S. Clarke 684, (NSW 934280); 14 Oct. 2015, S. Clarke 685, (NSW 934281); 14 Oct. 2015, S. Clarke 686, (NSW 934282); eastern side of Canobola Creek, on ledge 200 m above stream bed, 10 Oct. 2019, M.A.M. Renner 9164 & S. Clarke, (NSW 1058833); 10 Oct. 2019, M.A.M. Renner 9165 & S. Clarke, (NSW 1058834); 10 Oct. 2019, M.A.M. Renner 9166 & S. Clarke, (NSW 1058835); 10 Oct. 2019, M.A.M. Renner 9167 & S. Clarke, (NSW 1058836); 10 Oct. 2019, M.A.M. Renner 9168 & S. Clarke, (NSW 1058837); 10 Oct. 2019, M.A.M. Renner 9169 & S. Clarke, (NSW 1058838); Wolgan Valley, track to Glow Worm tunnel, from Wolgan Valley Road via Old Coach Road, 7 Oct. 2019, M.A.M. Renner 9162, (NSW 1058831); 7 Oct. 2019, M.A.M. Renner 9163, (NSW 1058832); Newnes State Forest: track leading to Rocky Creek tributary, 15 May 1990, G. D’Aubert 733 & K. Marriott, (NSW 227748).
5. Pultenaea aculeata M.A.M.Renner, P.H.Weston & S.Clarke, sp. nov.
Diagnosis
Distinct from other members of the P. glabra complex in the combination of short (3.6–14.0 mm), inrolled linear leaves whose adaxial surface is largely enclosed, and is glaucous owing to the dense cuticular columnar papillae, the leaf apex with a long, pungent apiculus, the dense covering of spreading hairs on the stem, the densely villous calyx, and the flowers borne in the axils of leaves on otherwise leafy shoots that continue vegetative growth.
Woody shrub up to 4 m tall, but usually up to 2 m; stems fawn–brown, mostly obscured by stipules, where exposed, clothed in antrorse, spreading hairs that are longer than those on the leaves. Branching irregular. Stipules red–brown, darkening with age; inserted on a projection from the stem, which is obtriangular in section; furcate, lobes long and narrow, and taper gradually to an acuminate apex, divergent and usually recurved; the basal half of the stipule is lighter in colour, and has ragged margins bearing a few irregular teeth on each side. Leaves linear 3.6–14.0 (average 6.7) mm long by 0.4–0.8 (average 0.6) mm wide, tightly inrolled, adaxial surface enclosed; apex aristate and pungent, discolourous; abaxial surface bears antrorse, appressed, short hairs on its sides and ventral surfaces, the hairs are denser in younger and smaller leaves; adaxial surface is glaucous, covered by dense cuticular pillbox papillae; the abaxial surface bears low triangular mamillae formed by each epidermal cell; leaf anatomy with a subepidermis of pigmented cells on the lower surface; the ventral epidermis is hyaline. Inflorescence flowers in the axils of leaves, which occur throughout the terminal cluster of flowers, internodes not contracted, shoots continue vegetative growth after flowering; stipules associated with flowers are larger than those behind sterile leaves. Flowers shortly pedicellate, pedicel villous; orange–brown trichomes present in flower axil. Calyx outer surface clothed by antrorse, appressed, straight hairs, continuous over from base of tube to near the lobe apex; lobes apices pungent, as for leaf apices. Bracteoles nearly glabrous or with a few basal, medial, or marginal hairs; lanceolate–triangular, apex long, narrow and sharp, margins entire or sparsely denticulate. Corolla keel vibrant deep red. Anthers charcoal grey or pale yellow, filaments pale yellow, pollen cream. Ovary glabrous, except for stiff hairs at the apex and on the lower surface of the style. Pod 5–6 mm long (Fig. 23, 24).
Distribution and ecology
Pultenaea aculeata is restricted to the ‘pagoda country’ east of Rylstone, and as currently understood is confined to an area ~20 km north to south by 6 km east to west, extending from the head of Brymair Creek east of the Capertee Valley northward to the Coxs Crown/Spring Log Ridge area. One of us (SC) has also undertaken extensive walking beyond the currently known limits of distribution but not encountered the taxon in otherwise suitable habitat. Therefore, we consider the geographic distribution of P. aculeata to be fairly well circumscribed. Pultenaea aculeata is confined to scattered pagoda formations within a matrix of dry eucalypt woodland, sometimes with Callitris. The Eucalyptus canopy is usually sparse to medium, with E. oreades among others over a dense heath, including Acacia hamiltoniana, Acacia obtusifolia, Acacia terminalis, Banksia penicillata, Boronia anethifolia, Calytrix tetragona, Dracophyllum secundum, Epacris reclinata, Isopogon dawsonii, Leionema lamprophyllum subsp. orbiculare, Leptospermum arachnoides, Leucopogon microphyllus, Styphelia mutica, Pimelea linifolia subsp. linoides, Phebalium squamulosum subsp. gracile, Platysace lanceolata, Prostanthera hindii and Zieria cytisoides. Pultenaea aculeata grows in crevices and cracks high on pagodas, on ledges and slopes bearing shallow soils between pagodas, among rocks, and on the top of steep slopes forming gullies separating pagoda complexes. The soils are sandy loams.
Pultenaea aculeata individuals exhibit the capacity to resprout following fire, including after fires that cause 100% canopy scorch (Fig. 25). However, following the Gospers Mountain fire over the summer of 2019–2020, no signs of resprouting were observed from pre-fire adults at sites visited up to July 2021. All sites contained sizable crops of vigorous, healthy post-fire seedlings. It is possible that the combination of severe drought, and extreme fire intensity, compromised the ability of individuals to resprout, and the fires killed all individuals. Adult plants observed during the height of the drought immediately preceding the fires were severely stressed. However, we have since observed post-fire resprouting in Pultenaea aculeata in multiple individuals across multiple sites throughout the distribution of the species during the summer of 2021–2022, possibly promoted by the La Nina weather pattern.

|
Recognition
Pultenaea aculeata possesses a combination of characters encompassing many aspects of form, from leaf micromorphology and anatomy to flower colour, that facilitate ready identification of plants, in both herbarium and field. The leaves of P. aculeata are particularly distinctive, and perhaps even diagnostic in their own right, given their short length (3.6–14.0 mm), inrolled margins with the adaxial surface largely enclosed, the adaxial surface glaucous owing to the dense cuticular columnar papillae, the long, pungent, often upwardly curved, apiculus, and the triangular mamillae present on the abaxial leaf surface. Other distinctive features of P. aculeata include the dense covering of spreading hairs on the stem; the stipules with divergent lobes, the flowers borne on the axils of leaves on otherwise leafy shoots that continue vegetative growth; the densely villous calyx; and the blood-red keel.
Conservation status
Pultenaea aculeata is currently listed as Endangered in New South Wales. It qualifies for ranking as Endangered under the IUCN criteria because of the extent of occurrence, being less than 5000 km2, the extreme fluctuations in numbers of mature individuals, decline in habitat quality as a result of severe fire events as occurred over the summer of 2019–2020 and browsing by feral herbivores including goats and deer. Inappropriate fire regimes, and soil-borne diseases also constitute threats to this species.
Etymology
From the Latin aculeata, for sting or spine, in reference to the long pungent leaf apices of this remarkable species.
Specimens examined
NEW SOUTH WALES. Central Tablelands: Wollemi National Park: above tributary of Ganguddy Creek, ~4 km SSE of Dunns Swamp, 30 Apr. 2005, S. Clarke 41, (NSW 867309); 30 Apr. 2005, S. Clarke 42, (NSW 867310); 30 Apr. 2005, S. Clarke 43, (NSW 867311); above tributary of Ganguddy Creek, ~4 km SSE of Dunns Swamp, 30 Apr. 2005, S. Clarke 44, (NSW 867307); 30 Apr. 2005, S. Clarke 45, (NSW 867308); on crest of pagoda ridge above Ganguddy Creek, ~3 km SSE of Dunns Swamp, 1 May 2005, S. Clarke 54, (NSW 867305); gully between pagodas above Currant Creek, 4 May 2005, S. Clarke 60, (NSW 867321); high pagoda ridge jutting into broad flat valley, tributary of Ganguddy Creek, 4 May 2005, S. Clarke 62, (NSW 867318); ~4 km NNE of Olinda, ~500 m west of Nullo Mountain Road, 7 Apr. 2013, S. Clarke 358, (NSW 977194); 7 Apr. 2013, S. Clarke 359, (NSW 977195); 7 Apr. 2013, S. Clarke 360, (NSW 977196); ~4 km NNE of Olinda, ~500 m west of Nullo Mountain Road, 7 Apr. 2013, S. Clarke 363, (NSW 977199); 7 Apr. 2013, S. Clarke 364, (NSW 977200); ~3.5 km N of Olinda, ~1 km W of Nullo Mountain Road, on ridge approaching Mt Coolcalwin, 21 Apr. 2013, S. Clarke 365, (NSW 977201); ~4.5 km N of Olinda, ~1 km W of Nullo Mountain Road, 26 Apr. 2013, S. Clarke 369, (NSW 977205); 26 Apr. 2013, S. Clarke 370, (NSW 977206); ~4.5 km N of Olinda, ~1 km W of Nullo Mountain Road, 26 Apr. 2013, S. Clarke 371, (NSW 977218); 26 Apr. 2013, S. Clarke 373, (NSW 977224); ~4 km N of Olinda, ~1 km W of Nullo Mountain Road, 26 Apr. 2013, S. Clarke 374, (NSW 977225); 26 Apr. 2013, S. Clarke 375, (NSW 977226); 26 Apr. 2013, S. Clarke 377, (NSW 977228); 26 Apr. 2013, S. Clarke 378, (NSW 977229); 26 Apr. 2013, S. Clarke 379, (NSW 977230); ~3.5 – 4 km N of Olinda, ~1 km W of Nullo Mountain Road, 6 Apr. 2013,S. Clarke 380, 2 (NSW 977231); 26 Apr. 2013, S. Clarke 381, (NSW 977232); 26 Apr. 2013, S. Clarke 382, (NSW 977233); 25 May 2013, S. Clarke 385, (NSW 977236); 25 May 2013, S. Clarke 386, (NSW 977239); 25 May 2013, S. Clarke 387, (NSW 977240); ~6 km ENE of Olinda, 500 m N of Kandos Weir, 1 km SE of Mt Touwouwan, 8 June 2013, S. Clarke 410, (NSW 977280); 8 June 2013, S. Clarke 412, (NSW 977282); 8 June 2013, S. Clarke 413, (NSW 977283); 8 June 2013, S. Clarke 414, (NSW 977284); 8 June 2013, S. Clarke 415, (NSW 977285); Currant Mountain Gap, ~4.5 km ESE of Olinda, ~200 m N of Narrango Road, 13 June 2013, S. Clarke 416, (NSW 977286); 13 June 2013, S. Clarke 417, (NSW 977287); ~4.5 km ESE of Olinda, ~500 m NE of Currant Mountain Gap, ~2.5 km S of Kandos Weir, 13 June 2013, S. Clarke 420, (NSW 977289); 13 June 2013, S. Clarke 421, (NSW 977290); 13 June 2013, S. Clarke 422, (NSW 977291); 13 June 2013, S. Clarke 423, (NSW 977292); ~5 km ESE of Olinda, ~2.5 km S of Kandos Weir, 13 June 2013, S. Clarke 425, (NSW 977295); ~5 km ESE of Olinda, ~2.5 km S of Kandos Weir, 13 June 2013, S. Clarke 433, (NSW 977309); 13 June 2013, S. Clarke 434, (NSW 977310); 13 June 2013, S. Clarke 435, (NSW 977311); 13 June 2013, S. Clarke 436, (NSW 977312); ~5.5 km ESE of Olinda, ~2.5 km S of Kandos Weir, 13 June 2013, S. Clarke 439, (NSW 977315); 13 June 2013, S. Clarke 440, (NSW 977317); 13 June 2013, S. Clarke 441, (NSW 977319); ~5.5 km SE of Olinda, ~1 km SE of Mt Towinhingy, 22 June 2013, S. Clarke 451, (NSW 977340); 22 June 2013, S. Clarke 453, (NSW 977377); ~7 km SE of Olinda, ~7 km S of Dunn’s Swamp, 22 June 2013, S. Clarke 464, (NSW 977471); ~6.5 km E of Olinda, ~2.5 km S of Kandos Weir, 27 June 2013, S. Clarke 480, (NSW 977504); ~5.5 km E of Olinda, ~2 km S of Kandos Weir, 27 June 2013, S. Clarke 485, (NSW 977510); ~11 km NE of Rylstone, 1 km E of Cox’s Crown, 1 km N of Cox’s Creek Road, 1 Aug. 2013, S. Clarke 493, (NSW 977519); 1 Aug. 2013, S. Clarke 494, (NSW 977520); ~11 km NE of Rylstone, 1 km E of Cox’s Crown, 1 km N of Cox’s Creek Road, 1 Aug. 2013, S. Clarke 495, (NSW 977521); ~11 km NE of Rylstone, ~1.5 km E of Cox’s Crown, ~1 km N of Cox’s Creek Road, 1 Aug. 2013, S. Clarke 496, (NSW 977522); 1 Aug. 2013, S. Clarke 497, (NSW 977523); ~12 km NE of Rylstone, ~2 km E of Cox’s Crown, ~1.5 km N of Cox’s Creek Road, Aug. 2013, S. Clarke 498, 1 (NSW 977524); ~3 km E of Kandos Weir, 18 Aug. 2013, S. Clarke 499, (NSW 853499); 18 Aug. 2013, S. Clarke 501, (NSW 853503); 18 Aug. 2013, S. Clarke 502, (NSW 853509); ~3 km E of Kandos Weir., 18 Aug. 2013, S. Clarke 503, (NSW 853510); ~4.5 km E of Olinda, 1.75 km SW of Kandos Weir, 25 Aug. 2013, S. Clarke 504, (NSW 853526); ~7 km SE of Olinda, ~4 km S of Kandos Weir, 1 Sep. 2013, S. Clarke 522, (NSW 853576); 1 Sep. 2013, S. Clarke 523, (NSW 853579); 1 Sep. 2013, S. Clarke 524, (NSW 853582); ~6 km SE of Olinda, ~3.5 km S of Kandos Weir, 1 Sep. 2013, S. Clarke 531, (NSW 853598); 1 Sep. 2013, S. Clarke 532, (NSW 853599); ~8.5 km NE of Olinda, ~3 km NE of Kandos Weir, 14 Sep. 2013, S. Clarke 543, (NSW 853638); 14 Sep. 2013, S. Clarke 544, (NSW 853639); ~8.5 km NE of Olinda, ~3 km NE of Kandos Weir, 14 Sep. 2013, S. Clarke 545, (NSW 853643); 14 Sep. 2013, S. Clarke 546, (NSW 853644); ~5 km SE of Olinda, ~5 km S of Kandos Weir, 15 Sep. 2013, S. Clarke 547, (NSW 853645); ~5 km SE of Olinda, ~5 km S of Kandos Weir, 15 Sep. 2013, S. Clarke 548, (NSW 853646); ~4.5 km SE of Olinda, ~5 km S of Kandos Weir, 15 Sep. 2015, S. Clarke 549, (NSW 853647); 15 Sep. 2013, S. Clarke 551, (NSW 853650); 15 Sep. 2013, S. Clarke 552, (NSW 853651); ~4.5 km SE of Olinda, ~5 km S of Kandos Weir, 15 Sep. 2013, S. Clarke 553, (NSW 853659); 15 Sep. 2013, S. Clarke 554, (NSW 853662); ~5 km E of Olinda, ~400 m S of Kandos Weir, 21 Sep. 2013, S. Clarke 556, (NSW 853665); 21 Sep. 2013, S. Clarke 557, (NSW 853667); ~6 km E of Olinda, ~750 m E of Kandos Weir, ~500 m N of Ganguddy camp site, 21 Sep. 2013, S. Clarke 565, (NSW 853723); ~7 km SE of Olinda, ~4.5 km SE of Kandos Weir, 29 Sep. 2013, S. Clarke 572, (NSW 853737); ~10 km SE of Olinda, ~9 km SE of Kandos Weir, 5 Oct. 2013, S. Clarke 579, (NSW 853766); ~5 km SE of Olinda, ~20 km E of Kandos, on ridge between Towinhingy Creek and Teatree Creek, 20 Sep. 2014, S. Clarke 582, (NSW 883653); ~7 km SE of Olinda, 22 km E of Kandos, ridge between Towinhingy Creek and Teatree Creek, 27 Sep. 2014, S. Clarke 591, (NSW 931249); ~6 km SE of Olinda, 20 km E of Kandos, 2.5 km NE of ‘Dunville’ homestead, ridge above Teatree Creek, 27 Sep. 2014, S. Clarke 593, (NSW 883643); 27 Sep. 2014, S. Clarke 595, (NSW 883645); 1.2 km SE of Mt Towinhingy, ~3.5 km S of Kandos Weir, 2 Jun. 2017, S. Clarke 723, (NSW 1002586); ~23 km E of Kandos, ~4.5 km E of Kandos Weir & 1.2 km S of Narrango Road, 16 June 2017, S. Clarke 736, (NSW 1002582); ~18 km E of Kandos, ~2.5 km SW of Kandos Weir, Spur running NW off Mt Towinhingy, 30 June 2017, S. Clarke 739, (NSW 1002579); 30 June 2017, S. Clarke 740, (NSW 1002580); 30 June 2017, S. Clarke 741, (NSW 1002581); ~25 km E of Kandos, ~3 km N of ‘Ingle Wood’, 16 July 2017, S. Clarke 749, (NSW 1002585); ~25 km E of Kandos, ~3 km N of ‘Ingle Wood’, 16 July 2017, S. Clarke 750, (NSW 1002583); 16 July 2017, S. Clarke 751, (NSW 1002584); Spring Log Ridge, Growee River Catchment, W of Heffron’s Hole., 4 Dec. 2003, D.M. Crayn 772, H.G. Washington, J.M. Allen, B.M. Horton, S. Clarke & C. Pavich, (NSW 716933); near fire trail, ‘Mycumbene’; E of Kandos (W of ‘Mycumbene’), 28 Oct. 1992, P. Goulter 931 & A.J. Ford 931, (NSW 264742); Currant Mountain Gap, ~24 km ESE of Rylstone, 11 Mar. 1969, R.G. Coveny 961 & J. Pickard, (NSW 467005); Currant Mountain Gap, ~16 miles [~26 km] E of Rylstone, 26 Apr. 1965, D.J. McGillivray 1174 & A.N. Rodd, (NSW 77999); Currant Mountain Gap, near Olinda, E of Rylstone, 14 Nov. 1966, D.J. McGillivray 1650, (NSW 94501); ~600 m S of road heading E from Dunn’s Swamp (walk south from a point 2.3 km E of Dunn’s Swamp turnoff on main road), 18 Dec. 2012, R.L. Johnstone 3215 & S. Clarke, (NSW 904279); about 6 miles [~9.7 km] E of Olinda on road to Mount Coricudgy, 2 Sep. 1969, T.M. Whaite 3317 & J.L. Whaite, (NSW 467938); N side of Currant Mountain Gap, 25.5 km E of Rylstone, 28 Sep. 1996, P.C. Jobson 4373, (NSW 415239); ~4 miles [~6.4 km] E of Nullo Mountain on the Rylstone–Mount Coricudgy road, Oct. 1967, L. Williams s.n., (NSW 108536); ‘Kyber’ [Khyber Pass], Currant Mountain Gap, Sep. 1951, P. Althofer s.n., (NSW 38971); Currant Mountain Gap, on top of Pagodas, May 1998, H.G. Washington, (NSW 440197); Dunn Swamp, 26 km NE of Kandos, 10 Dec. 1989, A. Ford s.n., (NSW 247272); Currant Mountain Gap, 21 Sep. 1952, H.S. McKee s.n., (NSW 38969); 3 miles [~4.8 km] E of Currant Mountain Gap (i.e. 8 miles [~12.9 km] E of Olinda), 31 Aug. 1951, L.A.S. Johnson s.n., (NSW 38970); Currant Mountain Gap E of Rylstone, 28 Aug. 1952, C.K. Ingram s.n., (NSW 47121); Narrabri West, Oct. 1914, J.L. Boorman s.n., (NSW 67001); 1 mile [~1.6 km] SE of Currant Mountain Gap, 6 Aug. 1961, B.G. Briggs s.n., (NSW 108535); Olinda, Coricudgy Road, 26 Sep. 2018, M.A.M. Renner 9036, R.L. Barrett, J.M. Allen & H.G. Washington, (NSW 1052810); Nullo Mountain State Forest: ~4.5 km NE of Olinda, ~1.5 km east of Nullo Mountain Road, ~1 km SE of ‘Spring Hill’, 1 June 2013, S. Clarke 388, (NSW 977244); 1 June 2013, S. Clarke 389, (NSW 977245); ~4.5 km NE of Olinda, ~1.75 km E of Nullo Mountain Road, ~1.5 km SE of ‘Spring Hill’, 1 June 2013, S. Clarke 392, (NSW 977248); 1 June 2013, S. Clarke 394, (NSW 977252); 1 June 2013, S. Clarke 395, (NSW 977253); ~4 km NE of Olinda, ~2 km E of Nullo Mountain Road, ~1.5 km SE of ‘Spring Hill’, 1 June 2013, S. Clarke 396, (NSW 977254); 1 June 2013, S. Clarke 397, (NSW 977259); 1 June 2013, S. Clarke 398, (NSW 977260; ~4 km NE of Olinda, ~2 km E of Nullo Mountain Road, ~1.5 km SE of ‘Spring Hill’, 1 June 2013, S. Clarke 399, (NSW 977261); ~4 km NE of Olinda, ~2 km E of Nullo Mountain Road, ~1.5 km SE of ‘Spring Hill’, 1 June 2013, S. Clarke 402, (NSW 977269); 1 June 2013, S. Clarke 403, (NSW 977270); 1 June 2013, S. Clarke 404, (NSW 977271); ~4 km NE of Olinda, ~2 km E of Nullo Mountain Road, ~1.5 km SE of ‘Spring Hill’, 1 June 2013, S. Clarke 405, (NSW 977274); 1 June 2013, S. Clarke 406, (NSW 977275); 1 June 2013, S. Clarke 407, (NSW 977276); 1 June 2013, S. Clarke 408, (NSW 977277); 1 June 2013, S. Clarke 409, (NSW 977278).
6. Pultenaea praecipua M.A.M.Renner & P.H.Weston, sp. nov.
Diagnosis
Pultenaea praecipua is distinct from other members of the P. glabra complex in having the adaxial leaf surface densely covered in tall papillae, such that the dorsal leaf surface appears glaucous to almost white; the abaxial leaf surface bears triangular mamillae; the leaf apex has a distinct apiculus, and the flowers are produced in inflorescences the internodes of which are not contracted in relation to those of vegetative branches; each flower is subtended by a normal sized, or nearly normal sized, leaf.
Etymology
From the Latin praecipua, distinguished, in reference to the striking appearance of flowering individuals of this taxon.
6a. Pultenaea praecipua M.A.M.Renner & P.H.Weston subsp. praecipua
Diagnosis
Pultenaea praecipua subsp. praecipua is distinguished from P. praecipua subsp. temperata by having wider oblanceolate leaves 0.6–2.1 (average 1.2) mm wide whose adaxial surface is mostly exposed, such that the glaucous colour imparted by the dense covering of columnar papillae presents as a striking feature of the plants in the field.
Densely branched, clump-forming shrub up to ~1 m tall, branches horizontally spreading from multi-stemmed base; strikingly coloured with two-tone leaves whose upper surface is wholly glaucous, stems yellow–green, bearing short antrorse appressed hairs. Branching irregular. Stipules black–red, aging black; furcate, narrowest at sinus apex, from there disc is flared below and lobes curve laterally above; lobes eccentrically keeled, apex acuminate but not attenuate, disc margins irregularly dentate, teeth close set. Leaves oblanceolate, 9.2–21.6 (average 13.9) mm long by 0.6–2.1 (average 1.2) mm wide, broadening towards the apex from a narrow base; incurved but not enclosing the adaxial surface, which remains visible; nearly glabrous, hairs present on the leaf base and the petiole only, the hairs antrorse, straight, appressed; abaxial leaf surface mamillose, mamillae formed by single epidermal cells, triangular to dome-shaped; adaxial leaf surface with dense tall columnar papillae that imparts a white–glaucous colour to the adaxial leaf surface; apex pungent, orange–brown; leaf anatomy with five vascular traces, outermost faint, with two enlarged cells on the abaxial side of the medial vascular trace, without orange-pigmented cells, the abaxial epidermis hyaline. Inflorescence with little or no internode contraction, flowers in axils of normal vegetative leaves, stem continuing vegetative growth flowering; stipules associated with flowers similar to, or shorter than, those of sterile leaves. Flowers pedicellate, pedicel glabrous. Calyx lobes broad, apex apiculate; ventral lobes auriculate at junction with dorsal lobes; calyx tube and lobes sparsely hairy, with short antrorse hairs; margins of ventral lobes hyaline. Bracteoles short, triangular, not exceeding adjacent calyx sinus, with sparse short hairs. Corolla keel blood-red, other segments yellow. Anthers pale apricot, filaments cream, with yellow hue. Pollen cream, with slight yellow hue. Ovary glabrous. Pods not seen (Fig. 26, 27).
Distribution and ecology
Pultenaea praecipua subsp. praecipua is known from a single population of ~12 plants at Genowlan Point, the northern-most extension of the plateau within Mugii Murum-ban, where they grow on a flat sandstone pavement with skeletal soil on the edge of a precipice, fully exposed to the north, with scattered Eucalyptus sparsifolia to immediate south-east. The pavement community comprises discontinuous sparse low shrubs including Hibbertia, Isopogon, Leptospermum, Calytrix, Leucopogon, Boronia, Acacia and scattered sedges. The Pultenaea plants occur as scattered, usually isolated, individuals in fully exposed situations.
Recognition
Pultenaea praecipua subsp. praecipua can be recognised by the combination of oblanceolate leaves 0.6–2.1 mm wide with a distinct apiculus, crowded, tall, pillbox papillae on the adaxial surface, and triangular mamillae on the abaxial surface. No other Pultenaea species in the Greater Blue Mountains has this combination of characters. Other distinctive features include the low dense shrub growth habit and the inflorescences on leafy shoots that continue vegetative growth. Pultenaea praecipua subsp. praecipua has a unique field aspect imparted by the broad oblanceolate leaves the upper surface of which bears crowded, tall, pillbox papillae. These papillae cause the adaxial leaf surface to appear glaucous, to the extent that they are nearly white. As such the leaves appear striped, with the glaucous–white adaxial surface between the mid-green abaxial surfaces, and from above the shrubs appear almost wholly glaucous. Flowering plants, with their combination of vibrant two-toned orange and red flowers overlaying the strikingly contrasting white and green leaves arranged in radiating series presents a remarkable plant. The leaves of P. praecipua subsp. praecipua are generally broader than those of P. praecipua subsp. temperata, up to 2.1 mm wide, whereas in P. praecipua subsp. temperata the leaves are up to 1.2 mm wide.
Conservation status
Pultenaea praecipua subsp. praecipua qualifies for listing as Critically Endangered under the IUCN criteria, as it is known from one population comprising fewer than 50 individuals. The taxon has an exceptionally small area of occupancy and extent of occurrence (both ~0.15 ha), a small population size, and is threatened by exotic herbivores including goats and deer, and by stochastic processes to which small populations are susceptible, particularly those that increase mortality rates and reduce recruitment, including severe drought, and intensified fire-regimes. Proximity to the cliff edge may mean that this taxon is also at risk from cliff-fall, through weathering or seismic events and ex situ conservation of all genotypes should be a priority for conservation of this rare taxon.
Specimens examined
New South Wales: Central Tablelands: Mujii Murum-ban National Park: the very tip of Genowlan Point, Capertee valley, 29 Nov. 1997, J.M. Allen & H.G. Washington, (NSW 417813); Oct. 1997, H.G. Washington, (NSW 417816); 24 May 1998, H.G. Washington, (NSW 423425); 17 Oct. 2018, M.A.M. Renner 9056, (NSW 1052717); 17 Oct. 2018, M.A.M. Renner 9057, (NSW 1052718); 17 Oct. 2018, M.A.M. Renner 9058, (NSW 1052719); 7 Oct. 2018, M.A.M. Renner 9060, 1 (NSW 1052721); 17 Oct. 2018, M.A.M. Renner 9061, (NSW 1052722).
6b. Pultenaea praecipua subsp. temperata M.A.M.Renner & R.L.Barrett, subsp. nov.
Diagnosis
Pultenaea praecipua subsp. temperata is distinguished from P. praecipua subsp. praecipua by having narrower linear leaves 0.6–1.2 (average 1.8) mm wide the adaxial surface of which is enclosed by the inrolled leaf, such that the glaucous colour imparted by the dense covering of columnar papillae is not visible on plants in the field.
Irregular, upright, woody shrub up to 0.8 m tall, stems yellow–green with short antrorse–erect hairs when young, brown when mature. Branching irregular. Stipules black–red, aging black; furcate, narrowest at sinus apex, lamina flared below and lobes curve laterally above; lobes eccentrically keeled, apex acuminate but not attenuate, disc margins irregularly dentate, teeth close set. Leaves dark green on under surface and glaucous above; linear, 6–21.2 (average 14.1) mm long by 0.6–1.2 (average 0.8) mm wide; strongly inrolled, the adaxial surface is enclosed and obscured, and bears tall pill-box papillae, these are not as tall as in P. praecipua; subsp. praecipua; leaf apex pungent, short, orange to orange–brown; leaves bearing a few scattered hairs, these are dense at the leaf base and on the petiole, abaxial surface bears triangular mamillae formed by bulging epidermal cells; ventral epidermis hyaline there are two enlarged cells beneath the medial vascular trace, separating it from the ventral epidermis; there are three vascular traces, the medial is largest, orange-pigmented cells occur in the outer extremity of the abaxial leaf. Inflorescence with no internode compression, flowers in axils of normal vegetative leaves, which occur throughout flower-bearing portion of stem, which continues vegetative growth after flower production; stipules associated with flowers are shorter than the vegetative stipules. Flowers shortly pedicellate, pedicel glabrous, with five or six orange–brown trichomes present in the leaf axil below each flower, long or short, not glandular at apex. Calyx lobes broad, short triangular (shorter for their width than subsp. praecipua), apices apiculate, base of ventral calyx lobes auriculate; lobes and tube sparsely hairy, concentrated at top of the tube but also extending onto the ventral lobes, which are hairier than the dorsal lobes; margins of ventral calyx lobes hyaline. Bracteoles short, triangular, apex not reaching the vertex of the adjacent calyx lobe sinus; with a medial band of short antrorse hairs. Corolla not seen. Pods not seen (Fig. 28, 29).
Distribution and ecology
The only known population of P. praecipua subsp. temperata grows on a sandstone shelf with shallow pale grey sandy loam soil, surrounded by dry sclerophyll forest including Eucalyptus oreades, Acacia obtusifolia, A. terminalis, Allocasuarina sp., Banksia cunninghamii, Callitris rhomboidea, C. endlicheri, Leptospermum sphaerocarpum and Persoonia linearis.
Recognition
Pultenaea praecipua subsp. temperata can be recognised by the combination of linear leaves 0.6–1.2 mm wide with a distinct apiculus, crowded, pillbox papillae on the adaxial surface, and triangular mamillae on the abaxial surface. The leaves of P. praecipua subsp. temperata are narrower than those of P. praecipua, up to 1.2 mm wide, whereas in P. praecipua subsp. praecipua the leaves are up to 2.1 mm wide. Pultenaea praecipua subsp. praecipua is also unusual in having five vascular traces in the leaf transverse section, P. praecipua subsp. temperata has three.
Notes
The recognition of two subspecies within a species known by two populations that differ by but few characters is justified by their allopatry, their genetic distinctiveness, and the consistent manifestation of those morphological differences in all individuals. We have examined all known extant individuals of both subspecies, and there are no morphologically intermediate individuals. Leaf width is of consistent expression within individuals, and within populations, and is therefore a consistent difference between the two subspecies., each having a different mean and modal leaf width. The degree of molecular difference between these two populations could be argued as being insufficient to support subspecific status, but then what are subspecies if they are not morphologically and genetically distinct allopatric populations of a species? The situation here with P. praecipua is straight forward in part because of the fact that each taxon is known by a single population, such that we have more or less complete knowledge of patterns of morphological and molecular variation. This contrasts with the situation in P. mutabilis, wherein we have a relatively limited population-level sampling, one that is sufficient, perhaps, to suggest that patterns of morphological and genetic variation are complex, and more sampling to fully understand these is required; hence, our different approaches to the treatment of intra-specific variation in these two species.
Conservation status
Pultenaea praecipua subsp. temperata warrants listing as Critically Endangered under the IUCN criteria. Pultenaea praecipua subsp. temperata is known from a single population that has experienced a decline in the number of mature individuals in recent years, as evidenced by the number of dead standing individuals observed during our site visit in 2018. The species is threatened by exotic herbivores including goats and deer, and by stochastic processes to which small populations are susceptible, particularly those that increase mortality rates and reduce recruitment, including severe drought, and intensified fire-regimes. The species may be susceptible to Phytophthora; ~200 dead individuals were observed in 2018 beneath a more or less closed Eucalyptus canopy; however, whether mortality was caused by shading, senescence, or disease was not established.
Etymology
From the Latin temperata, tempered or modest, in reference to the narrower leaves that enclose the adaxial leaf surface, reducing the conspicuousness of the striking contrast between adaxial and abaxial leaf surfaces.
Specimens examined
NEW SOUTH WALES. Central Tablelands: Nullo Mountain, ridge E of NW escarpment, Lee Creek catchment, Oct. 2003, H.G. Washington, (NSW 612468); H.G. Washington, 31 Aug. 2003, (NSW 608966); 14 Nov. 2003, H.G. Washington, (NSW 704525); Nullo Mountain, W of 2515 Nullo Mountain Road, narrow ridges above Lees Creek catchment, 24 Sep. 2018, M.A.M. Renner 9029, et al. (NSW 1052790); 24 Sep. 2018, M.A.M. Renner 9030 et al. (NSW 1052798); 24 Sep. 2018, M.A.M. Renner 9032, et al., (NSW 1052800); 24 Sep. 2018, M.A.M. Renner 9033, et al., (NSW 1052801); 24 Sep. 2018, M.A.M. Renner 9034 et al., (NSW 1052802).
7. Pultenaea percussa M.A.M.Renner & P.H.Weston, sp. nov.
Diagnosis
Pultenaea percussa is distinct among members of the P. glabra complex in its tightly inrolled–clavate leaves 2.3–3.9 mm long that lack an apiculus and the abaxial surface of which is ornamented with triangular mamillae formed by single epidermal cells; the flowers on normal leafy shoots, and the spreading growth habit.
Spreading woody shrub up to 60 cm tall and 180 cm across; branching irregular; branchlets hairy, hairs long, spreading or antrorse. Stipules furcate, lobes diverging and recurving slightly from a parallel-sided and appressed lamina; lobes acuminate, eccentrically keeled; margins denticulate. Leaves inrolled–clavate, 2.3–3.9 (average 3.3) mm long by 0.7–1.0 (average 0.8) mm wide, narrow-based then widening above, widest close to apex; apex rounded, muticous (without apiculus, and not even a hint of a nerve protruding from the leaf lamina); lamina and petiole glabrous; lamina folded dorsally in halves, adaxial surface almost completely enclosed; adaxial leaf surface glaucous, adorned with dense, tall, columnar papillae; abaxial leaf surface mamillose, with triangular mamillae formed by single epidermal cells. Leaf anatomy with abaxial epidermis orange, almost peach, in colour; below the abaxial epidermis is a layer of palisade-like cells extending nearly the breadth of the abaxial leaf, they are absent only from the very margin, these palisade cells are intensely orange–brown or orange–red pigmented; one vascular trace is present. Inflorescence on leafy shoots, internodes not contracted; shoot continuing vegetative growth after flower production; stipules associated with flowers similar to those subtending vegetative leaves, but with shorter lobes and ciliolate margins. Flowers pedicillate, pedicel short, sparsely hairy. Calyx glabrous, surface with low papillae but smaller than those on leaves; calyx lobe apex acute. Bracteoles triangular, with ciliolate margins, keeled in upper half, not exceeding the sinus between calyx lobes. Corolla colour unknown; standard obovate to slightly obcordate; wings narrow obovate from a broad stipe, basal auricle indistinct with a rounded to obtuse apex; keel linear on upper margin, apex evenly and continuously rounded, lower margin shallowly and continuously curved above a basal stipe, auricle indistinct and obtuse; keel asymmetrically narrow elliptic, subrectangular in outline, with linear upper margin from a truncate, nearly square basal auricle, apex broadly rounded, and ventral margin shallowly curved. Ovary not observed. Pods unknown (Fig. 30).
Distribution and ecology
Pultenaea percussa is known only from the type gathering, which was made from a population of plants of unknown extent on ‘shale slopes’ in the catchment of the Hollanders River, to the south of Shadowgraph Bluff.
Recognition
Pultenaea percussa is distinct in its short inrolled–clavate leaves (2.3–3.9 mm long) that bear triangular mamillae on the abaxial leaf surface. The leaf size, shape, and ornamentation present a unique combination of characters and wholly distinctive aspect within Pultenaea known from the eastern Australia.
Conservation status
Pultenaea percussa is data deficient, pending search and survey effort to relocate this species in the wild.
Etymology
From the Latin percussa ‘thrust through’ or ‘pierced’, but also referencing the modern use of this root in ‘percussion’. The hollow, club-shaped leaves of this species could either stike, or be struck. This species is dedicated to everyone who lives, or has lived, with a traumatic brain injury, and especially to everyone who supports them. Traumatic brain injuries diminish capacity and reduce quality of life, and may involve a deep and personal grief for the loss of self, which may never be fully recovered. Though traumatic brain injuries are varied in their effect, they are generally isolating and disorientating events from which recovery may take a great deal of support.
Specimens examined
Known only from the type gathering.
8. Pultenaea weindorferi Reader, Vict. Naturalist 22(3): 51 (1905)
Erect branched shrub up to 2 m tall, branchlets glabrous. Stipules linear–lanceolate, divided, plane, lobes not reflexed or divergent, divided to ~0.5 of their length, lobes keeled, keels running through lamina to base, margins irregularly denticulate. Leaves linear–lanceolate 5.9–15 (average 11.1) mm long, 0.6–1.4 (average 0.9) mm wide, navicular at apex, apex rounded to obtuse, cucullate; abaxial leaf surface mamillose, each mamilla formed by a single epidermal cell, triangular to dome-shaped; adaxial surface with columnar papillae that are tapered towards their apex; leaf lamina and petiole glabrous. Leaf anatomy with orange–red pigmented cells scattered throughout on the dorsal and ventral surfaces. Ventral epidermis orange-pigmented, 3 veins present, Inflorescences terminal, internodes contracted, terminating short lateral shoots, occasionally branching below the inflorescence; leaves among flowers reduced, stipules enlarged, broader and less divided than stipules in sterile shoot sectors. Flowers in clusters of 10 or more, pedicellate, pedicels glabrous. Calyx glabrous, except for cilia on inner margin of lobes; lobes cucullate with three veins, abaxial surface mamillose, mamillae formed by single epidermal cells, triangular to dome shaped as on the abaxial leaf surface. Bracteoles exceeding sinus between calyx lobes, coriaceous, nearly equal to calyx lobes in length. Corolla orange–yellow, with pronounced red semicircular marking at base of standard, otherwise lacking red markings, wings suffused with red towards their base; keel slightly paler than rest of the flower; greenish-cream to pale yellow particularly towards the apex; standard ovate above a basal stipe, folded not flat at flower maturity, apex with a shallow notch defined by the left and right sides of the standard; wing orientation variable, from embracing the keel to slightly laterally splayed, broadly rectangular–obovate above a long basal stipe, upper margin straight, lower margin curved, apex rounded, basal auricle a broad rounded triangle. Keel asymmetrically elliptic, upper margin linear, lower margin continuously curved, deepest at mid point, apex broadly rounded. Ovary glabrous. Pods not seen, turgid and glabrous fide Corrick (1996) (Fig. 31).
Distribution and ecology
Pultenaea weindorferi is endemic to central Victoria, including the Gippsland Plain, Central Victorian Uplands, and the Northern and Southern Fall Highlands, in association with drainage lines and swamps at a scattering of localities including near Daylesford, Kinglake, and Tonimbuk.
Recognition
Pultenaea weindorferi can be recognised by its combination of flowers in a terminal head-like inflorescence thee internodes of which are contracted, the linear leaves with a cuculllate apex, and abaxial leaf surface bearing conspicuous triangular mamillae.
Variation
A form with long soft hairs on the calyx and leaves, and a tuft of hairs on the summit of the ovary that occurs in Kinglake National Park was noted by de Kok and West (2002). Specimens in broad agreement with this description included in our study also had broader leaves (0.8–2.0 mm wide). These forms may represent hybrids with other co-occurring Pultenaea, such as P. humilis Benth. ex Hook.f., variation within P. weindorferi, as suggested by de Kok and West (2002), or an undescribed taxon, and further work on their status should be undertaken.
Notes
Multiple specimens derived from Weindorfer’s September 1903 gathering(s) exist. Variously, these are associated with three different identifiers, some specimens are associated with the number 1966, others with 2252; the remaining specimens have no unique identifier. Reader’s citation of original material refers to Weindorfer’s gathering(s) (Reader 1905), which is therefore citation of the included specimens (Art. 9.6). The association of different specimens with different numbers may suggest that Weindorfer made more than one gathering, but we do not think this is the case. Rather, we suspect that the numbers are Weindorfer’s own distribution numbers, recording the specimens he has sent to other people. Part of the evidence for this interpretation is that the numbers associated with Weindorfer’s specimens are not consecutive, and, further, different numbers are applied to what appear to be the same gathering. In support of this interpretation is the fact that the material in NSW, which was sent to Maiden in 1903, bears the number 1966. This material is accompanied by a note from Weindorfer that the specimen was ‘found by me at said locality’ and that Maiden believed the plants were likely new, and would describe them as such if so. One of the specimens in MEL bears the number 2252 in Weindorfer’s hand, along with a specimen label also written by Weindorfer, which has the species name ‘Pultenaea weindorferi F.M.Reader species nova’, suggesting that Weindorfer distributed this specimen closer to the publication date of 1905, and after he had sent material to Maiden in Sydney. Reader (1905) makes no mention of collection numbers. We infer that all the specimens gathered by Weindorfer near Wandin in September 1903 comprise a single gathering, which have different histories of distribution from Weindorfer’s personal herbarium. We designate the large specimen in MEL as lectotype, the other specimens are therefore isolectotypes.
Conservation status
Pultenaea weindorferi is listed as Endangered on the Victorian Government’s current Flora and Fauna Guarantee Act 1988 list. As noted in the introduction, the state of Victoria did not accept the synonymisation of P. weindorferi with P. glabra, a position supported by this study.
Specimens examined
VICTORIA. Wandin, G. Weindorfer s.n., Oct. 1904, (NSW 37200); Wandin, Oct. 1905, G. Weindorfer 5847/15, (NSW 37203); Wandin (~43 km E of Melbourne), 11 Oct. 1907, P.R.H. St John s.n., (NSW 424887); eastern Highlands, Tynong North, Button grass walking track near the intersection of Camp Road and the Gembrook–Tonimbuk road, 29 Nov. 2007, J.A. Jeanes 1803, G. Lay & D. Wilson, (NSW 831384); eastern Highlands, Kinglake National Park, ~1 km NW of Mt Everard, at end of track that leaves W side of Mt Everard to Mt Beggary track ~1.5 km S of Mt Beggary, 28 Dec. 1996, M.G. Corrick 11489, (NSW 624283); Midlands, Lerderderg State Park (N central part of park), O’Briens Rd, 24 Oct. 1999, V. Stajsic s.n., (NSW 675907).
Revised synonymy for Pultenaea flexilis
Pultenaea flexilis Sm., Annals of Botany (König & Sims) 1(3): 502 (1805)
Notes
Material at BM (BM000544616), annotated by F. A. W. Sieber as his unpublished ‘Dillwynia teucrioides’ is mixed, comprising two separate shoots, one of P. mutabilis var. mutabilis, the other of P. flexilis. These plants were evidently both gathered at Mount Wilson, which is likely, given the two species are today known to co-occur at nearby Bilpin.
Bentham cites only Brown’s gathering in the protologue of P. villosa var. glabrescens, which is taken as a reference to all the included specimens derived from that gathering (Art. 9.6), which are held at several herbaria. We designate the specimen from Brown’s herbarium in K as the lectotype, to preserve the current type citation and reflect Bentham’s locus labori. The lectotype bears Brown’s original label, reading ‘Rivulet near Gin’, gin being a little dam with a water wheel and mill in Highland parlance. This specimen comprises four shoots from possibly two different sources, as the specimen also has Bennett’s label (John Bennett was a compiler of the public set from derived from Brown’s herbarium).
The type of P. villosa var. glabrescens is a short-leaved morphotype of P. flexilis. One other specimen held by NSW from the Cumberland Plain and Georges River also has the same short leaves. The status of these plants and their relationships with the typical morphotype of P. flexilis warrants further investigation.
Data availability
The morphological and molecular data that support this study are available in the accompanying online supplementary material associated with this article (Supplementary Tables S1, S2).
Conflicts of interest
Dr Russell Barrett is an editor for Australian Systematic Botany but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Australian Systematic Botany encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
This study was funded internally within the Royal Botanic Garden, Sydney.
Supplementary material
Supplementary material is available online.
Acknowledgements
We thank two reviewers for comments that improved the submitted manuscript; Lesley Elkan and Catherine Wardrop for composing the illustrations of each taxon; Steve Douglas for sending specimens and extensive field observations of P. mutabilis var. mutabilis at Mount Irvine; Shawn Ryan for sending photos of Pultenaea glabra from Newnes Plateau, which led to the discovery of P. furcata; John and Jan Allen for company and Hayden Washington for guidance in the field while relocating Pultenaea praecipua subsp. temperata; and Sam Yap for her help trying to set up TreeMix. Author contributions: M. A. M. Renner conceived the study, conducted fieldwork, completed sample preparation for DNA and morphological data collection, collected morphological data, performed all analyses of morphological and molecular data, interpreted results, prepared taxon treatments, and wrote and revised the paper; R. L. Barrett conducted fieldwork, interpreted the data, contributed to the interpretation of types and synonymy, and wrote the paper; S. Clarke conducted fieldwork, making extensive collections of Pultenaea glabra complex, which facilitated species concept refinement by Peter Weston, and were the basis for morphological and molecular data collection, and wrote the paper; J. Clugston trouble-shot many aspects of the molecular data analysis and wrote the paper; T. C. Wilson conducted fieldwork, performed exploratory analyses of the molecular data, and wrote the paper; P. H. Weston formulated species concepts within the P. glabra complex to test, interpreted results, and wrote the paper.
References
Barrett RL, Clugston JAR, Cook LG, Crisp MD, Jobson PC, Lepschi BJ, Renner MAM, Weston PH (2021) Understanding diversity and systematic in Australian Fabaceae tribe Mirbelieae. Diversity 13, 391| Understanding diversity and systematic in Australian Fabaceae tribe Mirbelieae.Crossref | GoogleScholarGoogle Scholar |
Binks RM, Wilkins CF, Markey AS, Lyons MN, Byrne M (2020) Genomic data and morphological re-assessment reveals synonymy and hybridisation among Seringia taxa (Lasiopetaleae, Malvaceae) in remote north-western Australia. Taxon 69, 307–320.
| Genomic data and morphological re-assessment reveals synonymy and hybridisation among Seringia taxa (Lasiopetaleae, Malvaceae) in remote north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Bryant D, Moulton V (2004) Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Molecular Biology and Evolution 21, 255–265.
| Neighbor-net: an agglomerative method for the construction of phylogenetic networks.Crossref | GoogleScholarGoogle Scholar | 14660700PubMed |
Buchmann R, Hazelhurst S (2014) Genesis Manual. (University of the Witwatersrand: Johannesburg, South Africa) Available at http://www.bioinf.wits.ac.za/software/genesis/Genesis.pdf
Coates D, Byrne M, Moritz C (2018) Genetic diversity and conservation units: dealing with the species-population continuum in the age of genomics. Frontiers in Ecology and Evolution 6, 165
| Genetic diversity and conservation units: dealing with the species-population continuum in the age of genomics.Crossref | GoogleScholarGoogle Scholar |
Corrick MG (1996). Pultenaea. In ‘Flora of Victoria Vol. 3, Dicotyledons Winteraceae to Myrtaceae’. (Eds NG Walsh, TJ Entwisle) pp. 765–793. (Inkata Press: Melbourne, Vic., Australia)
de Kok RPJ, West JG (2002) A revision of Pultenaea (Fabaceae) 1. Species with ovaries glabrous and/or with tufted hairs. Australian Systematic Botany 15, 81–133.
| A revision of Pultenaea (Fabaceae) 1. Species with ovaries glabrous and/or with tufted hairs.Crossref | GoogleScholarGoogle Scholar |
de Kok RPJ, West JG (2003) A revision of Pultenaea (Fabaceae) 2. Eastern Australian species with velutinous ovaries and incurved leaves. Australian Systematic Botany 16, 229–273.
| A revision of Pultenaea (Fabaceae) 2. Eastern Australian species with velutinous ovaries and incurved leaves.Crossref | GoogleScholarGoogle Scholar |
de Kok RPJ, West JG (2004) A revision of Pultenaea (Fabaceae) 3. The eastern species with recurved leaves. Australian Systematic Botany 17, 273–326.
| A revision of Pultenaea (Fabaceae) 3. The eastern species with recurved leaves.Crossref | GoogleScholarGoogle Scholar |
de Queiroz K (1998) The general lineage concept of species, species criteria, and the process of speciation. In ‘Forms: Species and Speciation’. (Eds DJ Howard, SH Berlocher) pp. 57–75. (Oxford University Press: New York, NY, USA)
de Queiroz K (2007) Species concepts and species delimitation. Systematic Biology 56, 879–886.
| Species concepts and species delimitation.Crossref | GoogleScholarGoogle Scholar | 18027281PubMed |
Department of Environment and Conservation (2006) The Vegetation of the Western Blue Mountains including the Capertee, Coxs, Jenolan & Gurnang Areas. Volume 1: Technical Report (Final V1.1). (Hawkesbury–Nepean Catchment Management Authority, DEC: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/resources/nature/vegofwbluemtsvol1tech.pdf
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulations study. Molecular Ecology 14, 2611–2620.
| Detecting the number of clusters of individuals using the software STRUCTURE: a simulations study.Crossref | GoogleScholarGoogle Scholar | 15969739PubMed |
Goldbery R (1972) Geology of the western Blue Mountains. Geological Survey of New South Wales Bulletin 20, 1–172.
Goldbery R, Holland WN (1973) Stratigraphy and sedimentation of redbed facies in Narrabeen Group of Sydney Basin Australia. American Association of Petroleum Geologists Bulletin 57, 1314–1334.
Gruber B, Unmack PJ, Berry OF, Georges A (2018) dartR: an R package to facilitate analysis of SNP data generated from reduced representation genome sequencing. Molecular Ecology Resources 18, 691–699.
| dartR: an R package to facilitate analysis of SNP data generated from reduced representation genome sequencing.Crossref | GoogleScholarGoogle Scholar | 29266847PubMed |
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximul-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59, 307–321.
| New algorithms and methods to estimate maximul-likelihood phylogenies: assessing the performance of PhyML 3.0.Crossref | GoogleScholarGoogle Scholar | 20525638PubMed |
Hager T, Benson D (2010) The eucalypts of the Greater Blue Mountains World Heritage Area: distribution, classification and habitats of the species of Eucalyptus, Angophora and Corymbia (family Myrtaceae) recorded in its eight conservation reserves. Cunninghamia 11, 425–444.
Harden GJ (2002) Fabaceae [family description]. In ‘Flora of New South Wales. Vol. 2’, 2nd edn. (Ed. GJ Harden) p. 363. (New South Wales University Press: Sydney, NSW, Australia)
Herbert C (1997) Sequence stratigraphic analysis of Early and Middle Triassic alluvial and estuarine facies in the Sydney Basin, Australia. Australian Journal of Earth Sciences 44, 125–143.
| Sequence stratigraphic analysis of Early and Middle Triassic alluvial and estuarine facies in the Sydney Basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35, 518–522.
| UFBoot2: Improving the ultrafast bootstrap approximation.Crossref | GoogleScholarGoogle Scholar | 29077904PubMed |
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23, 254–267.
| Application of phylogenetic networks in evolutionary studies.Crossref | GoogleScholarGoogle Scholar | 16221896PubMed |
IUCN (2012) ‘IUCN Red List Categories and Criteria: Version 3.1’, 2nd edn. (IUCN: Gland, Switzerland; and Cambridge, UK)
Johnson LA, Cairns-Heath H (2010) Decryptic cryptic species: morphological and molecular evidence for recognizing Navarretia linearifolia as distinct from N. sinistra (Polemoniaceae). Systematic Botany 35, 618–628.
| Decryptic cryptic species: morphological and molecular evidence for recognizing Navarretia linearifolia as distinct from N. sinistra (Polemoniaceae).Crossref | GoogleScholarGoogle Scholar |
Jombart T (2008) adegenet: an R package for the multivariate analysis of genetic markers. Bioinformatics 24, 1403–1405.
| adegenet: an R package for the multivariate analysis of genetic markers.Crossref | GoogleScholarGoogle Scholar | 18397895PubMed |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587–589.
| ModelFinder: fast model selection for accurate phylogenetic estimates.Crossref | GoogleScholarGoogle Scholar | 28481363PubMed |
Killan A, Wenzl P, Huttner E, Carling J, Xia L, Blois H, Caig V, Heller-Uszynska K, Jaccoud D, Hopper C, Aschenbrenner-Kilian M, Evers M, Peng K, Cayla C, Hok P, Uszynski G (2012) Diversity Arrays technology: a generic genome profiling technology on open platforms. In ‘Data production and analysis in population genomics: methods and protocols’. (Eds F Pompanon, A Bonin) pp 67–89. (Humana Press: New York, NY, USA)
Lischer HEL, Excoffier L (2012) PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 28, 298–299.
| PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs.Crossref | GoogleScholarGoogle Scholar |
Martyn J (2018) ‘Rocks and trees: a photographic journey through the rich and varied geology, scenery and flora of the Sydney region.’ (STEP Inc.: Sydney, NSW, Australia)
Maslin BR (2001) Introduction to Acacia. Flora of Australia 11A, 3–13.
Mayne SJ, Nicholas E, Bigg-Wither AL, Rasidi JS, Raine MJ (1974) ‘Geology of the Sydney Basin: a review. Bulletin 149.’ (Department of Minerals and Energy: Canberra, ACT, Australia)
Melville J, Haines ML, Boysen K, Hodkinson L, Kilian A, Smith Date KL, Potvin DA, Parris KM (2017) Identifying hybridization and admixture using SNPs: application of the DArTseq platform in phylogeographic research on vertebrates. Royal Society Open Science 4, 161061
| Identifying hybridization and admixture using SNPs: application of the DArTseq platform in phylogeographic research on vertebrates.Crossref | GoogleScholarGoogle Scholar | 28791133PubMed |
Millar MA, Byrne M (2013) Cryptic divergent lineages of Pultenaea pauciflora M.B.Scott (Fabaceae: Mirbelieae) exhibit different evolutionary history. Biological Journal of the Linnean Society. Linnean Society of London 108, 871–881.
| Cryptic divergent lineages of Pultenaea pauciflora M.B.Scott (Fabaceae: Mirbelieae) exhibit different evolutionary history.Crossref | GoogleScholarGoogle Scholar |
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution 32, 268–274.
| IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies.Crossref | GoogleScholarGoogle Scholar | 25371430PubMed |
Orthia LA, Crist MD, Cook LG, de Kok RPJ (2005a) Bush peas: a rapid radiation with no support for monophyly of Pultenaea (Fabaceae: Mirbelieae). Australian Systematic Botany 18, 133–147.
| Bush peas: a rapid radiation with no support for monophyly of Pultenaea (Fabaceae: Mirbelieae).Crossref | GoogleScholarGoogle Scholar |
Orthia LA, Cook LG, Crisp MD (2005b) Generic delimitation and phylogenetic uncertainty: an example from a group that has undergone an explosive radiation. Australian Systematic Botany 18, 41–47.
| Generic delimitation and phylogenetic uncertainty: an example from a group that has undergone an explosive radiation.Crossref | GoogleScholarGoogle Scholar |
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000) Association mapping in structured populations. American Journal of Human Genetics 67, 170–181.
| Association mapping in structured populations.Crossref | GoogleScholarGoogle Scholar | 10827107PubMed |
Reader FM (1905) Contributions to the Flora of Victoria. No. 14. Victorian Naturalist 22, 51
Rutherford S, Rossetto M, Bragg JG, McPherson H, Benson D, Bonser SP, Wilson PG (2018) Speciation in the presence of gene flow: population genomics of closely related and diverging Eucalyptus species. Heredity 121, 126–141.
| Speciation in the presence of gene flow: population genomics of closely related and diverging Eucalyptus species.Crossref | GoogleScholarGoogle Scholar | 29632325PubMed |
Sansaloni CP, Petroli CD, Carling J, Hudson CJ, Steane DA, Myburg AA, Grattapaglia D, Vaillancourt RE, Kilian A (2010) A high-density Diversity Arrays Technology (DArT) microarray for genome-wide genotyping in Eucalyptus. Plant Methods 6, 16
| A high-density Diversity Arrays Technology (DArT) microarray for genome-wide genotyping in Eucalyptus.Crossref | GoogleScholarGoogle Scholar | 20587069PubMed |
Wang J (2017) The computer program structure for assigning individuals to populations: easy to use but easier to misuse. Molecular Ecology Resources 17, 981–990.
| The computer program structure for assigning individuals to populations: easy to use but easier to misuse.Crossref | GoogleScholarGoogle Scholar | 28028941PubMed |
Weston PH (1991) Pultenaea. In ‘Flora of New South Wales, Vol. 2’. (Ed. GJ Harden) pp 481–497. (New South Wales University Press: Sydney, NSW, Australia)
Wiley EO (1978) The evolutionary species concept reconsidered. Systematic Biology 27, 17–26.




























